Abstract
Mutations in ATP13A2 (PARK9) are causally linked to the rare neurodegenerative disorders Kufor-Rakeb syndrome, hereditary spastic paraplegia and neuronal ceroid lipofuscinosis. This suggests that ATP13A2, a lysosomal cation-transporting ATPase, plays a crucial role in neuronal cells. The heterogeneity of the clinical spectrum of ATP13A2-associated disorders is not yet well understood and currently, these diseases remain without effective treatment. Interestingly, ATP13A2 is widely conserved among eukaryotes, and the yeast model for ATP13A2 deficiency was the first to indicate a role in heavy metal homeostasis, which was later confirmed in human cells. In this study, we show that the deletion of YPK9 (the yeast orthologue of ATP13A2) in Saccharomyces cerevisiae leads to growth impairment in the presence of Zn2+, Mn2+, Co2+ and Ni2+, with the strongest phenotype being observed in the presence of zinc. Using the ypk9Δ mutant, we developed a high-throughput growth rescue screen based on the Zn2+ sensitivity phenotype. Screening of two libraries of Food and Drug Administration-approved drugs identified 11 compounds that rescued growth. Subsequently, we generated a zebrafish model for ATP13A2 deficiency and found that both partial and complete loss of atp13a2 function led to increased sensitivity to Mn2+. Based on this phenotype, we confirmed two of the drugs found in the yeast screen to also exert a rescue effect in zebrafish—N-acetylcysteine, a potent antioxidant, and furaltadone, a nitrofuran antibiotic. This study further supports that combining the high-throughput screening capacity of yeast with rapid in vivo drug testing in zebrafish can represent an efficient drug repurposing strategy in the context of rare inherited disorders involving conserved genes. This work also deepens the understanding of the role of ATP13A2 in heavy metal detoxification and provides a new in vivo model for investigating ATP13A2 deficiency.
Keywords: ATP13A2, budding yeast, drug screening, heavy metals, zebrafish
ATP13A2 deficiency is causally linked to rare neurodegenerative disorders that remain without effective treatment. In this study, we have developed and combined high-throughput and in vivo phenotypic assays in yeast and zebrafish to screen for compounds with therapeutic potential in ATP13A2 deficiency. N-Acetylcysteine and furaltadone emerged as lead compounds for follow-up studies.
Graphical Abstract
Graphical Abstract.
Introduction
ATP13A2, also known as PARK9 or CLN12 (Ramirez et al., 2006; Bras et al., 2012), belongs to the poorly characterized P5-type ATPase family of transport proteins (van Veen et al., 2014). The ATP13A2 gene encodes a lysosomal and late endosomal transmembrane protein of 1180 amino acids involved in cation transport, whose precise molecular function remains unknown. ATP13A2 is widely expressed by many neuronal populations, and more particularly in the dopaminergic neurons of the substantia nigra (Schultheis et al., 2004; Ramirez et al., 2006). Recessive mutations in the ATP13A2 gene were initially described to cause Kufor-Rakeb syndrome (KRS; OMIM# 606693), an atypical juvenile Parkinsonism with dementia and progressive brain atrophy (Ramirez et al., 2006). However, with the advent of new sequencing technologies and bioinformatics tools, the genetic and clinical spectra of ATP13A2 deficiencies have been expanded. Namely, ATP13A2 mutations have been additionally causally associated with a juvenile form of neuronal ceroid lipofuscinosis (OMIM# 606693; Farias et al., 2011; Bras et al., 2012) and a complicated form of hereditary spastic paraplegia-78 (OMIM# 617225; Kara et al., 2016; Estrada-Cuzcano et al., 2017). Juvenile neuronal ceroid lipofuscinosis is classified as a fatal lysosomal storage disorder characterized by the accumulation of an autofluorescent hydrophobic material called lipofuscin (Palmer et al., 1992). In contrast, hereditary spastic paraplegia-78 is considered as an adult neurodegenerative disorder, in which a main clinical feature is the progressive spasticity of the lower limbs due to axonopathy of the corticospinal neurons (Estrada-Cuzcano et al., 2017). Even more recently, the phenotypic spectrum associated with variants in the ATP13A2 gene was further expanded to also include a juvenile-onset form of amyotrophic lateral sclerosis (Spataro et al., 2019).
Although these four neurodegenerative diseases differ substantially from a clinical point of view, the cellular phenotypes associated with ATP13A2 deficiency converge. Studies in fibroblasts derived from KRS and hereditary spastic paraplegia-78 patients showed similar lysosomal and mitochondrial dysfunction phenotypes (Grunewald et al., 2012; Usenovic et al., 2012b; Estrada-Cuzcano et al., 2017). Furthermore, animal models of juvenile neuronal ceroid lipofuscinosis and KRS both featured age-related motor dysfunction, protein aggregation and lipofuscinosis (Farias et al., 2011; Wohlke et al., 2011; Schultheis et al., 2013; Kett et al., 2015). Interestingly, the ATP13A2 p.(T512I) missense variant has been described in patients diagnosed with KRS and hereditary spastic paraplegia-78, suggesting that environmental factors can determine the development of the clinical phenotype (Dehay et al., 2012; Usenovic et al., 2012a; Estrada-Cuzcano et al., 2017).
In fact, ATP13A2 polymorphisms have been identified in one study as potential risk markers for neurotoxic effects of manganese in humans and two KRS patients have been reported so far with increased iron levels in the brain (Behrens et al., 2010; Schneider et al., 2010; Rentschler et al., 2012). These two metals are currently the major metals associated with secondary Parkinsonism and increasing evidence suggests that heavy metal exposure is involved in several neurodegenerative conditions (Grubman et al., 2014; Cicero et al., 2017). Interestingly, the deletion of YPK9 (yeast orthologue of ATP13A2) in Saccharomyces cerevisiae led to decreased resistance against heavy metal toxicity (Gitler et al., 2009; Schmidt et al., 2009). The link between ATP13A2 and heavy metal homeostasis has received further support by subsequent studies in various mammalian cell models (Tan et al., 2011; Kong et al., 2014; Park et al., 2014).
Despite the strong evidence for a role of ATP13A2 in heavy metal homeostasis, the precise function of ATP13A2 remains unclear and there is currently no effective treatment to cure or reverse the symptoms of any of the ATP13A2-associated disorders. Others have previously shown that a 2-step phenotypic drug-screening approach in a more simple followed by a more complex model organism can be a promising strategy to identify potential lead compounds for inherited human disorders (Cotticelli et al., 2012; Soma et al., 2018; Vincent et al., 2018). Similarly, we took advantage of the apparent functional conservation of ATP13A2 across species to develop a drug-screening pipeline combining yeast (S. cerevisiae) and zebrafish (Danio rerio) models of ATP13A2 deficiency. Rapid growth, low cultivation costs and good reproducibility make budding yeast an excellent eukaryotic cell model for performing high-throughput (HTP) primary drug screens. HTP phenotypic screens in yeast models have, for instance, revealed compounds protecting against types of proteotoxicity relevant for human neurodegenerative disorders (Tardiff et al., 2012, 2013; Vincent et al., 2018). In this study, we screened >2500 compounds, including many approved drugs, for their ability to rescue growth in yeast ypk9Δ strains in the presence of toxic levels of zinc. Eleven validated hits were further tested for their ability to rescue a decreased manganese resistance phenotype identified in atp13a2 deficient zebrafish larvae. As an outcome, N-acetylcysteine (NAC) and furaltadone (FTD), a nitrofuran antibiotic, emerged as compounds that actively protect against increased metal sensitivity induced by ATP13A2 deficiency, in both yeast and zebrafish models. More generally, our study further supports that coupling large-scale primary drug screening in yeast followed by a more focused validation screen in the whole-organism zebrafish model represents a powerful approach to discover lead compounds for inherited human disorders involving conserved disease genes.
Materials and methods
Yeast strains
ypk9Δ strains were generated in the BY4742 and FY4 backgrounds. The BY4742ypk9Δ strain was obtained by crossing the BY4741ypk9Δ strain from the gene deletion collection (EUROSCARF) with wild-type (WT) BY4742, followed by tetrad dissection to isolate the genotype of interest. The FY4ypk9Δ strain was created via PCR-mediated gene replacement by the kanMX4 cassette (Brachmann et al., 1998) in the FY4 parental strain, which was a kind gift from Joseph Schacherer. To generate rescue strains, the YPK9 gene was amplified from yeast genomic DNA and cloned into the pAG303GPD-ccdB plasmid using Gateway technology (Alberti et al., 2007). Both the recombinant and empty plasmids were integrated into the HIS3 locus of BY4742ypk9Δ cells and positive clones were selected on synthetic complete medium lacking histidine. To generate the ypk9Δpdr5Δ double mutant, the PDR5 gene was replaced by the URA3 marker in the BY4742ypk9Δ strain, followed by selection on synthetic complete medium lacking uracil. Transformations were performed using a standard polyethylene glycol/lithium acetate protocol as previously described (Gietz and Woods, 2002), and correct gene disruption or gene integration was verified by PCR. The strains, plasmids and primers used in this study are listed in Supplementary Tables 1–3.
