Abstract
Purpose
To investigate the association between aqueous flare and progression of visual field loss using the Humphrey Field Analyzer in patients with retinitis pigmentosa (RP).
Methods
We examined a total of 101 eyes of 101 patients who were diagnosed with typical RP. Sixty-one percent of the patients were female, and the mean age of the total group was 47.4 years. Aqueous flare, visual field (by an Humphrey Field Analyzer, the central 10-2 SITA-Standard program), and optical coherence tomography measurements were obtained for all patients. The slope, which was derived from serial values of mean deviation, macular sensitivity, or foveal sensitivity for each eye with univariate linear regression, was used for analysis.
Results
Aqueous flare values were significantly correlated with the mean deviation slope (r = −0.20, P = 0.046), macular sensitivity slope (r = −0.28, P = 0.005) and foveal sensitivity slope (r = −0.20, P = 0.047). The values of the retinal sensitivity slope significantly decreased as the aqueous flare level increased (all P < 0.05). These associations remained unchanged after adjustment for age, sex, and posterior subcapsular cataract, and epiretinal membrane.
Conclusions
Elevation of aqueous flare is a risk factor for the decline of central visual function in RP. Aqueous flare may be a useful marker for disease progression in RP.
Keywords: aqueous flare, intraocular inflammation, retinitis pigmentosa
Retinitis pigmentosa (RP) is a group of inherited retinal degeneration diseases resulting from photoreceptor death, and individuals with RP typically suffer from impaired night vision and a gradual loss of the visual field.1 Several clinical studies have shown that chronic intraocular inflammation plays a role in the pathology of RP, supported by the results that inflammatory cells and proinflammatory cytokines are substantially increased in the vitreous of patients with RP.2,3 Aqueous flare has been used as a sensitive marker of blood–retina barrier breakdown and intraocular inflammation in ocular diseases such as diabetic retinopathy, AMD, and RP, as well as for the disease activity in these conditions.4–7
Assessing and protecting the central visual function is crucial for maintaining the quality of vision in individuals with RP.8 RP is a chronic progressive disease, and it is difficult to measure the slight changes of visual function. There are several methods for evaluating the progression of visual function, namely, perimetry tests and electroretinography. It was reported that the average sensitivity of 12 and 4 central points on the Humphrey Field Analyzer (HFA) 10-2 program were correlated with the best-corrected visual acuity (BCVA), especially in advanced RP.9 In previous reports, we defined the average sensitivity of 12 and 4 central points as macular sensitivity (MS) and foveal sensitivity (FS) and revealed that the slopes calculated by MS and FS value were more sensitive and suitable parameters for use in evaluating central visual function and representing disease progression than the slope of mean deviation (MD) values, which is usually used for progression of visual funciton.10,11
Aqueous flare is increased in patients with RP, and increased aqueous flare is correlated with worse visual function.12,13 However, there have been no studies assessing the association between aqueous flare and longitudinal visual function. In the present study, we investigated the association between aqueous flare and decline of central visual function in patients with RP using the HFA 10-2 program.
Methods
Study Design and Ethics Statement
We retrospectively studied the records of patients with RP and obtained examination results including age and sex, visual acuity, HFA measurements, and spectral domain optical coherence tomography findings (Cirrus HD-OCT; Carl-Zeiss Meditec, Dublin, CA).
This study was approved by the Institutional Review Board of Kyushu University Hospital (Fukuoka, Japan) and was conducted in accord with the tenets of the Declaration of Helsinki on Biomedical Research Involving Human Subjects. The review board waived the need for written informed consent because the study design was a retrospective chart review.
Patients
Patients were enrolled at Kyushu University Hospital from 2008 to 2015: 123 patients with a diagnosis of typical RP underwent the measurement of aqueous flare and automated static perimetry (HFA) testing. Patients who consecutively underwent at least three HFA examinations were included in the present study. The HFA measurement intervals were 3 to 12 months irregularly. The eyes of patients who had received treatments that were shown to affect aqueous flare values (e.g., topical steroid, topical dorzolamide, or oral acetazolamide), those of patients who had less than three HFA examinations and those of patients with CME, a history of other ocular diseases, or intraocular surgery (e.g., cataract surgery) during follow-up were excluded. After these exclusions, 101 (age range, 17–73 years) of the 123 patients were enrolled. The examination results of the right eye of each patient were used for analysis. In cases in which the right eye could not be examined owing to exclusion criteria, we used the left eye for analysis.
