Abstract
Intracerebral haemorrhage in the elderly is a severe manifestation of common forms of cerebral small vessel disease. Nearly 60% of intracerebral haemorrhage survivors will develop clinical manifestations of small vessel disease progression including recurrent haemorrhage, ischaemic stroke, dementia, late-life depression and gait impairment within 5 years. Blood pressure measurements following intracerebral haemorrhage are strongly associated with this risk. However, aggressive blood pressure lowering in the elderly carries substantial risks. In order to determine whether there might be an opportunity to select individuals at the highest risk for small vessel disease progression for aggressive blood pressure reduction, we investigated whether APOE gene variants ɛ2/ɛ4 modify the association between blood pressure and small vessel disease clinical progression after intracerebral haemorrhage. We conducted a single-centre longitudinal study at a tertiary care referral centre (Massachusetts General Hospital in Boston, MA, USA), analysing 716 consecutive survivors of acute intracerebral haemorrhage, enrolled from January 2006 to December 2016. We conducted research interviews at the time of enrolment and obtained APOE genotypes from peripheral venous blood samples. We followed patients longitudinally by means of validated phone-based research encounters, aimed at gathering measurements of systolic and diastolic blood pressure, as well as information on small vessel disease clinical outcomes (including recurrent haemorrhage, incident ischaemic stroke, incident dementia, incident depression and incident gait impairment). APOE ε4 and systolic blood pressure were associated with the risk of recurrent haemorrhage, ischaemic stroke and post-haemorrhage dementia, depression and gait impairment (all P < 0.05). APOE ε4 and systolic blood pressure interacted to increase the risk of recurrent haemorrhage, ischaemic stroke, dementia and gait impairment (all interaction P < 0.05). Among patients with elevated blood pressure following intracerebral haemorrhage (average systolic blood pressure 120–129 mmHg and diastolic blood pressure <80 mmHg) only those with one or more APOE ε4 copies were at increased risk for one or more small vessel disease outcomes (hazard ratio = 1.97, 95% confidence interval 1.17–3.31). Among haemorrhage survivors with hypertension (stage 1 and beyond) APOE genotype also stratified risk for all small vessel disease outcomes. In conclusion, APOE genotype modifies the already strong association of hypertension with multiple small vessel disease clinical outcomes among intracerebral haemorrhage survivors. These data raise the possibility that genetic screening could inform blood pressure treatment goals in this patient population.
Keywords: intracerebral haemorrhage, hypertension, APOE
Intracerebral haemorrhage is a severe manifestation of cerebral small vessel disease, alongside ischaemic stroke, dementia, depression and gait impairment. We found that systolic blood pressure and APOE ε4 interact to increase risk of small vessel disease disorders. Genetic screening may, therefore, inform hypertension treatment goals for intracerebral haemorrhage survivors.
Graphical Abstract
Graphical Abstract.
Introduction
Intracerebral haemorrhage (ICH) is the most severe form of stroke, accounting for 10–15% of all acute cerebrovascular events, and for ∼50% of stroke-related mortality and disability (Qureshi et al., 2001; Poon et al., 2014). Most spontaneous ICH cases are the acute manifestation of age-related cerebral small vessel disease (SVD; Pantoni, 2010; Biffi and Greenberg, 2011). ICH survivors are, therefore, at high risk for all manifestations of progressive SVD: recurrent ICH, ischaemic stroke (especially small vessel infarcts), cognitive impairment, late-life depression and gait impairment (Pantoni, 2010; Benedictus et al., 2015; Biffi et al., 2016; Moulin et al., 2016). The APOE gene has been robustly associated with SVD and with ICH risk; indeed, APOE variants ɛ2 and ɛ4 represent by far the most potent genetic risk factors for ICH (Greenberg et al., 1995; Tzourio et al., 2008; Biffi et al., 2010b).
While blood pressure (BP) control is widely advocated as effective for reducing ICH risk, the optimal degree of BP reduction remains controversial. Published ICH management guidelines recommend achieving goals of Systolic BP (SBP) <130 mmHg and Diastolic BP (DBP) <80 mmHg for secondary prevention (Hemphill et al., 2015). However, findings from randomized trials and a large meta-analysis suggest that individuals at high risk for cardiovascular diseases, as ICH survivors often are, benefit from achieving normal BP (i.e. SBP < 120 and DBP < 80 mmHg; Ettehad et al., 2016; Group et al., 2015). Indeed, the revised ACC/AHA guidelines recently proposed more stringent BP control goals for the general population (Whelton et al., 2018). We ourselves reported increased ICH recurrence risk among individuals with average SBP 120–129 mmHg (Biffi et al., 2015). On the other hand, pharmacological BP reduction in the elderly (the population most at risk for ICH) has been associated with increased risk of ischaemic stroke, cognitive impairment and gait impairment/falls (Eigenbrodt et al., 2000; Hyman and Taffet, 2009; Rose et al., 2010; Butt and Harvey, 2015).
Because of substantial variation in ICH risk based on APOE genotype, this genetic information may be of assistance in guiding BP management among ICH survivors. We, therefore, sought to test whether APOE genetic variation influences the association between BP and ICH recurrence risk, as well as the risk of other clinical manifestations of progressive SVD (ischaemic stroke, dementia, late-life depression and gait impairment) in a cohort of consecutive ICH survivors.
