Abstract
As the number of COVID-19 cases emerge, new complications associated with the disease are recognized. We present three cases of spontaneous pneumothorax in patients with COVID-19. They show that a pneumothorax can occur during different phases of disease, in patients without a pulmonary disease history and is not necessarily associated to positive pressure ventilation or severity of COVID-19. Although the exact causative mechanisms remain unknown, this observation might imply that extensive alveolar destruction due to COVID-19 may lead to bulla formation resulting in subsequent pneumothorax.
Keywords: COVID-19 pneumonia, SARS-CoV-2, Pneumothorax
Abbreviations: COVID-19, Coronavirus disease 2019; PCR, Polymerase Chain Reaction; MERS, Middle-East Respiratory Syndrome; SARS, Severe Acute Respiratory Syndrome; DAD, Diffuse Alveolar Damage; ARDS, Acute Respiratory Distress Syndrome
1. Introduction
Several recent autopsy studies published showed that vascular disease, diffuse alveolar damage and lymphocyte infiltration are present in lungs of patients that succumbed to COVID-19 disease [1,2]. We hypothesize that those pathological findings associated with COVID-19 pneumonitis may lead to bulla formation and thereby redispose to spontaneous pneumothorax. Here, we present three subsequent cases of sudden respiratory deterioration caused by primary spontaneous pneumothorax in COVID-19 patients.
2. Case reports
The first patient, a 63-year old man with no history of pulmonary disease, had been admitted for a confirmed Coronavirus Disease 2019 (COVID-19) pneumonia with symptoms of dyspnea, cough and fever for 15 days. During the admission, he required oxygen suppletion with High Flow Nasal Cannula for severe respiratory distress. Eleven days after discharge, after an initially prosperous recovery, he returned to hospital with thoracic pain and dyspnea. Chest X-ray showed a pneumothorax and a persistent perihilar consolidation (Fig. 1A). At readmission, an intercostal thoracic tube was placed, which was removed after pleural talcage five days later (Fig. 1B). The patient was discharged the following day in good clinical condition.
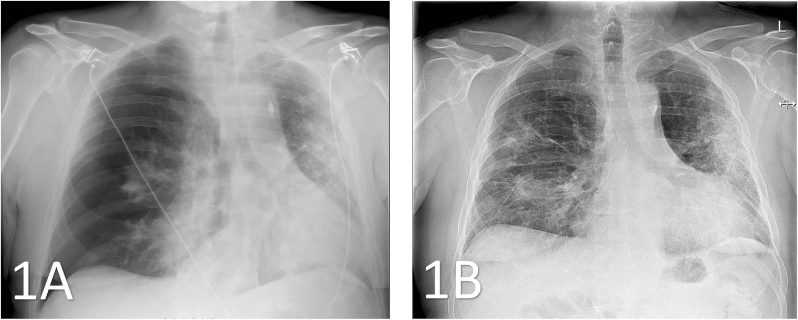
Fig. 1.
1A: Thoracic X-ray (anterior-posterior) from patient 1, showing a large right-sided pneumothorax with collapsed lung. Bilateral patchy pulmonary infiltrates due to COVID-19 are present. 1B: Imaging obtained a day after chest drainage showing an inflated lung with only a small apical pneumothorax left. Chest tube and infiltrates are still present.
The second patient, a 76-year old man with a history of centrilobular emphysema, was brought in after outpatient emergency placement of an intercostal tube for suspected tension pneumothorax. Upon admission, he tested positive for SARS-CoV-2 antibodies, thereby confirming recent COVID-19 infection. X-ray showed unilateral pneumothorax, no CT-scan was performed. He had not been admitted for pneumonia previously, nor had he experienced any respiratory complaints recently. The thoracic tube was removed three days after admission, followed by discharge from hospital in acceptable clinical condition.
The third patient, a 72-year old man with a history of COPD and asthma, presented at the Emergency Room with ten days of progressive dyspnea, fatigue, cough and fever. COVID-19 was confirmed by PCR. In hospital, the patient was treated with prednisolone, bronchodilators, ceftriaxone and oxygen suppletion. A thoracic CT-angiography was performed, revealing a pulmonary embolus. Furthermore, prominent bilateral ground-glass opacifications with consolidations and crazy paving congruent with COVID-19 were observed (Fig. 2A and B). After initial clinical improvement), an acute worsening of dyspnea and hypoxemia occurred. A chest X-ray revealed a right-sided pneumothorax. On a CT-scan performed after intercostal tube placement, multiple new bullae were seen in the middle and right lower lobe (Fig. 2C and D). Despite intercostal drainage, the pneumothorax did not resolve and the patient underwent video-assisted thoracic surgery. During surgery, multiple new bullae were observed and bullectomy and talcage were performed. More than a month after admission, the patient was discharged from hospital, significantly weakened by the admission.
