Highlights
-
•
Neutralizing antibody evaluation helps to assess the risk of re-infection.
-
•
Some COVID-19 patients may not develop neutralizing antibody after recovery.
-
•
SARS-CoV-2 neutralizing antibody levels are correlated with COVID-19 disease severity.
Keywords: SARS-CoV-2, COVID-19, Neutralizing antibody, Antibody-dependent enhancement, Humoral immunity, Pneumonia
Abstract
The emerging coronavirus disease 2019 (COVID-19) has become a serious global public health threat. With more and more recovered patients, it is urgently needed for evaluation of the neutralizing antibody (NAb) in these patients. In this study, we collected blood samples from 49 patients recently recovered from COVID-19. Serum NAbs were measured using a novel surrogate virus neutralization test (sVNT). Factors associated with NAb titers were analyzed using Ordinary Least Squares regression model. The median age of the study participants was 37 years (IQR, 30.0–54.5) and 55.1 % (27/49) of which were male. The median time to blood collection (for NAb analysis) from illness onset, viral clearance and discharge were 43.0 days (IQR, 36.0–50.0), 27.0 days (IQR, 20.5–37) and 17.0 days (IQR, 15.0–33.0), respectively. Patients had a median NAb titer of 1: 40 (IQR, 1:15–1:120). NAbs were not detected in two asymptomatic children who quickly cleared the virus. NAb titers were higher in patients with older age (p = 0.020), symptomatic infection (p = 0.044), more profound lung involvement (p<0.001), abnormal C-reactive protein level (p<0.01) and elevated lactate dehydrogenase (p = 0.019). Multivariable analysis revealed that severity of pneumonia and having comorbidity positively correlated with NAb titers in recovered patients (p = 0.02), while use of corticosteroids negatively impacted NAb titers (p = 0.01). Our study suggests that some COVID-19 patients may not have detectable NAb after recovery. SARS-CoV-2 NAb titers are positively correlated with severity of COVID-19 pneumonia.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is a major global crisis for human being, which has spread worldwide with such devastating speed and has caused a huge death toll and economic loss [1]. The causative pathogen, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), belongs to the subgenus Sarbecovirus of the genus Betacoronavirus and shares 79.6 % sequence identity to SARS-CoV [2]. Unlike SARS-CoV which generally causes severe lung injury, SARS-CoV-2 infection has wider disease spectrum ranged from asymptomatic infection to life-threatening respiratory failure [3,4]. The asymptomatically infected COVID-19 patients pose great public concern as they could also transmit SARS-CoV-2 and are difficult to be recognized without extensive testing [3,5,6]. Serologic surveillance of COVID-19 is attracting great interest because it not only helps to identify the affected cases but may also provide important information of herd immunity. During SARS-CoV infection, viral specific IgG peaks at month 4 after disease onset and decreases markedly after 16 months [7]. Although antibody responses are generally regarded as protective, detrimental effects, known as antibody-dependent enhancement (ADE), may also occur in the course of SARS-CoV infection [[8], [9], [10]]. It is unclear whether ADE play a role in the pathogenesis of COVID-19. A recent study showed that critically ill patients with COVID-19 had higher total SARS-CoV-2 antibody titers compared with patients with less severe diseases [11]. However, whether higher neutralizing antibody (NAb) titers are associated with more profound lung damage has yet to be elucidated.
In this study, we analyzed the SARS-CoV-2 NAb titers in patients recently recovered from COVID-19 using a novel SARS-CoV-2 surrogate virus neutralization test (sVNT) [12]. Also, we retrospectively measured and semi-quantified the lung injury of patients based on their chest computed tomography (CT) at admission through a previously described grading system [13]. We found the NAb levels among recovered patients varied. Remarkably, the NAbs were not detected in two recovered COVID-19 children. Taken together, NAb levels in recovered COVID-19 patients were positively correlated with the severity of lung injury. Our study suggested that recovered patients of COVID-19 may incur reinfection, and ADE could possibly contribute to progression of COVID-19 pneumonia.
