Abstract
Purpose:
Randomized studies have demonstrated a survival benefit for consolidative thoracic radiotherapy (TRT) in extensive stage (ES) small cell lung cancer (SCLC), however the radiation dose and optimal selection criteria are often debated.
Methods:
We analyzed 3280 stage IV SCLC treated with double-agent chemotherapy and TRT within the National Cancer Data Base (NCDB) and evaluated the differences in selection patterns and survival outcomes for patients who received at least 45 Gy of TRT and those who received < 45 Gy. Univariable and multivariable analyses identified characteristics predictive of overall survival. Propensity-adjusted Cox proportional hazard ratios for survival were used to account for indication bias between the two dose arms.
Results:
There were 1621 patients in the < 45 Gy group (most common 30 Gy) and 1659 patients in the 45 Gy or higher group (most common 45 Gy). White patients, T1-T3 lesions, an absence of brain/liver/bone metastases, and starting TRT after 12 weeks of chemotherapy were associated with the higher dose group.
With multivariable analysis, TRT to at least 45 Gy was an independent predictor of improved survival (HR = 0.78, P < 0.001) along with female gender, age < 65, lower comorbidity score, starting TRT 12 weeks after chemotherapy, and the absence of brain/liver/bone metastases (P < 0.01). Propensity adjusted regression model showed a persistent correlation between a higher dose and survival (HR = 0.74, P < 0.001). Survival at 1 and 2 years for the 45 Gy or higher arm was 58.1% and 25.2% compared to 43.8% and 15.1% for the < 45 Gy arm (P < 0.001).
Conclusion:
In the largest analysis of consolidative thoracic radiotherapy in ES-SCLC to date, dose escalation to at least 45 Gy was an independent predictor for increased survival. These findings may be validated in ongoing prospective studies.
Keywords: Small cell lung cancer, Extensive stage, Dose escalation, Thoracic radiotherapy, National Cancer Database, SCLC
1. Introduction
With a median survival of 6–12 months, small cell lung cancer (SCLC) most commonly presents with metastatic or extensive stage (ES) disease [1]. Cisplatin-based doublet chemotherapy is the mainstay of therapy with high rates of initial response. However, a proportion of patients harbor residual intrathoracic disease, precipitating the hypothesis that consolidative thoracic radiotherapy (TRT) can improve outcomes [2]. Several prospective and retrospective studies have demonstrated a survival benefit with the addition of thoracic radiation in ES-SCLC, including the recent phase III CREST trial which reported a 10% 2-year overall survival benefit for consolidative TRT to a dose of 30 Gy in 10 fractions [3-9].
Notably, the rate of intrathoracic progression in the CREST trial was 43.7%, leading the authors to propose that dose escalation may further improve outcomes [6]. Yet, given the aggressive nature of SCLC and potentially increased toxicity profile with TRT, the optimal selection characteristics for dose escalated consolidative radiotherapy is unknown [10]. Indeed, while consolidative TRT in ES-SCLC is included in the current National Comprehensive Cancer Network (NCCN) guidelines, the recommended dose ranges between 30 Gy in 10 fractions and 60 Gy in 30 fractions [11].
The recently closed NRG/RTOG 0937 phase II clinical trial compared prophylactic cranial irradiation with prophylactic cranial irradiation and consolidative TRT to 45 Gy, with reduced time to progression in the latter arm but without a difference in survival [12,13]. To date there have been no randomized trials to evaluate the impact of dose escalation in thoracic RT for ES-SCLC. We herein utilized the National Cancer Database (NCDB) to define factors affecting selection for dose escalation, and survival outcomes for stage IV SCLC patients who received chemotherapy and TRT in the modern era.
2. Methods
2.1. Patient selection
This study was exempt from institutional review board supervision due to the utilization of de-identified data provided by the NCDB, a tumor registry jointly managed by the American Cancer Society and American College of Surgeons. The database captures approximately 70% of cancer cases in the United States from over 1500 hospitals accredited by the Commission on Cancer. We queried the database to identify ES-SCLC patients treated with chemotherapy and TRT between the years 2004–2015, although ultimately only data between the years 2010–2014 met inclusion criteria. A complete CONSORT diagram depicting the cohort selection process is outlined on Fig. 1. Patient criteria included known stage IV disease, double-agent chemotherapy, and external beam radiotherapy to the lung or lung/thoracic lymph nodes to at least 30 Gy. Patients without known follow-up were excluded, as were cases with follow-up less than three months to account for immortal time bias.
Fig. 1.

CONSORT diagram. SCLC, small cell lung cancer. n, number of patients. Gy, Gray.
Ultimately, 3280 patients were eligible for final analysis with 1621 patients treated to less than 45 Gy and 1659 patients receiving at least 45 Gy. The median dose of the entire cohort was 45 Gy, dictating the two main comparison groups for propensity score-matched analysis to provide comparative balance, and to reflect the dose employed by the latest RTOG/NRG clinical trials [13]. Receiver operating characteristic curve analysis confirmed 45 Gy as the optimal a priori value. Fractionation was not incorporated in our analysis because the relative difference in dose per fraction between 1.5–3 Gy demonstrated no correlation with outcome compared to the total dose. This was confirmed by multivariable regression analysis on survival, where P < 0.001 for total dose, and P = 0.65 for dose/fraction. Additionally, there was no overlap in biologic equivalent dose10 between the < 45 Gy and ≥45 Gy groups. However, none of the patients were treated twice daily with a hyperfractionated regimen, as confirmed by the number of elapsed days between the start and end of radiotherapy.
