Abstract
Parkinson’s disease is prototypically a movement disorder. Although perceptual and motor functions are highly interdependent, much less is known about perceptual deficits in Parkinson’s disease, which are less observable by nature, and might go unnoticed if not tested directly. It is therefore imperative to seek and identify these, to fully understand the challenges facing patients with Parkinson’s disease. Also, perceptual deficits may be related to motor symptoms. Posture, gait and balance, affected in Parkinson’s disease, rely on veridical perception of one’s own motion (self-motion) in space. Yet it is not known whether self-motion perception is impaired in Parkinson’s disease. Using a well-established multisensory paradigm of heading discrimination (that has not been previously applied to Parkinson’s disease), we tested unisensory visual and vestibular self-motion perception, as well as multisensory integration of visual and vestibular cues, in 19 Parkinson’s disease, 23 healthy age-matched and 20 healthy young-adult participants. After experiencing vestibular (on a motion platform), visual (optic flow) or multisensory (combined visual–vestibular) self-motion stimuli at various headings, participants reported whether their perceived heading was to the right or left of straight ahead. Parkinson’s disease participants and age-matched controls were tested twice (Parkinson’s disease participants on and off medication). Parkinson’s disease participants demonstrated significantly impaired visual self-motion perception compared with age-matched controls on both visits, irrespective of medication status. Young controls performed slightly (but not significantly) better than age-matched controls and significantly better than the Parkinson’s disease group. The visual self-motion perception impairment in Parkinson’s disease correlated significantly with clinical disease severity. By contrast, vestibular performance was unimpaired in Parkinson’s disease. Remarkably, despite impaired visual self-motion perception, Parkinson’s disease participants significantly overweighted the visual cues during multisensory (visual–vestibular ) integration (compared with Bayesian predictions of optimal integration) and significantly more than controls. These findings indicate that self-motion perception in Parkinson’s disease is affected by impaired visual cues and by suboptimal visual–vestibular integration (overweighting of visual cues). Notably, vestibular self-motion perception was unimpaired. Thus, visual self-motion perception is specifically impaired in early-stage Parkinson’s disease. This can impact Parkinson’s disease diagnosis and subtyping. Overweighting of visual cues could reflect a general multisensory integration deficit in Parkinson’s disease, or specific overestimation of visual cue reliability. Finally, impaired self-motion perception in Parkinson’s disease may contribute to impaired balance and gait control. Future investigation into this connection might open up new avenues of alternative therapies to better treat these difficult symptoms.
Keywords: Parkinson’s disease, Bayesian, multisensory integration, self-motion perception
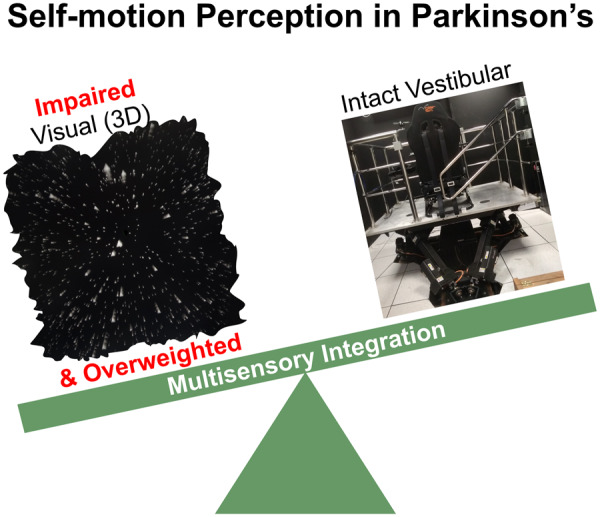
Motor functions, affected in Parkinson’s disease, rely on the ability to perceive one’s own motion in space. Here, we found impaired visual perception of self-motion in Parkinson’s disease, and visual overweighting during visual–vestibular integration. This may contribute to impaired motor control and can aid Parkinson’s disease diagnosis or subtyping.
Graphical Abstract
Graphical Abstract.
Introduction
Parkinson’s disease is primarily characterized by a decline in motor function, marked by the cardinal features of bradykinesia, akinesia, rigidity, tremor and postural instability (Parkinson, 1817; Jankovic, 2008; Postuma et al., 2015). Although James Parkinson already noted many of the non-motor aspects of Parkinson’s disease in his original seminal description (Parkinson, 1817), these are only recently receiving more substantial attention (Chaudhuri et al., 2006; Patel et al., 2014). In contrast to motor symptoms, non-motor symptoms (such as perceptual deficits) are by nature less observable, under-reported and may go unnoticed if not actively and directly tested (Shulman et al., 2002; Chaudhuri et al., 2010; Bonnet et al., 2012).
Veridical perception of one’s orientation and self-motion in space is fundamental for motor control, especially gait and balance (Dichgans and Brandt, 1978; Paillard, 1991; Horak and Macpherson, 1996; Lackner and DiZio, 2005; Cullen, 2012). Self-motion perception relies primarily on vestibular and visual (optic flow) cues (Dichgans and Brandt, 1978; Warren and Hannon, 1988; Fushiki et al., 2005; Gu et al., 2007; Fetsch et al., 2009, 2010; Butler et al., 2010; Zaidel et al., 2015), as well as other somatosensory cues, such as proprioception (Probst et al., 1985; Hlavačka et al., 1992; Mergner et al., 1993; Hlavacka et al., 1996; Mergner and Rosemeier, 1998; Schweigart et al., 2002; Durgin et al., 2005). Furthermore, these unisensory cues need to be integrated to form a unified and reliable percept of self-motion (Angelaki et al., 2009; Butler et al., 2010). It is currently not known: (i) whether vestibular/visual perception of self-motion is impaired in Parkinson’s disease or (ii) whether multisensory integration is affected in Parkinson’s disease (Halperin et al., 2020). In this study, we directly tested unisensory vestibular and unisensory visual performance, as well as multisensory integration of visual and vestibular cues for self-motion perception in Parkinson’s disease.
Vestibular deficits in Parkinson’s disease are a matter of debate. Reichert et al. (1982) demonstrated altered nystagmus responses in Parkinson’s disease to caloric stimulation, but this could reflect sensory-motor integration deficits, rather than vestibular deficits per se. Lithgow and Shoushtarian (2015) also report altered vestibular responses in Parkinson’s disease, but the technique used in that study (electrovestibulography, which measures vestibular responses from the ear canal) is esoteric and somewhat controversial (Brown et al., 2017). By contrast, Bertolini et al. (2015) did not find vestibular sensory impairment but rather deficits that likely reflect the faulty integration of vestibular signals in the brain. Thus, there is little evidence to support vestibular dysfunction in Parkinson’s disease. However, this requires further validation (see Smith, 2018 for a review).
Patients with Parkinson’s disease demonstrate altered navigational veering in response to visual self-motion (optic flow) stimuli (Davidsdottir et al., 2008; Lin et al., 2014), as well as reduced activation in visuomotor brain areas (van der Hoorn et al., 2014). While these studies imply visual deficits of self-motion perception, they do not isolate whether there is a specific perceptual (versus sensorimotor) deficit, because veering is a graded motor response. Hence, the need to perform an experiment that specifically tests visual self-motion perception.
A different type of visual motion stimulus, which is not designed to elicit a percept of self-motion, uses random dot kinematograms. Random dot kinematograms comprise dots moving in two-dimensions, presented through an aperture on a flat screen in front of the observer, to test the coherent perception of visual motion in the environment. Parkinson’s disease performance in random dot kinematogram experiments did not differ from controls (Putcha et al., 2014; Jaywant et al., 2016). However, Putcha et al. (2014) did find an association between increased discrimination thresholds and disease severity. There are also many other visual impairments described in Parkinson’s disease, including delays in visual evoked responses, abnormalities in contrast, spatiotemporal and colour sensitivity and altered perception of visual orientation (Bodis and Yahr, 1978; Bodis-Wollner et al., 1987; Montse et al., 2001; Gullett et al., 2013; Weil et al., 2016, 2017, 2018). Thus, visual motion perception in Parkinson’s disease requires further investigation.
Intriguingly, despite these abovementioned visual perception deficits, patients with Parkinson’s disease seem to be functionally more dependent on vision (Cooke et al., 1978; Bronstein et al., 1990; Azulay et al., 1999, 2002; Almeida and Lebold, 2010; Cowie et al., 2010). Thus, in contrast to healthy humans (Jacobs, 1999; Landy and Kojima, 2001; Ernst and Banks, 2002; Alais and Burr, 2004; Fetsch et al., 2009; Raposo et al., 2012) and animals (Gu et al., 2008; Raposo et al., 2012) and even other clinical populations (tested in autism, Zaidel et al., 2015), that largely follow Bayesian predictions of multisensory integration, we hypothesized that patients with Parkinson’s disease may have a specific (and perhaps unique) multisensory integration impairment, with visual overweighting. Here, we tested this hypothesis directly, within the Bayesian framework of multisensory integration.
Using a multisensory motion simulator, we tested visual, vestibular and combined (visual–vestibular ) perception of self-motion in patients with Parkinson’s disease and controls. To avoid confounding effects of motor dysfunction, participants performed a binary two-alternative forced choice psychophysics task. Specifically, we used a well-established task known as ‘heading discrimination’ (Gu et al., 2007; Fetsch et al., 2009; Angelaki et al., 2011; Zaidel et al., 2011, 2013) that has not been previously tested in Parkinson’s disease. In this task, participants experience linear self-motion stimuli (primarily in a forward direction, with slight deviations to the right or left) and are required to discriminate whether their perceived heading was to the left or to the right of straight ahead. Vestibular stimuli comprise linear translations of the motion platform, visual stimuli simulate linear translations of self-motion using optic flow and combined stimuli present the two together. This heading discrimination task tests linear (not rotational) self-motion perception.
