Abstract
The gastrointestinal (GI) tract microbiota is an environmental factor that regulates host immunity in allo-transplantation (allo-Tx). It is required for the development of resistance against pathogens and the stabilization of mucosa-associated lymphoid tissue. The gut-microbiota axis may also precipitate allograft rejection by producing metabolites that activate host cell-mediated and humoral immunity. Here, we discuss new insights into microbial immunomodulation, highlighting ongoing attempts to affect commensal colonization in an attempt to ameliorate allograft rejection cascade. Recent progress on the use of antibiotics to modulate GI microbiota diversity and innate-adaptive immune interface are discussed. Our focus on the microbiota’s influence of endoplasmic reticulum (ER) stress and autophagy signaling through hepatic EP4/CHOP/LC3B platforms reveals a novel molecular pathway and potential biomarkers determining the progression of allo-Tx damage. Understanding and harnessing the potential of microbiome/bacteriophage therapies may offer safe and effective means for personalized treatment to reduce risks of infections and immunosuppression in allo-Tx.
Introduction
Scientific discoveries continue to propel organ transplantation (Tx) to become the definitive treatment option for patients with end-stage organ diseases. Though the evolution of Tx success is marked by technical advancements in experimental approaches that include enhanced surgical techniques, medical diagnosis, selection process, and pharmacotherapy, there remains a significant imbalance between the supply of donor organs and the number of patients awaiting the life-saving transplant. The fulfillment of organ Tx premise requires methodological refinement based on novel, targeted and titratable immunosuppression to reduce risks and complications associated with early (acute) and late (chronic) graft rejection as well as minimizing harmful side effects of post-Tx therapies. Although donor – recipient MHC genetic disparity remains the key to the strength of alloimmune response, an essential role for environmental factors, such as “microbiota” of the gastrointestinal tract only recently has emerged as an important regulator of host immunity in allo-Tx (1).
The term microbiota refers to the microorganisms found in an environment, including bacteria, viruses, and fungi. In the gastrointestinal tract, a complex ecosystem exists where microbes compete and cooperate in symbiosis with the host. The community of human commensal microorganisms is a diverse ecosystem of ca. 100 trillion bacteria (1–2 kg in mass), with a genome of ca. 150-fold more genes than the human genome itself. It has been suggested that more than 1000 types of microorganisms occur in the human gut (2) with a significant proportion of all metabolites circulating systematically as microbiota products (3). Indeed, numerous studies establish the gut microbiome as an important physiological regulator of pathogen colonization, dietary nutrient metabolism, and immune regulation (4). As the role of microbiome in organ Tx remains largely unknown, there is unmet scientific and clinical need to develop a better appreciation of its unique mechanisms in order to build a foundation for development of improved and clinically-relevant novel treatment protocols to improve Tx outcomes. With the current limited knowledge on the role of microbiota in organ Tx, it is impossible to elucidate whether organ dysbiosis will eventually be linked to microbes in a causal role or represent an epiphenomenon related to allograft rejection or survival. In this review, we discuss the current knowledge and identify gaps that need further in-depth investigation, as it pertains to transplantation.
Microbiota considerations in allo-Tx
The microbiota can modify local and distal immune responses in Tx recipients (5–7) raising the possibility that it might fine-tune host alloreactivity. This hypothesis is supported by the fact that organs colonized with microbial communities, such as lung, or intestine have inferior outcomes than organs considered “sterile”, such as kidney or heart. In addition, the survival of minor MHC antigen mismatched skin grafts was prolonged when donors and recipients were pretreated with broad-spectrum antibiotics (Abx), at least in part by decreasing priming of alloreactive T cells (6). Emerging clinical evidence also suggests that acute rejection of intestinal and lung allografts associate with shifts in bacterial composition of the intestinal microbiome (8, 9). This might be even more pronounced in the skin component of vascularized composite allografts (VCA), such as face and extremity transplantation. As the human skin is densely colonized by a highly diverse microbiota comprising all three domains of life, it is obviously influenced by host and environmental factors and interacts with the skin immune system itself (10). However, how this response may ultimately affect VCA outcomes remains unknown. Of note, by modulating the activation of skin-draining lymph node APCs and their ability to prime alloreactive T cells, gut microbiome was identified as an important factor in skin allograft survival (7); while skin-restricted commensal colonization was shown to accelerate skin graft rejection (11). Hence, future studies on microbiota in VCA settings should involve both, the effects of recipient gut microbiome as well as skin-specific microbiome of the VCA donor.
The development of culture-independent methods such as 16S ribosomal RNA-tagged sequencing has improved the ability to detect the different types of microorganisms associated with various diseases. The use of shotgun metagenomics sequencing, transcriptomics, metabolomics and proteomics has enabled the identification of microbe’s functional capacity within the mammalian host. Based on the comprehensive characterization of 16S ribosomal RNA (rRNA) bacterial gene sequencing, the gut of humans and many other vertebrae is mostly dominated by four bacterial phyla: Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria with Bacteroidetes and Firmicutes representing most endemic bacteria in the GI tract (12). Imbalances of the intestinal microbiota, however, can be disrupted by several factors such as medications, diet, obesity, metabolic stress, and be essential for innate immune-dominated organ ischemia-reperfusion injury (IRI) (13). Indeed, IR stress-triggered tissue damage represents a significant and ongoing challenge in organ Tx because it causes an imbalance in metabolic supply and demand within the ischemic organ, resulting in tissue hypoxia and microvascular dysfunction. This is accompanied by elaboration of danger-associated molecular patterns from damaged tissue, targeted migration and infiltration of circulating monocyte-derived macrophages, neutrophils and T cells that generate reactive oxygen species and proinflammatory cytokine/chemokine programs (14). Hence, dampening the inflammatory response by targeting microbiota-derived mediators could lead to improvement in Tx outcomes (15).