Race was defined as either white, African American, or other/unknown. Comorbidity was quantified via Charlson/Deyo comorbidity index, and stage was defined by American Joint Cancer Committee 7th edition clinical staging with stage IV used as a surrogate for extensive stage disease. Income data in the patients’ residence census tract were provided as quartiles and reported here as above or below the median. Population classification was based on typology published by the USDA Economic Research Service, facility type was assigned according to Commission on Cancer accreditation category, and insurance status was reported on the admission page. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
2.2. Statistics
Statistical analysis was performed via SPSS version 20. Summary statistics were reported for discrete variables and Chi square tests were used to compare socioeconomic, clinical, and treatment characteristics between the < 45 Gy and ≥45 Gy groups. Bivariate logistic regression models were used to evaluate the association between independent variables of interest and dose group. To account for immortal time bias, survival analysis was restricted to patients with a survival time of at least 3 months (the allowed time for TRT to be considered “consolidative”). Overall survival (OS) was calculated from the date of diagnosis to the date of last contact or death using Kaplan Meier curves to present the cumulative probability of survival, and log-rank statistics to assess statistical significance between groups. Univariable survival analysis was performed for all characteristics listed on Table 1, and statistically significant factors were then entered in a hierarchical fashion using “enter” selection of the covariates’ likelihood ratios. Since the total number of candidate covariates were small relative to the total patient population, a confirmatory multivariable analysis using a stepwise backward elimination and forward selection procedures were performed and the same results were obtained. Adjusted hazard ratios (HR) and 95% confidence interval (Cl) are reported, with α = 0.05 used to indicate statistical significance.
Table 1.
Patient and Treatment Characteristics (N = 3280).
| Characteristic | No. (% or range) |
Characteristic | No. (% or range) |
|---|---|---|---|
| Demographics | Disease characteristics | ||
| Sex | Clinical T stage | ||
| Male | 1558 (47.5) | T1/T2 | 924 (28.2) |
| Female | 1722 (52.5) | T3 | 628 (19.1) |
| Age | T4 | 1361 (41.5) | |
| Median | 63.6 (23–90) | Unknown | 367 (11.2) |
| < 65 | 1743 (53.1) | Clinical N stage | |
| > 65 | 1537 (46.9) | N0/N1 | 493 (15) |
| Race | N2 | 1717 (52.3) | |
| White | 2918 (89) | N3 | 885 (27) |
| African American | 285 (8.7) | Unknown | 185 (5.6) |
| Other/Unknown | 77 (2.3) | Clinical M stage | |
| Comorbidity score | M0 | 40 (1.2) | |
| 0 | 1899 (57.9) | M1a | 788 (24.0) |
| 1 | 968 (29.5) | M1b | 1616 (49.3) |
| > 2 | 413 (12.6) | M1 (not specified) | 806 (24.6) |
| Insurance | Extrapulmonary metastases | ||
| Not insured | 169 (5.2) | Brain metastases | 580 (17.7) |
| Government | 1988 (60.6) | Liver metastases | 735 (22.4) |
| Private payer | 1082 (33.0) | Bone metastases | 843 (25.7) |
| Unknown | 41 (1.3) | Metastases at 1 site | 1702 (51.9) |
| Treatment facility type | Metastases > 1 site | 389 (11.9) | |
| Community cancer program | 470 (14.3) | ||
| Comprehensive community cancer program | 1625 (49.5) | Treatment characteristics | |
| Academic/research program | 832 (25.4) | Radiation dose, Gy | |
| Integrated network cancer program/Other | 341 (10.4) | Median (Gy) | 45 (30–75) |
| Treatment facility location | < 45 Gy | 1621 (49.4) | |
| Metro counties | 2482 (75.7) | > 45 Gy | 1724 (50.6) |
| Urban counties | 617 (18.8) | Timing of chest radiotherapy | |
| Rural counties | 110 (3.4) | < 12 weeks of starting CTX | 2077 (63.3) |
| Unknown | 71 (2.2) | > 12 weeks of starting CTX | 1203 (36.7) |
| Income, US dollars | |||
| < 38,000 | 707 (21.6) | Duration of radiotherapy | |
| 38,000–47,999 | 901 (27.5) | < 30 days | 1416 (43.2) |
| 48,000–62,999 | 944(28.8) | < 30–60 days | 1600 (48.8) |
| > 63,000 | 712 (21.7) | < 60–90 days | 264 (8.0) |
| Distance to treatment facility, miles | |||
| < 8 | 1429 (43.6) | ||
| > 8 | 1851 (56.4) | ||
| Year of Diagnosis | |||
| 2010–2011 | 1257 (38.3) | ||
| 2012–2013 | 1243 (37.9) | ||
| 2014 | 780 (23.8) | ||
LEGEND: No, number. CTX, chemotherapy.