2. Patients and methods
2.1. Patients
This study enrolled 49 patients who recently recovered from COVID-19, and were scheduled for their first follow-up visits in outpatients of the second hospital of Nanjing, China, from March 5, 2020 to March 16, 2020. The diagnosis of COVID-19 in those patients was based on positive nucleic acid test for SARS-CoV-2 from throat swab sample during their previous hospitalization. During inpatient days, SARS-CoV-2 viral loads from throat swab specimens were evaluated every other day using quantitative reverse transcription polymerase chain reaction (qRT-PCR) kits (BGI Genomics, Beijing, China) following WHO guidelines, as previously described [3]. When two-consecutive throat-swab samples obtained at least 24 h apart were negative for SARS-CoV-2 RNA, the patient was considered to have cleared the virus [14]. Blood samples were collected at the time of the first follow-up visit and serum samples were stored at -80℃ until NAb analysis. The medical records, including demographic data, medical history, underlying comorbidities, symptoms, laboratory parameters, radiological features and treatments, were collected from electronic health record system, and were compared between patients with different NAb titers. This study was approved by the ethics committee of the second hospital of Nanjing (reference number: 2020-LS-ky003). Written informed consent was obtained from patients in this study.
2.2. Semi-quantification of the lung injury at admission
A semi-quantitative method was applied to grade the severity of COVID-19 pneumonia, as described previously [13]. Based on the area of lung abnormalities on chest computed tomography, each lung lobe was visually scored from 0 to 5 that no lesion was assigned a score of 0. Involvements of<5 %, 5–25 %, 26 %–49 %, 50 %–75 % and >75 % of the lobe were assigned scores of 1, 2, 3, 4 and 5, respectively. The score of each chest CT was the sum of individual lobar score, and therefore could range from 0 to 25. Two experienced doctors with more than 10-year experience in thoracic radiology reviewed the CT images and determined final scores by consensus.
2.3. Surrogate virus neutralization test
Serum NAbs against receptor binding domain (RBD) of SARS-COV-2 were measured by a sVNT with the commercial kit provided by GenScript (Catalog number, L00847) as described recently with modification [12]. Samples were tested in duplicate. Briefly, 200 ng/well hACE2 was coated in the carbonate-bicarbonate coating buffer. The serum samples were pre-inactivated at 56℃ for 30 min, diluted with 2-fold serial gradients, and incubated with equal volume of horseradish peroxidase-conjugated RBD (HRP-RBD) at 37℃ for 30 min. Then, the serum/HRP-RBD mix (100 μl) was added to each well and incubated at 37℃ for 15 min. Unbound HRP-RBD were removed by four washes, followed by addition of chromogenic substrate TMB and incubation at 25℃ for 15 min. Colorimetric reaction was stopped by addition of stop solution. The absorbance reading at 450 nm was measured using microplate reader (BioTek Synergy H1). Inhibition (%) = (1 - Sample OD450 value/Negative Control OD450 value) × 100. The reciprocal of the maximal dilution with 50 % inhibition was counted as the titer of neutralizing antibody in tested serum sample.
2.4. Statistical analysis
Continuous variables were expressed as the medians and interquartile ranges (IQR). Categorical variables were summarized as the counts and percentages in each category. Comparison between groups was done using the Mann–Whitney U test, and Chi-Square test or Fisher's exact test for categorical variables as propriate. The Jonckheere-Terpstra test was used to analyze the trend of median NAb titers with age or pulmonary radiological abnormalities. Correlation was measured using Spearman's rank correlation coefficient. For the multivariable analyses, we developed Ordinary Least Squares (OLS) regression model to identify the clinical variables associated with the NAb titers after controlling for age and gender. Since the antibody NAb titer was a skewed distribution, we standardized it and used its Z score (mean = 0 and Std = 1) as the dependent variable. In our regression model, comorbidity, corticosteroid treatment, and intravenous immunoglobulin usage were dichotomized into “Yes vs. No”, and C-reactive protein (CRP) was dichotomized into “>10 mg/l vs. < = 10 mg/L”. CT score, lymphocyte count, and days from virus clearance used their original values. The statistical analysis was done by SPSS version 22.0 (IBM). A P value <0.05 is considered statistically significant. Distributions of NAb titers among different patient groups were plotted by graphpad prism version 6.
3. Results
3.1. Clinical characteristics of the patients
Of the 49 patients, 2 had severe illness and 8 had asymptomatic infection. The median age was 37 years (IQR, 30.0–54.5). 55.1 % (27/49) were male, 8.2 % (4/49) were daily smokers, and 22.4 % (11/49) had underlying comorbidities, including hypertension (12.2 %, 6/49) and diabetes (2.0 %, 1/49). The median time to blood collection (for NAb analysis) from illness onset, viral clearance and discharge were 43.0 days (IQR, 36.0–50.0), 27.0 days (IQR, 20.5–37) and 17.0 days (IQR, 15.0–33.0), respectively (Table 1 ).