The diagnosis of typical RP was based on a history of night blindness, visual field constriction and/or ring scotoma, and markedly reduced or nonrecordable a- and b-wave amplitudes on electroretinography testing, in addition to ophthalmoscopic findings (e.g., bone spicule-like pigment clumping in the mid-peripheral and peripheral retina and attenuation of retinal vessels).
Visual Field Testing
All patients underwent automated static perimetry before pupillary dilation with the central 10-2 SITA-Standard program. The lens was corrected as appropriate for the test distance. Visual field testing was repeated if the test reliability was not satisfactory (i.e., fixation loss >20%, false positive >15%, or false negative >33%). The calculation of the MD value involved averaging the differences between the measured sensitivities and the age-adjusted normal sensitivities (total deviations) at each test point. The MS was calculated as the average of the 12 central points as described previously.14,15 We also calculated the FS as the average of the four central points, excluding foveal points.10 If reliable data were not obtained in repeated tests, the data were not included in the study.
Simple linear regression has been well-recognized as an effective method for obtaining estimates of individual reductions in vision.8,16 For analysis of the parameters of MD, MS, and FS, we used the slope derived from the serial values of MD, MS, or FS obtained for each eye by univariate linear regression as the MD slope, MS slope, and FS slope, respectively. If reliable data were not obtained in repeated tests, the data were not included in the study.
Laser Flare Photometry
The aqueous flare was measured with a Kowa FM-600 laser flare meter (Kowa, Nagoya, Japan). We performed a single follow-up measurement in each patient, and the time of measurement was different for each patient, as described in our previous studies.12,17,18 Flare values were obtained 30 minutes after pupillary dilation with 0.5% tropicamide and 0.5% phenylephrine hydrochloride. Five measurements that were taken at the same time and averaged in each eye. The results are expressed as photon counts per millisecond. Calibration was performed on each day of aqueous flare measurement to ensure the measurement quality.
Measurements and Definitions of Confounding Factors
BCVA was measured with a standard Japanese decimal BCVA chart and was converted to the logMAR units.
The presence of posterior subcapsular cataract (PSC) was defined as a Lens Opacification Classification System III score of 1 or greater with the aid of a slit-lamp biomicroscope after dilation with tropicamide 0.5% and phenylephrine 0.5%.19 Epiretinal membrane (ERM) was detected by fundus examination and by spectral domain optical coherence tomography (Cirrus; Carl Zeiss Meditec). The diagnosis of ERM using spectral domain optical coherence tomography is based on the presence of a hyperreflective line or band over the retinal surface, frequently associated with wavy changes in the underlying retina.20,21 ERM was diagnosed when it was detected on both fundus photographs and optical coherence tomography images.
Statistical Analysis
The data are presented as the mean ± standard deviation. Geometric means and 95% prediction intervals are shown for aqueous flare values owing to their skewed distributions. The aqueous flare values were converted to a logarithmic scale to better approximate a normal distribution. The relationship between aqueous flare values and visual parameters was examined by Pearson's rank correlation coefficient. The association between aqueous flare values and decline of retinal sensitivities was tested using a linear regression model. In the multivariable-adjusted analysis, we included the following possible confounding factors: age, sex, PSC, and ERM. Age was treated as a continuous variable, and the others as categorical variables. Each categorical variable was coded as either 1 or 0 depending on the presence or absence of the factor. All of the statistical analyses were performed with SAS software, ver. 9.4 (SAS Institute, Cary, NC). Two-sided P values of less than 0.05 were considered significant.
Results
The demographic data of the study patients are summarized in Table 1. Sixty-one percent of the patients were female, the mean age of the total group was 47.4 years old, and the mean follow-up was 5.3 years. Table 1 also shows the mean values or frequencies of visual factors in the total of 101 patients with RP.
Table 1.
