Abstract
Purpose of the Review
Fermentable oligosaccharides disaccharides monosaccharides and polyols (FODMAP) dietary restriction ameliorates irritable bowel syndrome (IBS) symptoms; however, not all IBS subjects respond. Given the gut microbiome’s role in carbohydrate fermentation, investigators have evaluated whether the gut microbiome may predict low FODMAP diet efficacy.
Recent Findings
Gut microbiome fermentation, even to the same carbohydrate, is not uniform across all individuals with several factors (e.g., composition) playing a role. In both children and adults with IBS, studies are emerging suggesting the gut microbiome may predict low FODMAP diet efficacy. However, there is significant heterogeneity in the approaches (study population, microbiome assessment methods, statistical techniques, etc.) used amongst these studies.
Summary
The gut microbiome holds promise as a predictor of low FODMAP diet efficacy. However, further investigation using standardized approaches to evaluate the microbiome while concomitantly assessing other potential predictors are needed to more rigorously evaluate this area.
Keywords: Irritable bowel syndrome, metabolome, bacteria, fructose
INTRODUCTION
Irritable bowel syndrome (IBS) is a highly prevalent functional gastrointestinal pain disorder; it affects up to 20% of the world’s (both adult and pediatric) population.(1) It is characterized by chronic abdominal pain related to defecation or changes in stool form or stool frequency.(1) As a functional disorder, IBS is often best viewed within the context of the biopsychosocial model.(2) As such, several potentially inter-related factors may play a role including: gut inflammation, psychosocial factors (e.g. anxiety), genetics, visceral hypersensitivity, dysbiosis (altered gut microbiome) and diet.(3) Diet is an important factor in IBS symptom generation as more than 80% of both children, adolescents, and adults with IBS identify at least one food which worsens their IBS symptoms.(4–6) In both children and adults with IBS, a higher number of perceived dietary food intolerances is associated with increased IBS symptom severity.(4, 6) Dietary components which have gained significant recent attention as potential symptom generation culprits in those with IBS are fermentable oligosaccharide disaccharide monosaccharide and polyol (FODMAP) carbohydrates.
FODMAPs
FODMAP carbohydrates include: fructose, lactose, fructans, galactans, and polyols (Table 1). FODMAP carbohydrates as a group may be malabsorbed and rapidly fermented, thereby leading to symptoms seen in functional bowel disorders including abdominal pain, bloating, and increased flatulence.(7) In both adults and children with IBS, double-blind placebo controlled trials have demonstrated FODMAPs may exacerbate IBS gastrointestinal symptoms.(8) (9) In addition, in both adults and children with IBS, a FODMAP restriction diet (or low FODMAP diet) ameliorates IBS gastrointestinal symptoms.(10, 11) However, not all subjects with IBS benefit from a FODMAP restriction diet: Studies suggest up to 50% do not benefit from this dietary intervention.(12, 13)
Table 1:
Fermentable Oligosaccharide Monosaccharide Disaccharide and Polyol (FODMAP) Carbohydrates: Type, Structure, Factors Related to Malabsorption, Example of Potentially Relevant Microbiome Enzymes/Pathways, and Examples of Common Foods Containing the Delineated FODMAP
| FODMAP Carbohydrate | Structure | Factors Related to Malabsorption | Example of Associated Microbial Enzyme/Pathway | Examples of Common Foods Containing the Carbohydrate |
|---|---|---|---|---|
| Fructose | Monosaccharide | Passive absorption | Fructose-6-phosphate shunt | Honey, apples |
| Lactose | Disaccharide | Diminishing lactase enzyme expression in majority | β-galactosidase | Dairy products, milk, cheese |
| Fructans | Oligosaccharide – primarily fructose polymers | Lack hydrolases to metabolize | β-fructofuranosidase | Wheat, onions |
| Galactans | Oligosaccharide – primarily galactose polymers | Lack hydrolases to metabolize | α-galactosidase | Beans, legumes |
| Polyols | Sugar alcohol | Passive absorption | Sorbitol-6-phosphate dehydrogenase | Watermelon, pears |
Studies, often using magnetic resonance imaging, have demonstrated that FODMAPs such as fructose significantly increase small bowel water content following ingestion.(14) In contrast, FODMAPs such as fructans primarily increase colonic distention via microbial fermentation and subsequent gas production.(14) These physiologic responses were believed to be the primary mechanisms by which FODMAPs induced symptoms.(14, 15) However, investigators have not been able to differentiate those with IBS who develop symptoms vs do not develop symptoms during a FODMAP-related challenge based on these physiologic changes (e.g., gas production) alone.(9, 16) Moreover, healthy controls given FODMAPs have the same physiologic changes occur to the same degree as those with functional bowel disorders.(16) These data suggest additional mechanisms need to be determined for FODMAP induced IBS symptoms.