Yeast cultivation media and growth conditions
Rich media (YPD) used for yeast transformation experiments contained 10 g/l yeast extract, 20 g/l peptone and 20 g/l glucose. For all other cultivations in this study (metal ion measurements, growth phenotyping and drug screening), yeast cells were grown in YNB (6.7 g/l yeast nitrogen base without amino acids, 5 g/l ammonium sulfate, pH 5) supplemented with 20 g/l glucose, 0.08 g/l histidine, uracil, methionine, lysine and 0.24 g/l leucine. When needed for selection, the media were supplemented with geneticin (200 µg/ml). Metal solutions were sterilized by filtration and added at indicated concentrations to YNB medium.
Growth phenotyping assay in yeast
Yeast growth phenotyping was performed as previously described (Jung et al., 2015). Growth rate, final biomass and lag time were analysed using the GATHODE software (Jung et al., 2015). The growth conditions tested in this study are listed in Supplementary Table 4.
Drug screening in yeast
Two fresh single colonies of the ypk9Δpdr5Δ strain were inoculated from solid YPD plates into 5 ml YNB media with specific supplementation (as described above) and incubated overnight with shaking (200 rpm) at 30°C. After 16 h, 400 µl of overnight culture was inoculated into 4 ml fresh media and cultivated until the optical density at 600 nm (OD600) reached about 0.5. Compound plates were prepared by dispensing 25 µl growth media containing 15 mM ZnCl2 into sterile 384-well microplates using an electronic 384-channel VIAFLO pipette (INTEGRA). 0.05 and 0.5 µl droplets of 10 mM drug stock solutions in dimethyl sulfoxide (DMSO) were added using an ECHO550 contactless acoustic nanolitre dispensing system (Labcyte). The first and last two columns of each test plate were reserved for various controls. Finally, 25 µl of cell suspension diluted to a starting OD600 of about 0.0125 in growth medium supplemented with 15 mM ZnCl2 were added to each well. Positive, negative, viability and contamination control wells contained 4.5 mM EDTA, 1% or 0.1% DMSO instead of drug solution, water instead of ZnCl2 and water instead of cell inoculate, respectively. Microplates were incubated at 30°C and OD600 measurements were recorded using a TECAN M200 plate reader just after the inoculation of the cells as well as 48 h and 96 h later. The screening was performed as a biological duplicate, meaning that each drug was tested twice in two independent experiments. The statistical methods applied to the drug-screening data for quality assessment and hit identification are described in the Supplementary material.
Metal ion measurements by inductively coupled plasma—mass spectrometry
Intracellular Zn2+ concentrations were measured by inductively coupled plasma—mass spectrometry (Agilent 7900) in samples prepared from three independent yeast cultivations as described in the Supplementary material.
Zinquin chelation assay
A solution of 20 µM Zinquin (Sigma-Aldrich) was mixed with 5, 10, 25 and 50 µM of selected drugs with or without 5 µM ZnCl2 in phosphate-buffered saline at pH 7.0. Mixtures were incubated for 1 h at 25°C and shaking at 300 rpm. The samples were then transferred into a 96-well plate for fluorescence measurements in a microplate reader, at an excitation wavelength of 370 nm and an emission wavelength of 490 nm. Three independent replicates were measured per condition tested. The fluorescence intensities of the ZnCl2-containing samples were background-corrected by subtracting the fluorescence intensity of the corresponding samples incubated in the absence of ZnCl2.
Zebrafish strains
The atp13a2sa18624+/− and atp13a2sa14250+/− zebrafish lines (TLF background, F3 generation) were obtained from the European Zebrafish Resource Center and kept in our aquatic facility according to standard protocols (Westerfield, 2000). These atp13a2 lines were further outcrossed with WT (AB) or nacre lines.
Zebrafish embryos were obtained by natural spawning and then incubated in the dark at 28°C, in Danieau’s solution [17 mM NaCl, 2 mM KCl, 0.12 mM MgSO4, 1.8 mM Ca(NO3)2, 1.5 mM HEPES pH 7.5 and 1.2 µM methylene blue]. If needed, to inhibit pigmentation, 24 h post-fertilization embryos were incubated in Danieau’s solution with 0.2 mM 1-phenyl-2-thiourea (PTU, Sigma). For live imaging, larvae were anaesthetized with buffered MS222 (0.016% w/v).
Ethics statement concerning zebrafish manipulations
The Aquatic Facility at the Luxembourg Centre for Systems Biomedicine is registered as an authorized breeder, supplier and user of zebrafish with Gran-Ducal decree of 20 January 2016. All practices involving zebrafish were performed in accordance with European laws, guidelines and policies for animal experimentation, housing and care (European Directive 2010/63/EU on the protection of animals used for scientific purposes). Authorization number LUPA 2017/04 allowed the performance of fin biopsies for genotyping purposes. Besides this, the present study did not involve any additional procedures within the meaning of Article 3 of Directive 2010/63/EU and as such it was not subjected to authorization by an ethics committee.
Genotyping of zebrafish lines
The atp13a2sa18624 and atp13a2sa14250 lines were fin-clipped and genotyped by PCR followed by AluI restriction analysis or sequencing, respectively. Detailed protocols and primer sequences are given in the Supplementary material and Supplementary Table 3.
Gene knockdown in zebrafish using morpholino antisense oligonucleotides
Translation and splice-blocking morpholinos (MOs) for atp13a2 and non-targeting control MOs were purchased from Gene Tools. Manual microinjections of MOs were performed as previously described (Rosen et al., 2009). Morpholino sequences and the microinjection procedure are provided in the Supplementary material.
RNA extraction and quantitative PCR to study the tissular distribution of zebrafish atp13a2
Gene expression was analysed by quantitative PCR (qPCR) using the LightCycler480 (Roche). For each tissue, qPCR reactions were run for three biological replicates and two technical replicates were performed for each qPCR reaction. Expression levels of atp13a2 in indicated tissues were calculated relative to the eye tissue using the 2−△△Ct method (Livak and Schmittgen, 2001). ef1α and rpl13α were used as reference genes for normalization. Tissular RNA extraction and cDNA synthesis protocols are detailed in the Supplementary material and the qPCR primer sequences are listed in Supplementary Table 3.
Heavy metal toxicity assay and drug screening in zebrafish
Two days post-fertilization (dpf) atp13a2−/− mutant embryos or atp13a2 knockdown morphants were incubated in a 48-well plate with Danieau’s solution containing varying concentrations of MnCl2. The Mn2+-containing solution was renewed after 24 h and the toxicity assessed at 5 dpf. Experiments were performed with 12 larvae per well in quadruplet (n = 48) for each tested condition. For the drug screening, a similar procedure was applied but with drugs or vehicle (DMSO or water) added at 3 dpf and here experiments were performed with 12 or 15 larvae per well in triplicate. For consistency in the phenotypic scoring, all the experiments were performed by a single person, but the final hit confirmation for NAC and FTD in zebrafish was performed in ‘single-blinded’ experiments, in which the experimentalist did not know which larvae received which treatment, to avoid biased results.
Statistical analysis
All data are presented as means ± standard deviations (SDs). The statistical tests used for data analysis are described in the corresponding figure legends. Two-way ANOVA analyses with the respective post hoc tests were performed using the GraphPad Prism software (version 8.2.0) and extended statistics for the ANOVA tests are provided in Supplementary Table 5. The likelihood ratios for the zebrafish experiments were calculated using RStudio (version 1.0.143).
Data availability
The authors confirm that the data supporting the findings of this study are nearly fully available within the article and its supplementary documents. Additional supporting data are available from the corresponding author upon request.
Results
Deletion of the ATP13A2 homologue in yeast triggers metal hypersensitivity
Under standard yeast cultivation conditions, ypk9Δ strains grew similarly to WT control strains. In order to identify a phenotype suitable for implementing an HTP drug screen under YPK9 deficiency, we assayed the growth of WT and ypk9Δ strains in >200 different conditions in liquid microcultivation (Supplementary Table 4). For a subset of the growth conditions, including different carbon sources, pH values, stressors, drugs and defined concentrations of various heavy metals, we tested WT and knockout strains in two different genetic backgrounds (BY4742 and FY4) and additionally a YPK9 rescue strain (YPK9OE) in the BY4742 background (Fig. 1A and Supplementary Table 4). In both backgrounds, YPK9 deficiency led to a slowed growth phenotype only in the presence of certain metals (Zn2+, Mn2+, Ni2+ and Co2+, but not Ca2+, Li+ and Cu2+) in the cultivation medium. Although we were not able to detect a previously reported growth defect of the ypk9Δ strain in the presence of Cd2+ (Schmidt et al., 2009), we found that overexpression of YPK9 enhanced growth under Cd2+exposure (Fig. 1A). Subsequently, high-density growth curves (allowing for specific growth rate and yield of biomass calculation) were determined in the presence of the divalent metals Zn2+, Mn2+, Ni2+ and Co2+ for the WT, ypk9Δ and YPK9OE strains (BY4742 background), at concentrations that we had previously shown to significantly impair the growth of ypk9Δ cells (Supplementary Fig. 1 A and Fig. 1B). Overexpression of YPK9 in the ypk9Δ strain rescued the growth defects induced by the metal ion exposures, supporting that these phenotypes were gene-specific (Fig. 1B). These results fully support that Ypk9, as previously suggested by others, is mainly involved in metal homeostasis (Gitler et al., 2009; Schmidt et al., 2009) and shed some light on the specificity of this effect.