Propensity score analysis was used to account for indication bias caused by lack of randomization [14,15]. Propensity scores were calculated by multivariable logistic regression to provide a score reflecting the conditional probability of receiving less than 45 Gy or at least 45 Gy. The propensity model included observable variables significantly associated with dose-group selection on multivariable logistic regression, including race, timing of TRT, clinical N and T stage, and the presence of brain, liver, and/or bone metastases. Using the inverse probability of treatment weight, we then created a pseudopopulation with a distribution of confounding variables in each dose-group arm that was identical to the entire cohort. Subsequently we constructed a Cox proportional hazards model adjusting for propensity score with inverse probability weighting [16]. To avoid overcorrection, only factors significant on univariable survival analysis not included in the propensity score were included in the propensity-adjusted model. To strengthen the assumption of balance between groups, the propensity-adjusted score was validated by stratification into propensity score-based quintiles, which demonstrated that standardized difference between the treatment groups was less than 0.10 [16].
3. Results
3.1. Patient characteristics
Baseline patient characteristics for the entire cohort are shown on Table 1. In summary, the median age was 64 years with a slight female predominance (52.5%). Most patients had T4 (41.5%), N2 (52.3%), and M1b (49.3%) malignancies. Brain, liver, and bone metastases were present in 17.7%, 22.4%, and 25.7% of patients, respectively. Patients with extrathoracic metastasis were limited to 1 site in 77.2% of cases (51.9% overall) and more than one site in 22.8% of cases (11.9% overall). The median time from diagnosis to any treatment (chemotherapy or TRT) was 14 days (0–90 days) and median time to chemotherapy was 17 days. The median time from the initiation of chemotherapy to the start of TRT was 41 days, and the timing of TRT was partitioned into two groups: during the first 12 weeks of chemotherapy (63%), or at/beyond 12 weeks of chemotherapy (37%). The 12-week mark was used as a surrogate for the 4th cycle of chemotherapy, the timing of which constitutes “consolidative” thoracic radiotherapy. The median dose for all patients was 45 Gy, with most patients receiving exactly 30 Gy (21%), 45 Gy (13%), or 60 Gy (9%).
The most common doses in the low-dose arm were 30 Gy and 36 Gy, with a median dose of 35 Gy, and the high dose arm was most commonly 45 Gy or 60 Gy with a median of 54 Gy. Table 2 conveys the differences in demographic and tumor characteristics between those who received less than 45 Gy and those treated with at least 45 Gy. African Americans, T4 lesions, the presence of brain/liver/bone metastases, multiple extrathoracic metastases, and patients undergoing TRT before chemotherapy (or with the 1st cycle) were all less likely to be treated to 45 Gy or higher.
Table 2.
Comparative Use of Dose Escalated Chest Radiation by Baseline Characteristic.
| Characteristics | < 45 Gy N = 1621 |
≥45 Gy N = 1659 |
OR | 95% CI | P value |
|---|---|---|---|---|---|
| - | - | - | |||
| Gender | |||||
| Male | 783 (50.3) | 775 (49.7) | 1 | Reference | - |
| Female | 838 (48.7) | 884 (51.3) | 1.06 | 0.93–1.22 | 0.36 |
| Age | |||||
| < 65 | 862 (53.2) | 881 (53.1) | 1 | Reference | |
| 65 | 759 (46.8) | 778 (46.9) | 1.0 | 0.87–1.15 | 0.97 |
| Race | |||||
| White | 1417 (89.8) | 1501 (92.4) | 1 | Reference | |
| Black | 161 (10.2) | 124 (7.6) | 0.73 | 0.57–0.93 | 0.01 |
| Insurance | |||||
| Uninsured | 88 (5.5) | 81 (4.9) | 1 | Reference | |
| Government | 992 (62.1) | 996 (60.7) | 1.09 | 0.80–1.49 | 0.59 |
| Private | 518 (32.4) | 564 (34.4) | 1.18 | 0.86–1.64 | 0.31 |
| Income | |||||
| < 48,000 | 819 (50.7) | 789 (47.8) | 1 | Reference | |
| ≥48,000 | 795 (49.3) | 861 (52.2) | 1.12 | 0.98–1.29 | 0.10 |
| Facility | |||||
| Community | 233 (14.4) | 237 (14.4) | 1 | Reference | |
| Comprehensive Community | 796 (49.2) | 829 (50.2) | 1.02 | 0.83–1.26 | 0.82 |
| Academic | 417 (25.8) | 415 (25.1) | 0.98 | 0.78–1.23 | 0.85 |
| Integrated network cancer program | 171 (10.6) | 170 (10.3) | 0.98 | 0.74–1.29 | 0.87 |
| Population | |||||
| Metro | 1233 (77.6) | 1249 (77.1) | 1 | Reference | |
| Urban | 304 (19.1) | 313 (19.3) | 1.02 | 0.85–1.21 | 0.86 |
