Abstract
Purpose
COVID-19 pandemic has created havoc all over the globe and spared no one regardless of status, gender, location and ethnicity. There were questions raised if trauma and orthopaedic (T&O) procedures actually generated aerosols? The need for a review of literature highlighting the nature and impact of aerosol generation within T&O surgery was noted.
Methods
A comprehensive online search was performed for all published articles in the English language, evaluating AGPs in T&O surgery and the relevant personal protection equipment used.
Results
The search strategy populated 43 studies. Six studies were identified as duplicates. The shortlisted 37 studies were screened and nine studies were included in the review. An additional four studies were included from the bibliography review.
Conclusion
Most orthopaedic procedures are high-risk aerosol generating procedures (AGPs). Conventional surgical masks do not offer protection against high-risk AGPs. In the current era of COVID-19 pandemic, there is a significant risk to the transmission of infection to the theatre staff. For protection against airborne transmission, appropriate masks should be used. These need proper fitting and sizing to ensure full protection when used.
Keywords: AGP, Aerosol generating procedures, Covid-19, Pandemic, Corona virus
Introduction
In December 2019, an outbreak of the novel coronavirus disease (Covid-19) occurred in Wuhan, China.1 , 2 This spreads rapidly to other areas in China and worldwide.3 Common complications of the disease included acute respiratory distress syndrome [ARDS], arrhythmia, shock, acute cardiac injury, secondary infection, acute kidney injury, and death in severe cases. Its' course is long, and is highly contagious, even during the incubation period.4 The Covid-19 pandemic has spread rapidly, leading to a high death count worldwide. The mortality rate among healthcare professional is constantly evolving and worrying. This has been postulated to be multifactorial. Healthcare professionals are at a higher risk of catching the disease due to their exposure to higher viral loads, especially if the virus is aerosolized.5 , 6
The potential risk to the operating room personnel to exposure to infected material, such as blood or tissue debris, is well described.7 Ocular or mucocutaneous exposure bears an underestimated hazard of infection.8 This contamination risk is higher in orthopaedic surgery during trauma, spinal and arthroplasty procedures.9, 10, 11 Orthopaedic procedures, often involve the use of thermal energy tools, such as surgical lasers and electrocautery, and mechanical high-speed power tools, such as bone saws, reamers, and drills.12 The use of these tools generates large amount of tissue debris. This has been extensively reported in the field of dentistry, however only few studies conducted in orthopaedic surgery, as yet to the best of our knowledge, corroborate this.7 , 13
The likelihood of infection transmission for healthcare workers to Covid-19 is more than three times as high as the general population.14 Consequently, the attention has shifted towards discussion on how to optimally protect healthcare workers. However, recommendations for protection for healthcare workers differ globally. In 2007, the World Health Organization (WHO) released list of aerosol generating procedures (AGPs).5 WHO and Public Health England (PHE) laid down guidance for the use of N95 masks, when performing any AGP, on a suspected COVID-19 positive patient.6 , 15 These guidelines are constantly evolving; there is uncertainty regarding the optimal personal protection equipment (PPE) for AGPs. There has also been confusion regarding the definition of AGPs in T&O. Recommendations for PPE have been influenced by the availability of adequate masks, gloves, gowns, helmets and goggles rather than the science for their use.14 Therefore, a review of literature that highlights the nature and impact of aerosol generating procedures within T&O surgery, and its significance on surgeons and other operating room personnel is warranted.
Materials and methods
Literature search and study selection
A comprehensive online search of PUBMED, MEDLINE, EMBASE, SCOPUS, and GOOGLE SCHOLAR was performed for all published articles in the English language, evaluating AGPs in T&O surgery and the PPE used.
The search was conducted using the following Medical Subject Heading (MESH) terms: “surgical procedures” AND “aerosol” AND “orthopaedic” AND “PPE” AND “aerosol generating procedure” AND “AGP”. The ‘related articles’ function was used to expand the search from each relevant study identified. Bibliographies of retrieved papers were further screened for any additional eligible studies. All identified citations and abstracts were thoroughly reviewed. The latest search was performed on the 15th of June 2020. All studies reporting on AGPs in T&O surgery were included. The primary end-points of the study were: use of power tools or instruments, and orthopaedic surgical procedures leading to aerosol generation. The secondary endpoint included use of any PPE. When the same institution reported two studies, we included either the one of better quality (increased sample size), the most recent publication, or both if the studies described different patient cohorts. Studies were excluded from the analysis if they were studying aerosol generation in procedures in other surgical disciplines, aside from orthopedic surgery.