Materials and methods
Patient recruitment and baseline data collection
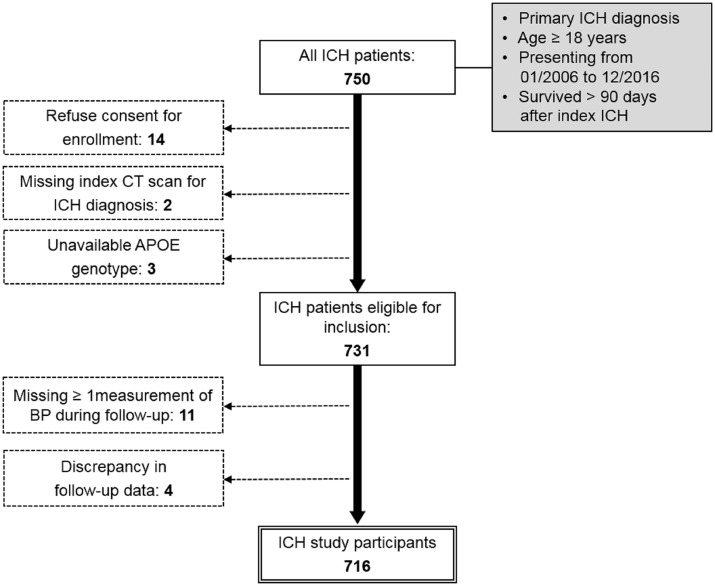
All participants were enrolled in an ongoing single-centre longitudinal cohort study of ICH as previously described (Biffi et al., 2010a; Biffi et al., 2012; Biffi et al., 2015), and selected based on the following inclusion criteria: (i) age ≥18 years; (ii) admitted to Massachusetts General Hospital from January 2006 to December 2016; (iii) diagnosed with spontaneous ICH confirmed by CT scan (Fig. 1); and (iv) survived at least 90 days after index ICH (Biffi et al., 2015). APOE genotypes for variants ɛ2 and ɛ4 were determined from DNA samples derived from peripheral venous blood, drawn at time of enrolment (Biffi et al., 2010b; Biffi et al., 2011). The study protocol was approved by the Massachusetts General Hospital Institutional Review Board. Written informed consent was obtained from all study participants or their surrogates. Additional information on recruitment and data collection can be found in the Supplementary Appendix.
Figure 1.
Study inclusion/exclusion criteria and enrolment flow diagram. Figure presents study design, inclusion/exclusion criteria and composition of patient group retained for analysis. Solid single-line boxes represent subjects meeting criteria for inclusion in the present study at each step. Eligibility criteria for inclusion in the study are listed in grey-background boxes. Dashed lines connect to dashed-bordered boxes listing criteria for exclusion and the number of subjects excluded. The double-line bordered box indicates the final study group selected for analyses mentioned in the Results section. ICH = intracerebral haemorrhage.
Longitudinal follow-up
ICH survivors and/or their caregivers were contacted and interviewed by dedicated study staff at 3, 6, 12 months after index ICH, and every 6 months thereafter, based on established protocols (Biffi et al., 2015). Investigators obtained information about ICH recurrence, ischaemic stroke (and subtype based on the TOAST method; Adams et al., 1993), cognitive impairment, gait impairment, mood impairment, death and medication use/dosing. Cognitive performance was evaluated using the Modified Telephone Interview for Cognitive Status (TICS-m) test and the 16-item (short) version of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE-16; Brandt et al., 1988; Jorm, 1994; de Jager et al., 2003; Barber and Stott, 2004; Knopman et al., 2010; Seo et al., 2011; Pendlebury et al., 2013). Incident depression was identified using the four-item version of the Geriatric Depression Scale (GDS-4; Almeida and Almeida, 1999; Pocklington et al., 2016). Gait impairment was defined as a newly developed requirement for assistance (caregiver or device) for everyday ambulation, as determined by the patient and/or caregiver report. Additional information on follow-up methodology can be found in the Supplementary Appendix. At each follow-up, time-point study staff also captured information on BP measurements as previously described (Biffi et al., 2015). In brief, study staff inquired about the most recent BP measurements obtained in a medical setting by medical personnel. If participants could not provide reliable BP measurements, medical records were obtained for review. We pre-specified data capture targets of ≥1 BP measurement per 6-month period.
Variables’ definitions
APOE genotype was represented by two dichotomous variables indicating presence versus absence of at least one copy of ɛ2 or ɛ4. We defined incident dementia for outcome analyses as meeting at least one of these criteria: (i) subjects assigned TICS-m scores <20 (Barber and Stott, 2004; Pendlebury et al., 2013); and (ii) subjects assigned average IQCODE-16 score >3.3 (Harrison et al., 2014, 2015). We defined incident depression as GDS-4 score >2 (Almeida and Almeida, 1999). Gait impairment was defined as described above. To assess the role of BP in ICH recurrence we initially analysed two time-varying variables: (i) SBP as a continuous variable; and (ii) DBP as a continuous variable (Biffi et al., 2015). We also analysed the following hypertension categories based on the 2017 American College of Cardiology/American Heart Association (ACC/AHA) high BP guidelines (Whelton et al., 2018): (i) normal BP (SBP <120 mmHg and DBP <80 mmHg); (ii) elevated BP (SBP 120–129 mmHg and DBP <80 mmHg); (iii) hypertension stage 1 (SBP 130–139 mmHg or DBP 80–90 mmHg); (iv) hypertension stage 2 (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg).
