Abstract
Serum uric acid level has been found to be associated with cerebrovascular diseases. However, whether serum uric acid level is a risk factor for arterial stiffness in the hypertension population is unclear. This study was designed to determine the relationship between serum uric acid level and arterial stiffness in the hypertension population. A total of 10450 participants were evaluated for the risk of arterial stiffness. Brachial-ankle pulse wave velocity (baPWV) was assessed, and high baPWV was determined as the highest quartile of baPWV values in a sex-specific manner. We evaluated the association between serum uric acid level and baPWV through multivariate-adjusted linear and logistic regression analyses. There was a significant difference on high baPWV between patients with quartiles of serum uric acid level in females and males (p<0.01), respectively. The odds ratios (95% CI) of the highest baPWV quartile across the sex-specific serum uric acid level were 1.0, 1.71 (1.35, 2.17), 1.75 (1.38, 2.23), and 1.95 (1.51, 2.51) in female, and 1.0, 1.33 (1.09, 1.64), 1.36 (1.11, 1.67), and 1.67 (1.36, 2.04) in male after adjusting for potential confounders. In conclusion, serum uric acid level could be considered as an important risk factor for arterial stiffness in Chinese hypertension population.
Keywords: arterial stiffness, pulse wave velocity, serum uric acid, hypertension, multivariate analysis
INTRODUCTION
Higher serum uric acid is a potential independent risk factor for cardiovascular disease, stroke, metabolic syndrome and arteriosclerosis [1–3]. The Framingham Study, which was the first to evaluate the association between serum uric acid level and cardiovascular disease in general population, demonstrated that higher serum uric acid level was a risk factor of cardiovascular disease [4]. Additionally, a prospective cohort study has shown that hyperuricemia could be considered an important risk factor for the mortality from all causes, total cardiovascular disease, and ischemic stroke in general population [5].
Brachial-ankle pulse wave velocity (baPWV), widely applied to assess arteriosclerosis during health screening in a non-invasive manner, is an important indicator for arterial stiffness [6, 7]. Several previous studies have suggested that higher serum uric acid, which could increase the risk of arteriosclerosis, was associated with increased arterial stiffness estimated by baPWV in general population [8, 9].
However, previous epidemiologic and prospective studies on baPWV were only limited in healthy individuals and general population, and the value of high baPWV in the gender reported in those studies was controversial [10, 11]. In addition, higher serum uric acid level increased the risk for hypertension, which has a positive correlation with arterial stiffness [12–14]. Based on current evidence, the association of serum uric acid level and arterial stiffness remains unclear in the hypertension population and whether higher serum uric acid level is an important risk factor for arterial stiffness in the hypertension population needs to be further investigated. Thus, we conducted this cross-section study, aiming to assess the association between serum uric acid level and arterial stiffness in the hypertension population. To our knowledge, no such large-scale study has previously reported on the association between serum uric acid level and arterial stiffness in the hypertension population.
RESULTS
Baseline characteristics
A total of 13110 candidates were recruited for the cross-section study at the time of the final survey in July 2017. Among these candidates, those who had missing data related to serum uric acid, baPWV, sex and age were excluded from the eligible candidates for this study (n=2528). Those with 0 value of baPWV (n=126) and those with implausible values of serum uric acid (<1mg/dl) (n=6) were also excluded from the pool of eligible candidates for this study. As a result, a total of 10450 subjects were included for the final analyses. A flowchart of the study is shown in Figure 1.
Figure 1.
Flowchart of the study.
Among 10450 study subjects, males accounted for 46.30% (n=4838) and females for 53.70% (n=5612). The age of the enrolled subjects ranged from 44 to 83 yr (female, 44–83 yr; male, 45–83 yr) with a mean age of 64.71±7.45 yr (female, 63.77±7.38 yr; male, 65.79±7.38 yr). The mean serum uric acid level was found to be significantly lower in female (5.29±1.44 mg/dL) than in male (6.24±1.47 mg/dL, p<0.001). Therefore, sex-specific quartiles of serum uric acid were used. The median (range) serum uric acid values of 3.63 (1.55- 4.20), 4.72 (4.22- 5.11), 5.65 (5.13- 6.25) and 6.94 (6.27-12.55) mg/L were used for female, and 4.57 (2.29- 5.23), 5.63 (5.24- 6.17), 6.61 (6.18- 7.19) and 7.95 (7.21-11.76) mg/L were used for male (Table 1).
The mean baPWV was significantly different among each serum uric acid quartile group in female and male, and the mean baPWV in the fourth quartile was more than that in the first quartile (both p<0.001). Among each serum uric acid quartile group, the body mass index (BMI), systolic blood pressure, diastolic blood pressure, heart rate, fasting glucose, total-cholesterol, high-density lipoprotein (HDL) cholesterol, and smoking status was significantly different in female (all p=0.672) but not significantly different in male (all p>0.001). The other baseline characteristics shown in Table 1 were significantly different among each serum uric acid quartile group in male and female participants (p<0.001 for all parameters).
