ABSTRACT
Background
Atrial Fibrillation (AFib) is the most common cardiac arrhythmia, occurring in ≈1% of the general population. An increased risk of malignancy among patients with AFib would be of substantial public health importance, given the high prevalence and associated economic burden of both disorders.
Objectives
To evaluate the relationship between atrial fibrillation (AFib) and cancer.
Methods
We conducted an extensive database search on PubMed, Google Scholar, ScienceDirect, and SEER Database from their inception to September 2019 for any study that evaluated the association between AFib and cancer.
Results
In the first 3 months of AFib diagnosis, Ostenfeld et al. reported an absolute cancer risk of 2.5% with a standardized incidence ratio of 7.02 and 3.53 for metastatic and localized cancer, respectively. Likewise, Saliba et al. detected an increase in the odds of cancer diagnosis in first 90 days after AF diagnosis with OR of 1.85. Moreover, in another study new-onset breast and colorectal cancer was especially associated with AF in the first 90 days after diagnosis with HR of 3.4 but not thereafter (HR 1.0). Similarly, Conen et al. reported high relative risk of cancer with HR of 3.54 in the first 3 months after new-onset AFib. However, beyond the initial 90 day period, the risk of cancer in AFib is only slightly increased.
Conclusion
Based on our review, there appears to be an increase in risk of subsequent diagnosis of cancer in patients with AF, likely owing to the shared risk factors between the two conditions. While the results of this study raise interesting questions for future search, they are not currently strong enough to justify initiating cancer screening for an occult cancer in a patient with AF. Regardless, measures to target modification of these shared risk factors remains an important consideration.
KEYWORDS: Atrial fibrillation, cancer(s), neoplasm(s), malignancy(ies)
1. Introduction
Atrial Fibrillation (AFib) is the most common cardiac arrhythmia, occurring in ≈1% of the general population [1]. AFib is associated with fourfold to fivefold increased risk of stroke, a twofold to threefold increased risk of congestive heart failure, a twofold increased risk of thromboembolism, impaired quality of life, and leads to increased mortality [2,3]. According to the Framingham study, even in the absence of preexisting cardiovascular disease, AFib doubles the mortality risk in both men (multivariate OR, 2.4 [95% CI, 1.8 to 3.3]) and women (multivariate OR, 2.2 [95% CI, 1.6 to 3.1], suggesting a close association between noncardiovascular diseases and AFib [4]. Among such disorders, malignancies are accountable for a substantial proportion (12%) of these deaths [5,6]. Cross-sectional studies showed that AFib was more prevalent in patients with cancer than those without cancer (3.6% in patients with cancers vs. 1.6% controls who had no diagnosis of malignancy) (odds ratio 1.19, 95% CI 1.02 to 1.38 [7,8]. A recent study showed that overall prevalence of AFib in cancer patients is approximately 14.1%. Although a formal association cannot be determined from cross-sectional studies, it has been suggested that cancer could be a risk factor for AFib. Alternatively, studies also suggest that AFib could promote the development of cancer; as both Women’s Health Study (WHS) and Danish National Registry study detected a significant increased risk of cancer after AFib diagnosis [9,10]. However, recent studies by Saliba et al., and Wassertheil-Smoller et al., paradoxically did not support the notion of AFib being a risk marker for cancer [11,12]. An increased risk of malignancy among patients with AFib would be of substantial public health importance, given the high prevalence and associated economic burden of both disorders. Therefore, we conducted this in-depth literature review to further evaluate the relationship between AFIB and cancer. A total of nine cohort studies were included in our review.
2. Materials and method
2.1. Search strategy and data sources
We conducted an extensive database search on PubMed, Google Scholar, ScienceDirect, and SEER Database from their inception to September 2019 for any study that evaluated the association between AFib and cancer. To identify any additional studies, the bibliographies of relevant articles were also searched. The search used the following keywords: Atrial fibrillation, cancer(s), neoplasm(s), and malignancy(ies).
