Abstract
Diabetes is an important risk factor for the development of cardiovascular disease including atherosclerosis and ischemic heart disease. Vascular complications including macro- and micro-vascular dysfunction are the leading causes of morbidity and mortality in diabetes. Disease mechanisms at present are unclear and no ideal therapies are available, which urgently calls for the identification of novel therapeutic targets/agents. An altered nucleotide- and nucleoside-mediated purinergic signaling has been implicated to cause diabetes-associated vascular dysfunction in major organs. Alteration of both purinergic P1 and P2 receptor sensitivity rather than the changes in receptor expression accounts for vascular dysfunction in diabetes. Activation of P2X7 receptors plays a crucial role in diabetes-induced retinal microvascular dysfunction. Recent findings have revealed that both ecto-nucleotidase CD39, a key enzyme hydrolyzing ATP, and CD73, an enzyme regulating adenosine turnover, are involved in the renal vascular injury in diabetes. Interestingly, erythrocyte dysfunction in diabetes by decreasing ATP release in response to physiological stimuli may serve as an important trigger to induce vascular dysfunction. Nucleot(s)ide-mediated purinergic activation also exerts long-term actions including inflammatory and atherogenic effects in hyperglycemic and diabetic conditions. This review highlights the current knowledge regarding the altered nucleot(s)ide-mediated purinergic signaling as an important disease mechanism for the diabetes-associated vascular complications. Better understanding the role of key receptor-mediated signaling in diabetes will provide more insights into their potential as targets for the treatment.
Keywords: Extracellular nucleotides, Up4A, Purinergic receptor, Endothelial dysfunction, Atherosclerosis, Diabetes
1. Introduction
Diabetes is an important risk factor for the development of cardiovascular disease including atherosclerosis and ischemic heart disease. Diabetes-induced macro- and micro-vascular dysfunction in heart, kidney, retinal microvasculature and peripheral vascular beds are the leading causes contributing to the increased morbidity and mortality in diabetes [1]. The pathogenesis of these vascular dysfunctions mainly comes from the imbalances between anti-inflammatory factors/vasodilators such as nitric oxide (NO) and adenosine triphosphate (ATP), and inflammatory factors/vasoconstrictors such as endothelin, reactive oxygen species (ROS) and ATP, as well as receptor-mediated signaling activated by certain endothelium-derived factors e.g. ATP and/or adenosine-activated purinergic signaling [2,3]. Despite the evidence showing that hyperglycemia has been a crucial factor driving vascular complications, recent clinical studies demonstrated that hyperglycemic control has not convincingly reduced the mortality among diabetic patients at high risk for cardiovascular events [4]. Apparently, the underlying causes of vascular dysfunction are multifactorial and complex, there is an unmet need to identify key disease mechanisms behind vascular complications in diabetes in order to develop novel therapies that specifically target such complications.
Increasing evidence suggests that extracellular nucleotides and nucleosides contribute to homeostasis of cardiovascular systems [5–7]. Nucleotides (such as ATP and UTP), nucleosides (such as adenosine), and dinucleotides (such as uridine adenosine tetraphosphate (Up4A)), can be released from endothelial cells, adventitial nerves or circulating cells like platelets, immune cells and erythrocytes to regulate vascular tone, vascular remodeling, vascular permeability, and inflammation through purinergic receptors [3,8]. Of importance, nucleot(s)ide-mediated purinergic signaling is altered in diabetes, which accounts for the development of vascular dysfunction [3]. Therefore, the nucleot(s) ides and nucleot(s)ide-mediated purinergic signaling would represent an innovative and valuable target for the treatment of diabetes-associated vascular complications. In this review, we summarize important aspects of purinergic dysfunction involving not only the vascular effect of purinergic activation at the cellular and the intact organ levels but also the trophic effect of nucleotide-mediated purinergic signaling in hyperglycemic and diabetic conditions. The main focus is on the evidence addressing altered involvement of nucleot(s)ides and nucleot(s) ide-regulated purinergic network as key mediators of diabetes-associated vascular dysfunction in major organs.
1.1. Nucleot(s)ides and its mediated purinergic signaling
Nucleot(s)ides predominantly exert their biological effects through purinergic receptors. Purinergic receptors have been classified into P1 and P2 categories [9,10]. Four subtypes of P1 receptors (also termed adenosine receptors) have been identified, namely A1R, A2AR, A2BR and A3R. A1R and A3R are negatively coupled to adenylyl cyclase through the Gi/o protein alpha-subunits and activation of those receptors decreases cAMP levels, whereas A2AR and A2BR are positively coupled to adenylyl cyclase through Gs and enhance cAMP levels [11,12]. The P2 receptors are further classified into two major families: ionotropic P2XRs and metabotropic P2YRs [10,13]. At least 7 P2XRs (P2X1-7R), and 8 P2YRs (P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R and P2Y14R) have been identified to date [14] (Fig. 1). Activation of P2XRs increases sodium and calcium permeability, while activation of P2YRs either affects adenylyl cyclase and alters cAMP levels or activates phospholipase C and releases intracellular calcium [14] (Fig. 1). Distribution and expression of purinergic receptors are ubiquitous throughout the body, with sub-type distributions varying regionally across tissues as well as across species [10,11,15]. In contrast to the activation of P1 receptors by adenosine only, P2 receptors can be activated by several nucleotides and have overlapping ligand preferences. P2X1_7Rs are mainly activated by ATP; P2Y4R, P2Y12R and P2Y13R are activated by ADP, while P2Y4R are sensitive to both ATP and ADP. Additionally, P2Y2R and P2Y4R are preferably sensitive to UTP, while P2Y6R preferably responds to UDP stimulation [10] (Table 1). Upon stimulation by these extracellular nucleot(s)ides, purinergic signaling is initiated, resulting in various physiological responses, including platelet aggregation, cellular proliferation, angiogenesis, immune responses and vascular tone regulation [7,10–12,15]. Such purinergic action is regulated by ecto-nucleotidases such as nucleoside triphosphate diphosphohydrolase (NTPDase, also known as ecto-ATPDase CD39), ecto-5′-nucleotidase (CD73), nucleotide pyrophosphatase/phosphodiesterases (NPP) and adenosine deaminase [8]. For instance, CD39 phosphohydrolyzes ATP and ADP to AMP, which is further dephosphorylated to adenosine by CD73. Thus, ecto-enzymes play a critical role in maintaining extracellular nucleotides and adenosine homeostasis.
Fig. 1.
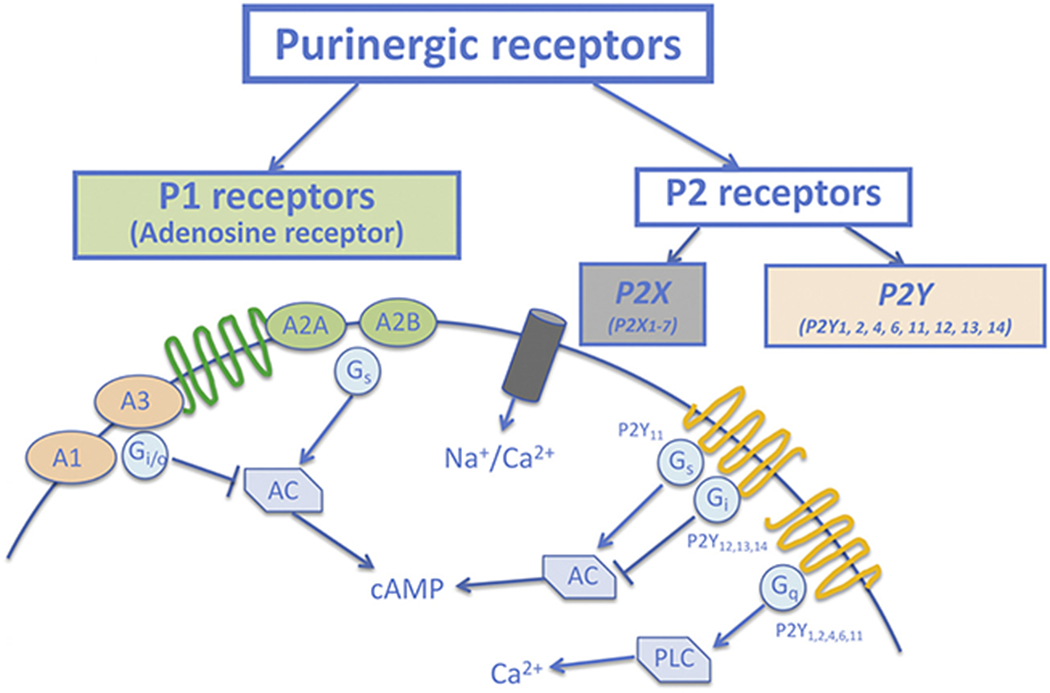
Purinergic receptor classification and their activated intracellular components. Purinergic receptors are divided to P1 and P2 classes. P1 receptors consist of A1, A2A, A2B and A3 receptors. A1 and A3 receptors are negatively coupled to adenylyl cyclase (AC) through the Gi/o and activation of those receptors decreases cAMP levels, whereas A2A and A2B receptors are positively coupled to AC through Gs and enhance cAMP levels. The P2 receptors are further classified into two major families: ionotropic P2X and metabotropic P2Y receptors. Activation of P2X receptors increases sodium and calcium permeability, while activation of P2Y receptors either affects AC and alter cAMP levels or activates phospholipase C (PLC) and release intracellular calcium.