Figure 1.
Large-scale phenotyping of ypk9Δ strains and validation of growth conditions for drug screening. (A) Heat map representing OD600 values measured 48 h after the inoculation of liquid microcultures in the indicated conditions with WT, ypk9Δ and YPK9 overexpression (YPK9OE) strains (BY4742 and/or FY4 background). The deletion of YPK9 resulted in growth defects in media containing Co2+, Mn2+, Zn2+ and Ni2+ (red arrows) and YPK9 overexpression rescued the growth defects (red and blue arrows). (B) Representative growth curves of WT, ypk9Δ and YPK9OE strains (BY4742 background) obtained in an independent experiment in the presence of Zn2+, Mn2+, Ni2+ and Co2+ at the indicated concentrations. (C) Biomass (at 48 h) and growth rates determined from the growth curves shown in B. (D) Growth curves of the same strains in minimal growth medium supplemented or not with 5 mM ZnCl2. Red boxes indicate the sampling points for metal ion analysis (1: early post-diauxic phase; 2: late stationary phase). (E) Intracellular Zn2+ concentrations measured by inductively coupled plasma—mass spectrometry in the indicated strains at the indicated growth phases. During the late stationary phase, WT and YPK9OE were able to significantly reduce the intracellular concentration compared to the early post-diauxic phase. In contrast, Δypk9 showed similar Zn levels, suggesting that Zn compartmentalization/externalization is affected in these cells. In C and E, values shown are means ± SDs of three biological replicates. Statistically significant differences between strains were determined using the two-way ANOVA test followed by Tukey’s multiple comparisons test (**P < 0.01, ***P < 0.001, ****P < 0.0001).
Zn2+, Ni2+ and Co2+ induced strong growth impairments in the ypk9Δ strain, as reflected in both the yield of biomass (60–80% lower compared with the WT strain) and in the growth rate (38–50% lower than WT; Fig. 1C). In contrast, Mn2+ decreased the growth rate of the ypk9Δ strain without significantly affecting the biomass. As Zn2+ is physiologically more relevant than Ni2+ and Co2+, we were interested to test the effect of YPK9 deficiency on intracellular Zn2+ pools. In the absence of supplemented zinc, the WT, ypk9Δ and YPK9OE strains showed similar intracellular Zn2+ concentrations (≈80–90 µg/g dry weight) at the two measurement time points (early post-diauxic and stationary phase; Fig. 1D and E). Supplementation of 5 mM ZnCl2 increased the intracellular Zn2+ concentrations about 100 times (≈7000–8000 µg/g dry weight). Interestingly, WT cells, but not ypk9Δ cells, were able to reduce the intracellular Zn2+ levels by about 2-fold; this decrease was even more drastic (14-fold) when YPK9 was overexpressed (Fig. 1E). These results suggest that YPK9 is not essential for cell proliferation in the presence of subtoxic levels of Zn2+, but that it plays an important role in the stress response induced by overexposure to this and probably some other metals in post-mitotic and/or aged cells. The observation that YPK9 overexpression led to intracellular Zn2+ levels that are lower than in the WT strain is consistent with the growth assays under divalent metal overexposure, where YPK9 overexpression enhanced growth rate and yield of biomass beyond the WT parameters (Fig. 1B and C), indicating that basal YPK9 expression levels do not provide optimal protection against metal ion stress.
High-throughput compound screen in an ATP13A2-deficient yeast model
HTP drug screening in the yeast model was performed in the 384-well microplate format where cells were cultivated in the presence of 15 mM ZnCl2 at 30°C, with OD600 measurements being taken just after inoculation as well as 48 h and 96 h later. To increase the probability of positive hits in the presence of lower drug concentrations, we additionally deleted the PDR5 gene in our ypk9Δ strain. PDR5 encodes the most pleiotropic drug export pump in budding yeast and its deletion has been shown to increase the susceptibility to externally added compounds (Rogers et al., 2001). We confirmed that PDR5 deletion did not cause any additional growth impairment in standard conditions or in the presence of ZnCl2, be it in the WT or in the ypk9Δ background (Supplementary Fig. 1B). For assay quality assessment, the Zʹ-factor was calculated for each tested plate based on negative and positive control wells containing DMSO and EDTA, respectively. EDTA restores WT growth by extracellular zinc chelation (Supplementary Fig. 1C) and, given the absence of any currently known drug to treat ATP13A2 deficiencies, served as a reference point for hit identification. In addition, cell vitality was tested in control wells containing medium devoid of Zn2+ and each plate also contained microbial contamination control wells (Fig. 2A).
Figure 2.
Drug screening of the Prestwick and Tocris chemical libraries in a yeast ATP13A2 deficiency model. (A) Pipeline for drug screening in 384-well plate format. Positive controls (4.5 mM EDTA, n = 16) and negative controls (0.1 and 1% DMSO, n = 16) were alternated in the first and second columns of each plate. The last two columns of each plate were reserved for 16 vitality (no metal) and 16 contamination (no cell inoculation) control wells. Drugs (n = 2553) were tested at 10 and 100 μM in biological duplicates. OD600 was measured 48 and 96 h after inoculation and standard statistical analysis for HTP drug-screening data was performed for each time point in order to determine the indicated parameters. (B) Z-scores for the screening plates with acceptable Zʹ-factor. From both libraries, 24 drugs were identified as positive hits (Z-score > cut-off value). Of those, 10 drugs were positive at 10 μM, 21 drugs at 100 μM and 14 were FDA-approved drugs. (C) From the 24 hits, 11 were validated in independent dose-response assays. Data shown are means ± SDs for three biological replicates. Statistically significant differences between treated and non-treated ypk9Δ cells were determined by the two-way ANOVA test followed by Dunnet’s multiple comparison post hoc test. Except when otherwise indicated (ns = not significant), the drug concentrations tested had significant effects (P < 0.01). WT, pdr5Δ strain; KO, ypk9Δpdr5Δ strain.
To identify lead compounds with potential therapeutic value for ATP13A2 deficiencies, the Prestwick and Tocris chemical libraries, which contain 1280 and 1273 compounds, respectively (of which 815 and 160 correspond to FDA/EMA-approved drugs), were screened using the yeast growth rescue assay. In total, 117 compounds are contained in both libraries and were thus tested twice. In total, 2553 compounds (of which 2436 unique compounds) were tested in duplicate and at two different concentrations (10 and 100 µM). In total, 24 compounds were identified as positive hits in both replicates (Fig. 2B and Supplementary Fig. 2 and Table 6). Of these, 17 compounds tested positive at one concentration only and seven compounds were positive at both concentrations. Of the 24 hit compounds, 22 compounds were re-tested (only three of the five cephalosporin antibiotic hits were further tested so far) and 11 could be validated by showing a reproducible protective effect against Zn2+-induced toxicity in a dose-response assay in the ypk9Δpdr5Δ strain, resulting in a validated hit rate of 0.5% (Fig. 2C). These 11 compounds can be grouped into three categories: (i) non-FDA-approved drugs (VU152100, VU10010, ML-365 and AMG-9810), (ii) antibiotics (moxalactam, cefmetazole, cefazolin and FTD) and (iii) direct or indirect chelators [NAC, deferoxamine and tetraethylenepentamine (TEPA)].
As seen in Fig. 2C, all validated drugs slightly alleviated the Zn2+-induced stress in WT cells, at least at the lower concentrations tested. In contrast, a stronger and dose-dependent positive effect was observed in YPK9 deficient cells. With the non-FDA-/EMA-approved compounds, virtually full rescue effects were reached at relatively low concentrations. These drugs precipitated at higher concentrations (>300 µM) and were, therefore, not tested at higher doses. Moreover, foam formation in microcultivations containing AMG9810 interfered with the OD measurements, leading to high variations in the results obtained with this compound. The remaining (FDA-approved) drugs were more soluble in water and were tested at higher concentrations without toxic effect, except for two antibiotics (FTD and cefazolin) that impaired growth of both WT and YPK9 deficient cells at the higher doses tested. Within the antibiotic group, containing cephalosporin antibiotics from different generations, cefmetazole exerted the strongest rescue effect. In the chelator group, a nearly full rescue effect was reached with NAC and TEPA, whereas only a moderate effect was observed with the lower concentrations tested for deferoxamine.
