Abstract
Purpose
Cyclic adenosine monophosphate (cAMP) and peroxisome proliferator-activated receptor alpha (PPARα) levels mediate extracellular matrix (ECM) changes by altering the levels of hypoxia-inducible factor 1-alpha (HIF-1α) in various tissues. We aimed to determine, in the sclera of guinea pigs, whether a prostanoid receptor (EP2)-linked cAMP modulation affects PPARα and HIF-1α signaling during myopia.
Methods
Three-week-old guinea pigs (n = 20 in each group), were monocularly injected with either an EP2 agonist (butaprost 1 µmol/L/10 µmol/L), an antagonist (AH6809 10 µmol/L/30 µmol/L) or a vehicle solution for two weeks during normal ocular growth. Separate sets of animals received these injections and underwent form deprivation (FD) simultaneously. Refraction and axial length (AL) were measured at two weeks, followed by scleral tissue isolation for quantitative PCR (qPCR) analysis (n = 10) and cAMP detection (n = 10) using a radioimmunoassay.
Results
Butaprost induced myopia development during normal ocular growth, with proportional increases in AL and cAMP levels. FD did not augment the magnitude of myopia or cAMP elevations in these agonist-injected eyes. AH6809 suppressed cAMP increases and myopia progression during FD, but had no effect in a normal visual environment. Of the diverse set of 27 genes related to cAMP, PPARα and HIF-1α signaling and ECM remodeling, butaprost differentially regulated 15 of them during myopia development. AH6809 injections during FD negated such differential gene expressions.
Conclusions
EP2 agonism increased cAMP and HIF-1α signaling subsequent to declines in PPARα and RXR mRNA levels, which in turn decreased scleral fibrosis and promoted myopia. EP2 antagonism instead inhibited each of these responses. Our data suggest that EP2 suppression may sustain scleral ECM structure and inhibit myopia development.
Keywords: EP2, HIF-1α, PPARα, myopia and sclera
Myopia presents with excessive ocular elongation resulting in a mismatch between the eye size and the refractive power of the cornea and lens, thus obscuring normal vision. Global myopia prevalence has risen rather rapidly in the past 50 years. In East Asian countries such as Taiwan, Hong Kong, China, Singapore and Japan, its incidence now ranges between 85% and 95%.1,2 The severity of this rise is exemplified by the fact that the prevalence of high myopia (over −6.00 diopters [D]) ranges approximately between 5% and 20% in some Asian populations, although it is also on the rise in western countries.3 In the year 2000, the global prevalence of myopia and high myopia was estimated to be 22.9% and 2.9%, respectively, which is predicted to increase dramatically to 49.8% and 9.8%, respectively, by 2050.4 The cause of myopia remains unclear, even though its incidence is lower among individuals who spend more time outdoors compared with those exposed to less daylight. Also, a genetic component cannot be ruled out, as highlighted by studies on parental myopia, genetic linkage, and candidate gene analyses and genome-wide association studies.5,6
Animal models exposed to visual distortions to induce myopia are often interrogated for molecular cues underlying this condition.7 Irrespective of its cause, increased eye size accompanying extensive extracellular matrix (ECM) remodeling of the sclera, resulting from changes in the scleral architecture and composition, are common features in humans and animal models of myopia. Such effects ultimately lead to excessive ocular elongation.8 Among other changes to the scleral ECM, degradation of collagen and proteoglycans, reduced levels of growth factors, such as transforming growth factor–β (TGF-β) isoforms and increased expression and activity of matrix metalloproteinases (MMPs) are observed during myopia.7,9
Adenylyl cyclase (AC) stimulation increases cyclic adenosine monophosphate (cAMP) content, which in turn degrades scleral collagen content during myopia in guinea pigs.10,11 We also reported that AC activation and presumed increases in scleral cAMP levels promote myopia development through inhibition of genes that promote collagen synthesis and ECM fibrosis in guinea pigs.12 Although numerous studies focus on studying the roles of various signaling pathways involved in myopia development, we investigated the role of prostaglandins (PGs) in this process. These lipid mediators are synthesized from arachidonic acid by cyclooxygenases (COX).13,14 Prostaglandin E2 (PGE2) is a naturally occurring major PG subtype that is most abundant in the human body.15 PGs interact with prostanoid E receptor subtypes (EP1-4) to perform specific functions.16,17 EP1 activation increases intracellular Ca2+ levels, while EP2 and EP4 elevate cAMP levels and EP3 reduces cAMP.18 PGE2 agonism promotes proliferation of NIH/3T3 and 3T6 cells, but it inhibits the proliferation of lung fibroblasts and the synthesis of collagen,19,20 thus highlighting a cell type-dependent response of its EP receptors. Such differences also exist between EP2 and EP4 receptors, both of which elevate cAMP levels21 that in turn affect collagen levels and fibroblast proliferation. In the eye, EP2 activation reduced intraocular pressure (IOP) in glaucomatous monkeys by increasing the uveoscleral outflow.22 A significant body of research has shown a strong relation between myopia and glaucoma with myopic individuals having a higher risk of developing glaucoma.23 The role of EP2 in mediating cAMP, and thus collagen levels combined with their role in reducing the IOP intrigued us to study the role of this particular receptor in myopia development.
In HEK-293 cells, cAMP-induced protein kinase A (PKA) activation enhanced the activity of peroxisome proliferator-activated receptor alpha (PPARα), which is an antifibrotic transcription factor.24 PPARα forms a heterodimer with retinoid X receptor (RXR) and regulates gene transcription and inhibits ECM accumulation in various tissues.25–27 However, in the guinea pig sclera, PPARα agonism had a profibrotic effect that suppressed myopia progression, whereas PPARα antagonism inhibited fibrosis and promoted myopia.28,29 We also earlier reported that a hypoxic environment in the sclera promotes myopia in mice and guinea pigs, while an inhibitor of hypoxia-inducible factor 1-alpha (HIF-1α), a known hypoxia marker, suppressed myopia development. Such myopia inhibitions were accompanied by COL1α1 downregulations in the latter model.30 Hypoxia has also been shown to decrease RXR levels in cardiac myocytes, which inhibits the formation of a PPARα:RXR complex,31 thus suppressing the transcriptional ability of PPARα.32 In HeLa and C33a cervical carcinoma cells, hypoxia increased cAMP levels by increasing AC activity.33 Thus we predicted that the scleral EP2 modulates cAMP levels and myopia development in guinea pigs through a crosstalk with PPARα and HIF-1α signaling pathways.
In this study, EP2 activity modulation had corresponding effects on cAMP accumulation and myopia progression. The mechanisms underlying such EP2-involvement were studied by monitoring the expression profiles of a few major scleral ECM genes and downstream genes in the PPARα and HIF-1α signaling pathways. The results allowed us to conclude that EP2 activation in a normal visual environment increased cAMP levels, which activated HIF-1α. These effects in turn inhibited fibrosis and PPARα/RXR signaling events that led to myopia development. Alternatively, EP2 antagonism during induced myopia suppressed the myopia-associated cAMP increases and negated the inhibition and activation of PPARα/RXR and HIF-1α signaling, respectively. These reciprocal effects offset declines in scleral ECM content during form deprivation (FD), ultimately preventing myopia progression. Such findings highlight the essential role of EP2 in altering scleral cAMP content, which affects both PPARα and HIF-1α levels that are key modulators of scleral ECM changes during myopia development.