Table 1.
Nucleot(s)ide ligand-receptor preference and purinergic receptor-mediated vascular action.
| Ligand | Preferable receptors | Vascular effect | Post-receptor signaling | Reference |
|---|---|---|---|---|
| ATP | P2X1-7R, P2Y1R | Relaxation (endothelial activation: P2X1R, P2X2R, P2X3R, P2X4R, P2X7R, P2Y1R, P2Y2R, P2Y4R, P2Y11R); Contraction (SMC activation: P2X1R, P2X2R, P2X4R, P2Y1R, P2Y2R, P2Y6R) | NO, Prostacyclin, EDHF (relaxation); ROS, TxA2 (contraction) | [10] |
| ADP | P2Y1R, P2Y12R, P2Y13R | [10] | ||
| UTP | P2Y2R, P2Y4R | [7,16] | ||
| UDP | P2Y6R | [10] | ||
| ADO | A1, A2A, A2B, A3 | Relaxation (A2AR, A2BR) and contraction (A1R, A3R) independent of receptor location | [11] |
ADO: adenosine; EDHF: endothelium-derived hyperpolarization factor; NO: nitric oxide; ROS: reactive oxygen species; TxA2: thromboxane.
In the vascular system, all four P1 receptor subtypes are expressed in both endothelial cells and smooth muscle cells [11,12]. With respect to regulation of vascular tone, activation of A1R and A3R typically results in vascular contraction, whereas activation of A2AR and A2BR leads to vascular relaxation [11,12]. The P1 receptor-mediated vascular tone regulation appears to be independent of the locations of receptor subtypes [11], whereas the effects of the activation of P2 receptor subtypes are tissue–/cell-dependent. For example, activation of P2 receptor subtypes on endothelial cells typically leads to vasodilation, while activation of P2 receptor subtypes on smooth muscle cells results in vasoconstriction [7,16]. Thus, activation of P2X4R, P2X2R, P2X4R, P2Y4R, P2Y2R and P2Y6R on smooth muscle cells results in vasoconstriction [7] via a mechanism involving production of ROS or thromboxane (TXA2) [8], while activation of P2X4R, P2X2R, P2X3R, P2X4R P2X7R, P2Y1R, P2Y2R, P2Y4R and P2Y11R on endothelial cells has been shown to produce NO, prostacyclin and endothelium-hyperpolarization factors leading to vasodilation [7,16] (Table 1), although there are also observations to suggest that activation of P2X1R on endothelial cells contributes to vascular contraction [17,18].
2. Purinergic dysfunction in diabetes
2.1. Alteration of purinergic signaling and endothelial/smooth muscle cell dysfunction
Endothelial dysfunction plays a crucial role in the development of vascular complications in diabetes [2]. Induction of endothelial dysfunction, caused by hyperglycemia, dyslipidemia and hyperinsulinemia results in impaired vascular regulation, oxidative stress, and inflammation. High glucose can stimulate the release of nucleotides thereby initiating purinergic signaling. Indeed, adenosine transport and adenosine receptor expression pattern have been found to be altered in human umbilical vein endothelial cells (HUVECs) derived from humans with gestational diabetes. HUVECs derived from gestational diabetic pregnancies exhibited a higher concentration of adenosine and higher protein levels for A1R but lower protein levels for A2BR [19,20]. Such alterations are attributed partially to the macro- and micro-vascular endothelial dysfunction in the placenta of humans with gestational diabetes [20,21]. Upon stimulation with high glucose and palmitate, ATP was released from HUVECs activating both P2X4R and P2X7R [22]. Such activation led to upregulation of various inflammatory genes, formation of ROS and reduction of NO bioavailability [22]. In aortas isolated from type 2 Goto-Kakizaki (GK) rats, endothelium-dependent relaxation was attenuated by not only the non-selective P1 and P2 receptor antagonists but also by the A1R, the P2X7R and the P2Y6R antagonists via a mechanism involving alteration of receptor sensitivity [23] (Table 2). Taken together, these studies indicate an altered purinergic signaling accounting for diabetes-associated endothelial dysfunction. In addition to endothelial cells, altered purinergic signaling may also be present in smooth muscle cells accounting for diabetes-induced vascular smooth muscle injury. Existing evidence demonstrated that increase in adenosine turnover affected iNOS levels in smooth muscle cells isolated from aortas of type 1 diabetic rats. The subsequent functional evidence has been observed in endothelium-denuded aortas in which NOS inhibition abolished adenosine-induced relaxation in diabetic but not control rats [24] (Table 2).
Table 2.
Purinergic receptor-mediated vascular function in the peripheral vasculature.
| Organ | Species | Diabetic model | Nucleot(s)ide/stimuli | Purinergic receptor | Vascular effect | Reference |
|---|---|---|---|---|---|---|
| Rat | STZ with endothelium-denudation | ADO+LNMA | Vascular relaxation↓ | [24] | ||
| Aorta | Rat | GK | Up4A | A1R, P2X7R | Vascular contraction↑ | [23] |
| ACh | A1R, P2X7R, P2Y6R | EDR↓ | ||||
| Rat | STZ with endothelial dysfunction | ADO | A2AR | EDR↓ | [26] | |
| ADO analogue/ the A2AR agonist | Vascular relaxation↓ | |||||
| Rat | OLETF | UDP | P2Y6R-sensitive | Vascular relaxation↑ | [27] | |
| P2Y6R-insensitive | Vascular contraction↑ | |||||
| Mouse | STZ without endothelial dysfunction | The A1R agonist | A1R | Vascular contraction↓ | [25] | |
| The A2AR agonist | A2AR | Vascular relaxation ↓ | ||||
| Mesenteric artery | Rat | GK | Up4A | Vascular contraction - | [23] | |
| ACh | EDR↓ | |||||
| Rat | STZ | ADP | P2Y1R | EDR↓ | [28] | |
| Rat | GK | ATP, UTP | P2Y6R | Vascular contractionf | [29] | |
| Mouse | STZ without endothelial dysfunction | The A1R agonist The A2BR agonist The A3R agonist |
Vascular contraction/relaxation - | [25] | ||
| Femoral circulation | Human | Type 2 diabetes | ATP, UTP, ADO | P2X1R, P2Y2R | LBF↓ | [31] |
ADO: adenosine; EDR: endothelium-dependent relaxation; GK: Goto Kakizaki; LBF: leg blood flow; LNMA: NG-monomethyl-L-arginine; OLETF: Otsuka Long-Evans Tokushima Fatty; STZ: streptozotocin; Up4A: uridine adenosine tetraphosphate; ↑: increased effect; ↓: decreased effect; −: no effect vs. control group.
2.2. Alteration of purinergic signaling in the peripheral vasculature (aorta, mesenteric artery and femoral circulation)
As mentioned above, activation of purinergic receptors located on endothelium vs. smooth muscle cells leads to different vascular responses. Under diabetic conditions, the location and distribution of purinergic receptors are altered and can even be shifted between endothelial cells and smooth muscle cells, which may induce different net vascular reactivity [8]. In aortas isolated from type 1 diabetic mice without obvious endothelial dysfunction, the AlR-mediated vascular contraction was decreased, while the A2AR-mediated vascular relaxation was impaired [25] (Table 2). The altered A1R-mediated vascular effect was likely due to a change in the receptor sensitivity, as the protein expression of A1R in aortas was comparable between diabetes and control [25]. In a type 1 diabetic rat model with endothelial dysfunction, adenosine-mediated NO production in endothelium was impaired [26]. Thus, the adenosine analogue- and the A2AR agonist-mediated aortic relaxations were reduced as compared to control rats [26] (Table 2). Such difference between diabetes and control rats in the adenosine analogue- and the A2AR agonist-mediated relaxations were abolished by endothelium denudation [26]. Likewise, NOS inhibition attenuated the adenosine analogue-induced relaxation more in aortas from control rats than those from diabetic rats [26]. Further, upon stimulation by the novel dinucleotide Up4A that activates both P1 and P2 receptors, Up4A-enhanced aortic contraction in a type 2 diabetic GK rat model was attenuated by the non-selective P1 receptor and the A1R antagonists without alteration of the receptor expression [23]. Taken together, these observations indicate that the A1R-mediated vascular contraction and the A2AR-mediated vascular relaxation in aortas are altered in diabetes likely through changes in receptor sensitivity.