Table 1.
Comparison of the clinical, laboratory and radiological characteristics among the patients with different SARS-CoV-2 neutralizing antibody titers.
| SARS-CoV-2 neutralizing antibody titers |
||||
|---|---|---|---|---|
| All (n = 49) | <1:80 (n = 32) | ≥1:80 (n = 17) | p value | |
| Demographics and clinical characteristics | ||||
| Age, years | 37 (30.0–54.5) | 35.5 (27.0–49.8) | 49 (33.0–59.0) | 0.047* |
| ≥60 | 7 (14.3) | 3 (9.4) | 4 (23.5) | 0.358 |
| Sex | … | … | … | 0.153 |
| Female | 22 (44.9) | 12 (37.5) | 10 (58.8) | … |
| Male | 27 (55.1) | 20 (62.5) | 7 (41.2) | … |
| Currently smoking | 4 (8.2) | 4 (12.5) | 0 (0) | 0.331 |
| Comorbidity | 11 (22.4) | 4 (12.5) | 7 (41.2) | 0.054 |
| Hypertension | 6 (12.2) | 2 (6.3) | 4 (23.5) | 0.194 |
| Diabetes | 1 (2.0) | 0 (0) | 1 (5.9) | 0.347 |
| Other | 5 (10.2) | 2 (6.3) | 3 (17.6) | 0.448 |
| At the time of admission | ||||
| White blood cell count, ×109 /L | 4.7 (3.91–5.49) | 4.71 (4.04–5.50) | 4.43 (3.38–5.55) | 0.674 |
| < 4 | 13 (26.5) | 7 (21.9) | 6 (35.3) | 0.501 |
| Lymphocyte count, × 109 /L | 1.47 (1.01–1.92) | 1.56 (1.16–2.02) | 1.16 (0.91–1.58) | 0.071 |
| < 0·8 | 6 (12.2) | 4 (12.5) | 2 (11.8) | 1 |
| Lactate dehydrogenase, IU/L | 203.0 (154–252) | 180.5 (148–227.3) | 247 (170–293) | 0.017* |
| >245 | 15 (30.6) | 6 (18.8) | 9 (52.9) | 0.013* |
| C-reactive protein, mg/L | 8.1 (1.43–21.5) | 4.95 (0.60–12.87) | 21 (5.37–30.19) | 0.016* |
| >10 | 21 (42.9) | 10 (31.3) | 11 (64.7) | 0.024* |
| D-dimer, μg/L | 0.20 (0.14-0.32) | 0.19 (0.11-0.32) | 0.25 (0.16-0.35) | 0.280 |
| > 0.55 | 7 (14.3) | 4 (12.5) | 3 (17.6) | 0.951 |
| Viral load (Ct value) | 32 (27.5–36.0) | 32 (25.3–36) | 32 (29.5–38.5) | 0.416 |
| CT score | 4 (1.5–6) | 4 (1–5) | 5 (3–8.5) | 0.020* |
| Treatments during hospitalization | ||||
| Lopinavir/ritonavir or darunavir/cobicistat | 46 (93.9) | 31 (96.9) | 15 (88.2) | 0.565 |
| Arbidol | 24 (49.0) | 14 (43.8) | 10 (58.8) | 0.315 |
| Corticosteroids | 12 (24.5) | 8 (25.0) | 4 (23.5) | 1.0 |
| Intravenous immunoglobulin | 22 (44.9) | 14 (43.8) | 8 (47.1) | 0.825 |
| At the time of follow-up | ||||
| Time from illness onset, days | 43.0 (36.0–50.0) | 44.0 (39.0–51.0) | 40.0 (29.0–46.0) | 0.035* |
| Time from viral clearance, days | 27.0 (20.5–37) | 34.5 (21.0–37.8) | 24 (20.0–28.0) | 0.085 |
| Time from discharge, days | 17.0 (15.0–33.0) | 30.0 (16.0–34.0) | 15.0 (14.0–17.0) | 0.002* |
| White blood cell count, ×109 /L | 5.77 (5.16–6.92) | 5.54 (5.11–6.87) | 5.82 (5.46–7.31) | 0.419 |
| < 4 | 1 (2.0) | 1 (3.1) | 0 (0) | 1 |
| Lymphocyte count, × 109 /L | 1.74 (1.38–2.04) | 1.76 (1.48–2.19) | 1.59 (1.28–1.91) | 0.143 |
| < 0·8 | 0 (0) | 0 (0) | 0 (0) | … |
| Lactate dehydrogenase, IU/L | 179 (145.6–209.0) | 173.0 (142.3–195.8) | 193.0 (158.5–237.5) | 0.081 |
| >245 | 5 (10.2) | 3 (9.4) | 2 (11.8) | 1 |
| C-reactive protein, mg/L | 0.72 (0.18–1.73) | 0.70 (0.22–1.41) | 0.73 (0.07–3.27) | 0.779 |
| >10 | 2 (4.1) | 1 (3.1) | 1 (5.9) | 1 |
| D-dimer, μg/L | 0.22 (0.19-0.37) | 0.19 (0.19-0.35) | 0.29 (0.19-0.72) | 0.131 |