Data extraction
Two reviewers (MM and KM) independently extracted data from each study; a third independent evaluator resolved any discrepancies (MI). Study characteristics (first author, year of publication, study design), population characteristics, type of surgical procedure, type of tool used and outcomes of interest as aerosol generation, were recorded. This systematic review was conducted in accordance with the established guidelines from Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA). Heterogeneity calculation was considered unsuitable owing to the inclusion criteria of including studies with methodological heterogeneity. Due to the heterogeneity of the available data it was decided to present the review in a narrative manner.
Results
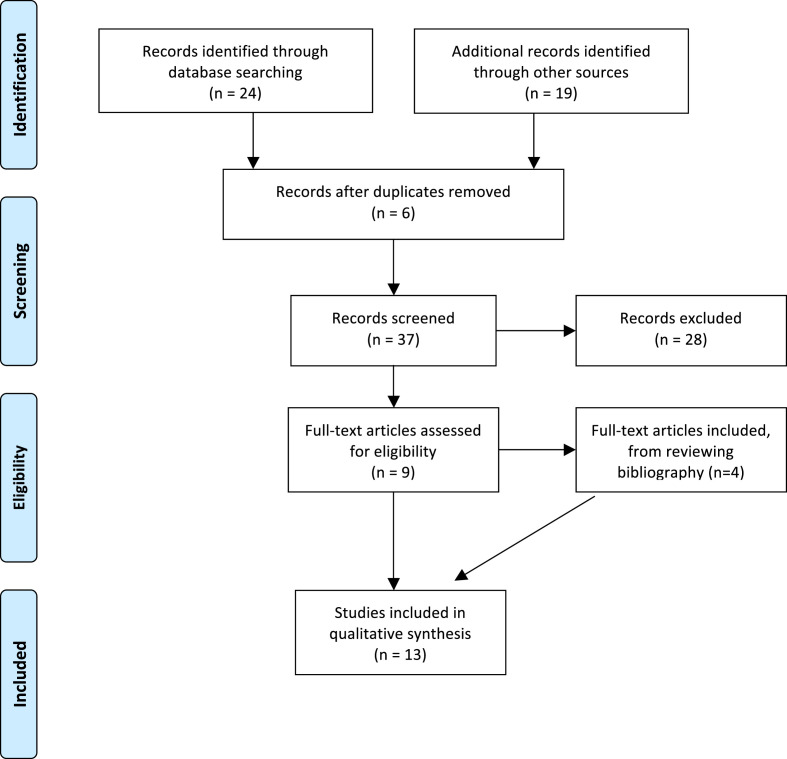
The search strategy populated 24 studies from PubMed, 16 studies from Scopus and 3 studies from Web of Science. Six studies were identified as duplicates and were excluded using Endnote X8 program (Thompson Reuter, USA). The shortlisted 37 studies were screened and 9 studies were included in the review. An additional 4 studies were included from the bibliography review, PRISMA flow-chart (Fig. 1 ).
Fig. 1.
PRISMA flow-chart for the review.
Characteristics of included studies
Thirteen studies met our inclusion criteria and were included in this review. The studies were conducted under different circumstances and used different design and populations. The included studies and their characteristics are described in Table 1 . A further detailed review, in terms of AGP, has been presented below depending on the tool used in the surgical setting.
Table 1.
