Abstract
Purpose
To explore the influence of indoleamine 2,3-dioxygenase (IDO) on macrophage recruitment, polarization and phagocytosis in Aspergillus fumigatus keratitis.
Methods
A murine model of A. fumigatus keratitis and peritoneal macrophages incubated with the hyphae of A. fumigatus were used. Macrophage recruitment in corneas was evaluated using immunofluorescence staining. The polarization of macrophages, which was stimulated by A. fumigatus and pretreatment with or without 1-methyltryptophan (1-MT), interferon gamma (IFNG), extracellular regulated protein kinases (ERK) antagonist, and p38 antagonist, was determined using reverse-transcription polymerase chain reaction and flow cytometry. P38 and ERK levels were determined using Western blotting. Macrophage phagocytosis was examined using colony-forming units.
Results
Compared with the A.F. group, recruitment of macrophages increased, tumor necrosis factor–α (TNF-α) and inducible nitric oxide synthase (iNOS) expression decreased, whereas arginase-1 (Arg-1) and interleukin-10 (IL-10) expression increased in the mouse corneas of the 1-MT+A.F. group. The ratio of CD206+/CD86+ macrophages in the corneas and spleens of 1-MT+A.F. group increased. Furthermore, in peritoneal macrophages stimulated by A. fumigatus, 1-MT promoted Arg-1 and IL-10 expression while upregulating the ratio of CD206+/CD86+ macrophages. Conversely, IDO agonist IFNG promoted TNF-α and iNOS expression, inhibited Arg-1 and IL-10 expression and downregulated the ratio of CD206+/CD86+ macrophages. The role of IFNG was reversed by the antagonist of P38 or ERK. P38 and ERK levels were downregulated in corneas of 1-MT+A.F. group. Besides, IFNG inhibited macrophage phagocytosis.
Conclusions
IDO inhibited macrophage recruitment and phagocytosis in A. fumigatus keratitis. Mechanistically, IDO is involved in M1 macrophage polarization in A. fumigatus keratitis through a MAPK/ERK-dependent pathway.
Keywords: indoleamine 2,3-dioxygenase; keratitis, Aspergillus fumigatus; macrophage; polarization; phagocytosis; recruitment
Fungal keratitis (FK) is an infectious and sight-threatening disease that may result in vision loss.1 It may affect those who suffer corneal trauma, chronic ocular surface disease, prior corneal surgery, topical corticosteroids, or through contact lens wear.2 Aspergillus is a common pathogenic fungus that can cause fungal keratitis.3 Inflammatory responses, such as vasodilation, exudation of active immune cells and secretion of immunoreactive substances, occur during the process of Aspergillus fumigatus keratitis. During these inflammatory responses, macrophages act as an immunomodulatory agent and play a major role in the phagocytosis of fungal spores and hyphae, as well as antigen cross-presentation.4 In addition, macrophages can modulate their phenotype to respond to the local microenvironment. Two functionally distinct subsets of macrophages have been recognized: proinflammatory (M1) and anti-inflammatory (M2) macrophages. They respond to different immune challenges and are essential for the pathogenesis of fungal keratitis.5,6
Indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme in the kynurenine pathway of tryptophan degradation, performs an important role in actuating protective tolerance against A. fumigatus.7–9 Our previous studies have shown that IDO acts as an immunomodulator and is involved in the inflammatory response related to A. fumigatus keratitis, while playing an important part in actuating the protective tolerance against A. fumigatus.10 IDO has been found to be upregulated in infected tissues, including corneal, lung, small intestine and placenta tissues, and can be expressed by professional antigen-presenting cells (APCs), for example, macrophages and dendritic cells (DCs) in the immune system, as well as epithelial cells and vascular endothelial cells.11 In addition, it has been reported that the inhibition of IDO mediated by 1-methyltryptophan (1-MT) may reduce the inflammatory response of macrophages in mice with chronic viral myocarditis.12 Overall, these findings have suggested that IDO acts as an immune modulator and may also be involved in the inflammatory reaction in macrophages resistance against A. fumigatus in corneal infections. However, the importance of IDO for the inflammatory reaction of macrophages infected by A. fumigatus has not yet been clarified. The elucidation of this effect will allow for the mechanism by which IDO participates in fungal inflammation to be more holonomic, and IDO may be a new effective therapeutic target for fungal keratitis.
Materials and Methods
Animal Models and IDO Antagonist Treatment
All treatments on animals were administered in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research guidelines. C57BL/6 mice (8-week-old females) were purchased from Jinan Peng yue Laboratory Animal Co. Ltd (Jinan, China). The models of A. fumigatus keratitis in C57BL/6 mice were established based on the methods developed by Jiang et al.13 Only the right cornea of each animal was treated, whereas the other cornea was harvested and used as the control. As described by Mo et al.,14 oral 1-MT (Sigma, St. Louis, MO, USA) suspended in water (1 mg/mL) was administered by gavage to exert an inhibitory effect on IDO during A. fumigatus infection. From the date of infection until death, mice in the 1-MT group drank a quantitative filter-sterilized 1-MT suspension every day, whereas mice in the control group received the same volume of sterilized phosphate-buffered saline solution (PBS). Thereafter, the corneas of healthy C57BL/6 mice were randomly divided into four groups: control group (treated with corneal epithelial scrape only), the 1-MT group (pretreated with 1-MT intragastric administration and treated with corneal epithelial scrape only), the A. fumigatus (A.F.) group (established the animal model of A. fumigatus keratitis), and the 1-MT + A.F. group (pretreated with 1-MT before establishing the animal model of A. fumigatus keratitis). The corneas were monitored under a slit lamp microscope with a digital camera, and the disease score of the cornea of each mouse after infection (1, 3 and 5 days) was recorded. The corneal clinical score was evaluated based on a 12-point scoring system.15
Acquisition of Mouse Peritoneal Primary Macrophages
Thioglycolate powder (3 g) was liquefied in 100 ml of ddH2O, and the solution was heated and stirred in a water bath until the powder had completely dissolved. The thioglycolate medium was autoclaved using high-temperature steam at 121°C for 15 minutes and then injected intraperitoneally with 1 mL of solution into each C57BL/6 mouse. On the seventh day after injection, the mice were sacrificed. Then the skin of the abdominal wall of the mice was cut while keeping the peritoneum intact. Then, 5 mL of RPMI-1640 medium was injected into the abdominal cavity of the mice using syringes. By gently massaging the abdomen for two minutes and extracting the culture medium, primary macrophages were obtained. To extract more macrophages, peritoneal lavage was repeated once more.
