Abstract
Purpose
We verified whether fetal RPE (fRPE) cells and mesenchymal stem cells (MSCs) cotransplantation can combine the features of these two cell types and alleviate retinal degeneration in a retinal degenerative disease mouse model.
Methods
Tail vein injection of sodium iodate (NaIO3) was conducted to establish the retinal degenerative disease mouse model. MSCs and fRPE cells were transplanted either separately or combined in the subretinal space of retinal degenerative disease animals. ERG, optical coherence tomography, histologic, and immunofluorescence analyses were performed. Furthermore, the expression level of Crx, rhodopsin, Iba1, F4/80, Caspase 3, nerve growth factor, and brain-derived neurotrophic factor were assessed to investigate the mechanisms involved in cell transplantation effects.
Results
Cotransplantation of fRPE and MSC cells promoted significant improvements in ERG results and in the survival rate of transplanted cells. In addition, MSC and fRPE cell cotransplantation resulted in an increase in the thickness of the total retina, as well as in the outer and inner nuclear layers. Combined transplantation also upregulated the expression level of Crx and rhodopsin and downregulated caspase 3 expression, highlighting its better photoreceptor rescue effect in relation to the single cell type transplantation. Finally, combined transplantation suppressed the expression of Iba1 and F4/80 factors while increasing the endogenous expression of nerve growth factor and brain-derived nerve growth factor neurotrophic factors. These data suggest that MSC and fRPE cell cotransplantation is able to suppress immunoreactions and promote neurotrophic factor excretion.
Conclusions
Combined transplantation of MSCs and fRPE cells results in a better retinal rescue effect than single cell type transplantation in NaIO3-induced retinopathy.
Keywords: age-related macular degeneration, mesenchymal stem cell, retinal pigment epithelium, cell transplantation
AMD is a type of retinal degenerative disease (RDD) characterized by progressive atrophy of the RPE and photoreceptors. Dysfunction of the RPE and subsequent photoreceptor degeneration lead to progressive visual impairment and, ultimately, blindness. These effects make AMD the leading cause of visual loss in aging populations (age >50 years).1 Geographic atrophy (or the “dry form”) and choroidal neovascularization (or the “wet form”) are the two advanced AMD forms, with geographic atrophy accounting for 90% of AMD case.2 Recently, anti-VEGF therapy has been discovered to be effective for wet AMD,4 whereas currently there are no treatments available for dry AMD.3
AMD progression is mediated by several pathways, such as oxidative stress, production of inflammatory cytokines, and malfunction of the immune system.5 RPE is a vital homeostatic controller of the retina to maintain its proper functions. It is assumed that preventing or delaying RPE degeneration may impair or halt AMD development. In recent years, RPE cell replacement therapy has achieved good results in visual improvement both in animal experiments and in clinical trials, which becomes a hopeful path for dry AMD treatment.6–9 In preclinical studies, several types of RPE have been applied as sources of RPE transplantation, including embryonic stem cells–derived RPE, induced pluripotent stem cells–derived RPE, adult RPE, and fetal RPE (fRPE). Nevertheless, most transplanted cells were eliminated by chronic inflammation and/or damaged by surgical procedures. Thus, further studies are needed to improve graft survival and transplant effectiveness. Mesenchymal stem cells (MSCs) are adult stem cells with great self-renewal and differentiation capabilities. Their long-term anti-inflammatory and immune regulation effects make them a promising new source of transplantation.10 Cytokines and neurotrophic factors secreted by MSCs can support retinal cells, promote graft survival, regulate immune rejection, and delay the retinal degeneration process in AMD patients. Patients who received MSC transplants presented recovery of photoreceptors, increased electrophysiologic response, and improved visual function.11,12 The effectiveness of transplanting a single cell type is limited, and the combined transplantation of different cells seems to be a useful way to assemble features from various cell sources. Recent studies have proven the feasibility of MSC cotransplantation in several diseases, including diabetes, spinal cord damage, and myocardial infarction.13–15 The outcomes have been satisfactory so far, considering the increased therapeutic effect and the absence of side effects. In our previous study, MSC coculturing with ARPE19 cells rescued NaIO3-induced cell death by inhibiting nuclear factor κB signaling pathways.16 Therefore, we assume that MSCs cotransplantation with human fRPE cells may have a beneficial effect on the degenerated retina. In the present study, the MSCs and fRPE cells were transplanted separately or combined in the subretinal space of a mouse model with retinal degeneration. ERG, optical coherence tomography (OCT), histologic, and immunofluorescence analyses and quantitative real-time PCR (qPCR) results confirmed a better photoreceptor rescue effect in mice that underwent fRPE and MSCs combined cell transplantation than in mice that received a single cell type transplantation. This study demonstrates the feasibility of MSCs and fRPE cells cotransplantation and also provides another avenue for future treatment strategies for dry AMD.
Methods
Animals and RDD Mouse Model
Wild-type mice (C57BL/6J) were purchased from the Institute of Animal Research in Nanjing Medical University. The animals were kept at the Nanjing Medical University Laboratory Animal Unit. All experimental events were agreed by the Nanjing Medical University Ethics Committee and implemented in accordance with the recommendations of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals.