In addition to altered P1 receptor-mediated vascular function in aortas, P2X7R inhibition attenuated Up4A-enhanced vascular contraction in GK rats [23] (Table 2). This attenuation is through alteration of receptor sensitivity, as the P2X7R expression was not affected by diabetes [23]. Of interest, UDP activated vasoconstrictor prostanoids in aortas from control Long–Evans Tokushima Otsuka (LETO) rats limiting vascular relaxation as compared to type 2 diabetic model of obese Otsuka Long-Evans Tokushima Fatty (OLETF) rats [27]. The UDP-induced relaxation in aortas from OLETF rats was partially attenuated by inhibition of MRS2578-sensitive P2Y6R [27]. On the other hand, UDP enhanced vascular contraction in endothelium-denuded aortas from OLETF rats vs. LETO rats likely through activation of MRS2578-insensitive P2Y6R [27] (Table 2).
In contrast to conduit aortas, purinergic signaling seems not to be affected in mesenteric arteries by diabetes in the same models mentioned above. Thus, adenosine-agonist (A1R, A2BR and A3R)-mediated vascular responses did not differ between type 1 diabetic mice and control mice [25] (Table 2). Moreover, impaired endothelium-dependent relaxation in mesenteric arteries isolated from GK rats was not affected by either the non-selective P1 or the non-selective P2 receptor antagonists [23]. On the other hand, the Up4A-induced vascular contraction was comparable in mesenteric arteries between GK and control rats [23] (Table 2). In an older type 1 diabetic rat model, P2Y1R-mediated relaxation was impaired in superior mesenteric arteries from long-term type 1 diabetic rats [28]. Such impairment was caused by the reduced P2Y1R-mediated NO signaling rather than reduced receptor expression [28] (Table 2). Similarly, in an aged and later-stage type 2 diabetic rat model, both ATP- and UTP-induced vascular contraction were increased in mesenteric arteries of GK rats [29] (Table 2). The enhancement in contraction was through endothelium-derived vasoconstrictor prostanoids/thromboxane and was mediated by activation of P2YR located on endothelial cells [29]. These observations suggest that aging and diabetic progression may play a determining role in regulation of purinergic signaling in resistance mesenteric arteries. In type 1 diabetic rats, perfusate of endothelium-denuded mesenteric arteries at basal condition or by electronic field stimulation as well as primary cultured endothelial cells by mechanical stimulation exhibited increased ATP and adenosine [30], providing insights into diabetes-induced neuropathy. However, the subsequent purinergic receptors activated by enhanced ATP and adenosine remain to be determined.
Alterations of vascular purinergic regulation observed in experimental diabetic models can be extrapolated into humans. Intrafemoral artery infusion of adenosine, ATP and UTP all resulted in impaired vasodilation in patients with type 2 diabetes as compared to control subjects [31]. Distribution and expression of the putative P2X1R and P2Y2R were comparable between patients with type 2 diabetes and control subjects [31], suggesting a possible altered receptor sensitivity that accounts for impaired vascular responses in diabetes (Table 2).
2.3. Alteration of vascular purinergic signaling in the kidney
Purinergic activation is actively involved in blood flow regulation in the kidney [32–34]. Altered purinergic signaling in diabetes may result in renal vascular dysfunction. Increased adenosine levels have been observed not only in streptozotocin-induced type 1 diabetic rats (vs. control rats) [35,36] but also in diabetic patients with diabetic nephropathy vs. diabetic patients without nephropathy or healthy subjects [37]. Moreover, mice lacking adenosine deaminase exhibited increased circulating adenosine and developed renal dysfunction [38]. These studies suggest that increased adenosine may have detrimental effect on renal function in diabetes. This is supported by several observations that injection of adenosine decreased renal blood flow more in streptozotocin-induced type 1 diabetic rats than in control rats [39]. Likewise, renal vascular response to endogenous adenosine, assessed by post-occlusive reduction of renal blood flow following renal artery occlusion, was significantly blunted in this model [39]. The adenosine-mediated vasoconstrictor effect could be attenuated by the A1R antagonist in both groups [39] (Table 3). In contrast, i.p. injection of the adenosine analogue 5′-(N-ethylcarboxamido)-adenosine (NECA) into streptozotocin-induced type 1 diabetic rats reduced diabetes-induced oxidative stress, inhibited the expression of proinflammatory genes and deactivated JNK-MAPK signaling pathways [40]. Administration of adenosine into streptozotocin-induced type 1 diabetic rats prevented kidney histopathological changes, decreased plasma kidney injury factor-1 and tumor necrosis factor-α [41]. The different outcomes induced by adenosine or adenosine receptor stimulation may depend on which specific adenosine receptor subtypes are activated. Adenosine receptor expression and distribution have been shown to be altered in type 1 diabetic rats [42], implying a functional role of adenosine receptors in the control of renal vascular function in diabetes. In contrast to the blocking effect of the A1R antagonist on adenosine-induced renal vasoconstriction in diabetic rats [39], diabetic mice lacking A1R augmented hyperfiltration and glomerular injury [43]. However, no differences in glomerular filtration between alloxan-induced diabetic A1R knockout and wild-type mice were observed (Table 3) [44]. Thus, the exact role for A1R in regulation of renal function in diabetes remains unclear and warrants further investigations. Activation of A2AR with the A2AR agonist in streptozotocin-induced type 1 diabetic rats attenuated glomerular inflammation and injury [45,46] (Table 3), indicating a protective role of activation of A2AR in diabetes-induced renal injury. Adenosine-mediated renal injury in diabetes has been report to be linked to the activation of A2BR, as A2BR inhibition prevented early alteration seen in diabetic glomerulopathy and fibrosis [47,48] (Table 3). In contrast, studies have reported that activation of endothelial A2BR corrected glomerular size and extracellular matrix deposition in type 1 diabetic mice [49] (Table 3). The role for A2BR is still controversial and future studies are needed to investigate the functional role of A2BR in the development of vascular dysfunction in diabetic kidney.
Table 3.
Purinergic receptor-mediated vascular function in kidney.
| Organ | Species | Diabetic model | Nucleot(s)ide/stimuli | Purinergic receptor | Vascular effect | Reference |
|---|---|---|---|---|---|---|
| Kidney | Rat | STZ | ADO/renal occlusion | A1R | RBF↓ | [39] |
| The A2BR antagonist | A2BR | Glomerulopathy and fibrosis↓ | [47,48] | |||
| The A2AR agonist | A2AR | Glomerular inflammation and injury↓ | [45,46] | |||
| The P2X7R agonist | P2X7R | Cortical blood flow↑, GFR↓ | [36] | |||
| Rat | GK | Up4A | P2R | vascular contraction↑ | [51] | |
| Mouse | Ins2+/−/A1AR−/− mutants | A1R | GFR↑, Glomerular Injury↑ | [43] | ||
| Mouse | alloxan-induced A1AR−/− | A1R | Glomerular Injury - | [44] | ||
| Mouse | STZ | The A2BR agonist | Endothelial A2BR | Glomerular Injury↓ | [49] | |
| Mouse | STZ with CD73−/− | Adenosine | Glomerular Injury↑ | [49] | ||
| Mouse | STZ with CD39−/− | ATP/UTP | Glomerular inflammation↑ | [50] | ||
| Mouse | HFD | The P2X7R agonist | P2X7R | Renal inflammation and injury↑ | [54] | |
| P2X7R−/− | Renal inflammation and injury↓ |
ADO: adenosine; CD39: ectonucleoside triphosphate diphosphohydrolase; CD73: ecto-5′-nucleotidase; GFR: glomerular filtration rate; GK: Goto Kakizaki; HFD: high fat diet; Ins2: also known as Akita+/−, a naturally occurring diabetic model; RBF: renal blood flow; STZ: streptozotocin; Up4A: uridine adenosine tetraphosphate; ↑: increased effect; ↓: decreased effect; −: no effect vs. control group.
Ecto-nucleotidases regulating nucleot(s)ide concentrations and turnover play an important role in diabetes-induced renal injury. CD73 expression was shown to be increased in the kidney of both type 1 and 2 diabetic mice [49]. Deletion of CD73 was associated with more severe nephropathy in streptozotocin-induced type 1 diabetic mice [49] (Table 3). Diabetic mice induced by streptozotocin lacking CD39 developed increased glomerular inflammation in association with higher levels of monocyte chemoattractant protein-1 (MCP-1) expression and showed much more glomerular fibrin deposition and stronger glomerular plasmingogen activator inhibitor-1 (PAI-1) staining as compared to control mice [50] (Table 3). CD39 phosphohydrolyzes ATP to ADP and AMP [8], the detrimental renal effects observed in diabetic mice with CD39 deficiency are likely related to increased accumulation of ATP/UTP, as PAI-1 and MCP-1 expression could be upregulated by ATP and UTP but not ADP or adenosine in vitro [50]. This may suggest an alteration of P2 receptor-mediated purinergic signaling in regulation of vascular function in diabetic kidney. Indeed, the renal vascular responses to UP4A stimulation were enhanced in type 2 diabetic GK rats via P2 receptor-mediated increase in thromboxane receptor sensitivity [51] (Table 3). Existing studies demonstrate that P2X7R levels were increased in the glomerular region of type 1 diabetic rats [52,53] and in both cortex and glomerular regions from patients with type 2 diabetes [54]. An alteration of P2X7R-mediated increased cortical blood flow, and decreased glomerular filtration rate and glomerular insulin space in type 1 diabetic rats were also observed [36] (Table 3). P2X7R stimulation evoked and P2X7R deletion attenuated renal inflammation and injury induced by high-fat diet in type 2 diabetes [54] (Table 3). All evidence may suggest not only P2R but also ecto-nucleotidases as potential therapeutic targets for the treatment of diabetes-associated renal vascular disease.