2.2. Study selection
Studies were considered eligible if they met all of the following criteria: an original study comparing AFib in adults >18 years of age with an inactive control (general population or age-matched controls), incidence reported for cancers of any type, follow-up over 1 year, provided hazard ratios or relative risks and the corresponding 95% CIs. Extra studies were identified by a hand search of all the references of the retrieved articles Studies were assessed as having low, high, or unclear level of bias using the Cochrane Risk of Bias assessment tool.
2.3. Data extraction and quality assessment
Two reviewers (NL, VK) independently identified all the retrieved studies based on the aforementioned selection criteria. We also performed a cross-reference search of eligible articles to identify studies, which we did not find in the computerized search. The following information were extracted from the studies: study design, the name of the first author; publication year; regions of study; study period; sample size; case size; duration of follow-up; gender; mean age or age range; comorbidities; and pharmacological treatment. Any disagreement was resolved through discussion and consensus.
3. Results
3.1. Studies and patient population
A total of nine cohort studies (N = 670, 733) were included [8–11]. Summary of this selection process is given in PRISMA flow chart (Figure 1). PICO questions for our review are mentioned as follows: Population: Adults >18 with AFib; Intervention: Investigations like CXR, CBC/CMP, ECG, or tumor-specific labs for cancer diagnosis; Comparison: General population or age matched controls without AFib diagnosis; Outcomes: The outcome of interest was incidence of any type of cancers in each study with hazard ratios or relative risks and the corresponding 95% CI’s to prove association of AFib and cancer.
Figure 1.
PRISMA flowsheet showing literature search and study selection details.
3.2. Studies investigating AFib (exposure) and cancer (outcome) association
Saliba et al. conducted two large prospected population-based case-control studies (the molecular Epidemiology of Colorectal Cancer (MECC) in March 1998, and the Breast Cancer in Northern Israel Study (BCINIS) in January 2000) to assess the association between cancer and AFib. They assessed an association between exposure (AFib) and outcome (cancer) using a case-control approach. Cases included 9264 incident cancer patients while 10,727 individuals who were cancer free at the time of recruitment were controls. A previous history of AFib was identified in 3.8% (N = 352) cases and 5.0% (N = 538) controls. The immediate period (90 days) after an AF event was associated with a 1.85 times increased risk of cancer. Cancer odds was 2.11 in the first 3 months of AF diagnosis, and 0.78 with AF of longer than 3 months.
Ostenfeld et al. conducted a population-based cohort study from 1980 through 2011 to determine the risk of localized or metastatic cancer following diagnosis of AFib (N = 269,742). Median follow-up was 3.4 years. They reported an absolute risk of 2.5% for cancer in AFib patients (N = 665) within 3 months of follow-up. A total of 2948 cases of metastatic cancers were diagnosed with standardized incidence ratio (SIR) of 7.02, while 2129 cases of nonmetastatic cancers were diagnosed with SIR of 3.53.
Wassertheil-Smoller et al. conducted a large trial (N = 93,676 postmenopausal women) named Women’s Health Initiative (WHI) Observational study in the United States from 1994 through 1998 to determine an association between AFib and development of invasive breast or colorectal cancer. 4376/86,046, patients had baseline diagnosis of AFib (5.1%). Median time of follow-up was 15.3 years and 15.9 years for breast and colorectal cancer, respectively. They found that AFib was associated with a 19% higher risk of incident breast cancer; however, they noticed that this association may be related to baseline use of cardiac glycosides (digoxin). Baseline use of cardiac glycosides showed 68% higher risk of invasive breast cancer with HR of 1.68. However, use of cardiac glycoside was not associated with risk of colorectal cancer (HR = 1.08).
Conen et al. conducted a large prospective cohort trial (N = 3461 women) from 1993 through 2014 to study the relationship between AFib and cancer. Follow-up was conducted for a median of 19.1 years. New onset AFib and malignant cancer was confirmed among 4.2% (N = 1467) and 14.8% (N = 5130) individuals, respectively. Risk of cancer was noted to be highest within the first 3 months of AFib with an incidence of 3.8 per 100 person-years (HR 3.54; p < 0.001), but it remained significantly elevated beyond 1 year as well (incidence 1.3 per 100 person-years; HR 1.42, p < 0.001).