Chelation activity of the yeast phenotypic hit compounds
The discrepancy between the concentrations at which the yeast phenotypic hit compounds at least started to exert rescue effects (10–100 µM) and the ZnCl2 concentration present in the cultivation medium (15 mM) for the drug-screening and dose-response assays suggested that the main mechanism of action of the hit compounds was not extracellular metal ion chelation. To consolidate this assumption, a fluorometric chelation assay using zinquin was performed to assess the chelation activity of the hit compounds. Zinquin forms a fluorescent complex with Zn2+ ions and the complex is UV-excitable. As positive controls, we used EDTA and clioquinol, both well-known Zn2+ chelators. The strong chelator EDTA completely sequestered the Zn2+ (20 µM) bound to zinquin already at the lowest concentration tested (10 µM), whereas clioquinol showed an increasing chelation activity between 5 and 50 µM. Except for VU152100 and VU10010 (which interfered with the zinquin chelation assay, due to their strong inherent fluorescence), the chelation activity of all the validated yeast phenotypic hit compounds was assessed in this same concentration range (Fig. 3A). Only TEPA, an industrial iron chelator, showed a relatively strong Zn2+ chelation activity, comparable with clioquinol at the highest concentration tested. From the eight remaining compounds, only the nitrofuran antibiotic FTD displayed also a dose-dependent, but much more moderate chelation activity (Fig. 3A). These results thus strongly suggest that our positive hits, except perhaps for TEPA, did not simply rescue the zinc sensitivity phenotype by ‘sweeping up’ the zinc added extracellularly to challenge our yeast model, but rather acted intracellularly to compensate more or less fully for the ATP13A2 deficiency.
Figure 3.
Chelation activity of validated yeast phenotypic rescue compounds. (A) Chelation activity was measured based on the Zn2+-selective fluorescent probe zinquin. Background corrected fluorescence intensities (excitation at 370 nm and emission at 490 nm) measured at the indicated drug concentrations are shown. H2O and DMSO were used as negative controls, EDTA and clioquinol as positive controls. Fluorescence intensities are negatively correlated with Zn2+ chelation activity. Data shown are means ± SDs for three independent replicates. AU, arbitrary units.
atp13a2 loss-of-function leads to decreased resistance against manganese toxicity in zebrafish
As ATP13A2-related disorders will predictably respond mostly to drugs which are able to reach their target site within the central nervous system, we established an atp13a2 deficient zebrafish model to further validate our positive hit compounds in vivo. As the ATP13A2 protein, which shares 50% amino acid sequence identity between human and zebrafish, has been studied only very little in the latter model (Lopes da Fonseca et al., 2013; Spataro et al., 2019), we first analysed the tissue distribution of the atp13a2 transcript in adult fish using qPCR. atp13a2 showed the highest expression in both female and male animals in the gonads, the brain and the eyes (Supplementary Fig. 3), further supporting the potential relevance of zebrafish in ATP13A2-linked disease modelling. It should be noted that rpl13α is more stably expressed across tissues in both males and females than ef1α ; therefore, we believe that the relative gene expression results based on the rpl13α reference gene are more reliable (McCurley and Callard, 2008; Xu et al., 2016).
Zebrafish lines carrying atp13a2 mutant alleles (atp13a2sa14250 or atp13a2sa18624) generated by N-ethyl-N-nitrosourea mutagenesis (Howe et al., 2013) were obtained from the European Zebrafish Resource Center. Each allele carries a single non-sense mutation (2153 T > A or 2457 T > G) resulting in a premature stop codon in exon 20 or exon 22, respectively (Fig. 4A). Both mutations can be predicted to cause a loss-of-function, as they lead to the elimination of important sequences of the P-domain, responsible for the binding of the Mg2+-ATP complex via Mg2+ coordination, as well of the transmembrane domains M5-M10 (Sorensen et al., 2010; Fig. 4A). The point mutation in the atp13a2sa18624 allele creates a new AluI restriction site, allowing to easily follow its transmission through generations by genotyping (Fig. 4B). We, therefore, focused on the atp13a2sa18624+/- line, which was backcrossed to a WT line to reduce unspecific mutations generated by N-ethyl-N-nitrosourea mutagenesis. Heterozygotes of the F5 generation were mated and their offspring (n = 37) were genotyped after 2 months; 35% of the progeny was found to be homozygous for the mutation, showing that atp13a2 deletion in zebrafish is not lethal during development (Fig. 4B). Furthermore, homozygous mutants were able to reach adulthood without any obvious morphological or behavioural abnormalities.
Figure 4.
Genotyping and phenotyping of atp13a2−/− zebrafish mutants. (A) Schematic illustration of the zebrafish atp13a2 gene structure. Exons are represented as numbered boxes and homologous positions of human disease-associated mutations that are close to mutations present in the zebrafish mutant lines used in this study are indicated (in black, non-sense mutations in zebrafish mutant lines; in blue, KRS-associated mutations; in green, NCL-associated mutation). In red, important domains for the ATP13A2 function are also indicated. (B) atp13a2sa18624−/+ heterozygous carriers were crossed and the offspring was raised to adulthood. AluI restriction analysis showed that 35% of the F6 population were homozygous mutants. Genomic DNA sequencing confirmed the expected mutations in heterozygous and homozygous zebrafish. (C) 2 dpf atp13a2sa18624+/+, atp13a2sa18624−/+ and atp13a2sa18624−/− larvae were incubated without or with 2 and 3 mM MnCl2 for 3 days. All 5 dpf larvae incubated in the presence of MnCl2 lacked the swimming bladder (moderate phenotype), but homozygous atp13a2sa18624−/− larvae, in addition, showed increased occurrence of pericardial oedemas, spine curvature, darkening of the brain tissue (strong phenotype; see arrows) and lethality. Data shown are means ± SDs for 60 larvae incubated and phenotyped in four separate wells (15 larvae per well). A likelihood ratio test was applied to verify the statistical significance of the phenotypic differences between WT (and heterozygous) larvae and homozygous atp13a2 larvae (****P < 0.0001). (D) A TUNEL assay was used to detect apoptotic cells in 5 dpf larvae exposed or not to MnCl2 as described under B. (E) Quantification of the larvae with extensive apoptotic areas in the CNS (indicated with a dotted circle and red arrowheads in D) as based on the TUNEL assay.
As ATP13A2 seems to play an important role in heavy metal homeostasis (this study; Tan et al., 2011; Park et al., 2014), we tested the effect of manganese on atp13a2sa18624+/+, atp13a2sa18624−/+and atp13a2sa18624−/− larvae. Exposure to MnCl2 has previously been described to cause neurodegeneration in zebrafish, and in humans, it leads to manganism, a disorder featuring symptoms that resemble those of Parkinson’s diseases (Bakthavatsalam et al., 2014; Roth, 2014). In this study, we exposed zebrafish larvae to 2 and 3 mM MnCl2 and compared the morphological abnormalities at 5 dpf. In the presence of Mn2+, WT and heterozygous larvae showed a moderate phenotype characterized mainly by an underdeveloped swimming bladder. In contrast, homozygous atp13a2 mutants were highly sensitive to Mn2+ and displayed multiple abnormalities, including pericardial oedemas, movement loss and spine curvature in addition to the underdevelopment of the swimming bladder (Fig. 4C and Supplementary Fig. 4). The homozygous mutant larvae also frequently featured darkening of the brain region, suggesting that this organ might be particularly affected by Mn2+ exposure in the absence of functional ATP13A2. Similar results were obtained in the second mutant line (atp13a2sa14250) upon exposure to Mn2+ (Supplementary Fig. 5). To uncover possible reasons for the darkening of the brain in mutant atp13a2sa18624−/− larvae, a TUNEL assay was performed in 5 dpf larvae exposed or not to Mn2+. Strikingly, 64% of the homozygous atp13a2 mutants showed large apoptotic areas throughout the central nervous system (brain and dorsal spine) after exposure to Mn2+, a phenotype that did not develop in the great majority of the WT control larvae in that same condition (Fig. 4D and E). As for the yeast ypk9Δ model, these observations show that atp13a2 deficiency renders zebrafish larvae much more sensitive to manganese exposure, with the interesting additional information from the zebrafish model that this sensitivity seems to be particularly pronounced in nerve cells in vertebrates.
