Abstract
Background
The incidence of venous thromboembolic events (VTE) in patients with COVID-19 is generally high but varies markedly. However, the relationship between anticoagulation and mortality in patients with COVID-19 is still unclear.
Methods
We performed a systematic review and meta-analysis to determine the incidence of VTE and evaluate the role of anticoagulation in patients with COVID-19. Random effects models were used to determine overall pooled estimates and 95% confidence intervals (CIs).
Results
After a database search, 25 observational studies (20 on VTE incidence and 5 on the relationship between anticoagulation and mortality) were included. The pooled incidence rates of VTE, pulmonary embolism (PE), and deep vein thrombosis (DVT) in hospitalised COVID-19 patients were 21% (95% CI 15–27%), 15% (95% CI 10–20%), and 27% (95% CI 19–36%), respectively. A meta-analysis of five studies found that anticoagulation was not associated with an increased risk of mortality in hospitalised COVID-19 patients (RR = 0.86, 95% CI, 0.69–1.09, P = 0.218; I2 = 47.4%).
Conclusions
In conclusion, the incidence of VTE among hospitalised COVID-19 patients was high. Clinical trials are urgently needed to evaluate the roles of prophylactic and therapeutic anticoagulation in COVID-19.
Keywords: COVID-19, coagulation, antithrombotic, heparin
Introduction
Since the outbreak of COVID-19 in December 2019, more than 6 million confirmed cases and 392,000 deaths have been reported worldwide as of June 1, 2020 (WHO, 2020). Aside from the lungs, this disease may also cause severe injury to the heart (Li et al., 2020), kidneys (Ronco et al., 2020), and liver (Mao et al., 2020) that can lead to death.
Emerging data suggest that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) also targets the haematological system (Iba et al., 2020). Venous thromboembolic events (VTE) were reported in a case series of COVID-19 patients (Koumoutsea et al., 2020, Zhou et al., 2020, Giacomelli et al., 2020). In a very recent study (Wichmann et al., 2020), autopsies performed on 12 consecutive COVID-19-positive patients revealed that pulmonary embolism (PE) was the direct cause of death in 4 patients. These observations have led to concerns that COVID-19 is associated with a risk of VTE. Increasingly, studies (Al-Samkari et al., 2020, Artifoni et al., 2020, Bompard et al., 2020, Cui et al., 2020, Demelo-Rodriguez et al., 2020, Desborough et al., 2020, Helms et al., 2020, Hippensteel et al., 2020, Klok et al., 2020, Leonard-Lorant et al., 2020, Llitjos et al., 2020, Lodigiani et al., 2020, Middeldorp et al., 2020, Nahum et al., 2020, Poissy et al., 2020, Ren et al., 2020, Stoneham et al., 2020, Thomas et al., 2020, Voicu et al., 2020, Zhang et al., 2020) have evaluated the incidence of VTE in COVID-19 patients, which tended to be higher among those in the intensive care unit (ICU). However, the results have been very inconsistent. COVID-19 has been observed to be associated with elevated d-dimer levels and coagulopathy in patients, which increases the risk of death. This suggests that COVID-19 patients without medical contraindications may benefit from anticoagulant treatment. Several observational studies (Ayerbe et al., 2020, Paranjpe et al., 2020, Russo et al., 2020, Tang et al., 2020, Tremblay et al., 2020) have investigated the association of anticoagulation with risk of death in COVID-19 patients, with varying results. Therefore, we reviewed the literature and performed a meta-analysis pertaining to this association. This report extends current knowledge by assessing (WHO, 2020) the pooled incidence of VTE [PE or deep vein thrombosis (DVT)] in hospitalised patients with COVID-19 and (Li et al., 2020) determining whether anticoagulant treatment affected mortality.
Methods
To ensure that the work was of high quality, we followed the Preferred Reporting Items of Systematic Reviews and Meta-analysis (PRISMA) guidelines (Table S1). All steps were performed independently by two investigators with different specialties. Any disagreements were resolved by discussion between the two reviewers, or by a third reviewer.
Search strategy
The PubMed, EMBASE, and Cochrane Library databases were searched to identify all relevant articles published between Jan 1, 2020 and June 4, 2020. The World Health Organization (WHO) database and medRxiv.org were also searched for potentially relevant publications, including accepted articles yet to be published. The following keywords, and combinations thereof, were searched for: (“Corona Virus Disease-2019” OR “2019 novel coronavirus” OR “SARS-CoV-2” OR “COVID-19” OR “2019-nCoV”) AND (“VTE” OR “PE” OR “DVT” OR “thromboembolism” OR “venous thrombosis” OR “pulmonary embolism” OR “deep venous thrombosis” OR “thrombotic” OR “anticoagulants” OR “factor Xa inhibitors” OR “heparinoids” OR “dabigatran” OR “rivaroxaban” OR “edoxaban” OR “apixaban” OR “heparin”). Reference lists of relevant articles were searched manually.