Characteristics of the 101 Patients With RP
| Variable | All |
|---|---|
| Sex, female (%) | 62 (61) |
| Follow-up, years | 5.3 ± 2.0 |
| Time between follow-ups, months | 6.2 ± 2.6 (2.7–14.2) |
| HFA measurements, times | 7.4 ± 3 |
| PSC | 36 (36) |
| ERM | 12 (12) |
| Aqueous flare, pc/ms | 7.7 (6.9–8.6) |
| Parameters at baseline | |
| Age, years | 47.4 ± 14 |
| VA, logMAR | 0.16 ± 0.3 |
| MD, dB | −12.7 ± 10.7 |
| MS, dB | 27.4 ± 8.3 |
| FS, dB | 29.2 ± 7.2 |
| Inheritance mode | |
| AD | 13 (13) |
| AR | 20 (20) |
| X-linked | 0 (0) |
| Sporadic | 68 (67) |
Values are given as the mean ± standard deviation or number (%).
Geometric means and 95% prediction intervals are shown for aqueous flare values owing to their skewed distributions.
VA, visual acuity; dB, decibel; AD, autosomal dominant RP; AR, autosomal recessive RP.
The MD, MS, and FS slopes at each flare level are plotted for each patient in Figure. We evaluated the correlation coefficient between aqueous flare values and visual factors in patients with RP. Aqueous flare values were significantly correlated with the MD slope (r = −0.20, P = 0.046), MS slope (r = −0.28, P = 0.005) and FS slope (r = −0.20, P = 0.047; Table 2).
Figure.
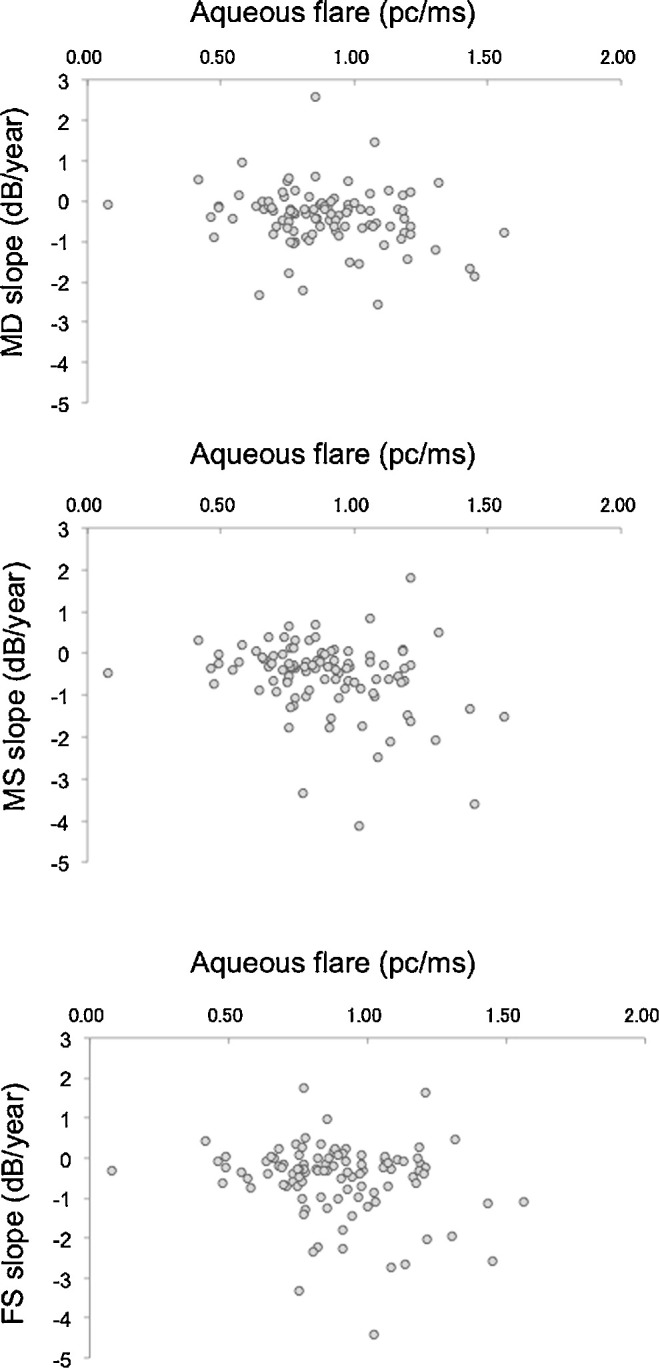
Scatterplot of aqueous flare and slope of visual function on the HFA 10-2 program in 101 patients with RP. The slope of the MDs is shown in the top row, the MS in the second row, and the FS in the third row. Aqueous flare was transformed to logarithmic scale. dB, decibel.