| > 0.55 | 10 (20.4) | 5 (15.6) | 5 (29.4) | 0.443 |
| CD4 T-cell count, cells/uL | 699 (490–829) | 716 (533–916) | 670 (446–783) | 0.150 |
| CD4 T-cell percentage, % | 35.0 (31.0–39.0) | 34.5 (30.3–40.0) | 35.0 (33.0–38.0) | 0.825 |
| CD8 T-cell count, cells/uL | 560 (376–667) | 557 (383–695) | 560 (361–631) | 0.644 |
| CD8 T-cell percentage, % | 27.0 (21.0–30.5) | 25.0 (21.0–30.0) | 27.0 (22.0–33.5) | 0.366 |
| CD4/CD8 ratio | 1.32 (0.95–1.92) | 1.35 (1.01–1.97) | 1.31 (0.84–1.86) | 0.529 |
| B cell count, cells/uL | 179 (123–266) | 196 (130–287) | 158 (108–189) | 0.057 |
| B cell percentage, % | 9.5 (7.5–12.8) | 9.9 (7.7–13.4) | 7.9 (6.5–10.6) | 0.071 |
Data were expressed as median (IQR, interquartile ranges), or n (%, n/N). Comparison between groups was done using Mann-Whitney U test, Chi-Square test or Fisher’s exact test, as appropriate.
indicated p<0.05.
At the time of admission, 26.5 % (13/49) of the patients had leukopenia and 12.2 % (6/49) of the patients had lymphocytopenia. Elevated lactate dehydrogenase (LDH), CRP and d-dimer levels were found in 30.6 % (15/49), 42.9 % (21/49) and 14.3 (7/49) of the patients, respectively. The median CT score of the patients was 4 (IQR, 1.5–6) (Table 1). During hospitalization, the vast majority of the patients received antiviral treatment with protease inhibitors (lopinavir/ritonavir or darunavir/cobicistat). Corticosteroids were administered in a quarter of the patients (Table 1). At the time of follow-up, the patients did not show obvious symptoms. Almost all patients had normal blood white blood cell count, lymphocyte count and CRP level. Elevated LDH and d-dimer levels were found in 10.2 % (5/49) and 20.4 % (10/49) of the patients (Table 1).
3.2. NAb levels in different patient groups
To determine the NAb titers, we utilized the sVNT kit, which targeted the RBD of SARS-CoV-2. Among the 49 recovered patients, the median NAb titer was 1: 40 (IQR, 1:15-1:120). 24.5 % (12/49) of the patients had Nab titers less than 1:20. To explore the possible factors associated with NAb titers, variables were compared between patients with relatively higher NAb titers (≥1:80) and patients with lower NAb titers (<1:80). There was no statistical difference with regard to sex, smoking history, underlying comorbidities, and blood parameters at the time of admission (white blood cell count, lymphocyte count and d-dimer) (Table 1). Treatment during hospitalization (antiviral treatment, corticosteroid administration, and intravenous use of immunoglobulin) between these two groups had no significant difference. In addition, lymphocyte subgroups were similar between the two groups (Table 1). Compared with the patients who had lower NAb titers, patients with higher NAb titers were older, and had more advanced illness, as reflected by higher LDH and CRP levels, and more extensive lung abnormalities at the time of admission (p<0.05). The time to NAb analysis from discharge or illness onset was significant shorter in patients with higher NAb titers (p<0.05, Table 1).