The characteristics of the studies included in the review.
| Study ID | Type of surgery | Tool used that caused aerosol generation | Study subject∖setting | Particle size |
|---|---|---|---|---|
| Nogler 2011 | Orthopaedic (Robodoc) | High-speed cutter | Stimulation | The same size of Staphylococcus aureus |
| Putzer 2017 | Orthopaedic (Lumbar spine) | Hydro-surgery debridement including a full surgical setup such as draping. | Cadaver | The same size of Staphylococcus aureus |
| Pereira 2014 | Orthopaedic | Cleaning/moving the patient Use of electrosurgical apparatus Movement of the surgical team) |
Operating theatre | Ranging from 0.3 µm–10 µm |
| Kucukdurmaz 2012 | NA | Domestic electric drills (DED) | Stimulation in empty operating room (OR) | Size of Staphylococcus epidermidis, Micrococcus luteus, and Staphylococcus capitis |
| Heinsohn 1991 | NA | Oscillating bone saw, Hall drill, and a Shea drill were used on bone, and an electrocautery | Bovine tissue | Between 0.07 to 14 µm |
| Jewett 1992 | NA | Protocol 1: an oscillating bone saw, cast saw, a Hall drill and a Shea drill were used to operate on and a bovine electro cautery. Protocol 2: same tools with HIV infected blood | Stimulation | Between 0.28 to 14 µm |
| Nogler 2001 | Orthopaedic (Lumbar Spine) | High-speed bone cutter. | Cadaver | The same size of Staphylococcus aureus |
| Nogler 2002 | Revision Hip Arthroplasty | Ultrasound device and a high-speed cutter | Cadaver | The same size of Staphylococcus aureus |
| Wendland 2016 | Hip and Knee arthroplasty | Tools used in Arthroplasty. | Artificial foam bone | NA |
| Yeh 1994 | Total Hip Arthroplasty | Total Hip Arthroplasty instruments | Dogs with 51 Cr-lablled blood | 60% of the RBCs associated with particles large than 10 µm |
| Yeh 1995 | Total Hip Arthroplasty, Orthopaedic (Spine), Total Knee Arthroplasty |
Scalpel, electrocautery, and irrigation/suction, bone drill, saw, acetabular reamer, hammer, sprayer | Operating theatre | < 0.3 µm to 3 µm |
| Nogler 2001 | Orthopaedic (Cervical spine) | High-speed cutter | Male human cadaver | The same size of Staphylococcus aureus |
| Heinsohn 1993 | Arthroplasty/Aneurysmal Resection/Prostatectomy/Ventricular Malformation Repair/Nephrectomy/Caesarean Section/Vaginal Delivery | Use of power surgical tools | Operating theatre | 14.8 µm, 3.5 µm and 0.52 µm |
Use of high-speed cutter
Nogler et al. showed that use of high-speed tools generate an aerosol cloud of approximately 6 m × 3.8 m.7 The cloud covered the entire work area and extended over to the members of the operating team outside the sterile field. The authors recommended the use of sufficient protection for all medical workers in the operating theatres.
Nogler et al. described aerosol generation with use of high-speed cutter, during spinal laminectomy at L2–L4 levels, in a human cadaveric study.16 Staphylococcus aureus (ATCC 12600) was introduced to contaminate the aerosol produced. This was detected in the operating room at an extension of 5 × 7 m. The surgical team showed extensive face and body contamination with S. aureus. Despite protection by a barrier drape, similar contamination was observed on both the cadaver's head and the anesthesiologist.
Nogler et al. measured the extent of the environmental and body contamination with S. aureus (ATCC 12600) caused by an ultrasound device and a high-speed cutter used during hip arthroplasty, tested on human cadavers.11 They reported environmental contamination was present in an area of 6 × 8 m for both devices. The concentration of contamination was lower for the ultrasound device. Both the ultrasound and the high-speed cutter contaminated all members of the surgical team. The devices tested produced aerosols, which covered the whole operating theatre and all personnel present during the procedure. Nogler et al. in a similar human cadaveric study concluded that with the use of high-speed cutters in surgery of the cervical spine, staphylococci were detected in the operating room at an extension of 5 × 7 m. The use of use of high-speed cutters produced an aerosol cloud that spread over the whole surgical room and contaminated the operating room and all personnel present.17
Hydro-surgery debridement
Putxer et al. performed a complete hydro-surgery debridement including a full surgical setup such as draping on human cadavers.18 The irrigation fluid was artificially contaminated with S. aureus (ATCC 6538). This study evaluated the spread of contaminated aerosols in hydro-surgery debridement with and without an additional draping device (surgical tent). Without the surgical tent, the hydro-surgery device contaminated all individuals in the operating room (OR) and all parts of the OR to some extent. Additional protection provided by a surgical tent was seen to produce significantly less contamination of the operating room. The surgeon and the surgical assistant showed the greatest decrease in colony-forming units on their person. For both test setups, environmental contamination was observed in an area of 6 × 8 m. Both test setups caused contamination of all personnel present during the procedure and of the whole operating room.