Table 2.
Correlation Coefficients Between Aqueous Flare and Slope of Visual Field Loss in 101 Eyes With RP
| Aqueous Flare, pc/ms (n = 101) | ||
|---|---|---|
| Variable | r | P Value |
| MD slope, dB/year | −0.20 | 0.046 |
| MS slope, dB/year | −0.28 | 0.005 |
| FS slope, dB/year | −0.20 | 0.047 |
Aqueous flare was transformed to the logarithmic scale.
dB, decibel.
We next investigated the age-, sex-, and multivariable-adjusted decrease in the retinal sensibility slope per every 1-log-transformed aqueous flare value (Table 3). The values of the retinal sensitivity slope significantly decreased as the aqueous flare level increased (all P < 0.05). These associations remained unchanged after adjustment for sex, age and PSC, and ERM.
Table 3.
Change in Slope for Retinal Sensitivities per Every 1-Log-Transformed Aqueous Flare in Eyes With RP
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Change in Slope, dB/year | P Value | Change in Slope, dB/year | P Value | |
| sMD slope | ||||
| Per every 1-log-transformed aqueous flare | −0.36 (0.1) | 0.02 | −0.34 (0.2) | 0.03 |
| MS slope | ||||
| Per every 1-log-transformed aqueous flare | −0.52 (0.2) | 0.004 | −0.48 (0.2) | 0.01 |
| FS slope | ||||
| Per every 1-log-transformed aqueous flare | −0.39 (0.2) | 0.04 | −0.34 (0.2) | 0.09 |
dB, decibel. Values are shown as the β estimate and (SE).
Aqueous flare was transformed to the logarithmic scale.
Model 1: adjustment was made for age and sex.
Model 2: adjustment was made for the variables used in model 1 and PSC and ERM.
Discussion
We studied the association between aqueous flare and the progression rate of visual field loss in patients with RP using automated static perimetry (the HFA 10-2 program). To our knowledge, this study is the first to identify an association between aqueous flare and progression of central visual function loss based on long-term follow-up data. In a multivariable analysis to control for possible confounding factors, aqueous flare was found to be an independent and significant factor for progression of central visual field loss.
Our previous studies showed that proinflammatory cytokines/chemokines such as IL-1α, IL-6, IL-8, and IFN-γ are elevated in the vitreous of patients with RP compared with that of patients with idiopathic ERM.3 On the basis of this finding, it is apparent that chronic intraocular inflammation occurs in RP. Sawa et al.22 showed that aqueous flare is caused by the breakdown of the blood–retinal barrier owing to intraocular inflammation. Aqueous flare values are increased in patients with RP compared with normal patients, supporting the association between chronic intraocular inflammation and RP.12 Several cross-sectional studies have assessed the association between intraocular inflammation and visual field loss in RP. Nishiguchi et al.13 reported that aqueous flare was negatively correlated with the visual field area calculated by Goldmann perimetry. We also reported that increases in aqueous flare were negatively correlated with BCVA and the MD value.12
The precise mechanisms underlying the association between intraocular inflammation and progression of visual field loss are unknown, but several potential mechanisms have been proposed. It has been reported that neuroinflammation may play a role in the progression of many chronic neurodegenerative disorders, including Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and multiple sclerosis.23 Intraocular inflammation in RP is thought to be caused by the death of rod photoreceptors followed by the gradual death of cone photoreceptors. Peng et al.24 reported that microglia neurotoxicity contributes to photoreceptor degeneration in the rd10 mouse model of RP with the involvement of proinflammatory cytotoxic mediators such as TNF-α, cyclo-oxygenase-1, and cyclo-oxygenase-2. We also demonstrated that increases of proinflammatory factors, such as monocyte chemotactic protein -1, IL-1β, and TNF-α, were observed in rd10 mice, and that the suppression of inflammatory responses via antioxidant production may protect photoreceptors against cell death in these animals.25 The influence of intraocular inflammation represented by aqueous flare may have an effect on photoreceptor degeneration in RP. However, because it remains unclear whether a causal relationship exists between intraocular inflammation and RP-associated photoreceptor degeneration, further investigations of this relationship are needed.