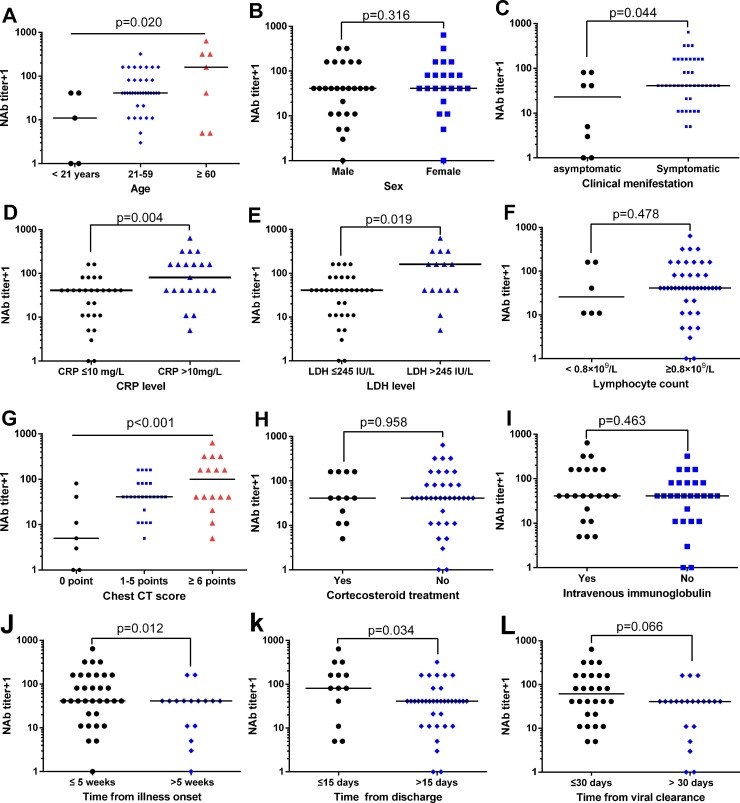
The distribution of NAb titers in patients with COVID-19 were then plotted based on the variables of age, sex, symptom, laboratory parameters and chest CT findings at the time of admission, treatment during hospitalization and the time of blood collection for antibody analysis (Fig. 1 ). There was a statistically significant trend of higher median NAb titers with higher levels of age (p = 0.020, Fig. 1A). Male patients had similar NAb tiers as compared with female patients (p = 0.316, Fig. 1B). The NAb titers were lower in patients with asymptomatic infection (p = 0.044, Fig. 1C). Patients with elevated CRP (>10 mg/L) or elevated LDH (>245 IU/L) level at the time of admission had significantly higher NAb titers during recovery (p = 0.004 and p = 0.019, respectively, Fig. 1D and E). Patients with lymphocytopenia (lymphocyte count <0.8 × 109 cells/L) had similar NAb titers as compared with those without lymphocytopenia (p = 0.478, Fig. 1F).
Fig. 1.
Distribution of neutralizing antibody titers in different patient groups.
NAb, neutralizing antibody; CT, computed tomography; CRP, C-reactive protein; LDH, lactate dehydrogenase.
There was a statistically significant trend of higher median NAb titers with higher levels of pulmonary abnormalities, as reflected by larger CT scores (p<0.001, Fig. 1G). NAb titers were not significantly different between patients with or without corticosteroid treatment (or intravenous immunoglobulin) (p = 0.958 and p = 0.463, respectively, Figure H and I). Time of blood collection for NAb analysis was likely to influence the NAb titers. Lower NAb titers were found in patients who had cleared the virus for more than 30 days, exceeded 15 days after discharge and more than 5 weeks after illness onset (Fig. 1J–L).