Table 1. Baseline characteristics of participants by UA.
| Index | Q1 | Q2 | Q3 | Q4 | p value |
| Female | |||||
| Number | 1387 | 1385 | 1418 | 1422 | |
| UA median (min-max) (mg/dL) | 3.63 (1.55-4.20) | 4.72 (4.22-5.11) | 5.65 (5.13-6.25) | 6.94 (6.27-12.55) | <0.001 |
| baPWV (cm/s) | 1583.86±289.68 | 1662.40±329.18 | 1738.36±327.81 | 1770.38±361.16 | <0.001 |
| Age (years) | 62.02±7.17 | 62.99±7.24 | 64.31±7.19 | 65.69±7.40 | <0.001 |
| BMI (kg/m2) | 24.24±3.55 | 25.45±3.77 | 26.28±3.85 | 26.85±3.84 | <0.001 |
| SBP (mmHg) | 137.09±12.79 | 139.78±13.38 | 141.21±13.55 | 143.22±15.45 | <0.001 |
| DBP (mmHg) | 82.02±7.64 | 82.98±8.20 | 83.42±8.14 | 83.38±8.53 | <0.001 |
| Heart rate (bpm) | 76.66±10.49 | 76.86±10.97 | 78.05±11.52 | 78.80±11.75 | <0.001 |
| Dietary data (%) | |||||
| Staple food (rice) | 681 (49.42) | 639 (46.47) | 638 (45.15) | 635 (44.91) | 0.066 |
| Vegetarian diet | 688 (49.93) | 708 (51.53) | 716 (50.89) | 722 (51.17) | 0.855 |
| Laboratory data | |||||
| GGT (U/L) | 14.70 (12.10-19.20) | 15.70 (12.50-20.22) | 17.50 (14.00-23.80) | 19.60 (14.90-26.50) | <0.001 |
| Crea (μmol/L) | 55.07±10.79 | 59.17±16.27 | 61.73±12.82 | 70.96±26.29 | <0.001 |
| Glu (mmol/L) | 6.21±2.29 | 6.12±1.76 | 6.25±1.84 | 6.52±1.85 | <0.001 |
| Serum lipid data | 5.18±1.03 | 5.38±1.02 | 5.48±1.11 | 5.68±1.20 | <0.001 |
| TCHO (mg/dL) | 1.31±0.31 | 1.27±0.29 | 1.24±0.28 | 1.22±0.29 | <0.001 |
| HDL (mg/dL) | 1.54±1.08 | 1.81±1.13 | 2.06±1.54 | 2.48±1.91 | <0.001 |
| TG (mg/dL) | 1.29 (0.94-1.82) | 1.52 (1.10-2.15) | 1.73 (1.24-2.46) | 2.00 (1.41-3.00) | <0.001 |
| Smoking (%) | 0.004 | ||||
| Never | 1344 (97.53) | 1321 (96.35) | 1348 (95.47) | 1340 (94.70) | |
| Former | 10 (0.73) | 17 (1.24) | 28 (1.98) | 33 (2.33) | |
| Current | 24 (1.74) | 33 (2.41) | 36 (2.55) | 42 (2.97) | |
| Medication use (%) | |||||
| Antihypertensive drugs | 1237 (89.83) | 1261 (91.98) | 1329 (94.06) | 1344 (95.39) | <0.001 |
| Lipid lowering drugs | 18 (1.31) | 17 (1.25) | 32 (2.28) | 30 (2.14) | 0.070 |
| Antiplatelet drugs | 20 (1.54) | 34 (2.68) | 36 (2.77) | 39 (3.10) | 0.063 |
| Male | |||||
| Number | 1201 | 1204 | 1223 | 1210 | |
| UA median (min-max) (mg/dL) | 4.57 (2.29-5.23) | 5.63 (5.24-6.17) | 6.61 (6.18-7.19) | 7.95 (7.21-11.76) | <0.001 |
| baPWV (cm/s) | 1657.92±270.04 | 1709.63±197.65 | 1710.55±255.66 | 1731.16±352.50 | <0.001 |
| Age (years) | 65.38±7.42 | 66.05±7.26 | 65.63±7.28 | 66.12±7.55 | 0.043 |
| BMI (kg/m2) | 23.77±3.58 | 23.99±3.54 | 24.09±3.61 | 24.09±3.66 | 0.100 |
| SBP (mmHg) | 139.24±11.48 | 139.79±13.02 | 140.45±12.41 | 140.42±13.67 | 0.073 |
| DBP (mmHg) | 83.88±8.39 | 83.82±8.60 | 84.14±8.29 | 84.01±8.59 | 0.809 |