Study selection
We included observational studies that reported the incidence of VTE in hospitalised patients with confirmed COVID-19. Studies not reporting clinical characteristics or clinical experience were excluded, as were case reports.
For studies that evaluated the effects of anticoagulation on mortality in patients with COVID-19, the following inclusion criteria were applied (WHO, 2020): case–control or cohort study (Li et al., 2020); no subjects in the reference group receiving anticoagulants (Ronco et al. (2020)); odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) of subsequent mortality reported (Mao et al., 2020); inclusion of adequate data to allow risk estimation; and (Iba et al., 2020) written in English.
Data extraction and quality assessment
For studies that evaluated the rate of VTE in patients with COVID-19, the following information was extracted using a standardised data collection method: author, study origin, design, period, site, baseline characteristics, and number of COVID-19 cases and VTE. Methodological quality was assessed using the instrument of Udina et al., which comprises 10 questions examining research quality. Studies with scores ≥ 15 were considered high quality.
For studies that investigated the impact of anticoagulation on mortality, the following information was extracted using a standardised data collection method: author, study origin, design, period and site, baseline characteristics, number of cases receiving and not receiving anticoagulants, measurement of anticoagulation, and statistical adjustments. The methodological quality was assessed using the Newcastle–Ottawa Scale (NOS) (Higgins, 2014), which has eight criteria and yields scores ranging from 0 (high risk of bias) to 9 (low risk of bias). Studies with scores ≥ 7 were regarded as high quality.
Statistical analysis
The meta-analysis of the rate of VTE was performed using a random-effects model with the Freeman–Tukey double arcsine transformation applied. The data on mortality risk were pooled using a random effects model with the generic inverse variance method, as described by DerSimonian and Laird (Greenland, 1987). We used the I² statistic to assess statistical heterogeneity; an I2 value > 50% was considered to indicate significant heterogeneity (Higgins and Thompson, 2002, Higgins et al., 2003). Owing to the anticipated heterogeneity of the included studies, we used a random-effects model to estimate effect sizes, which would provide more conservative estimates of the 95% CIs. The statistical analyses were performed using Stata software (ver. 12.0; Stata Corp., College Station, TX, USA).
Results
Search results
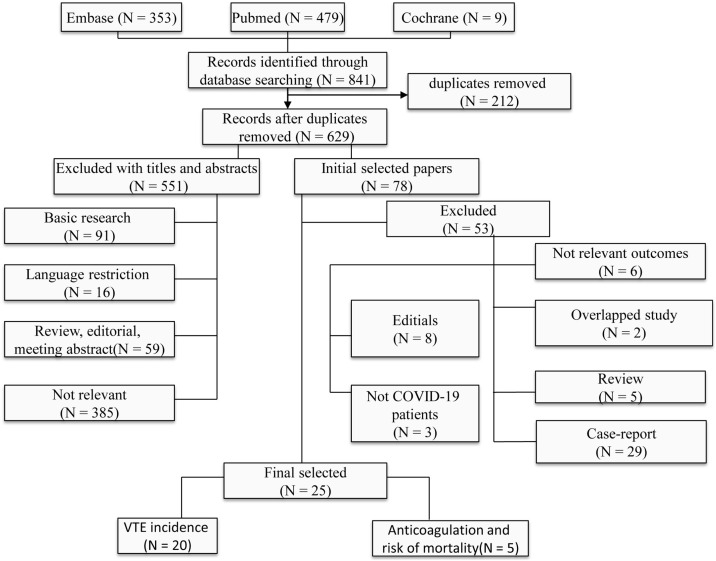
The electronic database and manual searches of the reference lists of relevant articles yielded 841 unique articles, and 212 duplicates. In total, 551 articles were excluded after reading the title and abstract. The full text of the remaining 78 articles was assessed in terms of suitability for the meta-analysis. Ultimately, our meta-analysis included 25 studies: 20 on DVT incidence and 5 on anticoagulation in COVID-19 patients. Figure 1 summarises the number of articles by reason for exclusion at each stage of the eligibility assessment.
Figure 1.
Flow chart of the studies considered and finally selected for review.
Characteristics of studies reporting the rate of VTE in COVID-19 patients
Table 1 summarizes the characteristics of the included studies reporting the rate of VTE. Fifteen studies were performed in Europe, two in USA and three in Chinese. The number of COVID-19 patients ranged from 26 to 400. Ten, four and six studies assessed the rate of VTE in patients only in the ICU, in both the ICU and general wards, and only in general wards, respectively. The mean age of the subjects ranged from 57 to 68 years and the proportion of males ranged from 52% to 81%. Table S2 presents the quality assessment results.
Table 1.