To establish the RDD mouse model, tail vein injection of 35 mg/kg sterile 1% sodium iodate (NaIO3) in saline was conducted. Mice were anesthetized by intraperitoneal injection of ketamine (37.5 mg/kg) and xylazine (5 mg/kg). Mice were randomly allocated to five groups: wild-type (healthy mice without treatment), an RDD model (NaIO3 mouse model without transplantation), fRPE (RDD mouse model transplanted with 1 µL of fRPE cell suspension), fRPE + MSC (RDD mouse model transplanted with 1 µL of fRPE and MSC cell suspension), and MSC (RDD mouse model transplanted with 1 µL of MSC cell suspension). After 30 days postoperatively, mice were anesthetized by intraperitoneal injection of ketamine (37.5 mg/kg) and xylazine (5 mg/kg) and then euthanized by cervical dislocation to perform eye sectioning, histologic evaluation, and total RNA extraction.
Cell Culture
Cell culture was performed in the laboratory of the National Stem Cell Clinical Trial Base at Jiangsu Province People's Hospital. The fRPE cells were gathered from eyes of aborted human fetuses between 11 and 14 weeks of gestation. Informed consent was obtained from the parents after explanation of the nature and possible consequences of this study. This procedure was approved by the Ethics Committee of Nanjing Medical University and followed the tenets of the Declaration of Helsinki.
After removal of the anterior segment, the vitreous, the retina and the pigment RPE monolayer were carefully separated in 10-mm petri dishes using a stereo microscope. Then, the RPE layer was digested in 1 mL of 0.25% trypsin for about 10 minutes. Finally, fRPE cells were cultured and nourished every 3 days. The materials for fRPE culture are listed as follows: MEM (α modification), N1 supplement, nonessential amino acids, taurine, hydrocortisone, triiodo-thyronin (Sigma, St. Louis, MO), glutamin 3, penicillin-streptomycin (Invitrogen, Carlsbad, CA), tryple select enzyme (Gibco, Grand Isle, NY), basement membrane matrix (Corning, Corning, NY), fetal bovine serum and heat inactivated (Thermo Scientific, Waltham, MA).
Human adipose MSCs were purchased from ATCC (PCS-500-011) and cells before 10 passages were used. The culture medium used was the MSC medium (MSCM; ScienCell Research Laboratories, Carlsbad, CA), which includes 1% MSC growth supplement (MSCGS; ScienCell Research Laboratories), 1% penicillin-streptomycin, and 5% FBS. The cells were trypsinized with 0.05% trypsin-EDTA sodium (Trypsin-EDTA, Gibco) when reaching 90% confluence, terminated by FBS. The fRPE and human adipose MSC were grown at 37°C in a humidified atmosphere with 5% CO2 in their respective standard culture media.
Cell Transplantation to RDD Animal Model
Cell transplantation was performed 7 days after NaIO3 infusion. Before surgery, the animals were anesthetized and their pupils were dilated with 1% tropicamide. A 32-gauge syringe (Hamilton, Reno, NV) containing 5 µL of cell suspension was inserted into the subretinal space. The limbus was punctured by a sterile 30-gauge ½ needle to relieve intraocular pressure and decrease cell reflux at the injection site. The fRPE cells and MSCs or their combination were implanted in the subretinal space of RDD-induced mice. Cell suspensions (5 µL/eye, total 6 × 105 cells/eye) were gently implanted in the subretinal space. In the fRPE + MSCs cotransplantation group, cell suspension fusions cell contained fRPE (5 × 105 cells/eye) and MSCs (1 × 105 cells/eye) cells. The ratio of fRPE to MSC in cotransplantation group was determined according to our cell experiment (Supplementary Fig. S1). After the operations, fundus examinations were performed by direct visualization on the ophthalmoscope.
ERG
ERG was performed at 7, 30, and 90 days after surgery to assess changes in retinal function. Mice were dark adapted for 1 hour and then anesthetized before ERG. Corneas were anesthetized with 0.5% Alcaine (Alcon, Rijksweg 14, 2870 Puurs, Belgium). Two active gold electrodes humidified by an ophthalmic gel (Lacrinorm 0.2%, Bausch & Lomb, Rochester, NY) were placed in each cornea. The ground and reference electrodes were placed on the forehead and tail, respectively. In accordance with the ISCEV full-field flash ERG standard, the ERG was performed as follows: dark-adapted ERG with a −25 dB (0.0095 cds/m2), −15 dB (0.095 cds/m2), and −5 dB (0.95 cds/m2) LED flash stimulus (rod system response); dark-adapted 3.0 ERG with a 0 dB (3.00 1626 cds/m2) LED flash stimulus (mixture of rod and cone system response); dark-adapted 3.0 OPS with a 0 dB (3.00 cds/m2) LED flash stimulus (oscillation potentials); and light-adapted 3.0 ERG with a 0 dB (3.00 cds/m2) LED flash stimulus (cone system response). The bandpass filter was set between 1 and 300 Hz. All the procedures were completed in a dark room with slight red safety lights. The results were recorded as the a-wave and b-wave amplitudes in each group.