An additional factor which may play a role in FODMAP induced symptoms relates to visceral sensation. Visceral hypersensitivity is common in both adults and children with IBS:(17–19) For example, up to 83% of children with IBS have visceral hypersensitivity as measured by rectal barostat.(17, 20) In adults with IBS given the fermentable CHO lactulose, the presence of baseline visceral hypersensitivity predicted more severe abdominal pain after lactulose ingestion.89 In another study in lactase deficient adults with IBS, visceral hypersensitivity (as measured via rectal barostat) was worsened by lactose ingestion.90 An MRI-based study in adults with IBS implicated visceral hypersensitivity rather than the amount of gas produced in the induction of abdominal pain.(16)
Identifying the mechanism(s) by which FODMAPs induce functional symptoms and predicting those with IBS who may benefit from a FODMAP restriction diet has garnered significant attention.(21) In clinical practice it is recommended that the FODMAP diet be applied in three phases: comprehensive restriction (to determine efficacy of the diet), re-introduction (to determine which FODMAPs may be able to be reintroduced into the diet), and personalized maintenance.(13) Potential challenges with following a low FODMAP diet include: it does not work in all participants; education challenges such as incongruence between available dietary FODMAP-related listings; potential association with unintended weight loss; unknown long term effects given that it removes foods considered healthy (e.g., fiber, certain fruits); and initial reduction in the abundance of microorganisms associated with health (e.g., Bifidobacterium).(22–25) It is recommended that subjects with IBS implementing a FODMAP diet work with a dietitian knowledgeable about the diet to mitigate these challenges. Based in part on these challenges and lack of clear pathophysiologic factors related to FODMAP induced IBS symptoms, experts in the field of functional bowel disorders have determined that identifying the mechanism by which FODMAP carbohydrates induce symptoms as an important area for future research.(21)
Potential Role of the Gut Microbiome
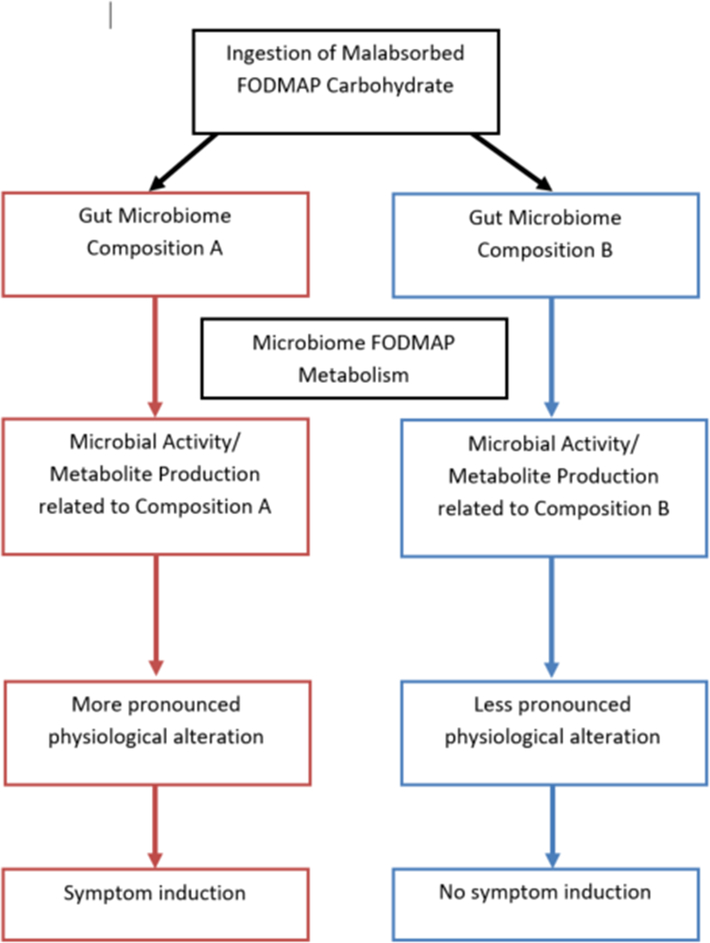