Culture of Macrophages and A. fumigatus Stimulation
A certain amount of 1-MT powder was dissolved in 1 mmol/L NaOH, and then adjusted to a PH 7.4 by adding 5 mmol/L HCl. The solution was protected from light and stored at 4°C for filtration and sterilization. Macrophages were cultured following previously described methods.16,17 The macrophages were pretreated with an IDO antagonist (1-MT, 1 mmol/L), IDO agonist (interferon gamma [IFNG], 400 U/mL; R&D Systems, Minneapolis, MN, USA), p38 antagonist (SB203580, 10 µmol/L; MCE, Monmouth Junction, NJ, USA) and extracellular regulated protein kinases (ERK) antagonist (U0126, 20 µmol/L, MCE), respectively, for two hours, then stimulated by inactivated A. fumigatus hypha (5 × 106 colony-forming units [CFU]/mL) for a specified duration (zero, four, eight, and 12 hours).
RT-PCR
The corneal tissue and mouse peritoneal primary macrophages were obtained and stored at −80°C. The total RNA of the isolated cells and tissues were extracted using RNAiso plus (TaKaRa, Dalian, China) and quantified using spectrophotometry. The first-strand cDNA was synthesized through the reverse transcription of 2 µg of total RNA. The product of cDNA was diluted with diethylpyrocarbonate-treated water. The reverse-transcription polymerase chain reaction (RT-PCR) Master Mix (Takara, Dalian, China) along with SYBR Green and specific primers were used to perform the RT-PCR analysis. The cycle parameters of the reactions were as follows: 95°C for 30 seconds, then 40 cycles of 95°C for 5 seconds, followed by 60°C for 30 seconds, with the final steps at 95°C for 15 seconds, 60°C for 30 seconds, and 95°C for 15 seconds. The quantitative expression of β-actin was used as the internal control. The primers used are given in Table.
Table.
Nucleotide Sequences of Mouse Primers Using For RT-PCR Analysis
| Gene | GenBank No. | Primer Sequence (5′-3′) |
|---|---|---|
| mβ-actin | NM_007393.5 | F: GAT TAC TGC TCT GGC TCC TAG C R: GAC TCA TCG TAC TCC TGC TTG C |
| mIDO1 | NM_008324.2 | F: TCC TGG CAA ACT GGA AGA AA R: CAC CAA TAG AGA GAC GAG GAA GAA G |
| mTNF-α | NM_013693.2 | F: ACC CTC ACA CTC AGA TCA TCT T R: GGT TGT CTT TGA GAT CCA TGC |
| miNOS | NM_010927.3 | F: TGT CTG CAG CAC TTG GAT CAG R: AAA CTT CGG AAG GGA GCA ATG |
| mArg-1 | NM_007482.3 | F: TGG GTG ACT CCC TGC ATA TCT R: TTC CAT CAC CTT GCC AAT CC |
| mIL-10 | NM_010548.2 | F: TGC TAA CCG ACT CCT TAA TGC AGG AC R: CCT TGA TTT CTG GGC CAT GCT TCT C |
Western Blotting Analysis
Corneal proteins were extracted using a RIPA lysis buffer (Solarbio, Beijing, China) plus 1 mmol/L PMSF (Solarbio) and a 1 mmol/L phosphatase inhibitor (MCE) at 4°C for 120 minutes. The lysate was repeatedly shaken a few times every 15 minutes, then spun in a centrifuge at 4°C at 12,000 rpm for five minutes, followed by determination of the concentration of the supernatant protein using BCA protein analysis reagent (Solarbio). The supernatant protein was denatured using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer at 109.8°C for 10 minutes. The protein (60 µg/well) was separated using 12% SDS-PAGE in a Tris/glycine/SDS buffer and electroblotted onto PVDF membranes (Millipore, Billerica, MA, USA). After blocking with a blocking buffer (Solarbio) at room temperature for 1.5 hours, the membranes were incubated with a beta Tubulin Polyclonal Antibody (1:2000; Elabscience, Wuhan, China), Phospho-p38 MAPK antibody, p38 MAPK antibody, Phospho-ERK antibody and ERK antibody (1:1000; CST, Danvers, MA, USA) at 4°C overnight, followed by incubation with an anti-rabbit secondary antibody (1:2000; Elabscience, Wuhan, China) for one hour. The blots were detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore).
Immunofluorescence Staining
The eyeballs of the C57BL/6 mice were removed and placed in an optimum cutting temperature (OCT) compound (Sakura Tissue-Tek, Torrance, CA, USA) and frozen using liquid nitrogen. The eyeball specimens were cut into 10-mm slices. After being dried at 37°C for six hours, the sections were finished using acetone for five minutes. After the sections were cleaned using PBS, a sealing solution (PBS containing 10% donkey serum) was used for 30 minutes at room temperature to seal the sections. After the blocking solution was removed, the slides were incubated overnight with anti-F4-80 antibody (1:600; Abcam, Cambridge, UK) at 4°C and afterward incubated with an Alexa Fluor 647-conjugated donkey anti-rat secondary antibody (1:500; Abcam). Then, an antifade reagent (Solarbio) was applied to the slides. Finally, digital images were captured using a Zeiss Axiovert microscope at magnification × 400. This experiment was repeated three times to count the number of macrophages recruited to the cornea in each group and compare the differences between the groups.