| Heart rate (bpm) | 75.57±11.79 | 75.61±11.72 | 75.95±11.91 | 76.21±11.81 | 0.509 |
| Dietary data (%) | |||||
| Staple food (rice) | 566 (47.68) | 552 (46.58) | 594 (49.21) | 617 (51.98) | 0.050 |
| Vegetarian diet | 386 (32.60) | 395 (33.36) | 398 (33.11) | 396 (33.36) | 0.977 |
| Laboratory data | |||||
| GGT (U/L) | 21.75 (16.50-31.50) | 23.00 (17.00-34.60) | 23.00 (17.20-34.50) | 24.60 (17.80-39.00) | <0.001 |
| Crea (μmol/L) | 75.78±14.61 | 79.55±22.98 | 81.63±32.87 | 85.47±47.18 | <0.001 |
| Glu (mmol/L) | 6.19±1.87 | 6.15±1.76 | 6.17±1.91 | 6.17±1.74 | 0.981 |
| Serum lipid data | |||||
| TCHO (mg/dL) | 4.99±0.99 | 4.99±1.03 | 5.04±1.05 | 5.07±1.05 | 0.153 |
| HDL (mg/dL) | 1.28±0.33 | 1.28±0.34 | 1.29±0.34 | 1.27±0.33 | 0.546 |
| TG (mg/dL) | 1.14 (0.85-1.57) | 1.16 (0.83-1.71) | 1.24 (0.86-1.77) | 1.26 (0.90-1.86) | <0.001 |
| Smoking (%) | 0.065 | ||||
| Never | 290 (24.49) | 293 (24.79) | 306 (25.37) | 321 (27.09) | |
| Former | 283 (23.90) | 310 (26.23) | 290 (24.05) | 327 (27.59) | |
| Current | 611 (51.60) | 579 (48.98) | 610 (50.58) | 537 (45.32) | |
| Medication use (%) | |||||
| Antihypertensive drugs | 1107 (93.34) | 1121 (94.76) | 1134 (94.34) | 1116 (94.74) | 0.404 |
| Lipid lowering drugs | 9 (0.76) | 14 (1.19) | 21 (1.75) | 13 (1.11) | 0.167 |
| Antiplatelet drugs | 42 (3.69) | 46 (4.15) | 41 (3.60) | 45 (4.03) | 0.889 |
UA: Uric Acid, baPWV: Brachial-ankle pulse wave velocity, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, GGT: gamma-glutamyl transpeptidase, Crea: Creatinine, Glu: glucose, TCHO: total cholesterol, HDL: high-density lipoprotein, TG: triglycerides.
Comparison of baPWV according to the UA quartile and gender
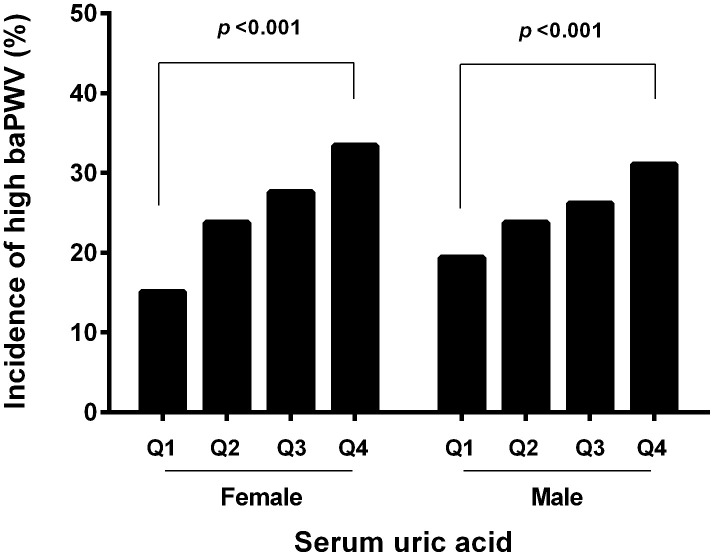
The mean baPWV value was 1689.54±335.90 cm/s in female, which was significantly lower than that in male (1702.41±275.87, p=0.034). The sex-specific inter quartile cutoff points for baPWV were 1453, 1629, and 1876 cm/s in female, and those of 1519, 1681, 1851 cm/s in male. High baPWV was defined as the highest quartile of the values among the study subjects. Thus, the high baPWV was defined as equal to more than 1876 cm/s in female and 1851 cm/s in male. The incidence of high baPWV in the fourth quartile was more than that in the first quartile both in female (33.40% vs 15.07%, p<0.001) and in male (31.07% vs 19.40%, p<0.001). The prevalence of the high baPWV in the first, second, third and fourth serum uric acid quartiles in each gender is shown in Figure 2.
Figure 2.
Incidence of high baPWV according to the UA quartile and gender. The incidence of high baPWV was increased with graded serum uric level in both genders.