Characteristics of the Included Studies.
| Author | Country (city) | Study design | Study period | Study site | Age (year) | Male | Outcomes | Number of COVID-19 case | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Al-Samkari et al, 2020 | USA | Retrospective multi-center | Mar 1 to Apr 5 2020 | ICU and GW | 62.5 | 57% | VTE | 400 | 17 |
| Artifoni et al, 2020 | French (Nantes) | Retrospective multi-center | Mar 25 to Apr 10 2020 | GW | 64 (46-75) | 60% | PE, DVT | 71 | 14 |
| Bompard et al, 2020 | French (Paris) | Retrospective multi-center | Mar 1 to Apr 116 2020 | ICU and GW | 64 (54-76) | 70% | PE | 135 | 16 |
| Cui et al, 2020 | China (Wuhan) | Retrospective single-center | Jau 30 to Mar 22, 2020 | ICU | 60 (14.1) | 46% | VTE | 81 | 17 |
| Demelo-Rodríguez et al, 2020 | Spain (Madrid) | Prospective single-center | First half of April 2020 | GW | 68.1 (14.5) | 65% | DVT | 156 | 16 |
| Desborough et al, 2020 | UK (London) | Retrospective single-center | Mar 3 to 31 2020 | ICU | 59 (49-66) | 73% | VTE | 66 | 14 |
| Helms et al, 2020 | French (Paris) | Prospective multi-center | Mar 3 to 31 2020 | ICU | 63 (53-71) | 81.00% | PE, DVT | 150 | 17 |
| Hippensteel et al, 2020 | USA (Aurora) | Retrospective single-center | Mar 18 to Apr 14 2020 | GW | 56 | 58.00% | PE, VTE, DVT | 61 | 14 |
| Klok et al, 2020 | Netherlands (Paris) | Retrospective multi-center | Mar 7 to Apr 5 2020 | ICU | 64 (12) | 76% | PE, DVT | 184 | 18 |
| Leonard-Lorant et al, 2020 | French (Paris) | Retrospective multi-center | March 1 to 31 2020 | ICU and GW | 64 (22) | 66% | PE | 106 | 14 |
| Llitjos et al, 2020 | French (Paris) | Retrospective multi-center | Mar 19 to Apr 11 2020 | ICU | 68 (51-74) | 77% | PE, VTE | 26 | 13 |
| Lodigiani et al, 2020 | Italy (Milan) | Retrospective single-center | Feb 13 to Apr 10 2020 | ICU | 66 (55-85) | 68% | PE, VTE, DVT | 388 | 16 |
| Middeldorp et al, 2020 | Netherlands (Amsterdam) | Retrospective single-center | Mar 2 to Apr 12, 2020 | ICU and GW | 61 (14) | 66% | PE, VTE, DVT | 198 | 16 |
| Nahum et al, 2020 | Germany (Nord) | Retrospective single-center | Mar to Apr 2020 | ICU | 62 (8.6%) | 78% | DVT | 34 | 13 |
| Poissy et al, 2020 | French (Lille) | Retrospective single-center | Feb 27 to Mar 31, 2020 | ICU | 57 (29-80) | 59% | PE, DVT | 107 | 15 |
| Ren et al, 2020 | China (Wuhan) | Retrospective single-center | Feb 27 to Mar 31, 2020 | ICU | 57 (62-80) | 54% | DVT | 48 | 10 |
| Stoneham et al, 2020 | UK (Brighton) | Retrospective multi-center | Mar 20 to Apr 16 2020 | ICU and GW | NA | NA | PE, VTE, DVT | 274 | 16 |
| Thomas et al, 2020 | UK (Cambridge) | Retrospective single-center | to Apr 14, 2020 | ICU | 20-89 | NA | PE, VTE | 63 | 14 |
| Voicu et al, 2020 | French (Paris) | Prospective single-center | Mar 13 to Apr 3 2020 | ICU and GW | NA | NA | DVT | 56 | 14 |
| Zhang et al, 2020 | China (Wuhan) | Retrospective single-center | Jan 29 to Feb 29, 2020 | GW | 63 (14) | 52% | DVT | 143 | 16 |
DVT, deep vein thrombosis; GW, general ward; ICU, intensive care unit; NA, not available; PE, pulmonary embolism; VTE, venous thromboembolic event.