Flow Cytometry
Mouse eyeballs were collected and stored in Hanks’ solution. The corneas were dissected and cut into pieces along the corneal limbus using microscissors. Each corneal tissue was placed in 100 µL of liberase (0.04 mg/mL; Roche, Basel, Switzerland) and heated in a water bath at 37°C for 60 minutes, with a gentle shock exerted every 15 minutes. Then, the undigested tissues were removed using cell-strainer filters. Macrophages cultured in vitro and molar mouse spleen cells were also filtered using a filter. The cell suspension was finally collected in Mouse Fc Block (BD Pharmingen, Franklin Lake, NJ, USA) for 15 minutes at 4°C, and then incubated with fluorescein-conjugated anti-mouse antibodies for 20 minutes at 4°C. Flow cytometry was performed using a flow cytometer (Beckman Coulter, Krefeld, Germany) and analyzed using FlowJo software. The gate was set on the CD45+ population. The main antibodies used were CD 45-PERCP, F4/80-PE-cy7, CD206-FITC and CD 86-PE (Biolegend, San Diego, CA, USA). All experiments were performed in triplicate.
Phagocytosis
The phagocytosis of the macrophages was evaluated using previously described procedures.18,19 A total of 100 µL of macrophages (1 × 106 cells/mL), 100 µL of conidia of Aspergillus spp. (1 × 106 CFU/mL), and 5% fetal bovine serum were suspended and incubated together at 37°C for rotation (4 rev./min). At different time points (zero minutes and 120 minutes), 50 µL of the suspension was taken out and added to 150 µL of cold Hank's to suspend phagocytosis. The sample was spun in a centrifuge at a rotating speed of 110g for four minutes, and the ectocytic conidia were left in the liquid supernatant and made into a diluent. The diluent 100 µL was tiled on glucose agar medium and incubated at 37°C for 36 hours, and the number of clonal formation units (ClFU) were counted. Phagocytosis was represented by a reduction in the percentage of ectocytic conidia at a given time point relative to the initial number of ectocytic conidia: phagocytic index P(t) = (1-Nt/N0) × 100%; where N0 and Nt are the number of living ectocytic conidia at times t = 0 and t = t, respectively. The experiment was repeated twice using three samples from each group. We calculated P(120min) (the phagocytic index at 120 minutes) to detect the intensity of macrophage phagocytosis in each group.
Statistical Analysis
To compare the differences between two groups, significance was determined using a t-test (GraphPad Prism). The data are presented as the mean ± SEM, and a P value < 0.05 was considered to indicate a significant difference.
Results
Effects of IDO on the Inflammatory Response of A. fumigatus Keratitis
To explore the influence of IDO inhibition on corneal infection in the mouse corneas inoculated with A. fumigatus, we first infected the corneas with A. fumigatus. Previous research studies have reported that the protein expression of IDO and the clinical score of the corneas were the highest on the third day after infection.13 Therefore the corneas were harvested on the third day after infection for further study.
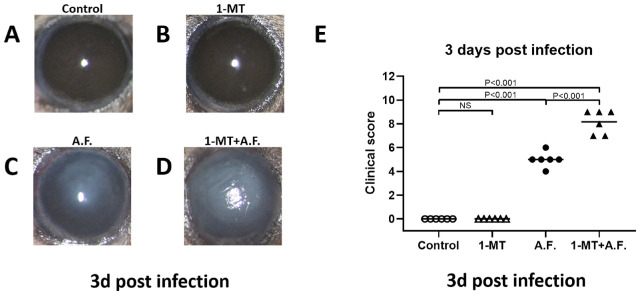
The corneas of healthy C57BL/6 mice were randomly divided into four groups, as described in the methods section. The effects of IDO on inflammatory responses were demonstrated using photographs of the anterior segment of the eyes (Figs. 1A–1D) and the clinical score (Fig. 1E). The results showed that the severity of A. fumigatus keratitis in the A.F. group was obviously higher than that of control group, and the severity of keratitis in the 1-MT+A.F. group increased, in comparison with that of the A.F. group. The clinical score of the 1-MT+A.F. group was obviously higher than that of the A.F. group (P < 0.01). Additionally, there was no obvious inflammation in the corneas of the 1-MT group, and the difference was not statistically significant, compared with the control group.
Figure 1.
Effects of IDO on the inflammatory response of A. fumigatus keratitis. The corneas of C57BL/6 mice were inoculated with A. fumigatus. The corneas were monitored, photographed under a slit lamp microscope (A–D), and evaluated using the scoring system (E). On the third day after A. fumigatus infection, the severity of corneal disease in the 1-MT+A.F. group (D) was found to be worse than that in the A.F. group (C), although there was no obvious inflammation in the corneas of the 1-MT group (B), compared with the control group (A). The clinical score of the 1-MT+A.F. group was higher than that of A.F. group (P < 0.01), and there was no significant difference between the 1-MT group and the control group (E).
IDO Inhibited the Macrophage Recruitment in A. fumigatus Keratitis
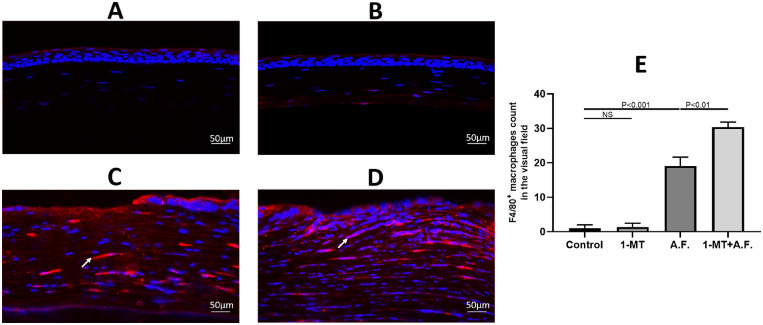
To confirm the effects of IDO on macrophage recruitment, the four aforementioned groups of mouse eyeballs underwent immunofluorescence staining. The quantity and localization of the macrophages were detected in mouse corneal tissues using an immunostaining F4/80 antibody. As shown in Figure 2, few macrophages were observed in the uninfected corneas. On the third day after A. fumigatus infection, the recruitment of macrophages in the corneal stroma increased, and the quantity of macrophages in 1-MT+A.F. group increased significantly, compared with A.F. group, as shown in Figure 2E.