| Rural | 51 (3.2) | 59 (3.6) | 1.14 | 0.78–1.68 | 0.50 |
| Distance to facility | |||||
| < 8miles | 723 (44.6) | 706 (42.6) | 1 | Reference | |
| ≥8 miles | 898 (55.4) | 953 (57.4) | 1.09 | 0.95–1.25 | 0.24 |
| Comorbid (Charlson-Deyo) | |||||
| 0 | 920 (56.8) | 979 (59) | 1 | Reference | |
| 1 | 488 (30.1) | 480 (28.9) | 0.92 | 0.79–1.08 | 0.32 |
| 2 or higher | 213 (13.1) | 200 (12.1) | 0.88 | 0.71–1.09 | 0.25 |
| Clinical T stage | |||||
| T1-T3 | 717 (50.1) | 835 (56.4) | 1 | Reference | |
| T4 | 715 (49.9) | 646 (43.6) | 0.78 | 0.67–0.90 | 0.001 |
| Clinical N stage | |||||
| N0/N1 | 228 (15.1) | 265 (16.7) | 1 | Reference | |
| N2 | 825 (54.7) | 892 (56.2) | 0.93 | 0.76–1.14 | 0.48 |
| N3 | 456 (30.2) | 429 (27) | 0.81 | 0.65–1.01 | 0.06 |
| Brain metastases | |||||
| No | 1291 (79.6) | 1409 (84.9) | 1 | Reference | |
| Yes | 330 (20.4) | 250 (15.1) | 0.69 | 0.58–0.83 | < 0.001 |
| Liver metastases | |||||
| No | 1134 (70.4) | 1398 (84.4) | 1 | Reference | |
| Yes | 477 (29.6) | 258 (15.6) | 0.44 | 0.37–0.52 | < 0.001 |
| Bone metastases | |||||
| No | 1094 (67.8) | 1334 (80.5) | 1 | Reference | |
| Yes | 519 (32.2) | 324 (19.5) | 0.51 | 0.44–0.60 | < 0.001 |
| Metastatic site | |||||
| Single site | 891 (76.9) | 811 (87) | 1 | Reference | |
| ≥1 site | 268 (23.1) | 121 (13) | 0.50 | 0.39–0.63 | < 0.001 |
| Timing of chest radiotherapy | |||||
| < 12 weeks of starting chemotherapy | 1020 (62.9) | 1057 (63.7) | 1 | Reference | |
| ≥12 weeks of starting chemotherapy | 601 (37.1) | 739 (36.3) | 0.84 | 0.73 - 0.97 | 0.02 |
| Years | |||||
| 2010–2011 | 607 (37.4) | 650 (39.2) | 1 | Reference | |
| 2012–2013 | 590 (36.4) | 653 (39.4) | 1.03 | (0.88–1.21) | 0.68 |
| 2014 | 424 (26.2) | 356 (21.5) | 0.78 | (0.66–0.94) | 0.008 |
LEGEND: OR, odds ratio. CI, confidence interval.
Bold indicates statistical significance.
3.2. Survival
At a median follow-up of 12 months, the Kaplan Meier survival for all patients was 51%, 20.3%, and 7.4% at 1, 2, and 5 years. High-dose TRT, female gender, age less than 65, non-government insurance, non-rural population, TRT after 12 weeks of chemotherapy (consolidative), N0-2 disease, and the absence of brain/liver/bone metastases were all significant predictors of longer survival on univariable analysis. With the exception of insurance and population, all these variables remained independent predictors upon multivariable analysis. Propensity score matched analysis revealed the high dose arm to remain a strong independent predictor of improved survival (HR = 0.74, 95% CI 0.69–0.80) (Table 3).
Table 3.
Multivariable Cox Proportional Hazards Models for Overall Survival.
| Characteristics | Without Propensity Score |
Propensity Score-Adjusted |
||
|---|---|---|---|---|
| Hazard of Death (95% CI) |
P Value | Hazard of Death (95% CI) |
P Value | |
| Thoracic radiotherapy dose | ||||
| < 45 Gy | Reference | Reference | ||
| ≥45 Gy | 0.78 (0.72–0.84) | < 0.001 | 0.74 (0.69–0.80) | < 0.001 |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.83 (0.77–0.90) | < 0.001 | 0.82 (0.76–0.88) | < 0.001 |
| Age | ||||
| < 65 | Reference | Reference | ||
| ≥65 | 1.28 (1.17–1.40) | < 0.001 | 1.24 (1.13–1.35) | < 0.001 |
| Comorbid (Charlson-Deyo) | ||||
| 0 | Reference | Reference | ||
| 1 | 1.19 (1.01–1.30) | < 0.001 | 1.20 (1.10–1.31) | < 0.001 |
| 2 or higher | 1.24 (1.10–1.39) | < 0.001 | 1.21 (1.08–1.37) | < 0.001 |
| Insurance | ||||
| Uninsured | Reference | Reference | ||
| Government | 1.10 (0.92–1.15) | 0.30 | 1.03 (0.86–1.25) | 0.74 |
| Private | 0.99 (0.82–1.19) | 0.88 | 0.93 (0.77–1.12) | 0.45 |
| Population | ||||
| Metro | Reference | Reference | ||
| Urban | 1.05 (0.95–1.15) | 0.37 | 1.06 (0.96–1.17) | 0.25 |
| Rural | 1.20 (0.98–1.46) | 0.08 | 1.23 (1.01–1.52) | 0.04 |
| Timing of chest radiotherapy | ||||
| Prior to/first cycle of CTX | Reference | |||
| < 12 weeks of starting CTX | 0.94 (0.85–1.04) | 0.23 | ||
| ≥12 weeks of starting CTX | 0.79 (0.71–0.87) | < 0.001 | ||
| Brain metastases | ||||
| No | Reference | |||
| Yes | 1.31 (1.14–1.52) | < 0.001 | ||
| Liver metastases | ||||
| No | Reference | |||
| Yes | 1.61 (1.38–1.87) | < 0.001 | ||
| Bone metastases | ||||
| No | Reference | |||
| Yes | 1.55 (1.34–1.80) | < 0.001 | ||
| Clinical N stage | ||||
| N0/N1 | Reference | |||
| N2 | 1.08 (0.96–1.21) | 0.21 | ||
| N3 | 1.22 (1.08–1.38) | 0.002 | ||
LEGEND: Gy, Gray. CI, confidence interval. CTX, chemotherapy.