Relationship between UA and baPWV
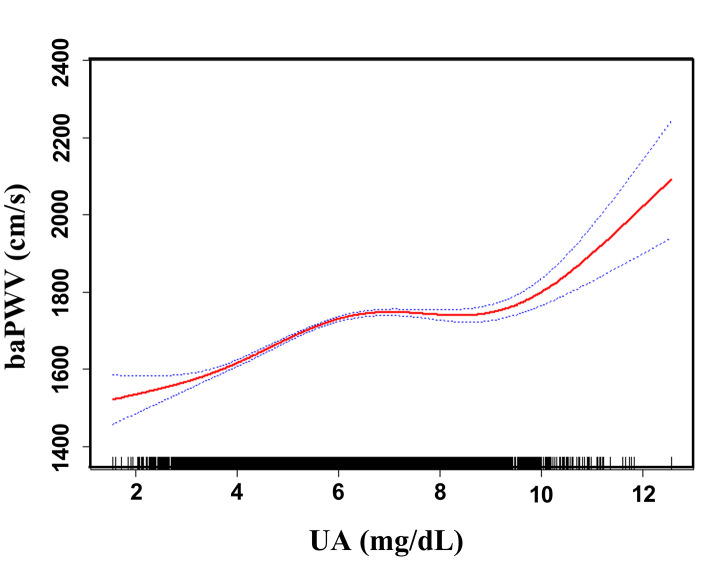
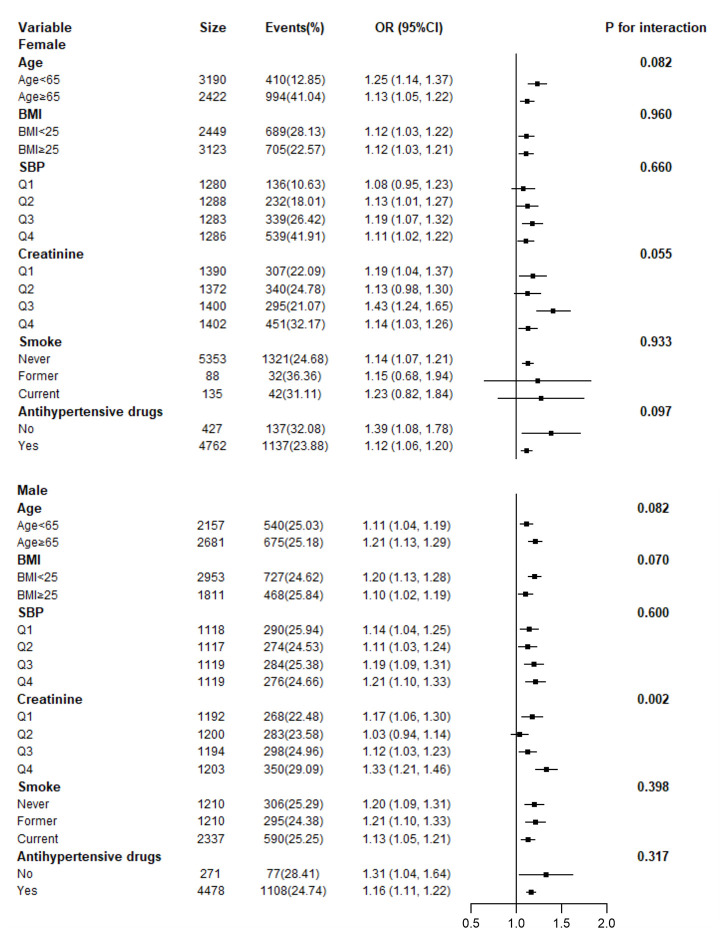
Adjusted smoothed plots suggest that there are linear relationships between the serum uric acid level and baPWV (Figure 3). Pearson’s correlation coefficients for the relationship between serum uric acid level and baPWV were 0.2048 (p<0.001) in female and 0.1284 (p=0.0001) in male. Scatter diagram between serum uric acid level and baPWV is shown in Supplementary Figure 1, and single factor logistic regression analysis of high baPWV is shown in Supplementary Table 1. Multiple logistic regression analysis without adjusting for confounder factors showed that the odds ratios (95%) for the relationship between serum uric acid and high baPWV were 1.27 (1.22-1.33, p<0.001) in female and 1.20 (1.15, 1.26, p<0.001) in male. When multivariate logistic analysis was performed after adjusting for age, BMI, systolic blood pressure, diastolic blood pressure, heart rate, gamma-glutamyl transpeptidase (GTP), creatinine, fasting glucose, total and HDL-cholesterol, triglycerides, smoking status, and antihypertensive drugs, the odds ratios (95%) for the relationship between serum uric acid and high baPWV were 1.13 (1.07- 1.21, p<0.001) in female and 1.16 (1.11- 1.22), p<0.001) in male, which showed that the association between serum uric acid level and high baPWV was statistically significant, and the odds ratios (95%) were 1.0, 1.71 (1.35, 2.17), 1.75 (1.38, 2.23), and 1.95 (1.51, 2.51) in female, and 1.0, 1.33 (1.09, 1.64), 1.36 (1.11, 1.67), and 1.67 (1.36, 2.04) in male after subdividing subjects according to the serum uric acid quartile and gender (Table 2.), which showed that the statistical significance was maintained. Hierarchical analysis, according to age, BMI, systolic blood pressure, triglycerides, smoking status, and antihypertensive drugs, also showed that the association between serum uric acid level and high baPWV was statistically significant (Figure 4, and Supplementary Table 2).
Figure 3.
Association between UA and baPWV. A linear relationship between UA and baPWV was detected after adjusting for age, BMI, systolic blood pressure, diastolic blood pressure, heart rate, gamma-GTP, creatinine, fasting glucose, total cholesterol, HDL-cholesterol, triglycerides, smoking status, and antihypertensive drugs. Solid lines represent the fitting curve, and dotted lines represent the corresponding 95% CI.