Microbiome and immune regulation
Immunopathologic mechanisms in allo-Tx rejection may be regulated by discrete changes in the microbiota diaspora and involve cell-mediated, humoral immunity and aberrant immunoregulation. The idea that microbes regulate alloimmunity originates from studies of germ-free mice that exhibit poor lymphoid organogenesis or reduced number of lacteals, whereas conventionalization of germ-free mice restore such immune deficits and lacteal maturation and integrity (16, 17). Similarly, early exposure to germs modified kidney damage and inflammation in a mouse model of IRI (18). The interaction of epithelial cells and the microbiota can affect the differentiation of innate and adoptive immune cells not only in the gut but also in the systemic circulation through the metabolites produced (Figure 1). For example, short-chain fatty acids (SCFAs), bile acids, tryptophan metabolites and amines from the microbiota can modulate neutrophil functions. SCFAs which are an important energy source of intestinal epithelial cells (19) can serve as an inhibitors of histone deacetylases (HDACs), leading to the suppression of proinflammatory cytokine expressions through the inhibition of NF-kB signaling (19–21). Innate lymphoid cells (ILCs) are recently discovered innate immune cells that belong to the lymphoid system, but do not show antibody-specific response and do not have B-cell and T-cell receptors. Although ILCs develop normally in the absence of the microbiota (22), interaction with the bacterial flora is required for their normal functioning (23). As Type 3 innate lymphoid (ILC3) cells are abundant at mucosal sites (24) much attention has been paid to their interaction with the microbiota.
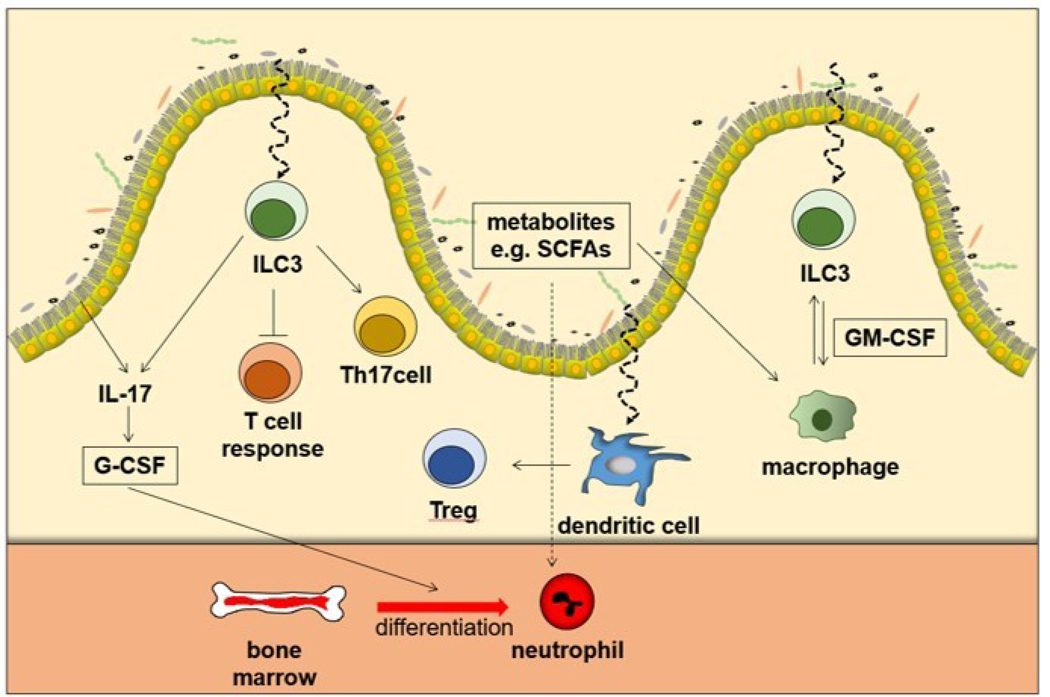
Figure 1. Dynamic interplay between gut microbiota and host immune repertoire.
Microorganisms promote IL17 secretion from intestine leading to production of granulocyte-macrophage colony-stimulating factor (GM-CSF), which regulates neutrophil differentiation. Type 3 innate lymphoid cells (ILC3), which are abundant in the mucosa, can suppress commensal-specific T-cell responses, license IL17 production from Th17 cells and regulate macrophage through GM-CSF. Dendritic cells can promote activation of mucosal Treg, which allows organ dysbiosis. Metabolites, such as short-chain fatty acids (SCFAs) can affect the differentiation, maturation and activation of immune cells, directly or indirectly.
Obviously, the role of the microbiota in T lymphocyte differentiation is of major importance in organ Tx. Microorganisms that colonize in the specific gut, create their own microenvironments, and provide signals that render dendritic cell (DCs) interactions with T-cells. Tolerogenic DC can induce immunotolerance by T cell anergy, or a lack of reaction by the body’s defense mechanisms to foreign substances, and regulatory T cell activation. DCs can interact with commensal bacteria as a luminal antigen and readily induce lamina propria resident macrophages to promote local Treg cells expansion (25). Moreover, the interaction between inflammatory monocytes and microbiota in the acute gastrointestinal tract infection provokes production of prostaglandin-2, which in turn suppress the activation of tissue damaging neutrophils (26). Taken together, these data indicate that the microbiota may affect not only local immunity but also organ Tx outcomes by interacting with local immune cells as well as releasing metabolites to induce distal immunity.
