Abstract
Transgenic corn expressing insecticidal proteins derived from the bacterium Bacillus thuringiensis (Bt) is an important pest management tool. Western corn rootworm, Diabrotica virgifera virgifera LeConte, is a key pest of corn in the midwestern United States that has developed field-evolved resistance to all available Bt traits. The first Bt trait to be commercialized for management of rootworm was Cry3Bb1 in 2003, and field-evolved resistance appeared in 2009. In this study, we examined fields in counties where greater-than-expected injury to Cry3 (Cry3Bb1 or mCry3A) corn roots (>1 node) had previously been reported (problem counties) and counties where injury had not been reported (non-problem counties). Four to eight fields were sampled per county in 2015, 2016, and 2017 to quantify rootworm abundance, root injury, Cry3Bb1resistance, and rootworm management strategies. Rootworm abundance, root injury, and resistance to Cry3Bb1 did not differ between county types. Management tactics differed between county types, with problem counties growing more corn, using more soil insecticide, and growing more Cry34/35Ab1 corn. Additionally, a comparison of root injury to Bt and non-Bt corn within fields indicated that farmers derived an economic benefit from planting Bt corn to manage corn rootworm. Our results suggest that rootworm populations are similar between problem and non-problem counties in Iowa due to similar levels of selection pressure on Cry3 corn, but problem county fields have applied more management tactics due to previous rootworm issues in the area.
Keywords: integrated pest management, landscape, resistance management, rootworm, transgenic crop
Western corn rootworm, Diabrotica virgifera virgifera LeConte, is an economically important pest of corn in the United States. Injury to corn primarily occurs when larvae feed on root tissue, which can result in a reduction in the ability of the corn plant to take up water and nutrients (Kahler et al. 1985, Godfrey et al. 1993). Plants may lodge when injury is severe, reducing yield by interfering with mechanical harvest (Riedell 1990, Spike and Tollefson 1991). The lifecycle of this pest is tightly linked to corn; eggs overwinter in cornfields, and larvae feed on corn root tissue that is present upon hatching the following spring (Chiang 1973, Spencer et al. 2009). Thus, fields where corn is grown for multiple consecutive years provide ideal habitat for this pest.
Management of western corn rootworm has historically included rotation to a non-host crop, such as soybean, and the use of soil insecticides at planting (Meinke et al. 2009). More recently, transgenic corn producing insecticidal proteins derived from the bacterium Bacillus thuringiensis (Bt) has become an important management tool for farmers in the midwestern United States. The first Bt trait targeting rootworm, Cry3Bb1, was introduced in 2003 (U.S. EPA 2003). There are currently four traits available for rootworm management either singly or as a pyramid of two toxins (Cry3Bb1, mCry3A, Cry34/35Ab1, and eCry3.1Ab) (Gassmann 2016). However, field-evolved resistance by western corn rootworm has been documented for all four toxins (Gassmann et al. 2011, 2014, 2020; Jakka et al. 2016).
The primary method by which farmers attempt to delay the development of resistance to Bt crops is the refuge strategy. This strategy relies on the planting of non-Bt plants in addition to Bt plants in a field. When the relatively rare individuals that survive on Bt plants mate with the Bt-susceptible individuals originating from non-Bt plants, the offspring are heterozygous for resistance to Bt (Gould 1998). Several factors increase the ability of this strategy to effectively delay resistance. These include a Bt dose that is sufficient to render the resistance trait functionally recessive (‘high dose’), a low initial resistance allele frequency in the population, and the presence of fitness costs associated with resistance (i.e., when resistant individuals experience lower fitness than susceptible individuals in the absence of Bt) (Carrière and Tabashnik 2001, Tabashnik et al. 2008, Gassmann et al. 2009, Carrière et al. 2010). None of the current Bt toxins meet the ‘high dose’ threshold for western corn rootworm (Gassmann 2016). Fitness costs of resistance to Bt in this species are often minor, and have not been detected in some populations (Hoffmann et al. 2015, Ingber and Gassmann 2015, Paolino and Gassmann 2017). These characteristics make western corn rootworm particularly capable of evolving resistance to Bt in spite of the refuge strategy. The first cases of field-evolved resistance to Cry3Bb1 corn occurred in 2009, only 6 yr after the initial commercialization of this trait (Gassmann et al. 2011).