OCT
OCT was performed 30 days after cell transplantation. Mice were anesthetized and their pupils were dilated. Scans centered on the optic nerve were obtained (Cirrus HD-OCT 5000, Carl Zeiss, 07745 Jena, Germany). The mean internal limiting membrane to RPE thickness was used to draw a three-dimensional map of the entire retina. Two central sections, namely, nasal to temporal and ventral to dorsal, were separated to normalize the data.
Histologic Analysis
One month after transplantation, mice were anesthetized by intraperitoneal injection of ketamine and xylazine and then killed by cervical dislocation and the eyes were enucleated and fixed in 4% paraformaldehyde for 2 hours. The eyes were then embedded in paraffin and sectioned 5 µm thick. Sections containing the optic nerve head were collected and stained with HE. The images were captured by an optical microscope (Olympus 1X71, Tokyo, Japan) and thicknesses of the whole retina, outer nuclear layer (ONL) and inner nuclear layer (INL) were measured at the same distance from the optic disc. These measurements were obtained by a single observer, who was unaware of the different experimental groups.
Immunofluorescence and Cell Count
The primary antibodies used were mouse anti-STEM121 (1:250; Cellartis, Gothenburg, Sweden, Catalog Y40410) and anti-BEST1 (1:200; Abcam, Cambridge, UK, Catalog ab2182). Immunofluorescence was performed according to the manufacturer's instructions. Briefly, the sections were washed with PBS and blocked for 1 hour in 1% BSA (Sigma) solution at room temperature. Then, the sections were incubated with primary and secondary antibodies. Cell nuclei were contrasted with DAPI. The confocal images were taken by a confocal fluorescence microscope (ZEISS, LSM700). The numbers of STEM121- and BEST1-positive cells were manually calculated and averaged in each eye to quantitatively assess the number of transplanted cells. For cell counting, a total of 60 sections were collected from each eye. One for every 5 sections were counted, that is, 12 sections were analyzed in each eye.
qPCR Assays
Total mRNA was extracted by the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA) and cDNA was synthesized with the PrimeScript RT Reagent Kit (Takara, Japan, Shiga). Quantitative real-time PCR was conducted by SYBR Premix Ex Taq (Takara) in a thermal cycler dice real time System (Takara). Cycle time values were acquired after analysis with the Sequence Detection System and analysis software (Applied Biosystems, Foster City, CA). The 2−ΔΔCt calculation method was applied to analyze the data. Data were reported as fold change over the control. Primers are shown in the Supplementary Table S1.
Statistical Analysis
All results were assessed by one-way ANOVA test or unpaired Student t test using GraphPad Prism 6 software (GraphPad Software Inc, La Jolla, CA). Data were presented as mean ± SEM. A P value of less than 0.05 was considered statistically significant.
Results
Cotransplantation of MSCs and fRPE Cells Promotes Retinal Function Restoration
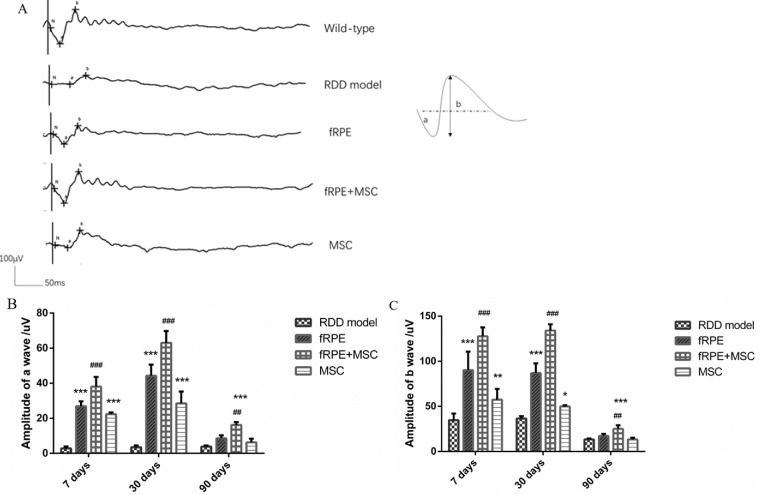
As a visual electrophysiologic examination, ERG can display retinal function by evaluating the amplitudes of a- and b-waves. To establish the appropriate NaIO3 treatment concentration that affects the retinal function, mice were injected with increasing dilutions of NaIO3. After NaIO3 injection, mice were examined by ERG. Scotopic ERG amplitudes of a- and b-waves showed a rapid decline and decreased in a dose-dependent manner (Supplementary Fig. S2). We used a 35 mg/kg dilution of NaIO3 in this study to mimic the pathogenic environment of AMD. Then, we investigated the