Given the strong zinc sensitivity phenotype observed in the yeast model, we also tested the effect of zinc exposure on zebrafish larvae. At lower concentrations of Zn2+ (up to 0.3 mM), we could not observe any dysmorphology or changes in locomotor behaviour in either control or homozygous atp13a2 mutant larvae (data not shown). However, at 1 mM ZnCl2, we observed higher lethality and strong tissue damage (often starting in the caudal region) in both the control and mutant larvae (Supplementary Fig. 6). As it was very difficult to detect significant differences between control and atp13a2 mutant larvae in this condition, we decided to focus on the manganese sensitivity phenotype of the atp13a2 mutant zebrafish model for the rest of this study.
atp13a2 zebrafish morphants phenocopy atp13a2 knockout mutants
In a previous study, transient downregulation of atp13a2 by microinjection of an exon1-intron1 splice-blocking morpholino antisense oligonucleotide (e1i1-MO) was reported to be lethal in zebrafish larvae (Lopes da Fonseca et al., 2013). This suggested that compensatory mechanisms (not induced in the atp13a2 knockdown larvae) may explain the absence of an overt phenotype (under control conditions) of our atp13a2 knockout mutants. Another explanation for the apparently contradictory results could be a gene-independent toxicity of the e1i1-MO used previously and we started testing this MO by injection into our atp13a2sa18624−/− and atp13a2sa14250−/− mutant embryos. At 3 dpf, all embryos were dysmorphic and showed locomotor impairments. In contrast, homozygous mutants injected with a control morpholino did not display any abnormality, indeed suggesting a possible atp13a2 independent effect elicited by the e1i1-MO (Supplementary Fig. 7). Therefore, for validation of the Mn2+ phenotype in atp13a2 morphants (as opposed to mutants), we designed a translation (ATG-MO) and a different splice-blocking (e2i2-MO) morpholino to inhibit the expression and processing of newly synthesized atp13a2 mRNA, respectively (Supplementary Fig. 8A). No obvious phenotypes were observed up to 5 dpf upon injection of 8 ng of either morpholino into WT embryos (Supplementary Fig. 8B). The lack of antibodies against the zebrafish Atp13a2 protein prevented the verification of decreased Atp13a2 levels in the morphants generated by injection of the ATG-MO. However, the efficiency of our splice-blocking morpholino was confirmed at 3 dpf by qPCR and sequencing of cDNA from these morphants provided evidence for aberrant atp13a2 splicing leading to an early stop codon (Supplementary Fig. 8A).
Subsequently, we incubated atp13a2 morphant larvae (generated with ATG-MO and e2i2-MO) in the presence of 1 and 3 mM MnCl2 and analysed them morphologically at 5 dpf. atp13a2 morphants were substantially more sensitive to Mn2+ than the control larvae (Supplementary Fig. 8C) and surviving larvae displayed similar phenotypes to those previously described for the atp13a2 mutant lines (pericardial oedemas, darkening of areas in the brain, spine curvature; Supplementary Fig. 8D). Actually, atp13a2 morphants showed even higher sensitivity to Mn2+ compared with the mutant larvae as exposure to 1 mM Mn2+ had a similar impact on the morphants as exposure to 3 mM Mn2+ on the mutants. Morphants were generated from the commonly used AB zebrafish strain whereas the mutant lines had been created in the Tüpfel long fin strain. A different genetic background may, therefore, at least partially explain that a partial gene knockdown led to a stronger phenotype than a null mutation. Overall, the results obtained in the atp13a2 morphants (created with our newly designed MOs) and atp13a2 knockout models were, however, in good agreement and our observations suggest that functional ATP13A2 protects multicellular organisms against manganese toxicity, but that it is not essential for zebrafish development under standard conditions.
Validation of yeast phenotypic hit compounds in atp13a2 knockout zebrafish
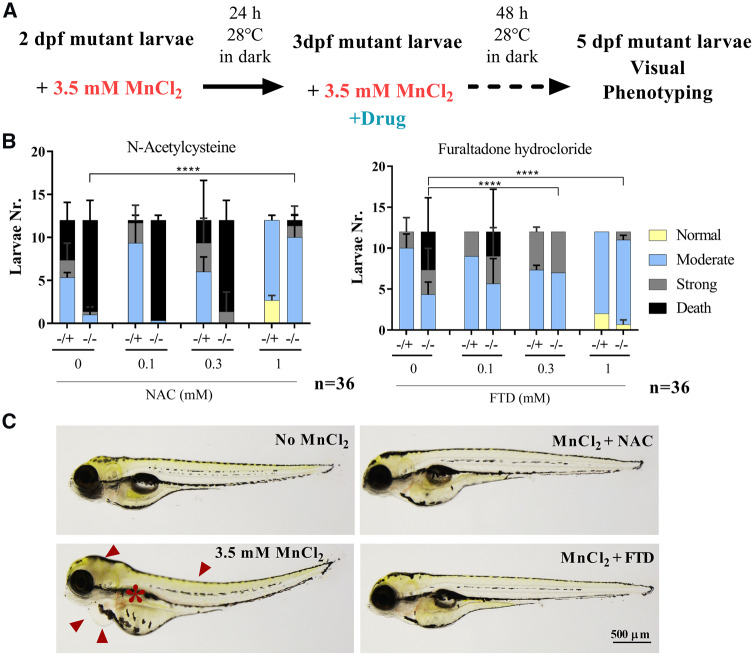
To further validate the hits obtained in the primary yeast screen in a more complex organism, we proceeded to their in vivo testing in our atp13a2 zebrafish mutant line (except for the industrial chelator TEPA). For each compound class, the maximum tolerated concentration was first determined in WT embryos in the absence of Mn2+ and sublethal drug concentrations were then tested for their phenotypic rescue potential (Supplementary Fig. 9A). atp13a2sa18624−/+ and atp13a2sa18624−/− larvae at 2 dpf were incubated in medium containing 3.5 mM MnCl2, followed 24 h later by an exchange for medium containing different concentrations of the drugs (0.1–1000 µM) in addition to MnCl2. Two of the 10 hit compounds tested (NAC and FTD) protected dose-dependently against Mn2+ toxicity, with an apparently more pronounced effect in mutant than in control larvae (5 dpf). Considering, for instance, the highest drug concentration tested (1 mM), >80% of the atp13a2 mutant larvae presented merely a moderate phenotype (absence of the swimming bladder) whereas >65% of these larvae either died or presented severe morphological abnormalities in the absence of drug (Fig. 5B and C). NAC and FTD also protected against Mn2+ toxicity in the WT larvae, but their relative effect was less pronounced in these animals given a weaker phenotypic impact of Mn2+ in the absence of the drug. The remaining drugs tested failed to rescue the phenotype induced by manganese exposure, be it in the WT or mutant larvae (Supplementary Fig. 9B).
Figure 5.
Secondary drug screening in atp13a2sa18624−/− zebrafish larvae. (A) Schematic representation of the drug-screening methodology. Two dpf larvae were incubated with 3.5 mM MnCl2. After 24 h, media was replaced by new media containing 0.1, 0.3 and 1 mM of each tested drug in addition to MnCl2. At 5 dpf, the phenotype was visually scored for each animal. (B) Of the 10 drugs tested in zebrafish larvae, NAC and FTD showed a protective effect against Mn2+ toxicity. Data shown are means ± SDs for 36 larvae incubated and phenotyped in three separate wells (12 larvae per well). A likelihood ratio test was applied to verify the statistical significance of the phenotypic differences observed between homozygous atp13a2 larvae treated or not with the drug hit compounds (****P < 0.0001). (C) Representative images of atp13a2sa18624−/− larvae at 5 dpf. In the absence of MnCl2, mutant larvae showed a normal development, whereas treatment with 3.5 mM MnCl2 led to a severe phenotype featuring pericardial oedemas, spine curvature and darkening of the brain region (arrowheads) in addition to the absence of the swimming bladder (*). This phenotype was clearly ameliorated by treatment with 1 mM NAC or FTD.
Discussion
ATP13A2 and heavy metal stress response in budding yeast and zebrafish
In this study, of around 200 growth conditions tested for ATP13A2 deficient yeast models in two different genetic backgrounds, only the presence of Mn2+, Co2+, Zn2+ and Ni2+ led to significant growth impairments in both ypk9Δ strains. We also found that, under Zn2+-repletion conditions, post-mitotic ypk9Δ cells contained considerably higher concentrations of this ion than WT cells. Accordingly, human cells derived from the olfactory mucosa (hONs) of ATP13A2−/− patients showed decreased viability in the presence of Zn2+ (Kong et al., 2014; Park et al., 2014). Also, patient cells showed higher intracellular Zn2+ levels than control cells only when challenged with ZnCl2. Interestingly, vesicular zinc levels were, however, lower in the ATP13A2 deficient cells (Kong et al., 2014; Park et al., 2014). Together with our data, all studies indicate that ATP13A2 is critically involved in zinc compartmentalization or externalization under conditions where storage compartments (vacuole/lysosomes) become overloaded. Further supporting the involvement of ATP13A2 in metal detoxification, we observed that deletion or transient knockdown of atp13a2 in zebrafish caused increased sensitivity to MnCl2 exposure, resulting in pericardial oedemas, spine curvature and higher mortality. As in the yeast model, we could only observe an obvious phenotype in zebrafish when we challenged the model with relatively high metal ion concentrations.