The intensity of selection is an important factor in the evolution of Bt resistance, and in the case of Cry3Bb1 resistance by western corn rootworm, continuous cultivation of Cry3Bb1 corn in a field is a key factor (Gassmann 2016). The level of resistance to Cry3Bb1 was shown to be correlated to the number of years a field had been planted to this trait (Gassmann et al. 2011). Iowa had the highest farmer adoption rates of Bt corn of any Midwestern state between 2003 and 2008, and areas of the state with intensive corn production would have exerted strong selection pressure for resistance (Scandizzo and Savastano 2010). The earliest cases of Cry3Bb1 resistance occurred in northeastern Iowa in 2009, an area of high corn cultivation (Gassmann et al. 2011). In the following year, 2010, several more fields exhibited injury to Cry3 corn and commensurate resistance levels by rootworm populations, and these fields were located in the northeast and northwest areas of Iowa (Gassmann et al. 2012). The distribution of Cry3Bb1-resistant populations expanded geographically between 2010 and 2013 (Gassmann et al. 2012, 2014; Dunbar et al. 2016; Jakka et al. 2016).
In the current study, our goal was to understand differences in rootworm populations and management strategies on a regional scale. Understanding regional management approaches may be especially informative for understanding what drives resistance in areas around the state. Other work has suggested that considering management tactics on a county-level scale may be useful for this reason (O’Rourke et al. 2011, Martinez and Caprio 2016). We examined fields in counties where injury to Cry3Bb1 corn by western corn rootworm had previously been reported, where western corn rootworm resistance to Cry3Bb1 had also been confirmed (‘problem counties’), and compared them to fields in counties where similar issues had not been reported in previous peer-reviewed publications (‘non-problem counties’). We hypothesized that fields in problem counties would have higher root injury to corn, higher abundance of western corn rootworm, and higher levels of resistance to Cry3Bb1 corn compared to non-problem counties. We also hypothesized that problem county fields would have different management strategies for rootworm than fields in non-problem counties.
Materials and Methods
County and Field Selection
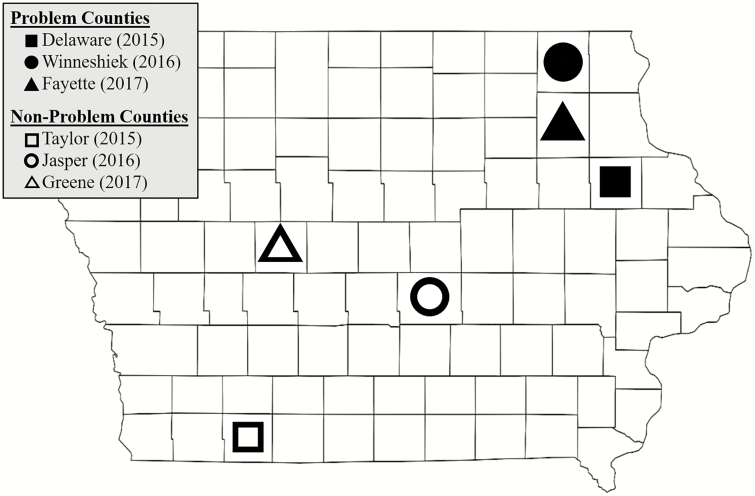
Counties in Iowa, United States, were selected based on the presence or absence of fields where greater-than-expected injury (>1 node of injury; U.S. EPA 2011) to Cry3Bb1 corn had been previously reported. ‘Problem counties’ were chosen from the pool of counties where injury had been reported, primarily located in northeastern Iowa. ‘Non-problem counties’ were chosen from counties in the central and southern areas of Iowa, where resistance issues had not been documented in previously published studies (Gassmann et al. 2011, 2012, 2014, 2016; Dunbar et al. 2016; Jakka et al. 2016). Resistance to Cry3Bb1 had also been confirmed in problem counties (Gassmann et al. 2011, 2014; Dunbar et al. 2016). One problem county and one non-problem county were sampled in 2015, 2016, and 2017 (Fig. 1). The number of fields sampled in problem counties were n = 6 (2015; Delaware County), n = 8 (2016; Winneshiek County), and n = 6 (2017; Fayette County). The number of fields sampled in non-problem counties were n = 4 (2015; Taylor County), n = 6 (2016; Jasper County), and n = 7 (2017; Greene County). Fields studied in both problem and non-problem counties were identified by regional agronomists and local cooperators. None of the fields of this study were sampled in Gassmann et al. (2011, 2012, 2014, 2016), Dunbar et al. (2016), or Jakka et al. (2016).