Statistical analysis
Separate statistical models were created for each outcome of interest, as well as for a composite outcome including all SVD-related clinical diagnoses (recurrent ICH, ischaemic stroke, dementia, depression and gait impairment). We determined factors associated with each outcome using log-rank tests (univariable analyses) and Cox regression (multivariable analyses). Additional details on multivariable modelling are provided in the Supplementary Appendix. We conducted interaction analyses for SBP/DBP with APOE ɛ2/ɛ4 if primary terms were found to be significant in multivariable analyses. We then separately performed analyses stratifying subjects by both hypertension severity (based on the 2017 ACC/AHA guidelines) and APOE genotype. We estimated yearly risk for SVD outcomes of interest for graphical plotting purposes, by combining the Nelson-Aalen cumulative hazard function with the Cox model determined statistical risk effects using the predictSurvProb function in the pec R package. Estimated risks were graphically subdivided based on: (i) number of APOE ɛ4 copies and (ii) hypertension severity (per 2017 ACC/AHA guidelines) during follow-up. We found that SVD outcomes showed significant correlation with each other (Supplementary Table 1), and therefore, did not meet the criteria for Bonferroni adjustment. We, therefore, addressed multiple testing burden by adopting the false discovery rate method (Keselman et al., 2002.). All significance tests were two-tailed, and the significance threshold was set at P < 0.05 (after false discovery rate adjustment). All analyses were performed with R software v 3.5.2 (The R Foundation for Statistical Computing). Additional information on the statistical methodology can be found in the Supplementary Appendix.
Literature review and attempted replication of results
To attempt external replication of our findings, we conducted a search of published literature and publicly available data, to identify suitable datasets for analysis. We searched PubMed, Embase, Ovid, Google Scholar, Dryad, Figshare, Zenodo and OSF for articles and data published prior to August 2018, using a dedicated electronic search strategy (see details in Supplementary Appendix). We selected for further manual review studies that (i) included only patients diagnosed with primary (i.e. spontaneous) ICH; and (ii) studies that had either APOE genotype or BP data available in the original dataset.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Study participants
A total of 750 patients met the initial criteria for inclusion (Fig. 1). Patients who declined consent (n = 14) were missing an index CT scan (n = 2) or had no available APOE genotype (n = 3) were excluded from all analyses. A total of 11 participants were missing BP measurements for one or more 6-month periods (Fig. 1), and were removed from all analyses; their forced re-introduction did not alter results substantially (Supplementary Table 2). Discrepancies between telephone-collected and EMR-collected follow-up data resulted in the removal of four patients from the present study (Fig. 1), and their removal did not alter results substantially (Supplementary Table 3). A total of 716 patients (Table 1) were, therefore, included in our analyses.
Table 1.
Participants’ characteristics
| Variable | No. of subjects | % |
|---|---|---|
| 716 | 100 | |
| Demographics | ||
| Age (mean, SD) | 70.5 (12.2) | |
| Sex (male) | 385 | 53.8 |
| Race/ethnicity | ||
| European American | 599 | 83.7 |
| African American | 47 | 6.6 |
| Asian American | 27 | 3.8 |
| Hispanic | 38 | 5.3 |
| Other | 5 | 0.7 |
| Education (≥10 years) | 418 | 58.4 |
| ICH location | ||
| Lobar | 323 | 45.1 |
| Non-lobar | 393 | 54.9 |
| Pre-ICH medical history | ||
| Hypertension | 531 | 74.2 |
| Ischaemic heart disease | 129 | 18.0 |
| Atrial fibrillation | 131 | 18.3 |
| Diabetes | 138 | 19.3 |
| Pre-ICH dementia | 64 | 8.9 |
| Pre-ICH mood disorder | 99 | 13.8 |
| Pre-ICH gait impairment | 34 | 4.8 |
| Prior ICH (before index event) | 37 | 5.2 |
| Prior ischaemic stroke/TIA | 85 | 11.9 |
| APOE genotype | ||
| APOE ɛ2 (frequency) | 0.09 | |
| APOE ɛ4 (frequency) | 0.19 | |
| Post-ICH medication use | ||
| Antiplatelet agents | 115 | 16.0 |
| Warfarin | 75 | 10.5 |
| Statins | 278 | 38.8 |
| Antihypertensive agents | 509 | 71.0 |
| SSRI | 236 | 32.9 |
ICH = intracerebral haemorrhage; SSRI = selective serotonin reuptake inhibitors.
Follow-up information and post-ICH outcome rates
During a median follow-up time of 52.8 months [inter-quartile range (IQR) 29.8–69.5), we observed an average rate of loss to follow-up of 1.4% per year. We observed a total of 89 recurrent ICH events among 716 study participants, corresponding to a recurrence rate of 3.4%/year (95% CI 2.1–5.4%), and 59 ischaemic stroke events, corresponding to an incidence rate of 2.1%/year (95% CI 1.4–3.0%). Among ischaemic stroke events, 21 (36%) were categorized as small vessel stroke by TOAST criteria, corresponding to an incidence rate of 0.9%/year (95% CI 0.3–1.4%). A total of 122/716 (17%) ICH survivors developed new-onset dementia, corresponding to an incidence rate of 5.2%/year (95% CI 4.6–5.7%). We observed that 182/716 (25%) ICH survivors developed new-onset depression, corresponding to an incidence rate of 6.4%/year (95% CI 5.7–6.9%). Finally, 95/716 (13%) participants developed new-onset gait impairment, corresponding to an estimated incidence rate of 3.6%/year (95% CI 3.1–4.0%). We present detailed information on study sample size, mortality, loss to follow-up and post-ICH outcomes during the first 5 years of follow-up in Supplementary Table 4.
APOE genotype, hypertension and outcome after intracerebral haemorrhage
In univariable analyses (Supplementary Table 5), SBP and APOE ɛ4 were associated with risk of recurrent ICH, small vessel ischaemic stroke, incident dementia, incident depression and incident gait impairment. These findings were confirmed in multivariable analyses (Table 2), after adjustment for relevant covariates (see Supplementary Appendix for additional details).
Table 2.