2.4. Alteration of purinergic signaling in the retinal microvasculature
Diabetic retinopathy is the most common complication of diabetes and one of the leading causes of blindness worldwide [55]. One of the most important characteristics is the death of microvascular pericytes and endothelial cells [56,57]. During chronic hyperglycemia, multiple factors including increased oxidative stress, upregulation of VEGF signaling and formation of advanced glycation end products have been shown to account for diabetes-induced dysfunction in retinal microvasculature [55,58]. Regarding the role of purinergic regulation in the development of retinopathy in diabetes, much emphasis is put on P2X7R based on its ability to induce inflammation. High glucose induced a significant release of IL-1β and lactate dehydrogenase in human retinal pericytes, which was inhibited by the P2X7R antagonist [59]. Streptozotocin-induced type 1 diabetic rats exhibited higher mRNA and protein levels of P2X7R in retina [60]. In pericyte-containing microvessels of such model, both ATP and the P2X7R agonist induced microvascular cell death, which could be inhibited by the P2X7R antagonist [56] (Table 4). Diabetes increased the vulnerability of retinal microvessels to apoptosis induced by P2X7R activation [56]. Activation of P2X7R was also involved in extracellular nicotinamide adenosine dinucleotide-increased vulnerability of retinal microvascular death in such diabetic model [61] (Table 4). As a functional consequence, a significant lower concentration of the P2X7R agonist was enough to reduce retinal blood velocity in alloxan-induced diabetic rabbits to a similar extent as compared to control group [62] (Table 4), indicating a more vulnerable retinal microvascular function regulated by activation of P2X7R in diabetes. Of interest, chronic i.p. injection with either the potent small molecule P2X7R inhibitor A740003 or AZ10606120 in type 1 diabetic rats reversed the increased retina microvascular permeability, fully obliterated elevated VEGF mRNA and protein levels, as well as IL-6 levels in retina [60] (Table 4). Strategies using various selective small molecule P2X7R inhibitors to combat chronic inflammatory diseases have been carried out in Phase I/II clinical studies [63] . With application of those small molecule inhibitors in the future, P2X7R may serve as a potential target for the treatment of retinal microvascular dysfunction in diabetic patients.
Table 4.
Purinergic receptor-mediated vascular function in the retinal microvasculature.
| Organ | Species | Diabetic model | Nucleot(s)ide/stimuli | Purinergic receptor | Vascular effect | Reference |
|---|---|---|---|---|---|---|
| Retinal microvasculature | Rat | STZ | ATP, the P2X7R agonist | P2X7R | Micro vascular death↑ | [56] |
| NAD + | P2X7R | Micro vascular death↑ | [61] | |||
| The P2X7R antagonist | P2X7R | Micro vascular permeability↓ | [60] | |||
| Rabbit | Alloxan | The P2X7R agonist | P2X7R | Retinal blood velocity↓ | [62] |
NAD+: nicotinamide adenosine dinucleotide; STZ: streptozotocin; ↑: increased effect; ↓: decreased effect.
2.5. Alteration of vascular purinergic signaling in the heart
Purinergic activation plays a pivotal role in the regulation of coronary microcirculation [8,11]. In isolated hearts of type 1 diabetic mice, infusion of the non-selective adenosine receptor agonist and the selective A2AR agonist both increased coronary flow without obvious effects on heart rate and cardiac function when compared to the control mice [64]. Further, in vivo bolus injection of the A2AR agonist increased coronary blood flow more in type 1 diabetic mice than control mice [64] (Table 5). Based on the similar baseline coronary flow measured ex vivo and in vivo between the two groups, these findings indicate a greater cardiac reserve in type 1 diabetic mice. This functional evidence is in line with the higher levels of A2AR protein expression in the coronary arteries from type 1 diabetic mice compared to that of control mice [64]. These observations suggest that adenosine and its A2AR selective agonist (regadenoson, Lexiscan®), routinely used in clinics, may not be appropriate for the detection of coronary artery diseases in an early phase of diabetes in which the change in adenosine receptor-mediated vascular effect may mask an underlying disease state.
Table 5.
Purinergic receptor-mediated vascular function in the heart.
| Organ | Species | Diabetic model | Nucleot(s)ide/stimuli | Purinergic receptor | Vascular effect | Reference |
|---|---|---|---|---|---|---|
| Heart | Swine | STZ and high-fat diet | Up4A | A2AR, P2X7R, P2Y1R | Coronary relaxation - | [70] |
| Ossabaw miniature swine | Metabolic syndrome | ADO/the ADO analogue | A2BR | CBF/coronary relaxation - | [65] | |
| Mouse | STZ | The A2AR agonist | A2AR | CBF/coronary relaxation↑ | [64] |
ADO: adenosine; CBF: coronary blood flow; STZ: streptozotocin; Up4A: uridine adenosine tetraphosphate; ↑: increased effect; ↓: decreased effect; −: no effect vs. control group.
In a swine model with early-stage metabolic syndrome and hyperglycemia, despite both adenosine-induced increase in coronary blood flow in vivo and the adenosine analogue-mediated relaxation in isolated coronary arterioles did not differ from control swine [65] (Table 5), there was a shift from the A2AR-mediated coronary relaxation, a primary mechanism for adenosine-induced vasodilation in healthy coronary microcirculation, to enhanced A2BR-mediated coronary relaxation in swine with early-stage metabolic syndrome [65]. This shift is likely due to the increased sensitivity of A2BR upon stimulation by the adenosine analogue, as the A2BR expression level was lower in coronary arterioles isolated from swine with metabolic syndrome [65]. Activation of KATP and Kv channels are main final effectors through which adenosine acts to hyperpolarize and relax smooth muscle in coronary microcirculation [66–68]. Involvement of Kv channels in adenosine receptor-mediated coronary relaxation was not affected by early-stage metabolic syndrome, whereas there was a reduced KATP channel function [65]. We previously demonstrated that activation of A2A receptors is coupled to KATP channel to regulate coronary microcirculation [68,69]. The reduced KATP function by early-stage metabolic syndrome is likely influenced by the shift of vasodilator A2AR.
Up4A produced potent coronary relaxation in normal swine, which was maintained in swine with metabolic derangement (diabetes induced by streptozotocin in combination with high fat diet), despite impaired coronary endothelial function in swine with metabolic derangement [70] (Table 5). The maintained relaxation by Up4A results from a balanced but altered cross-talk among purinergic signaling and endothelium-derived vasoactive factors. Thus, mRNA expression for eNOS, as well as expression of the vasodilator A2AR and P2X7R was reduced in isolated coronary small arteries isolated from swine with metabolic derangement [70]. Further, there was a decreased P2X7R- and NO-mediated vasodilator effect of Up4A, while a TxA2-mediated vasoconstrictor effect was unmasked [70]. All these vasoconstrictor effects were balanced by an increased vasodilator influence via P2Y4R and cytochrome P450 2C9 switched from producing vasoconstrictor to vasodilator metabolites in swine with metabolic derangement [70] (Table 5).
Taken together, existing evidence demonstrates that there is indeed an altered purinergic signaling regulating coronary microcirculation in diabetes. The vasodilator influence seems to be maintained by such altered purinergic signaling in early-phase of diabetes or metabolic syndromes. It is of interest and importance to further investigate the effect of disease progression e.g. late-stage of diabetes on the purinergic regulation of coronary microcirculation.
2.6. Alteration of purinergic signaling in other vascular beds
Existing evidence also demonstrated an altered purinergic signaling in diabetes accounting for vascular dysfunction in other vascular beds. Glucose-induced nucleotide-release increased L-type calcium channel activity and vasoconstriction in mouse cerebral arteries via activation of P2YnR [71] (Table 6). In perfused pancreas isolated from streptozotocin-induced type 1 diabetic rats, adenosine-mediated increase in vascular flow rate was impaired, which was restored by a chronic insulin treatment [72] (Table 6). Cutaneous vascular conductance during administration of ATP at three intradermal forearm skin sites of patients with type 2 diabetes was attenuated as compared to healthy subjects [73] (Table 6). Electrical stimulation in tail arteries isolated from streptozotocin-induced type 1 diabetic rats produced greater contraction, which was attenuated to a greater extent by the P2XR antagonist and the α-adrenergic blocker when comparing to control [74] (Table 6). Accordingly, ATP and norepinephrine produced a greater contraction in tail arteries of type 1 diabetic rats as compared to controls [74] (Table 6), providing evidence for diabetes-induced neuropathy in such arterial preparation.
Table 6.