protective effect of cell transplantation on retinal function after oxidative damage. Mice were injected with 35 mg/kg of NaIO3 through the tail vein and then the subretinal transplantation of fRPE, MSC, and fRPE + MSC cells was performed. ERGs were performed on mice at 7, 30, and 90 days after cell transplantation to reveal the functional improvements of the injured retina. The results showed that, compared with the RDD model group, all cell transplanted groups presented a significant increase in amplitudes of a- and b-waves at 7 and 30 days after cell transplantation (Fig. 1). In the fRPE + MSC transplantation group, the amplitudes of the a- and b-waves (38.03 ± 5.51 µV and 127.67±9.93 µV, respectively; P < 0.01) increased significantly 7 days after cell transplantation, compared with the fRPE (a-wave, 26.87 ± 2.85 µV; b-wave, 90.17 ± 20.53 µV; P < 0.01) and MSC transplantation groups (a-wave, 22.37 ± 1.03 µV; b-wave, 57.48 ± 11.89 µV: P < 0.01; Fig. 1). At the end of 30 days, the amplitudes of a- and b-waves in the cotransplantation group (a-wave, 63.00 ± 6.78 µV; b-wave, 134.00 ± 6.90 µV; P < 0.01) were also drastically higher than those in the transplanted groups of a single cell type (fRPE transplanted group presented a-wave, 44.22 ± 6.42 µV; b-wave, 86.97 ± 10.76 µV; P < 0.01; MSC transplanted group presented a-wave, 28.52 ± 6.72 µV; b-wave, 49.98 ± 1.38 µV; P < 0.01) (Fig. 1). However, after 90 days, no significant difference was found between the fRPE and MSC transplanted and RDD model groups. Meanwhile, although smaller, the amplitudes of a- and b-waves in the cotransplantation group (a-wave, 16.17 ± 1.67 µV; b-wave, 25.05 ± 3.97 µV; P < 0.01) still exhibited a considerably better effect on transplanted groups of a single cell type (fRPE transplanted group presented a-wave, 8.54 ± 1.74 µV; b-wave, 17.23 ± 2.29 µV; P < 0.01; MSC transplanted group presented a-wave, 6.30 ± 2.08 µV; b-wave, 13.27 ± 2.07 µV; P < 0.01; Fig. 1).
Figure 1.
Cotransplantation of MSCs and fRPE cells promotes retinal function restoration. (A) Representative scotopic ERG records 30 days after transplantation in the five experimental groups. Quantification of (B) a-wave and (C) b-wave amplitudes of RDD model, fRPE, fRPE + MSC and MSC mice after 7 days, 30 days and 90 days of cell transplantation. Data are shown as the mean ± SEM, n = 6. One-way ANOVA followed by Tukey's multiple comparison test. * P < 0.05, ** P < 0.01, ***P < 0.001 versus the RDD model group; ## P < 0.01, ### P < 0.001 versus the fRPE group.
Cotransplantation of MSCs and fRPE Cells Promotes a Better Photoreceptor Preservation Effect Than Single Cell Type Transplantation
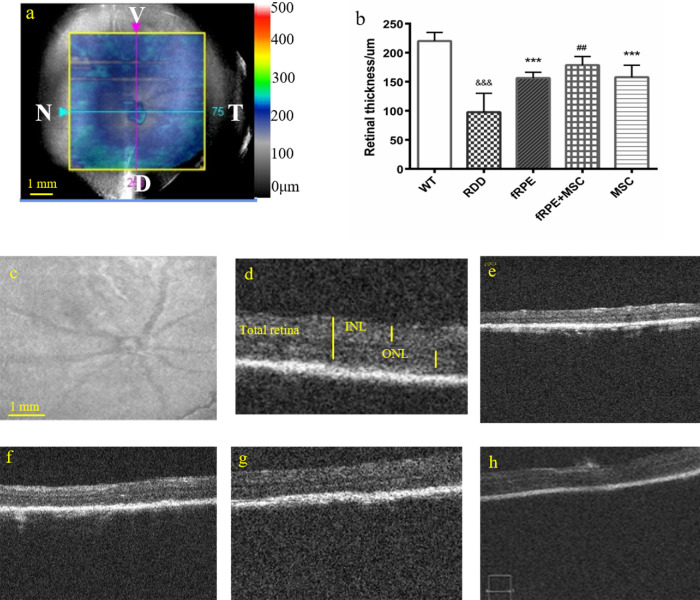
The pathology of dry AMD is characterized by the loss of retinal cells, especially RPE cells and photoreceptors. Therefore, the retinal structure was also examined to verify the photoreceptor rescue effect caused by cell cotransplantation. One month after transplantation, OCT was performed and the eyes of the mice were sectioned and stained with hematoxylin and eosin (HE). According to the results of the three-dimensional maps and the optical sections of the OCT, the retinal thickness (from internal limiting membrane to RPE) was obviously reduced after NaIO3 treatment from 201 to 241 µm in wild-type eyes to 45 to 112 µm in RDD mouse model eyes (Fig. 2). The HE-stained sections showed an attenuation of the retinal thickness, an irregular OS, and a disrupted RPE layer after NaIO3 treatment (Fig. 3c). The ONL, INL, and total retina thickness were measured to investigate the morphologic protective effect of different groups of cell transplantation.
Figure 2.
