Abstract
Purpose
To correlate carotid dose and risk of carotid blowout syndrome (CBOS) after stereotactic body radiation therapy (SBRT), hypothesizing that carotid dose does not correlate with CBOS.
Methods and Materials
We retrospectively reviewed 186 patients with recurrent, previously irradiated head and neck cancer treated between January 2008 and March 2013. Patients treated early in our experience with incomplete dosimetry were excluded from analysis (n = 111). A total of 75 patients were identified, providing 150 carotid arteries for analysis. Median follow-up was 8 months (range, 1–91 months) for all patients, and 37 months for surviving patients (range, 31–91 months). Patients were treated with linear accelerator–based SBRT to a median dose up to 44 Gy (range, 40–50 Gy) in 5 fractions delivered on a twice-weekly basis. Concurrent cetuximab was used in 63 patients (84%). The bilateral common, internal, and external carotid arteries were delineated 2 cm above and below the planning target volume. The maximum dose to 0.1 cm3 (D0.1cc), 1 cm3 (D1cc), and 2 cm3 (D2cc) of the carotid and the mean carotid dose from SBRT were recorded and analyzed for association with carotid bleeding events, using binary logistic regression.
Results
Median reirradiation interval was 20 months (range, 3–423 months), and median prior radiation dose was 70 Gy (range, 52.5–140 Gy). Sixteen patients (21.3%) received more than 1 course of SBRT, and the cumulative carotid doses from fused summary plans were recorded. The overall median D0.1cc, D1cc, D2cc, and mean carotid doses were 40.8 Gy (interquartile range [IQR], 21.6–47.6 Gy), 26.8 Gy (IQR, 14.1–42.1 Gy), 15.4 Gy (IQR, 8.4–32.7 Gy), and 15.0 Gy (IQR, 8.9–23.3 Gy), respectively. There were a total of 4 bleeding events (5.3%): 2 patients (2.7%) had mucosal bleeds that resolved after embolization of carotid branches, and 2 patients (2.7%) died from complications of CBOS. In the 2 patients with CBOS the D0.1cc was 48.4 Gy and 47.6 Gy, respectively. There was no significant association between bleeding events and D1cc (P = .280), D2cc (P = .571), or mean dose (P = .568). There was a trend toward increased risk of bleeding and D0.1cc (P = .080).
Conclusions
These results demonstrate a low risk of bleeding after reirradiation with SBRT when 5 fractions are delivered on nonconsecutive days, even when tumor is completely encasing the carotid artery. Although limited by the low number of events, no significant association was found between dose-volume parameters and the risk of carotid bleeding. No CBOS was noted when D0.1cc was <47.6 Gy.
Introduction
Stereotactic body radiation therapy (SBRT) has emerged as a viable treatment modality for patients with unresectable, locally recurrent, previously irradiated head and neck cancer (rHNC) (1). Compared with other modern reirradiation techniques, SBRT offers comparable disease outcomes with shorter treatment time and decreased acute toxicity (2). Limiting the widespread use of SBRTare concerns of carotid blowout syndrome (CBOS), an often fatal complication of head and neck cancer in which the carotid artery or one of its major branches rupture(s) (3). A number of early reports suggested comparatively higher rates of CBOS after SBRT than had been previously reported with conventional reirradiation techniques (3–5). However, with continued study, both tumor- and treatment-related factors have been identified to reduce the risk of CBOS after SBRT, including increasing the length of the treatment delivery period from daily to every other day and avoiding SBRT in patients with skin invasion or tumor-related ulceration (6–8).
Some of the earlier series also noted that the risk of CBOS may be higher in patients with >180° carotid involvement or carotid artery dose >100% of prescription (4, 5). This has prompted some practitioners to exclude patients with carotid encasement from SBRT or aggressively spare the carotid when using SBRT in such patients. Others, including our institutional practice, have not excluded patients from SBRT on the basis of extent of carotid involvement or carotid dose. As the potential outreach of SBRT is expanded into the cooperative group setting, such as the NRG KEYSTROKE trial examining SBRT plus pembrolizumab, consistent factors for patient selection and validated dose constraints to guide treatment planning are needed. Thus, we aimed to correlate carotid dose and risk of CBOS after SBRT, hypothesizing that carotid dose does not correlate with CBOS.