Testing the same task (heading discrimination) with the same categorical responses for visual or vestibular cues, and in the same participants, allowed us to isolate specific visual and/or vestibular perceptual (dys)function. Strikingly, we found impaired visual self-motion perception in Parkinson’s disease, whereas vestibular performance was unimpaired. Finally, by introducing a small discrepancy between the visual and vestibular cues (in the combined condition), we tested multisensory integration and found that patients with Parkinson’s disease overweighted the visual cues (under-weighting vestibular) despite the visual impairment, exposing suboptimal multisensory integration.
Materials and methods
Participants and procedures
We tested 20 patients with (early-stage) idiopathic Parkinson’s disease, recruited through the Movement Disorders Institute at Sheba Medical Center, 24 age-matched controls (recruited from the general public, spouses of the Parkinson’s disease participants and staff at Bar Ilan University) and 21 young-adult controls (recruited from the student body at Bar Ilan University). One participant from each group was excluded due to inadequate task performance as evidenced by close to arbitrary heading choices. This resulted in 19 Parkinson’s disease, 23 age-matched and 20 young-adult participants for further analysis. This study was approved by the Internal Review Boards of Bar Ilan University and Sheba Medical Center. All participants signed informed consent prior to partaking in the study and received compensation for participation. Exclusion criteria for recruitment to the study included: neurological or psychiatric conditions (apart from Parkinson’s disease), inability to walk independently or to climb stairs safely unassisted, poor corrected vision, deafness, dementia or vestibular dysfunction. Cognitive function was assessed in all participants using the Montreal Cognitive Assessment test (Nasreddine et al., 2005).
Individual participant details of the Parkinson’s disease, age-matched and young-adult groups are presented in Supplementary Tables 1–3, respectively (and each group’s data are summarized in Table 1). The Parkinson’s disease and age-matched groups did not differ significantly in age (t(40) = −0.08, P = 0.93), gender (χ2(1) = 0.04, P = 0.82) or cognitive function (Montreal Cognitive Assessment scores; t(40) = −1.56, P = 0.12). Disease severity in Parkinson’s disease participants was measured according to the motor part of the Unified Parkinson’s Disease Rating Scale (UPDRS) and is reported in Supplementary Table 1.
Table 1.
Summarized participants’ details
| Group | Age (years) | Gender (% male) | MoCA | TUG1 (s) | TUG2 (s) |
|---|---|---|---|---|---|
| Parkinson’s disease | 64.4 ± 8.0 | 68 | 25.8 ± 3.1 | 14.9 ± 4.9 | 14.0 ± 2.7 |
| Age-matched control | 62.7 ± 6.9 | 65 | 24.5 ± 2.3 | 12.6 ± 2.3 | 12.7 ± 3.4 |
| Young control | 23.9 ± 3.0 | 50 | 27.5 ± 1.5 | 13.0 ± 3.5 |
Age, MoCA and TUG scores presented as mean ± SD.
MoCA = Montreal Cognitive Assessment (range 0–30, higher scores reflect better cognition); TUG1 = timed up and go, Visit 1; TUG2 = timed up and go, Visit 2.
Parkinson’s disease participants and age-matched controls performed the same experiment twice (on two separate visits, 1 week apart). The primary aim of this was to test for a Parkinson’s disease medication effect. Although it would have been preferable to randomly counterbalance on and off medication states across the two visits, many patients with Parkinson’s disease requested to first experience and perform the experiment in the ‘on-state’. Hence, for the sake of patient comfort and consistency, we tested all patients with Parkinson’s disease first in the ‘on-state’ and, a week later, in the ‘off-state’ (at least 12 h since their last anti-Parkinson medication dose). Accordingly, for better comparison, the age-matched control participants also performed the same experiment twice. Young controls performed the experiment only once (the primary aim of testing young controls was to establish a baseline and to provide perspective for better understanding the Parkinson’s disease versus age-matched group results). All Parkinson’s disease participants and all but one age-matched participants returned for the second visit.
Gait function was assessed with a ‘timed up and go’ (TUG) test using a smartphone application (Madhushri et al., 2016). Individual TUG results are presented in the respective group tables (Supplementary Tables 1–3) and summarized per group in Table 1. All participants performed the TUG test twice in succession, prior to the psychophysics task on each visit (TUG scores reflect the average, per visit). Parkinson’s disease participants performed the TUG test more slowly than age-matched controls (14.9 ± 4.9 versus 12.6 ± 2.4 s on the first visit and 14.0 ± 2.7 versus 12.7 ± 3.4 s on the second visit, F(1,75) = 5.6, P = 0.02), with no significant effect of visit or visit–group interaction (consistent with the early stage of Parkinson’s disease in our cohort). We therefore did not find TUG results useful for further analyses (we used UPDRS scores to correlate task performance with disease severity).
Stimuli and task
The experiments were run in the motion simulator at the Gonda Brain Research Center, Bar Ilan University. Vestibular (inertial motion) stimuli were generated using a six-degrees-of-freedom motion platform (MB-E-6DOF/12/1000KG; Moog Inc.), upon which a car seat was mounted (Fig. 1A). Participants were seated comfortably in the car seat and restrained safely with a four-point seatbelt. Although additional somatosensory or proprioceptive cues may also be used during inertial motion (e.g. cutaneous sensation and muscle proprioception), we refer to this condition as ‘vestibular’ because performance strongly depends on intact vestibular labyrinths (Gu et al., 2007). In addition, even if considered collectively a vestibular–somatosensory–proprioceptive (non-visual) cue, the same rules of cue integration still apply when testing integration with visual cues.
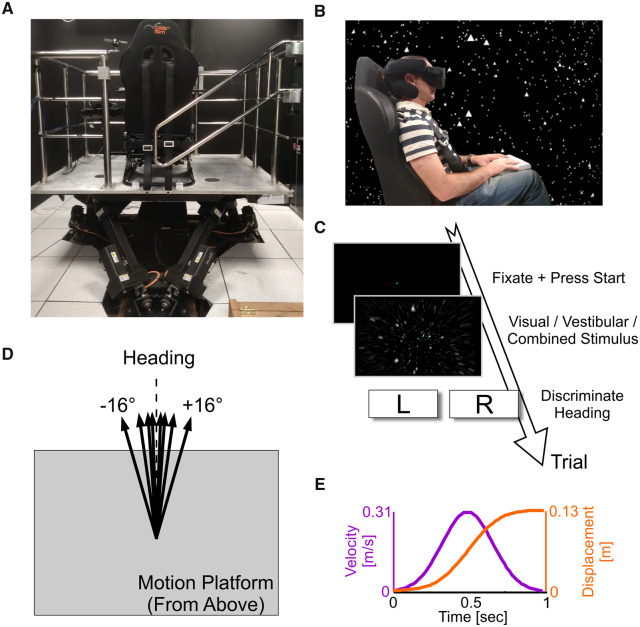
Figure 1.
Experimental set-up.
(A) The six-degrees-of-freedom motion platform, with mounted chair (viewed from behind). (B) A participant sitting in the chair (viewed from the side) wearing a head-mounted display (Oculus Rift) for visual optic flow stimuli. The background stars depict what the participant sees in virtual reality (shown here for illustrative purposes only). (C) Flow of a single trial: after trial initiation, a 1-s (visual, vestibular or combined visual–vestibular) motion stimulus is experienced, after which the participant reports his/her heading discrimination (left or right). (D) Schematic representation of various heading directions. (E) Motion profile (the same for all stimuli; only heading direction was varied).
The visual stimulus simulated self-motion through a three-dimensional cloud of ‘stars’ that was 130-cm wide, 130-cm tall and 100-cm deep and centred at 66 cm in front of the participant (before motion). Star density was 0.00125/cm3, with each star being a 0.5 cm × 0.5 cm white triangle. The visual stimulus was generated using OpenGL and presented via a virtual reality head-mounted display (Oculus Rift CV1; Fig. 1B) with a field of view that spanned 88° horizontally and 90° vertically. A clipping plane was set at 5 cm in front of the eyes to prevent stars from getting too close/large. The star field in the background of Fig. 1B reflects the visual experience of the participant in virtual reality (schematically).
Participants with corrected vision either wore their own glasses (or contact lenses) under the head-mounted display, or we inserted prescription lenses to match the participant’s prescription, from a set made specifically for the Oculus Rift (VR Lens Lab). The participant’s head was supported by a head support with lateral extensions to limit head movement (Black Bear; Matrix Seating Ltd.). Participants wore the head-mounted display, which covered their field of vision, throughout the experiment, and the room was kept dark to avoid any other visual cues. An intercom system enabled the participants and the experimenter to communicate throughout the duration of the experiment.
The (vestibular and visual) self-motion stimuli followed a linear path trajectory (0.13 m displacement) in the horizontal plane. These were primarily in the forward direction, but with slight deviations to the right or left of straight ahead (Fig. 1D). Stimulus velocity followed a Gaussian profile (peak velocity 0.31 m/s and peak acceleration 1.14 m/s2) and lasted 1 s (Fig. 1E). A single-interval stimulus was presented on each trial, which was either unisensory vestibular (inertial motion in darkness), unisensory visual (optic flow), or multisensory visual–vestibular (inertial motion with simultaneous optic flow). A central fixation point was displayed via the head-mounted display on all trials, rendered at a fixed distance of 66 cm straight in front of the participant. It remained at this location (in relation to the participant) also during the motion stimuli. Participants were instructed to maintain fixation on this point throughout each trial.