Use of domestic electric drills
Kucukdurmaz et al. studied the use of domestic electric drills in orthopaedic surgery. Although the study aimed at looking at risk of surgical site infection, one of the secondary outcomes demonstrated drills produced statistically significantly higher levels of particles than the ambient air (p < 0.01).19
Aerosol generating surgical activities
Pereira et al. showed that the concentration and size of aerosols present during orthopedic surgery were measured, and the potential sources were identified. Measurements of particle concentration and size were carried out with a portable particle counter. The activities performed within the operating theatre were recorded. The results showed that the concentration of particles varied considerably depending on the type of activity performed. A total of 32 events were identified as being associated with elevated particle concentrations. These events were classified into 13 different types of activities. It was observed that particles above 0.5 μm–1.0 μm had much greater peaks and wider spread than those below 0.5 μm–1.0 μm. The study reported that most events inside the room generate particles above 0.5 μm–1.0 μm. During surgery, the use of a bone saw was an important source of particles. The particle concentration remained high throughout the period in which the saw was used. This event generated particles in all of the size ranges that were considered.20
Heinsohn et al. assessed aerosol generation with bovine blood slowly dripped onto the working area to simulate operating scenario. Tests were performed using an oscillating bone saw, a hall drill, a shea drill on bone, and an electrocautery (Bovie), used in both the cutting and coagulation modes, on tendon. They concluded that surgical power tools generate blood-containing aerosols composed of particles small enough to be inhaled and deposited in the pulmonary region of the respiratory tract. Inspirable blood aerosols were detected in the surgeons' breathing zone during test operations.21
Jewett et al. evaluated aerosol generation with the same protocol as Heinsohn et al. They used a 10-stage low-pressure cade impactor to determine the particle size distribution of each aerosol and Hemastix was used to assess the hemoglobin content of each particle size. They did the same for another series of blood aerosol, which previously showed the ability to infect human T-cell cultures. They concluded that all of the tools tested produced blood-containing aerosol particles in the respirable size range (<5 microns). Surgical masks offered little protection against such particles.22
Yeh et al. evaluated the generation of aerosol with use of a scalpel, electrocautery, irrigation/suction, reamers, bone drill, and an oscillating saw. They found that the concentration and size distribution of these particles depended on the procedure being performed. Some of these particles contained hemoglobin. Quartz crystal microbalance cascade impactor system (QCM) data indicated that the aerosol concentration was highest (although the absolute values were low) when the surgical site was opened; electrocautery was being used primarily, and with occasional applications of irrigation/suction. They compared data obtained between a knee replacement procedure, in which a tourniquet was applied to reduce the blood losses, and other procedures, such as a hip replacement, suggested that the irrigation/suction procedure used during operations was one of the major sources of blood-associated aerosols.12
Jewett et al. evaluated the exposure to blood containing aerosols in orthopaedics, urology, cardiothoracic and obstetric surgery, in the operating theatre. They studied procedures involving use of power surgical tools. Data showed that the mucous membrane lining of the upper respiratory tract and the alveolar macrophages in the gas-exchange region are likely to be exposed to aerosolized blood in the operating theatre.23
Surgical tools used during hip and knee arthroplasty
Wendlandt et al. evaluated use of surgical helmet systems for protecting surgeons from droplets generated during orthopaedic procedures. They quantified the contamination of the surgeon by droplets during orthopaedic procedures by an in vitro simulation of hip and knee arthroplasty, while wearing surgical helmet systems versus conventional surgical clothing. They concluded that the contamination risk was 30% while wearing conventional clothing whereas none of the 20 subjects using the surgical helmet system reported any contamination after removal of the protective clothing.8
Yeah et al. evaluated the characterization of aerosols produced during total hip replacement surgery in dogs with Cr-labeled blood. Results confirmed that blood-associated aerosols were produced during orthopedic surgery. The time-averaged mass concentration near the surgical site, as measured by the personal impactor, was 0.37 mg m−3. 6.5 μg m−3 (1.8% of the total mass concentration) was attributed to red blood cells (RBCs). The estimated number of RBCs or hemoglobin that might be inhaled by a surgeon without any respiratory protection during the course of an orthopedic surgery was about 2.9 × 10 s RBCs or 8.7 pg of hemoglobin. About 60% of the RBCs were associated with particles larger than 10 pm in aerodynamic diameter, and about 8% of the RBCs were associated with particles less than 0.5 pm. The number ratio between the RBCs and lymphocytes for humans is about 2200:1; thus, the estimated number of lymphocytes that might be inhaled by the surgeon, without any respiratory protection, intra-operatively would be less than 135.24
To assess the significance of these findings on the potential risk to health care workers will require further studies of the relationship between pathogens and particle sizes and the viability of pathogens associated.