Methods and Materials
Following appropriate institutional review board approval, we retrospectively reviewed 186 patients with rHNC treated between January 2008 and March 2013 as a part of our prospectively maintained institutional radiosurgery registry. Patients treated early in our experience with incomplete dosimetry or treated with <5 fractions to doses <40 Gy were excluded from analysis, as were patients treated with primary SBRT without prior irradiation. Patients were treated with linear accelerator–based SBRT using Varian Triology or TrueBeam treatment platforms (Varian Medical Systems, Palo Alto, CA) to 40 to 50 Gy in 5 fractions delivered on an alternating-day basis (maximum of 3 fractions per week). Patients treated with <40 Gy were excluded, given the importance of doses >35 to 40 Gy noted in prior publications (2, 9). Patients with squamous cell histology also received concurrent cetuximab with SBRT, administered at 400 mg/m2 on day −7 (loading dose) then 250 mg/m2 days 0 and +8. Radiation dose selection was based on gross tumor volume (GTV): those >25 cm3 received 44–50 Gy, and those <25 cm3 received 40 Gy in 5 fractions (9). Positron emission tomography/computed tomography (CT) or magnetic resonance imaging was used along with contrast-enhanced CT simulation with 1.25-mm slice thickness to define the GTV; the GTV to planning target volume (PTV) expansion included 0 to 5 mm, with no expansion for clinical target volume and no elective nodal irradiation (10). We initially used no margin early in our experience, but review of patients with recurrences after SBRT demonstrated that the addition of a small margin up to 5 mm may reduce the risk of failure, though this can be reduced if there is concern for toxicity to critical structures, such as the spinal cord or carotids (10). Treatment localization included at minimum daily image guidance with cone beam CT imaging, with Brainlab ExacTrack (Brainlab, Munich, Germany) also commonly used daily, especially in patients with lesions close to the base of skull. Patients treated in our previously reported phase 2 study examining SBRT and cetuximab were included in this analysis (11).
Our institutional practices, including our institutional protocols, inclusion criteria, and treatment planning parameters, have not excluded patients on the basis of the extent of carotid involvement, and no specific dosimetric goals were used in treatment planning for the carotid artery. Thus, we retrospectively delineated the bilateral common, internal, and external carotid arteries 2 cm above and below the PTV. The maximum dose to 0.1 cm3 (D0.1cc), 1 cm3 (D1cc), and 2 cm3 (D2cc) of the carotid and the mean carotid dose from SBRT were recorded. Because of the wide capture of our SBRT program and inconsistent recording/changes in treatment planning systems over the often long reirradiation interval, prior radiation therapy dose from external beam radiation was not included in this analysis. However, if patients were treated with multiple courses of SBRT after prior external beam irradiation, the cumulative doses to the carotids from SBRT courses were summated and included for this analysis.
Carotid blowout syndrome was defined as rupture and hemorrhage from the carotid artery or its major branches after reirradiation in the absence of residual or progressive local disease. Other previously established risk factors for CBOS were collected, including contact angle of the tumor with the carotid artery (0°, <180°, ≥180°, 360°), skin invasion, diagnosis of infection/necrosis (according to imaging), treatment site (mucosal vs neck), presence of ulceration, and PTV size (5, 6). The carotid dosimetric parameters and other risk factors were analyzed for association with carotid bleeding events using the Kaplan-Meier method and log-rank test for significance for categorical variables and Cox regression for continuous variables. The low number of events limited formation of valid multivariable analysis. Statistical analyses were completed using IBM SPSS, version 23 (IBM, Armonk, NY).
Results
Of the initial 186 patients identified, a total of 75 patients with complete dosimetric data available were identified, providing 150 carotid arteries for analysis. Median follow-up was 8 months (range, 1–91 months) for all patients and 37 months for surviving patients (range, 31–91 months). Median reirradiation interval was 20 months (range, 3–423 months), and median prior radiation dose was 70 Gy (range, 52.5–140 Gy). Eight patients (10.7%) received more than 1 course of SBRT, and the cumulative carotid doses from fused summary plans were recorded. The median SBRT prescription dose was 44 Gy (range, 40–50 Gy). Complete patient and treatment characteristics are included in Table 1.