Moreover, we showed that there was a significant correlation between high-sensitivity C-reactive protein, which is used as a marker for systemic inflammation, and the MD changes over the prior 3 years.26 These findings were in concordance with our present findings. These results indicated that both intraocular and systemic inflammation in RP are implicated in the pathology of RP.
RP is frequently complicated with PSC,18,27–29 CME,14,15,30,31 and ERM.9,32,33 It is well-known that central visual function is affected by PSC and macular complications. We demonstrated that there was a marginal trend of association between the MS or FS slope between the eyes with and without macular complications, but this association was not statistically significant.10 Taking into consideration the effects of PSC, macular complications as potential confounding factors, although there were no CME patients in this study because of the exclusion criteria, we assessed the association between aqueous flare values and the progression of visual field loss (Table 3). After adjusting for these confounding factors, aqueous flare was significantly associated with advanced progression, suggesting that aqueous flare is an appropriate marker for the assessment of disease progression in RP.
A previous study showed that there was no significant difference in aqueous flare values in a mydriatic group from 0.5 hours to 1.0 hour after the application of 0.5% phenylephrine–0.5% tropicamide, and the flare values decreased significantly 2 hours after the application.34 Thus, the manufacturer recommended that aqueous flare values be measured using a laser flare meter from 0.5 hours to 1.0 hour after pupillary dilation. However, there is a possibility that pupillary size can affect the values of aqueous flare. Oshika et al.34 reported that change in pupillary size had little influence on the measurement results for the following reasons. (1) The influence of 1% pilocarpine on flare intensity was assessed in normal human eyes, and pupillary size in itself had little influence on aqueous flare.35 (2) Flare meter measurements are made within a sampling window located in the anterior chamber. Signals above and below the sampling window are counted and subtracted from the flare signals to cancel background noise.36 Therefore, considering these adjustments made for the two background signals, we consider that any measurement contamination owing to the size of constricted pupils would have been excluded.
We have no data about the value of aqueous flare in similar age groups with no disease in this study. However, in our previous study, we reported that the aqueous flare values were significantly higher in the patients with RP compared with the null disease cohort (10.6 ± 7.9 vs. 5.0 ± 2.1 pc/ms, P < 0.0001, mean age 54.0, range 22–89 years). Kuchle et al.4 showed that aqueous flare was observed in patients with mild-stage RP with a remaining visual field of 40° or greater to patients with severe-stage RP with only a small remaining central island of less than 10°. Additionally, we reported that aqueous flares are negatively correlated with central visual function assessed by the HFA 10-2 program. These results show that aqueous flares are present when most of the photoreceptors are lost.
Our present findings are significant because of the study's large sample size and long follow-up time. Nevertheless, there are some limitations that should be discussed. First, although potential confounders were included in our analyses, we cannot rule out the possibility of unknown confounding factors for the development of retinal sensitivity slope. Second, we analyzed only single measurement data regarding aqueous flare values in the follow up, but only 29 of the 101 patients were reexamined at two time points for aqueous flare longitudinally, and the average increase per year was 33.4%. It was difficult to accurately measure the change of aqueous flare owing to this small sample size and limited number of measurements. Therefore, further studies are needed to compare the efficacy of single measurement of aqueous flare versus change in aqueous flare to clarify which measure is more accurate and sensitive for representing the progression of central visual function in patients with RP.
In conclusion, elevation of aqueous flare, which represents the intraocular inflammation, is a risk factor for the decline of central visual function in RP. Aqueous flare may be a useful marker for disease progression in RP.