Fig. 1.
Map of Iowa, United States showing location of counties used in the study. Open shapes represent non-problem counties, while closed shapes represent problem counties. Fields in each county were assessed for abundance of western corn rootworm, root injury to corn, and resistance to Cry3Bb1 corn.
Adult Rootworm Abundance and Root Injury
Abundance of western corn rootworm in all fields was measured by placing 10 unbaited yellow sticky traps (Pherocon, Trécé Inc., Adair, OK) in each field. Traps were placed on plants at ear-level, in two parallel transects of five traps each (Dunbar et al. 2016). Individual traps were 30 m apart, with approximately 15 m between transects. All traps were placed a minimum of 30 m from any field border. Three sampling periods were conducted for each field, which ranged from 6 to 14 d (mean ± SD = 7.84 ± 1.85). Sampling periods began 21 July in 2015, 25 July in 2016, and 24 July in 2017.
Root injury for all fields was assessed by digging roots of corn plants and rating them on a 0–3 node injury scale (Oleson et al. 2005). Ten roots were taken from rows adjacent to where the sticky traps were placed and were washed and rated within 48 h. A sample of leaf tissue was taken from each plant and used to determine the presence and identity of Bt proteins targeting western corn rootworm using an ELISA-based test kit (Envirologix Inc., Portland, ME).
Single-Plant Bioassays
Live adult rootworm were collected from fields using a manual aspirator (BioQuip Products Inc., Part #1135A, Rancho Dominguez, CA). For all years, the mean number of adults collected per field was 70 ± 136.7 (mean ± SD), ranging from 0 to 607. At least one adult rootworm was collected from six non-problem county fields and 10 problem county fields. After transporting to the laboratory, all rootworm adults from an individual field were placed in an 18 × 18 × 18 cm plastic insect cage (MegaView Science Co. Ltd., Taichung, Taiwan) in an environmental chamber (I41-LL, Percival Scientific, Perry, IA) at 16:8 (L:D) h cycle, 25°C, 65% RH. Each population was supplied with a corn leaf, Petri dish with a complete adult diet (Frontier Agricultural Sciences, Newark, DE), and a water source of 1.5% agar solid (Thermo Fisher Scientific Inc., Waltham, MA), all of which were changed three times weekly. A Petri dish of oviposition substrate (soil sieved to particle size <180 µm, moistened, with grooves for rootworm oviposition) was also provided for collection of eggs. These were collected and replaced once per week until all adults were dead. For all fields, mean number of eggs produced was 1,818.9 ± 4,190.6 (mean ± SD), and ranged from 0 to 22,000.
Upon collection from the cages, oviposition dish substrate was covered with soil and the Petri dish lid, and held in the environmental chamber for 2 wk. After that time, eggs were washed from the substrate and placed in soil, and then put into cold storage (4°C) for at least 5 mo to break diapause. After that time, eggs were removed from cold storage and placed in a dark environmental chamber at 25°C until hatch (approximately 2 wk). Upon hatching, larvae were used in 17 d single-plant bioassays following Gassmann et al. (2014).
Bioassays consisted of two corn types: Cry3Bb1 corn (DKC 43–48) and its non-Bt genetic isoline (DKC 43–46). Plants were grown to the V4-V5 growth stage (Abendroth et al. 2011) in 0.95-liter plastic cups (Placon, Madison, WI); there were 12 replicates of each corn type for each population tested (n = 7 controls, n = 5 non-problem, n = 5 problem). Twelve neonate larvae (>24 h old) were added to each plant by placing them gently with a paintbrush on exposed root tissue. Plants were then cut to approximately 20 cm in order to fit in environmental chambers, with leaves trimmed to approximately 10 cm. An adhesive was applied to the inside rim of the cups to prevent larvae from escaping (Tanglefoot Insect Barrier, The Ortho Group, Marysville, OH). The plants were placed in environmental chambers (24°C, 16:8 (L:D) h, 65% RH). Cups were examined three times weekly and 50 ml deionized water was added if the soil surface was dry.