Methods
Three-week-old pigmented guinea pigs from Bi Kai experimental animal farm (Danyang, Jiangsu) were reared in standard cages under a 12-hour light/dark cycle with food and water provided ad libitum. Right eye of each animal was used for drug injections and/or FD, while the fellow eye was untreated. The treatment and care of animals were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol for handling animals was approved by the animal care and ethics committee at Wenzhou Medical University (Wenzhou, P.R. China).
Drug Injections and Myopia Induction
Daily peribulbar injections of either an EP2 agonist (butaprost), an EP2 antagonist (AH6809) or a vehicle (0.1% dimethyl sulfoxide [DMSO]), were administered monocularly in mildly restrained guinea pigs. The drugs were dissolved in DMSO, diluted in Milli-Q water to a final working concentration of 0.1%, and 100 µL of the drug or vehicle was injected for two weeks. Animals in groups 1 and 2 received the agonist, “N + Butaprost (1 µmol/L)” and “N + Butaprost (10 µmol/L),” respectively, whereas groups 3 and 4 received the antagonist, “N + AH6809 (10 µmol/L)” and “N + AH6809 (30 µmol/L),” respectively. Group 5 was injected with a vehicle solution of 100 µL Milli-Q water in 0.1% DMSO (“N + DMSO”). Groups 1 through 5 were exposed to normal visual environment. Group 6 was subjected to monocular FD using latex facemasks, whereas groups 7 through 11 received butaprost, AH6809 or vehicle injections along with FD for two weeks (Table 1). Although butaprost has been studied extensively as a therapeutic agent in the management of glaucoma,34 AH6809 is a potent EP2 antagonist35,36 that acts via suppressing cAMP elevations in other tissues/cells such as in lung fibroblasts37 and in the hippocampus of rats during brain injury.38 Hence, this drug was used in our study to identify the antagonistic effects of EP2 receptor in the sclera during myopia development. These aforementioned drug concentrations were chosen on the basis of studies showing they were effective in mediating ECM remodeling and cell proliferation without causing cellular toxicity in various tissue systems.37,39 A separate set of untreated animals (Group 12) served as age-matched (AM) controls (Table 1).
Table 1.
Groups and Treatment Paradigms for Agonist, Antagonist, and Vehicle Injections in Guinea Pigs
| Group Number | Group Name | Treatment | Sample Size |
|---|---|---|---|
| 1 | N+Butaprost (1 µmol/L) | EP2 agonist injections in a normal visual environment | 17 |
| 2 | N+Butaprost (10 µmol/L) | EP2 agonist injections in a normal visual environment | 18 |
| 3 | N+AH6809 (10 µmol/L) | EP2 antagonist injections in a normal visual environment | 18 |
| 4 | N+AH6809 (30 µmol/L) | EP2 antagonist injections in a normal visual environment | 18 |
| 5 | N+DMSO | Vehicle injection in a normal visual environment | 18/19*(in butaprost/ AH6809 groups, respectively) |
| 6 | FD | Form deprivation | 19 |
| 7 | FD+Butaprost (1 µmol/L) | EP2 agonist injections with form deprivation | 15 |
| 8 | FD+Butaprost (10 µmol/L) | EP2 agonist injections with form deprivation | 12 |
| 9 | FD+AH6809 (10 µmol/L) | EP2 antagonist injections with form deprivation | 18 |
| 10 | FD+AH6809 (30 µmol/L) | EP2 antagonist injections with form deprivation | 17 |
| 11 | FD+DMSO | Vehicle injection with form deprivation | 15/18* (in Butaprost/ AH6809 groups, respectively) |
| 12 | AM | Untreated age-matched controls | 20 |
*Butaprost and AH6809 injection groups had separate sets “N+DMSO” and “FD+DMSO” controls for parallel comparison across drug and vehicle injection treatment paradigms.
Ocular Measurements and Sclera Isolation
At the end of two weeks, the refraction was measured in minimal lighting using a custom-built eccentric infrared photoretinoscope.40 Each eye was measured thrice, and the mean value was considered the final refraction. Axial length (AL) and vitreous chamber depth (VCD) were estimated using an A-scan ultrasonograph (AVISO Echograph Class I-Type Bat, frequency:11 MHz; Quantel Medical, Clermont-Ferrand, France) as previously described.29 The refraction and axial length measurements were also obtained at the start of the experiment. After completing the two-week ocular measurements, the guinea pigs were terminally anesthetized with an overdose of chloral hydrate and euthanized via cervical dislocation. Their eyes were immediately enucleated on ice with cornea, iris, lens, vitreous body, retina, and choroid discarded to isolate the sclera. Some scleral tissues were stored in RNAlater for gene expression analysis (n = 10), and some were snap-frozen in liquid nitrogen for cAMP assay (n = 10) and stored at −80°C.
Scleral cAMP Assay
The scleral samples were weighed and homogenized in a ball mill (MM400; Retsch, Haan, Germany) with 200 µL acetate buffer (50 mmol/L Na+ acetate, 50 mmol/L H+ acetate, and 4 mmol/L ethylenediaminetetraacetic acid, pH 4.75) for 10 minutes at a frequency of 30 Hz. The homogenized samples were spun in a centrifuge at 13,000g for two minutes at 4°C onto which a 900-µL aliquot of acetate buffer was added and further lysed at 200 watts for 15 seconds in an ultrasonic crusher (JY92-2D; Ningbo Scientz Biotechnology, China) on ice. The homogenate was spun in a centrifuge at 13,000g for five minutes at 4°C to collect the supernatant. Acetate buffer (1 mL) and 100% ethanol (2 mL) were added to the supernatant and spun in a centrifuge at 13,000g for 15 minutes at 4°C. The supernatants were collected and dried at 60°C overnight. The dried and powdered residue was dissolved in 1 mL acetate buffer with 0.1 mL being assayed using the 125I-cAMP radioimmunoassay kit (Institute of Isotopes Ltd, Budapest, Hungary) according to the manufacturer's protocol. The cAMP concentrations were estimated using a gamma scintillation counter (xh6080; Xi'an Nuclear Instrument Factory, Xi'an, Shaanxi, China) and presented as fmol/mg.
RNA Extraction, cDNA Synthesis and Gene Expression Analysis
Total RNA was isolated using the RNeasy Fibrous Tissue Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Samples with an A260/A280 ratio of at least 1.8 as measured with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) were used for gene expression analysis. Total RNA (0.5 µg) was converted to cDNA using M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA) as described earlier.12 Guinea pig–specific primers (Table 2) were designed using primer3 software41 and quantitative polymerase chain reaction (qPCR) was performed in duplicates using a SYBR Green master mix (Applied Biosystems, Carlsbad, CA, USA) on an Applied Biosystems ViiATM 7 PCR System. The mRNA level of each target gene was estimated separately in treated and control samples and normalized to the reference gene, 18S rRNA (that was amplified in a separate well to that of the target genes). Relative gene expression values were converted to fold change estimates using the 2−ΔΔCt formula.42,43 Also the results of gene expression data was compared with the already existing data from Guo et al.44 that used a similar targeted gene expression analyses from diverse pathways using the PCR technique that we used here. The differentially expressed gene list, along with their fold changes, from four days of FD in tree shrews was used to perform a network analysis using STRING (v10) database45 that shows interactions between molecules (Supplementary Fig. S2).