Acknowledgments
Supported by Grants from the Bayer Retina Award (to YI) and the Japanese Ministry of Education, Culture, Sports, Science, and Technology (grant #16H06268 to YM, #16K15735 to KS, #18K16960 to KF).
Disclosure: K. Fujiwara, None; Y. Ikeda, None; Y. Murakami, None; T. Tachibana, None; J. Funatsu, None; Y. Koyanagi, None; S. Nakatake, None; S. Shimokawa, None; N. Yoshida, None; S. Nakao, None; T. Hisatomi, None; T. Ishibashi, None; K.-H. Sonoda, None
References
- 1. Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006; 368: 1795–1809. [DOI] [PubMed] [Google Scholar]
- 2. Newsome DA, Michels RG.. Detection of lymphocytes in the vitreous gel of patients with retinitis pigmentosa. Am J Ophthalmol. 1988; 105: 596–602. [DOI] [PubMed] [Google Scholar]
- 3. Yoshida N, Ikeda Y, Notomi S, et al.. Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013; 120: 100–105. [DOI] [PubMed] [Google Scholar]
- 4. Kuchle M, Nguyen NX, Martus P, et al.. Aqueous flare in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 1998; 236: 426–433. [DOI] [PubMed] [Google Scholar]
- 5. Kubota T, Motomatsu K, Sakamoto M, Honda T, Ishibashi T. Aqueous flare in eyes with senile disciform macular degeneration: correlation with clinical stage and area of neovascular membrane. Graefes Arch Clin Exp Ophthalmol. 1996; 234: 285–287. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen NX, Schonherr U, Kuchle M. Aqueous flare and retinal capillary changes in eyes with diabetic retinopathy. Ophthalmologica. 1995; 209: 145–148. [DOI] [PubMed] [Google Scholar]
- 7. Schroder S, Muether PS, Caramoy A, et al.. Anterior chamber aqueous flare is a strong predictor for proliferative vitreoretinopathy in patients with rhegmatogenous retinal detachment. Retina. 2012; 32: 38–42. [DOI] [PubMed] [Google Scholar]
- 8. Birch DG, Locke KG, Felius J, et al.. Rates of decline in regions of the visual field defined by frequency-domain optical coherence tomography in patients with RPGR-mediated X-linked retinitis pigmentosa. Ophthalmology. 2015; 122: 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iijima H. Visual loss and perimetric sensitivity in eyes with retinitis pigmentosa. Jpn J Ophthalmol. 2013; 57: 563–567. [DOI] [PubMed] [Google Scholar]
- 10. Fujiwara K, Ikeda Y, Murakami Y, et al.. Assessment of Central Visual Function in Patients with Retinitis Pigmentosa. Sci Rep. 2018; 23: 8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sayo A, Ueno S, Kominami K, et al.. Longitudinal study of visual field changes determined by Humphrey Field Analyzer 10-2 in patients with retinitis pigmentosa. Sci Rep. 2017; 27: 16383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murakami Y, Yoshida N, Ikeda Y, et al.. Relationship between aqueous flare and visual function in retinitis pigmentosa. Am J Ophthalmol. 2015; 159: 958–963. [DOI] [PubMed] [Google Scholar]
- 13. Nishiguchi KM, Yokoyama Y, Kunikata H, et al.. Correlation between aqueous flare and residual visual field area in retinitis pigmentosa. Br J Ophthalmol. 2018; 0: 1–6. [DOI] [PubMed] [Google Scholar]
- 14. Ikeda Y, et al.. The clinical efficacy of a topical dorzolamide in the management of cystoid macular edema in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 809–814. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda Y, Hisatomi T, Yoshida N, et al.. Therapeutic effect of prolonged treatment with topical dorzolamide for cystoid macular oedema in patients with retinitis pigmentosa. Br J Ophthalmol. 2013; 97: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 16. Iannaccone A, Kritchevsky SB, Ciccarelli ML, et al.. Kinetics of visual field loss in Usher syndrome type II. Invest Ophthalmol Vis Sci. 2004; 45: 784–792. [DOI] [PubMed] [Google Scholar]