Larvae were allowed to feed undisturbed for 17 d, after which time the aboveground plant tissue was removed and the contents of the cups were placed on Berlese funnels for 4 d. Larvae were collected in vials filled with 85% ethanol and counted using a dissecting microscope (MZ6, Leica, Microsystems, Wetzlar, Germany). Head capsule width was measured using a microscope camera and imaging software (Moticam 2500, Motic Images Plus 3.0; Motic North America, Richmond, British Columbia, Canada), and this measurement was used to determine larval instar following Hammack et al. (2003).
Bioassays began (i.e., larvae were added to plants) 19 April 2016 for field populations collected in 2015, and the last vials were collected from Berlese funnels on 9 May 2016. For 2016 populations, bioassays began 8 May 2017, and ended 11 June. For 2017 populations, bioassays began 23 April 2018, and ended 10 June. Bioassays were conducted weekly, with either one or two populations assayed simultaneously with a susceptible, diapausing control population. Identical procedures were used for controls as field populations. In total, seven control replicates were assayed, each with 12 non-Bt and 12 Bt plants, with each plant receiving 12 larvae. The control population used in 2016 was a Bt-susceptible, diapausing strain obtained from the United States Department of Agriculture, Agricultural Research Service (USDA-ARS), North Central Agricultural Research Laboratory in Brookings, South Dakota. This population was originally collected in Butler County, NE in 1999. In 2017 and 2018, all controls were multiple replicates of a single Bt-susceptible, diapausing population also obtained from USDA-ARS, which had been collected in Moody County, SD, in 1986. Both strains of rootworm had been brought into laboratory culture prior to 2003, the year Bt corn was commercialized for rootworm management, and were never exposed to Bt corn in the laboratory.
Bioassay data for a rootworm population from a specific field were excluded from analysis if the population did not produce enough larvae to run the assay on at least five plants of both corn types. Populations from 10 fields produced sufficient replication in bioassays, while adults were collected from six fields, which did not produce enough larvae. The number of adults collected from the field populations used in bioassays ranged from 33 to 607, with a mean of 248.8 ± 157.7 (mean ± SD). Eggs produced from these populations ranged from 400 to 22,000, with a mean of 6,370 ± 5,977 (mean ± SD). Adult numbers for fields that did not produce sufficient larvae ranged from 2 to 68 (17.7 ± 23.2 [mean ± SD]), with corresponding egg numbers of 0 – 3000 (600 ± 1,077 [mean ± SD]).
Field Management History
Field management history was obtained from farmers and cooperators, which included Bt traits used (Cry3Bb1, mCry3A, eCry3.1Ab, Cry34/35Ab1, or a pyramid of multiple toxins) and soil insecticide use for a total of 6 yr (the year of sampling and five previous years). In some cases, data on field history could not be obtained. The mean number of years for which Bt use could be obtained was 4.6 ± 1.7 (mean ± SD), and for soil insecticide use, the mean number of years was 5.1 ± 2.1 (mean ± SD) (Supp Tables S1–S3 [online only]).
The total number of years that corn had been grown consecutively in each field was calculated using CropScape Data Layer (National Agricultural Statistics Service, United States Department of Agriculture; available at: https://nassgeodata.gmu.edu/CropScape) from 2003, the year Bt corn was first introduced for rootworm management. Using the Cropscape Data Layer information and the field management histories, the following metrics were calculated for each field: 1) the total number of years of continuous corn, 2) the proportion of years the field was planted to corn in the last 6 yr, 3) the proportion of years soil insecticide had been used on corn out of the previous 6 yr, 4) the proportion of years non-Bt corn was planted out of the previous 6 yr, 5) the proportion of years single-trait Cry3 corn was planted (Cry3Bb1 or mCry3A) out of the previous 6 yr, 6) the proportion of years single-trait Cry34/35Ab1 corn was planted out of the previous 6 yr, 7) the proportion of years pyramided Bt corn was planted in the last 6 yr (either Cry34/35Ab1 + Cry3Bb1 or Cry34/35Ab1 + mCry3A), 8) the proportion of years soil insecticide was used on Bt corn in the last 6 yr, and 9) proportion of years soil insecticide was used on non-Bt corn in the last 6 yr. No fields in the experiment had planted any corn hybrids expressing eCry3.1Ab as part of the field management history.