Table 1.
Patient and treatment characteristics
| Factor | Number | Percentage | Carotid bleed, n (%) | Association of factor with carotid bleeding (P) |
|---|---|---|---|---|
| Age (y), median (IQR) | 65 (57–72) | - | - | .978 |
| Sex | .286 | |||
| Male | 46 | 61.3 | 3 (6.5) | |
| Female | 29 | 38.7 | 1 (3.4) | |
| Prior surgery | .235 | |||
| Yes | 50 | 66.7 | 0 (0) | |
| No | 25 | 33.3 | 4(8) | |
| Concurrent cetuximab | .430 | |||
| Yes | 63 | 84.0 | 4 (6.4) | |
| No | 12 | 16.0 | 0 (0) | |
| Interval from EBRT to SBRT (mo), median (IQR) | 20 (8.6–56.25) | - | - | .477 |
| Courses of SBRT | .352 | |||
| 1 | 67 | 89.3 | 3 (4.5) | |
| 2 | 5 | 6.7 | 0(0) | |
| 3 | 3 | 4.0 | 1 (33.3) | |
| Site of recurrence | .624 | |||
| Mucosal | 46 | 61.3 | 2 (4.3) | |
| Neck | 29 | 38.7 | 2 (6.9) | |
| Carotid encasement with tumor | .868 | |||
| 0° | 59 | 78.7 | 1 (1.7) | |
| >0°, ≤180° | 4 | 5.3 | 3 (50.0) | |
| >180°, <360° | 5 | 6.7 | 1 (20.0) | |
| 360° | 7 | 9.3 | 0(0) | |
| Skin invasion | .167 | |||
| Yes | 4 | 5.3 | 1 (25) | |
| No | 71 | 94.7 | 3 (4.2) | |
| Necrosis/infection | .318 | |||
| Yes | 19 | 25.3 | 0 (0) | |
| No | 56 | 74.7 | 4 (7.1) | |
| Ulceration | .315 | |||
| Yes | 7 | 9.3 | 1 (14.3) | |
| No | 68 | 90.3 | 3 (4.4) | |
Abbreviations: EBRT = external beam radiation therapy; IQR = interquartile range; SBRT = stereotactic body radiation therapy.
The overall median D0.1cc, D1cc, D2cc, and mean carotid doses were 40.8 Gy (interquartile range [IQR], 21.6–47.6 Gy), 26.8 Gy (IQR, 14.1–42.1Gy), 15.4Gy (IQR, 8.4–32.7 Gy), and 15.0 Gy (IQR, 8.9–23.3 Gy), respectively (Table 2). There were a total of 4 bleeding events (5.3%): 2 patients (2.7%) had mucosal bleeds that resolved after embolization of carotid branches, and 2 patients (2.7%) died from complications of CBOS (Fig. 1). The median time to carotid bleeding events was 12 months (range, 5–13 months). In the 2 patients with CBOS, the D0.1cc was 48.4 Gy and 47.6 Gy, respectively. There was no significant association between bleeding events and PTV size (P = .685), D1cc (P = .280), D2cc (P = .571), or mean dose (P = .658). There was a trend toward greater likelihood of carotid bleeding with increasing D0.1cc (odds ratio 1.03, 95% confidence interval 0.996–1.067, P = .080). Additionally, no significant association was found between bleeding events and other baseline risk factors, including male versus female gender (P = .286), neck versus mucosal site of recurrent disease (P = .624), skin involvement (P = .167), diagnosis of necrosis/infection (P = .318), presence of ulceration (P = .315), or contact angle of tumor with the carotid artery (P = .868). Table 1 includes the number of patients with each risk factor experiencing carotid bleeding and the association between bleeding events and risk factors on univariate analyses.
Table 2.
Carotid dosimetry and association with bleeding events
| Parameter | Median | IQR | OR (95% CI) | P |
|---|---|---|---|---|
| D0.1cc | 40.8 Gy | 21.6–47.6 Gy | 1.031 (0.996–1.067) | .080 |
| D1cc | 26.8 Gy | 14.1–42.1 Gy | 1.022 (0.982–1.064) | .280 |
| D2cc | 15.4 Gy | 8.4–32.7 Gy | 1.013 (0.968–1.061) | .571 |
| Mean dose | 15.0 Gy | 8.9–23.3 Gy | 1.017 (0.943–1.098) | .658 |
| PTV size | 39.3 cm3 | 17.2–76.6 cm3 | 0.992 (0.952–1.033) | .685 |
Abbreviations: CI = confidence interval; IQR = interquartile range; OR = odds ratio; PTV = planning target volume.