One potential mechanism being actively investigated by which FODMAP carbohydrates induce IBS symptoms may be related to the gut microbiome (Figure 1). The microbiome is composed of several types of microorganisms (including bacteria, archaea, fungi, and viruses) and their genetic material.(26) Though the majority of these microorganisms cannot be cultured, new generation sequencing methods allow for culture independent elucidation of both microbial taxonomy and microbial genes (metagenomics).(27–29) Additional ‘omics technologies may provide insight into the gut microbiome activity. This includes metabolomics, which is the study of the metabolites (metabolome) in a biological sample. The gut metabolome comprises numerous small metabolites produced by the human; however, the majority of gut metabolites seen in feces are produced by the gut microbiome.(30, 31) Metabolomic studies have identified metabolites resulting from colonic microbiome metabolism of dietary products within 2 hr. of consumption(32, 33) and changes in stool metabolites within 24 hours of an antibiotic intervention.(34) These data suggest microbiome activity is readily altered by foods and/or other interventions which are ingested.
Figure 1:
Hypothetical Framework of the Potential Role of Microbiome Composition and/or Microbiome Activity (Metabolite Production) as it Relates to Fermentable Oligosaccharide Disaccharide Monosaccharide and Polyol (FODMAP) Carbohydrate Metabolism and Subsequent Symptom Development in Functional Bowel Disorders
The intestinal microbiome contains over 9 million genes: This number significantly outnumbers the number of human genes.(35) These microbial genes allow the microbiome to serve essential functions in maintaining human health. One important health related gut microbiome function includes the colonic fermentation of dietary carbohydrates – traditionally believed to be of great importance for energy salvage.(36) Though humans may lack the necessary enzymes to hydrolyze the bonds within certain FODMAP carbohydrates (e.g., fructans), microbial genes allow the microbiome to metabolize malabsorbed carbohydrates such as FODMAPs (Table 1).(37)
When comparing IBS subjects vs. healthy subjects, microbiome composition differences have been identified; however, the same differences have not been consistently identified across all studies.(38, 39) In children with IBS, using a combination of microbiome composition, microbiome metabolic pathways, and fecal metabolites may help differentiate children with IBS from healthy controls with high accuracy.(40) These types of alterations in microbiome composition (dysbiosis) may be relevant with FODMAPs as microbiome composition determines how luminal polysaccharides are degraded based on the interactions between microorganisms.