Data Analysis
All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC). For rootworm abundance, values were calculated as the total number of adults caught/trap/sampling day. The values used in analyses are those of the single sampling period for a field when abundance was highest, and thus reflect peak measured abundance of western corn rootworm for the year within each field. To test the hypothesis that root injury and rootworm abundance differed between problem counties and non-problem counties, two-way analysis of variance was used (PROC GLM), with either root injury or abundance as the response, and field type (problem county or non-problem county), year of sampling, and field type × year as explanatory variables. Root injury and abundance were transformed using a square root transformation to improve normality of the residuals. Individual plants that tested negative for Bt traits in a field planted to Bt corn (refuge plants) were removed from the analysis of variance (mean number of refuge plants per field = 0.43 ± 0.79 [mean ± SD]). However, a difference in mean injury to Bt versus non-Bt (refuge) plants within each field was examined using a paired t-test (PROC TTEST).
Rootworm survival in plant-based bioassays was analyzed separately by year using analysis of variance (PROC GLM) with proportion survival as the response, and population, corn type (Cry3Bb1 corn vs. non-Bt corn), and their interaction as explanatory variables. Proportion survival was transformed by the arcsine of the square root to improve normality of the residuals. Linear contrasts (CONTRAST statement) were used to compare survival in each field population to all susceptible control replicates from the same year, and this comparison was made for Cry3Bb1 corn and non-Bt corn. Significantly higher survival on Cry3Bb1 corn by a field population compared to the susceptible control (P = 0.05) would indicate resistance to this Bt toxin. A difference in survival on non-Bt corn between a field population and the control would suggest variation in assay survival not caused by Cry3Bb1 corn.
Resistance was further characterized by examining survival on Cry3Bb1 corn and non-Bt corn within each field population and control replicate. A one-tailed t-test (PROC TTEST) was used to test for a lower proportion of survival on Cry3Bb1 corn compared to non-Bt corn. A one-tailed test was used because survival would be expected to be lower on Cry3Bb1 corn compared to non-Bt corn, but not higher. The null hypothesis was that survival did not differ between the two corn types, with the alternative hypothesis that survival was lower on Cry3Bb1 corn than on non-Bt corn. A rejection of the null hypothesis would indicate incomplete resistance to the Cry3Bb1 toxin. The proportion of larvae surviving to the third, and final, instar on each plant (i.e., the number of larvae collected at the end of the assay that reached the third instar stage divided by the total number of larvae collected from the plant) was also examined. This metric was likewise analyzed using a one-tailed t-test (PROC TTEST), as a lower proportion of third instars could be expected on Cry3Bb1 corn compared to non-Bt corn, but not a higher proportion. The null hypothesis was that the proportion of third instars would be equal between the two corn types within a field population, indicating a similar rate of development on Cry3Bb1 corn and non-Bt corn. The alternative hypothesis was that the proportion of third instars was lower on Cry3Bb1 corn compared to non-Bt corn, suggesting slower development on Cry3Bb1 corn. A rejection of the null would indicate a lower level of adaptation to Cry3Bb1. Proportion of third instars was not analyzed for control populations because survival on Cry3Bb1 corn was generally low (mean proportion survival on Cry3Bb1 corn = 0.02 ± 0.03 [mean ± SD]). Proportion survival and proportion of third instars were transformed by the arcsine of the square root to improve normality of the residuals. For all t-tests, pooled variances were used when variances were equal in the two groups, and the Satterthwaite method was used when variances were unequal. Equality of variances was assessed using the folded F-statistic and associated P-value (i.e., variances are unequal when P ≤ 0.05), as reported in the output of PROC TTEST.