OCT assays demonstrate that the retinal thickness recovers significantly after 30 days of fRPE, fRPE + MSC and MSC cell transplantation. (a) Representative retinal image of OCT three-dimensional maps in wild-type control mice, with a scale bar of 1 mm. OCT scanned a 6 × 6-mm2 area of the mouse retina as marked by a yellow square. Blue lines represent nasal (N) to temporal (T) axes and pink lines represent ventral (V) to dorsal (D) axes. The point of intersection of two lines represents the optic nerve of each eye. (b) Measurement of total retina thickness in the five experimental groups 30 days after cell transplantation. (c) Representative retinal image of red-free fundus photographs in wild-type control mice. (d–h) Optical sections in the optic nerve in the temporal area of normal wild-type mice, RDD model, fRPE, fRPE + MSC and MSC transplanted mice, respectively. Data are shown as mean ± SEM, n = 6. Significant differences were calculated using one-way ANOVA followed by Tukey's multiple comparison test. &&& P < 0.01 versus wild type; *** P < 0.001 versus RDD model group; ## P < 0.01 versus fRPE group.
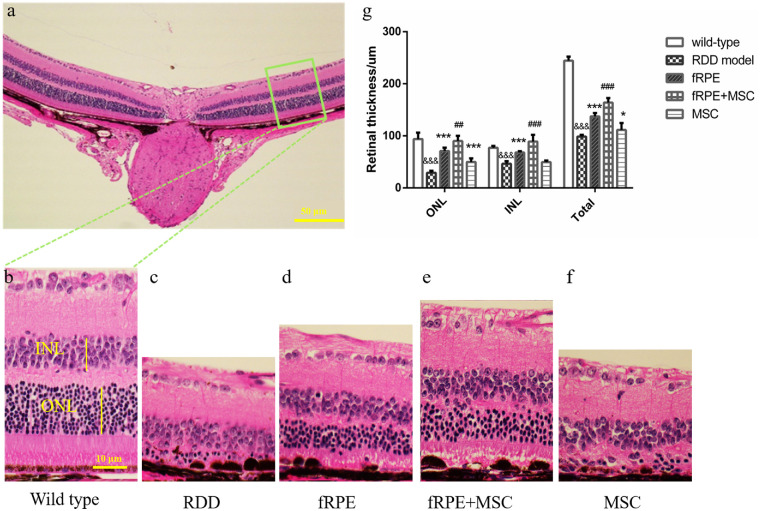
Figure 3.
fRPE, fRPE + MSC, and MSC cell transplantation prevents retinal thinning in histologic analysis. (a) Representative image of the HE stained retina of wild-type control mice with a 50 µm scale bar. (b–f) Retinal segments between 400 and 600 µm from the optic nerve in the temporal area of wild-type mice, RDD model, fRPE, fRPE + MSC and MSC transplanted mice, respectively, with a scale bar of 10 µm (green box in a). (g) Measurement of the ONL, INL, and total retina thickness in the five experimental groups after 30 days of cell transplantation. Data are shown as mean ± SEM, n = 6. Significant differences were calculated using one-way ANOVA followed by Tukey's multiple comparison test. &&& P < 0.001 versus wild type; * P < 0.05, *** P < 0.001 versus RDD model group; ## P < 0.01, ### P < 0.001 versus the fRPE group.
OCT results showed that retinal thickness was better preserved in all cell transplantation groups compared with the RDD model group. In addition, retinal resuscitation was considerably improved in the fRPE + MSC cotransplantation group (178.6 ± 4.956 µm) than in the fRPE (156.3 ± 3.3 µm; P < 0.01) and MSC (157.8 ± 7.32 µm; P < 0.05) transplantation groups. HE results also showed that the total retinal thickness was greater in all cell transplantation groups than in the RDD model group (Fig. 3). Moreover, the ONL rescue effect was more robust in the fRPE + MSC (90.15 ± 9.98 µm) cotransplantation group than in the fRPE (70.97 ± 6.52 µm; P < 0.01) and MSC transplantation groups (49.80 ± 7.04 µm; P < 0.01). Significant recovery of INL thickness was also observed 1 month after transplantation in the fRPE (68.24 ± 2.34 µm; P < 0.01) and fRPE + MSC (89.04 ± 12.86 µm; P < 0.01) transplanted eyes, compared with the untreated group (46.25 ± 5.17 µm).
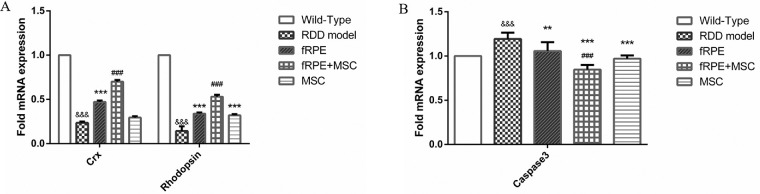
The qPCR assays were conducted 30 days after transplantation to verify the photoreceptor rescue effect caused by different cells transplantations. The results showed greater expression of rod photoreceptor marker (Crx and rhodopsin) in all cell transplantation groups than in the RDD model group. In addition, the level of expression of this marker was significantly higher in the fRPE + MSC cotransplantation group than in the fRPE and MSC transplanted groups (Fig. 4A). These data indicate that the fRPE + MSC cotransplantation exhibits a better photoreceptor protection effect than transplanted groups of a single cell type.