- 17. Fujiwara K, Ikeda Y, Murakami Y, et al.. Association between aqueous flare and epiretinal membrane in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2016; 57: 4282–4386. [DOI] [PubMed] [Google Scholar]
- 18. Fujiwara K, Ikeda Y, Murakami Y, et al.. Risk factors for posterior subcapsular cataract in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2017; 58: 2534–2537. [DOI] [PubMed] [Google Scholar]
- 19. Chylack LJ, Wolfe JK, Singer DM, et al.. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993; 111: 831–836. [DOI] [PubMed] [Google Scholar]
- 20. Testa F, Rossi S, Colucci R, et al.. Macular abnormalities in Italian patients with retinitis pigmentosa. Br J Ophthalmol. 2014; 98: 946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen J, Lee L.. Clinical applications and new developments of optical coherence tomography: an evidence-based review. Clin Exp Optom. 2007; 90: 317–335. [DOI] [PubMed] [Google Scholar]
- 22. Sawa M. Clinical application of laser flare-cell meter. Jpn J Ophthalmol. 1990; 34: 346–363. [PubMed] [Google Scholar]
- 23. Glass CK, Saijo K, Winner B, et al.. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010; 140: 918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peng B, Xiao J, Wang K, et al.. Suppression of microglial activation is neuroprotective in a mouse model of human retinitis pigmentosa. J Neurosci. 2014; 34: 8139–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshida N, Ikeda Y, Notomi S, et al.. Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013; 120: e5–e12. [DOI] [PubMed] [Google Scholar]
- 26. Murakami Y, Ikeda Y, Nakatake S, et al.. C-Reactive protein and progression of vision loss in retinitis pigmentosa. Acta Ophthalmol. 2018; 96: e174–e179. [DOI] [PubMed] [Google Scholar]
- 27. Fishman GA, Anderson RJ, Lourenco P.. Prevalence of posterior subcapsular lens opacities in patients with retinitis pigmentosa. Br J Ophthalmol. 1985; 69: 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merin S, Auerbach E. Retinitis pigmentosa. Surv Ophthalmol. 1976; 20: 303–346. [DOI] [PubMed] [Google Scholar]
- 29. Pruett RC. Retinitis pigmentosa: clinical observations and correlations. Trans Am Ophthalmol Soc. 1983; 81: 693–735. [PMC free article] [PubMed] [Google Scholar]
- 30. Hirakawa H, Iijima H, Gohdo T, et al.. Progression of defects in the central 10-degree visual field of patients with retinitis pigmentosa and choroideremia. Am J Ophthalmol. 1999; 127: 436–442. [DOI] [PubMed] [Google Scholar]
- 31. Hajali M, Fishman GA, Anderson RJ. The prevalence of cystoid macular oedema in retinitis pigmentosa patients determined by optical coherence tomography. Br J Ophthalmol. 2008; 92: 1065–1068. [DOI] [PubMed] [Google Scholar]
- 32. Hagiwara A, Yamamoto S, Ogata K, et al.. Macular abnormalities in patients with retinitis pigmentosa: prevalence on OCT examination and outcomes of vitreoretinal surgery. Acta Ophthalmol. 2011; 89: e122–e125. [DOI] [PubMed] [Google Scholar]
- 33. Testa F, Rossi S, Colucci R, et al.. Macular abnormalities in Italian patients with retinitis pigmentosa. Br J Ophthalmol. 2014; 98: 946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oshika T, Nishi M, Mochizuki M, et al.. Quantitative assessment of aquaoue flare and cells in uveitis. Jpn J Ophthalmol. 1989; 33: 279–287. [PubMed] [Google Scholar]
- 35. Mori M, Araie M, Sakurai M, Oshika T. Effects of pilocarpine and tropicamide on blood-aqueous barrier permeability in man. Invest Ophthalmol Vis Sci. 1992; 33: 416–423. [PubMed] [Google Scholar]
- 36. Sawa M, Tsurimaki Y, Tsuru T, Shimizu H. New quantitative method to determine protein concentration and cell number in aqueous in vivo. Jpn J Ophthalmol. 1988; 32: 132–142. [PubMed] [Google Scholar]