VTE incidence
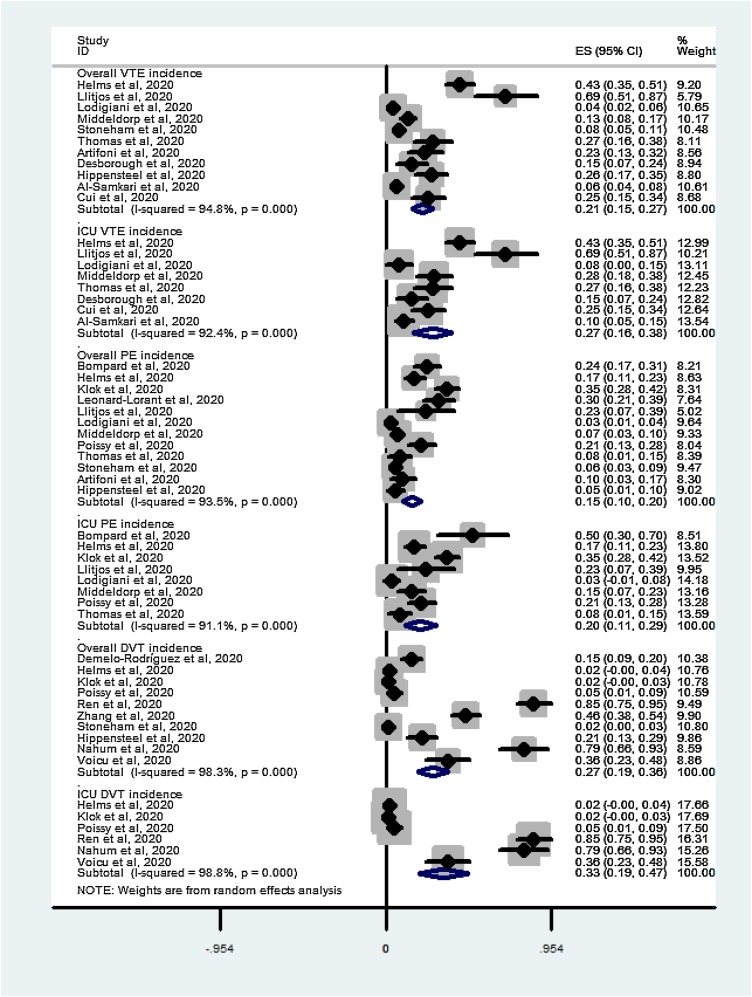
Eleven studies (Al-Samkari et al., 2020, Artifoni et al., 2020, Cui et al., 2020, Desborough et al., 2020, Helms et al., 2020, Hippensteel et al., 2020, Llitjos et al., 2020, Lodigiani et al., 2020, Middeldorp et al., 2020, Stoneham et al., 2020, Thomas et al., 2020) reported the overall incidence of VTE (ICU and general wards), which ranged from 4% to 42%. VTE occurred in 255 of 1,808 hospitalised patients. The meta-analysis revealed a pooled incidence rate of VTE of 21% (95% CI 15–27%, I2 = 94.8%; Figure 2 A) among all hospitalised patients. Eight (Al-Samkari et al., 2020, Cui et al., 2020, Desborough et al., 2020, Helms et al., 2020, Llitjos et al., 2020, Lodigiani et al., 2020, Middeldorp et al., 2020, Thomas et al., 2020) studies reported the incidence of VTE in the ICU setting. VTE occurred in 169 of 656 ICU patients. A meta-analysis of the proportions revealed a pooled incidence of VTE of 27% (95% C.I. 16–38%, I2 = 92.4%; Figure 2B) among ICU patients.
Figure 2.
Summary of pooled VTE incidence in COVID-19 patients (A) VTE in all hospitalised patients; (B) VTE in ICU patients; (C) PE in all hospitalised patients; (D) PE in ICU patients; (E) DVT in all hospitalised patients; (F) DVT in ICU patients.
Twelve studies (Artifoni et al., 2020, Bompard et al., 2020, Helms et al., 2020, Hippensteel et al., 2020, Klok et al., 2020, Leonard-Lorant et al., 2020, Llitjos et al., 2020, Middeldorp et al., 2020, Poissy et al., 2020, Stoneham et al., 2020, Thomas et al., 2020) reported the overall incidence of PE, which varied from 2% to 35%. PE occurred in 238 out of 1,793 hospitalised patients. The meta-analysis revealed a pooled incidence rate of PE of 15% (95% CI 10% - 20%, I2 = 93.5%; Figure 2C) among all hospitalised patients. Eight studies (Bompard et al., 2020, Helms et al., 2020, Klok et al., 2020, Llitjos et al., 2020, Lodigiani et al., 2020, Middeldorp et al., 2020, Poissy et al., 2020, Thomas et al., 2020) reported the incidence of PE in the ICU setting. PE occurred in 148 of 690 ICU patients. A meta-analysis of the proportions revealed a pooled incidence of PE of 20% (95% CI 9–31%, I2 = 49.6%; Figure 2D) among ICU patients.