Table 2.
Primer Sequences for qPCR Analysis
| Genes | Sequence (5′- 3′) | Tm (°C) | Product Size |
|---|---|---|---|
| Creb1_F | GATCTTAGTGCCCAGCAACC | 58.62 | 175 bp |
| Creb1_R | TAGGCGAACTTCTCGCTTTC | 58.01 | |
| Creb2_F | GGGGCTGAAGAAAGCTTAGG | 58.24 | 329 bp |
| Creb2_R | GGGGGCTCCGTATTAGTCTC | 59.03 | |
| Creb3_F | CTCCAGGCCATGGTAATTGA | 57.27 | 97 bp |
| Creb3_R | CAGGTACAAAGAGGAGGCAGA | 59.10 | |
| Pparα_F | GGTGAGGACTTCGGCTTTTA | 57.53 | 110 bp |
| Pparα_R | AGTACTGCCGCTTCCACAAG | 60.32 | |
| Cpt1a_F | ATGTGGAAGTGGATGAAGTGC | 58.56 | 176 bp |
| Cpt1a_R | TTATCAGAGCCTTGGCGATT | 56.99 | |
| Rara_F | GTGAGGAACGACCGCAATAA | 58.28 | 247 bp |
| Rara_R | CGGTCTTGATGATGCACTTG | 57.18 | |
| Rarb_F | GCTACGAGATGACTGCTGAGT | 59.60 | 111 bp |
| Rarb_R | ATGCACTTGGTGGCCAGTTC | 61.18 | |
| Rarg_F | CGAGCTCAGAGGAGATGGTT | 58.89 | 215 bp |
| Rarg_R | CCTGGTCACCTTGTTGATGA | 57.43 | |
| Rxra_F | CTCTCCCATGAACCCTGTGA | 58.72 | 170 bp |
| Rxra_R | AGCTGTACACGCCATAGTGC | 60.46 | |
| Rxrb_F | GCTCCTTATCGCCTCCTTTT | 57.67 | 178 bp |
| Rxrb_R | CATCCTCATGTCACGCATTT | 56.50 | |
| Rxrg_F | GAATGACCTGGTCCTCCAAG | 57.59 | 205 bp |
| Rxrg_R | TGAAGACATGCCTGTGGAGA | 58.65 | |
| Hif1α_F | AGCTGCTGGAGACACAATCAT | 59.72 | 212 bp |
| Hif1α_R | CTTGATTGAGTGCAGGGTCA | 57.81 | |
| Bmp6 _F | AAGAAGGCTGGCTGGAATTT | 57.68 | 205 bp |
| Bmp6 _R | ACGTGTACCTCGCTCACCTT | 61.18 | |
| Gata4_F | AGCTTCATGTAGAGGCCACAG | 59.79 | 180 bp |
| Gata4_R | TGGCCTCTACCACAAGATGA | 58.05 | |
| Abca1_F | ACCCGCGTATTTTTCTCCAT | 57.59 | 121 bp |
| Abca1_R | GAGGGAGCATGTGGAGTTCT | 59.09 | |
| Pml_F | GTGCCCATCTATGCCTTCTC | 58.11 | 110 bp |
| Pml_R | ATGATCTTTCGGGAGCACTG | 57.68 | |
| Ptprn2_F | AAGCTTCGCACCAGAAAGTC | 58.77 | 80 bp |
| Ptprn2_R | GGCTCCAACCTCTACCACAT | 59.09 | |
| Chd4_F | AGGCATGTCCTACTGGCACT | 60.91 | 144 bp |
| Chd4_R | CGGCTCTTCTCTTCATCACC | 58.07 | |
| Lrpap1_F | AGGCTGACGACCTATACGACA | 60.41 | 188 bp |
| Lrpap1_R | CTCCAGCTCCTTCTTGGTGA | 59.02 | |
| Col1a1_F | GGTCCTGATGGCAAAACTG | 56.50 | 121 bp |
| Col1a1_R | CACCTTTAGGTCCAGGGAAT | 56.50 | |
| Col2a1_F | CTGTGACGAAGGGATTGTCCT | 59.72 | 287 bp |
| Col2a1_R | GGAGGTCCTTTGGGTCCTACA | 60.83 | |
| Col6a5_F | TGGCCACGTCTCAGTTTCAT | 59.60 | 424 bp |
| Col6a5_R | ACACGAGGTCAGCAAGTGAAT | 59.93 | |
| Col12a1_F | AGGCGAAAGGAAATCAGCCAC | 61.22 | 355 bp |
| Col12a1_R | AGCAGGGCATTTTGCTTCATC | 59.79 | |
| Acta1_F | GAAGGAGTAGCCACGCTCAG | 60.18 | 319 bp |
| Acta1_R | TGCTGTCCCTCTATGCCTCT | 60.03 | |
| TGF-β1_F | ATGAATAGCAGCCAGGTCAC | 57.66 | 95 bp |
| TGF-β1_R | GTGCTCACTGCTCCTGTGAT | 60.04 | |
| TGF-β2_F | ATCCCGCTTGAAATCAATGT | 55.69 | 206 bp |
| TGF-β2_R | AGACCCCACATCTCCTGCTA | 59.66 | |
| TGF-β3_F | GAGAGTTGCTCCACCTTTGG | 58.47 | 209 bp |
| TGF-β3_R | GTCCACGAACCTAAGGGCTA | 58.81 |
Statistical Analysis
Mean differences in refraction, VCD, and AL between the treated and fellow eyes (i.e., interocular difference) at the end of two weeks were determined for each group and presented as mean ± standard deviation (SD). Statistical significance of the change in ocular refraction and biometrics between groups was estimated using one-way analysis of variance (ANOVA) and corrected for multiple testing using Bonferroni correction, unless otherwise stated. Significant changes in cAMP levels and gene expression levels were assessed using paired sample t-test for animals in the same group and independent sample t-test for intergroup comparisons.
Results
There were no significant differences in refraction and axial length between the right and left eyes of guinea pigs in each group before the start of the experiment, which is consistent with our earlier published work.46,47 Guinea pigs subjected to FD, with or without vehicle (0.1% DMSO) injections, developed significant amounts of myopia characterized by increased VCD and AL compared to their fellow eyes (P < 0.0001, paired sample t test) and AM controls (P < 0.0001, independent sample t test). Interocular differences in ocular refraction and biometrics of animals in group 6 were not significantly different from those in group 11 (FD vs. FD + DMSO: P > 0.05, one-way ANOVA), suggesting a lack of vehicle effect. Data on group 6 are not shown, whereas those on group 11 are presented in comparisons involving the effects of drug injections on myopia.