Purinergic receptor-mediated vascular function in other vascular beds.
| Organ | Species | Diabetic model | Nucleot(s)ide/stimuli | Purinergic receptor | Vascular effect | Reference |
|---|---|---|---|---|---|---|
| Cerebral artery | Mouse | Hyperglycemia | Glucose | P2Y11R | Vascular contraction↑ | [71] |
| Pancreas | Rat | STZ | ADO | Vascular flow rate↓ | [72] | |
| Tail artery | Rat | STZ | ATP/Electrical stimulation | P2XR | Contraction↑ | [74] |
| Skin | Human | Type 2 diabetes | ATP | Vascular conductance↓ | [73] | |
| Muscle arteriole | Hamster | RBC from patients with type 2 diabetes | Decreased ATP release under hypoxia | Vascular relaxation↓ | [88,90] |
ADO: adenosine; RBC: red blood cells; STZ: streptozotocin; ↑: increased effect; ↓: decreased effect.
2.7. Role of erythrocyte-mediated purinergic signaling
Emerging studies have revealed that erythrocytes not only act as regulators of normal physiological function to maintain cardiovascular homeostasis and integrity, but also act as important triggers for the development of various cardiovascular diseases [75–79]. Erythrocytes undergo functional alterations including reduced NO-like bioactivity and/or enhanced oxidative stress in various diseases including diabetes [77,80]. Such altered function of erythrocytes may subsequently affect cardiovascular function thereby accounting for cardiovascular complications. Indeed, recent studies have shown that erythrocytes from patients with type 2 diabetes induce endothelial dysfunction and aggravate cardiac injury following ischemia reperfusion [75,79,81], indicating erythrocyte as a novel disease mediator for the development of cardiovascular complications in diabetes.
Erythrocytes serve as an ATP pool in the circulation. The release of ATP from erythrocytes, particularly when subjected to reduced oxygen tension or shear stress, has been suggested to regulate the matching of oxygen delivery with need in skeletal muscle [82,83]. ATP release from erythrocytes in response to low oxygen tension or mechanical deformation requires increases in cAMP, resulting from the activity of Gistimulated adenylyl cyclase. In these pathways, cAMP can be hydrolyzed by phosphodiesterase (PDE) 3 [84,85]. ATP is suggested to be exported in response to exposure of erythrocytes to reduced oxygen tension via pannexin 2 channels [86]. In contrast, ATP release in response to activation of prostacyclin receptors on erythrocytes was reported to occur via the voltage-dependent anion channel [87]. Once released into the microcirculation, ATP activates purinergic receptors e.g. P2Y4R on vascular walls to generate vasodilators such as NO and PGI2 [82]. These vasodilators are released extraluminally where they act on vascular smooth muscle to induce vasodilation. NO and PGI2 are also released into the vascular lumen where they interact with erythrocytes. NO has been shown to inhibit hypoxia-induced ATP release (negative feedback regulation), while prostacyclin stimulates receptor-mediated ATP release (positive feedback regulation) [82]. Further, once released in the circulation, ATP can be degraded by various ecto-nucleotidases to adenosine contributing to vasodilation [8]. Of note, ATP release in response to exposure of erythrocytes to low oxygen tension was markedly impaired in patients with type 2 diabetes as compared to healthy subjects [88–90]. As a functional consequence, erythrocytes from patients with type 2 diabetes failed to dilate resistance vessels under hypoxic conditions in contrast to erythrocytes from healthy subjects [88,90] (Table 6). The impairment in ATP release was associated with deceased Gi2 expression and cAMP production [89], the latter of which was restored by PDE3 inhibition in erythrocytes from patients with type 2 diabetes [89–91]. Further, the same group found that the impairment in ATP release could be rescued by addition of insulin and c-peptide in erythrocytes from patients with type 2 diabetes via a mechanism stimulating PKC and cGMP pathways [92,93]. Of functional importance, PDE3 inhibition in erythrocytes from patients with type 2 diabetes rescued vasodilator ability in hamster skeletal muscle arterioles [90], indicating a detrimental effect of erythrocytes in diabetes on vascular function due to attenuated ATP release. All together, these findings strongly suggest that erythrocytes and erythrocyte-mediated purinergic signaling may serve as potential therapeutic targets for the treatment of vascular complications in diabetes.
2.8. The trophic action of purinergic signaling in diabetes
As mentioned above, diabetes provokes an exaggerated stimulation of endothelium resulting in increased ROS and decreased NO. Diabetic patients also experience vascular remodeling characterized by increased wall-lumen ratio, mainly reflecting an increase in vascular smooth muscle cells. Together with involvement of other inflammatory cells, this remodeling eventually leads to the development of vascular disease including atherosclerosis [94]. Diabetic patients are at increased atherothrombotic risk and have elevated rates of ischemic recurrence [95]. Activation of both P1R and P2R by adenosine, ATP or UTP exerts various long-term actions resulting in vascular proliferation and growth [3,8]. These purinergic receptor-mediated long-term actions may be dysregulated in diabetes accounting for diabetic vascular disease. HUVECs derived from gestational diabetic pregnancies exhibited a higher concentration of adenosine and higher protein levels for A1R but lower protein levels for A2BR [19,20] (Table 7). Further, high extracellular glucose induced release of ATP and/or UTP and reduced adenosine transport in endothelial cells [22,96]. The high glucose-induced ATP release led to upregulation of various inflammatory genes, formation of ROS and reduction of NO bioavailability via activation of P2X4R and P2X7R [22] (Table 7). High glucose was also shown to induce UTP release in smooth muscle cells which subsequently activated P2Y2R and P2Y6R resulting in increases in the proatherogenic nuclear factor of activated T-cells (NFAT) [97] (Table 7). Aortic smooth muscle cells obtained from rats with streptozotocin-induced type 1 diabetes exhibited a greater susceptibility to the inhibitory effects of adenosine on cell proliferation, suggesting a role for adenosine regulation on diabetic atherosclerosis [98]. It was also reported that there is an association of increased P2X7R expression in monocytes of patients with type 2 diabetes with high plasma C-reactive protein that may play a role in the vascular dysfunction in type 2 diabetes [99] (Table 7). P2Y12R inhibition has been recommended in the management of myocardial infarction. P2Y12R inhibition combined with aspirin reduced ischemic events also in diabetic patients with acute coronary syndrome undergoing percutaneous coronary intervention. More clinical trials are currently under investigations and will provide understandings into optimizing the beneficial effects and minimizing bleeding risks [95]. Collectively, these studies indicate that extracellular nucleotide release is a potential metabolic sensor for the vascular trophic response to hyperglycemia and may provide a link between diabetes and diabetic vascular disease.
Table 7.
Purinergic receptor-mediated vascular trophic action.
| Organ | Species | Diabetic model | Nucleot(s)ide/stimuli | Purinergic receptor | Vascular effect | Reference |
|---|---|---|---|---|---|---|
| HUVECs | Human | GDP | A1R, A2BR | ADO↑ | [19,20] | |
| High glucose | ATP, UTP | P2X4R, P2X7R | Inflammatory genes↑; ROS↑; NO↓ | [22,96] | ||
| Monocytes | Human | Type 2 diabetic patients | P2X7R | Plasma C-reactive protein↑ | [99] | |
| Aorta SMCs | Mouse | High glucose | UTP | P2Y2R, P2Y6R | NFAT↑ | [97] |
ADO: adenosine; GDP: gestational diabetic pregnancy; HUVECs: human umbilical endothelial cells; NFAT: nuclear factor of activated T-cells; NO: nitric oxide; ROS: reactive oxygen species; ↑: increased effect; ↓: decreased effect.
3. Conclusions and perspectives
Emerging evidence has revealed the importance of nucleot(s)ide-mediated purinergic signaling in diabetes-associated vascular complications in major organs including heart, kidney, retinal microvasculature and many other peripheral vascular beds. Hyperglycemia plays a role in initiating disease progress, while aging and diabetes progression may further affect purinergic signaling and deteriorate vascular complications particularly in resistance vessels. A1R, A2AR and A2BR among P1 receptors, P2X4R, P2X7R, P2Y4R, P2Y6R, P2Y41R and P2Y12R among P2 receptors are mainly involved in diabetes-associated vascular dysfunction. It seems that the altered receptor sensitivity rather than the changes in receptor expression accounts for vascular dysfunction in diabetes. Activation of P2X7R plays a crucial role in diabetes-induced retinal microvascular injury. Application of the small molecule P2X7R inhibitor A740003 and AZ10606120 has already provided promising outcome for the treatment of retinal microvascular dysfunction in diabetes and future clinical studies are needed to confirm the therapeutic potential. Recent findings have elucidated CD39 as a key enzyme protecting the kidney from renal vascular injury in diabetic animals, while CD73 is induced in diabetic kidney and deletion of CD73 is associated with severe nephropathy. Furthermore, erythrocyte dysfunction in diabetes acts as a trigger by decreasing ATP release that affects vascular function. Nucleot(s)ide-mediated purinergic activation also exerts long-term vascular actions including inflammatory and atherogenic effects in hyperglycemic and diabetic conditions. However, the role of vascular purinergic signaling in the development of atherosclerosis, hypertension and calcification secondary to diabetes remains unclear, which warrants future investigations. Targeting nucleod(s)ide-mediated purinergic signaling may serve as a potential therapeutic target for the treatment of vascular complications in diabetes.