3.3. Multivariable analysis of the factors associated with NAb titers
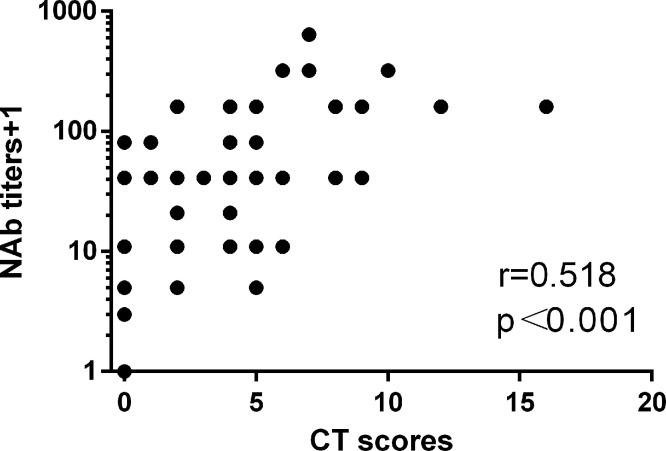
It’s worthy mentioning that patients with higher NAb titers had significant higher levels of pulmonary abnormalities (p<0.001, Fig. 1G). To further analyze their correlation, we first used Spearman's rank correlation coefficient to measure the strength of correlation between NAb levels and pulmonary injury. As shown in Fig. 2 , there was a positive correlation between NAb titers and chest CT score (r = 0.518, p<0.001). For multivariable analysis, we used the OLS model (multiple linear regression) to adjust the confounding factors. The selection of confounding factors was mainly based on clinical judgement. Independent variables included in the OLS model included age, sex, CT score, comorbidity, laboratory parameters that associated with disease severity (CRP level and lymphocyte counts), treatment that may influence immune response to pathogen (corticosteroids and intravenous immunoglobulin) and time of blood collection for NAb analysis. As there was multicollinearity among three variables related to the time of blood collection, which were the “time from illness onset”, “time from viral clearance”, and “time from discharge”, we only included one variable (time from viral clearance) in the OLS model.
Fig. 2.
Correlation between neutralizing antibody titers and CT scores.
In this OLS model, we found three clinical variables were significantly associated with the Z score of NAb titers: CT score, comorbidity, and corticosteroid treatment (Table 2). Every 1 CT score increase was associated with 0.10 point increase in antibody Z score. Those who had at least 1 comorbidity were 0.67 points higher in antibody Z score than those without comorbidity. Corticosteroid treatment was associated with 0.83 points decrease in antibody Z score. The standardized coefficients in Table 2 compared independent variables’ effect strength on the dependent variable. The CT score had the highest absolute value of standardized coefficient (0.36) in the model, which indicated its strong association with the antibody Z score. The model fit was good with 0.52 R2 value. When the variable of “time from illness onset” or “time from discharge” was included in the OLS model, the results were similar that CT score, comorbidity, and corticosteroid treatment remained significantly associated with the Z score of NAb titers. The CT score had the standardized coefficient of 0.39 and 0.36, respectively (Supplemental Tables 1 and 2).
Table 2.
OLS regression model for neutralizing antibody titer Z score.
| Unstandardized coefficient | Standardized coefficient | p-value | |
|---|---|---|---|
| Intercept | −0.29 | 0.00 | 0.59 |
| Age | 0.01 | 0.17 | 0.24 |
| Male (vs. Female) | −0.29 | −0.15 | 0.22 |
| CT score | 0.10 | 0.36 | 0.02 |
| Comorbidity (vs. No) | 0.67 | 0.28 | 0.02 |
| Corticosteroid treatment (vs. No) | −0.83 | −0.36 | 0.01 |
| Intravenous Immunoglobulin (vs. No) | 0.23 | 0.12 | 0.41 |
| CRP>10 mg/l (vs. CRP< = 10) | 0.33 | 0.16 | 0.28 |
| Lymphocyte count | −0.03 | −0.14 | 0.25 |
| Days from virus clearance | −0.02 | −0.16 | 0.18 |
R2 = 0.52.
3.4. Characteristics of two patients with no detectable NAb
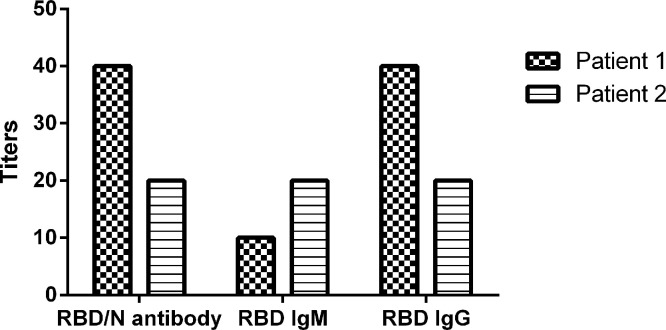
For our interest, among the 49 patients, two pediatric patients had no detectable NAbs. They were 5 and 6 years old. Consistently, their total antibodies as well as the anti-RBD antibody titers were also relatively low, ranged from 1:20 to 1:40 (Fig. 3 ). Both children had asymptomatic infections, and were identified by screening of the close contacts by local Centers for Disease Control and Prevention. Before being transferred to the second hospital of Nanjing for quarantine, nucleic acid tests for SARS-CoV-2 from throat swab samples were positive. However, the tests became negative after admission. Routine blood laboratory parameters of the patients, including complete blood count, liver and renal function, creatine kinase, lactate dehydrogenase, myocardial enzymes, coagulation profile, interleukin-6, procalcitonin, C-reactive protein and erythrocyte sedimentation rate, were all within the normal range. The chest CT scans were also normal.