To account for variation in survival on non-Bt corn among the populations (see Results), a second, complementary, analysis was performed using corrected survival. Corrected survival was based on Abbott (1925) and was calculated for each population as: proportion of larvae surviving on Cry3Bb1 corn in a replicate ÷ mean proportion surviving larvae on non-Bt corn in all replicates of a population. One-way analysis of variance (PROC GLM) was performed for each year, with corrected survival as the response variable and population as the explanatory variable. Linear contrasts (CONTRAST statement) were used to compare corrected survival in each field to the control replicates of the same year. Significantly higher corrected survival in a field population compared to control replicates would be indicative of resistance to Cry3Bb1 corn. An additional test was conducted using analysis of variance (PROC GLM) to test for a difference in corrected survival between problem county fields and non-problem county fields, which used corrected survival as the response and field type as a factor in the model. Corrected survival was transformed using an arcsine of the square root transformation to improve normality of the residuals.
Field management in the year of sampling was examined using χ 2 analysis (PROC FREQ) to compare fields in non-problem and problem counties. To examine differences in field management strategies between problem and non-problem counties, t-tests were conducted for past field management metrics (PROC TTEST). For some metrics, a priori assumptions could be made. Continuous corn growth and failure to rotate from corn are contributing factors to rootworm presence and resistance to Bt (Gassmann et al. 2011). Additionally, in past studies, farmers have responded to rootworm issues by increasing soil insecticide use (Dunbar et al. 2016). Thus, it is reasonable to infer that these metrics will be higher in areas where rootworm have historically been problematic (i.e., the problem counties of our experiment) compared to other areas of the state. Thus, years of continuous corn, proportion corn grown, and proportion soil insecticide use were analyzed with a one-tailed t-test with the null hypothesis of no difference between county types and the alterative hypothesis that the metric was higher in problem counties than non-problem counties. Likewise, proportion non-Bt corn grown was examined with a one-tailed test with the null hypothesis of no difference, and the alternative hypothesis that this metric was higher in non-problem counties. Proportion Cry3 corn, proportion Cry34/35Ab1, and proportion pyramided corn were analyzed with a two-tailed test. Proportion of soil insecticide used on non-Bt corn was not examined with a t-test, as the mean of this metric in non-problem counties was zero. Years of continuous corn was transformed by ln(y) to improve normality of the residuals, and all other metrics were transformed by the arcsine of the square root to improve homogeneity of variances. In cases where homogeneity of variances could not be achieved, the Satterthwaite method was used to calculate degrees of freedom and test statistics. Data points for a field were excluded if the relevant information for that field was unavailable for more than 3 of the 6-yr field history. In cases where the information was known for more than three but fewer than 6 yr, proportions were calculated from the total available data (e.g., if soil insecticide use was known for 4 yr, but was unknown for 2 yr, the proportion would have been calculated out of a total of 4 yr) (Supp Tables S1–S3 [online only]).
Multiple regression was used to examine the effects of field management on rootworm abundance and root injury (PROC REG) (Sokal and Rohlf 1995). All current and historical field management metrics were analyzed using correlation analysis (PROC CORR) to determine the presence of collinearity among the variables (Pearson’s correlation coefficient > 0.8). Collinearity among variables indicates that variation in the response could be potentially explained by multiple variables in the model. Cry3 corn grown in the year of sampling was collinear with the proportion of years Cry3 corn was grown in 6 yr, and was removed from analysis (Supp Table S4 [online only]). The remaining variable represented all Cry3 corn growth in the year of sampling and over the 6-yr management history (referred to as ‘Cry3 corn grown’). Soil insecticide used in the year of sampling was collinear with proportion of years soil insecticide was used in 6 yr and the proportion of years soil insecticide was used on Bt corn in 6 yr (Supp Table S4 [online only]). The proportion of years soil insecticide was used in 6 yr was retained, and the other two variables were removed. The remaining variable represented soil insecticide use in the year of sampling and over the management history when Bt corn was present (referred to as ‘soil insecticide use’).