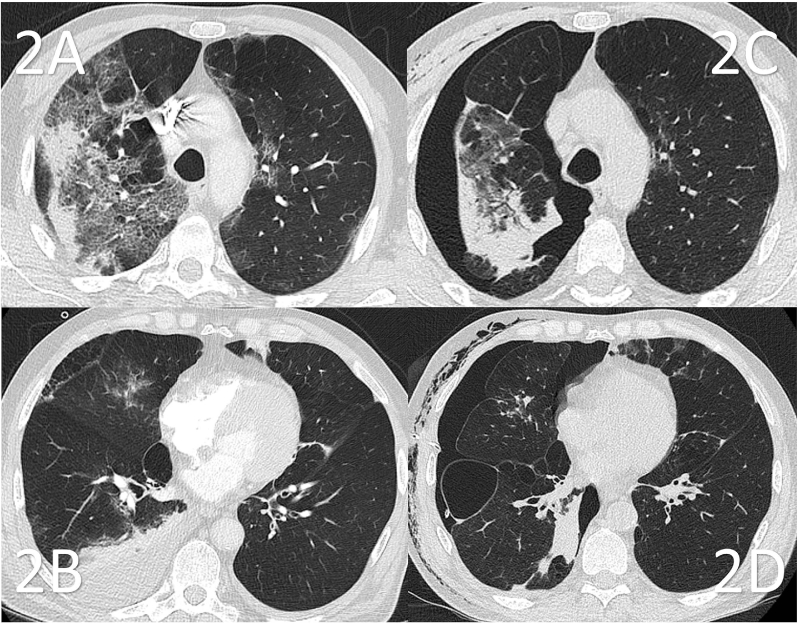
Fig. 2.
Transversal slides of 2 different CT scans of patient 3, obtained before (2A, 2B) and after (2C, 2D) occurrence of the pneumothorax with 2 weeks in between. Note: the presented slides represent different anatomic landmarks, but the presented lung coupes represent the same area due to shifting of the lung by the pneumothorax. 2A: Severe ground glass opacities (GGO) congruent with COVID-19. 2B: A pre-existent bulla observed in the middle lobe. Pleural effusion is present. 2C: The subsequently performed CT scan shows pneumothorax, atelectasis and subcutaneous emphysema. In comparison to Fig. 2A, progressive fibrotic reaction with honeycombing formation is seen after diminution of the GGO. 2D: Newly formed bullae are present laterobasally in the right lower lobe. Intercostal thorax tube in situ.
3. Discussion
To our knowledge, no relationship between SARS-CoV-2 infection and pneumothorax has been described before. A few cases of pneumothorax after COVID-19 have been reported, including another case in the Netherlands [3,4]. The latter reports a case of a formerly healthy 31-year old male with COVID-19. This patient was re-admitted to hospital for a right-sided pneumothorax which occurred after admission for COVID-19. A subsequent CT-scan revealed extensive bulla formation where first ground-glass opacities had been observed. We developed a hypothesis that alveolar damage caused by SARS-CoV-2 promotes severe destruction of alveolar tissue resulting in bulla formation, thereby enhancing the risk of pneumothorax.
Middle-East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) are caused by different strains of coronavirus. In all three diseases, ground-glass opacities and consolidations are commonly found [5]. In SARS, these abnormalities are more often unilateral and can be either focal or multifocal, while in MERS and COVID-19 bilateral multifocal abnormalities are most common. In follow-up studies, fibrosis and pleural effusion occur in one-third of patients with MERS, but are rarely seen in SARS [6]. Long-term follow up studies investigating patients suffering from the former outbreak of SARS in 2002–2003, showed that ground-glass opacities remained for several months, which slowly progressed into diffuse fine reticulation rather than bulla formation [7]. While pneumothorax is rare in both SARS and MERS, it has been associated in MERS with poor prognosis.
Although other types of viral pneumonitis, such as Influenza, measles, hantavirus and cytomegalovirus are also associated with bilateral ground-glass opacification and consolidation, bulla formation has not been reported [8]. Pneumonitis caused by influenza viruses causes mostly ground-glass attenuation with lobar distribution and consolidation, which usually resolve after three weeks. No association between these infections, bulla formation and pneumothorax has been described [9,10].