EP2 Agonist Promotes Myopia Development and Axial Elongation During Normal Ocular Growth
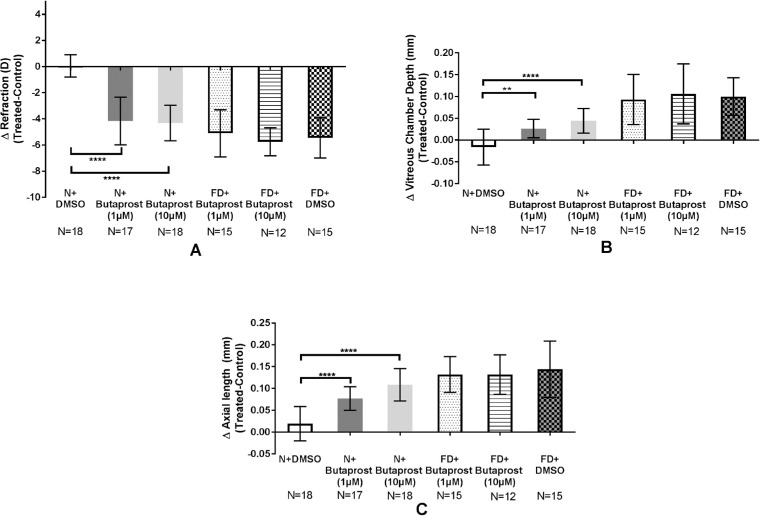
Guinea pigs injected with butaprost (1 µmol/L and 10 µmol/L) and exposed to an unobstructed visual environment progressed towards myopic refractions compared to the vehicle-injected eyes (N + Butaprost (1 µmol/L) and N + Butaprost (10 µmol/L) versus N + DMSO: −4.15 ± 1.82 D and −4.3 ± 1.36 D versus 0.06 ± 0.85 D, respectively; P < 0.0001, Fig. 1A). Butaprost or vehicle injections during FD similarly resulted in a significant myopic shift compared to group 5 (N + DMSO) (Fig. 1A). Consistent with refractive error changes, VCD and AL were significantly elongated after 1 µmol/L and 10 µmol/L butaprost injections during normal ocular growth (Figs. 1B and 1C) compared with group 5. During FD, either dose of the drug did not significantly alter the magnitude of myopia induced or ocular elongations compared those injected with the vehicle (Groups 7 vs. 8 vs. 11: −5.09 ± 1.80 D vs. −5.74 ± 1.07D vs. −5.44 ± 1.53 D, P > 0.05, Figs. 1A through 1C).
Figure 1.
Butaprost induces myopia development. Significant myopic shift in refraction (A) combined with increases in VCD (B) and AL (C) were associated with butaprost injections and FD. Data presented as mean interocular difference between treated and fellow eyes ± SD. **P < 0.01 and ****P < 0.0001; one-way ANOVA.
EP2 Antagonist Inhibits Myopic Shift and Axial Elongation During FD
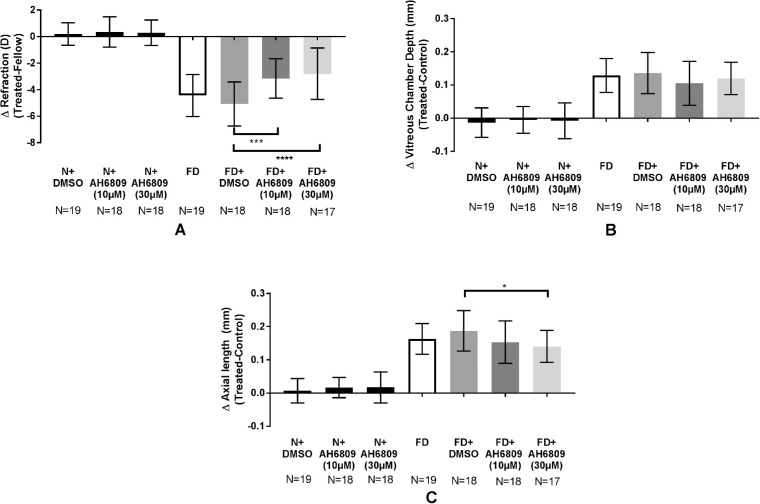
Refraction, VCD and AL were not significantly different between the AH6809-injected and their respective fellow eyes during normal ocular growth (data not shown). In contrast, AH6809 injections during FD (Groups 9 and 10) significantly inhibited the progression of myopia compared with the vehicle-injected group (FD + DMSO vs. FD + AH6809 [10 µmol/L] vs. FD + AH6809 [30 µmol/L]: −5.07 ± 1.66D vs. −3.16 ± 1.48 D vs. −2.79 ± 1.94 D, at least P < 0.0001, Fig. 2A). Although both dosages suppressed the myopic shift in refraction, only the highest dosage significantly inhibited AL elongations (FD + DMSO vs. FD + AH6809 [30 µmol/L]: 0.19 ± 0.06 mm vs. 0.14 ± 0.05 mm, P < 0.05, Fig. 2C). Refraction and ocular length in the fellow eyes of guinea pigs in treatment groups that developed myopia or in those that presented with myopia inhibition were not significantly different from each other (Supplementary Fig. S1).
Figure 2.
AH6809 suppresses FD-induced myopia progression. Myopia development was significantly inhibited after injections with an EP2 antagonist, AH6809 (A). VCD was unchanged (B), but 30 µmol/L AH6809 during FD inhibited AL elongation (C). The antagonist did not alter these parameters in an unobstructed visual environment. Data presented as mean interocular difference between treated and fellow eyes ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; one-way ANOVA).
EP2 Agonism Increases Scleral cAMP Levels
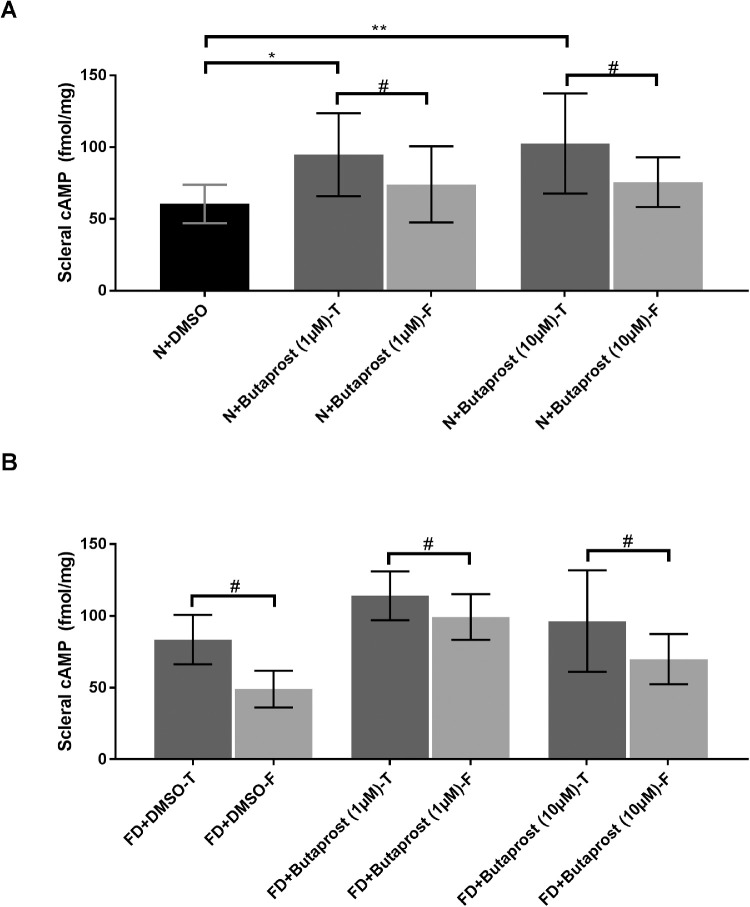
Compared to the vehicle injected eyes, scleral cAMP levels were significantly higher in eyes injected with butaprost (N+DMSO vs. N + Butaprost (1 µmol/L) and N + Butaprost (10 µmol/L): 60.41 ± 13.35 vs. 94.75 ± 28.9 and 102.6 ± 34.89 fmol/mg, respectively, at least P < 0.05, Fig. 3A, one-way ANOVA). Intragroup comparison showed significant cAMP increases (P < 0.05, paired sample t-test) in eyes injected with 1 µmol/L and 10 µmol/L butaprost (“T”) compared with their respective fellow (“F”) eyes during normal ocular growth (Fig. 3A) and FD (Fig. 3B). Similarly, FD increased cAMP levels relative to their fellow controls (FD + DMSO-T vs. F: 83.47 ± 17.24 vs. 49.01 ± 12.83 fmol/mg, P < 0.05, Fig. 3B). Intergroup comparison among the fellow eyes of the drug and DMSO-injected groups showed increased cAMP levels on 1 µmol/L of butaprost injection alone (FD + Butaprost-F [1 µmol/L] vs. FD + DMSO-F: 99.2 ± 15.84 vs. 49.01 ± 12.83 fmol/mg, P < 0.05, one-way ANOVA with Bonferroni correction, Fig. 3B). A possible yoking effect between the treated and fellow eyes of each treatment group, as reported earlier in guinea pigs,12,48 could account for such a finding. Consistent with the lack of significant refractive and biometric changes among groups 7, 8, and 11, butaprost injections during FD did not significantly alter the cAMP levels relative to vehicle injected eyes (P > 0.05 one-way ANOVA with Bonferroni's correction).