Acknowledgment
This work was supported by the Karolinska Institutet Grant (2016 and 2018) (to ZZ), the Loo and Hans Ostermans Stiftelse (2018- 01213) (to ZZ), the Sigurt and Elsa Goljes Memorial Foundation (to ZZ), the Lars Hiertas Minne Foundation (FO2018-0156) (to ZZ), the Swedish Heart and Lung Foundation Fellowship (20190341) (to ZZ) and NIH-HL027339 (to SJM).
Footnotes
Declaration of Competing Inteterest
None.
References
- [1].Paneni F, Beckman JA, Creager MA, Cosentino F, Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I, Eur. Heart J 34 (31) (2013) 2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shi Y, Vanhoutte PM, Macro- and microvascular endothelial dysfunction in diabetes, J. Diabetes 9 (5) (2017) 434–449. [DOI] [PubMed] [Google Scholar]
- [3].Burnstock G, Purinergic signaling in the cardiovascular system, Circ. Res 120 (1) (2017) 207–228. [DOI] [PubMed] [Google Scholar]
- [4].Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF, Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis, Jama 316 (3) (2016) 313–324. [DOI] [PubMed] [Google Scholar]
- [5].Ralevic V, Burnstock G, Involvement of purinergic signaling in cardiovascular diseases, Drug News Perspect. 16 (3) (2003) 133–140. [DOI] [PubMed] [Google Scholar]
- [6].Ralevic V, Dunn WR, Purinergic transmission in blood vessels, Auton. Neurosci 191 (2015) 48–66. [DOI] [PubMed] [Google Scholar]
- [7].Burnstock G, Control of vascular tone by purines and pyrimidines, British J. Pharmacol 161 (3) (2010) 527–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhou Z, Matsumoto T, Jankowski V, Pernow J, Mustafa SJ, Duncker DJ, Merkus D, Uridine adenosine tetraphosphate and purinergic signaling in cardiovascular system: an update, Pharmacol. Res 141 (2019) 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dalziel HH, Westfall DP, Receptors for adenine nucleotides and nucleosides: subclassification, distribution, and molecular characterization, Pharmacol. Rev 46 (4) (1994) 449–466. [PubMed] [Google Scholar]
- [10].Burnstock G, Purine and pyrimidine receptors, Cell. Mol. Life Sci 64 (12) (2007) 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Headrick JP, Ashton KJ, Rose’meyer RB, Peart JN, Cardiovascular adenosine receptors: expression, actions and interactions, Pharmacol. Ther 140 (1) (2013) 92–111. [DOI] [PubMed] [Google Scholar]
- [12].Mustafa SJ, Morrison RR, Teng B, Pelleg A, Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology, Handbook of Experimental Pharmacology, 193 2009, pp. 161–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA, International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy, Pharmacol. Rev 58 (3) (2006) 281–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burnstock G, Introduction: P2 receptors, Curr. Top. Med. Chem 4 (8) (2004) 793–803. [DOI] [PubMed] [Google Scholar]
- [15].Erlinge D, Burnstock G, P2 receptors in cardiovascular regulation and disease, Purinergic Signal 4 (1) (2008) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Burnstock G, Purinergic signalling and endothelium, Curr. Vase. Pharmacol 14 (2) (2016) 130–145. [DOI] [PubMed] [Google Scholar]
- [17].Zhou Z, Sun C, Tilley SL, Mustafa SJ, Mechanisms underlying uridine adenosine tetraphosphate-induced vascular contraction in mouse aorta: role of thromboxane and purinergic receptors, Vase. Pharmacol 73 (2015) 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Judkins CP, Sobey CG, Dang TT, Miller AA, Dusting GJ, Drummond GR, NADPH-induced contractions of mouse aorta do not involve NADPH oxidase: a role for P2X receptors, J. Pharmacol. Exp. Ther 317 (2) (2006) 644–650. [DOI] [PubMed] [Google Scholar]
- [19].Guzman-Gutierrez E, Armella A, Toledo F, Pardo F, Leiva A, Sobrevia L, Insulin requires A1 adenosine receptors expression to reverse gestational diabetes-increased L-arginine transport in human umbilical vein endothelium, Purinergic Signal 12 (1) (2016) 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guzman-Gutierrez E, Arroyo P, Salsoso R, Fuenzalida B, Saez T, Leiva A, Pardo F, Sobrevia L, Role of insulin and adenosine in the human placenta microvascular and macrovascular endothelial cell dysfunction in gestational diabetes mellitus, Microcirculation 21 (1) (2014) 26–37. [DOI] [PubMed] [Google Scholar]
- [21].Subiabre M, Villalobos-Labra R, Silva L, Fuentes G, Toledo F, Sobrevia L, Role of insulin, adenosine, and adipokine receptors in the foetoplacental vascular dysfunction in gestational diabetes mellitus, Biochim. Biophys. Acta Mol. basis Dis 165370 (2019). [DOI] [PubMed] [Google Scholar]
- [22].Sathanoori R, Sward K, Olde B, Erlinge D, The ATP receptors P2X7 and P2X4 modulate high glucose and palmitate-induced inflammatory responses in endothelial cells, PLoS One 10 (5) (2015) e0125111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mahdi A, Jiao T, Tratsiakovich Y, Yang J, Ostenson CG, Pernow J, Zhou Z, Altered purinergic receptor sensitivity in type 2 diabetes-associated endothelial dysfunction and Up(4)A-mediated vascular contraction, Int. J. Mol. Sci 19 (12) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nassi A, Malorgio F, Tedesco S, Cignarella A, Gaion RM, Upregulation of inducible NO synthase by exogenous adenosine in vascular smooth muscle cells activated by inflammatory stimuli in experimental diabetes, Cardiovasc. Diabetol 15 (2016) 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Labazi H, Teng B, Mustafa SJ, Functional changes in vascular reactivity to adenosine receptor activation in type I diabetic mice, Eur. J. Pharmacol 820 (2018) 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fahim M, Hussain T, Mustafa SJ, Relaxation of rat aorta by adenosine in diabetes with and without hypertension: role of endothelium, Eur. J. Pharmacol 412 (1) (2001) 51–59. [DOI] [PubMed] [Google Scholar]
- [27].Kobayashi S, Matsumoto T, Ando M, Iguchi M, Watanabe S, Taguchi K, Kobayashi T, UDP-induced relaxation is enhanced in aorta from female obese Otsuka Long-Evans Tokushima fatty rats, Purinergic Signal 14 (1) (2018) 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T, Mechanisms underlying reduced P2Y(1) -receptor-mediated relaxation in superior mesenteric arteries from long-term streptozotocin-induced diabetic rats, Acta Physiol (Oxford) 207 (1) (2013) 130–141. [DOI] [PubMed] [Google Scholar]
- [29].Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T, Mechanisms underlying altered extracellular nucleotide-induced contractions in mesenteric arteries from rats in later-stage type 2 diabetes: effect of ANG II type 1 receptor antagonism, Am. J. Physiol. Heart Circ. Physiol 301 (5) (2011) H1850–H1861. [DOI] [PubMed] [Google Scholar]
- [30].Donoso MV, Mascayano MJ, Poblete IM, Huidobro-Toro JP, Increased ATP and ADO overflow from sympathetic nerve endings and mesentery endothelial cells plus reduced nitric oxide are involved in diabetic neurovascular dysfunction, Front. Pharmacol 9 (2018) 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thaning P, Bune LT, Hellsten Y, Pilegaard H, Saltin B, Rosenmeier JB, Attenuated purinergic receptor function in patients with type 2 diabetes, Diabetes 59 (1) (2010) 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guan Z, Fellner RC, Van Beusecum J, Inscho EW, P2 receptors in renal autoregulation, Curr. Vase. Pharmacol 12 (6) (2014) 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hansen PB, Schnermann J, Vasoconstrictor and vasodilator effects of adenosine in the kidney, Am. J. Physiol. Ren. Physiol 285 (4) (2003) F590–F599. [DOI] [PubMed] [Google Scholar]
- [34].Menzies RI, Tam FW, Unwin RJ, Bailey MA, Purinergic signaling in kidney disease, Kidney Int. 91 (2) (2017) 315–323. [DOI] [PubMed] [Google Scholar]
- [35].Roa H, Gajardo C, Troncoso E, Fuentealba V, Escudero C, Yanez A, Sobrevia L, Pastor-Anglada M, Quezada C, San Martin R, Adenosine mediates transforming growth factor-beta 1 release in kidney glomeruli of diabetic rats, FEBS Lett. 583 (19) (2009) 3192–3198. [DOI] [PubMed] [Google Scholar]
- [36].Kreft E, Kowalski R, Jankowski M, Szczepanska-Konkel M, Renal vasculature reactivity to agonist of P2X7 receptor is increased in streptozotocin-induced diabetes, Pharmacol. Rep 68 (1) (2016) 71–74. [DOI] [PubMed] [Google Scholar]