Figure 2.
Effects of IDO on the recruitment of macrophages in mouse corneas. The corneas of C57BL/6 mice were inoculated with A. fumigatus. Macrophages (red) were labeled with the F4/80 marker, and immunofluorescence staining showed there were few macrophages in corneas of the control group (A) and the 1-MT group (B), whereas the quantity of macrophage recruitment in the 1-MT+A.F. group (D) was obviously increased, compared with the A.F. group (C). (E) The comparison of macrophage count in the visual field of the corneas. Scale bar: 50 µm.
IDO Inhibited the Phagocytosis of Macrophages
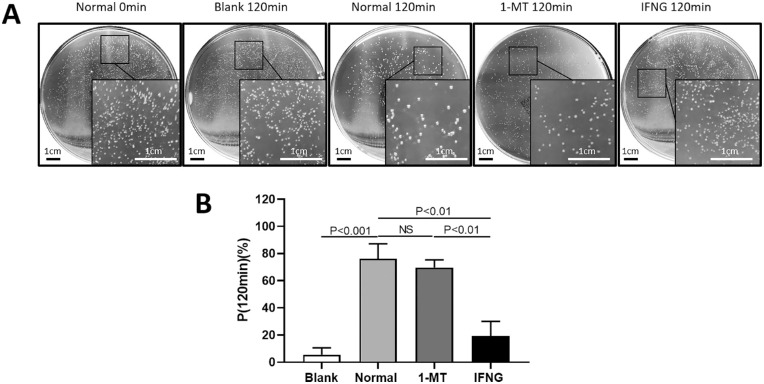
To further assess the effect of IDO on the phagocytosis of macrophages, we detected the count of CFU. P(120min) of macrophages in the blank group which was close to 0%, indicating that other factors other than macrophages had little effect on A. fumigatus conidia. P(120min) of macrophages in the 1-MT group and the normal group did not change significantly, whereas P(120min) of macrophages was lower in the IFNG group, compared with the normal group (Fig. 3).
Figure 3.
Effects of IDO on the phagocytosis of macrophages. (A) The CFU of the ectocytic live A. fumigatus conidia. Scale bar for the plates images: 1 cm. (B) P(120min) of macrophages in each group. Compared with the normal group, the effect of 1-MT on the phagocytic index of the macrophages was not significantly different, and IFNG decreased the phagocytic index (P < 0.01).
IDO had an Impact on Macrophage Polarization
In order to confirm the effect of IDO on the polarization of macrophages in A. fumigatus keratitis, the four aforementioned groups of mouse corneas or spleens underwent RT-PCR or flow cytometry analyses, and the polarization of peritoneal primary macrophages was detected by RT-PCR.
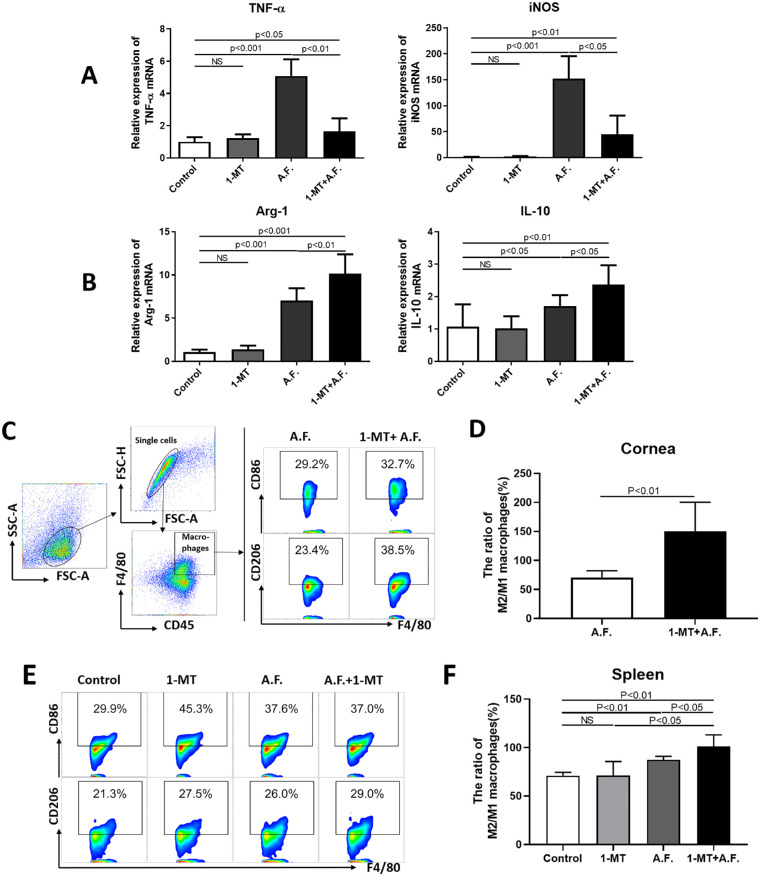
The mRNA expression of tumor necrosis factor–α (TNF-α), inducible nitric oxide synthase (iNOS), interleukin-10 (IL-10), and arginase-1 (Arg-1) in the corneas of the A.F. group were higher than that of the control group (P < 0.05). Compared with the A.F. group, 1-MT increased IL-10 and Arg-1 expression (Fig. 4B) and decreased TNF-α and iNOS expression (Fig. 4A). We further detected the changes in the M2/M1 macrophage ratio in the corneas and spleens of mice using flow cytometry. Compared with the A.F. group, the ratio of CD206+/ CD86+ macrophages increased in the corneas of the 1-MT+A.F. group (Figs. 4C, 4D); meanwhile the ratio also increased in mouse spleens (Figs. 4E, 4F).