Figure 3.
Butaprost increases cAMP levels. Butaprost increased scleral cAMP relative to the fellow and vehicle-injected eyes during normal ocular growth (A). FD increased scleral cAMP relative to their fellow eyes with no significant differences amongst the treated (“T”) eyes (B). Mean cAMP data presented as femtomole/milligram of the tissue (± SD). #P < 0.05, paired sample t-test, *P < 0.05; **P < 0.01; one-way ANOVA with Bonferroni correction.
EP2 Antagonism Suppresses FD-Induced Scleral cAMP Elevations
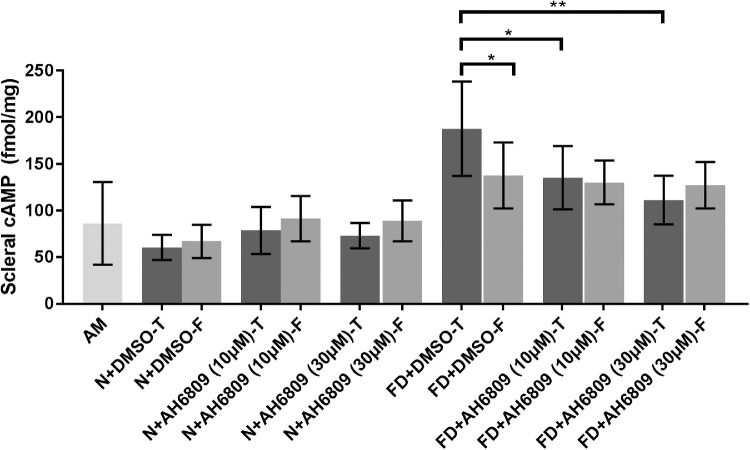
A separate set of experiments was carried out to determine the effect of AH6809 injections on scleral cAMP levels compared to that of vehicle, during FD. As reported above, scleral cAMP levels were significantly increased during myopia development relative to fellow controls (FD + DMSO-T vs. FD + DMSO-F: 187.8 ± 50.61 vs. 137.7 ± 35.37 fmol/mg, mean ± SD, P < 0.05, paired sample t-test, Fig. 4). In contrast, compared to the vehicle injected eyes (187.8 ± 50.61 fmol/mg), scleral cAMP levels were significantly decreased in response to 10 µmol/L (135.3 ± 34.01 fmol/mg, P < 0.05) and 30 µmol/L AH6809 (111.3 ± 26.06 fmol/mg, P < 0.01, one-way ANOVA with Bonferroni's correction, Fig. 4) during FD. On the other hand, intragroup comparison showed no significant changes in cAMP levels between the treated (“T”) and fellow (“F”) eyes of animals receiving either dose of AH6809 (P > 0.05, paired samples t-test, Fig. 4) during FD. A possible yoking effect in guinea pigs as reported earlier12,48 could account for increases in cAMP levels in the fellow eyes of drug/vehicle injected groups during FD relative to AM controls. However, an intergroup comparison showed no significant changes in cAMP levels across the fellow eyes of vehicle and drug-injected groups (P > 0.05, one-way ANOVA with Bonferroni's correction). Consistent with the lack of refractive and AL changes, cAMP levels in AH6809-injected eyes (“T”) did not significantly differ from those of the vehicle-injected ones (“T”) during normal ocular growth (Fig. 4). Similarly, no significant changes in cAMP levels were observed among their fellow eyes (N + DMSO-F vs. N+AH6809-F (10 µmol/L) and N + AH6809-F (30 µmol/L): P > 0.05, one-way ANOVA).
Figure 4.
AH6809 suppresses scleral cAMP increases during FD. Myopic scleral cAMP increases were inhibited by AH6809 injections during FD. AH6809 did not significantly affect cAMP levels during normal ocular growth. Mean cAMP data presented as femtomole/milligram of the tissue (± SD). *P < 0.05; **P < 0.01; one-way ANOVA with Bonferroni correction.
Differential Gene Expression Patterns Underlie Butaprost-Mediated Myopia Development
Scleral gene expression changes associated with butaprost-induced myopia development were evaluated. Given that no significant changes were observed in refraction, AL and cAMP levels between the butaprost and vehicle-injected eyes during FD, gene expression analysis was not carried out in groups 7 and 8.
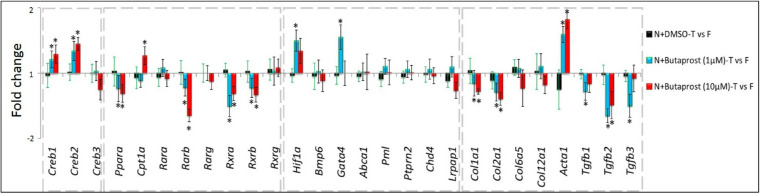
Butaprost increased the mRNA levels of cAMP Responsive Element Binding Protein, Creb1 and Creb2, in parallel with scleral cAMP elevations. On the other hand, Pparα expression was downregulated with 1 and 10µM of butaprost. Two subtypes of RXR receptors, Rxra and Rxrb, were also downregulated (Fig. 5). Among the retinoic acid receptors, butaprost injections only downregulated Rarb. Overall, such declines may hinder formation of a PPARα:RXR complex that is critical to gene transcription. Also, butaprost increased the Hif1α expression levels. Out of the hypoxic signaling pathway genes that earlier showed significant interactions with human myopia genes,30 butaprost (1 µmol/L) only upregulated Gata4 (Fig. 5). Among the scleral ECM-related genes, only Acta1, which encodes the smooth muscle protein, α-smooth muscle actin (α-SMA), was upregulated. On the other hand, butaprost (1 µmol/L) downregulated collagen subtypes (Col1a1 and Col2a1) and TGF-β isoforms (TGF-β1, -β2, -β3). Although collagens were similarly downregulated, only TGF-β2 mRNA was reduced significantly with 10 µmol/L butaprost (Fig. 5). In a normal visual environment, the expression levels of all target genes were unaltered between the DMSO-injected and fellow control eyes.