- [37].Xia JF, Liang QL, Liang XP, Wang YM, Hu P, Li P, Luo GA, Ultraviolet and tandem mass spectrometry for simultaneous quantification of 21 pivotal metabolites in plasma from patients with diabetic nephropathy, J. Chromatogr. B Anal. Technol. Biomed. Life Sci 877 (20–21) (2009) 1930–1936. [DOI] [PubMed] [Google Scholar]
- [38].Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y, A2B adenosine receptor-mediated induction of IL-6 promotes CKD, J. Am. Soc. Nephrol 22 (5) (2011) 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pflueger AC, Schenk F, Osswald H, Increased sensitivity of the renal vasculature to adenosine in streptozotocin-induced diabetes mellitus rats, Am. J. Phys 269 (4 Pt 2) (1995) F529–F535. [DOI] [PubMed] [Google Scholar]
- [40].Elsherbiny NM, Abd El Galil KH, Gabr MM, Al-Gayyar MM, Eissa LA, El-Shishtawy MM, Reno-protective effect of NECA in diabetic nephropathy: implication of IL-18 and ICAM-1, Eur. Cytokine Netw 23 (3) (2012) 78–86. [DOI] [PubMed] [Google Scholar]
- [41].Taskiran E, Erbas O, Yigitturk G, Meral A, Akar H, Taskiran D, Exogenously administered adenosine attenuates renal damage in streptozotocin-induced diabetic rats, Ren. Fail 38 (8) (2016) 1276–1282. [DOI] [PubMed] [Google Scholar]
- [42].Antonioli L, Blandizzi C, Csoka B, Pacher P, Hasko G, Adenosine signalling in diabetes mellitus-pathophysiology and therapeutic considerations, Nat. Rev. Endocrinol 11 (4) (2015) 228–241. [DOI] [PubMed] [Google Scholar]
- [43].Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J, Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury, J. Am. Soc. Nephrol 19 (4) (2008) 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sallstrom J, Carlsson PO, Fredholm BB. Larsson E, Persson AE, Palm F, Diabetes-induced hyperfiltration in adenosine A(1)-receptor deficient mice lacking the tubuloglomerular feedback mechanism, Acta Physiol (Oxford) 190 (3) (2007) 253–259. [DOI] [PubMed] [Google Scholar]
- [45].Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD, Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy, Am. J. Physiol. Ren. Physiol 290 (4) (2006) F828–F837. [DOI] [PubMed] [Google Scholar]
- [46].Persson P, Friederich-Persson M, Fasching A, Hansell P, Inagi R, Palm F, Adenosine A2 a receptor stimulation prevents proteinuria in diabetic rats by promoting an anti-inflammatory phenotype without affecting oxidative stress, Acta Physiol (Oxford) 214 (3) (2015) 311–318. [DOI] [PubMed] [Google Scholar]
- [47].Quezada C, Alarcon S, Jaramillo C, Munoz D, Oyarzun C, San Martin R, Targeting adenosine signaling to treatment of diabetic nephropathy, Curr. Drug Targets 14 (4) (2013) 490–496. [DOI] [PubMed] [Google Scholar]
- [48].Cardenas A, Toledo C, Oyarzun C, Sepulveda A, Quezada C, Guillen-Gomez E, Diaz-Encarnacion MM, Pastor-Anglada M, San Martin R, Adenosine A(2B) receptor-mediated VEGF induction promotes diabetic glomerulopathy, Lab. Investig 93 (1) (2013) 135–144. [DOI] [PubMed] [Google Scholar]
- [49].Tak E, Ridyard D, Kim JH, Zimmerman M, Werner T, Wang XX, Shabeka U, Seo SW, Christians U, Klawitter J, Moldovan R, Garcia G, Levi M, Haase V, Ravid K, Eltzschig HK, Grenz A, CD73-dependent generation of adenosine and endothelial Adora2b signaling attenuate diabetic nephropathy, J. Am. Soc. Nephrol 25 (3) (2014) 547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Friedman DJ, Rennke HG, Csizmadia E, Enjyoji K, Robson SC, The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy, Diabetes 56 (9) (2007) 2371–2379. [DOI] [PubMed] [Google Scholar]
- [51].Matsumoto T, Watanabe S, Kawamura R, Taguchi K, Kobayashi T, Enhanced uridine adenosine tetraphosphate-induced contraction in renal artery from type 2 diabetic Goto-Kakizaki rats due to activated cyclooxygenase/thromboxane receptor axis, Pflugers Arch. 466 (2) (2014) 331–342. [DOI] [PubMed] [Google Scholar]
- [52].Vonend O, Turner CM, Chan CM, Loesch A, Dell’Anna GC, Srai KS, Burnstock G, Unwin RJ, Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models, Kidney Int. 66 (1) (2004) 157–166. [DOI] [PubMed] [Google Scholar]
- [53].Rodrigues AM, Bergamaschi CT, Fernandes MJ, Paredes-Gamero EJ, Buri MV, Ferreira AT, Araujo SR, Punaro GR, Maciel FR, Nogueira GB, Higa EM, P2X (7) receptor in the kidneys of diabetic rats submitted to aerobic training or to N-acetylcysteine supplementation [corrected], PLoS One 9 (6) (2014) e97452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Solini A, Menini S, Rossi C, Ricci C, Santini E, Blasetti Fantauzzi C, Iacobini C, Pugliese G, The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation, J. Pathol 231 (3) (2013) 342–353. [DOI] [PubMed] [Google Scholar]
- [55].Ventura ALM, Dos Santos-Rodrigues A, Mitchell CH, Faillace MP, Purinergic signaling in the retina: From development to disease, Brain Res. Bull 19 (2019) 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sugiyama T, Role of P2X7 receptors in the development of diabetic retinopathy, World J. Diabetes 5 (2) (2014) 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Robertson PL, Ar D, Goldstein GW, Phosphoinositide metabolism and prostacyclin formation in retinal microvascular endothelium: stimulation by adenine nucleotides, Exp. Eye Res 50 (1) (1990) 37–44. [DOI] [PubMed] [Google Scholar]
- [58].Wang W, Lo ACY, Diabetic retinopathy: pathophysiology and treatments, Int. J. Mol. Sci 19 (6) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Platania CBM, Giurdanella G, Di Paola L, Leggio GM, Drago F, Salomone S, Bucolo C, P2X7 receptor antagonism: implications in diabetic retinopathy, Biochem. Pharmacol 138 (2017) 130–139. [DOI] [PubMed] [Google Scholar]
- [60].Clapp C, Diaz-Lezama N, Adan-Castro E, Ramirez-Hernandez G, Moreno-Carranza B, Sarti AC, Falzoni S, Solini A, Di Virgilio F, Pharmacological blockade of the P2X7 receptor reverses retinal damage in a rat model of type 1 diabetes, Acta Diabetol. 56 (9) (2019) 1031–1036. [DOI] [PubMed] [Google Scholar]
- [61].Liao SD, Puro DG, NAD +-induced vasotoxicity in the pericyte-containing microvasculature of the rat retina: effect of diabetes, Invest. Ophthalmol. Vis. Sci 47 (11) (2006) 5032–5038. [DOI] [PubMed] [Google Scholar]
- [62].Sugiyama T, Oku H, Komori A, Ikeda T, Effect of P2X7 receptor activation on the retinal blood velocity of diabetic rabbits, Arch. Ophthalmol 124 (8) (2006) 1143–1149. [DOI] [PubMed] [Google Scholar]
- [63].Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S, The P2X7 receptor in infection and inflammation, Immunity 47 (1) (2017) 15–31. [DOI] [PubMed] [Google Scholar]
- [64].Labazi H, Teng B, Zhou Z, Mustafa SJ, Enhanced A2A adenosine receptor-mediated increase in coronary flow in type I diabetic mice, J. Mol. Cell. Cardiol 90 (2016) 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bender SB, Tune JD, Borbouse L, Long X, Sturek M, Laughlin MH, Altered mechanism of adenosine-induced coronary arteriolar dilation in early-stage metabolic syndrome, Exp. Biol. Med. (Maywood) 234 (6) (2009) 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hedegaard ER, Nielsen BD, Run A, Hughes AD, Kroigaard C, Mogensen S, Matchkov VV, Frobert O, Simonsen U, KV 7 channels are involved in hypoxia-induced vasodilatation of porcine coronary arteries, Br. J. Pharmacol 171 (1) (2014) 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz J, Bratz I, Lull J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FK, Janota D, Oyewumi MO, Logan S, Lindner JR, Chilian WM, Requisite role of Kv1.5 channels in coronary metabolic dilation, Circ. Res 117 (7) (2015) 612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sharifi-Sanjani M, Zhou X, Asano S, Tilley S, Ledent C, Teng B, Dick GM, Mustafa SJ, Interactions between A(2A) adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia, Am. J. Physiol. Heart Circ. Physiol 304 (10) (2013) H1294–H1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sanjani MS, Teng B, Krahn T, Tilley S, Ledent C, Mustafa SJ, Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to the KATP channel using A2B and A2A/2B double-knockout mice, Am. J. Physiol. Heart Circ. Physiol 301 (6) (2011) H2322–H2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhou Z, Sorop O, de Beer VJ, Heinonen I, Cheng C, Jan Danser AH, Duncker DJ, Merkus D, Altered purinergic signaling in uridine adenosine tetraphosphate-induced coronary relaxation in swine with metabolic derangement, Purinergic Signal 13 (3) (2017) 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Prada MP, Syed AU, Buonarati OR, Reddy GR, Nystoriak MA, Ghosh D. Simo S, Sato D, Sasse KC, Ward SM, Santana LF, Xiang YK, Hell JW, Nieves-Cintron M, Navedo MF, A Gs-coupled purinergic receptor boosts Ca(2+) influx and vascular contractility during diabetic hyperglycemia, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gross R, Hillaire-Buys D, Bertrand G, Ribes G, Loubatieres-Mariani MM, Diabetes and impaired response of glucagon cells and vascular bed to adenosine in rat pancreas, Diabetes 38 (10) (1989) 1291–1295. [DOI] [PubMed] [Google Scholar]