The main goal of this study was to perform a drug screen for ATP13A2 deficiencies, calling for acute exposure protocols yielding relevant phenotypes in a short time frame to allow for HTP screening. We did not evaluate long-term exposure to heavy metals, but considering that ATP13A2 deficiencies are linked to juvenile and adult rare neurodegenerative disorders, it might be that a slight dysregulation of heavy metal homeostasis leads to gradual accumulation and/or mis-localization of those metals over the years until the onset of disease symptoms. Heavy metals have been found to be part of lipofuscins, a hallmark of NCLs, as well as of abnormal aggregates containing mainly α-synuclein in the brain of Parkinson’s disease patients (Terman and Brunk, 1998; Gardner et al., 2017). The fact that these features have also been observed in ATP13A2-deficient patients and animal models, combined with ample evidence for the role of ATP13A2 in heavy metal homeostasis, suggest that heavy metals may play a pivotal role in the pathology of ATP13A2-associated disorders. Notably, the clinical heterogeneity observed in ATP13A2 patients may result from varying interactions between environmental metal exposures and genetic factors (Rentschler et al., 2012).
Primary and secondary drug screen for ATP13A2 deficiencies
In the present study, we developed and combined two phenotypic drug-screening assays, using budding yeast and zebrafish to identify drugs that could be used to treat ATP13A2-associated disorders. Currently, 32 pathogenic mutations have been described for ATP13A2 and most of these mutations appear to cause protein instability or functional disruption (Ramirez et al., 2006; Podhajska et al., 2012; Estrada-Cuzcano et al., 2017). We, therefore, opted for complete loss-of-function models, seeking to identify drugs with therapeutic potential for most of these disorders.
As the mechanism of action of ATP13A2 in heavy metal detoxification seems to be well-conserved (Gitler et al., 2009; Schmidt et al., 2009; Tan et al., 2011; Kong et al., 2014; Park et al., 2014), we used yeast as a HTP screening platform for chemicals that alleviate Zn2+-induced stress in YPK9 deleted cells. We focused the screen on libraries containing in large part FDA- and EMA-approved drugs and tested in total 2553 chemical compounds. From the primary screening, 11 hits were validated in a concentration-response assay. For further validation of the hits, an in vivo disease model in zebrafish was established using atp13a2 mutant larvae exposed to Mn2+. In addition to this in vivo evaluation of compound efficacy, zebrafish larvae allow to concomitantly address drug safety and drug delivery through the blood-brain barrier, which is a major challenge in drug discovery for neurodegenerative disorders (Eliceiri et al., 2011; Kanungo et al., 2014; Zeng et al., 2017).
Of the 11 hits identified in the yeast-based primary screen, NAC and FTD were validated as being able to alleviate Mn2+ toxicity in the zebrafish-based secondary screen. NAC was initially prescribed for the treatment of paracetamol-induced hepatoxicity by restoring hepatic concentrations of glutathione and also as a mucolytic agent (Bavarsad Shahripour et al., 2014). However, in the last year, several studies have reported a neuroprotective function for NAC by increasing the glutathione levels (Bavarsad Shahripour et al., 2014). Furthermore, glutathione deficiency has long been implicated in the development of Parkinson’s disease (Perry et al., 1982; Bjorklund et al., 2018). In fact, one ongoing clinical trial using NAC has been reported for Parkinson’s disease (Monti et al., 2015) and NAC treatment also seems to be beneficial for Alzheimer’s disease where it showed positive effects on some secondary outcome measures (cognitive tests; Adair et al., 2001). Interestingly, independently to this study, it has been reported that NAC mitigates Zn2+- and Fe2+-induced toxicity in ATP13A2−/− hONs and CHO cells (Park et al., 2014; Rinaldi et al., 2015). The positive effects of NAC in three different disease models for ATP13A2 deficiency, in addition to the described beneficial effects in Parkinson’s and Alzheimer’s disease, suggest that its nutritional supplementation could be of therapeutic value in ATP13A2-associated disorders. Interestingly, another mucolytic agent, Ambroxol, has emerged recently as a drug with beneficial effects in Parkinson’s disease and Gaucher’s disease. Not only does Ambroxol seem to be a potent chaperone for glucocerebrosidase, the enzyme deficient in Gaucher patients, but it has been shown to promote exocytosis, as well as lysosomal and mitochondrial biogenesis (Magalhaes et al., 2018). It remains to be explored whether the beneficial effects of NAC in neurodegenerative disorders may be mediated at least in part through similar properties.
In contrast, little is known about FTD, a nitrofuran antibiotic primarily used for bacterial infections in poultry (Nouws et al., 1987). Currently, nitrofurans are prohibited in the EU for livestock production due to their potential carcinogenicity. However, nitrofuran antibiotics can still be used in humans for the treatment of infections of the urinary tract and the skin as well as bacterial diarrhoea (Guay, 2008; Vasheghani et al., 2008). Interestingly, although five members of this family of antibiotics were present in one of the libraries screened in this study, only FTD was identified as a hit. In comparison to other nitrofurans, FTD has an additional morpholinomethyl modification, which is retained in FTD-derived metabolites (AMOZ; Supplementary Fig. 10; Vass et al., 2008). In the zinquin chelation assay, FTD exhibited a slight chelation activity at high concentrations, suggesting that intracellular metal ion chelation by FTD and/or one or several of its biotransformation product(s) could be a mechanism of action. Although the use of FTD is currently controversial, our study suggests that this compound possesses some favourable features in the context of heavy metal exposure, more particularly in combination with ATP13A2 deficiency, and that it could be used for chemical lead optimization.
Chelating agents have been proposed as effective secondary antioxidants by stabilizing the oxidative form of heavy metals and thereby decreasing metal ion induced oxidative stress that may, for instance, cause cell death (Turan et al., 2016). Moreover, KRS belongs to the group of neurodegenerative disorders with brain iron accumulation and several ATP13A2 deficiency models, including the yeast and zebrafish models used here, strongly implicate this protein in the compartmentalization and externalization of heavy metals. Taken together, these findings suggest that chelation therapy could be beneficial for the treatment of ATP13A2-associated disorders. Some clinical studies already support the therapeutic use of chelators for other neurodegenerative disorders, such as Friedreich’s ataxia (Boddaert et al., 2007; Dusek et al., 2016; Martin-Bastida et al., 2017). In this disease, the decrease of iron–sulphur clusters and haem formation cause iron accumulation in mitochondria, and administration of deferiprone to these patients over a 6-month period reduced iron content, resulting in improved clinical symptoms (Boddaert et al., 2007). However, larger clinical trials and studies are still needed, as chelation therapy remains controversial; important metals needed for normal cellular function could be unspecifically chelated, resulting in non-desired side effects (Nunez and Chana-Cuevas, 2018).
Most drugs identified in the yeast model failed to rescue the phenotype in the zebrafish model. Possible reasons for this could be: (i) a low penetration through the blood-brain barrier, (ii) a yeast-specific mechanism of action and/or (iii) a Zn2+-specific effect. Indeed, cephalosporin antibiotics are known to have a generally poor blood-brain barrier penetration (Lutsar and Friedland, 2000). Our finding that all cephalosporin antibiotics (belonging to the class of β-lactam antibiotics) contained in the Prestwick library were identified as hits in the yeast-based primary screen is nevertheless noteworthy. It has been demonstrated that β-lactams confer neuroprotection by decreasing synaptic glutamate levels through the increase of glutamate uptake in the cells. This effect was shown to be beneficial in an SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis (Rothstein et al., 2005), a disease that has also been recently linked to ATP13A2-deficiency (Spataro et al., 2019). In the mouse model, ceftriaxone (a third-generation β-lactam not contained in the chemical libraries screened in this study) treatment delayed the loss of muscle strength and body weight. In addition, β-lactams are also known as metal chelators such as Zn2+, Cu2+, Co2+, Ni2+ (Mukherjee and Ghosh, 1995), although they did not show a high affinity for Zn2+ using our zinquin assay.
Other interesting hits were VU152100 and VU10010, two selective activators of the M4 muscarinic acetylcholine receptor, which were able to rescue the yeast phenotype in the micromolar range. M4 receptors are G protein-coupled receptors that play an important role in regulating midbrain dopaminergic activity; they are attractive entry points for the treatment of disorders involving altered dopaminergic function in the basal ganglia, including Parkinson’s disease (Brady et al., 2008). However, the yeast genome does not encode any protein with appreciable homology to the human M4 receptor. Similarly, two additional non-approved drugs identified as hits in the yeast screen, AMG9810 and ML365, bind to proteins (vanilloid TRPV1 receptor and TASK-1 channel, respectively; Gavva et al., 2005; Zou et al., 2010) that are not conserved in yeast. Understanding the mechanism by which these drugs alleviate the Zn2+ toxicity in the yeast ypk9Δ mutant thus requires further investigation.