Nine studies (Demelo-Rodriguez et al., 2020, Helms et al., 2020, Hippensteel et al., 2020, Klok et al., 2020, Nahum et al., 2020, Poissy et al., 2020, Ren et al., 2020, Stoneham et al., 2020, Voicu et al., 2020, Zhang et al., 2020) reported the overall incidence of DVT, which varied from 2% to 85%. DVT occurred in 212 out of 1,243 hospitalised patients. The meta-analysis revealed a pooled incidence rate of DVT of 27% (95% CI 19%-36%, I2 = 98.3%; Figure 2E) among all hospitalised patients. Seven studies (Helms et al., 2020, Klok et al., 2020, Nahum et al., 2020, Poissy et al., 2020, Ren et al., 2020, Voicu et al., 2020) reported the incidence of DVT in the ICU setting. DVT occurred in 99 out of 579 ICU patients. Meta-analysis revealed a pooled incidence of DVT of 33% (95% CI 19% - 47%, I2 = 98.8%; Figure 2F) among ICU patients.
Characteristics of studies reporting the impact of anticoagulation on mortality in COVID-19 patients
Table 2 summarises the characteristics of the included studies reporting the impact of anticoagulation on mortality. Four studies were performed in Europe and the USA; only one was conducted in China. Although two studies evaluated the impact of pre-admission antithrombotic therapy, patients who discontinued antithrombotic drugs during hospitalisation were excluded. The number of COVID-19 patients ranged from 192 to 3,100. The mean age of the subjects ranged from 56 to 67 years, and the proportion of males ranged from 55% to 60%. Table S3 presents the quality assessment results (Figure 3 ).
Table 2.
Characteristics of the Included Studies.
| Author | Country (city) | Study design | Study period | Age (year) | Male | Measurement of anticoagulant treatment | Anticoagulant | Non- anticoagulant | Confounder adjustment | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Tang et al, 2020 | China (Wuhan) | Single-center retrospective cohort | Jan 1 to Feb 13 2020 | 65.1 ± 12 | 60% | Medical record review | 99 | 350 | No | 6 |
| Paranjpe et al, 2020 | USA (New York) | Multiple-center retrospective cohort | Mar 14 to Apr 11 2020 | NA | NA | Medical record review | 786 | 1987 | Yes | 8 |
| Vincenzo et al, 2020 | Italy (Lombardy region) | Multiple-center retrospective cohort | Feb to Apr 2020 | 67.7 ± 15.2 | 59.90% | Databases of health care use | 26 | 166 | Yes | 6 |
| Tremblay et al, 2020 | USA (New York) | Multiple-center retrospective cohort | Mar 1 to Apr 1 2020 | 56.6 ± 18.2 | 55% | Databases of health care use | 241 | 2859 | Yes | 8 |
| Luis et al, 2020 | Spanish | Multiple-center retrospective cohort | to Apr 24 2020 | 67.6 ± 15.5 | 61% | Databases of health care use | 1734 | 285 | Yes | 7 |
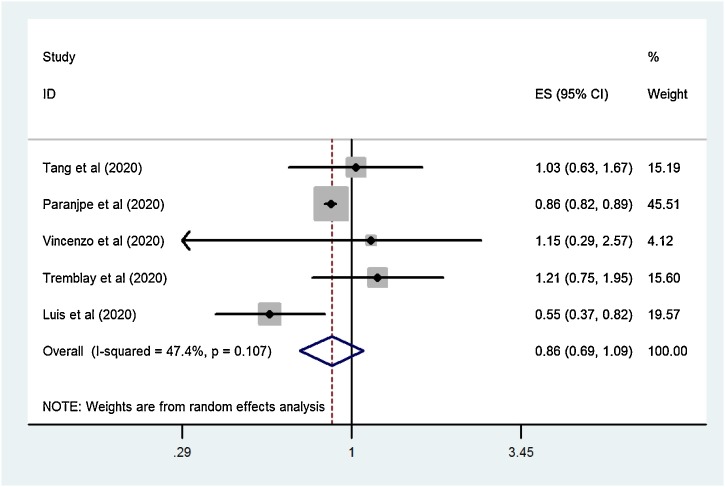
Figure 3.
Forest plot of anticoagulation and risk of mortality in COVID-19 patients.
Anticoagulation and risk of mortality in COVID-19 patients
This analysis included five studies (Ayerbe et al., 2020, Paranjpe et al., 2020, Russo et al., 2020, Tang et al., 2020, Tremblay et al., 2020), including 2,886 and 5,647 COVID-19 cases receiving and not receiving anticoagulants, respectively. Overall, the risk of mortality was similar between the anticoagulant-exposed and non-exposed COVID-19 patients (RR = 0.86, 95% CI, 0.69–1.09, P = 0.218; I2 = 47.4%; Figure 2). Limiting the analysis to the studies providing adjusted data, there was no significant decrease in mortality risk in patients receiving anticoagulant therapy (RR = 0.84, 95% CI, 0.63–1.13, P = 0.243; I2 = 57.6%). Excluding two studies that specified pre-admission antithrombotic therapy, the meta-analysis of the remaining three studies also found that anticoagulation was not associated with a lower risk of mortality (RR = 0.79, 95% CI, 0.48–1.31, P = 0.361; I2 = 55.8%).