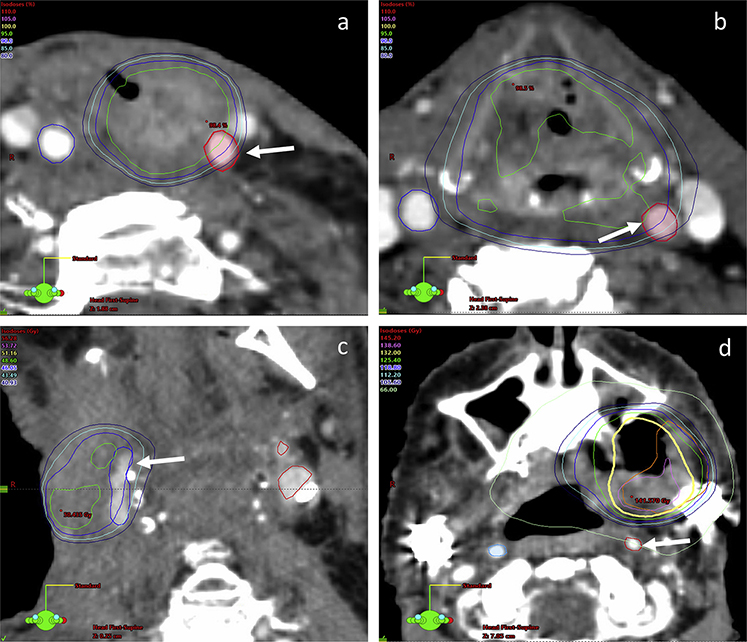
Fig. 1.
Axial view of treatment plans in patients with carotid bleeding events, with solid white arrows indicating carotid artery. (a) Stereotactic body radiation therapy (SBRT) plan of a 59-year-old man treated with a single course of SBRT (44 Gy in 5 fractions), who developed a mucosal bleed that stabilized after embolization. (b) Plan of a 64-year-old man treated with a single course of SBRT (44 Gy in 5 fractions), who developed rupture of the right carotid. (c) Plan of a 67-year-old man treated with a single course of SBRT (44 Gy in 5 fractions), who died after right carotid rupture. (d) Summary plan of a 70-year-old woman treated with 3 courses of SBRT (44 Gy in 5 fractions each), who developed bleeding that stabilized after embolization.
Discussion
Locoregional recurrence remains the predominant pattern of failure for locally advanced head and neck cancers treated with definitive radiation therapy (12, 13). Although the preferred treatment of rHNC is salvage surgery, the majority of patients are not candidates owing to either extent of local invasion or the presence of comorbidities, and survival rates remain poor (14). Reirradiation with conventional radiation therapy techniques has been shown to provide prolonged survival in some patients, though it is associated with severe acute and late toxicity rates of up to 78% and 37%, respectively (15–17). Modern reirradiation techniques, including intensity modulated radiation therapy and SBRT, have been used in an attempt to improve tumor control and reduce toxicity. Although no prospective comparisons of these techniques exist, a recent multi-institutional retrospective study demonstrated decreased acute grade ≥4 toxicity with SBRT and no difference in late effects (2). Although patients in this study with recursive partitioning analysis class II demonstrated improved survival with IMRT, survival was comparable when ≥35 Gy was delivered with SBRT (18).
Our institutional policy has been to treat patients with unresectable rHNC with SBRT delivered in 5 fractions over 2 weeks with concurrent cetuximab, with demonstrated 1-year locoregional progression-free survival and overall survival rates of 37% and 40%, respectively (11). Other institutions have also demonstrated the efficacy and feasibility of this treatment technique in prospective settings (19, 20). Prior analyses have shown a benefit to dose escalation to at least 40 Gy, on the basis of the volume of recurrence tumor, and this reirradiation technique is the subject of the upcoming NRG KEYSTROKE trial (9).