Participants held a control box (Cedrus RB-540) that rested on their lap. They initiated trials at their own pace by pressing the centre (start) button and reported their perceived heading direction, after the stimulus ended, by pressing the right or left button on the response box. Three different auditory tones were used to indicate: (i) that the system was ready for a new trial (i.e. to press start), (ii) that a choice was registered and (iii) a response time-out (2 s after the end of the stimulus, if a choice was not registered). Participants were instructed to avoid this time-out by making a timely response on every trial and to guess when unsure. No feedback was provided during the experiment regarding whether their answer was correct/incorrect.
All participants underwent brief training with practice trials and verbal feedback from the experimenter to confirm that they understood the instructions and performed the task adequately before starting the actual experiment. During this training, the participants were instructed to imagine themselves in a space ship and to envision themselves flying through the star field as they moved forward (to encourage a feeling of self-motion from the optic flow). Also, coupling of the visual stimulus to inertial (vestibular) self-motion in combined trials, further heightened the experience of self-motion from optic flow. The task for all (visual, vestibular and combined) stimuli was the same—to indicate whether their perceived heading was to the left/right of straight ahead. Notably, for the visual stimulus, this is different from reporting leftward/rightward motion of the stars (which would yield the opposite choice, e.g. a heading stimulus to the right of straight ahead would have more stars moving leftward optically). Thus, correct choices by the participants indicate that they understood the task to report self-motion perception.
Possible stimulus heading values were distributed logarithmically around straight ahead at angles ±16°, 8°, 4°, 2°, 1°, 0.5° or 0.25°, where zero represents straight ahead and positive (or negative) values represent a rightward (or leftward) deviation from straight ahead. The absolute heading magnitude was set according to a staircase procedure (Cornsweet, 1962), and the heading sign (positive or negative) was selected randomly for each trial. A separate staircase procedure was run per condition (with pseudo-randomly interleaved trials). Each staircase began with the easiest heading (±16°). After a correct response, heading magnitude was reduced (such that the task became more difficult) 30% of the time and remained unchanged 70% of the time. After an incorrect response, it was increased (such that the task became easier) 80% of the time and remained unchanged 20% of the time. This staircase rule converges at ∼73% correct responses, thereby sampling an information-rich region of the psychometric function, on an individual basis.
Visual cue reliability was controlled by manipulating visual motion coherence. Two coherence levels were used to test visual performance: (i) 100% coherence, in which all the stars moved coherently according to the direction of simulated self-motion, and (ii) 65% coherence, in which, on each frame (16.7 ms), 65% of the stars moved coherently and the remaining 35% were randomly displaced. For 65% coherence, it would not be feasible to solve the task by following individual stars, which would have a 35% chance of disappearing on each frame. For example, the probability of a single star remaining visible for 0.2 s (12 frames) at 65% coherence is 0.6512 < 1%. It should also be noted that the primary motion direction (forward) is marked by a radially expanding field (in three dimensions), where stars move in all directions optically. Thus, individual stars’ motions do not simply reflect rightward or leftward headings. Hence, task performance requires integration of motion from many stars in the dynamic scene.
We chose 65% coherence because multisensory integration is best studied when the unisensory (visual and vestibular) cue reliabilities are similar (Angelaki et al., 2009) and pilot experiments in our system showed that visual reliability at 65% coherence was roughly similar to vestibular performance. Accordingly, multisensory conditions were also tested at 65% coherence. Unisensory visual performance was also tested at 100% coherence, to disambiguate whether any observed visual deficit resulted from impaired visual perception per se, or from sensitivity to sensory noise (Zaidel et al., 2015). Vestibular reliability was not manipulated.
To test and quantify multisensory cue weighting, a slight discrepancy (Δ) between the visual and vestibular headings was introduced when they were presented in combination. By convention, Δ > 0 means that the vestibular headings were offset to the right and the visual headings were offset to the left, each by Δ/2 (and vice versa for Δ < 0). Having a set of discrepancy between the visual and vestibular headings allowed us to measure the relative cue weighting. For example, for evenly weighted cues, the multisensory percept should lie exactly in between the two. By contrast, when one cue is dominant, then the multisensory percept should lie closer to the dominant cue (this is explained further, quantitatively, below).
Five stimulus conditions were run (interleaved): (i) vestibular only, (ii) visual only with 100% visual coherence, (iii) visual only with 65% visual coherence, (iv) combined visual–vestibular with 65% visual coherence and Δ = +6° and (v) combined visual–vestibular with 65% visual coherence and Δ = −6°. Δ = ±6° was used, since it is well within the range of values that are integrated despite the discrepancy (Acerbi et al., 2018). A total of 400 trials were collected per visit (80 trials for each of the 5 stimulus conditions). The experiment was divided into two blocks of 200 trials, 20 min per block, to allow the participants to take a break in the middle.
Data analyses and statistics
Data analyses were performed with custom software using MATLAB R2013b (MathWorks) and ‘psignifit’ toolbox for MATLAB version 4 (Schütt et al., 2016). Psychometric plots represent the proportion of rightward choices as a function of heading angle and were calculated by fitting the data with a cumulative Gaussian distribution function (see examples in Fig. 2). Separate psychometric functions were fit per participant for each stimulus condition. The mean (µ) of the fitted cumulative Gaussian distribution function represents the point of subjective equality (namely, the heading for which the probability of choosing right or left is P = 0.5). And, the psychophysical ‘threshold’ is defined as the standard deviation (σ) of the fitted cumulative Gaussian distribution function. Lower threshold values reflect better (more precise) performance. Since thresholds are nonnegative values that scale geometrically, logarithmic values were used for statistics and plotting. For better threshold estimation, lapse rates were also simultaneously fit (with a narrow prior, up to 0.1) and the thresholds’ prior was extended to allow for high threshold values among some of the participants.
Figure 2.
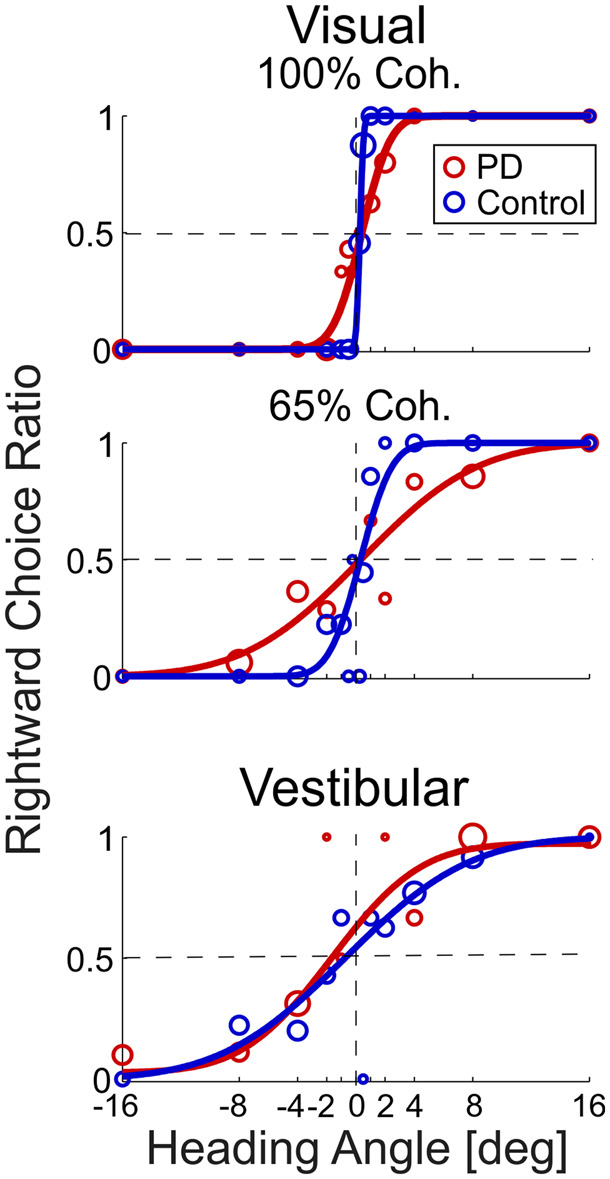
Example psychometric plots.
Behavioural responses to visual stimuli at 100% coherence (top plot), 65% coherence (middle plot) and vestibular stimuli (bottom plot) are presented for an example Parkinson’s disease participant (PD, red) and an example age-matched control participant (dark blue). Circle markers represent the ratio of rightward choices for a specific heading (marker size reflects the number of trials collected at that heading). The data were fitted with cumulative Gaussian distribution functions (solid lines). The vertical dashed line marks heading = 0°, and the horizontal dashed line marks y = 0.5 (equal probability of rightward and leftward choices).
Statistical analyses were performed using JASP (version 0.11.1) and MATLAB. To compare visual thresholds, we applied a three-way repeated measures ANOVA defining visual coherence (two levels: 100% and 65%) and visit (Visits 1 and 2) as within-subject factors and group (Parkinson’s disease and age-matched controls) as the between-subject factor. The same was done for vestibular thresholds, but without coherence as a factor. This allowed us to test for both main effects and interactions. The young controls were not part of those ANOVA comparisons because they were only tested in one visit (and the primary comparison in this study was Parkinson’s disease versus age-matched controls). Hence, further analyses, per visit, were also performed. Additional details of these specific statistical comparisons are presented together with the results below.
Bayesian multisensory integration
The Bayesian framework for multisensory integration has been well described previously (see Angelaki et al., 2009 for review). We briefly summarize it here, as it relates to our study. When presented with a visual or vestibular heading stimulus ( or , respectively), the participant will have a noisy internal measurement of that stimulus ( or , respectively). These measurements are assumed to be normally distributed around the stimulus: and , where and represent visual and vestibular noise distribution variances, respectively. Cue reliability (R) is defined as the inverse variance:
| (1) |
Thus, visual and vestibular cue reliabilities can be estimated from the unisensory thresholds (taken from the standard deviations, or , of the fitted Gaussian psychometric curves; see ‘Data analyses and statistics’ section).