Bold indicates statistical significance.
3.3. Dose-response
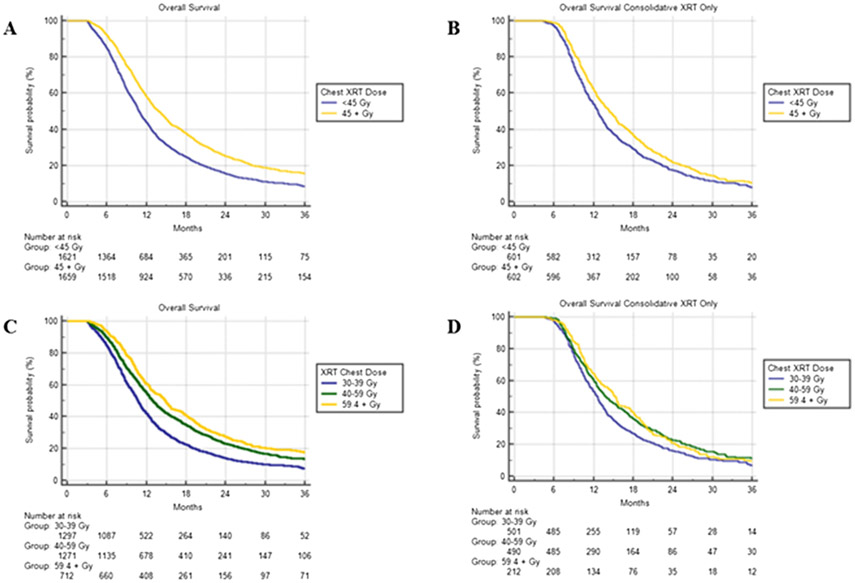
The 1 and 2-year survival for patients receiving at least 45 Gy was 58.1% and 25.2% compared to 43.8% and 15.1% among patients less than 45 Gy (HR = 0.70, 95% CI 0.65–0.76, P < 0.001). We further partitioned dose groups into low (30–39 Gy), intermediate (40–59 Gy), and high (59.4–75 Gy) dose categories, and KM median survivals were 10.7 (95% CI 10.3–11.1), 12.9 (95% CI 12.3–13.5), and 14.9 (95% CI 13.9–15.8) months, respectively (P < 0.001). We also performed a Kaplan Meier survival analysis among different dose groups for patients receiving TRT at least 12 weeks into chemotherapy (consolidative). The statistically significant discrepancies in survival persisted, albeit with a smaller margin. The Kaplan Meier curves are all depicted on Fig. 2.
Fig. 2.
Dose Response in TRT for ES-SCLC.
A-Survival all thoracic radiotherapy patients < 45 Gy vs. ≥45 Gy.
B-Survival thoracic radiotherapy patients after 12 weeks of chemotherapy < 45 Gy vs. ≥45 Gy.
C-Survival all thoracic radiotherapy patients low vs. intermediate vs. high dose.
D-Survival thoracic radiotherapy patients after 12 weeks of chemotherapy low vs. intermediate vs. high dose.
4. Discussion
Consolidative thoracic radiotherapy was first shown to confer a survival benefit by Jeremic et al. in 1999. The randomized trial reported a 10% 2-year overall survival benefit with the addition of TRT in extensive stage patients, thus introducing the concept of consolidative TRT in ES-SCLC. However, many clinicians failed to adopt this regimen which included hyperfractionated, twice daily radiotherapy with daily concurrent chemotherapy [3]. Decades later the CREST trial produced similar results with modern radiotherapy techniques and logistically favorable delivery of chemotherapy and radiation [6]. Despite this evidence, most metastatic SCLC is treated with chemotherapy alone. Within the NCDB dataset, only 13.5% of stage IV SCLC patients received TRT between the years 2010–2014. Interestingly, there was a considerable increase in the number of extensive stage patients with TRT in 2014 (n = 780) compared to years 2010–2013 (average n = 625), perhaps related to penetration of the CREST trial results reported in 2014 [6]. Accordingly, two national surveys published since the CREST trial report that the use of consolidative TRT in ES-SCLC is growing. However, the same surveys describe great variability in patient selection and radiotherapy doses [17,18]. While some argue to remain at 30 Gy to limit potential toxicity, others cite a high local failure rate as substantial evidence to investigate appropriate candidates for dose escalation [18-20]. Below we interpret the results of our NCDB analysis to examine which stage IV SCLC patients were selected for, and potentially benefited from, dose escalation in TRT.