Multivariable analyses of association between APOE, BP and post-ICH outcomes
| Risk factorsa | Post-ICH outcomes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICH recurrence |
Small vessel ischaemic stroke |
Incident dementia |
Incident depression |
Gait impairment |
||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| APOE genotype | ||||||||||
| APOE ε2(≥ 1 copy) | 1.26 (0.56—2.83) | 0.58 | 1.06 (0.84–1.33) | 0.62 | 1.32 (0.79–2.20) | 0.29 | 0.89 (0.45–1.78) | 0.74 | 1.21 (0.80–1.83) | 0.37 |
| APOE ε4(≥ 1 copy) | 1.87 (1.20–2.92) | 0.006 | 1.19 (1.01–1.41) | 0.047 | 1.85 (1.21–1.84) | 0.005 | 1.70 (1.10–2.63) | 0.018 | 1.56 (1.12–2.18) | 0.01 |
| BP measures | ||||||||||
| SBP (10 mmHg increase) | 1.33 (1.06–1.66) | 0.012 | 1.25 (1.01–1.57) | 0.039 | 1.69 (1.14–2.50) | 0.009 | 1.23 (1.01–1.50) | 0.049 | 1.45 (1.14–1.85) | 0.003 |
| DBP (10 mmHg increase) | 1.09 (0.99–1.20) | 0.080 | 1.20 (0.87–1.65) | 0.26 | 0.96 (0.88–1.04) | 0.33 | 1.29 (0.59–2.83) | 0.53 | 1.03 (0.92–1.15) | 0.60 |
All models included the following covariates for adjustment: self-reported race/ethnicity, history of prior ICH (lobar and/or non-lobar), educational level, ICH location, antiplatelet agent use and warfarin use.
DBP = diastolic blood pressure; ICH = intracerebral haemorrhage; SBP = systolic blood pressure.
We subsequently tested for interaction between SBP and APOE ɛ4 in multivariable analyses, and found association with increased risk of recurrent ICH, small vessel ischaemic stroke, incident dementia and incident gait impairment (Supplementary Table 6, interaction P < 0.05 for ICH recurrence, small vessel ischaemic stroke, dementia and gait impairment). We repeated multivariable analyses for dementia, depression and gait impairment for subjects who did not experience ICH or ischaemic stroke during follow-up (n = 566). In this subset, we identified consistent interaction between SBP and APOE ɛ4 in determining the risk of dementia and gait impairment (Supplementary Table 7).
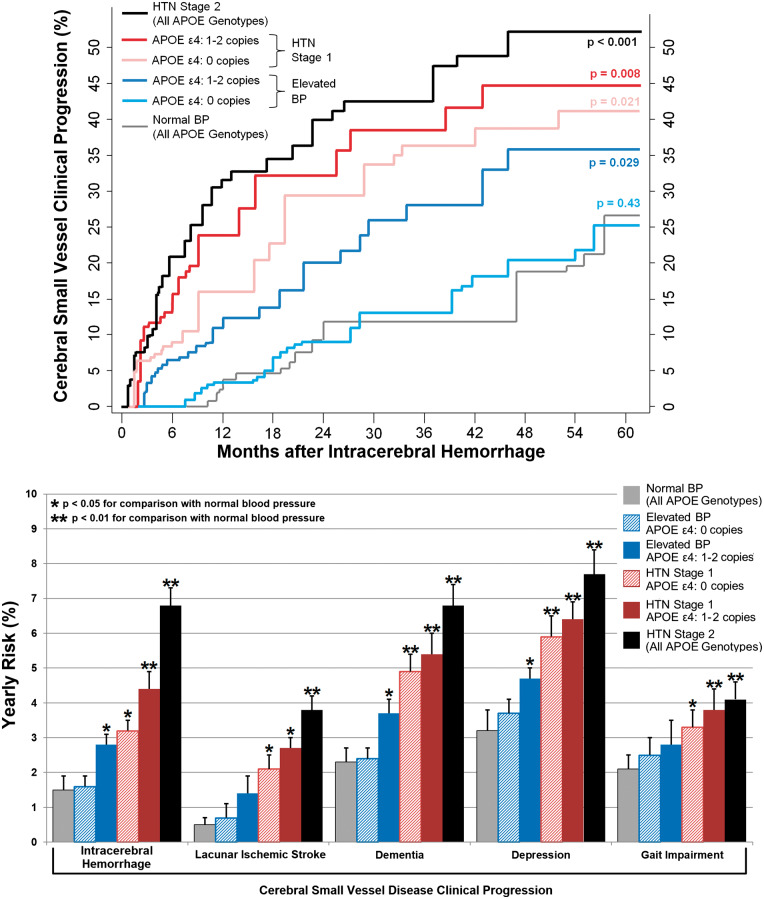
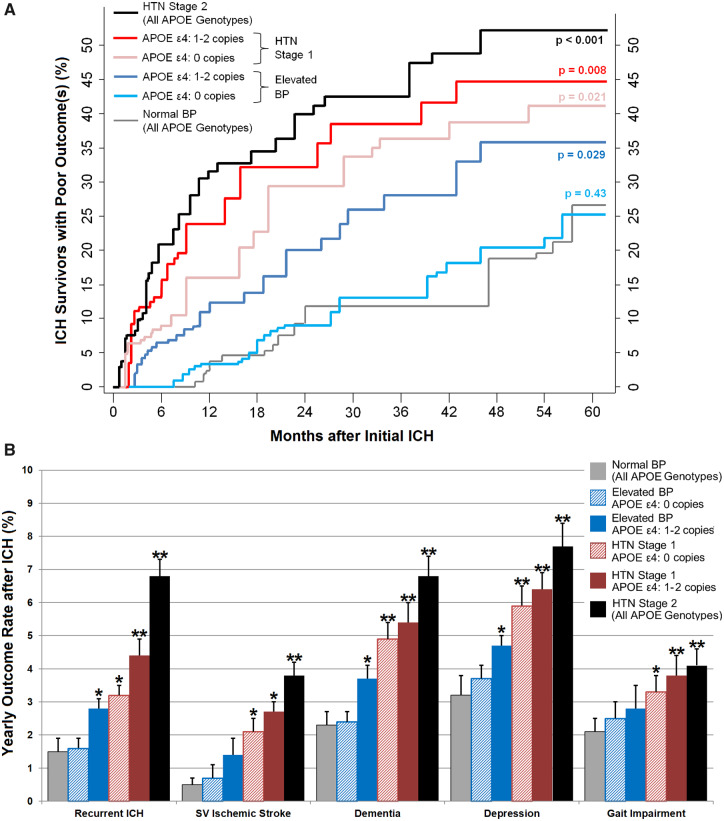
To quantify APOE-dependent effects on the relationship between BP and post-ICH outcomes, we first explored associations between APOE ɛ4 and the composite post-ICH poor outcome endpoint (including recurrent ICH, small vessel ischaemic stroke, dementia, depression and gait impairment) within each hypertension severity category. We found that APOE ɛ4 was associated with increased risk for poor outcome among patients with elevated BP, hypertension stages 1 and 2 (Supplementary Table 8). We then repeated all multivariable analyses after stratification for hypertension severity (according to 2017 ACC/AHA guidelines) and number of ɛ4 copies (Table 3). These analyses demonstrated that non-hypertensive ICH survivors with elevated BP (i.e. SBP of 120–129 mmHg and DBP <80 mmHg) were at increased risk for a composite endpoint of recurrent ICH, small vessel ischaemic stroke, dementia, depression and gait impairment only if they possessed one or