Vinter et al. conducted a population-based cohort of 270,000 patients with new-onset AF. Median follow-up time was 16–19 years. They found higher incidence of cancer within first 90 days after diagnosis of AFib with the standardized rate incidence ratio of cancer diagnosis to be 5.11 [13].
3.3. Studies investigating cancer (exposure) and AFib (outcome) association
Kattelus et al. conducted a long-term prospective study (OPERA study) (N = 1045) with a mean follow-up time of 16.3 years. They demonstrated that cancer patients had greater probability of developing AF for a HR of 2.47 [14].
Guzzetti et al. observed in a case-control study (cases/controls 456/791) that patients who were admitted for surgical management of colorectal neoplasm from 1987 to 1998 were more than twice as likely to have AFib than patients admitted for non-neoplastic surgery, with an adjusted odds ratio (OR) of 3.5 [15].
Likewise, another case-control study was performed by Guzzetti et al. in 2008 (N = 2339). In this study, they compared presurgery electrocardiograms of patients with cancer (colorectal/ breast cancer -> cases) to matched patients admitted for non-neoplastic surgery. They observed that AFib was present in 3.6% of the cases and 1.6% in controls with OR of 3.3 [7].
In 2012, Erichsen et al. conducted a population-based case control study including 28,333 patients. They observed after 90 days of colorectal cancer diagnosis patients were more likely to develop AFib than controls with OR of 12. This higher risk was only found in initial 90-day period [16].
Saliba et al. in aforementioned study also assessed the association between cancer as exposure and AFib as outcome using a prospective cohort approach analysis. In the first 90 days, the risk of AFib was 3.05, while it was 1.01 beyond first 90 days.
4. Discussion
In this review, we investigated the association between cancer and atrial fibrillation. Aforementioned results showed a markedly increased incidence of cancer diagnosis within the first 3 months following a diagnosis of atrial fibrillation. However, beyond 3 months the risk was only slightly increased.
The potential mechanisms underlying the association between AFib and increased long-term cancer risk remain unclear. Shared risk factors (e.g., advanced age, obesity, diabetes, and smoking) for both cancer and AFib possibly explain this association [8]. Also, antiarrhythmic medications are guideline recommended first-line management of AFib. Recent studies have shown increased cancer risk with antiarrhythmic medication use particularly Amiodarone. The study by Su et al. found that amiodarone use was associated with increased risk of incident cancer in male patients with (SIR, 1.18; 95% CI, 1.02 to 1.36 [p = 0.022]). Similarly, in the study by Lim et al. Amiodarone was associated with an increased risk of malignant neoplasm of liver and intrahepatic bile ducts with an odds ratio of 1.60 (1.45–1.77). The latter study also found an association of increased risk of cancer with other antiarrhythmic medications like Quinidine and Propafenone; however, the adjusted ratios were not significant for these two drugs [17,24]. Few case reports have described hepatotoxicity arising from the use of Propafenone. Steatohepatitis, hepatocellular injury, and fibrosis leading to cirrhosis and HCC is proposed mechanism of action [19]. Furthermore, as suggested by the prominence of colon cancer, increased detection can be due to gastrointestinal bleeding with anticoagulation use (Figure 2).
Figure 2.