Figure 4.
The influence of IDO on the polarization of corneal and splenic macrophages. The corneas of C57BL/6 mice were infected with A. fumigatus. Mouse corneas and spleens were collected three days after A. fumigatus infection. The mRNA expression of TNF-α, iNOS, Arg-1, and IL-10 in the A.F. group was significantly higher than that of the control group (A, B). Compared with A.F. group, 1-MT downregulated the expression of TNF-α, iNOS (P < 0.01) (A) and up-regulated the expression of Arg-1 and IL-10 (P < 0.05) (B) in the corneas. Flow cytometry gating strategies of M2 macrophages (CD45+F4/80+CD86+) and M1 macrophages (CD45+F4/80+CD206+). Furthermore, 1-MT upregulated the ratio of M2/M1 macrophages in the corneas (P < 0.01) (C, D) and spleens (P < 0.05) (E, F), compared with the A.F. group.
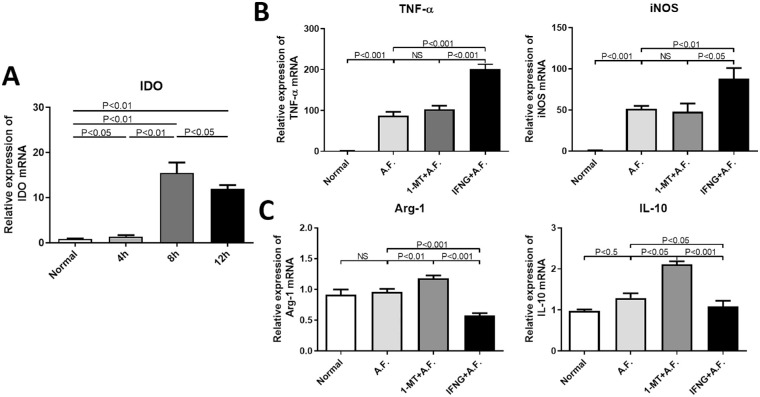
Next, we observed whether IDO affected the polarization of peritoneal primary macrophages. First, the results of the RT-PCR analysis indicated that IDO mRNA expression level was the highest at 8 hours after stimulation by inactivated A. fumigatus hypha (Fig. 5A). Then, the peritoneal primary macrophages were randomly divided into four groups: normal group, A.F. group, 1-MT+ A.F. group and IFNG+ A.F. group. The results of the RT-PCR analysis showed that, compared with A.F. group, IFNG increased the mRNA expression level of iNOS and TNF-α (P < 0.05) and decreased that of IL-10 and Arg-1 (P < 0.01), whereas 1-MT upregulated IL-10 and Arg-1 expression levels (P < 0.05) in macrophages (Fig. 5).
Figure 5.
Effects of IDO on the polarization of mouse peritoneal primary macrophages. Mouse peritoneal primary macrophages were stimulated by inactivated A. fumigatus hypha. Contrasted with the control group, the mRNA expression of IDO in mouse peritoneal macrophages increased significantly (P < 0.01), and reached the peak after eight hours (A). After the mouse peritoneal macrophages were stimulated by inactivated A. fumigatus hypha for eight hours, the results of the RT-PCR analysis showed that, compared with A.F. group, IFNG promoted the expression of TNF-α and iNOS (P < 0.01) (B) and inhibited the expression of Arg-1 and IL-10 (P < 0.05) (C), whereas the expression of Arg-1 and IL-10 were promoted by 1-MT (P < 0.05) (C).
IDO Affected the Protein Expression of P38 and ERK in A. fumigatus Keratitis
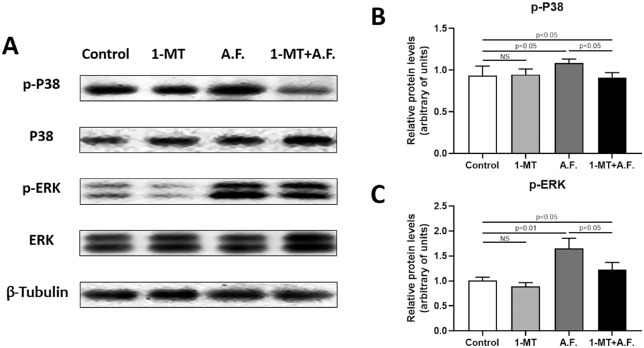
To clarify the mechanism by which IDO affects macrophage polarization, we detected MAPK/ERK signaling pathway related proteins using Western blotting analysis (Fig. 6). The phosphorylation levels of p38 and ERK increased after mouse corneas were infected with A. fumigatus. Compared with the A.F. group, 1-MT downregulated the phosphorylation levels of p38 and ERK. We also detected the total level of P38 and ERK, and their changes were not significant.
Figure 6.
Effects of IDO on the protein expression of P38 and ERK in A. fumigatus keratitis. The corneas of C57BL/6 mice were infected by A. fumigatus. Mouse corneas were collected three days after A. fumigatus infection. Western blotting was used to detect the phosphorylation and total level of P38 and ERK in the mouse corneas (A–C). It was shown that, compared with the control group, the phosphorylation level of p38 and ERK increased in the A.F. group (P < 0.01), whereas 1-MT decreased the phosphorylation level of p38 and ERK, compared with the A.F. group (P < 0.01). The changes in the total level of P38 and ERK were not significant.