Table 2. Association between UA and high baPWV.
| Variable | Model 1 | Model 2 | ||
| Incidence, n (%) | OR (95%CI) p | N | OR (95%CI) p | |
| Female | ||||
| UA (continuous) 5612 | 1404 (25.02) | 1.27 (1.22, 1.33) <0.001 | 4898 | 1.13 (1.07, 1.21) <0.001 |
| log UA (categorical) | ||||
| Q1 (0.19-0.62) 1387 | 209 (15.07) | 1 | 1 | |
| Q2 (0.63-0.71) 1385 | 329 (23.75) | 1.76 (1.45, 2.13) <0.001 | 1.71 (1.35, 2.17) <0.001 | |
| Q3 (0.71-0.80) 1418 | 391 (27.57) | 2.15 (1.78, 2.59) <0.001 | 1.75 (1.38, 2.23) <0.001 | |
| Q4 (0.80-1.10) 1422 | 475 (33.40) | 2.83 (2.35, 3.40) <0.001 | 1.95 (1.51, 2.51) <0.001 | |
| Male | ||||
| UA (continuous) 4838 | 1215 (25.11) | 1.20 (1.15, 1.26) <0.001 | 4248 | 1.16 (1.11, 1.22) <0.001 |
| log UA (categorical) | ||||
| Q1(0.36-0.72) 1201 | 233 (19.40) | 1 | 1 | |
| Q2 (0.72-0.79) 1208 | 286 (23.75) | 1.29 (1.06, 1.57) 0.009 | 1.33 (1.09, 1.64) 0.006 | |
| Q3 (0.79-0.86) 1205 | 320 (26.17) | 1.47 (1.22, 1.78) <0.001 | 1.36 (1.11, 1.67) 0.004 | |
| Q4 (0.86-1.07) 1215 | 376 (31.07) | 1.87 (1.55, 2.26) <0.001 | 1.67 (1.36, 2.04) <0.001 | |
Model 1: unadjusted
Model 2: adjusted for age, BMI, systolic blood pressure, diastolic blood pressure, heart rate, gamma-GTP, creatinine, fasting glucose, total cholesterol, HDL-cholesterol, triglycerides, smoking status, and antihypertensive drugs.
Figure 4.
Hierarchical analysis on relationship of UA and high baPWV. Each stratification adjusted for all the factors (age, BMI, systolic blood pressure, diastolic blood pressure, heart rate, gamma-GTP, creatinine, fasting glucose, total cholesterol, HDL-cholesterol, triglycerides, smoking status, and antihypertensive drugs) except the stratification factor itself.
DISCUSSION
This cross-section study showed that serum uric level was associated with arterial stiffness in the hypertension population. Our study found that a positive correlation between serum uric level and baPWV, and the incidence of high baPWV was increased with graded serum uric level in both genders. Furthermore, the association between serum uric level and arterial stiffness was independent after adjustment for other confounding atherogenic risk factors and hierarchical analysis. Therefore, these results demonstrated that higher serum uric acid level is a risk factor of arterial stiffness in the hypertension population.
In the present study, higher values of baPWV in both genders, including mean baPWV and high baPWV, were detected in our hypertension population than previous studies in general subjects. Our result showed that the mean baPWV values were 1689.54±335.90 cm/s in female and 1702.41±275.87 cm/s in male, which was higher than that of the Japanese study (1447±315 cm/s in female, n=297; 1549±342 cm/s in male, n=695), and the Korean study (1483.2±331.6 cm/s in female, n=3401; 1581.1±338.1 cm/s in male n=2167,) [8, 15]. High baPWV was defined as the highest quartile of the values among the study subjects. Therefore, the high baPWV values were 1876 cm/s in female and 1851 cm/s in male in our study, while the high baPWV values were 1594 cm/s in female and 1721 cm/s in male in the Japanese study [8]. Previous study has demonstrated that baPWV values over 1400 cm/s in female and 1600 cm/s in male could be a feasible cutoff point so as to assess aortic stiffness and predict cardiovascular mortality by the Framingham risk score [16]. Why higher baPWV values than before in gender were detected in our study? It may be explained by the main reason that participants in our study were hypertensives, while previous studies were performed in general subjects. It has been demonstrated that arterial stiffness was associated with hypertension, and arterial stiffness is increasingly recognized as an important index in patients with hypertension [17–19]. Therefore, it is reasonable to observe high baPWV in the hypertension population in our study. Besides, it is also related to the age of the enrolled subjects, ranged from 44 to 83 years, which increased the mean age. Age is an independent risk factor for arterial stiffness. Many epidemiological studies have demonstrated a significant relationship between age and arterial stiffness, and showed the baPWV increased with age [20–23]. Whether genetic factors influence baPWV values in various races are not clear, which needs to be investigated on genetic diversity and population structure.
Our study revealed that serum uric acid level is an important risk factor for arterial stiffness in the hypertension population. In the current study, we found that graded serum uric level does not have a uniform impact on arterial stiffness evaluated by baPWV, and high risk for arterial stiffness was observed in high serum uric level. This result was consistent with previous studies in general population [8, 9, 24]. Our study also demonstrated that the risk of serum uric acid on arterial stiffness differs in gender. The present finding suggested that the normal serum uric acid level plays a slight role for arterial stiffness in male, but its relative importance increases with the presence of hyperuricaemia. In contrast, serum uric acid is an important risk factor for arterial stiffness throughout the different graded serum uric acid levels in female, particularly when hyperuricaemia develops. Our results indicated that serum uric acid level plays a critical role in the progression of arterial stiffness in female than in male in the hypertension population. Previous studies have demonstrated that serum uric acid level does not have a uniform impact on both genders, it was significantly associated with increased aortic and peripheral arterial stiffness in males, while females with gout were at increased risk for acute myocardial infarction compared to males [25–28]. Moreover, it has been revealed that serum uric acid level is an independent predictor of worsening coronary endothelial function only in females [29]. Endothelial dysfunction and inflammatory role for serum uric acid in the hypertension population could be a hypothesis that can account for increased arterial stiffness [30–32]. Previous animal model studies have shown that serum uric acid first activates the renin-angiotension system, inhibits nitric oxide, and leads to systemic vascular resistance, followed by a uric acid- mediated vasculopathy, involving afferent arterioles, leading to delayed sodium sensitive hypertension [33]. In addition, Oxidative stress related to uric acid metabolism also plays a critical role in the pathogenesis of hypertension [34]. A cross-sectional and 5-year follow-up study showed that inflammatory gene polymorphisms were not associated with arterial stiffness, but large-scale prospective studies are warranted [35]. The explicit mechanism of serum uric acid in arterial stiffness and its gender- specific effects still remain to be determined.