Discussion
Covid-19 pandemic is the largest global health care crisis of this century. A large number of healthcare workers have succumbed to this virus, and the count is rising by the day.25 The PPE, at Work Regulations 1992, legislates that an employer should provide suitable protection and training in the use of equipment.6 Studies have recommended that orthopaedic surgeons wear adequate protective gowns and face/eye protection during procedures likely to generate splashes or sprays of body fluids. Despite higher cost, global demands during the pandemic, personal protection during surgical interventions is mandatory.8 The Center for Disease Control in both the US26 and equivalent organization in China, the Association of Spanish Surgeons,27 Australia's Department of Health specifically recommend the use of N95 respirators for surgeries involving AGPs on COVID-19 patients.28 In a time when there is limited information about transmission of COVID-19, aggressive protection with complete PPE for AGPs is in line with guidance from multiple national organizations, as well as the limited data available from published studies. Authors have gone far to recommend guidelines suggesting all theatre staff should wear enhanced PPE.25 , 29
During a standard procedure, the aerosol cloud produced extends over the area occupied by all sterile and non-sterile members of the operating team. Thus, it is necessary to provide sufficient protection for all medical workers in the operating room.7 The concentration of aerosol particles inside an operating room varies depending on the type of activity performed inside the theatre complex. Pereira et al. described that the particles generated by the use of electrosurgical apparatus represent an important source of air contamination. These small particles, gases, and vapors may contain potentially harmful contaminants, such as DNA viruses, aerosols, cell fragments, and other gaseous hydrocarbons, that can be inhaled by the occupants of the operating room.20
Another study demonstrates that contaminated aerosols produced during use of a high pressure pulsed lavage system can spread over the entire operating room, contaminating both the animate and the inanimate environment. This risk remains for the surgical team, especially if the contaminated aerosol is inhaled or comes into contact with conjunctival or mucous membranes.18
During laboratory simulations, it has been demonstrated that instruments can produce inhalable aerosols.12 An aerosol cloud consisting of a mixture of irrigation fluid and blood; is produced due to the high revolutions of high speed devices, while working around a basin of fluid or blood or by stream of fluid or blood.7 , 30 Schultz et al. reported that high speed cutters generate a large amount of free particles of tissue from patients, out of which 35% were contaminated with microbes.31 This aerosol cloud presents a risk of microbial contamination for the surgical team.7
The most common sources of infection are viral pathogens, bacterial, and fungal agents. There are several reports of infection from bacterial agents such as S. aureus, viral agents like hepatitis B/C, and Herpes simplex from injuries with sharp and high-speed tools.7 , 11 , 32 The contamination risk via this route of transmission is especially high in orthopaedic surgery. There is also a risk of infection for team members through inhalation of aerosols contaminated with pathogens such as Mycobacterium tuberculosis, legionella, hepatitis B, Varicella zoster, smallpox, influenza and S. aureus. 7 , 11 , 32 , 33 There is addition risk of infection for patients operated on in the same room after such surgery, or in contact with contaminated medical staff.34