more APOE ɛ4 copies: hazard ratio (HR) = 1.97, 95% confidence interval (CI) 1.17–3.31, P = 0.011 for comparison between participants with elevated BP with versus without APOE ɛ4 (Fig. 2A). Specifically, APOE ɛ4 carriers with elevated BP showed significant differences in risks for recurrent ICH (HR = 2.11, 95% CI 1.06–4.21, P = 0.036), dementia (HR = 1.89, 95% CI 1.05–3.41, P = 0.037) and depression (HR = 1.66, 95% CI 1.02–2.70, P = 0.044) as detailed in Fig. 2B. ICH survivors with hypertension Stage 1 and beyond were also at increased risk for poor outcomes after ICH, with APOE genotype further increasing risk among those with one or more ɛ4 copies (Fig. 2A and B). We provide detailed results of association analyses for the composite post-ICH poor outcome endpoint (including recurrent ICH, small vessel ischaemic stroke, dementia, depression and gait impairment), stratified by APOE genotype and hypertension severity, in Supplementary Table 9. Finally, we estimated whether modelling the SBP/APOE ɛ4 genotype interaction improved predictive ability for post-ICH outcomes, and found significant differences (compared with models including both variables but no interaction term) for prediction of future risk of ICH recurrence, ischaemic stroke, dementia, depression and gait impairment (Supplementary Table 10).
Table 3.
Hypertension severity and post-ICH outcomes, stratified by APOE ε4 genotype
| Risk factorsa | Post-ICH outcomes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICH recurrence |
Small vessel stroke |
Incident dementia |
Incident depression |
Gait impairment |
||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| APOE ε4: 0 copies | ||||||||||
| Normal BP | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Elevated BP | 1.68 (0.54–5.20) | 0.19 | 1.39 (0.92–2.10) | 0.12 | 1.23 (0.60–2.50) | 0.57 | 1.36 (0.98–1.88) | 0.07 | 1.12 (0.95–1.32) | 0.20 |
| Hypertension: Stage 1 | 2.21 (1.14–4.30) | 0.020 | 1.88 (1.02–3.46) | 0.044 | 2.14 (1.25–3.67) | 0.006 | 1.54 (1.10–2.16) | 0.013 | 1.81 (0.91–3.59) | 0.10 |
| Hypertension: Stage 2 | 3.54 (1.40–8.92) | 0.004 | 2.19 (1.07–4.47) | 0.033 | 2.58 (1.23–5.42) | 0.013 | 2.39 (1.26–4.53) | 0.009 | 2.35 (1.19–4.46) | 0.015 |
| APOE ε4: ≥1 copy | ||||||||||
| Normal BP | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Elevated BP | 2.11 (1.23–3.63) | 0.004 | 1.69 (1.08–2.64) | 0.022 | 1.90 (1.22–2.95) | 0.004 | 1.25 (0.88–1.78) | 0.22 | 1.74 (1.21–1.50) | 0.003 |
| Hypertension: Stage 1 | 3.05 (1.14–6.46) | 0.002 | 2.49 (1.25–4.95) | 0.01 | 2.81 (1.27–6.20) | 0.011 | 1.60 (1.15–2.23) | 0.007 | 2.84 (1.20–6.71) | 0.018 |
| Hypertension: Stage 2 | 4.21 (1.53–11.59) | 0.003 | 3.12 (1.41–6.92) | 0.006 | 4.08 (1.58–10.41) | 0.004 | 2.77 (1.23–6.21) | 0.015 | 3.33 (1.34–8.25) | 0.01 |
All models included the following covariates for adjustment: self-reported race/ethnicity, history of prior ICH, educational level, ICH location, antiplatelet agent use and warfarin use.
ICH = intracerebral haemorrhage; BP = blood pressure.
Figure 2.