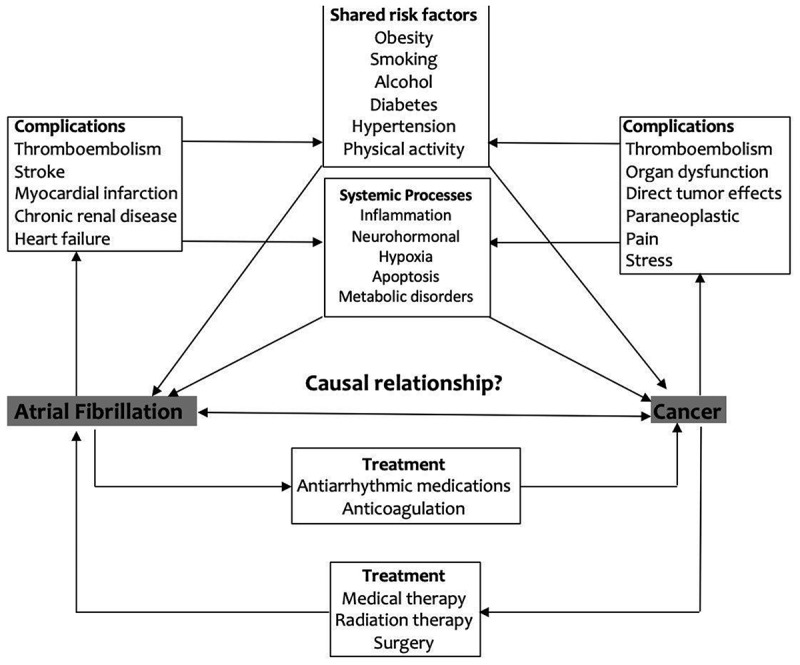
In our analysis, the most significant association between AFib and incident cancer was in the first 3 months of AFib diagnosis, hence it can be speculated that AFib may be an early sign of occult cancer. Further, in the Danish study, 57% of the reported cancers had metastasis, suggesting that the cancers were present and undetected at the time when AFib was diagnosed. This finding is in congruence to the study by Guzzetti et al., who reported a more than two-fold higher prevalence of AFib among cases with either colorectal or breast cancer as compared to cancer-naïve controls [7]. Multiple other studies have echoed this association indicating concomitant prevalence of AFib and cancer [15,16,18]. Cancer may increase risk of AFib via complex/systemic inflammatory responses leading to CRP elevation, hypercoagulability leading to pulmonary micro-embolism or autoantibody production by paraneoplastic syndromes.
In contrast with the relationship between AFib and heart failure, myocardial infarction, and venous thromboembolism, which are more likely to be truly causally bidirectional, the association between AFib and cancer is not as distinct. Women Health Study [12] suggested that AFib may be an initial manifestation of a systemic process which increases the risk of both the diseases and proposed a bidirectional association between the two entities with possible interlinking by shared risk factors (obesity, smoking, and alcohol) and shared systemic factors (inflammation, oxidative stress, and apoptosis) [26,27,28].
Our analysis did not show a significant association between AFib and breast cancer. The study by Wassertheil-Smoller et al. noted that the association of risk of breast cancer with AFib in postmenopausal women was related to the use of cardiac glycosides in these patients [12]. Similarly, a recent meta-analysis showed an overall 34% increase in breast cancer risk with use of cardiac glycosides, further validating this association [25].
The elevated medical surveillance among patients with new-onset AFib may result in detection bias as a potential cause of increased diagnosis of cancer in the immediate period as investigations like imaging studies (e.g., Chest X-ray, cerebral scan, CT, or MRI) are more prevalent in patients with AFib [20]. In addition, some patients with AF can be symptomatic and repeatedly seek care for managing these symptoms leading to an increased chance diagnosis of cancer [21] or possibly due to excessive radiations that AFib patients may already be undergoing which could lead to a potential cancer diagnosis. This aspect is not well reported in literature and thus can be explored in the future research. WHS in 2016 reported elevated relative risk for malignant cancer beyond 1 year of AFib diagnosis after adjustment for screening. In Danish study, AFib showed strong association with cancers that are sensitive to detection bias like nonmelanoma skin cancers [22] and advanced malignancies. Similarly, a case-control study by Muller et al. consisting of 12,304 veterans in the United States showed a positive association of AFib and atrial flutter with increased occurrence of colon cancer after 5–10 years after initial diagnosis [23], indicating AFib may act as a potential risk marker for cancer.
This analysis has patient management implications for clinicians and warrants future research to further delineate the relationship between these two entities. AFib is associated with increased mortality, concomitant cancer diagnosis further increasing mortality risk underscoring the need for cancer surveillance in such patients [29]. There are several shared risk factors between AFib and cancer, keeping this in mind some preventive strategies might be beneficial for both disorders, e.g., smoking cessation. Alternatively, AFib and cancer also share opposite risk factors, such as Atrial natriuretic peptide (ANP) levels are increased in AFib, and in some recent studies ANP has shown anti-proliferative activity against various forms of human cancers [30–32]. Identification of such factors in future studies may have relevant public health implications for cancer prevention. Finally, the interrelations between AFib and cancer are complex, which demands further investigations.