Standard surgical PPE includes a face shield, mask, and waterproof gown, double gloves, and shoe covers. There is some disagreement, however, regarding the type of respiratory protection. N95 respirators, powered air purifying respirators (PAPR), or standard surgical masks have been proposed for surgical procedures on patients with COVID-19.35 Electron microscopy has measured the COVID-19 virus to be between 70 and 90 nm in diameter.36 However, droplets less than 5 μm in size are typically produced by coughing and sneezing, during which the virus can travel up to 4.5 m, representing a risk to healthcare staff.37 Surgical facemasks were found to provide very little protection for particle sizes 10–80 nm.38 N95/FFP2 masks are at least 95% effective for particle sizes 0.1–0.3 μm, which increases to 99.5% or higher for particles that are 0.75 μm or larger.35 Therefore, over 95% protection is provided with an FFP2/N95 mask when performing an AGP.6 A surgical mask is capable of blocking gross inhalation of droplets, while a well-fitted N95 respirator is additionally capable of filtering aerosols. This is of particular interest to orthopaedic surgeons as aerosols generation have been identified from use of high-speed tools.30 Smoke from electrocautery devices has been shown to harbor intact bacterial and virus particles.39, 40, 41, 42
The incidence of infection with COVID-19 during the early stages of the outbreak, amongst orthopaedic surgeons in Wuhan, China ranged between 1.5% and 20.7%. The specific recommendation made by authors to prevent COVID-19 infection amongst the orthopaedic community, was to stay more vigilant and wear N95 respirators at all times.43 There have been questions raised regarding trauma and orthopaedic procedures being regarded as AGP. This review has confirmed that surgical power tools such as saws, burrs, drills as well as electrocautery in cutting and coagulation mode, used in T&O surgery lead to aerosol generation. Procedures involving these instruments place healthcare workers within the operating theatre at high risk for COVID-19 disease transmission. The limitation of this review is the constantly evolving scenario and the inability to perform a systematic review due to the heterogeneity of available information.
Conclusion
Most orthopaedic procedures produce aerosols. Conventional surgical masks do not offer protection against high-risk AGPs. In the current era of COVID19 pandemic, there is a significant risk to the transmission of infection to the theatre staff. For protection against airborne transmission, air-purifying respirator masks should be used. Proper fitting and sizing is essential to ensure protection whilst using these masks. This review helps to clarify the uncertainty surrounding the generation of aerosols with trauma and orthopaedic procedures.
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 Jan 22;382(10):929–936. doi: 10.1056/NEJMoa2001191. Massachusetts Medical Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdorf A., Porru F., Rugulies R. The COVID-19 (Coronavirus) pandemic: consequences for occupational health. Scand J Work Environ Health. 2020 May 1;46(3):229–230. doi: 10.5271/sjweh.3893. [DOI] [PubMed] [Google Scholar]
- 5.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS ONE. 2012 Jan 26;7(4) doi: 10.1371/journal.pone.0035797. Public Library of Science; e35797EP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herron J., Hay-David A., Gilliam A.D., Brennan P.A. Personal protective equipment and Covid 19 – a risk to healthcare staff? Br J Oral Maxillofac Surg. 2020 Jan 13 doi: 10.1016/j.bjoms.2020.04.015. Published by Elsevier Ltd on behalf of The British Association of Oral and Maxillofacial Surgeons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogler M., Wimmer C., Lass-Florl C., Mayr E., Trobos S., Gegenhuber C. Contamination risk of the surgical team through ROBODOC's high-speed cutter. Clin Orthop Relat Res. 2001 Jun;(387):225–231. doi: 10.1097/00003086-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Wendlandt R., Thomas M., Kienast B., Schulz A.P. In-vitro evaluation of surgical helmet systems for protecting surgeons from droplets generated during orthopaedic procedures. J Hosp Infect. 2016 Sep;94(1):75–79. doi: 10.1016/j.jhin.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Smith R.C., Mooar P.A., Cooke T., Sherk H.H. Contamination of operating room personnel during total arthroplasty. Clin Orthop Relat Res. 1991 Oct;(271):9–11. [PubMed] [Google Scholar]