4.1. Patient selection
With the exception of race, socioeconomic factors did not play a significant role in treatment selection between low dose and high dose arms. Unsurprisingly, patients with greater local and distant disease burden were more likely to receive a lower dose of TRT, likely due to larger radiotherapy treatment volumes and risk of toxicity at high doses. In our analysis, patients with extrathoracic metastases to more than one site were half as likely to receive the higher dose compared to those with 1 metastatic site. Multiple studies have proposed a role for more aggressive radiotherapy in patients with oligometastatic disease, including a secondary analysis of the CREST trial that reported a significantly improved survival for patients with 2 or fewer extrathoracic metastases [20,21]. The authors concluded that such patients should be the focus of dose escalated TRT in future trials [20].
TRT delivered before or with the first day of chemotherapy was also associated with a lower dose compared to those starting radiation weeks after starting chemotherapy. Presumably, patients who were treated with at least 12 weeks (4 cycles) of chemotherapy had comparatively less residual disease than patients who were treated immediately and therefore dose escalation may have been more feasible. However, it should be noted that 51% of patients undergoing TRT prior to 12 weeks of chemotherapy were in the high dose arm. Current guidelines and clinical trials recommend TRT only in the consolidative setting, although the NCDB data reflects that the majority of metastatic SCLC patients actually received TRT prior to the 12th week of chemotherapy. Some have investigated if the benefit of TRT may be greater if implemented earlier, as has been proven in early stage disease, however the authors concluded that timing had no association with survival [22,23]. In our analysis, dose escalation correlated with longer survival for TRT delivered prior to, concurrently with, or 12 weeks after, the start of chemotherapy.
4.2. Survival
Controlling for disease burden and treatment timing on multivariable propensity-adjusted models, dose escalated TRT was independently associated with longer survival. Slotman et al. proposed that patients with limited metastatic disease are the ideal candidates for aggressive radiotherapy, consistent with our analysis demonstrating a survival benefit (HR = 0.72, P < 0.001) for extensive stage patients limited to 1 extrathoracic metastatic site when treated to at least 45 Gy, and no benefit for multiple extrathoracic site of disease (P = 0.56). Males, older patients, and those presenting with brain/liver/bone metastases were all independent predictors of reduced survival on multivariable analysis, with liver and bone metastasis as the two strongest correlates, consistent with the literature [10,20,21,24,25]. We included brain metastases in this analysis to investigate the practice patterns of TRT in all forms of extensive stage disease and found that a considerable proportion of patients (17.7%) with TRT also had brain metastases, 43% of whom were in the higher dose group and correlated with longer survival (HR = 0.71, P < 0.001).
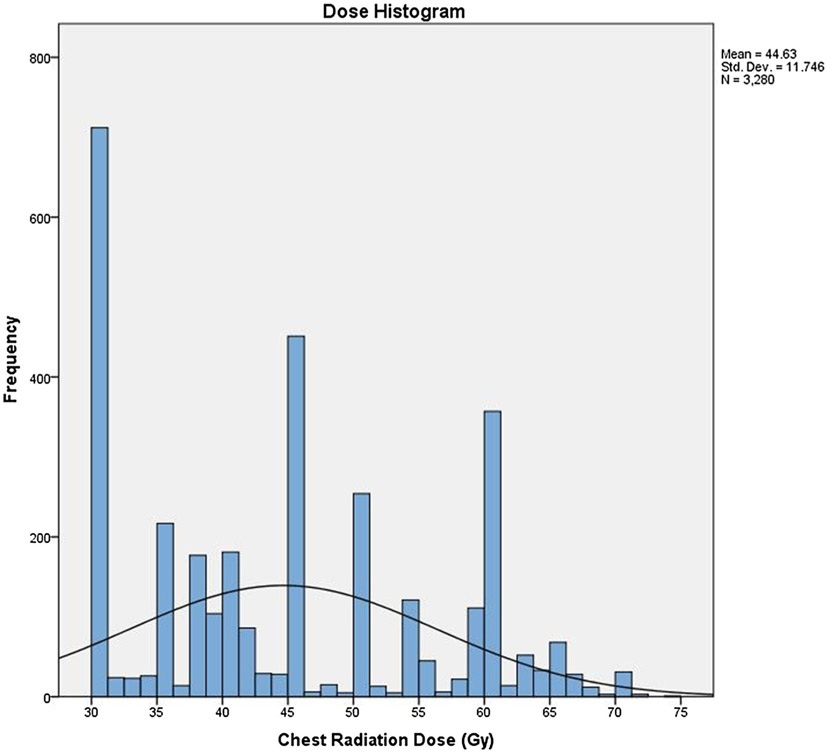
The TRT doses in stage IV SCLC varied in this dataset, with frequency peaks at 30 Gy, followed by 45 Gy, and 60 Gy (Fig. 3). One retrospective study indicated that a TRT biologic equivalent dose greater than 50 Gy10 (approximately equivalent to 45 Gy in 25 fractions) in ES SCLC correlated with improved control and survival [26]. As a continuous variable in Cox regression analysis, dose remained a strong predictor of survival (HR = 0.98, P < 0.001). It is possible that dose escalation beyond 45 Gy may be associated with even higher survival rates in this patient population, as suggested by increasing survival between the “30-39 Gy”, “40-59 Gy”, and “59.4 Gy and higher” groups. Notably, the intermediate and high dose survival curves overlap for consolidative TRT patients, suggesting that the survival difference may be less pronounced beyond a certain dose.