Microorganisms have several interactions with each other when metabolizing substrates such as malabsorbed (FODMAP) carbohydrates including: competition, syntrophy, and cross-feeding relationships.(36) These microbial interactions determine the availability of both the FODMAP carbohydrate itself and its subsequent metabolites. In addition, these interactions amongst microorganisms may influence how a microorganism metabolizes a FODMAP carbohydrate. For example, archaea may stimulate bacterial fermentation: Bacteroides thetaiotamicron expresses more fructofuranosidases (capable of metabolizing fructans) when co-cultured with the archaeal organism M. smithii - resulting in an increase in acetate production.(41) Another example can be seen in a humanized gnotobiotic rodent study where evaluation of three different human-derived gut microbiome communities of different composition resulted in markedly different responses in both magnitude and type of microbiota-derived metabolites.(42)
FODMAPs, once consumed, also modulate both microbial composition and microbial fermentation.(9, 15) These gut microbial responses, even to the same carbohydrate, are not uniform across all individuals with variations in both subsequent composition changes and fermentation byproducts. In one study in healthy adults receiving agave inulin (a type of fructan) supplementation the majority of subjects had a subsequent increase in Bifidobacterium; however, a sizeable subset ~ 25% had a decrease in Bifidobacterium. Even those who had an increase in Bifidobacterium with the supplementation had different amounts (percentages) of increase.(43) In a separate study evaluating production of short-chain fatty acids in healthy adults to different fibers (including inulin), the composition of an individual’s microbiota was an important factor in determining the production identified.(44) Ultimately, microbial fermentation response to CHO ingestion appears to be strongly influenced by an individual’s gut microbiome composition.(37, 42, 44)
Fermentation metabolites resulting from FODMAP metabolism may include (amongst many): short chain fatty acids (e.g. acetate), lactate, hydrogen, methane, hydrogen sulfide, and amines.(38) Though further studies are needed, these metabolites may alter the colonic environment or affect colonic physiology in such a manner as to lead to an exacerbation in IBS symptoms. For example, production of fermentation metabolites (e.g., short chain fatty acids) may lead to increased luminal acidity. In one rodent model of visceral sensation, intraluminal colonic acidic hypertonic saline (akin to what may occur in the setting of carbohydrate malabsorption) infusions increased visceral hypersensitivity.(45) Visceral hypersensitivity related to FODMAP intake has also been associated with increased gut immune activation with one rodent study associating this increase with increased intestinal SCFA production.(46) However, increases in gut immune activation from FODMAP carbohydrates are not uniformly seen in all rodent models.(47) Further investigation is needed to determine the potential role of microbiome-derived metabolites on FODMAP induced symptoms.
Microbiome Composition and Predicting FODMAP Diet Efficacy (Table 2)
Table 2:
Characteristics and Findings of Studies Evaluating Gut Microbiome Composition as a Predictor of low Fermentable Oligosaccharide Disaccharide Monosaccharide and Polyol diet efficacy for Irritable Bowel Syndrome
| Reference | IBS Population studied | Study Design | Diet Responder Definition | Microbiome Analysis | Discriminant Analysis | Key Baseline Microbiome Findings |
|---|---|---|---|---|---|---|
| Chumpitazi et al.12 | Children (ages 7–17 years), n=8 | Open-label: low FODMAP × 1 week | ≥50% decrease in abdominal pain frequency from baseline | 16s rRNA pyro-sequencing | Yes: LEfSE | Responders (n=4) enriched in Sporobacter, Subdoligranulum, Alistipes, and an unclassified Ruminococcaceae. Non-responders (n=4) enriched in Bacteriodes, unclassified Bacteriodales, and a different unclassified Ruminococcaceae. |
| Halmos et al.10 | Adults, n=30 | Cross-over RCT (low FODMAP vs. Australian diet) × 21 days | 10 mm decrease in visual analogue scale | Select PCR probes of total bacteria and 9 specific targets | No | No differences between Responders (n=21) vs. Non-responders (n=9). |