- 10.Giachino A., Profitt A., Taine W. Contamination of the conjunctiva of the orthopaedic surgeon. A technical note. J Bone Joint Surg Am. 1988 Jan;70(1):126–127. [PubMed] [Google Scholar]
- 11.Nogler M., Lass-Florl C., Wimmer C., Mayr E., Bach C., Ogon M. Contamination during removal of cement in revision hip arthroplasty. A cadaver study using ultrasound and high-speed cutters. J Bone Joint Surg Br. 2003 Apr;85(3):436–439. doi: 10.1302/0301-620x.85b3.12451. [DOI] [PubMed] [Google Scholar]
- 12.Yeh H.C., Turner R.S., Jones R.K., Muggenburg B.A., Lundgren D.L., Smith J.P. Characterization of aerosols produced during surgical procedures in hospitals. Aerosol Sci Tech. 1995;22(2):151–161. Taylor & Francis. [Google Scholar]
- 13.Shpuntoff H., Shpuntoff R.L. High-speed dental handpieces and spread of airborne infections. N Y State Dent J. 1993 Jan;59(1):21–23. [PubMed] [Google Scholar]
- 14.Hirschmann M.T., Hart A., Henckel J., Sadoghi P., Seil R., Mouton C. COVID-19 coronavirus: recommended personal protective equipment for the orthopaedic and trauma surgeon. Knee Surg Sports Traumatol Arthrosc. 2020 Apr:1–9. doi: 10.1007/s00167-020-06022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baller A. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. 2020 Apr;7:1–28. [Google Scholar]
- 16.Nogler M., Lass-Florl C., Ogon M., Mayr E., Bach C., Wimmer C. Environmental and body contamination through aerosols produced by high-speed cutters in lumbar spine surgery. Spine. 2001 Oct;26(19):2156–2159. doi: 10.1097/00007632-200110010-00023. [DOI] [PubMed] [Google Scholar]
- 17.Nogler M., Lass-Florl C., Wimmer C., Bach C., Kaufmann C., Ogon M. Aerosols produced by high-speed cutters in cervical spine surgery: extent of environmental contamination. Eur Spine J. 2001 Aug 1;10(4):274–277. doi: 10.1007/s005860100310. Official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. Springer-Verlag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putzer D., Lechner R., Coraca-Huber D., Mayr A., Nogler M., Thaler M. The extent of environmental and body contamination through aerosols by hydro-surgical debridement in the lumbar spine. Arch Orthop Trauma Surg. 2017 Jun;137(6):743–747. doi: 10.1007/s00402-017-2668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kucukdurmaz F., Imren Y., Akkoyunlu Y., Tuncay I., Sen C. Domestic electric drills in the service of orthopaedic surgery: a potential and preventable source of surgical site infections. Acta Orthop Traumatol Turc. 2012;46(6):455–459. doi: 10.3944/aott.2012.2794. [DOI] [PubMed] [Google Scholar]
- 20.Pereira M.L., Vilain R., Leivas T.P., Tribess A. Measurement of the concentration and size of aerosol particles and identification of the sources in orthopedic surgeries. HVAC&R Research. 2012;18(4):588–601. Taylor & Francis. [Google Scholar]
- 21.Heinsohn P., Jewett D.L., Balzer L., Bennett C.H., Seipel P., Rosen A. Aerosols created by some surgical power tools: particle size distribution and qualitative hemoglobin content. Appl Occup Environ Hyg. 1991;6(9):773–776. Taylor & Francis. [Google Scholar]
- 22.Jewett D.L., Heinsohn P., Bennett C., Rosen A., Neuilly C. Blood-containing aerosols generated by surgical techniques: a possible infectious hazard. Am Ind Hyg Assoc J. 1992 Apr;53(4):228–231. doi: 10.1080/15298669291359564. [DOI] [PubMed] [Google Scholar]
- 23.Heinsohn P., Jewett D.L. Exposure to blood-containing aerosols in the operating room: a preliminary study. Am Ind Hyg Assoc J. 1993 Aug;54(8):446–453. doi: 10.1080/15298669391354946. [DOI] [PubMed] [Google Scholar]
- 24.Yeh H.C., Muggenburg B.A., Guilmette R.A., Snipes M.B., Turner R.S., Jones R.K., et al. Characterization of aerosols produced during total hip replacement surgery in dogs with 51Cr-labeled blood. J Aerosol Sci. 1995;26(3):511–518. [Google Scholar]
- 25.Raghavan R., Middleton P.R., Mehdi A. Minimising aerosol generation during orthopaedic surgical procedures- Current practice to protect theatre staff during Covid-19 pandemic. J Clin Orthop Trauma. 2020 May-Jun;11(3):506–507. doi: 10.1016/j.jcot.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirator-use-faq.html