Risk of poor outcomes after ICH, based on APOE genotype and hypertension severity during follow-up. (A) Kaplan–Meier estimates of risk for composite poor post-ICH outcome, including: (i) recurrent ICH; (ii) ischaemic stroke (all subtypes); (iii) incident dementia; (iv) incident depression; and (v) incident gait impairment. Risk distributions are separated based on APOE genotype (ε4: 0 copies versus 1–2 copies) and hypertension severity during follow-up. P-values are calculated for each group in reference to normotensive subjects (regardless of APOE genotype) using the Log-rank test. (B) Estimates of yearly risk for individual post-ICH outcomes of interest, stratified by APOE genotype (ε4: 0 copies versus 1–2 copies) and hypertension severity during follow-up. Vertical error bars indicate one standard deviation in risk estimate. Single asterisk indicates P-value < 0.05 for comparison with normotensive subjects (regardless of APOE genotype) using the Log-rank test. Double asterisks indicate P-value < 0.01 for comparison with normotensive subjects (regardless of APOE genotype) using the Log-rank test. ICH = intracerebral haemorrhage; SV = small vessel.
Systematic review and attempted replication of results
After reviewing published literature and publicly available data, we identified 50 original reports of ICH survivors (see Supplementary Appendix). Of these, 16 were conducted at Massachusetts General Hospital and enrolled participants also included in the present study. Of the remaining 34 studies, 16 included APOE genotype for enrolled ICH survivors and only 2 had available BP data during follow-up. Of the latter, one included APOE genotype but only evaluated ICH recurrence as the outcome of interest. We, therefore, concluded that independent replication of our findings was not feasible at the time of our analyses.
Discussion
We demonstrate that APOE ɛ4 interacts with BP following primary ICH to increase the risk for recurrent ICH, small vessel ischaemic stroke, incident dementia and incident gait impairment. This effect extended to non-hypertensive ICH survivors with SBP of 120–129 mmHg and DBP <80 mmHg) who were at increased risk for a composite endpoint of recurrent ICH, small vessel ischaemic stroke, dementia, depression and gait impairment only if they possessed APOE ɛ4. Thus, APOE genotype identified high-risk individuals who would otherwise be deemed to be relatively low risk. These results, which represent a unique example of interaction between a common genetic variant and a modifiable risk factor (BP) to influence multiple outcomes for a prevalent, highly relevant neurological condition.
The finding that APOE ɛ4 modifies the association of BP with multiple common clinical manifestations of SVD among ICH survivors has clinical implications. First and foremost, counselling of ICH patients and their caregivers may benefit from the inclusion of APOE genotype. Carriers of ɛ4 might be selected for closer BP monitoring and/or more aggressive management. From a broader perspective, published guidelines for BP reduction following ICH may merit reconsideration and inclusion of genetic information. We also demonstrated that modelling the interaction between APOE ɛ4 and BP improved the predictive capability for most outcomes of interest. This is relevant for future research studies in the field of ICH and SVD; more accurate modelling of outcome risk would allow investigators to design randomized controlled trials focused on highest-risk individuals, thus maximizing success rate for identification of truly beneficial interventions (Stanley, 2007). Furthermore, clarification of the biological basis for the described interaction between APOE genotype and BP control may well highlight novel aspects of SVD pathophysiology, thus offering additional targets for potential intervention. Finally, the opportunity to act on a modifiable risk factor based on easily ascertained genetic data would represent an ideal ‘sandbox’ to explore psychological, economic and societal implications of potential upcoming advances in precision medicine (Gray et al., 2014; Collins and Varmus, 2015; Lander, 2015).
APOE genotype plays a crucial role in determining the risk and severity of cerebral amyloid angiopathy (CAA), a common form of cerebral SVD characterized by accumulation of amyloid-β (Aβ) in the CNS leptomeningeal medium and small arteries (Rannikmae et al., 2013). Common clinical manifestations of CAA include ICH, lacunar ischaemic stroke, cognitive and gait impairment (Biffi and Greenberg, 2011). As previously described for parenchymal Aβ accumulation, hypertension severity likely leads to worsening damage to CAA-prone arterial vessels among APOE ɛ4 carriers, increasing the risk for a variety of associated clinical outcomes (de Leeuw et al., 2004; de Frias et al., 2014; Kester et al., 2014). Due to the frequent co-existence of vascular Aβ pathology (in the form of CAA) and parenchymal Aβ pathology (in the form of Alzheimer’s disease), a proportion of the interaction effects we identified likely reflects known relationships between hypertension severity and Alzheimer’s disease progression (Smith and Greenberg, 2009). Taken together, these considerations reflect the established role of APOE in risk for Alzheimer’s disease/CAA by dint of their role in Aβ aggregation, deposition and clearance (Kanekiyo et al., 2014). However, APOE gene products also play a critical role in non-amyloid biological pathways, including inflammatory response (Tai et al., 2015), CNS lipid homeostasis (Mahley, 2016), neurogenesis and synaptic plasticity (Liu et al., 2013; Kim et al., 2014) and mitochondrial resistance to oxidative stress (Jofre-Monseny et al., 2008). As a result, APOE ɛ4 acts directly or in concert with age, head injury, oxidative stress, ischaemia and inflammation to alter disease onset, progression and prognosis in a variety of neurological disorders (Maiti et al., 2015). Finally, APOE ɛ4 has also been directly linked with cerebrovascular dysfunction via a variety of mechanisms, including pericyte migration/activation, astrocyte activation, smooth muscle cell damage, basement membrane degradation and alterations in brain endothelial cells (Zlokovic, 2013; Tai et al., 2016). We, therefore, hypothesize our findings to reflect more broadly the biological interaction between the damaging effects of hypertension and the pathological substrate represented by APOE ɛ4 across a multitude of mechanistic pathways.