| Chumpitazi et al.11 | Children, n=33 | Cross-over RCT Low FODMAP vs. Typical American childhood diet × 48 hours | 50% decrease in abdominal pain frequency during the low FODMAP diet alone. | 16s rRNA pyro-sequencing | Yes: LEfSE | Responders (n=8) were enriched in 63 microbial OTUs including: Bacteriodes, Ruminococcaceae, F. prausnitzii, Dorea and Clostridiales. Nonresponders (n=15) were uniquely enriched in Turicibacter. Placebo-responders (n=10) not included. |
| Bennet et al.49 | Adults, n=31 placed on low FODMAP | Parallel group RCT (low FODMAP vs. traditional IBS diet) × 4 weeks | Reduced IBS-Symptom Severity Score by ≥ 50 | 54 DNA probes targeting ≥ 300 bacteria. Calculation of Dysbiosis Index. | Yes: Multivariate factor orthogonal partial least square discriminant analysis | No bacteria found to be enriched in Responders (n=19). Nonresponders (n=12) enriched in several bacteria including Bacteroides stercoris, Parabacteriodes, Bacillus, Pseudomonas, Acineteobacter, Desulfitispora, Streptococcus, Dorea, Ruminococcus gnavus. Nonresponders had a higher dysbiosis index score at baseline. |
| Valeur et al.48 | Adults, n=61 | Open-label, 4 week FODMAP restriction | 50% reduction in IBS-Symptom Severity Score | 54 DNA probes targeting ≥ 300 bacteria. Calculation of Dysbiosis Index. | No | Responders (n=32) were enriched in Bacteroides fragilis, Acinetobacter, Ruminiclostridium, Streptococcus, and Eubacterium. Nonresponders (n=29) were enriched in Clostridia, Negativicutes, Bacilli; Actinomycetales, Anaerotruncus; Clostridiales; and Shigella, Escherichia. No difference in dysbiosis index between Responders and Nonresponders. |
IBS= irritable bowel syndrome; RCT = randomized controlled trial; LEfSE= Linear Discriminant Analysis Effect Size; OTU = operational taxonomic unit
Given the role of the microbiome in determining the metabolism and subsequent metabolite production with carbohydrate ingestion, several investigators have evaluated whether microbiome composition prior to starting a low FODMAP diet (baseline) may predict low FODMAP diet efficacy (Table 1). These investigations have been relatively heterogeneous in methodology in several areas including: study design (e.g. randomized control trial vs. open-label); population included (e.g., children vs. adults with IBS), number of subjects; method used to determine microbiome composition (e.g., select DNA probes vs. 16S rRNA sequencing); definition of clinical response (responder vs. non-responder) to a low FODMAP diet; and the statistical analyses (e.g., whether a discriminant analysis was conducted and if so, what type) employed.
With this heterogeneity amongst studies in mind, it is not surprising that the results have not been consistent amongst them. Nevertheless, four of the five studies identified microbiome signatures differentiating low FODMAP diet responders vs. non-responders. Two studies identified that those who responded to a low FODMAP diet had a microbiome composition enriched in bacteria with an increased ability to metabolize carbohydrates (saccharolytic).(11, 48) One study identified increased dysbiosis in those who did not respond to a low FODMAP diet; however, another study using a similar microbiome analysis technique did not identify dysbiosis as a contributing factor.(48, 49) Though promising, future studies which are larger and use more uniform design and microbiome evaluation approaches are needed to definitively determine if baseline microbiome composition may predict low FODMAP diet efficacy.
Microbiome Metabolic Pathways/ Metabolites and Predicting Low FODMAP diet Efficacy
In addition to microbiome composition, investigators have evaluated both microbiome metabolic potential and fecal metabolites which may be produced by the microbiome as a potential means to predict low FODMAP diet efficacy. In a short-term low FODMAP randomized controlled crossover trial, we identified two carbohydrate related metabolic pathways enriched in the gut microbiome of low FODMAP responders: LacI family transcription regulators and Alpha-N-arabinofuranosidase.(11) LacI family transcriptional regulators regulate microbiome carbohydrate utilization genes and facilitate expression of these genes depending on environmental/substrate availability. Alpha-N-arabinofuranosidase enzymes metabolize arabinogalactans which are a type of FODMAP found in wheat flour.(11) These data suggest microbial genetic potential to metabolize FODMAP carbohydrates may predict FODMAP diet efficacy. However, to date, additional studies investigating gut microbial genes/metabolic pathways and their relationship to the FODMAP diet remain to be completed.