In the multisensory condition, when both visual and vestibular measurements are attained, the optimal estimate of the stimulus (assuming a flat prior) is a linear weighted combination of the measurements:
| (2) |
where the cue weights:
| (3) |
reflect their relative reliabilities (note that the weights sum to 1; ). Intuitively, when , the visual cue is relatively more reliable ( > ). Hence, the visual measurement should be given more weight than the vestibular measurement, during multisensory integration.
To test whether patients with Parkinson’s disease indeed followed Bayesian optimal cue weighting, we compared the Bayesian predicted weights [Equation (3)] to the actual (empirical, observed) visual and vestibular weights, estimated from the multisensory conditions. To estimate these actual weights, a systematic discrepancy () was introduced between the cues in the multisensory condition (Δ = ±6° in our study). The actual visual weights could then be estimated as follows (Fetsch et al., 2009):
| (4) |
where µ+ and µ− are the point of subjective equalities of the combined cue conditions with positive and negative Δ, respectively. The vestibular weights are then calculated by:
| (5) |
Bayesian optimal integration also predicts that the multisensory (combined) cue threshold should be lower than, and can be quantitatively predicted from, the unisensory thresholds (Ma et al., 2006) as follows:
| (6) |
We also compared the Bayesian prediction for the combined thresholds to the actual (empirical) combined cue thresholds measured from the multisensory conditions. Since there were two combined conditions (Δ = ±6), the (geometric) mean of the two thresholds was used.
Data availability
The data are available upon request from the authors.
Results
In this study, we tested visual and vestibular (unisensory) self-motion perception, as well as visual–vestibular multisensory integration, in patients with Parkinson’s disease. We found impaired visual self-motion perception in Parkinson’s disease, but normal vestibular self-motion perception. Furthermore, in the multisensory condition, patients with Parkinson’s disease overweighted their (impaired) visual cues, deviating from Bayesian predictions of optimal integration. Details and expansion of these results are presented below.
Impaired visual self-motion perception in Parkinson’s disease
Unisensory visual and vestibular performance of an example patient with Parkinson’s disease and an example age-matched control are presented in Fig. 2. A steeper psychometric curve (one that approaches a step function) reflects better performance—namely, higher precision in discriminating rightward from leftward heading stimuli. While poorer performance is marked by a flatter (less steep) curve. In Fig. 2, the example Parkinson’s disease participant (red) demonstrates worse visual self-motion perception (flatter psychometric curve) compared to the control participant (dark blue)—for both 100% and 65% visual motion coherence conditions (top and middle plots, respectively). By contrast, they had similar vestibular self-motion perception (bottom plot). To compare performance quantitatively, and across groups, perceptual thresholds were measured from the psychometric data, per participant, condition and visit (see ‘Materials and methods’ section).
Patients with Parkinson’s disease had consistently higher visual thresholds (i.e. worse visual performance) in all conditions (Fig. 3, left column) —the red line (depicting visual thresholds in Parkinson’s disease) lies above the others for both 100% and 65% coherence levels, and for both the first (Fig. 3A) and second (Fig. 3B) visits. Comparing Parkinson’s disease to age-matched controls revealed that the increase in Parkinson’s disease visual thresholds is significant (F(1,39) = 4.9, P = 0.03, repeated measures three-way ANOVA: two groups × two coherences × two visits; young controls performed the task on one visit only and are therefore compared in a separate analysis below). A significant effect of coherence (F(1,39) = 60.3, P < 0.001) trivially reflects the manipulation of visual coherence (higher thresholds for 65% versus 100% coherence). No significant interactions (between group, coherence and visit) were found (P > 0.16). These results therefore indicate that visual self-motion perception is generally impaired in Parkinson’s disease.
Figure 3.
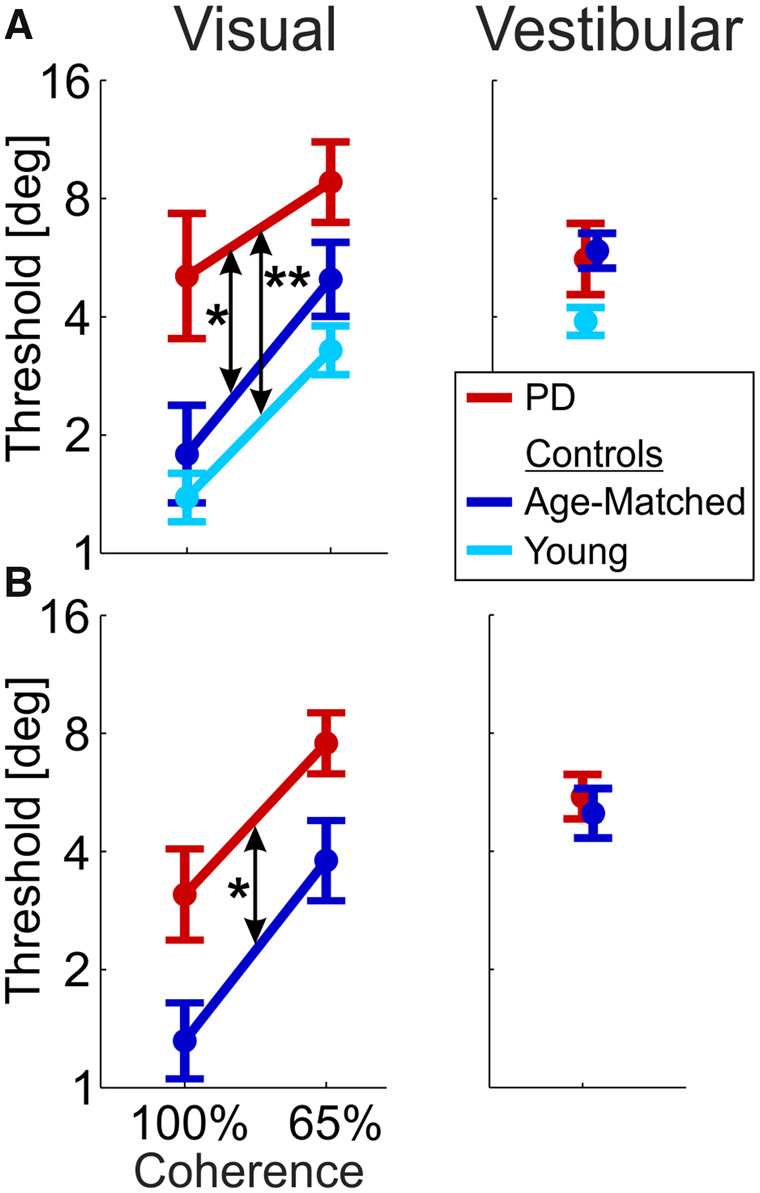
Impaired visual self-motion perception in Parkinson’s disease.
Visual and vestibular thresholds (left and right columns, respectively) are presented for the Parkinson’s disease (PD, red), age-matched (dark blue) and young control (light blue) groups. (A) Data from Visit 1 (Parkinson’s disease on medication). (B) Data from Visit 2 (Parkinson’s disease off medication). Young controls were tested only once (Visit 1). Data points and error bars represent mean ± SEM. *P < 0.05; **P < 0.01.
Further analysis, per visit, showed that patients with Parkinson’s disease had significantly impaired visual performance on both visits, independently. On the first visit, Parkinson’s disease visual thresholds were significantly higher versus both age-matched and young control groups (P = 0.04 and P = 0.003, respectively, post hoc comparison with Tukey correction; repeated measures two-way ANOVA: three groups × two coherences; Fig. 3A). Young healthy controls had slightly lower visual thresholds than age-matched controls (but this was not significant, PTukey = 0.55). Also, on the second visit, Parkinson’s disease visual thresholds were significantly higher versus age-matched controls (F(1,39) = 4.3, P = 0.045; repeated measures two-way ANOVA: two groups × two coherences; Fig. 3B). Finding the same result, of increased visual thresholds in Parkinson’s disease, on both visits (independently), strengthens the finding.
Unimpaired vestibular performance in Parkinson’s disease
By contrast, vestibular thresholds for the Parkinson’s disease and age-matched control groups were highly overlapping in both the first and second visits (red and dark blue, respectively; Fig. 3, right column) and did not differ statistically (F(1,39) = 0.033, P = 0.86; repeated measures two-way ANOVA: two groups × two visits). This indicates that vestibular self-motion perception was not impaired in the Parkinson’s disease group. Although there was a trend for lower vestibular thresholds in the young controls (first visit; Fig. 3A, right), this did not reach significance (F(2,59) = 2.74, P = 0.07; one-way ANOVA: three groups). Comparable performance of Parkinson’s disease and age-matched controls in the vestibular condition indicates that impaired performance in the visual condition did not arise from other difficulties in task performance (e.g. reporting choices) and validates the use of a two-alternative forced choice task to probe perceptual function in Parkinson’s disease. The stark difference between the visual and vestibular results (significantly impaired visual, but intact vestibular performance in Parkinson’s disease) tested in the same (interleaved) task, with the same participants, points to a specific impairment of visual self-motion perception in Parkinson’s disease.