Figure 4.
fRPE + MSC cell cotransplantation exhibits a photoreceptor protection effect and relieves NaIO3-induced cell apoptosis. (A) Crx and rhodopsin expression were significantly higher in the fRPE + MSC cotransplatantion group than in the transplanted groups of a single cell type (fRPE or MSC only) 30 days after transplantation. &&& P < 0.001 fRPE + MSC versus wild type; *** P < 0.001 versus RDD model group; ### P < 0.001 versus fRPE group (n = 6). (B) The expression of caspase 3 significantly decreased in cell transplantation groups, especially in fRPE + MSC cotransplantation group. &&& P < 0.001 versus wild type; ** P < 0.01, *** P < 0.001 versus RDD model group; ### P < 0.001 versus fRPE group (n = 6).
We assessed the relative expression level of caspase 3 with qPCR to elucidate whether the relief of cell apoptosis can be attributed to the protective effect of the cell. Our results showed that the increased expression of Caspase 3 induced by NaIO3 was reversed by cell transplantation, especially by the cotransplantation group (Fig. 4B).
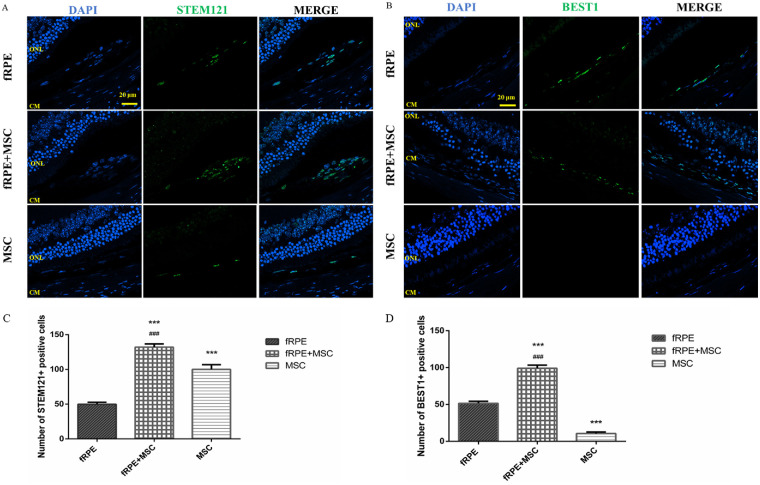
fRPE + MSC Cell Cotransplantation Improves Graft Survival
To elucidate the protective effect of MSCs on the support of transplanted fRPE cells, immunofluorescence was used 30 days after cell transplantation. As a marker for transplanted cells, human-specific marker STEM121 staining (Fig. 5A) was applied to label grafts and BEST1 staining (Fig. 5B) was used to distinguish fRPE cells from MSCs. By calculating the average number of STEM121+ cells per eye, we found that the STEM121+ cell number were notably higher in the fRPE + MSC cotransplantation group (132.00 ± 4.73 cells) than in the fRPE (49.83 ± 2.86 cells; P < 0.01) and MSC (100.00 ± 6.81 cells; P < 0.01) transplantation groups (Fig. 5C). To detect the survival of transplanted fRPE cells in different transplantation groups, we also calculated the average number of BEST1+ cells per eye. We found that the BEST1+ cell number was notably higher in the fRPE + MSC group (99.00 ± 4.29 cells) than in the fRPE (51.50 ± 2.66 cells; P < 0.01) group (Fig. 5D). These results indicated that the combined transplantation can facilitate the transplant survival, especially in relation to fRPE cells.
Figure 5.
Immunofluorescence staining using human STEM121 and BEST1 staining (both in green) was performed 30 days after cell transplantation. Scale bars = 20 µm. (A–B) Sections were stained using human STEM121 and BEST1 staining (both in green). Nuclei were visualized with DAPI counterstain (blue). (C) Quantification of STEM121+ and (D) BEST1+ cells in the mouse retina. After 30 days of cell transplantation, the average number of STEM121+ or BEST1+ cells in the whole retina were significantly higher in the fRPE + MSC cotransplantation group than in the fRPE transplantation group. Data are shown as the mean ± SEM, n = 6. One-way ANOVA followed by Tukey's multiple comparison test. *** P < 0.001 versus fRPE group; ### P < 0.001 versus MSC group. CM, choroid membrane.
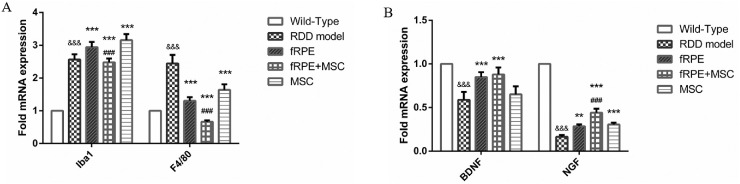
fRPE + MSC Cell Cotransplantation Suppresses the Expression of Iba1 and F4/80 and Increases the Endogenous Expression of Nerve Growth Factor and Brain-Derived Nerve Growth Factor in NaIO3-Induced Retinopathy
We analyzed the expression level of microglia/macrophage factors Iba1 and F4/80 by qPCR after 30 days of cell transplantation owing to the microglia cell playing a crucial role in retinal disfunction.17 Our data revealed that the relative level of expression of Iba1 and F4/80 microglial factors in the combined transplantation group (fRPE + MSC) decreased more than in the RDD model and in the fRPE transplantation groups (Fig. 6A). Remarkably, the relative Iba1 expression level in the single cell type transplantation groups increased more than that in the RDD model group. This effect can probably be attributed to the damage from surgery, which could be verified by an additional experimental group injected with PBS to replace cell transplantation after NaIO3 treatment. Additionally, we assayed the expression level of endogenous neurotrophic factors by q-PCR and we found that nerve growth factor and brain-derived nerve growth factor expression level increased after cell transplantation, especially in the cotransplantation group (Fig. 6B). Furthermore, the combined transplantation group showed a higher level of nerve growth factor expression than the fRPE group (P < 0.01).