One of the primary concerns with SBRT for treatment of rHNC has been the possibility of significant late toxicities, which can include dysphagia, osteonecrosis, laryngeal edema/stenosis, and trachea-esophageal fistula (21). Carotid blowout syndrome is a rare but often fatal complication of reirradiation of rHNC that has been reported in 1% to 4% of patients undergoing conventional reirradiation with daily or twice-daily fractionation (3). Early reports of reirradiation with SBRT demonstrated CBOS rates ranging from 8% to 17%, raising serious concerns with the feasibility of this modality (4, 5). Some previously identified risk factors have included carotid encasement >180° by tumor, skin invasion, presence of ulceration, diagnosis of infection/necrosis, treatment of neck recurrences, and increased PTV size (5–7). A later analysis, however, demonstrated that CBOS rates can be reduced substantially by delivering 5 fractions in an every-other-day course, which may allow for increased sublethal damage repair of normal tissues compared with daily irradiation (8).
In the present study we demonstrate an overall 5.3% risk of carotid bleeding events, including patients with bleeds of minor branches of the carotid that resolved after embolization. Only 2 patients (2.6%) in our cohort experienced true CBOS, a figure that is comparable to reported CBOS rates with conventional reirradiation (3). We sought to examine the potential association of dosimetric parameters on carotid bleeding, as well other previously established risk factors. A total of 75 patients were investigated, providing 150 carotid arteries for analysis, and no significant association was found between bleeding risk and any of the established baseline risk factors as well as the D0.1, D1cc, D2cc, or mean carotid dose. It should be noted, however, that in the 2 patients with CBOS the D0.1 values were 48.4 Gy and 47.6 Gy, which does exceed the typical marginal prescription dose. This suggests that although it is not necessary to avoid treating patients with complete carotid encasement provided an every-other-day fractionation scheme is used, it may be reasonable to avoid a hot spot within the carotid artery because no CBOS was noted when D0.1cc was <47.6 Gy. Carotid artery D0.1cc < 47.6 Gy represents a potential constraint to guide application of SBRT for future clinical trial design.
Some limitations of this study include its retrospective nature and unavoidable problems with patient selection bias. Additional toxicities may have been underreported in patients who were eventually lost to follow-up, though our minimum follow-up time of 31 months among living patients exceeds the normal interval at which CBOS occurs (3). Summary plans were created in patients who underwent multiple courses of SBRT, though registration errors are inevitable when fusing head and neck plans with significant time intervals between treatment courses. The fact that we were not able to confirm previously identified risk factors for CBOS could be due to the overall low number of events, which also limited multivariable analysis. Notwithstanding these limitations, our extensive experience does corroborate these results indicating a low risk of bleeding after reirradiation with SBRT when 5 fractions are delivered on nonconsecutive days, even when tumor is completely encasing the carotid artery. Though no significant associations with bleeding risk and dose parameters were found, both patients suffering CBOS received a carotid D0.1cc of at least 47.6 Gy. We would caution against allowing an excessive hot spot within the carotid artery, as a note of precaution. As the utilization of SBRT for reirradiation of rHNC continues to expand and future cooperative group protocols are planned, these data can help to guide future patient selection and dose constraints.
Summary.
Stereotactic body radiation therapy has emerged as a viable treatment option for recurrent head and neck cancers after prior irradiation, though carotid dose constraints are not defined. The maximum dose to 0.1 cm3, 1 cm3, and 2 cm3 of the carotid and the mean dose were analyzed for association with bleeding. No significant association was found between dose-volume parameters and risk of carotid bleeding, and no CBOS was noted when D0.1cc was <47.6 Gy.
Footnotes
Data were submitted for presentation at the Radiosurgery Society Annual Scientific Meeting, November 2–4, 2017, Las Vegas, NV.
Conflict of interest: J.A.V. receives speaking honoraria from Brainlab.