Microbiome challenges in allo-Tx
Infectious complications represent a serious challenge in clinical organ Tx that can result from disruptions of intestinal barriers. Gut dysbiotic changes involving multidrug-resistant organisms (MDROs) predispose human patients to end-stage liver complications and can lead to decreased microbiome diversity. This was shown recently when 16S rRNA sequencing was performed on fecal samples collected prior to liver transplant and periodically until one-year post-transplantation to test whether MDRO colonization was associated with decreased microbiome diversity (27). In another study, it was shown that the clinically important pathogen methicillin-resistant Staphylococcus aureus can cause infections in lung recipients during the first month post-Tx and vancomycin resistant Enterococcii (VRE) has been observed in 15% of solid organ transplantation (SOT) (28). Interestingly, differences in the use of Abx in Europe and America may explain why US centers show VRE in 32% of liver transplant recipients whereas numbers are minimal in Europe (29, 30). Many studies now show that the success of SOT and the prevention of graft-failure depends not only on the diagnosis, and management of infectious diseases but also on the patient’s immunosuppressive status, stemming from epidemiologic and commensal exposures of the microbiota (31). This is important when considering that long-term graft survival is much worse for patients that experience acute rejection episodes (32). Unfortunately, studies that detail the effects of the microbiota in allo-Tx remain in their infancy, and only associating putative correlations between graft outcomes and the gut or allograft microbiota profiles. However, as skin-restricted microbiota was shown to accelerate skin graft rejection (11), while gut microbiota impacted chronic lung allograft rejection (33), it is possible that the microbiota in both anatomical locations, skin and intestine, may independently influence alloreactivity towards distinct allo-Tx. For example, in a minor mismatch model of orthotopic lung transplantation, the priming phase of the alloresponse, or when T cells first encounter donor antigens, did not correlate with reduced lung allograft pathology. Instead, the authors propose a mechanism that involves diminished graft-reactive T cell activation (33). In another study, modulation of cutaneous S. epidermidis–painted monocolonization restricted to donor skin was sufficient to accelerate skin graft rejection, by a mechanism involving alloimmunity at the effector rather than during the priming phase of the alloresponse (11). Another consideration that has clinical relevance concerns the therapeutic levels of immunosuppressive drugs, such as tacrolimus, and their potential for altering distinct microbiota structures. Early studies from the kidney transplant field established the challenge of tacrolimus dosing because of interpatient and intrapatient variability in drug absorption, metabolism, and disposition. For example, in one study, fecal specimens were collected from kidney transplant patients maintained on tacrolimus during the first week of transplantation, compared to those on tacrolimus maintenance, or those who did not develop acute rejection in the first post-transplant month (34). Using deep sequencing of the PCR amplified 16S rRNA V4-V5 region, gut microbial analyses identified a novel association between fecal Faecalibacterium prausnitzii abundance and tacrolimus dosing requirements. Specifically, the authors show that fecal F. prausnitzii abundance in the first week of transplantation was 11.8% in the group that received an escalation of tacrolimus but only 0.8% in the group that did not require additional immunosuppression. Other studies imply that gut microbiota are capable of metabolizing tacrolimus, suggesting a mechanism explaining why tacrolimus exposure in kidney transplant recipients has differential efficacy (35). In sum, investigating communities of commensal microbes associated with different Tx outcomes should allow the identification of specific members of the microbiota that can either positively or negatively affect the allograft rejection cascade and clinical outcomes.
FMT as a viable bacterial replacement strategy in allo-Tx
In some chronic gut dysbioses, bacterial replacement therapy has shown great promise for human health. For example, fecal microbiota transplantation (FMT), which transfers fecal microbiota from healthy donors to the gut of diseased individuals has shown promising results in the treatment of recurrent Clostridium difficile infection (36). To date, only a handful of studies have investigated the consequences of FMT in allo-Tx. In one recent study, mice receiving vascularized murine cardiac allografts and FMT treatment were assessed for how discrete microbial populations influence allogeneic immunity (37). Fecal samples were sourced from normal, pregnant (immune-suppressed), or spontaneously colitic (proinflammatory) mice and when compositional differences were studied, they found that normal mice had higher relative abundance of Lactobacillus and Bifidobacterium whereas colitic mice had more Lachnospiraceae, Bacteroides, Desulfovibrio, and Mucispirillum. Importantly, transient immunosuppression with anti-CD40L mAb coupled with Abx treatment led to a graft survival advantage. Since colitic FMT in these experiments reduced graft survival in comparison with the normal or pregnant FMT, the authors concluded it was likely that Abx treatment attenuated the components of the microbiota that were detrimental to graft survival. Importantly, this study identified Bifidobacterium pseudolongum as an environmental factor that can influence allograft outcomes by altering anti-inflammatory cytokine IL-10 and chemokine CCL19 levels while lessening levels of proinflammatory cytokines, TNFα and IL-6. While B. pseudolongum was associated with potential anti-inflammatory functions, it is still not clear as to whether and how it alone can ameliorate allograft rejection. In another study, the effect of FMT was investigated on the immunosuppressive effect in mice that had first been treated with tacrolimus (38). Indeed, tacrolimus treatment significantly increased the relative abundance of Allobaculum, Bacteroides, and Lactobacillus and CD4+ CD25hiFoxP3+ regulatory T cells in the colonic mucosa and the circulation. Moreover, when low-dose tacrolimus was supplemented with FMT from high-dose tacrolimus–altered mice, skin allograft survival rate significantly improved, thus establishing that alteration of bacterial taxa of the gut microbiota has the potential to control allograft rejection.
Because colonized organs have worse transplant outcomes than sterile organs, future allo-Tx practices will need to understand how host and donor microbiota could serve as independent predictors for graft dysfunction. Already several studies have hinted at this possibility by showing how infections with Staphylococcus aureus (39) or Listeria monocytogenes (40) at the time of transplantation can increase alloreactivity and prevent the induction of immune tolerance. In one study, measuring pro-inflammatory cytokines led to the discovery that S. aureus infections impact T cell alloreactivity and confer resistance to anti-CD154/donor-specific transfusion through the production of IL-6 (39). After demonstrating that graft rejection was associated with S. aureus infection in a classic T cell-mediated response, mouse IL-6−/− recipients or wild-type counterparts with disrupted IL-6 signaling were utilized to demonstrate that S. aureus-induced IL-6 was critical for the ability of S. aureus to prevent skin allograft acceptance.