Discussion
Main findings
To our knowledge, this is the first systematic review and meta-analysis of the incidence of VTE and effects of anticoagulation on mortality in patients with COVID-19. We found that the overall rates of VTE, PE, and DVT were high (pooled incidence rates of 21%, 15%, and 27%, respectively). These rates were higher among patients admitted to the ICU, and antithrombotic therapy was not associated with a lower mortality risk.
Possible mechanisms underlying the findings
Although the relationship between SARS-CoV-2 and VTE was reported soon after the COVID-19 outbreak (Terpos et al., 2020, Wang et al., 2020), the underlying mechanism requires further exploration. The first possible mechanism is cytokine storm caused by viral infection. Several studies have reported significantly higher plasma cytokine concentrations in COVID-19 patients than in healthy adults (Han et al., 2020, McGonagle et al., 2020, Wan et al., 2020). Furthermore, an elevated IL-6 level was associated with more severe COVID-19 infection (Han et al., 2020, Lagunas-Rangel and Chavez-Valencia, 2020). Inflammatory cytokines, such as TNF-α and IL-6, strongly induce the expression of tissue factors on endothelial cell surfaces and leucocytes, particularly monocytes (de Jonge et al., 2003, Mutlu et al., 2007). Tissue factors are the primary initiator of the blood coagulation cascade and strongly contribute to the hypercoagulable state in COVID-19 infection. Inflammatory cytokines can also trigger the release of ultra-large von Willebrand factor multimers from the endothelium (Tomaske et al., 2011), causing thrombotic microangiopathy; this has been confirmed at autopsy in patients who died from COVID-19 (Ackermann et al., 2020). Finally, the concentrations of vascular heparin-like molecules are reduced by inflammation, which interferes with the natural anticoagulant pathways (Schmitt et al., 2019). The second potential mechanism is virus-induced endothelial dysfunction. SARS-CoV-2 is capable of directly infecting the vascular endothelium by entering cells via angiotensin-converting enzyme receptors (Zost et al., 2020), which results in the massive release of plasminogen activators and inhibition of fibrinolysis (Frantzeskaki et al., 2017). In SARS-CoV-1-infected patients, high plasma tissue-type plasminogen activator (t-PA) concentrations are observed (Giannis et al., 2020). The third putative mechanism is complement activation in viral pneumonia (Gralinski et al., 2018). Deposits of terminal complement components have been observed in the lungs of COVID-19 patients, indicating that dysregulated complement activation contributes to coagulopathy in COVID-19 patients (Magro et al., 2020). Other clinical factors, such as hypoxemia, hyperthermia, and hypovolemia, may also enhance the hypercoagulable state in COVID-19 (Henry et al., 2020).
Unfortunately, our findings suggest that anticoagulation is unlikely to protect against COVID-19-related mortality. A previous meta-analysis (Zheng et al., 2020) demonstrated that cardiovascular diseases are related to an unfavourable prognosis in COVID-19 patients, so any investigation of the impact of anticoagulation on mortality should consider cardiovascular conditions. In our analysis, two studies (Russo et al., 2020, Tremblay et al., 2020) evaluated the impact of pre-admission antithrombotic therapy, which indicates underlying cardiovascular disease in the COVID-19 cases receiving anticoagulants. Therefore, the protective effect of anticoagulation may have been underestimated due to confounding by indication. However, further sensitivity analysis did not show a significant decrease in mortality in the COVID-19 patients who received antithrombotic therapy. (Tang et al. (2020) observed that anticoagulant therapy appeared to be associated with a better prognosis in severe COVID-19 patients with markedly elevated d-dimer, implying that COVID-19 patients with other indications would benefit from anticoagulant therapy.
Implications for clinical practice
Our findings have important implications for clinicians. Hospitalised COVID-19 patients, particularly those admitted to the ICU, should have their coagulation function monitored through repeated measurements of d-dimer, prothrombin time, and platelet count. VTE should be suspected if patients have a high d-dimer or show rapid respiratory deterioration. The Padua or Caprini prediction score should be used to assess patients with mild symptoms of VTE; the use of standard-dose thromboprophylaxis is acceptable in hospitalized low-risk COVID-19 patients if they do not have medical contraindications. Furthermore, higher-dose VTE prophylaxis may benefit critically ill patients and seems to be associated with better outcomes.
Call for future studies
Considering the current controversies and challenges, more studies of COVID-19- coagulation are needed, including of the efficacy and safety of prophylactic and therapeutic anticoagulation. It is also necessary to develop a scoring system to estimate the risk of VTE in patients with COVID-19. Finally, the optimal dose of anticoagulant to prevent VTE in COVID-19 patients needs to be determined in controlled trials.