Fig. 3.
Antibody analysis in two patients with negative neutralizing antibody test results. RBD, receptor binding domain; N, nucleocapsid protein.
4. Discussion
According to the Johns Hopkins case-tracking dashboard, as of July 3, 2020, there are over 10 million confirmed cases of COVID-19, leading to more than 520 thousand deaths. In the meantime, more than 5.7 million people have officially recovered. One important question is whether recovered patients have protective immunity against re-infection. To answer this question, accurate measurement of NAb in recovered COVID-19 patients will be a necessary first step. However, NAb detection currently relies on virus neutralization test (VNT) or pseudovirus-based virus neutralization test (pVNT), which requires handling live viruses in high biosafety level containment laboratory and also needs well trained staff to perform the tedious and time consuming procedure [[15], [16], [17]]. Even in the most developed nations or regions, those could not be easily available. In the present study, a novel sVNT, which was demonstrated to correlate well with VNT [12], was used to evaluate the NAb in patients recently recovered from COVID-19. NAbs were not detected in two asymptomatic children who quickly cleared the virus. Although the exact reason for undetectable NAb was not known, the finding highlights the needs to further investigate the possibility of re-infection for those recovered COVID-19 patients with no detectable NAbs.
A recent study in Shanghai evaluated the SARS-CoV-2 NAb titers using pVNT, which found that NAb titers were higher in patients with older age, higher CRP levels and lower lymphocyte counts [17]. Of note, those parameters are associated with disease severity of COVID-19 [[18], [19], [20]]. Nevertheless, a direct evaluation of the relationship of NAb titers with severity of COVID-19 pneumonia has not yet been reported. In the present study, we used a previously described semi-quantitative method to grade the severity of COVID-19 pneumonia [13]. CT score was assigned to each chest CT. A score of 0 represented normal chest CT and a score of 25 generally represented the most severe lung damage. As shown in Table 1 and Fig. 1, patients with higher NAb titers indeed had poorer baseline chest CT scores (more profound pneumonia). In multivariate analyses, after adjustment for age, sex, comorbidity, corticosteroid treatment, CRP level, lymphocyte count and time of NAb analysis, baseline chest CT scores still strongly correlated with NAb titers in patients recovered from COVID-19 (Table 2, p = 0.02). The finding suggested that pneumonia progression related to antibody level can occur during COVID-19. However, whether this is a reflection of ADE needs much more research for COVID-19 pathogenesis.
ADE has been described in many virus infections, including dengue virus, Zika virus, Ebola virus, and coronavirus [21]. In the context of Middle Eastern respiratory syndrome coronavirus (MERS-CoV) infection, binding of specific neutralizing antibodies to the virus could facilitate the entry of virus into immune cells through Fcγ receptors [22]. Importantly, lung epithelial cells could also express functional neonatal Fc receptor [23]. An immunological therapy with intravenous immunoglobulin to block Fcγ receptors has been advised as a potentially therapeutic strategy for patients with COVID-19 [24]. In our study, corticosteroid treatment was found to negatively impact the generation of NAb (Table 2, p = 0.01). If ADE is indeed a major problem in the pathogenesis of COVID-19, it is possible that corticosteroid could be used to mitigate the progression of COVID-19 pneumonia. Recently, studies did suggest that COVID-19 patients with moderate-to-severe illness could benefit from corticosteroid-treatment [25,26].
It should be noted that “correlation” does not imply “causation”. The finding that NAb titers were positively correlated with baseline pulmonary abnormalities did not prove that NAb and the associated ADE caused lung damage. However, our study, to some extent, raised the concern regarding the antibody-based treatment for COVID-19 pneumonia. If antibody-based treatment was administered, the patient should be closely monitored for possible exacerbation of COVID-19 pneumonia due to ADE. Our current study was limited by relatively small sample size which was difficult to maximally adjust the confounding factors associated with NAb titers. Moreover, the vast majority of the patients had mild-to-moderate illness, and only two patients with severe COVID-19 were included in the study. A large-scale study, which include more patients with life-threatening COVID-19, is needed to further evaluate the findings in our study.