IDO Regulated Macrophage Polarization Through the MAPK/ERK-Dependent Pathway
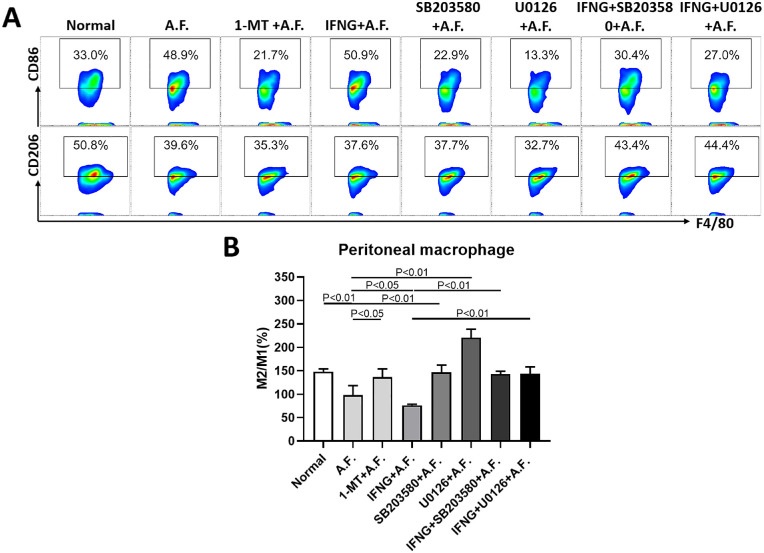
To further confirm that IDO regulates the polarization of macrophages through the MAPK/ERK pathway, we detected the ratio of M2/M1 (CD206+/CD86+) macrophages in the eight groups with or without 1-MT, IFNG, SB203580, and U0126 pretreatment (Fig. 7). The statistical results of flow cytometry showed that, compared with the normal group, the ratio of M2/M1 macrophages was downregulated after stimulation by A. fumigatus (A.F. group); compared with the A.F. group, the ratio in the 1-MT+A.F. group was upregulated and that in the IFNG+A.F. group was downregulated; whereas compared with the IFNG+A.F. group, the ratio in the SB203580+A.F. and U0126+A.F. groups were upregulated.
Figure 7.
Effects of IDO on macrophage polarization through the MAPK/ERK pathway. The mouse peritoneal primary macrophages were pretreated with 1-MT, IFNG, SB203580, or U0126 for two hours and were exposed to inactivated A. fumigatus spores for eight hours. The cultures were subjected to flow cytometry to quantify the percentage of M1 macrophages (CD45+F4/80+CD86+) and M2 macrophages (CD45+F4/80+CD206+) (A) and the ratio of M2/M1 macrophages (B). The results show that: compared with the normal group, the ratio of M2/M1 in the A.F. group decreased (P < 0.01); compared with the A.F. group, the ratio in the 1-MT+A.F. group increased (P < 0.05), the ratio in the IFNG+A.F. group decreased (P < 0.05), whereas compared with the IFNG+A.F. group, the ratio in the SB203580+A.F. and U0126 +A.F. groups increased (P < 0.01).
Discussion
FK is a serious and highly blinding infectious ophthalmic disease caused by pathogenic fungal infection, with an unclear idiographic pathogenesis. IDO, a rate-limiting enzyme involved in tryptophan decomposition metabolism along the kynurenine pathway, is renowned for its extensive participation in the immune regulation of infections, autoimmune diseases, immunologic escape of tumors and transplantation immunology.11,20 It has been reported that IDO may perform a pathogenic role in the progression of the inflammatory diseases.21–23 IDO can change the function of immune cells through the metabolic pathway of inflammatory reaction.24 Our preliminary studies have found that IDO was expressed in the cornea and is involved in the inflammatory response related to A. fumigatus keratitis.10 However, the specific mechanism by which IDO is involved in fungal-mediated inflammation responses is unclear.
The infection of A. fumigatus on cornea induced vasodilation of the surrounding tissues and recruitment of neutrophils and macrophages, which could secrete inflammatory factors to mediate the inflammatory response and initiate an immune response. Among them, macrophages—which possess the functions of phagocytosis and antigen presentation—have two major characteristics, i.e., diversity and plasticity.4,6,25 As the front line of defense cells, macrophages can activate the immune system to trigger an immune response. The results of immunofluorescence verified that the recruitment of macrophages was upregulated in the corneal stroma of mouse corneas after infection with A. fumigatus. In other studies, it has been found that IDO performs a corresponding regulatory effect on local tissues by acting on macrophages.26,27 However, it has not been clarified whether IDO plays a regulatory role in fungal keratitis by affecting macrophage function.
To further clarify the effects of IDO on macrophage recruitment in A. fumigatus keratitis, murine A. fumigatus keratitis models were established by scraping off the central epidermis of the cornea and smearing A. fumigatus. Through 1-MT gastric treatment, we competitively inhibited IDO. Immunofluorescence results clearly indicated that the quantity of macrophages increased in the cornea of mice following the inhibition of IDO, confirming that IDO can inhibit the recruitment of macrophages into the corneal stroma. Similarly, Polyzos et al.28 also confirmed that 1-MT pretreatment increases vascular inflammation and the recruiting quantity of CD68+ macrophages into the atheromatous plaques.
Furthermore, 1-MT and IFNG were used for the pretreatment of mouse peritoneal primary macrophages. CFU counting experiments confirmed that the ability of macrophages to phagocytose spores of A. fumigatus conidia significantly decreased after stimulation with IDO. Similarly, Poormasjedi-Meibod et al.29 found that in the islet-fibroblast composite grafts and celiac macrophage-fibroblasts co-culture system, IDO-induced tryptophan deficiency could restrain macrophage function, and local IDO significantly decreased macrophage infiltration. Lee et al.30 have proved that the expression of IDO in macrophages and the mesenchymal stromal cells was greatly enhanced in the inflamed human dental pulp tissue, and that dental pulp stem cells (DPSCs) could inhibit the function of macrophages through the IDO protein signaling pathway.
In fungal keratitis, the activity of IDO can inhibit the recruitment and phagocytosis of macrophages to a certain extent, thus promoting local metabolic changes in inflammatory response and inhibiting inflammation. Excessive inflammatory reactions can cause damage to corneal tissue; therefore it is essential for IDO to perform an immunomodulatory role by altering the function of macrophages in A. fumigatus keratitis.