Figure 5.
Differential gene expression patterns induced by butaprost during normal ocular growth. Significant gene expression changes, presented as fold changes, were observed in cAMP-related (A), PPAR/RXR-related (B), hypoxia-related (C), and ECM-related (D) genes in response to butaprost injections. Gene expression data of “N+DMSO” group are provided for visual comparison of the effect of vehicle and drug injections, with the other experimental conditions being the same (*P < 0.05, paired sample t-test).
AH6809-Induced Myopia Inhibition Accompanies Suppressed Gene Expression Changes
FD and FD + DMSO groups did not show any significant differences in refraction, AL and cAMP levels between them suggesting a lack of vehicle effect. Hence, the effect of AH6809 injections on gene expression was compared with animals that received vehicle injections in the same form-deprived environment (i.e., Group 11).
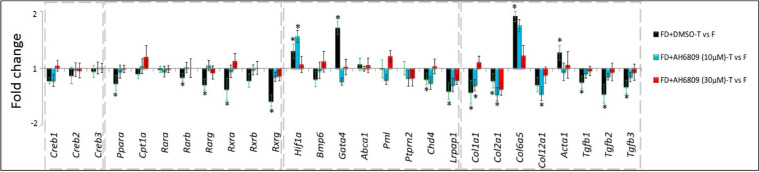
FD-induced (FD + DMSO) Pparα downregulations were suppressed with AH6809 injections (Fig. 6). Myopic downregulations of Rarb and RXR receptor subtypes (Rxra, Rxrb and Rxrg) also returned to baseline on myopia inhibition with the antagonist (Fig. 6). AH6809 (30 µmol/L) also suppressed the increases in Hif1α mRNA levels. Although Acta1 upregulation and TGF-β isoform downregulations in group 11 were inhibited by both doses of AH6809, only 30 µmol/L of the drug reduced Col1a1, Col2a1 and Col12a1 downregulations (Fig. 6). Overall, during FD, AH6809 negated significant myopic-gene expression changes. Such reversals are consistent with the inhibitory effects of AH6809 on both myopia development and cAMP increases. Given that this EP2 antagonist did not induce any significant changes in a normal visual environment, gene expression analyses were not carried out in groups 3 and 4.
Figure 6.
Differential gene expression patterns during AH6809-mediated myopia inhibition. Gene expression changes, presented as fold changes, in cAMP-PKA-related (A), PPAR/t RXR-related (B), hypoxia-related genes (C), and ECM-related (D) genes during myopia (FD+DMSO) were inhibited AH6809 injections during FD. *P < 0.05, paired sample t-test.
Discussion
A balance between ECM synthesis and degradation is critical in maintaining the structure and integrity of the sclera. During myopia development, this balance is disrupted by increased degradation and reduced ECM synthesis in mammalian models. We here showed that the activation of prostaglandin receptor subtype, EP2 induces myopic scleral ECM remodeling through increases in scleral cAMP levels and suppression of PPARα/RXR signaling. Such an EP2-mediated crosstalk mechanism stimulates the HIF-1α signaling pathway that promotes myopia development in guinea pigs.
Activation of EP2 led to scleral cAMP increases and myopia development in an unobstructed visual environment. Although FD-mediated myopia development also led to increased cAMP levels, such myopic shifts and cAMP increases were suppressed with an EP2 antagonist in a form-deprived environment. These results are consistent with our earlier study that showed increased cAMP levels to be associated with a myopic refraction, whereas declines in cAMP levels suppressed the progression of induced myopia in guinea pigs.10 Our findings suggest that the EP2 agonist can only increase baseline cAMP levels provided they are not pre-elevated by FD. On the other hand, the EP2 antagonist only reduced the FD-induced cAMP elevations to levels that were not lower than those seen in AM controls. Such a phenomenon is in agreement with “ceiling” and “floor’ effects of cyclic guanosine monophosphate (cGMP) levels observed in the sclera of guinea pigs during myopia.49 In the myopic model, we earlier showed that retinal prostaglandin F2α receptor (FP) expression levels declined during FD in guinea pigs. Treatment with a FP antagonist resulted in a myopic refraction, whereas one of its agonist inhibited FD-induced myopia development.47 However, we here found that butaprost induced a myopic shift whereas AH6809 inhibited the progression of FD-induced myopia. This could be because of the diverse roles of prostaglandins that are mediated by their receptors (e.g., EP and FP) that perform specific functions. Given that the sclera is mainly composed of collagen as opposed to retina that is multilayered with different cell types, the opposing effects of EP and FP receptors towards myopia development is not surprising. This is in agreement with tissue/cell-specific response of prostaglandins receptors reported earlier.19,20
To gain additional insights into EP2 mechanisms, some of the scleral gene expression changes associated with EP2 receptor agonism and antagonism were characterized. Butaprost injections upregulated Creb1 and Creb2, which is consistent with increased cAMP levels; however, FD did not alter their gene expression levels. This could be due to the mechanism of the action of butaprost that impacts directly on cAMP levels, whereas FD depends on visual cues, such as blur to induce myopia. This highlights possible differences between the type of myopic stimulus and the mechanisms underlying myopia development, similar to the diversities reported earlier between negative lens and diffuser-induced myopia.50,51 Butaprost upregulated Hif1α during FD, which is consistent with our earlier data that showed hypoxia to be a key regulator of myopia development in mice and guinea pig models.30 Also, hypoxia has been shown to increase cAMP levels in carcinoma cell lines33 and activate CREB in mice lungs52 and HeLa cell lines.53 Our current data showing upregulation of scleral Hif1α and increased cAMP levels suggest that hypoxia promotes myopia development through increased cAMP levels. Upregulation of the transcription factor, Pparα inhibits fibrosis in various tissues.27 However, we here found that downregulation of Pparα by butaprost instead promoted anti-fibrotic ECM changes. Although it is well established that the sclerae of humans and animal models are characterized by antifibrotic events during myopia, downregulation of scleral Pparα is consistent with an earlier study wherein PPARα antagonism induced myopia development in guinea pigs.29 Formation of the PPARα:RXR complex is critical in mediating the expression of downstream genes. However, we showed here that butaprost injections reduced Rxra and Rxrb mRNA levels as well. Studies in cardiac myocytes have shown activation of Hif1α also downregulated Rxrα that led to inhibited binding of PPARα/RXR complex and reduced Pparα expression.31,54 Furthermore, activation of Pparα inhibited Hif1α expression in cardiac myocytes.32
These data provide a possible mechanism by which PPARα activity could be inhibited during myopia development. Namely, declines in RXR mRNA levels suppresses PPARα:RXR binding in TGF hypoxic scleral tissue. Furthermore, downregulation of collagen (Col1a1 and Col2a1) and TGF-β subtypes (β1, β2 and β3) and upregulation of the myofibroblast marker, Acta1, during EP2 activation and FD-mediated myopia development reiterates the degenerative changes to the scleral ECM during myopia. The changes in cAMP levels and the differential regulation of Pparα, Rxra, Rxrb, and ECM-related genes were not significantly different from that of the fellow eyes on myopia inhibition with AH6809 injections during FD. Surprisingly, lower dose (10 µmol/L) of this EP2 antagonist during myopia induction increased Hif1α expression, whereas the highest dose suppressed it completely along with inhibited axial elongation. Further research into linked pathways, such as the Wnt signaling channel that is implicated in ocular growth55,56 and hypoxia,57,58 may be required to understand the mechanisms of Hif1α activation in the context of myopia development and axial elongation, given that only the highest dose of AH6809 suppressed Hif1α expression and ocular elongation.