The cellular protectant properties of the hits identified during our primary HTP screen demonstrate the strength of yeast for the identification of chemical suppressors of ATP13A2-associated disease phenotypes. However, in vivo secondary screening is clearly important for validating the bioactivity of the ‘primary’ hits in the context of a multicellular organism. In this study, we utilized the advantages of yeast and zebrafish ATP13A2 deficiency models, combining these two bioassay systems to create an integrated screening platform for drug repurposing. Ultimately, this platform might accelerate both the repurposing of existing drugs, as well as the discovery of new lead compounds for the treatment of neurodegenerative disorders associated with the dysregulation of heavy metal homeostasis. As a next step, both NAC and FTD, as well as some of the other hits with activity only in the yeast model, should be tested in other in vivo models of ATP13A2 deficiency, such as mouse or dog (Farias et al., 2011; Schultheis et al., 2013).
Supplementary Material
Acknowledgements
We thank the Clinical & Experimental Neuroscience group of LCSB and Semra Smajic for their help with performing automated and HTP procedures during the primary drug screening. The automated screening platform was funded through a PEARL grant of the Luxembourg National Research Fund (FNR) to Rejko Krüger. In addition, we want to thank Enrico Glaab and Todor Kondic for their help with statistical analyses, and Ibrahim Boussaad and Gerald Cruciani for their critical comments throughout the development of the project. We thank Cédric Guignard and Johanna Ziebel (LIST) for performing the inductively coupled plasma—mass spectrometry metal measurements in yeast cells. Finally, we are also grateful to the LCSB fish caretaker team for their valuable daily work.
Glossary
Abbreviations
- DMSO =
dimethyl sulfoxide
- dpf =
days post-fertilization
- EDTA =
ethylenediaminetetraacetic acid
- EMA =
European Medicines Agency
- FDA =
Food and Drug Administration
- FTD =
furaltadone
- HTP =
high-throughput
- KRS =
Kufor-Rakeb syndrome
- MO =
morpholino antisense oligonucleotide
- NAC =
N-acetylcysteine
- OMIM =
Online Mendelian Inheritance in Man
- PDR5 =
pleiotropic drug resistance 5
- qPCR =
quantitative PCR
- TEPA =
tetraethylenepentamine
Funding
This work was supported in part by donations from the ATOZ foundation, Mr. Norbert Becker and by a Pelican award from the Fondation du Pélican de Mie et Pierre Hippert-Faber to UHM. The inductively coupled plasma—mass spectrometry measurements were partially financed by a 2018 Biomedicine award to UHM of the Doctoral School in Systems and Molecular Biomedicine at the University of Luxembourg.
Competing interests
The authors report no competing interests.
References
- Adair JC, Knoefel JE, Morgan N.. Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology 2001; 57: 1515–7. [DOI] [PubMed] [Google Scholar]
- Alberti S, Gitler AD, Lindquist S.. A suite of Gateway cloning vectors for high-throughput genetic analysis in Saccharomyces cerevisiae. Yeast 2007; 24: 913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakthavatsalam S, Das Sharma S, Sonawane M, Thirumalai V, Datta A.. A zebrafish model of manganism reveals reversible and treatable symptoms that are independent of neurotoxicity. Dis Model Mech 2014; 7: 1239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavarsad Shahripour R, Harrigan MR, Alexandrov AV.. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav 2014; 4: 108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MI, Bruggemann N, Chana P, Venegas P, Kagi M, Parrao T, et al. Clinical spectrum of Kufor-Rakeb syndrome in the Chilean kindred with ATP13A2 mutations. Mov Disord 2010; 25: 1929–37. [DOI] [PubMed] [Google Scholar]
- Bjorklund G, Stejskal V, Urbina MA, Dadar M, Chirumbolo S, Mutter J.. Metals and Parkinson’s disease: mechanisms and biochemical processes. Curr Med Chem 2018; 25: 2198–214. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Le Quan Sang KH, Rotig A, Leroy-Willig A, Gallet S, Brunelle F, et al. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood 2007; 110: 401–8. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD.. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998; 14: 115–32. [DOI] [PubMed] [Google Scholar]
- Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther 2008; 327: 941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras J, Verloes A, Schneider SA, Mole SE, Guerreiro RJ.. Mutation of the parkinsonism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum Mol Genet 2012; 21: 2646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero CE, Mostile G, Vasta R, Rapisarda V, Signorelli SS, Ferrante M, et al. Metals and neurodegenerative diseases. A systematic review. Environ Res 2017; 159: 82–94. [DOI] [PubMed] [Google Scholar]
- Cotticelli MG, Rasmussen L, Kushner NL, McKellip S, Sosa MI, Manouvakhova A, et al. Primary and secondary drug screening assays for Friedreich ataxia. J Biomol Screen 2012; 17: 303–13. [DOI] [PubMed] [Google Scholar]
- Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron MH, Doudnikoff E, et al. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci USA 2012; 109: 9611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek P, Schneider SA, Aaseth J.. Iron chelation in the treatment of neurodegenerative diseases. J Trace Elem Med Biol 2016; 38: 81–92. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Gonzalez AM, Baird A.. Zebrafish model of the blood-brain barrier: morphological and permeability studies. Methods Mol Biol 2011; 686: 371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Cuzcano A, Martin S, Chamova T, Synofzik M, Timmann D, Holemans T, et al. Loss-of-function mutations in the ATP13A2/PARK9 gene cause complicated hereditary spastic paraplegia (SPG78). Brain 2017; 140: 287–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias FH, Zeng R, Johnson GS, Wininger FA, Taylor JF, Schnabel RD, et al. A truncating mutation in ATP13A2 is responsible for adult-onset neuronal ceroid lipofuscinosis in Tibetan terriers. Neurobiol Dis 2011; 42: 468–74. [DOI] [PubMed] [Google Scholar]
- Gardner B, Dieriks BV, Cameron S, Mendis LHS, Turner C, Faull RLM, et al. Metal concentrations and distributions in the human olfactory bulb in Parkinson’s disease. Sci Rep 2017; 7: 10454.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 2005; 313: 474–84. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA.. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 2002; 350: 87–96. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet 2009; 41: 308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman A, Pollari E, Duncan C, Caragounis A, Blom T, Volitakis I, et al. Deregulation of biometal homeostasis: the missing link for neuronal ceroid lipofuscinoses? Metallomics 2014; 6: 932–43. [DOI] [PubMed] [Google Scholar]
- Grunewald A, Arns B, Seibler P, Rakovic A, Munchau A, Ramirez A, et al. ATP13A2 mutations impair mitochondrial function in fibroblasts from patients with Kufor-Rakeb syndrome. Neurobiol Aging 2012; 33: 1843.e1–7. [DOI] [PubMed] [Google Scholar]
- Guay DR. Contemporary management of uncomplicated urinary tract infections. Drugs 2008; 68: 1169–205. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013; 496: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung PP, Christian N, Kay DP, Skupin A, Linster CL.. Protocols and programs for high-throughput growth and aging phenotyping in yeast. PLoS One 2015; 10: e0119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanungo J, Cuevas E, Ali SF, Paule MG.. Zebrafish model in drug safety assessment. Curr Pharm Des 2014; 20: 5416–29. [DOI] [PubMed] [Google Scholar]
- Kara E, Tucci A, Manzoni C, Lynch DS, Elpidorou M, Bettencourt C, et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain 2016; 139: 1904–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kett LR, Stiller B, Bernath MM, Tasset I, Blesa J, Jackson-Lewis V, et al. alpha-Synuclein-independent histopathological and motor deficits in mice lacking the endolysosomal Parkinsonism protein Atp13a2. J Neurosci 2015; 35: 5724–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum Mol Genet 2014; 23: 2816–33. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- Lopes da Fonseca T, Correia A, Hasselaar W, van der Linde HC, Willemsen R, Outeiro TF.. The zebrafish homologue of Parkinson’s disease ATP13A2 is essential for embryonic survival. Brain Res Bull 2013; 90: 118–26. [DOI] [PubMed] [Google Scholar]
- Lutsar I, Friedland IR.. Pharmacokinetics and pharmacodynamics of cephalosporins in cerebrospinal fluid. Clin Pharmacokinet 2000; 39: 335–43. [DOI] [PubMed] [Google Scholar]
- Magalhaes J, Gegg ME, Migdalska-Richards A, Schapira AH.. Effects of ambroxol on the autophagy-lysosome pathway and mitochondria in primary cortical neurons. Sci Rep 2018; 8: 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bastida A, Ward RJ, Newbould R, Piccini P, Sharp D, Kabba C, et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci Rep 2017; 7: 1398.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley AT, Callard GV.. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol Biol 2008; 9: 102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti DA, Newberg AB, Kremens D, 2015. Physiological Effects of Nutritional Support in Patients with Parkinson’s Disease. Clinicaltrialsgov. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02445651 (10 April 2019, date last accessed).