Figure 6.
fRPE + MSC cell cotransplantation suppresses the expression of Iba1 and f4/80 and increases the nerve growth factor and brain-derived nerve growth factor expression in NaIO3-induced retinopathy. (A) The expression level of Iba1 and F4/80 microglial factors significantly decreased in the fRPE + MSC cotransplantation group. &&& P < 0.001 versus wild type; ** P < 0.01, *** P < 0.001 versus RDD model group; ### P < 0.001 versus fRPE group (n = 6). (B) The expression level of nerve growth factor and brain-derived nerve growth factor neurotrophic factors significantly increased in all cell transplantation groups 1 month after the cell transplantation, especially in the fRPE + MSC cotransplantation group. &&& P < 0.001 versus wild type; ** P < 0.01, *** P < 0.001 versus RDD model group; ### P < 0.001 versus fRPE group (n = 6).
Discussion
The successful establishment of retinal degeneration mouse model by NaIO3 treatment and retinal function preservation after fRPE + MSC cell cotransplantation were observed by ERG analysis. Retinal function in all experimental groups decreased from 30 to 90 days, which was attested by the observation of the decrease in a- and b-waves over time. At 90 days after transplantation, neither fRPE nor MSCs cells alone retained retinal function. However, the cotransplantation of fRPE and MSC cells maintained some retinal function, implying a reciprocal effect between these two cell types. Over 3 months of follow-up, the a- and b-wave amplitudes in the fRPE + MSC cotransplantation group were constantly higher than in the fRPE and MSC transplantation groups. These results showed a greater protective effect of retinal function in fRPE + MSC transplanted eyes than in eyes transplanted with isolated fRPE and MSC cells. This effect was preserved for at least 3 months.
Subsequently, we demonstrated the structural preservation of the retina in RDD NaIO3-induced mouse model by HE and OCT methods after combined transplantation of fRPE and MSCs cells. The role of MSCs in supporting fRPE cells survival after transplantation was demonstrated by fluorescein staining and graft cell counting. The pathologic results confirmed the degeneration of the overall retina (including ONL and INL) in the RDD model group compared with the wild-type group. Pathologic data revealed that the cotransplantation group showed a thicker ONL and a greater number of surviving transplanted cells, in agreement with the ERG results. This finding can be attributed to the mutual effect of MSCs and fRPE cells, where MSC cells have created a better microenvironment and fRPE cells have specialized in restoring the retina. Similar results were obtained with INL and total retina thickness. In addition, the fRPE transplantation group showed better rescue effect than the MSC transplantation group. We evaluated the expression of photoreceptor function genes (Crx and rhodopsin) because the ONL thickness is normally used to assess the level of photoreceptor protection.18 We found that the expression level of these genes increased significantly in combined transplantation group, which is in line with the ONL thickness data obtained. Our previous study showed that the NaIO3 injury could lead to cell apoptosis and that coculture with MSCs was able to suppress this effect.16 To explore whether the alleviation of cell apoptosis was related to the cell protective effect, caspase 3 expression level was assessed by qPCR assays. We showed that caspase 3 expression was increased in the RDD model and decreased in cell transplantation groups, especially in the cotransplantation one. Taken together, our data demonstrated that MSC + fRPE cotransplantation group presented the best photoreceptor rescue effect among the experimental groups studied. However, it is necessary to further investigate the pathways involved in the MSC + fRPE cotransplantation effect to understand its mechanism in protecting retina cell death in a more comprehensive way.
Microglial activation is reported to play a vigorous role in the progression of retinal degeneration. Microglial activation serves as an inflammation strengthening factor in pathologic states,19 thus impairing the survival of transplanted cells.20 The elevation of expression level of Iba1 and F4/80 factors indicates the activation of macrophages or microglial cells. Our study found that the combined cell transplantation (MSC + fRPE) provided a greater gliosis-suppressing state than single cell type transplantation (fRPE or MSC only). These data highlight the benefits of the reciprocal effect between these two cell types in the cotransplantation strategy. Chemokines play a major role in helping the survival of transplanted cells. Thus, the level of expression of endogenous neurotrophic factors have been tested because the primary role of MSCs is to improve the cellular microenvironment.21 In this study, the cotransplantation group presented more excretion of neutrophic factors when compared with the transplanted groups with a single cell type. This effect can be attributed to the vitality of the transplanted cells.