In conclusion, we found that some COVID-19 patients may not have detectable NAb after recovery. Therefore, the possibility of re-infection could not be excluded. SARS-COV-2 neutralizing antibody titers were positively correlated with severity of COVID-19 pneumonia, and the possible contribution of ADE to disease severity needs much more research in the context of COVID-19 pneumonia.
Funding
This study was funded in part by project of Jiangsu province medical youth talent (QNRC2016059), N anjing medical science and technique development foundation (ZKX17040 and YKK17172), and ACS IRG ( IRG-19-137-20). The development of sVNT was supported by grants from Singapore National Medical Research Council (STPRG-FY19-001 and COVID19RF-003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Competing Interest
Authors XQ, ST and PL were employed by the company GenScript Biotech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgement
We thank Dr. Yue Cao at Medical University of South Carolina for assistance with statistical analysis.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.biopha.2020.110629.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Bedford J., Enria D., Giesecke J., Heymann D.L., Ihekweazu C., Kobinger G., Lane H.C., Memish Z., Oh M.-d., Sall A.A., Schuchat A., Ungchusak K., Wieler L.H. COVID-19: towards controlling of a pandemic. Lancet. 2020;395(10229):1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., Ma H., Chen W., Lin Y., Zheng Y., Wang J., Hu Z., Yi Y., Shen H. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China, science China. Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus I., Research T. A novel coronavirus from patients with pneumonia in China, 2019. New Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye F., Xu S., Rong Z., Xu R., Liu X., Deng P., Liu H., Xu X. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W., Fontanet A., Zhang P.-H., Zhan L., Xin Z.-T., Baril L., Tang F., Lv H., Cao W.-C. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J. Infect. Dis. 2006;193(6):792–795. doi: 10.1086/500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee N., Chan P.K., Ip M., Wong E., Ho J., Ho C., Cockram C.S., Hui D.S. Anti-SARS-CoV IgG response in relation to disease severity of severe acute respiratory syndrome. J. Clin. Virol. 2006;35(2):179–184. doi: 10.1016/j.jcv.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., Tang H., Nishiura K., Peng J., Tan Z., Wu T., Cheung K.W., Chan K.H., Alvarez X., Qin C., Lackner A., Perlman S., Yuen K.Y., Chen Z. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI insight. 2019;4(4) doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yip M.S., Leung H.L., Li P.H., Cheung C.Y., Dutry I., Li D., Daeron M., Bruzzone R., Peiris J.S., Jaume M. Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS. Hong Kong Med. J. 2016;22(3 Suppl 4):25–31. [PubMed] [Google Scholar]
- 11.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I.C., Tiu C., Hu Z., Chen V.C.-W., Young B.E., Sia W.R., Tan Y.-J., Foo R., Yi Y., Lye D.C., Anderson D.E., Wang L.-F. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020 doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 13.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., Zheng D., Wang J., Hesketh R.L., Yang L. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Chen W., Hu C., Huang L., Hu Z., Zeng Y., Wang W., Yi Y. 2020. Characterization of a Big Family Cluster Infection Associated With SARS-Cov-2 in Nanjing District. [Google Scholar]
- 15.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M., Lu Q., Li X., Sun Q., Liu J., Fan C., Huang W., Xu M., Wang Y. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020;9(1):680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., Ling Y., Zhang Y., Xun J., Lu L., Jiang S., Lu H., Wen Y., Huang J. medRxiv; 2020. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications. 2020.03.30.20047365. [Google Scholar]
- 18.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Jama; China: 2020. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eroshenko N., Gill T., Keaveney M.K., Church G.M., Trevejo J.M., Rajaniemi H. Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat. Biotechnol. 2020;38(7):789–791. doi: 10.1038/s41587-020-0577-1. [DOI] [PubMed] [Google Scholar]
- 22.Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z., Zhou Y., Du L., Li F. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 2020;94(5) doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiekermann G.M., Finn P.W., Ward E.S., Dumont J., Dickinson B.L., Blumberg R.S., Lencer W.I. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 2002;196(3):303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. New Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Z., Lv Y., Xu C., Sun W., Chen W., Peng Z., Chen C., Cui X., Jiao D., Cheng C., Chi Y., Wei H., Hu C., Zeng Y., Zhang X., Yi Y. Clinical use of short-course and low-dose corticosteroids in patients with non-severe COVID-19 during pneumonia progression. Front. Public Health. 2020;8(355) doi: 10.3389/fpubh.2020.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.