Several limitations should be considered in the interpretation of our results. First, this study was designed as a cross-sectional study, so the results do not infer causality and need to be further confirmed in a prospective study. Second, the enrolled subjects of our study were hypertension patients, and the use of different antihypertensive drugs and dosage may confound our findings, thus further research needs to examine the effect of different antihypertensive drugs and other concomitant medication on the association between serum uric acid level and arterial stiffness in the hypertension population. Despite the previous limitations, there are several strong points of this study. In particular, our analyzed data were obtained from a large sample of hypertension population, and the sample size of 10450 participants in this study can guarantee reliable conclusions. Additionally, all studied patients were enrolled from wards in hospitals where they information were kept with electronic medical records, which ensure the integrity and authenticity of clinical data. Moreover, because there is a difference in the normal values of uric acid in gender, and the number of females were more than that of males in this study, thus, we analyzed data by sex separately to reduce their bias and to arrive at a more reliable conclusion.
In conclusion, this study demonstrates that serum uric acid level could be considered as an important risk factor for arterial stiffness both in gender in the hypertension population. Further studies are required that have a larger number of patients and follow-up study to define the value of serum uric acid level in arterial stiffness.
MATERIALS AND METHODS
Ethics
This study was performed according to the principles of the Declaration of Helsinki, and was approved by the ethics committee of the Changhai Hospital, Naval Military Medical University, Shanghai, China. We obtained informed consent from patients prior to sample collection.
Design
This was a cross-sectional study, designed to explore the correlation between serum uric acid level and arterial stiffness in the hypertension population. Patients were recruited from the Changhai hospital, No.908 hospital, Baoshan and Chongming district community health service centers in China from January 2008 to June 2017. The data were collected by hospital interviews. Demographics, clinical profile and laboratory data were based on electronic medical records in hospital and health service centers to search for the key words hypertension, cholesterol, arterial stiffness, and serum uric acid to identify all hospitalized patients with hypertension.
Study subjects
Included subjects had a diagnosis of hypertension according to WHO/ISH (1999) diagnostic standards [36]. The inclusion criteria were (1) a blood pressure of ≥140/90 mmHg in individuals not on antihypertensive therapy, or (2) previously diagnosed hypertension in individuals taking antihypertensive therapy, (3) use of the following one or more antihypertensive drugs: diuretics, alpha- and beta-blockers, calcium channel blockers (CCB), angiotensin converting enzyme inhibitor (ACEI) and angiotensin II receptor blockers (ARB), and (4) aged ≥41 years.
The exclusion criteria were (1) unreliable to the questionnaire, (2) unavailable to write documentation, and (3) data were not available for review, including unintegrated clinical and laboratory data.
Clinical and laboratory data
All participants were administered a standardized questionnaire that provided information related to occupation, medical history, past and current medication use, and personal habits such as cigarette, alcohol consumption and dietary habit.
Fasting venous blood samples were collected on admission to measure serum uric acid, gamma-GTP, creatinine, fasting glucose, and lipids, including total cholesterol, HDL cholesterol, and triglycerides. Additionally, systolic blood pressure was taken using an automatic blood pressure cuff, and the value was obtained by averaging three consecutive blood pressure measurements. BMI was calculated from weight and height measurements.
Measurement of baPWV
The baPWV was automatically measured using form PWV/ABI instruments (PWV/ABI, BP-203RPE; Omron-Colin, Japan) as previously described [37, 38] by trained volunteers from local medical colleges. The validations of this automatic device and its reproducibility have been previously published [6]. The measurements of baPWV on both sides of arm and ankle were averaged to obtain the mean baPWV value. High baPWV was defined as the highest quartile of the values among the study subjects [8].
Statistical analysis
Categorical variables were presented as counts and percentages and were analyzed by Fisher’s exact tests or the chi-square tests. Continuous variables were reported as the means and standard deviations for data of normal distribution, which were analyzed by One-Way Anova analysis, and they were reported as medians and interquartile ranges for data of abnormal distribution, which were analyzed by Kruskal-Wallis tests. The association between serum uric acid and baPWV and high baPWV was assessed by linear curve fitting analysis and multiple logistic regression analysis, respectively. Both non-adjusted and multivariate adjusted models were applied, and interaction and stratified analyses were conducted. Serum uric acid level was log-transformed for analyses and evaluated by quartiles. High baPWV was evaluated as categorical variables divided by the highest quartile of the values. Statistical analyses were performed using Statistical Package of the Social Sciences Software version 21.0 (SPSS, Chicago, IL, USA), and statistical graphics were generated using GraphPad PRISM 6 (Graph Pad Software Inc., San Diego, CA, USA). The level of significance was set with a two-tailed p-value of <0.05.