Histopathology from autopsy studies in deceased COVID-19 patients report bilateral Diffuse Alveolar Damage (DAD), evident desquamation of pneumocytes and hyaline membrane formation. Thrombosis, microangiopathy and leukocyte infiltration, mostly consisting of lymphocytes, are also frequently reported [1,2,11]. In a recently published autopsy study, pathological findings in lung tissue were compared between patients with COVID-19, patients with ARDS caused by H1N1 infection and patients without pneumonia [12]. DAD was found in both diseases, while extensive endothelial and vascular damage as well as microthrombi were ubiquitous in COVID-19 infected lung specimen. These features were absent in uninfected lungs. Although pneumothorax can be a complication of positive airway pressure in patients with ARDS receiving mechanical ventilation, most of the COVID-19 patients, in contrast to the H1N1 group, did not receive any form of ventilation. The DAD seen in both ventilated and unventilated COVID-19 patients may cause development of bullae and thereby predispose to pneumothorax in different stages of disease. The pathological findings in patients with COVID-19 pneumonia resemble those found in post-mortem studies in SARS and MERS [1,11,12].
In conclusion, the presented cases suggest that some patients with COVID-19 are possibly at risk for pneumothorax, thereby providing a new insight in the COVID-19 care. Possibly, bullae formation and subsequent pneumothorax can occur in formerly healthy lungs, which is not necessarily related to the initial severity of COVID-19 and can occur during any stage of the disease. This demonstrates that the possibility of a pneumothorax should be kept in mind in patients with, or recovering from, COVID-19 disease with progressive dyspnea. Further research is required to assess the underlying mechanisms responsible for pneumothorax in COVID-19 patients. We suggest that extensive alveolar destruction may lead to bullae formation resulting in subsequent pneumothorax. Long-term follow up of COVID-19 patients is necessary to provide knowledge about the course of pulmonary sequelae and risk of pneumothorax.
Contributions to the manuscript
MLJ: Patient treatment, conception, substantial contribution to the draft manuscript, final approval.
MJGM: Patient treatment, substantial contribution to the draft manuscript, final approval.
SEC: Providing Figures with description, revising the draft, final approval.
GJB: Patient treatment, conception, revising the draft, final approval.
Declaration of competing interest
The before named authors report no conflict of interest.
No grants, funding, gifts, equipment, drugs or any other sort of (financial) support is associated with the realization of this manuscript.
This manuscript and its abstract were not presented or published before.
References
- 1.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloemen H., Hagmolen Of Ten Have W., Clappers-Gielen G.A.L. [Chest pain and dyspnea during the recovery period of COVID-19 pneumonia] Neth. J. Med. 2020:164. [PubMed] [Google Scholar]
- 4.Sun R., Liu H., Wang X. Mediastinal emphysema, giant Bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J. Radiol. 2020;21(5):541–544. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseiny M., Kooraki S., Gholamrezanezhad A., Reddy S., Myers L. Radiology perspective of Coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. AJR Am. J. Roentgenol. 2020;214(5):1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 6.Ketai L., Paul N.S., Wong K.T. Radiology of severe acute respiratory syndrome (SARS): the emerging pathologic-radiologic correlates of an emerging disease. J. Thorac. Imag. 2006;21(4):276–283. doi: 10.1097/01.rti.0000213581.14225.f1. [DOI] [PubMed] [Google Scholar]
- 7.Wu X., Dong D., Ma D. Thin-Section computed tomography manifestations during convalescence and long-term follow-up of patients with severe acute respiratory syndrome (SARS) Med Sci Monit. 2016;22:2793–2799. doi: 10.12659/MSM.896985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E.A., Lee K.S., Primack S.L., Yoon H.K., Byun H.S., Kim T.S. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics. 2002;22 doi: 10.1148/radiographics.22.suppl_1.g02oc15s137. Spec No:S137-S149. [DOI] [PubMed] [Google Scholar]
- 9.Franquet T., Lee K.S., Muller N.L. Thin-section CT findings in 32 immunocompromised patients with cytomegalovirus pneumonia who do not have AIDS. AJR Am. J. Roentgenol. 2003;181(4):1059–1063. doi: 10.2214/ajr.181.4.1811059. [DOI] [PubMed] [Google Scholar]
- 10.Koo H.J., Lim S., Choe J., Choi S.H., Sung H., Do K.H. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38(3):719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 11.Wichmann D., Sperhake J.P., Lutgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]