- Mukherjee G, Ghosh T.. Metal ion interaction with penicillins–Part VII: mixed-ligand complex formation of cobalt(II), nickel(II), copper(II), and Zinc(II) with ampicillin and nucleic bases. J Inorg Biochem 1995; 59: 827–33. [DOI] [PubMed] [Google Scholar]
- Nouws JF, Vree TB, Aerts MM, Degen M, Driessens F.. Some pharmacokinetic data about furaltadone and nitrofurazone administered orally to preruminant calves. Vet Q 1987; 9: 208–14. [DOI] [PubMed] [Google Scholar]
- Nunez MT, Chana-Cuevas P.. New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals (Basel) 2018; 11: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DN, Fearnley IM, Walker JE, Hall NA, Lake BD, Wolfe LS, et al. Mitochondrial ATP synthase subunit c storage in the ceroid-lipofuscinoses (Batten disease). Am J Med Genet 1992; 42: 561–7. [DOI] [PubMed] [Google Scholar]
- Park JS, Koentjoro B, Veivers D, Mackay-Sim A, Sue CM.. Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum Mol Genet 2014; 23: 2802–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TL, Godin DV, Hansen S.. Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci Lett 1982; 33: 305–10. [DOI] [PubMed] [Google Scholar]
- Podhajska A, Musso A, Trancikova A, Stafa K, Moser R, Sonnay S, et al. Common pathogenic effects of missense mutations in the P-type ATPase ATP13A2 (PARK9) associated with early-onset parkinsonism. PLoS One 2012; 7: e39942.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 2006; 38: 1184–91. [DOI] [PubMed] [Google Scholar]
- Rentschler G, Covolo L, Haddad AA, Lucchini RG, Zoni S, Broberg K.. ATP13A2 (PARK9) polymorphisms influence the neurotoxic effects of manganese. Neurotoxicology 2012; 33: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi DE, Corradi GR, Cuesta LM, Adamo HP, de Tezanos Pinto F.. The Parkinson-associated human P5B-ATPase ATP13A2 protects against the iron-induced cytotoxicity. Biochim Biophys Acta 2015; 1848: 1646–55. [DOI] [PubMed] [Google Scholar]
- Rogers B, Decottignies A, Kolaczkowski M, Carvajal E, Balzi E, Goffeau A.. The pleitropic drug ABC transporters from Saccharomyces cerevisiae. J Mol Microbiol Biotechnol 2001; 3: 207–14. [PubMed] [Google Scholar]
- Rosen JN, Sweeney MF, Mably JD.. Microinjection of zebrafish embryos to analyze gene function. J Vis Exp 2009; (25): e1115. doi: 10.3791/1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA. Correlation between the biochemical pathways altered by mutated Parkinson-related genes and chronic exposure to manganese. Neurotoxicology 2014; 44: 314–25. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005; 433: 73–7. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Wolfe DM, Stiller B, Pearce DA.. Cd2+, Mn2+, Ni2+ and Se2+ toxicity to Saccharomyces cerevisiae lacking YPK9p the orthologue of human ATP13A2. Biochem Biophys Res Commun 2009; 383: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SA, Paisan-Ruiz C, Quinn NP, Lees AJ, Houlden H, Hardy J, et al. ATP13A2 mutations (PARK9) cause neurodegeneration with brain iron accumulation. Mov Disord 2010; 25: 979–84. [DOI] [PubMed] [Google Scholar]
- Schultheis PJ, Fleming SM, Clippinger AK, Lewis J, Tsunemi T, Giasson B, et al. Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited alpha-synuclein accumulation and age-dependent sensorimotor deficits. Hum Mol Genet 2013; 22: 2067–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis PJ, Hagen TT, O’Toole KK, Tachibana A, Burke CR, McGill DL, et al. Characterization of the P5 subfamily of P-type transport ATPases in mice. Biochem Biophys Res Commun 2004; 323: 731–8. [DOI] [PubMed] [Google Scholar]
- Soma S, Latimer AJ, Chun H, Vicary AC, Timbalia SA, Boulet A, et al. Elesclomol restores mitochondrial function in genetic models of copper deficiency. Proc Natl Acad Sci USA 2018; 115: 8161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen DM, Buch-Pedersen MJ, Palmgren MG.. Structural divergence between the two subgroups of P5 ATPases. Biochim Biophys Acta 2010; 1797: 846–55. [DOI] [PubMed] [Google Scholar]
- Spataro R, Kousi M, Farhan SMK, Willer JR, Ross JP, Dion PA, et al. Mutations in ATP13A2 (PARK9) are associated with an amyotrophic lateral sclerosis-like phenotype, implicating this locus in further phenotypic expansion. Hum Genomics 2019; 13: 19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Zhang T, Jiang L, Chi J, Hu D, Pan Q, et al. Regulation of intracellular manganese homeostasis by Kufor-Rakeb syndrome-associated ATP13A2 protein. J Biol Chem 2011; 286: 29654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff DF, Jui NT, Khurana V, Tambe MA, Thompson ML, Chung CY, et al. Yeast reveal a “druggable” Rsp5/Nedd4 network that ameliorates alpha-synuclein toxicity in neurons. Science 2013; 342: 979–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff DF, Tucci ML, Caldwell KA, Caldwell GA, Lindquist S.. Different 8-hydroxyquinolines protect models of TDP-43 protein, alpha-synuclein, and polyglutamine proteotoxicity through distinct mechanisms. J Biol Chem 2012; 287: 4107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A, Brunk UT.. Lipofuscin: mechanisms of formation and increase with age. APMIS 1998; 106: 265–76. [DOI] [PubMed] [Google Scholar]
- Turan B, Şendil K, Şengül E, Gültekin MS, Taslimi P, Gulçin İ, et al. The synthesis of some beta-lactams and investigation of their metal-chelating activity, carbonic anhydrase and acetylcholinesterase inhibition profiles. J Enzyme Inhib Med Chem 2016; 31: 79–88. [DOI] [PubMed] [Google Scholar]
- Usenovic M, Knight AL, Ray A, Wong V, Brown KR, Caldwell GA, et al. Identification of novel ATP13A2 interactors and their role in alpha-synuclein misfolding and toxicity. Hum Mol Genet 2012; 21: 3785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D.. Deficiency of ATP13A2 leads to lysosomal dysfunction, alpha-synuclein accumulation, and neurotoxicity. J Neurosci 2012; 32: 4240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen S, Sorensen DM, Holemans T, Holen HW, Palmgren MG, Vangheluwe P.. Cellular function and pathological role of ATP13A2 and related P-type transport ATPases in Parkinson’s disease and other neurological disorders. Front Mol Neurosci 2014; 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasheghani MM, Bayat M, Rezaei F, Bayat A, Karimipour M.. Effect of low-level laser therapy on mast cells in second-degree burns in rats. Photomed Laser Surg 2008; 26: 1–5. [DOI] [PubMed] [Google Scholar]
- Vass M, Hruska K, Franek M.. Nitrofuran antibiotics: a review on the application, prohibition and residual analysis. Vet Med-Czech 2008; 53: 469–500. [Google Scholar]
- Vincent BM, Tardiff DF, Piotrowski JS, Aron R, Lucas MC, Chung CY, et al. Inhibiting stearoyl-CoA desaturase ameliorates alpha-synuclein cytotoxicity. Cell Rep 2018; 25: 2742–54.e31. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). 4th edn Eugene: University of Oregon Press; 2000. [Google Scholar]
- Wohlke A, Philipp U, Bock P, Beineke A, Lichtner P, Meitinger T, et al. A one base pair deletion in the canine ATP13A2 gene causes exon skipping and late-onset neuronal ceroid lipofuscinosis in the Tibetan terrier. PLoS Genet 2011; 7: e1002304.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Li C, Zeng Q, Agrawal I, Zhu X, Gong Z.. Genome-wide identification of suitable zebrafish Danio rerio reference genes for normalization of gene expression data by RT-qPCR. J Fish Biol 2016; 88: 2095–110. [DOI] [PubMed] [Google Scholar]
- Zeng A, Ye T, Cao D, Huang X, Yang Y, Chen X, et al. Identify a blood-brain barrier penetrating drug-TNB using zebrafish orthotopic glioblastoma xenograft model. Sci Rep 2017; 7: 14372.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou B, Flaherty DP, Simpson DS, Maki BE, Miller MR, Shi J, et al. ML365: development of bis-amides as selective inhibitors of the KCNK3/TASK1 two pore potassium channel In: Probe reports from the NIH molecular libraries program [Internet]. Bethesda, MD: National Center for Biotechnology Information (US); 2010. Available from: https://www.ncbi.nlm.nih.gov/books/NBK179826/ [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are nearly fully available within the article and its supplementary documents. Additional supporting data are available from the corresponding author upon request.