No observed medication effect on self-motion perception
One might have expected worse performance in the second visit versus the first visit in the Parkinson’s disease group (off versus on medication), but this was not observed. Rather, both groups had improved visual thresholds on the second visit (P = 0.02, three-way ANOVA presented above, Fig. 3 left) without any group × visit interaction (P = 0.93). Vestibular thresholds were unchanged for both groups (P = 0.4, two-way ANOVA presented above, Fig. 3 right) also with no group × visit interaction (P = 0.53). These results suggest that patients with Parkinson’s disease responded to the second visit in the same way as age-matched controls (with a small learning effect for visual cues) and that medication status on/off did not improve or impair self-motion perception. Additional studies, with counterbalanced medication state across visits (and larger sample size), might uncover subtle and specific medication effects.
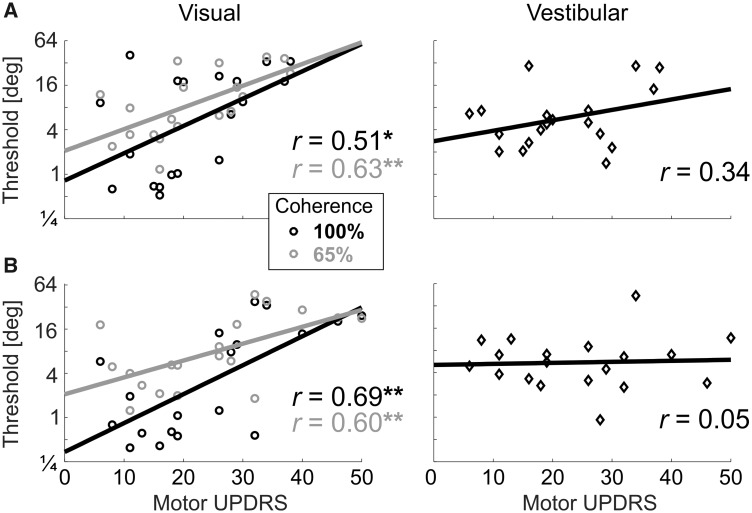
Visual thresholds correlate with motor impairment
To further investigate the relationship between impaired visual self-motion perception and Parkinson’s disease, we tested whether perceptual thresholds correlate with disease severity (motor UPDRS scores). For visual thresholds, four (Pearson) correlations were tested (two coherence levels × two visits). UPDRS scores on and off medication were used for the first and second visits, respectively. All four correlations were statistically significant: r = 0.51, P = 0.026 and r = 0.63, P = 0.004 (100% and 65% coherence, respectively, for the first visit; Fig. 4A, left) and r = 0.69, P = 0.002 and r = 0.60, P = 0.007 (100% and 65% coherence, respectively, for the second visit; Fig. 4B, left). By contrast, no significant correlation was seen between vestibular thresholds and UPDRS scores (r = 0.34, P = 0.15 and r = 0.05, P = 0.84 for the first and second visits, respectively; Fig. 4, right column).
Figure 4.
Visual self-motion perception deteriorates with Parkinson’s disease severity.
For the Parkinson’s disease participants, visual and vestibular thresholds (left and right columns, respectively) are presented versus their UPDRS motor scores. (A) Data from Visit 1 (on medication). (B) Data from Visit 2 (off medication). Black and grey circle markers represent visual thresholds at 100% and 65% coherence, respectively. Black diamonds present vestibular thresholds. Solid lines and ‘r’ values represent the linear regressions and correlation values of the respective plots. *P < 0.05; **P < 0.01. UPDRS = Unified Parkinson’s Disease Rating Scale.
Thus, visual (but not vestibular) self-motion perception deteriorates with disease severity. Furthermore, the correlation scores (r = 0.51–0.69) indicate that between 26% and 48% of the variance in visual thresholds can be attributed to Parkinson’s disease severity. This is striking in light of the high variance of perceptual thresholds typically observed across individuals. Although task performance could also correlate with cognitive function, this would not explain any of our results since the Parkinson’s disease and age-matched groups had comparable scores (presented above and in Table 1). We further confirmed this by adding Montreal Cognitive Assessment as a covariate to the three-way ANOVA presented above. We found that also when controlling for cognitive function, Parkinson’s disease visual self-motion performance was significantly impaired versus age-matched controls (P = 0.012). A trend for lower visual thresholds with better Montreal Cognitive Assessment scores was seen, but this was not significant (P = 0.06).
Visual overweighting in Parkinson’s disease
Our second main aim in this study (beyond testing unisensory visual and vestibular self-motion perception in Parkinson’s disease) was to examine how patients with Parkinson’s disease integrate information from visual and vestibular cues. Bayesian theory of multisensory integration provides two specific testable predictions of optimal integration: (i) the weights attributed by a participant to each cue, during integration, should equal the relative reliabilities of the respective cues, such that the more reliable cue is more heavily weighted. Quantitatively, the predicted weights can be calculated from the unisensory thresholds [using Equations (1) and (3)]. And these can be compared with the actual (empirically observed) cue weights, measured from the multisensory conditions [using Equations (4) and (5)]. (ii) A participant’s multisensory (combined cue) threshold should be lower (better) than each of his/her unisensory thresholds. Also, multisensory thresholds can be quantitatively predicted from the unisensory thresholds [using Equation (6)] and compared with the actual (empirically observed) multisensory thresholds. Thresholds (and thus predicted weights) are personal quantities, which depend on each individual’s function. Therefore, these two predictions were tested per participant.
For the first prediction (regarding cue weighting), it is sufficient to compare empirically observed versus predicted weights for one cue (cue weights sum to one, so results for the second cue are complementary). Here, we present this comparison for visual weights (from the first and second visits in Fig. 5A and B, respectively). Scatter plots (Fig. 5, left column) depict observed versus predicted visual weights for Parkinson’s disease, age-matched and young participants (red, dark blue and light blue ‘o’ markers, respectively) as well as group mean ± SEM (respectively coloured ‘+’ markers). Deviations from the diagonal black lines (which represent perfect predictions) to the upper left (or lower right) indicate visual (or vestibular) overweighting. These deviations were analysed, per group and visit, using two-tailed paired t-tests (with Bonferroni correction for five comparisons) and compared between Parkinson’s disease and age-matched controls across visits using a repeated measures ANOVA.
Figure 5.
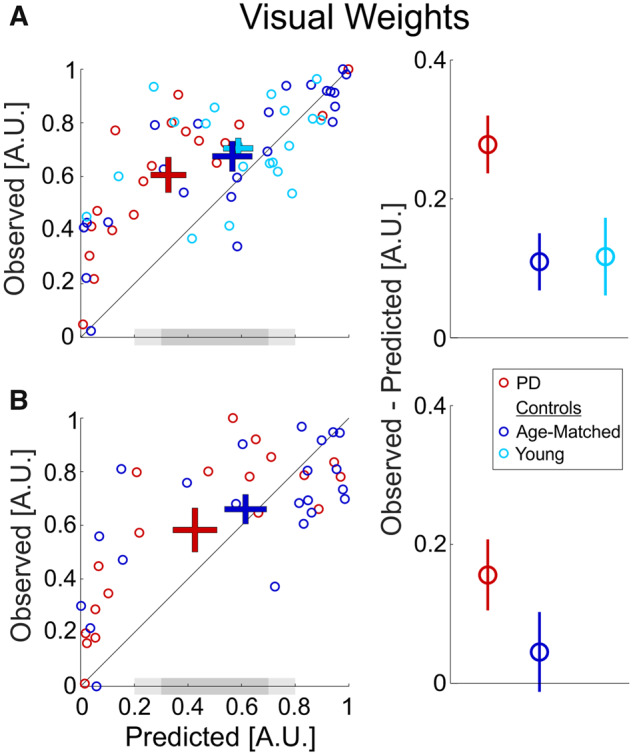
Visual overweighting in Parkinson’s disease.
(A) Data from Visit 1 (Parkinson’s disease on medication). (B) Data from Visit 2 (Parkinson’s disease off medication). Young controls were tested only once (Visit 1). Left column (scatter plot): each data point depicts the observed visual weight of an individual participant (extracted from the combined cue Δ conditions) versus the Bayesian predicted visual weights (estimated from the unisensory conditions). ‘+’ markers represent the mean ± SEM for each group (by respective colour). The diagonal black line (y = x) represents equality between observed and predicted weights. The dark grey-shaded region on the x-axes marks the range of predicted weights 0.5 ± 0.2. The light grey-shaded regions extend this to mark the range 0.5 ± 0.3. Right column: the difference between the observed and predicted visual weights (mean ± SEM) for each group (by respective colour). A.U. = arbitrary units, PD = Parkinson's disease.
Patients with Parkinson’s disease had the largest deviations from optimality, with significant visual overweighting, on both the first and second visits (t(18) = 6.7, P = 10−5 and t(18) = 3.14, P = 0.028, respectively). Although visual overweighting was also seen for the control groups, this was to a lesser degree, and did not reach significance (t(22) = 2.66, P = 0.07 and t(19) = 2.09, P = 0.25 for age-matched and young controls, respectively, on the first visit and t(21) = 0.78, P > 1 for age-matched controls on the second visit). The difference between the observed and predicted weights (Fig. 5, right column) was significantly larger in Parkinson’s disease versus age-matched controls (F(1,39) = 5.1, P = 0.03; repeated measures two-way ANOVA: two groups × two visits) indicating greater visual overweighting in Parkinson’s disease. Visual overweighting was reduced for both groups in the second visit (F(1,39) = 8.4, P = 0.006) with no significant group × visit interaction (F(1,39) = 0.6, P = 0.4). This indicates a possible practice effect for both Parkinson’s disease and age-matched controls (in line with improved visual thresholds, described above). And, like above, these data provide no evidence for a medication effect.
Since greater visual overweighting may be seen for the data with low predicted visual weights, we performed further analysis, limiting the data to the middle range of predicted weights (i.e. 0.5 ± 0.2; dark grey-shaded region in Fig. 5). The rationale here was to compare the three groups, where all participants in the comparison have similar predicted weights. Also, comparing the data around the middle zone of predicted weights (0.5) is most informative to test overweighting since it is less prone to ceiling or floor effects. We again used paired t-tests (with Bonferroni correction for five comparisons).