In another study revealing a role for metabolite-induced alloimmunity in skin transplants, genetically similar individuals [e.g., C57BL/6 mice from Jackson (Jax) laboratory or from Taconic (Tac) Farms] were investigated to determine whether variability observed in graft rejection was the result of differences in their commensal community structures (41). Notably, when fecal material from Jax mice was transferred to Tac recipients, prolongation of skin graft survival was observed. When the fecal microbiota composition was analyzed, it was discovered that the genus Alistipes (ASV_5969) could delay skin graft rejection. This led the authors to hypothesize that Alistipes-colonized Tx recipients benefit from alloimmunity-dampening by the presence of anti-inflammatory metabolite sulfobacin B (42). Supporting this supposition were the cumulative findings: 1/ TNF and NF-κB levels were diminished after co-culturing macrophages with sulfobacin B, in vitro (43); 2/ intraperitoneal in vivo injections of sulfobacin b diminished the inflammatory response to phorbol 12- myristate 13-acetate and LPS; and 3/ TNF transcript levels in the lymph nodes of skin transplanted Jax mice were significantly lower than in Tac mice (41). Whether these findings warrant a future consideration of mono-probiotic or FMT therapies in organ Tx remains unclear, but these studies and others show the scientific community is moving towards the goal of understanding how specific microbial communities cause/reduce disease-specific pathophysiology.
Moving towards mono-bacteriotherapy in allo-Tx
The use of gut microbes in the treatment of allo-Tx will remain a significant challenge until adequate monitoring of fecal material and long-term recipient safety outcomes can be ensured. Notably, in a demonstration of how little we understand the diversity, structure, and function of the microbes present in FMT, the Food and Drug Administration (FDA) has currently ceased all ongoing clinical trials as a result of two patients who suffered invasive infections, one of whom perished, from a common donor infected with extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli (44). In both cases, the donor stool had not been tested for ESBL-producing gram-negative organisms prior to use, leading to the unfortunate spread of MDROs. One solution to bypass the unwarranted spread of MDROs and the exhaustive necessity of cataloging the compositional complexity of FMT is for the organ transplantation field to move in the direction of those more mature fields that have already identified beneficial intestinal flora in their models. For example, early experiments from the ‘80s identified three species from the Bacteroides genus (B. ovatus, B. thetaiotaomicron and B. vulgatus) that contributes to remission of recurrent Clostridium difficile (rCDI) (45). In these experiments, a cocktail of ten different facultatively aerobic and anaerobic bacteria diluted in sterile saline were delivered to five patients presenting with recurrent Clostridium difficile (rCDI) with subsequent loss of C. difficile and its toxin from the patients’ stools. This led to the rapid bowel colonization of Bacteroides sp, which had not been present in pre-treatment stool samples (45). Three species from the Bacteroides genus (B. ovatus, B. thetaiotaomicron and B. vulgatus) were recognized to contribute to remission of rCDI following these studies and this led the FDA to approve an IND (Investigational New Drug) application to test the safety and efficacy of triple Bacteroides combinations in human patients (46). Recently, Ihekweazu et al. (46) refined their treatment of rCDI to a single monotherapy. Using colitis-inducing dextran sulfate sodium and coculturing with either a triple or single Bacteroides cocktail as compared to FMT from the stool of healthy donor mice, Bacteroides ovatus as a monotherapy was superior in mitigating weight loss, improved survival and reduced inflammation by increasing epithelial cell proliferation, goblet cell production and crypt depth. These data suggest that microbiota manipulation in organ Tx, as either a prebiotic and postbiotic, can offer a new means of prevention and possibly treatment of allo-Tx rejection.
Bacteriotherapy - a case study to improve allo-Tx outcomes in mice and humans
Given its unique anatomic location and vascular supply, the liver serves an important role in metabolism and immunological functions. The liver receives 70% of its blood supply through the portal vein and produces first-pass metabolism for the gastrointestinal luminal contents including dietary nutrients, toxins, xenobiotics, gut microbiota and its metabolites that translocate through the intestinal epithelium. Microbial diversity, therefore, is associated with the prognosis of orthotopic liver transplantation (OLT) (47, 48). Disturbed imbalances of the endogenous gut microbiota are associated with chronic liver conditions such as nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH), alcoholic liver disease (ALD), as well as complications from cirrhosis. It was reported that the differences in the intestinal microbiota of patients between pre-OLT and post-OLT showed a significant decrease in the abundance of Actinobacillus, Escherichia and Shigella, and a significant increase in the abundance of Micromonosporacease, Desulfobacrerales, the Sarcina genus of Eubacteriaceae and Akkermansia after liver transplantation (49). Others reported the family level Bacteroides, Enterobacteriaceae, Streptococcaceae and Bifidobacteriaceae were increased in patients with acute cellular rejection (ACR), while Enterococcaceae, Lactobacillaceae, Clostridiaseae, Ruminococcaceae and Peptostreptococcaceae were increased in none-ACR patients (48). However, few studies have elucidated the mechanism of how the intestinal microbiota may actually influence the prognosis of OLT.