- 27.https://www.aecirujanos.es/files/noticias/152/documentos/Recomendaciones_caso_cirugia.pdf
- 28.https://www.health.gov.au/sites/default/files/documents/2020/03/interim-recommendations-for-the-use-of-personal-protective-equipment-ppe-during-hospital-care-of-people-with-coronavirus-disease-2019-covid-19.pdf
- 29.Wong K.C., Leung K.S. Transmission and prevention of occupational infections in orthopaedic surgeons. J Bone Joint Surg Am. 2004 May;86(5):1065–1076. doi: 10.2106/00004623-200405000-00029. [DOI] [PubMed] [Google Scholar]
- 30.Eduard W., Heederik D. Methods for quantitative assessment of airborne levels of noninfectious microorganisms in highly contaminated work environments. Am Ind Hyg Assoc J. 1998 Feb;59(2):113–127. doi: 10.1080/15428119891010370. [DOI] [PubMed] [Google Scholar]
- 31.Bible J.E., Biswas D., Whang P.G., Simpson A.K., Grauer J.N. Which regions of the operating gown should be considered most sterile? Clin Orthop Relat Res. 2009 Mar 1;467(3):825–830. doi: 10.1007/s11999-008-0341-1. Springer-Verlag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harpaz R., Seidlein Von L., Averhoff F.M., Tormey M.P., Sinha S.D., Kotsopoulou K., et al. Transmission of hepatitis B virus to multiple patients from a surgeon without evidence of inadequate infection control. N Engl J Med. 1996 Feb;334(9):549–554. doi: 10.1056/NEJM199602293340901. [DOI] [PubMed] [Google Scholar]
- 33.Eickhoff T.C. Airborne nosocomial infection: a contemporary perspective. Infect Control Hosp Epidemiol. 1994 Oct;15(10):663–672. doi: 10.1086/646830. [DOI] [PubMed] [Google Scholar]
- 34.Isenberg H.D., Tucci V., Cintron F., Singer C., Weinstein G.S., Tyras D.H. Single-source outbreak of Candida tropicalis complicating coronary bypass surgery. J Clin Microbiol. 1989 Nov;27(11):2426–2428. doi: 10.1128/jcm.27.11.2426-2428.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian Y., Willeke K., Grinshpun S.A., Donnelly J., Coffey C.C. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am Ind Hyg Assoc J. 1998;59(2):128–132. doi: 10.1080/15428119891010389. Taylor & Francis. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.-M., Chung Y.-S., Jo H.J., Lee N.-J., Kim M.S., Woo S.H., et al. Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res Perspect. 2020 Jan 28;11(1):3–7. doi: 10.24171/j.phrp.2020.11.1.02. Korea Centers for Disease Control & Prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loh N.-H.W., Tan Y., Taculod J., Gorospe B., Teope A.S., Somani J., et al. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anaesth. 2020 Jul;67(7):893–894. doi: 10.1007/s12630-020-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bałazy A., Toivola M., Adhikari A., Sivasubramani S.K., Reponen T., Grinshpun S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Contr. 2006;34(2):51–57. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Kwak H.D., Kim S.-H., Seo Y.S., Song K.-J. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016 Dec;73(12):857–863. doi: 10.1136/oemed-2016-103724. [DOI] [PubMed] [Google Scholar]
- 40.Mellor G., Hutchinson M. Is it time for a more systematic approach to the hazards of surgical smoke?: reconsidering the evidence. Workplace Health & Saf. 2013 Jun;61(6):265–270. doi: 10.1177/216507991306100605. [DOI] [PubMed] [Google Scholar]
- 41.Schultz L. Can efficient smoke evacuation limit aerosolization of bacteria? AORN J. 2015 Jul;102(1):7–14. doi: 10.1016/j.aorn.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y., Song Y., Hu X., Yan L., Zhu X. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among the gynecologists. J Cancer. 2019;10(12):2788–2799. doi: 10.7150/jca.31464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo X., Wang J., Hu D., Wu L., Gu L., Wang Y., et al. Survey of COVID-19 disease among orthopaedic surgeons in Wuhan, People's Republic of China. J Bone Joint Surg Am. 2020 May 20;102(10):847–854. doi: 10.2106/JBJS.20.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]