Strengths and limitations
The strengths of this meta-analysis included its compliance with the PRISMA statement and comprehensive search strategy. This review also had a major limitation with important implications regarding the interpretation of the results: it included a broad range of COVID-19 patients with widely varying characteristics. Moreover, the studies differed in terms of country of origin, definition of anticoagulant exposure, and design. These factors may have introduced heterogeneity, which could affect the results.
Conclusions
In summary, we reported a high pooled incidence of VTE and the need for coagulation monitoring in patients diagnosed with COVID-19. Additional high-quality data are needed to understand the risk of VTE, and the effects of anticoagulation on prognosis and mortality, in COVID-19 patients.
Funding Source
This study was supported by Natural Science Foundation of Zhejiang Province (Grant No. LY20H090012).
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approve
No ethical approval was required for this review as all data were already published in peer‐reviewed journals. No patients were involved in the design, conduct or interpretation of our review.
Authors’ contributions
Y.F.L., L.Y.P., W.W.Z. and H.Y.J. conceived the study and revised the manuscript critically for important intellectual content. F.C. and S.S.H. made substantial contributions to its design, acquisition, analysis and interpretation of data. X.Z. participated in the design, acquisition, analysis and interpretation of data. All authors read and approved the final manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.08.023.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- WHO WHO. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Li J.W., Han T.W., Woodward M., Anderson C.S., Zhou H., Chen Y.D., Neal B. The impact of 2019 novel coronavirus on heart injury: A Systematic review and Meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C., Reis T., Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J., Shen J., Zhu L.R., Chen Y., Iacucci M., Ng S.C., Ghosh S., Chen M.H. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T., Levy J.H., Levi M., Connors J.M., Thachil J. Coagulopathy of Coronavirus Disease 2019. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumoutsea E.V., Vivanti A.J., Shehata N., Benachi A., Le Gouez A., Desconclois C., Whittle W., Snelgrove J., Malinowski A.K. COVID-19 and acute coagulopathy in pregnancy. J Thromb Haemost. 2020 doi: 10.1111/jth.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., She J., Wang Y., Ma X. A Case of Coronavirus Disease 2019 With Concomitant Acute Cerebral Infarction and Deep Vein Thrombosis. Front Neurol. 2020;11:296. doi: 10.3389/fneur.2020.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli E, Dorigo W, Fargion A, Calugi G, Cianchi G, Pratesi C. Acute Thrombosis of an Aortic Prosthetic Graft in a Patient with Severe COVID-19-Related Pneumonia. Ann Vasc Surg. 2020 doi: 10.1016/j.avsg.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann D., Sperhake J.P., Lutgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schroder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Puschel K., Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C., Fogerty A.E., Waheed A., Goodarzi K., Bendapudi P., Bornikova L., Gupta S., Leaf D., Kuter D.J., Rosovsky R.P. COVID and Coagulation: Bleeding and Thrombotic Manifestations of SARS-CoV2 Infection. Blood. 2020 doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artifoni M., Danic G., Gautier G., Gicquel P., Boutoille D., Raffi F., Neel A., Lecomte R. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompard F., Monnier H., Saab I., Tordjman M., Abdoul H., Fournier L., Sanchez O., Lorut C., Chassagnon G., Revel M.P. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J. 2020 doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demelo-Rodriguez P., Cervilla-Munoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macias M., Toledo-Samaniego N., Garcia-Garcia A., Garcia-Fernandez-Bravo I., Ji Z, de-Miguel-Diez J, Alvarez-Sala-Walther LA, Del-Toro-Cervera J, Galeano-Valle F. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desborough M.J.R., Doyle A.J., Griffiths A., Retter A., Breen K.A., Hunt B.J. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res. 2020;193:1–4. doi: 10.1016/j.thromres.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Angles-Cano E., Sattler L., Mertes P.M., Meziani F., Group C.T. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippensteel J.A., Burnham E.L., Jolley S.E. Prevalence of Venous Thromboembolism in Critically Ill Patients with COVID-19. Br J Haematol. 2020 doi: 10.1111/bjh.16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O., Schneider F., Labani A., Bilbault P., Moliere S., Leyendecker P., Roy C., Ohana M. Acute Pulmonary Embolism in COVID-19 Patients on CT Angiography and Relationship to D-Dimer Levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020 doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Alexia B., Sandri M.T., Barco S., C-TF Humanitas. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum J., Morichau-Beauchant T., Daviaud F., Echegut P., Fichet J., Maillet J.M., Thierry S. Venous Thrombosis Among Critically Ill Patients With Coronavirus Disease 2019 (COVID-19) JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S., Lille ICUHC-g Pulmonary Embolism in COVID-19 Patients: Awareness of an Increased Prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- Ren B., Yan F., Deng Z., Zhang S., Xiao L., Wu M., Cai L. Extremely High Incidence of Lower Extremity Deep Venous Thrombosis in 48 Patients with Severe COVID-19 in Wuhan. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047407. [DOI] [PubMed] [Google Scholar]