Fig. 3.
Histogram of thoracic radiation doses employed in the entire cohort with dose in Gray on the X axis and number of cases in the Y axis.
4.3. Limitations
The data presented here is powered by large numbers and supported by strong statistical analysis, but nevertheless is subject to several limitations including selection bias. Additionally, initial treatment response, salvage therapies, and disease recurrence are not included in the NCDB, all of which may affect the interpretation of results. For instance, while no patient was treated with whole brain radiation as part of their initial treatment plan, it is unknown if any received prophylactic or therapeutic brain radiation following initial treatment, both of which have proven to improve survival in randomized trials [2,27]. Also, while the interim between start of chemotherapy and start of radiotherapy was used as a surrogate for cycles of chemotherapy administered prior to thoracic TRT, it is unknown if all cycles were completed–just that they were initiated.
Efforts were made to account for confounding variables and selection bias with multivariable analysis, and the propensity score-matched model was further validated with balance diagnostics. A recent criticism of the propensity score analyses with large data was published in the Journal of Clinical Oncology, concluding that most of these studies lack statistical transparency and depth [28]. We therefore detailed our comprehensive statistical approach in the methods section, with appropriate justification cited in the literature. Nevertheless, propensity matching cannot substitute for the randomization conducted in phase III trials, which incorporates the unobservable variables that are unaccounted for with propensity matching.
5. Conclusion
In the largest analysis of TRT in ES-SCLC to date, dose escalation to 45 Gy or higher was an independent predictor of longer survival in this NCDB-based study. Other independent predictors of survival and thereby potentially appropriate candidates for consolidative TRT dose escalation in future clinical trials, include metastases limited to one site, adenopathy confined to the mediastinum, and limited comorbidity. These results warrant further investigation in prospective trials.
Acknowledgments
Obtained funding
None.
Footnotes
Prior presentation
None.
Financial disclosure for all authors
None.
Relationships relevant to this manuscript
None.
All other relationships
None.
References
- [1].PDQ Adult Treatment Editorial Board, Small Cell Lung Cancer Treatment (PDQ®): Health Professional Version, (2002) Accessed April 21, 2018 http://www.ncbi.nlm.nih.gov/pubmed/26389347. [Google Scholar]
- [2].Slotman B, Faivre-Finn C, Kramer G, et al. , Prophylactic cranial irradiation in extensive small-cell lung cancer, N. Engl. J. Med 357 (7) (2007) 664–672, 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- [3].Jeremic B, Shibamoto Y, Nikolic N, et al. , Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study, J. Clin. Oncol 17 (7) (1999) 2092–2099, 10.1200/JCO.1999.17.7.2092. [DOI] [PubMed] [Google Scholar]
- [4].Zhu H, Zhou Z, Wang Y, et al. , Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis, Cancer 117 (23) (2011) 5423–5431, 10.1002/cncr.26206. [DOI] [PubMed] [Google Scholar]
- [5].Luan Z, Wang Z, Huang W, et al. , Efficacy of 3D conformal thoracic radiotherapy for extensive-stage small-cell lung cancer: a retrospective study, Exp. Ther. Med 10 (2) (2015) 671–678, 10.3892/etm.2015.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Slotman BJ, Van Tinteren H, Praag JO, et al. , Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial, Lancet 385 (9962) (2015) 36–42, 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- [7].De Sanctis V, Agolli L, Visco V, et al. , Cytokines, fatigue, and cutaneous erythema in early stage breast cancer patients receiving adjuvant radiation therapy, Biomed Res. Int 2014 (2014), 10.1155/2014/523568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Giuliani ME, Atallah S, Sun A, et al. , Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic radiotherapy, Clin. Lung Cancer 12 (6) (2011) 375–379, 10.1016/j.cllc.2011.03.028. [DOI] [PubMed] [Google Scholar]
- [9].Yee D, Butts C, Reiman A, et al. , Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer, Radiother. Oncol 102 (2) (2012) 234–238, 10.1016/j.radonc.2011.08.042. [DOI] [PubMed] [Google Scholar]
- [10].Palma DA, Warner A, Louie AV, Senan S, Slotman B, Rodrigues GB, Thoracic radiotherapy for extensive stage small-cell lung Cancer: a meta-analysis, Clin. Lung Cancer 17 (4) (2016) 239–244, 10.1016/j.cllc.2015.09.007. [DOI] [PubMed] [Google Scholar]