We recently reported on the benefit of Abx pretreatment in OLT recipients, and identified a mechanistic underpinning of the gut – liver axis that involves attenuated endoplasmic reticulum (ER) stress and enhanced autophagy signaling (Figure 2) (50). While ER stress is known to promote various pathologies, including hepatic IRI (51), autophagy exerts largely cytoprotective functions against peri-transplant IR stress (52). In our study, mouse OLT recipients pretreated with oral amoxicillin for 10 days showed improved hepatocellular function as evidenced by decreased levels of serum aspartate aminotransferase (AST), attenuated histological pathology and decreased frequency of TUNEL positive (apoptotic), CD68 positive (macrophages) and cathepsin G positive (neutrophils) infiltrating cells. We discovered Abx pretreatment increased the expression of COX2, a responsive enzyme that produces Prostaglandin E2 (PGE2) in the intestine, PGE2 metabolite levels in portal and peripheral blood and PGE2 receptor 4 (EP4) in the liver. When we moved to in vitro studies with mouse primary hepatocytes co-cultured with exogenous PGE2, we were able to replicate suppression of ER stress (CHOP) and enhanced autophagy (LC3B) seen in our animal model. Moreover, EP4 antagonist adjunct restored ER stress (CHOP) and inhibited autophagy (LC3B) responses both in vitro and in vivo. These results indicate that the PGE2/EP4 axis plays a key role in ER stress and autophagy regulation and protection against IRI in OLT; while pro-inflammatory microbe communities may serve as stressors of innate responses in myeloid cells, and impact survival.
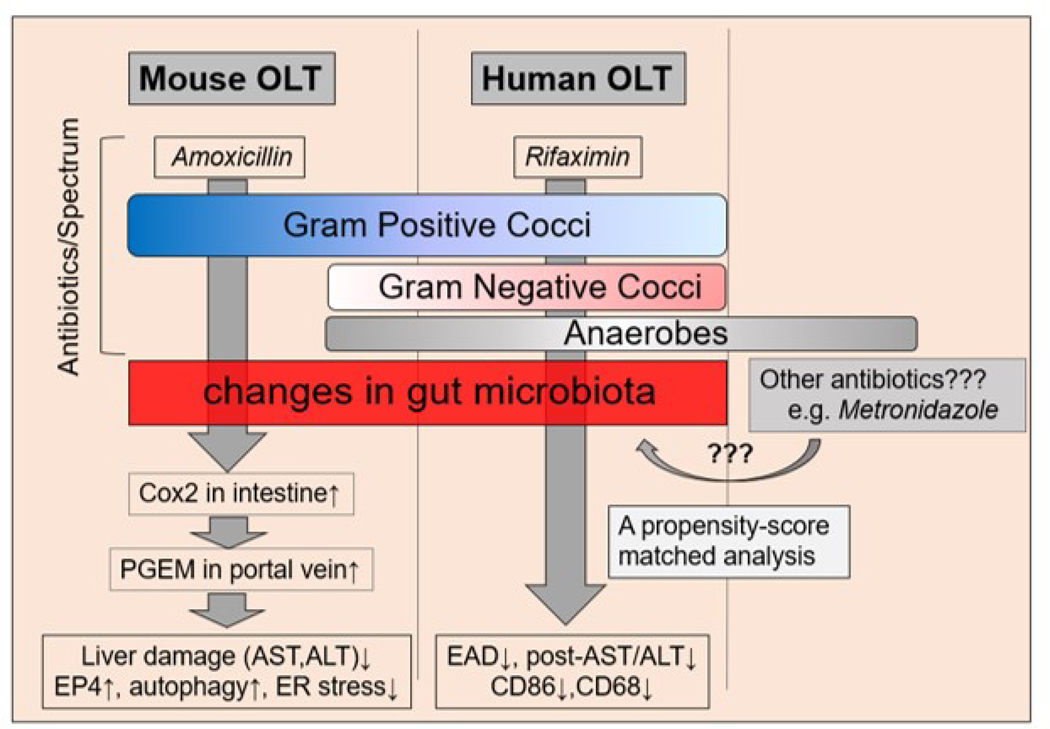
Figure 2. Abx treatment as bacteriotherapy in mouse and human OLT recipients.
Amoxicillin pretreatment attenuated hepatic IRI through gut-liver crosstalk in a mouse allo-OLT model. Recreation of classic IRI phenotype by fecal microbiota transplantation (FMT) from naïve mice implicates the pathogenic function of gut microbiota. Pre-transplant Rifaximin reduced hepatic IRI and improved hepatocellular function in human OLT patients. Future studies are warranted to clarify Abx-modulated gut microbial compositions and functions as well as other Abx with distinct activity spectrums.
In the clinical arm, a retrospective analysis of human OLT recipients with extended Abx treatment showed increased EP4, reduced ER stress (CHOP), increased autophagy (LC3B) and decreased CD68/cathepsin G expression, as well as decreased AST/ALT levels, all indicative of mitigated IR-mediated liver damage. Interestingly, patients with prolonged (>10 days) Abx treatment showed a significantly higher Model for End-Stage Liver Disease (MELD) score, longer hospital and ICU stays and required more blood transfusions during surgery. However, despite their higher acuities, the patient cohort under prolonged Abx regimen exhibited superior post-transplant hepatocellular function. Most importantly, multivariate analysis showed pre-OLT Abx free or Abx<10days was an independent predictive factor of early allograft dysfunction (EAD). By integrating mouse and human data, this study underscores the benefits of extended recipient Abx pretreatment in OLT recipients.
Our study has also raised the key question as to whether the modulation of hepatic EP4/CHOP/LC3B platforms were the consequence of microbiome disturbances or the association of Abx – liver direct crosstalk. For example, amoxicillin used in our experimental arm is rapidly absorbed after oral administration, with a half-life of 61.3 minutes; and being excreted unchanged in the urine. By contrast, in our clinical cohort, rifaximin (Rfx) was identified as the most frequently used Abx in patients pre-OLT. Rfx is an FDA-approved, minimally absorbed oral antimicrobial agent with broad activity towards enteric pathogens, including gram-positive, gram-negative, aerobic and anaerobic bacteria (52). First approved in the United States in 2004, Rfx is now widely used for the treatment of hepatic encephalopathy (53), improving survival and for reducing the risk of hospitalization and portal hypertension (54). Fujisaka et al. (55) also reported that the gut microbiome modification by a nonabsorbed Abx (vancomycin) in high-fat diet-fed mice depressed hepatic ER stress (55). However, in our studies, no discernable difference was observed with Abx-conditioning as EP4 antagonists diminished the cytoprotective features in both in vivo and in vitro studies. To better elucidate the influence of gut microbiota composition upon hepatocellular damage in IR-stressed OLT, we conducted a separate series of experiments in Abx-pretreated mice with or without adjunctive FMT. Since adjunctive FMT from untreated naïve mice recreated hepatic IRI in Abx-treated OLT group, we concluded that gut microbiota not only contributes to IRI pathophysiology but may also represent a potential target for therapeutic intervention to improve OLT clinical outcomes.