In summary, the present study indicated that (i) NaIO3 injection induced apoptosis of retinal cells, (ii) retinal cells apoptosis was followed by a reduction in retinal thickness and impaired retinal function, (iii) cotransplantation of fRPE + MSC cells was able to relieve the atrophy of photoreceptors and preserve the retinal function of RDD animals, and (iv) this effect was obtained by suppressing immunoreaction and promoting excretion of neurotrophic factors. Further studies are required to explore the molecular mechanisms of combined fRPE + MSCs transplantation in the rescue of retinal cells in NaIO3-induced retinopathy. Therefore, our study showed that combined transplantation of MSCs and fRPE cells results in a better retinal rescue effect than transplantation of a single cell type in NaIO3-induced retinopathy after 30 days of cell transplantation. As a result, combined transplantation can replace transplantation of a single cell type as a conceivable strategy to improve graft survival and the therapeutic effect in the treatment of degenerative retinal diseases. However, further studies, including prolonged observation times and the identification of fRPE functions, are needed to provide more evidence to guide future clinical applications.
Supplementary Material
Acknowledgments
Supported by Changzhou Sci&Tech Program (Grant No CJ20190067). Some work was supported by grants from the National Key Research and Development Program of China (2017YFA0104101) and the National Natural Science Foundation of China (81970821, 81770973, 81870694).
Disclosure: T. Pan, None; H. Shen, None; S. Yuan, None; G. Lu, None; Y. Zhang, None; H .Wang, None; Y. Zhao, None; X. Sun, None; Q. Liu, None
References
- 1. Milam A, Brantley Jr., Handa James T. Foreword: dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018; 59: 3–20. [DOI] [PubMed] [Google Scholar]
- 2. Stein JD, Vanderbeek BL, Talwar N, et al.. Rates of nonexudative and exudative AMD among Asian American ethnic groups. Invest Ophthalmol Vis Sci. 2011; 52: 6842–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel HR, Hariprasad SM, Eichenbaum D. Geographic atrophy: clinical impact and emerging treatments. Ophthalmic Surg Lasers Imaging Retina. 2015; 46: 8–13. [DOI] [PubMed] [Google Scholar]
- 4. Zampros I, Praidou A, Brazitikos P, et al.. Antivascular endothelial growth factor agents for neovascular age-related macular degeneration. J Ophthalmol. 2012; 2012: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaw PX, Stiles T, Douglas C, et al.. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci. 2016; 3: 196–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Binder S, Stanzel BV, Krebs I, et al.. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007; 26: 516–554. [DOI] [PubMed] [Google Scholar]
- 7. Mead B, Berry M, Logan A, et al.. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015; 14: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nazari Hossein, Zhang Li, Zhu Danhong, et al.. Stem cell-based therapies for age-related macular degeneration: the promises and the challenges. Prog Retina Eye Res. 2015; 48: 1–39. [DOI] [PubMed] [Google Scholar]
- 9. Qu Z, Guan Y, Cui L, et al.. Transplantation of rat embryonic stem cell-derived retinal progenitor cells preserves the retinal structure and function in rat retinal degeneration. Stem Cell Res Ther. 2015; 6: 219–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng TK, Fortino VR, Pelaez D, et al.. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells. 2014; 6: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oner A, Gonen ZB, Sinim N, et al.. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study. Stem Cell Res Ther. 2016; 7: 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doorn J, Moll J, Le Blanc K, et al.. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng. Part B Rev. 2012; 18: 101–115. [DOI] [PubMed] [Google Scholar]
- 13. Ohmura Y, Tanemura M, Kawaguhi N, et al.. Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation. 2010; 90: 1366–1373. [DOI] [PubMed] [Google Scholar]
- 14. Park DY, Mayle RE, Smith RL, et al.. Combined transplantation of human neuronal and mesenchymal stem cells following spinal cord injury. Global Spine J. 2013; 3: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Souza LC, Carvalho KA, Rebelatto C, et al.. Combined transplantation of skeletal myoblasts and mesenchymal cells (cocultivation) in ventricular dysfunction after myocardial infarction. Arq Bras Cardiol. 2004; 83: 288–299. [DOI] [PubMed] [Google Scholar]
- 16. Mao Xiying, Pan Ting, Shen Han, et al.. The rescue effect of mesenchymal stem cell on Sodium iodate-induced retinal pigment epithelial cell death through deactivation of NF-kB-mediated NLRP3 inflammasome. Biomed Pharmacother. 2018; 103: 517–523. [DOI] [PubMed] [Google Scholar]
- 17. Jin N, Gao L, Fan X, et al.. Friend or foe? Resident microglia vs bone marrow-derived microglia and their roles in the retinal degeneration. Mol Neurobiol. 2017; 54: 4094–4112. [DOI] [PubMed] [Google Scholar]
- 18. Luo J, Baranov P, Patel S, et al.. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014; 289: 6362–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng B, Xiao J, Wang K, et al.. Suppression of microglial activation is neuroprotective in a mouse model of human retinitis pigmentosa. J Neurosci. 2014; 34: 8139–8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hippert C, Graca AB, Pearson RA. Gliosis can impede integration following photoreceptor transplantation into the diseased retina. Adv Exp Med Biol. 2016; 854: 579–585. [DOI] [PubMed] [Google Scholar]
- 21. Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012; 3: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.