With respect to microbial-derived metabolites, a small open-label pilot feasibility trial using a global metabolomics approach in children with IBS identified several fecal metabolites enriched in low FODMAP diet responders including: gamma-tocopherol, alpha-ketoglutarate, and 1,3,7-trimethylurate.(12) In contrast, non-responders were enriched in several fecal metabolites at baseline including asparagylleucine, urobilinogen, and methylsuccinate.(12) Using a more focused fecal metabolite evaluation, Rossi et al. identified that baseline fecal volatile organic compound (VOCs) profiles differentiate low FODMAP responders vs. non-responders with a high accuracy.(50) The specific VOCs within the profiles which differentiated responders from non-responders VOCs are gaseous carbon based compounds: There are approximately 101 fecal VOCs in stools from healthy individuals.(51) VOCs are believed to be reflective, in part, of microbial composition and fermentation of dietary components such as carbohydrates and proteins.(51) Though these data suggest there are potential baseline differences in baseline microbiome activity differentiating responders vs. non-responders, further work clarifying the specific VOCs playing a role and/or other associated fecal metabolites which may predict low FODMAP diet response are needed.
Conclusion
FODMAP dietary restriction ameliorates IBS gastrointestinal symptoms in both children and adults with IBS. However, not all IBS subjects respond and there may be challenges with implementing a low FODMAP diet. The mechanism(s) by which FODMAP carbohydrates induce IBS symptoms is under active investigation. Given the role of the microbiome in fermenting malabsorbed carbohydrates and the individualized microbiome response (e.g., production of metabolites) which may occur based on an individual’s microbiome composition, there has been increasing attention on the potential role of the gut microbiome in predicting low FODMAP diet efficacy. In both children and adults with IBS, heterogeneous studies suggest that both microbiome composition and microbiome-related metabolomic profiles may predict FODMAP restriction efficacy. However, further investigation utilizing consistent and rigorous methodologic approaches and ideally incorporating other potential factors (e.g., visceral sensation) are needed to further elucidate the role of the gut microbiome as a baseline predictor of low FODMAP diet efficacy in functional bowel disorders.
KEY POINTS.
Gut microbiome fermentation, even to the same carbohydrate, is not uniform across all individuals with several factors (e.g., composition) playing a role.
In both children and adults with IBS, studies are emerging suggesting the gut microbiome may predict low FODMAP diet efficacy.
However, there is significant heterogeneity in the approaches (study population, microbiome assessment methods, statistical techniques, etc.) used amongst these studies.
Further investigation using standardized approaches to evaluate the microbiome while concomitantly assessing other potential predictors are needed to more rigorously evaluate this area.
ACKNOWLEDGEMENTS
Financial and/or Intellectual support was provided by NIH K23 DK101688; NIH R03 DK117219; and P30 DK056338 which funds the Texas Medical Center Digestive Disease Center.
Funding Support: NIH K23 DK101688; NIH R03 DK117219; and P30 DK056338 which funds the Texas Medical Center Digestive Disease Center.