- [73].Fujii N, Meade RD, McNeely BD, Nishiyasu T, Sigal RJ, Kenny GP, Type 2 diabetes specifically attenuates purinergic skin vasodilatation without affecting muscarinic and nicotinic skin vasodilatation and sweating, Exp. Physiol 103 (2) (2018) 212–221. [DOI] [PubMed] [Google Scholar]
- [74].Speirs L, Donnelly A, Lynch J, Scholfield CN, Johnson C, ATP and nor epinephrine contributions to sympathetic vasoconstriction of tail artery are altered in streptozotocin-diabetic rats, Am. J. Physiol. Heart Circ. Physiol 291 (5) (2006) H2327–H2333. [DOI] [PubMed] [Google Scholar]
- [75].Zhou Z, Mahdi A, Tratsiakovich Y, Zahoran S, Kovamees O, Nordin F, Uribe Gonzalez AE, Alvarsson M, Ostenson CG, Andersson DC, Hedin U, Hermesz E, Lundberg JO, Yang J, Pernow J, Erythrocytes from patients with type 2 diabetes induce endothelial dysfunction via arginase I, J. Am. Coll. Cardiol 72 (7) (2018) 769–780. [DOI] [PubMed] [Google Scholar]
- [76].Helms CC, Gladwin MT, Kim-Shapiro DB, Erythrocytes and vascular function: oxygen and nitric oxide, Front. Physiol 9 (2018) 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pernow J, Mahdi A, Yang J, Zhou Z, Red blood cell dysfunction: a new player in cardiovascular disease, Cardiovasc. Res 115 (11) (2019) 1596–1650, 10.1093/cvr/cvz156. [DOI] [PubMed] [Google Scholar]
- [78].Zhou Z, Yang J, Pernow J, Erythrocytes and cardiovascular complications, Aging (Albany NY) 10 (12) (2018) 3643–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yang J, Zheng X, Mahdi A, Zhou Z, Tratsiakovich Y, Jiao T, Kiss A, Kovamees O, Alvarsson M, Catrina SB, Lundberg JO, Brismar K, Pernow J, Red blood cells in type 2 diabetes impair cardiac post-ischemic recovery through an arginase-dependent modulation of nitric oxide synthase and reactive oxygen species, JACC Basic Transl. Sci 3 (4) (2018) 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cortese-Krott MM, Kelm M, Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol. 2 (2014) 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mahdi A, Jiao T, Yang J, Kovamees O, Alvarsson M, von Heijne M, Zhou Z, Pernow J, The effect of glycemic control on endothelial and cardiac dysfunction induced by red blood cells in type 2 diabetes, Front. Pharmacol 10 (2019) 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sprague RS, Ellsworth ML, Erythrocyte-derived ATP and perfusion distribution: role of intracellular and intercellular communication, Microcirculation 19 (5) (2012) 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gonzalez-Alonso J, ATP as a mediator of erythrocyte-dependent regulation of skeletal muscle blood flow and oxygen delivery in humans, J. Physiol 590 (20) (2012) 5001–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lohman AW, Billaud M, Isakson BE, Mechanisms of ATP release and signalling in the blood vessel wall, Cardiovasc. Res 95 (3) (2012) 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Adderley SP, Sprague RS, Stephenson AH, Hanson MS, Regulation of cAMP by phosphodiesterases in erythrocytes, Pharmacol. Rep 62 (3) (2010) 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes, Am. J. Physiol. Heart Circ. Physiol 299 (4) (2010) H1146–H1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sridharan M, Bowles EA, Richards JP, Krantic M, Davis KL, Dietrich KA, Stephenson AH, Ellsworth ML, Sprague RS, Prostacyclin receptor-mediated ATP release from erythrocytes requires the voltage-dependent anion channel, Am. J. Physiol. Heart Circ. Physiol 302 (3) (2012) H553–H559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sprague RS, Goldman D, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, Ellsworth ML, Divergent effects of low-0(2) tension and iloprost on ATP release from erythrocytes of humans with type 2 diabetes: implications for 0(2) supply to skeletal muscle, Am. J. Physiol. Heart Circ. Physiol 299 (2) (2010) H566–H573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sprague RS, Stephenson AH, Bowles EA, Stumpf MS, Lonigro AJ, Reduced expression of G(i) in erythrocytes of humans with type 2 diabetes is associated with impairment of both cAMP generation and ATP release, Diabetes 55 (12) (2006) 3588–3593. [DOI] [PubMed] [Google Scholar]
- [90].Sprague RS, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, Ellsworth ML, A selective phosphodiesterase 3 inhibitor rescues low P02-induced ATP release from erythrocytes of humans with type 2 diabetes: implication for vascular control, Am. J. Physiol. Heart Circ. Physiol 301 (6) (2011) H2466–H2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dergunov SA, Bowles EA, Gordon W, Green M, Bierman A, Ellsworth ML, Pinkhassik E, Sprague RS, Liposomal delivery of a phosphodiesterase 3 inhibitor rescues low oxygen-induced ATP release from erythrocytes of humans with type 2 diabetes, Biochem. Biophys. Rep 2 (2015) 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Richards JP, Yosten GL, Kolar GR, Jones CW, Stephenson AH, Ellsworth ML, Sprague RS, Low 02-induced ATP release from erythrocytes of humans with type 2 diabetes is restored by physiological ratios of C-peptide and insulin, Am. J. Phys. Regul. Integr. Comp. Phys 307 (7) (2014) R862–R868. [DOI] [PubMed] [Google Scholar]
- [93].Richards JP, Bowles EA, Gordon WR, Ellsworth ML, Stephenson AH, Sprague RS, Mechanisms of C-peptide-mediated rescue of low 02-induced ATP release from erythrocytes of humans with type 2 diabetes, Am. J. Phys. Regul. Integr. Comp. Phys 308 (5) (2015) R411–R418. [DOI] [PubMed] [Google Scholar]
- [94].Vecchie A, Montecucco F, Carbone F, Dallegri F, Bonaventura A, Diabetes and vascular disease: is it all about glycemia? Curr. Pharm. Des 25 (29) (2019) 3112–3127. [DOI] [PubMed] [Google Scholar]
- [95].Rivas Rios JR, Franchi F, Rollini F, Angiolillo DJ, Diabetes and antiplatelet therapy: from bench to bedside, Cardiovasc. Diagn. Ther 8 (5) (2018) 594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Parodi J, Flores C, Aguayo C, Rudolph MI, Casanello P, Sobrevia L, Inhibition of nitrobenzylthioinosine-sensitive adenosine transport by elevated D-glucose involves activation of P2Y2 purinoceptors in human umbilical vein endothelial cells, Circ. Res 90 (5) (2002) 570–577. [DOI] [PubMed] [Google Scholar]
- [97].Nilsson J, Nilsson LM, Chen YW, Molkentin JD, Erlinge D, Gomez MF, High glucose activates nuclear factor of activated T cells in native vascular smooth muscle, Arterioscler. Thromb. Vase. Biol 26 (4) (2006) 794–800. [DOI] [PubMed] [Google Scholar]
- [98].Pares-Herbute N, Hillaire-Buys D, Etienne P, Gross R, Loubatieres-Mariani MM, Monnier L, Adenosine inhibitory effect on enhanced growth of aortic smooth muscle cells from streptozotocin-induced diabetic rats, Br. J. Pharmacol 118 (3) (1996) 783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wu H, Nie Y, Xiong H, Liu S, Li G, Huang A, Guo L, Wang S, Xue Y, Wu B, Peng L, Song M, Li G, Liang S, P2X7 Receptor expression in peripheral blood monocytes is correlated with plasma C-reactive protein and cytokine levels in patients with type 2 diabetes mellitus: a preliminary report, Inflammation 38 (6) (2015) 2076–2081. [DOI] [PubMed] [Google Scholar]