- Stoneham S.M., Milne K.M., Nuttal E., Frew G.H., Sturrock B.R., Sivaloganathan H., Ladikou E.E., Drage S., Phillips B., Chevassut T.J., Eziefula A.C. Thrombotic risk in COVID-19: a case series and case-control study. Clin Med (Lond) 2020 doi: 10.7861/clinmed.2020-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W., Varley J., Johnston A., Symington E., Robinson M., Sheares K., Lavinio A., Besser M. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voicu S., Bonnin P., Stepanian A., Chousterman B.G., Le Gall A., Malissin I., Deye N., Siguret V., Mebazaa A., Megarbane B. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J., Zhang C., Li H., Xia X., Kong S., Liao J., Jia H., Pang X., Song Y., Tian Y., Wang B., Wu C., Yuan H., Zhang Y., Li Y., Sun W., Zhang Y., Zhu S., Wang S., Xie Y., Ge S., Zhang L., Hu Y., Xie M. Deep Vein Thrombosis in Hospitalized Patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- Ayerbe L., Risco C., Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe I., Fuster V., Lala A., Russak A., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., Zhao S., Nadkarni G.N. Association of Treatment Dose Anticoagulation with In-Hospital Survival Among Hospitalized Patients with COVID-19. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo V., Di Maio M., Attena E., Silverio A., Scudiero F., Celentani D., Lodigiani C., Di Micco P. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacol Res. 2020:104965. doi: 10.1016/j.phrs.2020.104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay D., van Gerwen M., Alsen M., Thibaud S., Kessler A.J., Venugopal S., Makki I., Qin Q., Dharmapuri S., Jun T., Bhalla S., Berwick S., Feld J., Mascarenhas J., Troy K., Cromwell C., Dunn A., Oh W.K., Naymagon L. Impact of anticoagulation prior to COVID-19 infection: a propensity score-matched cohort study. Blood. 2020 doi: 10.1182/blood.2020006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P. The Cochrane collaboration; 2014. Cochrane handbook for systematic reviews of interventions version 5.1.0. Available at: www.cochrane-handbook.org [6 December 2014] [Google Scholar]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A. Hematological findings and complications of COVID-19. Am J Hematol. 2020 doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Chen R., Liu C., Liang W., Guan W., Tang R., Tang C., Zhang N., Zhong N., Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F., Jiang Y., Cheng X., Zhu C., Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O’Regan A., Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y., Gao C. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol. 2020;189(3):428–437. doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas-Rangel F.A., Chavez-Valencia V. High IL-6/IFN-gamma ratio could be associated with severe disease in COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge E., Friederich P.W., Vlasuk G.P., Rote W.E., Vroom M.B, Levi M, van der Poll T. Activation of coagulation by administration of recombinant factor VIIa elicits interleukin 6 (IL-6) and IL-8 release in healthy human subjects. Clin Diagn Lab Immunol. 2003;10(3):495–497. doi: 10.1128/CDLI.10.3.495-497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu G.M., Green D., Bellmeyer A., Baker C.M., Burgess Z., Rajamannan N., Christman J.W., Foiles N., Kamp D.W., Ghio A.J., Chandel N.S., Dean D.A., Sznajder J.I., Budinger G.R. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117(10):2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaske M., Candinas R., Weiss M., Bauersfeld U. Safety and efficacy of paediatric outpatient radiofrequency catheter ablations. Int J Cardiol. 2011;148(3):276–279. doi: 10.1016/j.ijcard.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt F.C.F., Manolov V., Morgenstern J., Fleming T., Heitmeier S., Uhle F., Al-Saeedi M., Hackert T., Bruckner T., Schöchl H., Weigand M.A., Hofer S., Brenner T. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9(1):19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P., Schäfer A., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Martinez D.R., Williamson L.E., Chen E.C., Jones T., Day S., Myers L., Hassan A.O., Kafai N.M., Winkler E.S., Fox J.M., Shrihari S., Mueller B.K., Meiler J., Chandrashekar A., Mercado N.B., Steinhardt J.J., Ren K., Loo Y.M., Kallewaard N.L., McCune B.T., Keeler S.P., Holtzman M.J., Barouch D.H., Gralinski L.E., Baric R.S., Thackray L.B., Diamond M.S., Carnahan R.H., Crowe J.E., Jr. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respiration. 2017;93(3):212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., Whitmore A., Heise M.T., Baric R.S. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. mBio. 2018;9(5) doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Vikse J., Benoit S., Favaloro E.J., Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., Ye C., Zhang P., Xing Y., Guo H., Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.