- [11].Kalemkerian GP, Loo BW, Chair V, et al. NCCN Guidelines Version 2.2018 Panel Members Small Cell Lung Cancer Charles Florsheim Patient Advocate, Accessed April 21, 2018 https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. [Google Scholar]
- [12].Gore EM, Hu C, Sun AY, et al. , Randomized phase II study comparing prophylactic cranial irradiation alone to prophylactic cranial irradiation and consolidative extracranial irradiation for extensive-disease small cell lung cancer (ED SCLC): NRG oncology RTOG 0937, J. Thorac. Oncol 12 (10) (2017) 1561–1570, 10.1016/j.jtho.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gore EM, Hu C, Sun A, et al. , NRG oncology/RTOG 0937: randomized phase 2 study comparing prophylactic cranial irradiation (PCI) alone to PCI and consolidative extracranial irradiation for extensive disease small cell lung cancer (ED-SCLC), Int. J. Radiat. Oncol 94 (1) (2016) 5, 10.1016/j.ijrobp.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].D’Agostino RB, Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group, Stat. Med 17 (19) (1998) 2265–2281 doi:. [DOI] [PubMed] [Google Scholar]
- [15].Cohen J, Statistical power analysis for the behavioral sciences, Stat. Power Anal. Behav. Sci. 2nd (1988) 567, 10.1234/12345678. [DOI] [Google Scholar]
- [16].Austin PC, An introduction to propensity score methods for reducing the effects of confounding in observational studies, Multivariate Behav. Res 46 (3) (2011) 399–424, 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Post CM, Verma V, Mitin T, Simone CB 2nd, Practice patterns of thoracic radiotherapy for extensive-stage small-cell lung cancer: survey of US academic thoracic radiation oncologists, Clin. Lung Cancer (2016), http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medp&NEWS=N&AN=28087132. [DOI] [PubMed] [Google Scholar]
- [18].Mitin T, Jain A, Degnin C, Chen Y, Henderson M, Thomas CR, Current patterns of care for patients with extensive stage small cell lung cancer: survey of US radiation oncologists on their recommendations regarding thoracic consolidation radiotherapy, Lung Cancer 100 (2016) 85–89, 10.1016/j.lungcan.2016.08.005. [DOI] [PubMed] [Google Scholar]
- [19].Slotman BJ, van Tinteren H, Praag JO, et al. , Radiotherapy for extensive stage small-cell lung cancer - authors’ reply, Lancet (Lond., Engl.) 385 (9975) (2015) 1292–1293, 10.1016/S0140-6736(15)60679-1. [DOI] [PubMed] [Google Scholar]
- [20].Slotman BJ, Faivre-Finn C, van Tinteren H, et al. , Which patients with ES-SCLC are most likely to benefit from more aggressive radiotherapy: a secondary analysis of the Phase III CREST trial, Lung Cancer 108 (2017) 150–153, 10.1016/j.lungcan.2017.03.007. [DOI] [PubMed] [Google Scholar]
- [21].Xu L-M, Cheng C, Kang M, et al. , Thoracic radiotherapy (TRT) improved survival in both oligo-and polymetastatic extensive stage small cell lung cancer, Sci. Rep 7 (2017), 10.1038/s41598-017-09775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Luo J, Xu L, Zhao L, et al. , Timing of thoracic radiotherapy in the treatment of extensive-stage small-cell lung cancer: Important or not? Radiat. Oncol 12 (1) (2017), 10.1186/s13014-017-0779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. , Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer, J. Clin. Oncol 24 (7) (2006) 1057–1063, 10.1200/JCO.2005.02.9793. [DOI] [PubMed] [Google Scholar]
- [24].Pignon JP, Arriagada R, Ihde DC, et al. , A meta-analysis of thoracic radiotherapy for small-cell lung cancer, N. Engl. J. Med 327 (23) (1992) 1618–1624, 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- [25].Zhang X, Yu J, Zhu H, et al. , Consolidative thoracic radiotherapy for extensive stage small cell lung cancer, Oncotarget 8 (13) (2017) 22251–22261, 10.18632/oncotarget.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li-Ming X, jun Zhao L, Simone CB, et al. , Receipt of thoracic radiation therapy and radiotherapy dose are correlated with outcomes in a retrospective study of three hundred and six patients with extensive stage small-cell lung cancer, Radiother. Oncol 125 (2) (2017) 331–337, 10.1016/j.radonc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- [27].Lekic M, Kovac V, Triller N, Knez L, Sadikov A, Cufer T, Outcome of small cell lung cancer (SCLC) patients with brain metastases in a routine clinical setting, Radiol. Oncol 46 (1) (2012) 54–59, 10.2478/v10019-012-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang H, Li J-D, Zhang T-Y, Wu M-C, Yang T, Zheng Y-J, Propensity score analysis: more details, more credibility, J. Clin. Oncol 35 (28) (2017) 3265–3266, 10.1200/JCO.2017.74.3641. [DOI] [PubMed] [Google Scholar]