References
- 1.Wong SJ, Heron DE, Stenson K, et al. Locoregional recurrent or second primary head and neck cancer: Management strategies and challenges. Am Soc Clin Oncol Educ B 2016;36:284–292. [DOI] [PubMed] [Google Scholar]
- 2.Vargo JA, Ward MC, Caudell JJ, et al. A multi-institution comparison of SBRT and IMRT for definitive re-irradiation of recurrent or second primary head and neck cancer. Int J Radiat Oncol Biol Phys 2017; 10.1016/j.ijrobp.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald MW, Moore MG, Johnstone PA. Risk of carotid blowout after reirradiation of the head and neck: A systematic review. Int J Radiat Oncol Biol Phys 2012;82:1083–1089. [DOI] [PubMed] [Google Scholar]
- 4.Cengiz M, Özyiğit G, Yazici G, et al. Salvage reirradiaton with stereotactic body radiotherapy for locally recurrent head-and-neck tumors. Int J Radiat Oncol Biol Phys 2011;81:104–109. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki H, Ogita M, Kodani N, et al. Frequency, outcome and prognostic factors of carotid blowout syndrome after hypofractionated re-irradiation of head and neck cancer using CyberKnife: A multi-institutional study. Radiother Oncol 2013;107:305–309. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki H, Ogita M, Himei K, et al. Carotid blowout syndrome in pharyngeal cancer patients treated by hypofractionated stereotactic reirradiation using CyberKnife: A multi-institutional matched-cohort analysis. Radiother Oncol 2015;115:67–71. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki H, Ogita M, Himei K, et al. Reirradiation using robotic image-guided stereotactic radiotherapy of recurrent head and neck cancer. J Radiat Res 2016;57:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazici G, Sanl1 TY, Cengiz M, et al. A simple strategy to decrease fatal carotid blowout syndrome after stereotactic body reirradiaton for recurrent head and neck cancers. Radiat Oncol 2013;8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rwigema J-CM, Heron DE, Ferris RL, et al. The impact of tumor volume and radiotherapy dose on outcome in previously irradiated recurrent squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. Am J Clin Oncol 2011;34: 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K, Heron DE, Clump DA, et al. Target delineation in stereotactic body radiation therapy for recurrent head and neck cancer: A retrospective analysis of the impact of margins and automated PET-CT segmentation. Radiother Oncol 2013;106:90–95. [DOI] [PubMed] [Google Scholar]
- 11.Vargo JA, Ferris RL, Ohr J, et al. A prospective phase 2 trial of reirradiation with stereotactic body radiation therapy plus cetuximab in patients with previously irradiated recurrent squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2015;91: 480–488. [DOI] [PubMed] [Google Scholar]
- 12.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003;21:92–98. [DOI] [PubMed] [Google Scholar]
- 13.Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: A randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014;89:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temam S, Pape E, Janot F, et al. Salvage surgery after failure of very accelerated radiotherapy in advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2005;62:1078–1083. [DOI] [PubMed] [Google Scholar]
- 15.Kao J, Garofalo MC, Milano MT, et al. Reirradiation of recurrent and second primary head and neck malignancies: A comprehensive review. Cancer Treat Rev 2003;29:21–30. [DOI] [PubMed] [Google Scholar]
- 16.Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 2008;30:281–288. [DOI] [PubMed] [Google Scholar]
- 17.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: Results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol 2007;25:4800–4805. [DOI] [PubMed] [Google Scholar]
- 18.Ward MC, Riaz N, Caudell JJ, et al. Multi-institution analysis of intensity modulated radiation therapy–based reirradiation for head and neck cancer: Prognostic factors and recursive partitioning analysis for overall survival. Int J Radiat Oncol Biol Phys 2016;96:S115. [Google Scholar]
- 19.Lartigau EF, Tresch E, Thariat J, et al. Multi institutional phase II study of concomitant stereotactic reirradiation and cetuximab for recurrent head and neck cancer. Radiother Oncol 2013;109: 281–285. [DOI] [PubMed] [Google Scholar]
- 20.Comet B, Kramar A, Faivre-Pierret M, et al. Salvage stereotactic reirradiation with or without cetuximab for locally recurrent head-andneck cancer: A feasibility study. Int J Radiat Oncol Biol Phys 2012; 84:203–209. [DOI] [PubMed] [Google Scholar]
- 21.Ling DC, Vargo JA, Ferris RL, et al. Risk of severe toxicity according to site of recurrence in patients treated with stereotactic body radiation therapy for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 2016;95:973–980. [DOI] [PubMed] [Google Scholar]