For each of the three groups (in the first visit), seven participants (21 in total) lay within this range of predicted weights 0.5 ± 0.2. Also here, the Parkinson’s disease group still demonstrated significant overweighting—observed weights were significantly greater than predicted: 0.77 ± 0.08 versus 0.45 ± 0.09 (mean ± SEM; t(6) = −5.51, P = 0.005). By contrast, neither of the control groups showed significant differences between observed versus predicted weights: 0.58 ± 0.14 versus 0.50 ± 0.13 (t(6) = −0.99, P > 1) for age-matched controls and 0.65 ± 0.19 versus 0.50 ± 0.09 (t(6) = −1.88, P = 0.55) for young controls. For the second visit, few data lay within the range 0.5 ± 0.2 (five Parkinson’s disease and three age-matched controls; although, a trend was still seen for overweighting in Parkinson’s disease). Hence we applied a broader range (0.5 ± 0.3; including the light grey-shaded regions in Fig. 5) and found similar results: significant overweighting in Parkinson’s disease (P < 0.02 in both visits) but not for the control groups (P > 0.4 in both visits).
Multisensory thresholds
The second Bayesian prediction is a little more difficult to discern, since the largest expected reduction in multisensory (versus unisensory) thresholds is only by a factor of 1.4 () and occurs when the unisensory thresholds are equal [Equation (6)]. Visual (65% coherence) and vestibular thresholds were indeed roughly similar per group, but less so for the Parkinson’s disease group, who had larger visual versus vestibular thresholds (Fig. 6, right column) making it more difficult to discern an integration deficit. A trend was seen for larger observed versus predicted thresholds in Parkinson’s disease on their first visit (Fig. 6A, right plot, and red ‘+’ marker lying above the diagonal line in Fig. 6A, left plot); however, this was not significant (P = 0.33 after Bonferroni correction for 5 comparisons, paired t-test). Multisensory thresholds for the two control groups on the first visit were consistent with optimal integration (light blue and dark blue ‘+’ markers lying close to the diagonal in Fig. 6A, left plot, and comparable observed and predicted multisensory thresholds in Fig. 6A, right plot; P > 1 after Bonferroni correction, paired t-tests). Also, on the second visit, there were no significant differences between observed and predicted thresholds, for both groups (P > 0.16 after Bonferroni correction, paired t-tests).
Figure 6.
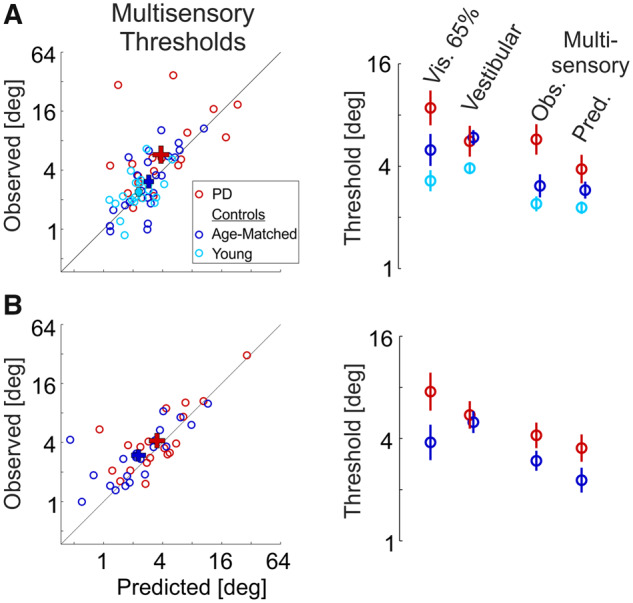
Multisensory thresholds.
(A) Data from Visit 1 (Parkinson’s disease on medication). (B) Data from Visit 2 (Parkinson’s disease off medication). Young controls were tested only once (Visit 1). Left column: each data point depicts the observed combined cue (multisensory) threshold of an individual participant versus the Bayesian prediction (estimated from the unisensory conditions). ‘+’ markers represent the mean ± SEM for each group (by respective colour). The diagonal black line (y = x) represents equality between observed and predicted thresholds. Right column: mean ± SEM unisensory (visual 65% coherence and vestibular) and multisensory (observed and predicted) thresholds, per group (by respective colour). PD = Parkinson's disease.
Discussion
In this study, we investigated the perception of self-motion in Parkinson’s disease. To measure unisensory visual and vestibular function, separately, and to test multisensory integration of visual and vestibular cues, we used a well-established psychophysics paradigm that has not been previously applied to study Parkinson’s disease. Advantages of this paradigm are: (i) it is not dependent on graded motor responses (which are themselves altered in Parkinson’s disease). Rather, it relies on categorical choices (right or left), which are analysed based on signal detection theory to provide a canonical metric for perceptual function. (ii) It allowed us to quantify multisensory integration within the principled framework of Bayesian inference. Our findings that visual and vestibular thresholds in young adults were only slightly (but not significantly) better than age-matched controls indicate that this paradigm is well suited also to an older population, as we tested here to investigate self-motion perception in Parkinson’s disease.
The study results provide several novel and interesting findings: (i) patients with Parkinson’s disease demonstrated impaired visual self-motion perception, which deteriorates with Parkinson’s disease severity; (ii) Parkinson’s disease vestibular function was indistinguishable from age-matched controls; and (iii) patients with Parkinson’s disease overweighted visual (versus vestibular) cues during multisensory integration. Together these results indicate that self-motion perception is affected in Parkinson’s disease both by impaired visual cues and by suboptimal multisensory integration. We further discuss these results and their implications here below.
Altered veering in response to visual optic flow has been described in Parkinson’s disease (Davidsdottir et al., 2008; Lin et al., 2014). However, with graded motor actions (veering) as responses, those studies do not dissociate perceptual from motor or sensorimotor dysfunction. Here, we found a specific impairment in visual self-motion perception in Parkinson’s disease. This does not reflect a general deficit of visual motion perception, since the ability to discriminate aggregate motion of a (flat) random dot kinematogram (dots moving on a two-dimensional screen; not simulating self-motion) is not impaired in Parkinson’s disease (Putcha et al., 2014; Jaywant et al., 2016). Thus, visual self-motion perception (a skill that is vital for proficient balance and gait) seems specifically impaired in Parkinson’s disease. This might reflect the higher complexity of perceptual processing required to differentiate one’s own motion from motion of objects in the environment (Dokka et al., 2015) and/or the three-dimensional nature of our stimuli and experiment.
Our concurrent observation of unimpaired performance with vestibular stimuli (in the same participants and task) first confirms that impaired visual performance was not due to difficulty in performing the task itself but rather reflects a specific impairment of visual self-motion perception. Notably, it also suggests that vestibular performance seems largely spared in Parkinson’s disease. This is in line with Bertolini et al. (2015) who also did not find vestibular sensory impairment (but rather found a central vestibular integration failure). Accordingly, altered nystagmus in Parkinson’s disease to caloric stimulation (Reichert et al., 1982) might reflect sensory-motor integration deficits, rather than vestibular deficits per se. However, it is also possible that vestibular impairment might only emerge at a later stage in Parkinson’s disease (our cohort was early stage). Nonetheless, the stark difference between the visual and vestibular results suggests a stronger visual self-motion impairment, seen already at an early stage.
We did not find any significant effects of medication status on self-motion perception in Parkinson’s disease. However, we should be careful not to conclude from this that there is no medication effect, for two main reasons. First, in these data, medication status is confounded with visit sequence (patients were on medication on the first visit and off medication on the second visit). Second, our cohort was mainly early-stage Parkinson’s disease and most (14 out of 19) were levodopa naive. Therefore, future research with more data (and counterbalancing on and off medication status, by visit) is required to further address this question.
Humans (and animals) have been shown, in many tasks and across different modalities, to integrate their senses in a near-optimal Bayesian manner (Jacobs, 1999; Landy and Kojima, 2001; Ernst and Banks, 2002; Alais and Burr, 2004; Butler et al., 2010; Raposo et al., 2012). Thus, visual overweighting to the extent that we find here in Parkinson’s disease is quite rare. Furthermore, in this same visual–vestibular self-motion task, humans (and monkeys) have been shown to dynamically reweight visual and vestibular cues in accordance with relative cue reliability, even on single trials (Fetsch et al., 2009). Yet, despite visual self-motion perception presumably deteriorating in Parkinson’s disease over a long time, their integration weights seem to reflect their original, unimpaired state.
There are several possible explanations to this finding, which may not be mutually exclusive. (i) It could reflect a general deficit in multisensory integration in Parkinson’s disease, perhaps related to general cognitive inflexibility (Cools et al., 2001). (ii) It may reflect a specific overestimation of visual cue reliability in Parkinson’s disease (i.e. not correctly estimating the current state of visual function), which would lead to visual overweighting. In support of this idea, patients with Parkinson’s disease seem to demonstrate increased visual dependence (Cooke et al., 1978; Azulay et al., 2002; Vaugoyeau et al., 2007; Davidsdottir et al., 2008; Barnett-Cowan et al., 2010; Funato et al., 2010) despite their many visual deficits (Weil et al., 2016). Also, patients with Parkinson’s disease (with dyskinesia) show increased sensitivity to unreliable visual input (Stevenson et al., 2014). Therefore, it is possible that patients with Parkinson’s disease specifically overestimate the reliability of their current visual function. (iii) Finally, we also need to bear in mind that results regarding multisensory integration rely on accurate estimation of the unisensory cues (e.g. underestimating visual reliability might lead to underestimating predicted visual weights, Shalom and Zaidel, 2018). Further research is needed to tease apart these options, by testing multisensory integration between other modalities, directly investigating Parkinson’s disease estimates of their own visual reliability and investigating the possible effects of medication on altered multisensory integration.