The impact of rifaximin in human OLT
Our recent translational study (50) was followed by assessing the impact of pre-transplant Rfx therapy on reducing hepatic IRI in human liver transplant patients (56). In this study, 520 OLT individuals were divided into two groups based on the duration of Rfx use: 1/ Rfx group (28 and >28 days) and 2/ control group (none or <28days). Of the 252 patients who received pretransplant Abx other than Rfx, 206 patients were included in the propensity score-matched analysis. This important difference was mitigated by use of a propensity score-matching analysis to verify the true effect of Rfx. Thirty-nine patients matched to the nearest scores, in each group, were selected. Serum ALT levels at POD0 and POD2–6 in the Rfx group were significantly lower than control group and the Rfx group had a significantly lower rate of EAD compared with the control group. Liver biopsies obtained after transplantation in the Rfx group were characterized by decreased activation of infiltrating macrophages (CD86) and neutrophils (cathepsin G) as compared with controls. It is interesting that Rfx was used only in the pre-transplant period and not continued after transplantation, suggesting that pretreatment with Rfx may alter the microbiome in prospective OLT patients. It is still unclear as to whether and how administration of Rfx in the postoperative period could further decrease the rate of EAD and improve long-term clinical outcomes. Although these two studies suggest the administration of Abx influences OLT function via gut – liver axis, future studies warrant clarity of the detailed gut microbial composition after Abx (Rfx or amoxicillin) treatment.
Bacteriophage-therapy (BT) in allo-Tx
Immunosuppressive agents obligatory for the prevention of allo-Tx rejection can also induce severe bacterial infections caused by MDROs that range in frequency and severity. Therefore, as the conventionality of using fecal intestinal cocktails (whether by FMT or monotherapies) becomes an emerging strategy in transplant recipients, management of gut microbiota will remain an ever-growing challenge. An emerging strategy in the management of MDROs is the bacteriophage therapy (BT). Though experience with BT is more than a century old, it was largely forgotten and abandoned in the antibiotics era. Phages (for short) are bacteria-infecting viruses that employ host translation machinery to replicate and upon cell lysis, new phage particles are released into the environment. As a result, they are prey specific, infecting a single species or strain, and making targeted BT possible. Although phages exist in the human body (especially in the intestinal tract) and contribute to maintenance of immune homeostasis at the level of both the intestines and other distant tissues, experience in transplantation remain limited to a few studies. For instance, Courtwright et al. (57) reported on the successful use of BT in four lung transplant patients presented with life threatening Pseudomonas aeruginosa and Burkholderia dolosa MDROs. Following treatment, the authors reported consolidative opacities improved with no treatment-related adverse events observed. In addition, Aslam et al. (58) also described the success of BT in the treatment of MDROs but highlighted specific challenges that will need to be overcome before BT becomes standardized treatment. For example, patients’ bacterial isolates will need to be identified by microbiome profiling and used in the selection of lytic phage viruses by “phage hunting”. This technical achievement was recently described by Duan et al. (59) when they isolated phages from untreated raw sewage water to target cytolytic E. faecalis, in the treatment of alcoholic hepatitis. Indeed, BT-targeting of this particular bacteria strain depressed ethanol-induced liver disease in germ-free mice colonized with E. faecalis, consistent with an idea that phage therapy might exert potent anti-inflammatory effects to attenuate progression of bacteria-induced early hepatic injury into end-stage organ insufficiency requiring liver Tx.
Other considerations for BT strategies will include genotypic characterization, safety studies, endotoxin removal to ensure patient safety, optimization of delivery parameters [timing, route, and frequency of administration (inhaled vs intravenous)], screening for the development of phage resistance isolates, understanding phage-directed allograft humoral immune responses, and preventing complications with transplant medications including antiviral therapies. Moreover, since phages are natural modulators of bacterial colonization, the need to monitor the cascading effects interbacterial interactions will be an important consideration, as was shown recently (60). Despite these challenges, BT in allo-Tx and graft-versus-host disease (GvHD) remains an attractive novel idea that warrants further evaluation in basic research studies and well-designed clinical trials (58, 61).
Conclusion
Future research that focuses on the molecular pathways by which microbial signals influence alloimmunity may lead the way towards identification of novel therapeutic targets to alleviate the harmful consequences of allo-Tx. However, many questions remain, among the most clinically relevant: How does FMT recreate organ damage in Abx-treated hosts? How does Abx treatment change the gut-microbiota composition and what species are most affected? What metabolites from the presence of beneficial or loss of harmful microbes influence post-transplantation functionally? Is there a role for pre- or post-probiotics in altering the diversity of gut microbiota? What organs should be the focus of future microbiota-centered study? How does altering microbiome diversity influence known immune responses to allo-Tx? and What are the long-term consequences of personalized bacterio (phage) therapies? Future research should also focus on the relationship between dysbiosis, altered bacterial communities and their metabolites as the knowledge from these advances in the field will continue to augment the lives of those affected by organ replacement therapies.
Highlights.