Abbreviations
- FODMAP
fermentable oligosaccharides disaccharides monosaccharides and polyols
- IBS
irritable bowel syndrome
- VOCs
volatile organic compounds
- RCT
randomized controlled trial
- LEfSE
Linear Discriminant Analysis Effect Size
- OTU
operational taxonomic unit
REFERENCES
- 1.Hyams JS, Di Lorenzo C, Saps M, et al. Functional Disorders: Children and Adolescents. Gastroenterology. 2016;150:1456–68. [DOI] [PubMed] [Google Scholar]
- 2.Harris LA, Umar SB, Baffy N, Heitkemper MM. Irritable Bowel Syndrome and Female Patients. Gastroenterol Clin North Am. 2016;45(2):179–204. [DOI] [PubMed] [Google Scholar]
- 3.Chumpitazi BP, Shulman RJ. Underlying molecular and cellular mechanisms in childhood irritable bowel syndrome. Molecular and cellular pediatrics. 2016;3(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chumpitazi BP, Weidler EM, Lu DY, et al. Self-Perceived Food Intolerances Are Common and Associated with Clinical Severity in Childhood Irritable Bowel Syndrome. Journal of the Academy of Nutrition and Dietetics. 2016;116(9):1458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed-Knight B, Squires M, Chitkara DK, van Tilburg MA. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohn L, Storsrud S, Tornblom H, et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–41. [DOI] [PubMed] [Google Scholar]
- 7.Muir JG, Gibson PR. The Low FODMAP Diet for Treatment of Irritable Bowel Syndrome and Other Gastrointestinal Disorders. Gastroenterology & hepatology. 2013;9(7):450–2. [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6(7):765–71. [DOI] [PubMed] [Google Scholar]
- 9.Chumpitazi BP, McMeans AR, Vaughan A, et al. Fructans Exacerbate Symptoms in a Subset of Children With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2018;16(2):219–25 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halmos EP, Power VA, Shepherd SJ, et al. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology. 2014;146(1):67–75 e5. [DOI] [PubMed] [Google Scholar]
- 11.Chumpitazi BP, Cope JL, Hollister EB, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. 2015;42(4):418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chumpitazi BP, Hollister EB, Oezguen N, et al. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes. 2014;5(2):165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelan K, Martin LD, Staudacher HM, Lomer MCE. The low FODMAP diet in the management of irritable bowel syndrome: an evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J Hum Nutr Diet. 2018. [DOI] [PubMed] [Google Scholar]
- 14.Murray K, Wilkinson-Smith V, Hoad C, et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109(1):110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25(8):1366–73. [DOI] [PubMed] [Google Scholar]
- 16.Major G, Pritchard S, Murray K, et al. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology. 2017;152(1):124–33 e2. [DOI] [PubMed] [Google Scholar]
- 17.Di Lorenzo C, Youssef NN, Sigurdsson L, et al. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr. 2001;139(6):838–43. [DOI] [PubMed] [Google Scholar]
- 18.Mertz H, Naliboff B, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–92. [DOI] [PubMed] [Google Scholar]
- 20.Ginkel RV, Voskuijl WP, Benninga MA, et al. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology. 2001;120:31–8. [DOI] [PubMed] [Google Scholar]
- 21.Chang L, Di Lorenzo C, Farrugia G, et al. Functional Bowel Disorders: A Roadmap to Guide the Next Generation of Research. Gastroenterology. 2018;154(3):723–35. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):707–17. [DOI] [PubMed] [Google Scholar]
- 23.Halmos EP, Christophersen CT, Bird AR, et al. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. [DOI] [PubMed] [Google Scholar]
- 24.Frieling T, Heise J, Krummen B, et al. Tolerability of FODMAP - reduced diet in irritable bowel syndrome - efficacy, adherence, and body weight course. Z Gastroenterol. 2019. [DOI] [PubMed] [Google Scholar]
- 25.McMeans AR, King KL, Chumpitazi BP. Low FODMAP Dietary Food Lists are Often Discordant. Am J Gastroenterol. 2017;112(4):655–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamady M, Knight R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009;19(7):1141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstock GM. Genomic approaches to studying the human microbiota. Nature. 2012;489(7415):250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Wang B, Zhang M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105(6):2117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oresic M Metabolomics, a novel tool for studies of nutrition, metabolism and lipid dysfunction. Nutr Metab Cardiovasc Dis. 2009;19(11):816–24. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd AJ, Fave G, Beckmann M, et al. Use of mass spectrometry fingerprinting to identify urinary metabolites after consumption of specific foods. Am J Clin Nutr. 2011;94(4):981–91. [DOI] [PubMed] [Google Scholar]
- 33.Roowi S, Stalmach A, Mullen W, et al. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. Journal of agricultural and food chemistry. 2010;58(2):1296–304. [DOI] [PubMed] [Google Scholar]
- 34.Yap IK, Li JV, Saric J, et al. Metabonomic and microbiological analysis of the dynamic effect of vancomycin-induced gut microbiota modification in the mouse. Journal of proteome research. 2008;7(9):3718–28. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Jia H, Cai X, et al. An integrated catalog of reference genes in the human gut microbiome. Nature biotechnology. 2014;32(8):834–41. [DOI] [PubMed] [Google Scholar]
- 36.Seth EC, Taga ME. Nutrient cross-feeding in the microbial world. Frontiers in microbiology. 2014;5:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flint HJ, Scott KP, Duncan SH, et al. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306.This manuscript provides an excellent reference foundation toward understanding the role of the microbiome in metabolism of carbohydrates including certain FODMAPs.