Perception and action are intricately interconnected (Gibson, 1966; Prinz, 1997; Goodale and Westwood, 2004; Warren, 2006; Merriam et al., 2007; Turvey, 2007). Sensory and perceptual deficits in Parkinson’s disease contribute to motor impairment (Konczak et al., 2009), and freezing of gait (Almeida and Lebold, 2010). Conversely, sensory input, such as auditory clicks, or a visual grid of tiles on the floor can aid Parkinson’s disease function (Bagley et al., 1991; Freeman et al., 1993; Lim et al., 2005). An important motivation for studying self-motion perception in Parkinson’s disease is to gain better insight into the mechanisms of balance and gait dysfunction, which are disabling, difficult to treat, and increase the risk of falling. Here, we did not directly test the connection between impaired self-motion perception and gait and balance disorders. However, a connection is likely and should be investigated in future studies.
Our results have implications for early detection of Parkinson’s disease. Certain non-motor symptoms including sensory, perceptual and cognitive impairments may be present even before the diagnosis of Parkinson’s disease. Identification of these impairments in the pre-diagnostic or prodromal phase of the disease would be highly relevant clinically, e.g. for possible neuro-protective treatments (Noyce et al., 2016). In our cohort, many (8 out of 19) patients were early in the course of the disease (disease duration <4 years) and most (14 out of 19) were levodopa naive. It is therefore possible that visual self-motion impairment and/or visual overweighting could be used as a biomarker that is abnormal at clinical Parkinson’s disease diagnosis or possibly even before.
An additional potential clinical application is for Parkinson’s disease subtyping. Impaired visual self-motion perception may be associated with certain Parkinson’s disease subtypes defined clinically (Lord et al., 2014; Mollenhauer et al., 2014) and/or genetically (Neumann et al., 2009; Alcalay et al., 2012; Wang et al., 2014; Zokaei et al., 2014). We did not find a significant correlation between impaired visual self-motion perception and cognitive function, but the trend may suggest a possible connection, in line with a relationship between other visual impairments and cognitive decline in Parkinson’s disease (Gagnon et al., 2009; Weil et al., 2016).
In summary, we have shown here that patients with Parkinson’s disease have impaired self-motion perception. This is specifically driven by a deficit in visual self-motion cues. Furthermore, these impaired visual cues of self-motion are overweighted when integrated with largely intact vestibular cues, leading to suboptimal multisensory integration. Defective self-motion perception and suboptimal multisensory integration may have a profound impact on function in Parkinson’s disease, and our understanding thereof.
Supplementary Material
Acknowledgements
We would like to thank Avraham Elkaras for programming assistance, David Swissa for mechanical assistance and Judith Sonn and Tamar Harpaz for management assistance. We would also like to thank the staff of the Movement Disorders Institute at Sheba Medical Center for help with patient recruitment.
Funding
This work was supported by grants from the National Institute for Psychobiology in Israel (NIPI, 235-17-18) and the Israeli Centers of Research Excellence (I-CORE, Center No. 51/11 to A.Z.).
Competing interests
The authors report no competing interests.
Glossary
- TUG =
timed up and go
References
- Acerbi L, Dokka K, Angelaki DE, Ma WJ.. Bayesian comparison of explicit and implicit causal inference strategies in multisensory heading perception. PLoS Comput Biol 2018; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alais D, Burr D.. The ventriloquist effect results from near-optimal bimodal integration. Curr Biol 2004; 14: 257–62. [DOI] [PubMed] [Google Scholar]
- Alcalay RN, Caccappolo E, Mejia-Santana H, Tang M-X, Rosado L, Reilly MO, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology 2012; 78: 1434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida QJ, Lebold CA.. Freezing of gait in Parkinson’s disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry 2010; 81: 513–8. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Gu Y, DeAngelis GC.. Multisensory integration: psychophysics, neurophysiology, and computation. Curr Opin Neurobiol 2009; 19: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, Gu Y, DeAngelis GC.. Visual and vestibular cue integration for heading perception in extrastriate visual cortex. J Physiol 2011; 589: 825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azulay JP, Mesure S, Amblard B, Blin O, Sangla I, Pouget J.. Visual control of locomotion in Parkinson’s disease. Brain 1999; 122: 111–20. [DOI] [PubMed] [Google Scholar]
- Azulay JP, Mesure S, Amblard B, Pouget J.. Increased visual dependence in Parkinson’s disease. Percept Mot Skills 2002; 95: 1106–14. [DOI] [PubMed] [Google Scholar]
- Bagley S, Kelly B, Tunnicliffe N, Turnbull GI, Walker JM.. The effect of visual cues on the gait of independently mobile Parkinson’s disease patients. Physiotherapy 1991; 77: 415–20. [Google Scholar]
- Barnett-Cowan M, Dyde RT, Fox SH, Moro E, Hutchison WD, Harris LR.. Multisensory determinants of orientation perception in Parkinson’s disease. Neuroscience 2010; 167: 1138–50. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Wicki A, Baumann CR, Straumann D, Palla A.. Impaired tilt perception in Parkinson’s disease: a central vestibular integration failure. PLoS One 2015; 10: e0124253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodis-Wollner I, Marx MS, Mitra S, Bobak P, Mylin L, Yahr M.. Visual dysfunction in Parkinson’s disease: loss in spatiotemporal contrast sensitivity. Brain 1987; 110: 1675–98. [DOI] [PubMed] [Google Scholar]
- Bodis I, Yahr MD.. Measurements of visual evoked potentials in Parkinson’s disease. J Neurol 1978; 101: 661–71. [DOI] [PubMed] [Google Scholar]
- Bonnet AM, Jutras MF, Czernecki V, Corvol JC, Vidailhet M.. Nonmotor symptoms in Parkinson’s disease in 2012: relevant clinical aspects. Parkinsons Dis 2012; 2012: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AM, Hood JD, Gresty MA, Panagi C.. Visual control of balance in cerebellar and Parkinsonian syndromes. Brain 1990; 113: 767–79. [DOI] [PubMed] [Google Scholar]
- Brown DJ, Pastras CJ, Curthoys IS.. Electrophysiological measurements of peripheral vestibular function—A review of electrovestibulography. Front Syst Neurosci 2017; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JS, Smith ST, Campos JL, Bülthoff HH.. Bayesian integration of visual and vestibular signals for heading. J Vis 2010; 10: 23. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AH.. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 2006; 5: 235–245. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Prieto-Jurcynska C, Naidu Y, Mitra T, Frades-Payo B, Tluk S, et al. The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord 2010; 25: 704–9. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Brown JD, Brooks VB.. Increased Dependence on Visual Information for Movement Control in Patients with Parkinson’s disease. Can J Neurol Sci 1978; 5: 413–5. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RAA, Sahakian BJJ, Robbins T.. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain 2001; 124: 2503–12. [DOI] [PubMed] [Google Scholar]
- Cornsweet TN. The staircase-method in psychophysics. Am J Psychol 1962; 75: 485–91. [PubMed] [Google Scholar]
- Cowie D, Limousin P, Peters A, Day BL.. Insights into the neural control of locomotion from walking through doorways in Parkinson’s disease. Neuropsychologia 2010; 48: 2750–7. [DOI] [PubMed] [Google Scholar]
- Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 2012; 35: 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsdottir S, Wagenaar R, Young D, Cronin-Golomb A.. Impact of optic flow perception and egocentric coordinates on veering in Parkinson’s disease. Brain 2008; 131: 2882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichgans J, Brandt T.. Visual-vestibular interaction: effects on self-motion perception and postural control In: Held R, Leibowitz HW, Teuber HL, editors. Perception. Springer; 1978. Vol. 80, p. 755–804. [Google Scholar]
- Dokka K, Deangelis GC, Angelaki DE.. Multisensory integration of visual and vestibular signals improves heading discrimination in the presence of a moving object. J Neurosci 2015; 35: 13599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgin FH, Pelah A, Fox LF, Lewis J, Kane R, Walley KA.. Self-motion perception during locomotor recalibration: more than meets the eye. J Exp Psychol Hum Percept Perform 2005; 31: 398–419. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS.. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 2002; 415: 429–33. [DOI] [PubMed] [Google Scholar]
- Fetsch CR, DeAngelis GC, Angelaki DE.. Visual-vestibular cue integration for heading perception: applications of optimal cue integration theory. Eur J Neurosci 2010; 31: 1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetsch CR, Turner AH, DeAngelis GC, Angelaki DE.. Dynamic reweighting of visual and vestibular cues during self-motion perception. J Neurosci 2009; 29: 15601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JS, Cody FW, Schady W.. The influence of external timing cues upon the rhythm of voluntary movements in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1993; 56: 1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato T, Aoi S, Oshima H, Tsuchiya K.. Variant and invariant patterns embedded in human locomotion through whole body kinematic coordination. Exp Brain Res 2010; 205: 497–511. [DOI] [PubMed] [Google Scholar]
- Fushiki H, Kobayashi K, Asai M, Watanabe Y.. Influence of visually induced self-motion on postural stability. Acta Otolaryngol 2005; 125: 60–4. [DOI] [PubMed] [Google Scholar]
- Gagnon J, Vendette M, Postuma RB, Desjardins C, Massicotte‐Marquez J, Panisset M, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Ann Neurol 2009; 66: 39–47. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The senses considered as perceptual systems. Boston: Houghton Mifflin; 1966.