The sclera is dynamic in nature, which maintains the ocular shape during ocular growth and myopia development. Scleral ECM undergoes rapid changes such as a significant decrease in its glycosaminoglycan content within a day after FD in tree shrews.59 Also microarray analysis revealed that four days of FD or AC activation was adequate to alter the scleral gene expression patterns in guinea pigs.12 Furthermore, 24 hours of FD increased the expression of α-SMA, a highly contractile protein in the sclera of tree shrews without affecting the ocular growth,60 suggesting that scleral molecular changes could act as a predecessor for scleral structural changes. Such rapid scleral ECM changes ultimately lead to ocular elongation as hypothesized in the retinoscleral signaling cascade.8 Our findings also implicate the presence of a dose-dependent response in the expression patterns of Creb1, Creb2, Rxra, Hif1α, Col1a1, Col2a1, Acta1 and TGF-β2 upon butaprost injection in normal eyes, without such a trend in ocular growth or refractive error development. Overall these findings reiterate that scleral ECM genes are capable of rapid remodeling that could precede excessive ocular elongation.
Although AH6809 interacts with the EP1 receptor,16 this drug also inhibits PGE2-mediated cAMP increases in various tissues. Therefore such cAMP declines are attributable to AH6809 acting as an EP2 antagonist, because EP2 activation by butaprost increases cAMP levels.38,61,62 Similar inhibition of cAMP increases in the sclera of guinea pigs reiterates the affinity of AH6809 towards EP2 receptor. It is tenable that the absence of data on refraction, gene expression and cAMP levels during recovery from myopia could be a limitation of this study. However, we did find that both doses of AH6809 injections during FD suppressed myopia progression, whereas only the highest dose (30 µmol/L) inhibited myopic axial length elongations that also negated FD-mediated gene expression changes. Longer periods of FD with AH6809 injections could potentially lead to significant VCD inhibition and AL suppression even with 10 µmol/L of AH6809. Yang et al.47 showed that even though prostaglandin F receptor (FP) agonist inhibited myopia development during FD in guinea pigs, significant suppression of axial length was evident only at four weeks, but not at the two-week timepoint. Inhibited VCD growth was also more pronounced and significant after four weeks of treatment.47 It must be noted that the guinea pigs used in our study were three weeks old compared with the two-week-old ones used by Yang et al.47 above. The age of the guinea pigs at the start of treatment could also be detrimental in causing a bidirectional change in ocular growth pattern, with this EP2 antagonist, thus demanding longer treatment durations in future. Further investigations into the involvement of other PGE receptor subtypes, for example, EP3 that inhibits cAMP levels, will broaden our understanding of the overall contribution of prostaglandins and their cognate receptor subtypes in controlling myopia development.
We conclude that EP2-mediated scleral cAMP modulation affects myopia development through a crosstalk between PPARα and HIF-1α signaling in guinea pigs. Such EP2 activations are mediated by increases in cAMP levels that inhibit PPARα/RXR activity and increase HIF-1α levels, which ultimately lead to myopia. However, inhibition of EP2 negates these changes and retards myopic ocular growth. Our study successfully identified the presence and significance of such an interaction in the sclera, which could be critical for myopia control.
Supplementary Material
Acknowledgments
Supported by the National Natural Science Foundation of China (NSFC) grants 81830027, 81670876, 81670886 and 81700868.
Disclosure: N. Srinivasalu, None; S. Zhang, None; R. Xu, None; P.S. Reinach, None; Y. Su, None; Y. Zhu, None; J. Qu, None; X. Zhou, None
References
- 1. Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012; 32: 3–16. [DOI] [PubMed] [Google Scholar]
- 2. Chen M, Wu A, Zhang L, et al.. The increasing prevalence of myopia and high myopia among high school students in Fenghua city, eastern China: a 15-year population-based survey. BMC Ophthalmol. 2018; 18: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morgan IG, He M, Rose KA. EPIDEMIC OF PATHOLOGIC MYOPIA: What Can Laboratory Studies and Epidemiology Tell Us? Retina. 2017; 37: 989–997. [DOI] [PubMed] [Google Scholar]
- 4. Holden BA, Fricke TR, Wilson DA, et al.. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 5. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012; 379: 1739–1748. [DOI] [PubMed] [Google Scholar]
- 6. Tedja MS, Haarman AEG, Meester-Smoor MA, et al.. IMI—myopia genetics report. Invest Ophthalmol Vis Sci. 2019; 60: M89–m105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Troilo D, Smith EL 3rd, Nickla DL, et al.. IMI—report on experimental models of emmetropization and myopia. Invest Ophthalmol Vis Sci. 2019; 60: M31–m88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009; 86: E23–E30. [DOI] [PubMed] [Google Scholar]
- 9. Metlapally R, Wildsoet CF. Scleral Mechanisms Underlying Ocular Growth and Myopia. Prog Mol Biol Transl Sci. 2015; 134: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tao Y, Pan M, Liu S, et al.. cAMP level modulates scleral collagen remodeling, a critical step in the development of myopia. PLoS One. 2013; 8: e71441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006; 362: 623–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srinivasalu N, Lu C, Pan M, et al.. Role of cyclic adenosine monophosphate in myopic scleral remodeling in guinea pigs: a microarray analysis. Invest Ophthalmol Vis Sci. 2018; 59: 4318–4325. [DOI] [PubMed] [Google Scholar]
- 13. Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989; 259: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001; 294: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 15. Serhan CN, Levy B.. Success of prostaglandin E2 in structure-function is a challenge for structure-based therapeutics. Proc Natl Acad Sci U S A. 2003; 100: 8609–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994; 46: 205–229. [PubMed] [Google Scholar]
- 17. Breyer MD, Zhang Y, Guan YF, Hao CM, Hebert RL, Breyer RM. Regulation of renal function by prostaglandin E receptors. Kidney Int Suppl. 1998; 67: S88–94. [DOI] [PubMed] [Google Scholar]
- 18. Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999; 79: 1193–1226. [DOI] [PubMed] [Google Scholar]
- 19. Watanabe T, Satoh H, Togoh M, Taniguchi S, Hashimoto Y, Kurokawa K. Positive and negative regulation of cell proliferation through prostaglandin receptors in NIH-3T3 cells. J Cell Physiol. 1996; 169: 401–409. [DOI] [PubMed] [Google Scholar]
- 20. Sanchez T, Moreno JJ.. Role of EP(1) and EP(4) PGE(2) subtype receptors in serum-induced 3T6 fibroblast cycle progression and proliferation. Am J Physiol Cell Physiol. 2002; 282: C280–288. [DOI] [PubMed] [Google Scholar]
- 21. Srivastava T, McCarthy ET, Sharma R, et al.. Fluid flow shear stress upregulates prostanoid receptor EP2 but not EP4 in murine podocytes. Prostaglandins Other Lipid Mediat. 2013; 104–105: 49–57. [DOI] [PubMed] [Google Scholar]
- 22. Nilsson SF, Drecoll E, Lütjen-Drecoll E, et al.. The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 2006; 47: 4042–4049. [DOI] [PubMed] [Google Scholar]