The robustness of our results is supported by the strengths of our design, which include consistent capture and incorporation of relevant neuroimaging, genetic and BP information and follow-up based on standardized procedures, capturing multiple relevant outcome endpoints of immediate clinical relevance. The study’s limitations derive most substantially from its small sample size. Furthermore, subjects were recruited and followed in an observational manner at a single academic tertiary care centre. These findings will, therefore, require replication in different centres and healthcare delivery settings. In our systematic review, we were unable to identify currently available resources that would allow for a ready replication of our findings. In addition, we were limited in our characterization of cognitive, mood and gait disorders. Specifically, our ability to better delineate cognitive impairment by sub-domains, capture severity of mood symptoms or describe patterns of gait impairment is minimal, due to the nature of the screening tools employed. Finally, BP data capture was non-standardized and likely resulted in imprecision in capturing hypertension severity. However, this is unlikely to have systematically affected individuals based on APOE genotype, and more likely eroded statistical power instead of generating false-positive associations.
In summary, we demonstrate that APOE genotype interacts with average SBP to influence long-term clinical outcomes following ICH. Among non-hypertensive ICH survivors with elevated BP, APOE genotype identified those at higher risk for poor outcome. Although these findings require replication, their incorporation in clinical practice and future clinical trials may guide precision approaches for BP control in this very high-risk population.
Supplementary Material
Acknowledgements
Study design: J.R., A.B.; data acquisition: all authors; statistical analysis: A.B.; study management: A.B., C.K., K.S., J.R.; manuscript preparation: A.B., M.P.M., P.K.; manuscript review: all authors. All statistical analyses performed by A.B., MD (Massachusetts General Hospital). Drs. A.B. and J.R. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The authors’ work on this study was supported by funding from the National Institute of Health (K23NS100816, R01NS093870 and R01AG26484). The funding entities had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests
Dr. A.B. is supported by K23NS100816. Dr. S.M.G. is supported by R01AG26484. Dr. A.V. is supported by R01AG047975, R01AG026484, P50AG005134, K23AG028726. Dr. C.D.A. is supported by K23NS086873, R01NS103924, AHA 18SFRN34110082, the MGH Center for Genomic Medicine, and has consulted for ApoPharma, Inc. Dr. J.R. is supported by R01NS036695, UM1HG008895, R01NS093870, R24NS092983, and has consulted for New Beta Innovations, Boehringer Ingelheim, and Pfizer Inc. The remaining authors report no disclosures.
Glossary
Abbreviations
- 95% CI
95% confidence interval
- Aβ
amyloid-β
- BP
blood pressure
- CAA
cerebral amyloid angiopathy
- DBP
diastolic blood pressure
- GDS-4
4-item version of the Geriatric Depression Scale
- HR
hazard ratio
- ICH
intracerebral haemorrhage
- IQR
inter-quartile range
- IQCODE-16
16-item (short) version of the Informant Questionnaire on Cognitive Decline in the Elderly
- SVD
small vessel disease
- SBP
systolic blood pressure
- TICS-m
Modified Telephone Interview for Cognitive Status
References
- Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Almeida SA.. Short versions of the Geriatric Depression Scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry 1999; 14: 858–65. [DOI] [PubMed] [Google Scholar]
- Barber M, Stott DJ.. Validity of the Telephone Interview for Cognitive Status (TICS) in post-stroke subjects. Int J Geriatr Psychiatry 2004; 19: 75–9. [DOI] [PubMed] [Google Scholar]
- Benedictus MR, Hochart A, Rossi C, Boulouis G, Henon H, van der Flier WM, et al. Prognostic factors for cognitive decline after intracerebral hemorrhage. Stroke 2015; 46: 2773. [DOI] [PubMed] [Google Scholar]
- Biffi A, Anderson C, Battey TW, Ayres A, Greenberg SM, Viswanathan A, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 2015; 314: 904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Anderson CD, Jagiella JM, Schmidt H, Kissela B, Hansen BM, et al. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. Lancet Neurol 2011; 10: 702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Bailey D, Anderson CD, Ayres AM, Gurol EM, Greenberg SM, et al. Risk factors associated with early vs. delayed dementia after intracerebral hemorrhage. JAMA Neurol 2016; 73: 969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Greenberg SM.. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol 2011; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010; 75: 693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Shulman JM, Jagiella JM, Cortellini L, Ayres AM, Schwab K, et al. Genetic variation at CR1 increases risk of cerebral amyloid angiopathy. Neurology 2012; 78: 334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Sonni A, Anderson CD, Kissela B, Jagiella JM, Schmidt H, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol 2010; 68: 934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein MF.. The telephone interview for cognitive status. Neuropsychiatry. Neuropsychol Behav Neurol 1988; 1: 111–8. [Google Scholar]
- Butt DA, Harvey PJ.. Benefits and risks of antihypertensive medications in the elderly. J Intern Med 2015; 278: 599–626. [DOI] [PubMed] [Google Scholar]
- Collins FS, Varmus H.. A new initiative on precision medicine. N Engl J Med 2015; 372: 793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Schaie KW, Willis SL.. Hypertension moderates the effect of APOE on 21-year cognitive trajectories. Psychol Aging 2014; 29: 431–9. [DOI] [PubMed] [Google Scholar]
- de Jager CA, Budge MM, Clarke R.. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry 2003; 18: 318–24. [DOI] [PubMed] [Google Scholar]
- de Leeuw F-E, Richard F, de Groot JC, van Duijn CM, Hofman A, van Gijn J, et al. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke 2004; 35: 1057–60. [DOI] [PubMed] [Google Scholar]
- Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D.. Orthostatic hypotension as a risk factor for stroke: the atherosclerosis risk in communities (ARIC) study, 1987-1996. Stroke 2000; 31: 2307–13. [DOI] [PubMed] [Google Scholar]
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387: 957–67. [DOI] [PubMed] [Google Scholar]
- Gray SW, Martins Y, Feuerman LZ, Bernhardt BA, Biesecker BB, Christensen KD, et al. Social and behavioral research in genomic sequencing: approaches from the Clinical Sequencing Exploratory Research Consortium Outcomes and Measures Working Group. Genet Med 2014; 16: 727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT.. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol 1995; 38: 254–9. [DOI] [PubMed] [Google Scholar]
- Group SR, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Fearon P, Noel-Storr AH, McShane R, Stott DJ, Quinn TJ.. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within a general practice (primary care) setting. Cochrane Database Syst Rev 2014; 7: CD010771. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Fearon P, Noel-Storr AH, McShane R, Stott DJ, Quinn TJ.. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within a secondary care setting. Cochrane Database Syst Rev 2015; 3: CD010772. [DOI] [PubMed] [Google Scholar]
- Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 2032–60. [DOI] [PubMed] [Google Scholar]
- Hyman DJ, Taffet GE.. Blood pressure control in the elderly: can you have too much of a good thing? Curr Hypertens Rep 2009; 11: 337–42. [DOI] [PubMed] [Google Scholar]
- Jofre-Monseny L, Minihane AM, Rimbach G.. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res 2008; 52: 131–45. [DOI] [PubMed] [Google Scholar]
- Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994; 24: 145–53. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, Xu H, Bu G.. ApoE and Abeta in Alzheimer’s disease: accidental encounters or partners? Neuron 2014; 81: 740–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keselman HJ, Cribbie R, Holland B.. Controlling the rate of Type I error over a large set of statistical tests. Br J Math Stat Psychol 2002; 55: 27–39. [DOI] [PubMed] [Google Scholar]
- Kester MI, Goos JD, Teunissen CE, Benedictus MR, Bouwman FH, Wattjes MP, et al. Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol 2014; 71: 855. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon H, Basak J, Kim J.. Apolipoprotein E in synaptic plasticity and Alzheimer’s disease: potential cellular and molecular mechanisms. Mol Cells 2014; 37: 767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJ, Petersen RC, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology 2010; 34: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES. Cutting the Gordian helix–regulating genomic testing in the era of precision medicine. N Engl J Med 2015; 372: 1185–6. [DOI] [PubMed] [Google Scholar]
- Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G.. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013; 9: 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW. Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arterioscler Thromb Vasc Biol 2016; 36: 1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti TK, Konar S, Bir S, Kalakoti P, Bollam P, Nanda A.. Role of apolipoprotein E polymorphism as a prognostic marker in traumatic brain injury and neurodegenerative disease: a critical review. Neurosurg Focus 2015; 39: E3.. [DOI] [PubMed] [Google Scholar]
- Moulin S, Labreuche J, Bombois S, Rossi C, Boulouis G, Henon H, et al. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol 2016; 15: 820–9. [DOI] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Welch SJ, Cuthbertson FC, Mariz J, Mehta Z, Rothwell PM.. Telephone assessment of cognition after transient ischemic attack and stroke: modified telephone interview of cognitive status and telephone Montreal Cognitive Assessment versus face-to-face Montreal Cognitive Assessment and neuropsychological battery. Stroke 2013; 44: 227–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocklington C, Gilbody S, Manea L, McMillan D.. The diagnostic accuracy of brief versions of the Geriatric Depression Scale: a systematic review and meta-analysis. Int J Geriatr Psychiatry 2016; 31: 837.. [DOI] [PubMed] [Google Scholar]
- Poon MT, Fonville AF, Al-Shahi Salman R.. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014; 85: 660–7. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF.. Spontaneous intracerebral hemorrhage. N Engl J Med 2001; 344: 1450–60. [DOI] [PubMed] [Google Scholar]
- Rannikmae K, Samarasekera N, Martinez-Gonzalez NA, Al-Shahi Salman R, Sudlow CL.. Genetics of cerebral amyloid angiopathy: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2013; 84: 901–8. [DOI] [PubMed] [Google Scholar]
- Rose KM, Couper D, Eigenbrodt ML, Mosley TH, Sharrett AR, Gottesman RF.. Orthostatic hypotension and cognitive function: the Atherosclerosis Risk in Communities Study. Neuroepidemiology 2010; 34: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo EH, Lee DY, Kim SG, Kim KW, Kim do H, Kim BJ, et al. Validity of the telephone interview for cognitive status (TICS) and modified TICS (TICSm) for mild cognitive impairment (MCI) and dementia screening. Arch Gerontol Geriatr 2011; 52: e26–30. [DOI] [PubMed] [Google Scholar]
- Smith EE, Greenberg SM.. Beta-amyloid, blood vessels, and brain function. Stroke 2009; 40: 2601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. Design of randomized controlled trials. Circulation 2007; 115: 1164–9. [DOI] [PubMed] [Google Scholar]
- Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, et al. APOE-modulated Abeta-induced neuroinflammation in Alzheimer’s disease: current landscape, novel data, and future perspective. J Neurochem 2015; 133: 465–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AW, et al. The role of APOE in cerebrovascular dysfunction. Acta Neuropathol 2016; 131: 709–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio C, Arima H, Harrap S, Anderson C, Godin O, Woodward M, et al. APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology 2008; 70: 1322–8. [DOI] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 2018; 71: 1269–324. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol 2013; 70: 440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.