Some important limitations to our review should be considered. More than 80% of the subjects in the included studies were female. Because of the paucity of the data, it is difficult to comment on the subgroup analyses to evaluate the association of AFib with cancers other than that of breast and colon. No adjustments for sex, comorbidities, or medication use were made which remains a concern. The numbers of included studies was small.
5. Summary
In conclusion, available data suggests that patients with AFib have an increased probability of a cancer diagnosis within 3 months after being diagnosed with AFib. After the initial 90-day period, the risk is only slightly increased. Screening for cancer at the time of AFib diagnosis might require extensive work-up, and it remains unclear whether earlier diagnosis will affect the prognosis. Regardless, optimal risk factor control in AFib patients seems justifiable.
Author Contributions
Concept and design: NL. Data analysis and interpretation: NL, VK, MJA, AL, UA, and MM. Drafting article: All authors. Critical revision of article: MM, FA, and MH. Approval of article: All authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. [DOI] [PubMed] [Google Scholar]
- [2].Friberg L, Bergfeldt L.. Atrial fibrillation prevalence revisited. J Intern Med. 2013. November;274(5):461–468. . Epub 2013 Aug 7. [DOI] [PubMed] [Google Scholar]
- [3].Ferreira C, Providência R, Ferreira MJ, et al. Atrial fibrillation and non-cardiovascular diseases: a systematic review. Arq Bras Cardiol. 2015. November;105(5):519–526. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation. 1998;98(10):946–952. [DOI] [PubMed] [Google Scholar]
- [5].Marijon E, Le Heuzey JY, Connolly S, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013. November 12;128(20):2192–2201. [DOI] [PubMed] [Google Scholar]
- [6].Fauchier L, Villejoubert O, Clementy N, et al. Causes of death and influencing factors in patients with atrial fibrillation. Am J Med. 2016. December;129(12):1278–1287. . Epub 2016 Jul 28. [DOI] [PubMed] [Google Scholar]
- [7].Guzzetti S, Costantino G, Vernocchi A, et al. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern Emerg Med. 2008;3:227–231. [DOI] [PubMed] [Google Scholar]
- [8].O’Neal WT, Lakoski SG, Qureshi W, et al. Relation between cancer and atrial fibrillation (from the Reasons for geographic and racial differences in stroke study). Am J Cardiol. 2015;115(8):1090–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Conen D, Wong JA, Sandhu RK, et al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016;1(4):389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ostenfeld EB, Erichsen R, Pedersen L, et al. Atrial fibrillation as a marker of occult cancer. PLoS One. 2014;9(8):e102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saliba W, Rennert HS, Gronich N, et al. Association of atrial fibrillation and cancer: analysis from two large population-based case-control studies. PLoS One. 2018. January 11;13(1):e0190324. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wassertheil-Smoller S, McGinn AP, Martin L, et al. The associations of atrial fibrillation with the risks of incident invasive breast and colorectal cancer. Am J Epidemiol. 2017. March 1;185(5):372–384. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vinter N, Christesen AMS, Fenger-Grøn M, et al. Atrial fibrillation and risk of cancer: a Danish population-based cohort study. J Am Heart Assoc. 2018;7(17):e009543–e009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kattelus H, Kesaniemi YA, Huikuri H, et al. Cancer increases the risk of atrial fibrillation during long-term follow-up (OPERA study). PLoS One. 2018;13(10):e0205454. Article e0205454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guzzetti S, Costantino G, Sada S, et al. Colorectal cancer and atrial fibrillation: a case-control study. Am J Med. 2002;112(7):587–588. [DOI] [PubMed] [Google Scholar]