The gut microbiota has great potential to regulate allograft rejection cascade
Microbial metabolites play complex roles in host cell-mediated and humoral immunity
Mono-bacteriotherapy can increase our understanding of microbial signaling
Antibiotic microbe replacement modulates ER stress/autophagy in mouse and human liver transplant recipients
Bacteriophage therapy is an emerging personalized treatment option in organ transplantation
Acknowledgments
Supported in part by: NIH Grants P01 AI120944; R01 DK062357, DK107533, and DK102110 (JWKW)
Abbreviations:
- Abx
antibiotics
- APC
antigen-presenting cells
- BT
Bacteriophage therapy
- ESBL
extended-spectrum beta-lactamase
- FMT
fecal microbiota transplantation
- ILC3
Type 3 innate lymphoid cells
- IRI
ischemia-reperfusion injury
- MDROs
multidrug-resistant organisms
- OLT
orthotopic liver transplantation
- (rCDI)
recurrent Clostridium difficile
- SOT
solid organ transplantation
- Tx
transplant
- VCA
vascularized composite allotransplantation
- VRE
vancomycin-resistant Enterococcii
Footnotes
Disclosures: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Bromberg JS, Fricke WF, Brinkman CC, Simon T, Mongodin EF. Microbiota-implications for immunity and transplantation. Nat Rev Nephrol 2015;11:342–353. [DOI] [PubMed] [Google Scholar]
- 2.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rook G, Backhed F, Levin BR, McFall-Ngai MJ, McLean AR. Evolution, human-microbe interactions, and life history plasticity. Lancet 2017;390:521–530. [DOI] [PubMed] [Google Scholar]
- 4.Purchiaroni F, Tortora A, Gabrielli M, Bertucci F, Gigante G, Ianiro G, Ojetti V, et al. The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci 2013;17:323–333. [PubMed] [Google Scholar]
- 5.Sepulveda M, Pirozzolo I, Alegre ML. Impact of the microbiota on solid organ transplant rejection. Curr Opin Organ Transplant 2019;24:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei YM, Chen L, Wang Y, Stefka AT, Molinero LL, Theriault B, Aquino-Michaels K, et al. The composition of the microbiota modulates allograft rejection. J Clin Invest 2016;126:2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng F, Klinger CN, Felix KM, Bradley CP, Wu E, Tran NL, Umesaki Y, et al. Gut Microbiota Drive Autoimmune Arthritis by Promoting Differentiation and Migration of Peyer’s Patch T Follicular Helper Cells. Immunity 2016;44:875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Li Q, Li J. Gut microbiota and its implications in small bowel transplantation. Front Med 2018;12:239–248. [DOI] [PubMed] [Google Scholar]
- 9.Beaume M, Lazarevic V, Kohler T, Gaia N, Manuel O, Aubert JD, Baerlocher L, et al. Microbial Communities of Conducting and Respiratory Zones of Lung-Transplanted Patients. Front Microbiol 2016;7:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunjani N, Hlela C, O’Mahony L. Microbiome and skin biology. Curr Opin Allergy Clin Immunol 2019;19:328–333. [DOI] [PubMed] [Google Scholar]
- 11.Lei YM, Sepulveda M, Chen L, Wang Y, Pirozzolo I, Theriault B, Chong AS, et al. Skin-restricted commensal colonization accelerates skin graft rejection. JCI Insight 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, Ugarte E, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol 2009;11:2574–2584. [DOI] [PubMed] [Google Scholar]
- 13.Khanna S, Tosh PK. A clinician’s primer on the role of the microbiome in human health and disease. Mayo Clin Proc 2014;89:107–114. [DOI] [PubMed] [Google Scholar]
- 14.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol 2013;10:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emal D, Rampanelli E, Stroo I, Butter LM, Teske GJ, Claessen N, Stokman G, et al. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol 2017;28:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005;122:107–118. [DOI] [PubMed] [Google Scholar]
- 17.Suh SH, Choe K, Hong SP, Jeong SH, Makinen T, Kim KS, Alitalo K, et al. Gut microbiota regulates lacteal integrity by inducing VEGF-C in intestinal villus macrophages. EMBO Rep 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang HR, Gandolfo MT, Ko GJ, Satpute S, Racusen L, Rabb H. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol 2009;297:F1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 2011;13:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, Kotani J. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res 2008;28:321–328. [DOI] [PubMed] [Google Scholar]
- 21.Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 2011;22:849–855. [DOI] [PubMed] [Google Scholar]
- 22.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science 2010;330:665–669. [DOI] [PubMed] [Google Scholar]
- 23.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008;29:958–970. [DOI] [PubMed] [Google Scholar]
- 24.Tait Wojno ED, Artis D. Emerging concepts and future challenges in innate lymphoid cell biology. J Exp Med 2016;213:2229–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol 2010;184:2026–2037. [DOI] [PubMed] [Google Scholar]
- 26.Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, et al. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med 2013;19:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annavajhala MK, Gomez-Simmonds A, Macesic N, Sullivan SB, Kress A, Khan SD, Giddins MJ, et al. Colonizing multidrug-resistant bacteria and the longitudinal evolution of the intestinal microbiome after liver transplantation. Nat Commun 2019;10:4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nellore A, Huprikar S, Practice AICo. Vancomycin-resistant Enterococcus in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13549. [DOI] [PubMed] [Google Scholar]
- 29.Orloff SL, Busch AM, Olyaei AJ, Corless CL, Benner KG, Flora KD, Rosen HR, et al. Vancomycin-resistant Enterococcus in liver transplant patients. Am J Surg 1999;177:418–422. [DOI] [PubMed] [Google Scholar]
- 30.McNeil SA, Malani PN, Chenoweth CE, Fontana RJ, Magee JC, Punch JD, Mackin ML, et al. Vancomycin-resistant enterococcal colonization and infection in liver transplant candidates and recipients: a prospective surveillance study. Clin Infect Dis 2006;42:195–203. [DOI] [PubMed] [Google Scholar]