- 38.Rajilic-Stojanovic M, Jonkers DM, Salonen A, et al. Intestinal Microbiota And Diet in IBS: Causes, Consequences, or Epiphenomena? Am J Gastroenterol. 2015;110(2):278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saulnier DM, Riehle K, Mistretta TA, et al. Gastrointestinal Microbiome Signatures of Pediatric Patients With Irritable Bowel Syndrome. Gastroenterology. 2011;141(5):1782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollister EB, Oezguen N, Chumpitazi BP, et al. Leveraging Human Microbiome Features to Diagnose and Stratify Children with Irritable Bowel Syndrome. J Mol Diagn. 2019;21(3):449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A. 2006;103(26):10011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smits SA, Marcobal A, Higginbottom S, et al. Individualized Responses of Gut Microbiota to Dietary Intervention Modeled in Humanized Mice. mSystems. 2016;1(5).This humanized gnotobiotic rodent study demonstrates the importance of microbial composition on subsequent microbe-derived metabolite production.
- 43.Holscher HD, Bauer LL, Gourineni V, et al. Agave Inulin Supplementation Affects the Fecal Microbiota of Healthy Adults Participating in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J Nutr. 2015;145(9):2025–32. [DOI] [PubMed] [Google Scholar]
- 44.Baxter NT, Schmidt AW, Venkataraman A, et al. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. MBio. 2019;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La JH, Feng B, Schwartz ES, et al. Luminal hypertonicity and acidity modulate colorectal afferents and induce persistent visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen BR, Du LJ, He HQ, et al. Fructo-oligosaccharide intensifies visceral hypersensitivity and intestinal inflammation in a stress-induced irritable bowel syndrome mouse model. World J Gastroenterol. 2017;23(47):8321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuck CJ, Caminero A, Jimenez Vargas NN, et al. The impact of dietary fermentable carbohydrates on a postinflammatory model of irritable bowel syndrome. Neurogastroenterol Motil. 2019:e13675. [DOI] [PubMed] [Google Scholar]
- 48.Valeur J, Smastuen MC, Knudsen T, et al. Exploring Gut Microbiota Composition as an Indicator of Clinical Response to Dietary FODMAP Restriction in Patients with Irritable Bowel Syndrome. Dig Dis Sci. 2018;63(2):429–36. [DOI] [PubMed] [Google Scholar]
- 49.Bennet SMP, Bohn L, Storsrud S, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2017. [DOI] [PubMed] [Google Scholar]
- 50.Rossi M, Aggio R, Staudacher HM, et al. Volatile Organic Compounds in Feces Associate With Response to Dietary Intervention in Patients With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2018;16(3):385–91 e1.This is a robust clinical related trial that demonstrates microbial metabolite (volatile organic compounds) at baseline may predict low FODMAP diet efficacy.
- 51.Garner CE, Smith S, de Lacy Costello B, et al. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21(8):1675–88. [DOI] [PubMed] [Google Scholar]