Supplementary Material
Footnotes
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: This study was supported by the Municipal Commission of Health and Family Planning Foundation of Shanghai (20164Y0277). The National Key research and Development Planning (2018YFC2002100, 2018YFC2002101). The National Medical Professional Degree Postgraduate Education Steering Committee Funding of China (B1-YX20180302-06)
REFERENCES
- 1.Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003; 34:1951–56. 10.1161/01.STR.0000081983.34771.D2 [DOI] [PubMed] [Google Scholar]
- 2.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007; 116:894–900. 10.1161/CIRCULATIONAHA.107.703389 [DOI] [PubMed] [Google Scholar]
- 3.Ramirez AJ, Christen AI, Sanchez RA. Serum uric acid elevation is associated to arterial stiffness in hypertensive patients with metabolic disturbances. Curr Hypertens Rev. 2018; 14:154–60. 10.2174/1573402114666180413143312 [DOI] [PubMed] [Google Scholar]
- 4.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the framingham heart study. Ann Intern Med. 1999; 131:7–13. 10.7326/0003-4819-131-1-199907060-00003 [DOI] [PubMed] [Google Scholar]
- 5.Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a chinese cohort study. Arthritis Rheum. 2009; 61:225–32. 10.1002/art.24164 [DOI] [PubMed] [Google Scholar]
- 6.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002; 25:359–64. 10.1291/hypres.25.359 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009; 27:2022–27. 10.1097/HJH.0b013e32832e94e7 [DOI] [PubMed] [Google Scholar]
- 8.Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Higher serum uric acid is associated with increased arterial stiffness in Japanese individuals. Atherosclerosis. 2007; 192:131–37. 10.1016/j.atherosclerosis.2006.04.016 [DOI] [PubMed] [Google Scholar]
- 9.Antonini-Canterin F, Di Nora C, Pellegrinet M, Vriz O, La Carrubba S, Carerj S, Zito C, Matescu A, Ravasel A, Cosei I, Popescu BA. Effect of uric acid serum levels on carotid arterial stiffness and intima-media thickness: A high resolution Echo-Tracking Study. Monaldi Arch Chest Dis. 2019; 89. 10.4081/monaldi.2019.1007 [DOI] [PubMed] [Google Scholar]
- 10.Kuo CF, Yu KH, Luo SF, Ko YS, Wen MS, Lin YS, Hung KC, Chen CC, Lin CM, Hwang JS, Tseng WY, Chen HW, Shen YM, See LC. Role of uric acid in the link between arterial stiffness and cardiac hypertrophy: a cross-sectional study. Rheumatology (Oxford). 2010; 49:1189–96. 10.1093/rheumatology/keq095 [DOI] [PubMed] [Google Scholar]
- 11.Canepa M, Viazzi F, Strait JB, Ameri P, Pontremoli R, Brunelli C, Studenski S, Ferrucci L, Lakatta EG, AlGhatrif M. Longitudinal association between serum uric acid and arterial stiffness: results from the baltimore longitudinal study of aging. Hypertension. 2017; 69:228–35. 10.1161/HYPERTENSIONAHA.116.08114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlachopoulos C, Xaplanteris P, Vyssoulis G, Bratsas A, Baou K, Tzamou V, Aznaouridis K, Dima I, Lazaros G, Stefanadis C. Association of serum uric acid level with aortic stiffness and arterial wave reflections in newly diagnosed, never-treated hypertension. Am J Hypertens. 2011; 24:33–39. 10.1038/ajh.2010.111 [DOI] [PubMed] [Google Scholar]
- 13.Antza C, Papakatsika S, Kotronis G, Mikoudi K, Stabouli S, Kotsis V. 9A.01: Hyperuricemia is an independent determinant of arterial stiffness. J Hypertens. 2015; 33 Suppl 1:e117 10.1097/01.hjh.0000467664.99650.46 [DOI] [PubMed] [Google Scholar]
- 14.Cicero AF, Rosticci M, Fogacci F, Grandi E, D’Addato S, Borghi C, and Brisighella Heart Study Group. High serum uric acid is associated to poorly controlled blood pressure and higher arterial stiffness in hypertensive subjects. Eur J Intern Med. 2017; 37:38–42. 10.1016/j.ejim.2016.07.026 [DOI] [PubMed] [Google Scholar]
- 15.Bae JS, Shin DH, Park PS, Choi BY, Kim MK, Shin MH, Lee YH, Chun BY, Kim SK. The impact of serum uric acid level on arterial stiffness and carotid atherosclerosis: the korean multi-rural communities cohort study. Atherosclerosis. 2013; 231:145–51. 10.1016/j.atherosclerosis.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 16.Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Hypertension is the most common component of metabolic syndrome and the greatest contributor to carotid arteriosclerosis in apparently healthy Japanese individuals. Hypertens Res. 2005; 28:27–34. 10.1291/hypres.28.27 [DOI] [PubMed] [Google Scholar]