- 31.Fishman JA. Infection in Organ Transplantation. Am J Transplant 2017;17:856–879. [DOI] [PubMed] [Google Scholar]
- 32.Koo EH, Jang HR, Lee JE, Park JB, Kim SJ, Kim DJ, Kim YG, et al. The impact of early and late acute rejection on graft survival in renal transplantation. Kidney Res Clin Pract 2015;34:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Q, Turturice B, Wagner S, Huang Y, Gupta PK, Schott C, Metwally A, et al. Gut Microbiota Can Impact Chronic Murine Lung Allograft Rejection. Am J Respir Cell Mol Biol 2019;60:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JR, Muthukumar T, Dadhania D, Taur Y, Jenq RR, Toussaint NC, Ling L, et al. Gut microbiota and tacrolimus dosing in kidney transplantation. PLoS One 2015;10:e0122399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y, Crnkovic CM, Won KJ, Yang X, Lee JR, Orjala J, Lee H, et al. Commensal Gut Bacteria Convert the Immunosuppressant Tacrolimus to Less Potent Metabolites. Drug Metab Dispos 2019;47:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011;53:994–1002. [DOI] [PubMed] [Google Scholar]
- 37.Bromberg JS, Hittle L, Xiong Y, Saxena V, Smyth EM, Li L, Zhang T, et al. Gut microbiota-dependent modulation of innate immunity and lymph node remodeling affects cardiac allograft outcomes. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Liu L, Tang H, Jiao W, Zeng S, Xu Y, Zhang Q, et al. Immunosuppressive effect of the gut microbiome altered by high-dose tacrolimus in mice. Am J Transplant 2018;18:1646–1656. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed EB, Wang T, Daniels M, Alegre ML, Chong AS. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant 2011;11:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang CR, Alegre ML, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant 2010;10:1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntosh CM, Chen L, Shaiber A, Eren AM, Alegre ML. Gut microbes contribute to variation in solid organ transplant outcomes in mice. Microbiome 2018;6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker A, Pfitzner B, Harir M, Schaubeck M, Calasan J, Heinzmann SS, Turaev D, et al. Sulfonolipids as novel metabolite markers of Alistipes and Odoribacter affected by high-fat diets. Sci Rep 2017;7:11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda J, Nishida M, Takikawa H, Yoshida H, Azuma T, Yoshida M, Mizushina Y. Inhibitory effects of sulfobacin B on DNA polymerase and inflammation. Int J Mol Med 2010;26:751–758. [DOI] [PubMed] [Google Scholar]
- 44.Soucheray S FDA issues alert after fecal microbiota transplant death In. CIDRAP - Center for Infectious Disease Research and Policy: University of Minnesota, Office of the Vice President for Research; 2019. [Google Scholar]
- 45.Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet 1989;1:1156–1160. [DOI] [PubMed] [Google Scholar]
- 46.Ihekweazu FD, Fofanova TY, Queliza K, Nagy-Szakal D, Stewart CJ, Engevik MA, Hulten KG, et al. Bacteroides ovatus ATCC 8483 monotherapy is superior to traditional fecal transplant and multi-strain bacteriotherapy in a murine colitis model. Gut Microbes 2019;10:504–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren Z, Jiang J, Lu H, Chen X, He Y, Zhang H, Xie H, et al. Intestinal microbial variation may predict early acute rejection after liver transplantation in rats. Transplantation 2014;98:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato K, Nagao M, Miyamoto K, Oka K, Takahashi M, Yamamoto M, Matsumura Y, et al. Longitudinal Analysis of the Intestinal Microbiota in Liver Transplantation. Transplant Direct 2017;3:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun LY, Yang YS, Qu W, Zhu ZJ, Wei L, Ye ZS, Zhang JR, et al. Gut microbiota of liver transplantation recipients. Sci Rep 2017;7:3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura K, Kageyama S, Ito T, Hirao H, Kadono K, Aziz A, Dery KJ, et al. Antibiotic pretreatment alleviates liver transplant damage in mice and humans. J Clin Invest 2019;129:3420–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology 2011;53:1752–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura K, Kageyama S, Yue S, Huang J, Fujii T, Ke B, Sosa RA, et al. Heme oxygenase-1 regulates sirtuin-1-autophagy pathway in liver transplantation: From mouse to human. Am J Transplant 2018;18:1110–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion 2006;73 Suppl 1:13–27. [DOI] [PubMed] [Google Scholar]
- 54.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362:1071–1081. [DOI] [PubMed] [Google Scholar]
- 55.Fujisaka S, Ussar S, Clish C, Devkota S, Dreyfuss JM, Sakaguchi M, Soto M, et al. Antibiotic effects on gut microbiota and metabolism are host dependent. J Clin Invest 2016;126:4430–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito T, Nakamura K, Kageyama S, Korayem IM, Hirao H, Kadono K, Aziz J, et al. Impact of Rifaximin Therapy on Ischemia/Reperfusion Injury in Liver Transplantation: A Propensity Score-Matched Analysis. Liver Transpl 2019;25:1778–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Courtwright A, Koval C, Lehman S, Morales S, Furr CL, Rosas F, Brownstein M, et al. Safety and Efficacy of Bacteriophage Therapy in Lung Transplant Candidates and Recipients. J Heart Lung Transplant;38:S11–S12. [Google Scholar]
- 58.Aslam S, Courtwright AM, Koval C, Lehman SM, Morales S, Furr CL, Rosas F, et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am J Transplant 2019;19:2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, White RC, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019;575:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsu BB, Gibson TE, Yeliseyev V, Liu Q, Lyon L, Bry L, Silver PA, et al. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 2019;25:803–814 e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorski A, Jonczyk-Matysiak E, Miedzybrodzki R, Weber-Dabrowska B, Borysowski J. “Phage Transplantation in Allotransplantation”: Possible Treatment in Graft-Versus-Host Disease? Front Immunol 2018;9:941. [DOI] [PMC free article] [PubMed] [Google Scholar]