- [16].Erichsen, R, Christiansen, CF, Mehnert, F, Weiss, NS, Baron, JA, and Sørensen, HT. 2012. Colorectal cancer and risk of atrial fibrillation and flutter: a population-based case–control study. Internal and emergency medicine, 7(5):431–438. [DOI] [PubMed] [Google Scholar]
- [17].Su VY, Hu YW, Chou KT, Ou SM, Lee YC, Lin EY, Chen TJ, Tzeng CH, Liu CJ. Amiodarone and the risk of cancer: a nationwide population-based study. Cancer. 2013. May 1;119(9):1699–1705. doi: 10.1002/cncr.27881. Epub 2013 Apr 8. [DOI] [PubMed] [Google Scholar]
- [18].Guzzetti S, Costantino G, Sada S, et al. Atrial fibrillation as a complication of colorectal tumors. Recenti Prog Med. 2003;94:260–263. [PubMed] [Google Scholar]
- [19].Younan LB, Barada KA, Faraj WG, Tawil AN, Jabbour MN, et al. Propafenone hepatotoxicity: report of a new case and review of the literature. Saudi J Gastroenterol. 2013;19:235–237. doi: 10.4103/1319-3767.118137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dillon P, Ghanbari H.. Diagnostic evaluation and follow-up of patients with atrial fibrillation. Cardiol Clin. 2014. November;32(4):507–519. doi: 10.1016/j.ccl.2014.07.008. Epub 2014 Aug 27. [DOI] [PubMed] [Google Scholar]
- [21].Clua-Espuny JL, Lechuga-Duran I, Bosch-Princep R, Roso-Llorach A, Panisello-TAfiballa A, Lucas-Noll J, López-Pablo C, Queralt-Tomas L, Giménez-Garcia E, González-Rojas N, Gallofré López M. Prevalence of undiagnosed atrial fibrillation and of that not being treated with anticoagulant drugs: the AFIBABE study. Rev Esp Cardiol (Engl Ed). 2013. July;66(7):545–552. doi: 10.1016/j.rec.2013.03.003. Epub 2013 May 31. [DOI] [PubMed] [Google Scholar]
- [22].Helfand M, Mahon S, Eden K. Screening for Skin Cancer [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2001. April. Report No.: 01-S002. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. [Google Scholar]
- [23].Müller AD, Sonnenberg A, Wasserman IH. Diseases preceding colon cancer. A case-control study among veterans. Dig Dis Sci. 1994;39(11):2480–2484. [DOI] [PubMed] [Google Scholar]
- [24].Lim YP, Lin CL, Lin YN, Ma WC, Chen WC, Hung DZ, Kao CH. Antiarrhythmic agents and the risk of malignant neoplasm of liver and intrahepatic bile ducts. PLoS One. 2015. January 15;10(1):e0116960. doi: 10.1371/journal.pone.0116960. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Karasneh RA, Murray LJ, Cardwell CR. Cardiac glycosides and breast cancer risk: a systematic review and meta-analysis of observational studies. Int J Cancer. 2017. March 1;140(5):1035–1041. Epub 2016 Nov 27. [DOI] [PubMed] [Google Scholar]
- [26].Li J, Solus J, Chen Q, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sørensen HT, Mellemkjær L, Steffensen FH, Olsen JH, Nielsen GL. The risk of a diagnosis of cancer Afibter primary deep venous thrombosis or pulmonary embolism. N Engl J Med. 1998;338:1169–1173. [DOI] [PubMed] [Google Scholar]
- [28].Vernino S, Adamski J, Krjzer TJ, Fealey RD, Lennon WA. Neuronal nicotinic Ach-receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology. 1998;50:1806–1813. [DOI] [PubMed] [Google Scholar]
- [29].Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case-control study. Eur Heart J. 2013. April;34(14):1061–1067. Epub 2013 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rossi A, Enriquez-Sarano M, Burnett JC Jr, et al. Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler-echocardiographic study. J Am Coll Cardiol. 2000;35(5):1256±1262. [DOI] [PubMed] [Google Scholar]
- [31].Vesely DL. Cardiac and renal hormones: anticancer effects in vitro and in vivo. J Investig Med. 2009. January;57(1):22–28. [DOI] [PubMed] [Google Scholar]
- [32].Vesely DL. Heart peptide hormones: adjunct and primary treatments of cancer. Anticancer Res. 2016. November;36(11):5693–5700. [DOI] [PubMed] [Google Scholar]