The macrophage immune mechanism consists of two classical parts: activated M1 and M2 macrophages. Classical M1 macrophages are proinflammatory macrophages that play a central role in host defense against infection, whereas alternative M2 macrophages are related to the anti-inflammatory response and tissue remodeling. M1 and M2 macrophages represent the two terminals of the total activation of macrophages. The transformation of macrophages with different phenotypes regulates the onset, development, and termination of inflammatory diseases. Moreover, they respond to different immune challenges and perform a significant role in the pathogenesis of fungal keratitis.5,6 Our experiments also proved that 1-MT pretreatment inhibited the expression of M1 inflammatory factors (TNF-α, iNOS) in the cornea of mice and promoted the expression of M2 inflammatory factors (IL-10, Arg-1), compared with cornea infected with A. fumigatus alone. More intuitively, flow cytometry proved that after IDO inhibition the ratio of CD206+/CD86+ macrophages improved in the mouse corneas and spleen, which demonstrated that IDO can facilitate macrophage polarization into the M2 phenotype in A. fumigatus keratitis. Meanwhile, it was recognized that IFNG can upregulate IDO in mice and human monocytes31 and macrophages.32 Based on the flow cytometry results, we can conclude that IFNG, as an IDO agonist, induced mouse peritoneal primary macrophage polarization into the M1 phenotype, whereas 1-MT, as an IDO antagonist, induced its polarization into the M2 phenotype. Tjiu et al.,32 Wang et al.,33 and Benoit et al.34 showed that IFNG could also upregulate the expression of IDO and induce monocyte macrophages to differentiate into the M1 phenotype and that the expression intensity of IDO could regulate the polarization of macrophages.32,33 M1 phenotypic macrophages exert antitumor activity,32 which is also detrimental for Coxiella burnetiid.34
Interestingly, our present results proved that the immunosuppressive factor, IDO, could promote the polarization of macrophages into the M1 phenotype, which formed proinflammatory cells. M1 macrophages secrete inflammatory mediators, such as TNF-α and iNOS. These proinflammatory factors form the inflammatory microenvironment, promote the transformation of unpolarized macrophages into the M1 phenotype and establish a defense mechanism of proinflammatory positive feedback activation of antibacterial properties, which contributes to the elimination of invading microorganisms.35 This regulation effect of IDO on macrophage polarization is harmful to A. fumigatus. On the one hand, in A. fumigatus keratitis IDO can lower the infiltration level of macrophages into the corneal stroma and prevent excessive harmful inflammatory reactions, on the other hand, IDO can also foster the polarization of macrophages recruited to the cornea into the M1 phenotype, which is beneficial for the elimination of invasive A. fumigatus. Accordingly, the presence of IDO is vital in keeping the balance between anti-inflammatory and proinflammatory effects.
To adequately determine the mechanism by which IDO regulates macrophage function, we conducted experiments in vivo and in vitro. Western blotting analysis showed that the expression of p-P38 and p-ERK in the cornea of C57BL/6 mice infected by A. fumigatus keratitis decreased after 1-MT pretreatment. In addition, the results of flow cytometry confirmed that IFNG, as the IDO agonist, promoted the polarization of macrophages toward the M1 phenotype, whereas P38 and ERK antagonists reversed the regulatory effect of IFNG on the polarization of macrophages. In the cornea, the MAPK/ERK signaling pathway is one of the pathways associated with the regulation of cells and cytokines for participation in inflammatory responses. Studies by Zhang et al.36 have also indicated that p38α promoted M1 polarization of macrophages in steatohepatitis. Thus, in A. fumigatus keratitis, IDO may catalyze the polarization of macrophages into the M1 phenotype through the MAPK/ERK-dependent pathway.
In conclusion, IDO inhibition can aggravate the disease response of A. fumigatus-infected mice. Our research demonstrated that IDO can inhibit macrophage recruitment and phagocytosis in A. fumigatus keratitis to a certain degree. Moreover, IDO may promote the polarization of macrophages into the M1 phenotype by activating the MAPK/ERK signaling pathway. It was also concluded that IDO is crucial for keeping the balance between anti-inflammatory and proinflammatory effects in A. fumigatus keratitis. Therefore to bolster its role in the prognosis of fungal keratitis, the mechanism of IDO regulation of the function of other immune cells needs to be investigated much more comprehensively.
Acknowledgments
Supported by the Youth National Natural Science Foundation of China (No. 81700800), the Natural Science Foundation of Shandong Province (No. ZR2017MH008) and the National Natural Science Foundation of China (No. 81870632).