- Goodale MA, Westwood DA.. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol 2004; 14: 203–11. [DOI] [PubMed] [Google Scholar]
- Gu Y, Angelaki DE, DeAngelis GC.. Neural correlates of multisensory cue integration in macaque MSTd. Nat Neurosci 2008; 11: 1201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, DeAngelis GC, Angelaki DE.. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci 2007; 10: 1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullett JM, Price CC, Nguyen P, Okun MS, Bauer RM, Bowers D.. Reliability of three Benton Judgment of Line Orientation short forms in idiopathic Parkinson’s disease. Clin Neuropsychol 2013; 27: 1167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin O, Israeli-Korn S, Yakubovich S, Hassin-Baer S, Zaidel A.. Self-motion perception in Parkinson’s disease. Eur J Neurosci 2020; doi: 10.1111/ejn.14716. [DOI] [PubMed] [Google Scholar]
- Hlavacka F, Mergner T, Bolha B.. Human self-motion perception during translatory vestibular and proprioceptive stimulation. Neurosci Lett 1996; 210: 83–6. [DOI] [PubMed] [Google Scholar]
- Hlavačka F, Mergner T, Schweigart G.. Interaction of vestibular and proprioceptive inputs for human self-motion perception. Neurosci Lett 1992; 138: 161–4. [DOI] [PubMed] [Google Scholar]
- van der Hoorn A, Renken RJ, Leenders KL, de Jong BM.. Parkinson-related changes of activation in visuomotor brain regions during perceived forward self-motion. PLoS One 2014; 9: e95861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM.. Postural orientation and equilibrium. Handb Physiol 1996; 1: 255–92. [Google Scholar]
- Jacobs RA. Optimal integration of texture and motion cues to depth. Vision Res 1999; 39: 3621–9. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008; 79: 368–76. [DOI] [PubMed] [Google Scholar]
- Jaywant A, Shiffrar M, Roy S, Cronin-Golomb A.. Impaired perception of biological motion in Parkinson’s disease. Neuropsychology 2016; 30: 720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, et al. Proprioception and motor control in Parkinson’s disease. J Mot Behav 2009; 41: 543–52. [DOI] [PubMed] [Google Scholar]
- Lackner JR, DiZio P.. Vestibular, proprioceptive, and haptic contributions to spatial orientation. Annu Rev Psychol 2005; 56: 115–47. [DOI] [PubMed] [Google Scholar]
- Landy MS, Kojima H.. Ideal cue combination for localizing texture-defined edges. J Opt Soc Am A 2001; 18: 2307–20. [DOI] [PubMed] [Google Scholar]
- Lim I, van Wegen E, de Goede C, Deutekom M, Nieuwboer A, Willems A, et al. Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic review. Clin Rehabil 2005; 19: 695–713. [DOI] [PubMed] [Google Scholar]
- Lin CC, Wagenaar RC, Young D, Saltzman EL, Ren X, Neargarder S, et al. Effects of Parkinson’s disease on optic flow perception for heading direction during navigation. Exp Brain Res 2014; 232: 1343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow BJ, Shoushtarian M.. Parkinson’s disease: disturbed vestibular function and levodopa. J Neurol Sci 2015; 353: 49–58. [DOI] [PubMed] [Google Scholar]
- Lord S, Galna B, Coleman S, Yarnall A, Burn D, Rochester L.. Cognition and gait show a selective pattern of association dominated by phenotype in incident Parkinson’s disease. Front. Aging Neurosci 2014; 6: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WJ, Beck JM, Latham PE, Pouget A.. Bayesian inference with probabilistic population codes. Nat Neurosci 2006; 9: 1432–8. [DOI] [PubMed] [Google Scholar]
- Madhushri P, Dzhagaryan AA, Jovanov E, Milenkovic A. A smartphone application suite for assessing mobility. In: Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. IEEE; 2016. p. 3117–20. [DOI] [PubMed]
- Mergner T, Hlavacka F, Schweigart G. Interaction of vestibular and proprioceptive inputs. J Vestib Res Equil 1993; 3: 41–57. [PubMed] [Google Scholar]
- Mergner T, Rosemeier T.. Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions—a conceptual model. Brain Res Rev 1998; 28: 118–35. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL.. Remapping in human visual cortex. J Neurophysiol 2007; 97: 1738–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B, Rochester L, Chen‐Plotkin A, Brooks D.. What can biomarkers tell us about cognition in Parkinson’s disease? Mov Disord 2014; 29: 622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montse A, Pere V, Carme J, Francesc V, Eduardo T.. Visuospatial deficits in Parkinsons disease assessed by judgment of line orientation test: error analyses and practice effects. J Clin Exp Neuropsychol 2001; 23: 592–8. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, BéDirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 2009; 132: 1783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce AJ, Lees AJ, Schrag A-E.. The prediagnostic phase of Parkinson’s disease. J Neurol Neurosurg Psychiatry 2016; 87: 871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard J. Motor and representational framing of space. In: Paillard J, editor. Brain space. Oxford, UK: Oxford University Press; 1991. p. 163–182. [Google Scholar]
- Parkinson J. An essay on the shaking palsy. London: Sherwood, Neely and Jones; 1817. [Google Scholar]
- Patel N, Jankovic J, Hallett M.. Sensory aspects of movement disorders. Lancet Neurol 2014; 13: 100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CWW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015; 30: 1591–601. [DOI] [PubMed] [Google Scholar]
- Prinz W. Perception and action planning. Eur J Cogn Psychol 1997; 9: 129–54. [Google Scholar]
- Probst T, Straube A, Bles W.. Differential effects of ambivalent visual-vestibular-somatosensory stimulation on the perception of self-motion. Behav Brain Res 1985; 16: 71–9. [DOI] [PubMed] [Google Scholar]
- Putcha D, Ross RS, Rosen ML, Norton DJ, Cronin-Golomb A, Somers DC, et al. Functional correlates of optic flow motion processing in Parkinson’s disease. Front Integr Neurosci 2014; 8: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo D, Sheppard JPP, Schrater PRR, Churchland A.. Multisensory decision-making in rats and humans. J Neurosci 2012; 32: 3726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert WH, Doolittle J, McDowell FH.. Vestibular dysfunction in Parkinson disease. Neurology 1982; 32: 1133. [DOI] [PubMed] [Google Scholar]
- Schütt HH, Harmeling S, Macke JH, Wichmann FA.. Painfree and accurate Bayesian estimation of psychometric functions for (potentially) overdispersed data. Vision Res 2016; 122: 105–23. [DOI] [PubMed] [Google Scholar]
- Schweigart G, Chien R-D, Mergner T.. Neck proprioception compensates for age-related deterioration of vestibular self-motion perception. Exp Brain Res 2002; 147: 89–97. [DOI] [PubMed] [Google Scholar]
- Shalom S, Zaidel A.. Better than optimal. Neuron 2018; 97: 484–7. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Taback RL, Rabinstein AA, Weiner WJ.. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2002; 8: 193–7. [DOI] [PubMed] [Google Scholar]
- Smith P. Vestibular functions and Parkinson’s disease. Front Neurol 2018; 9: 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J, Lee C, Lee BS, TalebiFard P, Ty EB, Aseeva K, et al. Excessive sensitivity to uncertain visual input in L-DOPA-induced dyskinesias in Parkinson’s disease: further implications for cerebellar involvement. Front Neurol 2014; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey MT. Action and perception at the level of synergies. Hum Mov Sci 2007; 26: 657–97. [DOI] [PubMed] [Google Scholar]
- Vaugoyeau M, Viel S, Assaiante C, Amblard B, Azulay JP.. Impaired vertical postural control and proprioceptive integration deficits in Parkinson’s disease. Neuroscience 2007; 146: 852–63. [DOI] [PubMed] [Google Scholar]
- Wang C, Cai Y, Gu Z, Ma J, Zheng Z, Tang B-S, et al. Clinical profiles of Parkinson’s disease associated with common leucine-rich repeat kinase 2 and glucocerebrosidase genetic variants in Chinese individuals. Neurobiol Aging 2014; 35: 725–e1. [DOI] [PubMed] [Google Scholar]
- Warren WH. The dynamics of perception and action. Psychol Rev 2006; 113: 358–89. [DOI] [PubMed] [Google Scholar]
- Warren WH, Hannon DJ.. Direction of self-motion is perceived from optical-flow. Nature 1988; 336: 162–3. [Google Scholar]
- Weil RS, Pappa K, Schade RN, Schrag AE, Bahrami B, Schwarzkopf DS, et al. The cats‐and‐dogs test: a tool to identify visuoperceptual deficits in Parkinson’s disease. Mov Disord 2017; 32: 1789–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR.. Visual dysfunction in Parkinson’s disease. Brain 2016; 139: 2827–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil RS, Schwarzkopf DS, Bahrami B, Fleming SM, Jackson BM, Goch TJC, et al. Assessing cognitive dysfunction in Parkinson’s disease: an online tool to detect visuo‐perceptual deficits. Mov Disord 2018; 33: 544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Goin-Kochel RP, Angelaki DE.. Self-motion perception in autism is compromised by visual noise but integrated optimally across multiple senses. Proc Natl Acad Sci USA 2015; 112: 6461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Ma WJ, Angelaki DE.. Supervised calibration relies on the multisensory percept. Neuron 2013; 80: 1544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Turner AH, Angelaki DE.. Multisensory calibration is independent of cue reliability. J Neurosci 2011; 31: 13949–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zokaei N, McNeill A, Proukakis C, Beavan M, Jarman P, Korlipara P, et al. Visual short-term memory deficits associated with GBA mutation and Parkinson’s disease. Brain 2014; 137: 2303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available upon request from the authors.