- 17.Smulyan H, Lieber A, Safar ME. Hypertension, diabetes type II, and their association: role of arterial stiffness. Am J Hypertens. 2016; 29:5–13. 10.1093/ajh/hpv107 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF. Arterial stiffness and hypertension. Hypertension. 2014; 64:13–18. 10.1161/HYPERTENSIONAHA.114.00921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verwoert GC, Franco OH, Hoeks AP, Reneman RS, Hofman A, V Duijn CM, Sijbrands EJ, Witteman JC, Mattace-Raso FU. Arterial stiffness and hypertension in a large population of untreated individuals: the rotterdam study. J Hypertens. 2014; 32:1606–12. 10.1097/HJH.0000000000000237 [DOI] [PubMed] [Google Scholar]
- 20.Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015; 65:252–6. 10.1161/HYPERTENSIONAHA.114.03617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility—reykjavik study. Brain. 2011; 134:3398–407. 10.1093/brain/awr253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the framingham heart study. Hypertension. 2004; 43:1239–45. 10.1161/01.HYP.0000128420.01881.aa [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Shi S, Zhao XJ, Wang JK, Liu ZW, Liu FQ, Zhu L, Zhu SM, Zhang Y, Pan S. Association between the lipid profile and renal dysfunction in the heart failure patients. Kidney Blood Press Res. 2019; 44:52–61. 10.1159/000498834 [DOI] [PubMed] [Google Scholar]
- 24.Shin JY, Lee HR, Shim JY. Significance of high-normal serum uric acid level as a risk factor for arterial stiffness in healthy korean men. Vasc Med. 2012; 17:37–43. 10.1177/1358863X11434197 [DOI] [PubMed] [Google Scholar]
- 25.Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis. 2003; 166:303–09. 10.1016/s0021-9150(02)00332-5 [DOI] [PubMed] [Google Scholar]
- 26.De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis. 2010; 69:1162–64. 10.1136/ard.2009.122770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsurer R, Afsar B. Serum uric acid and arterial stiffness in hypertensive chronic kidney disease patients: sex-specific variations. Blood Press Monit. 2014; 19:271–79. 10.1097/MBP.0000000000000056 [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Xiang G, Xiang L, Sun H. Serum uric acid is associated with arterial stiffness in men with newly diagnosed type 2 diabetes mellitus. J Endocrinol Invest. 2014; 37:441–47. 10.1007/s40618-013-0034-9 [DOI] [PubMed] [Google Scholar]
- 29.Kuwahata S, Hamasaki S, Ishida S, Kataoka T, Yoshikawa A, Orihara K, Ogawa M, Oketani N, Saihara K, Okui H, Shinsato T, Kubozono T, Ichiki H, et al. Effect of uric acid on coronary microvascular endothelial function in women: association with eGFR and ADMA. J Atheroscler Thromb. 2010; 17:259–69. 10.5551/jat.1594 [DOI] [PubMed] [Google Scholar]
- 30.Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension. 2010; 55:9–14. 10.1161/HYPERTENSIONAHA.107.090464 [DOI] [PubMed] [Google Scholar]
- 31.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005; 67:1739–42. 10.1111/j.1523-1755.2005.00273.x [DOI] [PubMed] [Google Scholar]
- 32.Wan QL, Fu X, Dai W, Yang J, Luo Z, Meng X, Liu X, Zhong R, Yang H, Zhou Q. Uric acid induces stress resistance and extends the life span through activating the stress response factor DAF-16/FOXO and SKN-1/NRF2. Aging (Albany NY). 2020; 12:2840–56. 10.18632/aging.102781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feig DI. Uric acid and hypertension. Semin Nephrol. 2011; 31:441–46. 10.1016/j.semnephrol.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 34.De Becker B, Borghi C, Burnier M, van de Borne P. Uric acid and hypertension: a focused review and practical recommendations. J Hypertens. 2019; 37:878–83. 10.1097/HJH.0000000000001980 [DOI] [PubMed] [Google Scholar]
- 35.Kheradmand M, Niimura H, Kuwabara K, Nakahata N, Nakamura A, Ogawa S, Mantjoro EM, Shimatani K, Nerome Y, Owaki T, Kusano K, Takezaki T. Association of inflammatory gene polymorphisms and conventional risk factors with arterial stiffness by age. J Epidemiol. 2013; 23:457–65. 10.2188/jea.je20130054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, Neal B, Rodgers A, Ni Mhurchu C, Clark T. 1999 world health organization-international society of hypertension guidelines for the management of hypertension. Guidelines sub-committee of the world health organization. Clin Exp Hypertens. 1999; 21:1009–60. 10.3109/10641969909061028 [DOI] [PubMed] [Google Scholar]
- 37.Chen SC, Chang JM, Liu WC, Tsai YC, Tsai JC, Hsu PC, Lin TH, Lin MY, Su HM, Hwang SJ, Chen HC. Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol. 2011; 6:724–32. 10.2215/CJN.07700910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura M, Yamashita T, Yajima J, Oikawa Y, Sagara K, Koike A, Kirigaya H, Nagashima K, Sawada H, Aizawa T, and Shinken Database Study Group. Brachial-ankle pulse wave velocity as a risk stratification index for the short-term prognosis of type 2 diabetic patients with coronary artery disease. Hypertens Res. 2010; 33:1018–24. 10.1038/hr.2010.126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.