Disclosure: N. Jiang, None; L. Zhang, None; G. Zhao, None; J. Lin, None; Q. Wang, None; Q. Xu, None; C. Li, None; L. Hu, None; X. Peng, None; F. Yu, None; M. Xu, None
References
- 1. Mahmoudi S, Masoomi A, Ahmadikia K, et al.. Fungal keratitis: An overview of clinical and laboratory aspects. Mycoses. 2018; 61: 916–930. [DOI] [PubMed] [Google Scholar]
- 2. Bourcier T, Sauer A, Dory A, Denis J, Sabou M. Fungal keratitis. J Fr Ophtalmol. 2017; 40: 882–888. [DOI] [PubMed] [Google Scholar]
- 3. Leal SM Jr., Pearlman E.. The role of cytokines and pathogen recognition molecules in fungal keratitis - Insights from human disease and animal models. Cytokine. 2012; 58: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang J, Yin S, Bi F, et al.. TIMAP repression by TGFbeta and HDAC3-associated Smad signaling regulates macrophage M2 phenotypic phagocytosis. J Mol Med (Berl). 2017; 95: 273–285. [DOI] [PubMed] [Google Scholar]
- 5. Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013; 8: e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu J, Wang Y, Xie L. Potential role of macrophages in experimental keratomycosis. Invest Ophthalmol Vis Sci. 2009; 50: 2087–2094. [DOI] [PubMed] [Google Scholar]
- 7. Carvalho A, Cunha C, Bozza S, et al.. Immunity and tolerance to fungi in hematopoietic transplantation: principles and perspectives. Front Immunol. 2012; 3: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Luca A, Bozza S, Zelante T, et al.. Non-hematopoietic cells contribute to protective tolerance to Aspergillus fumigatus via a TRIF pathway converging on IDO. Cell Mol Immunol. 2010; 7: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romani L, Bistoni F, Perruccio K, et al.. Thymosin alpha1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006; 108: 2265–2274. [DOI] [PubMed] [Google Scholar]
- 10. Jiang N, Zhao G, Lin J, et al.. Indoleamine 2,3-Dioxygenase Is Involved in the Inflammation Response of Corneal Epithelial Cells to Aspergillus fumigatus Infections. PLoS One. 2015; 10: e0137423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013; 34: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo G, Sun L, Yang L, Xu H. IDO1 depletion induces an anti-inflammatory response in macrophages in mice with chronic viral myocarditis. Cell Cycle. 2019; 18: 2598–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang N, Zhao GQ, Lin J, et al.. Expression of indoleamine 2,3-dioxygenase in a murine model of Aspergillus fumigatus keratitis. Int J Ophthalmol. 2016; 9: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bozza S, Fallarino F, Pitzurra L, et al.. A crucial role for tryptophan catabolism at the host/Candida albicans interface. J Immunol. 2005; 174: 2910–2918. [DOI] [PubMed] [Google Scholar]
- 15. Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003; 44: 210–216. [DOI] [PubMed] [Google Scholar]
- 16. He K, Yue LH, Zhao GQ, et al.. The role of LOX-1 on innate immunity against Aspergillus keratitis in mice. Int J Ophthalmol. 2016; 9: 1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu M, Li C, Zhao GQ, et al.. Boxb mediate BALB/c mice corneal inflammation through a TLR4/MyD88-dependent signaling pathway in Aspergillus fumigatus keratitis. Int J Ophthalmol. 2018; 11: 548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perkhofer S, Speth C, Dierich MP, Lass-Florl C. In vitro determination of phagocytosis and intracellular killing of Aspergillus species by mononuclear phagocytes. Mycopathologia. 2007; 163: 303–307. [DOI] [PubMed] [Google Scholar]
- 19. Novakowski KE, Loukov D, Chawla V, Bowdish DM. Bacterial binding, phagocytosis, and killing: measurements using colony forming units. Methods Mol Biol. 2017; 1519: 297–309. [DOI] [PubMed] [Google Scholar]
- 20. Iversen TZ, Andersen MH, Svane IM. The targeting of indoleamine 2,3 dioxygenase -mediated immune escape in cancer. Basic Clin Pharmacol Toxicol. 2015; 116: 19–24. [DOI] [PubMed] [Google Scholar]
- 21. Li F, Zhang R, Li S, Liu J. IDO1: an important immunotherapy target in cancer treatment. Int Immunopharmacol. 2017; 47: 70–77. [DOI] [PubMed] [Google Scholar]
- 22. Minhas PS, Liu L, Moon PK, et al.. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019; 20: 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee SM, Park HY, Suh YS, et al.. Inhibition of acute lethal pulmonary inflammation by the IDO-AhR pathway. Proc Natl Acad Sci U S A. 2017; 114: E5881–E5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb J. 1991; 5: 2516–2522. [PubMed] [Google Scholar]
- 25. Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012; 33: 3792–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bozec A, Luo Y, Engdahl C, Figueiredo C, Bang H, Schett G. Abatacept blocks anti-citrullinated protein antibody and rheumatoid factor mediated cytokine production in human macrophages in IDO-dependent manner. Arthritis Res Ther. 2018; 20: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cozzi A, Zignego AL, Carpendo R, et al.. Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients. J Viral Hepat. 2006; 13: 402–408. [DOI] [PubMed] [Google Scholar]
- 28. Polyzos KA, Ovchinnikova O, Berg M, et al.. Inhibition of indoleamine 2,3-dioxygenase promotes vascular inflammation and increases atherosclerosis in Apoe-/- mice. Cardiovasc Res. 2015; 106: 295–302. [DOI] [PubMed] [Google Scholar]
- 29. Poormasjedi-Meibod MS, Jalili RB, Hosseini-Tabatabaei A, Hartwell R, Ghahary A. Immuno-regulatory function of indoleamine 2,3 dioxygenase through modulation of innate immune responses. PLoS One. 2013; 8: e71044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee S, Zhang QZ, Karabucak B, Le AD. DPSCs from inflamed pulp modulate macrophage function via the TNF-alpha/IDO axis. J Dent Res. 2016; 95: 1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miwa N, Hayakawa S, Miyazaki S, et al.. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-gamma increase in normal pregnancy but decrease in spontaneous abortion. Mol Hum Reprod. 2005; 11: 865–870. [DOI] [PubMed] [Google Scholar]
- 32. Tjiu JW, Chen JS, Shun CT, et al.. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009; 129: 1016–1025. [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Wang H, Zhang F, Liu H, Du J. [Expression of indoleamine 2, 3-dioxygenase modulates macrophage polarization in THP-1 cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014; 30: 901–905. [PubMed] [Google Scholar]
- 34. Benoit M, Ghigo E, Capo C, Raoult D, Mege JL. The uptake of apoptotic cells drives Coxiella burnetii replication and macrophage polarization: a model for Q fever endocarditis. PLoS Pathog. 2008; 4: e1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weisser SB, McLarren KW, Kuroda E, Sly LM. Generation and characterization of murine alternatively activated macrophages. Methods Mol Biol. 2013; 946: 225–239. [DOI] [PubMed] [Google Scholar]
- 36. Zhang X, Fan L, Wu J, et al.. Macrophage p38alpha promotes nutritional steatohepatitis through M1 polarization. J Hepatol. 2019; 71: 163–174. [DOI] [PubMed] [Google Scholar]