- 23. Marcus MW, de Vries MM, Montolio FGJ, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011; 118: 1989–1994.e1982. [DOI] [PubMed] [Google Scholar]
- 24. Lazennec G, Canaple L, Saugy D, Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol Endocrinol. 2000; 14: 1962–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DiRenzo J, Söderstrom M, Kurokawa R, et al.. Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors. Mol Cell Biol. 1997; 17: 2166–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ijpenberg A, Tan NS, Gelman L, et al.. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 2004; 23: 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McVicker BL, Bennett RG. Novel anti-fibrotic therapies. Front Pharmacol. 2017; 8: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertrand E, Fritsch C, Diether S, et al.. Identification of apolipoprotein AI as a “STOP” signal for myopia. Mol Cell Proteomics. 2006; 5: 2158–2166. [DOI] [PubMed] [Google Scholar]
- 29. Pan M, Jiao S, Reinach PS, et al.. Opposing effects of PPARα agonism and antagonism on refractive development and form deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci. 2018; 59: 5803–5815. [DOI] [PubMed] [Google Scholar]
- 30. Wu H, Chen W, Zhao F, et al.. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci. 2018;201721443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huss JM, Levy FH, Kelly DP. Hypoxia inhibits the peroxisome proliferator-activated receptor alpha/retinoid X receptor gene regulatory pathway in cardiac myocytes: a mechanism for O2-dependent modulation of mitochondrial fatty acid oxidation. J Biol Chem. 2001; 276: 27605–27612. [DOI] [PubMed] [Google Scholar]
- 32. Zhou J, Zhang S, Xue J, et al.. Activation of peroxisome proliferator-activated receptor alpha (PPARalpha) suppresses hypoxia-inducible factor-1alpha (HIF-1alpha) signaling in cancer cells. J Biol Chem. 2012; 287: 35161–35169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simko V, Iuliano F, Sevcikova A, et al.. Hypoxia induces cancer-associated cAMP/PKA signalling through HIF-mediated transcriptional control of adenylyl cyclases VI and VII. Sci Rep. 2017; 7: 10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. B Toris C. Pharmacotherapies for glaucoma. Curr Mol Med. 2010; 10: 824–840. [DOI] [PubMed] [Google Scholar]
- 35. Ganesh T. Prostanoid receptor EP2 as a therapeutic target: Miniperspective. J Med Chem. 2014; 57: 4454–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woodward DF, Pepperl DJ, Burkey TH, Regan JW. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2 receptor antagonist. Biochem Pharmacol. 1995; 50: 1731–1733. [DOI] [PubMed] [Google Scholar]
- 37. Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007; 292: L405–413. [DOI] [PubMed] [Google Scholar]
- 38. Li P, Jiang H, Wu H, et al.. AH6809 decreases production of inflammatory mediators by PGE2 - EP2 - cAMP signaling pathway in an experimentally induced pure cerebral concussion in rats. Brain Res. 2018; 1698: 11–28. [DOI] [PubMed] [Google Scholar]
- 39. Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. The Journal of Immunology. 2004; 173: 559–565. [DOI] [PubMed] [Google Scholar]
- 40. Jiang L, Schaeffel F, Zhou X, et al.. Spontaneous axial myopia and emmetropization in a strain of wild-type guinea pig (Cavia porcellus). Invest Ophthalmol Vis Sci. 2009; 50: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 41. Untergasser A, Cutcutache I, Koressaar T, et al.. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012; 40: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 43. Schmittgen TD, Livak KJ.. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 44. Guo L, Frost MR, He L, Siegwart JT Jr., Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013; 54: 6806–6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Szklarczyk D, Franceschini A, Wyder S, et al.. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015; 43: D447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu F, Zhou X, Zhao H, et al.. Axial myopia induced by a monocularly-deprived facemask in guinea pigs: a non-invasive and effective model. Exp Eye Res. 2006; 82: 628–636. [DOI] [PubMed] [Google Scholar]
- 47. Yang J, Pan M, Reinach PS, et al.. Prostaglandin F2α Receptor Modulation Affects Eye Development in Guinea Pigs. Basic Clin Pharmacol Toxicol. 2018; 123: 263–270. [DOI] [PubMed] [Google Scholar]
- 48. Howlett MH, McFadden SA.. Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Res. 2006; 46: 267–283. [DOI] [PubMed] [Google Scholar]
- 49. Fang F, Pan M, Yan T, et al.. The role of cGMP in ocular growth and the development of form-deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci. 2013; 54: 7887–7902. [DOI] [PubMed] [Google Scholar]
- 50. Dong F, Zhi Z, Pan M, et al.. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011; 17: 2824–2834. [PMC free article] [PubMed] [Google Scholar]
- 51. Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011; 93: 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leonard MO, Howell K, Madden SF, et al.. Hypoxia selectively activates the CREB family of transcription factors in the in vivo lung. Am J Respir Crit Care Med. 2008; 178: 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakayama K. cAMP-response element-binding protein (CREB) and NF-kappaB transcription factors are activated during prolonged hypoxia and cooperatively regulate the induction of matrix metalloproteinase MMP1. J Biol Chem. 2013; 288: 22584–22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Belanger AJ, Luo Z, Vincent KA, et al.. Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor alpha/retinoid X receptor. Biochem Biophys Res Commun. 2007; 364: 567–572. [DOI] [PubMed] [Google Scholar]
- 55. Ma M, Zhang Z, Du E, et al.. Wnt signaling in form deprivation myopia of the mice retina. PLoS One. 2014; 9: e91086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fuhrmann S. Wnt signaling in eye organogenesis. Organogenesis. 2008; 4: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Genetos DC, Toupadakis CA, Raheja LF, et al.. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J Cell Biochem. 2010; 110: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hong C-F, Chen W-Y, Wu C-W. Upregulation of Wnt signaling under hypoxia promotes lung cancer progression. Oncol Rep. 2017; 38: 1706–1714. [DOI] [PubMed] [Google Scholar]
- 59. Moring AG, Baker JR, Norton TT. Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Invest Ophthalmol Vis Sci. 2007; 48: 2947–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jobling AI, Gentle A, Metlapally R, McGowan BJ, McBrien NA. Regulation of scleral cell contraction by transforming growth factor-β and stress: competing roles in myopic eye growth. J Biol Chem. 2009; 284: 2072–2079. [DOI] [PubMed] [Google Scholar]
- 61. Biddulph DM, Capehart AA, Beasley TC. Comparative effects of cytosine arabinoside and a prostaglandin E2 antagonist, AH6809, on chondrogenesis in serum-free cultures of chick limb mesenchyme. Exp Cell Res. 1991; 196: 131–133. [DOI] [PubMed] [Google Scholar]
- 62. Casibang M, Moody TW. AH6809 antagonizes non-small cell lung cancer prostaglandin receptors. Lung Cancer. 2002; 36: 33–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.