The field management metrics used as independent variables in multiple regression were: non-Bt corn planted in the year of sampling (0 = no, 1 = yes), Cry34/35Ab1 corn planted in the year of sampling (0 = no, 1 = yes), pyramided corn grown in the year of sampling (either Cry34/35Ab1 + Cry3Bb1 or Cry34/35Ab1 + mCry3A, 0 = no, 1 = yes), the total number of years the field was planted to corn continuously since 2003, the proportion of years the field was planted to corn in the previous 6 yr, soil insecticide use, the proportion of years non-Bt corn was planted in the past 6 yr, Cry3 corn grown, the proportion of years Cry34/35Ab1 corn was planted in the past 6 yr, the proportion of years a pyramid hybrid of corn had been grown in the past 6 yr (either Cry34/35Ab1 + Cry3Bb1 or Cry34/35Ab1 + mCry3A), and proportion of years soil insecticide was used on non-Bt corn in the past 6 yr. Of the 37 fields examined in this study, seven fields were missing information for one or more field metrics, thus a total of 30 fields were used in multiple regression (Supp Tables S1–S3 [online only]). All independent variables were included as potential variables in the regression model with either rootworm abundance or root injury as the response. Stepwise selection was used to select the best model, with the thresholds of P ≤ 0.25 for entry into the model and P ≤ 0.15 to be retained in the model, following Dunbar et al. (2016). Rootworm abundance and root injury were transformed using a square root transformation and years of continuous corn was transformed using ln(y) to improve normality of the residuals.
Results
Adult Rootworm Abundance and Root Injury
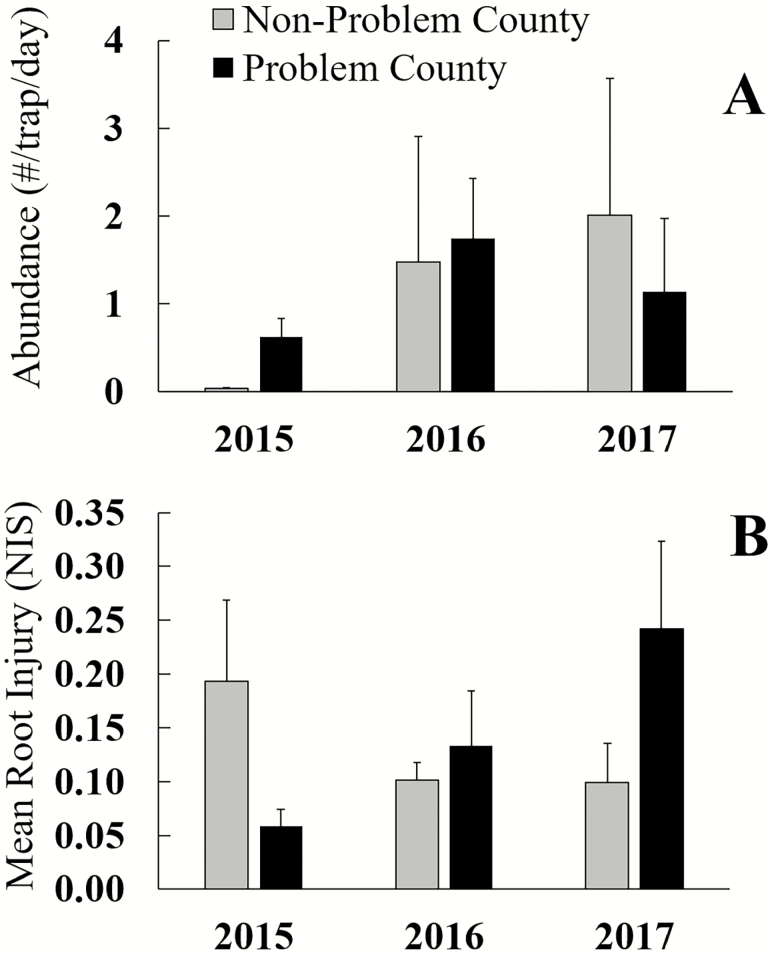
There was no effect of field type (problem county vs. non-problem county), year (2015, 2016, or 2017), or their interaction on rootworm abundance (Fig. 2A; Table 1). Mean abundance in all fields of the study was 1.2 rootworm/trap/d ± 0.4 (mean ± SE). There was likewise no effect of field type, year, or their interaction on root injury (Fig. 2B; Table 1). Mean root injury for all fields was 0.13 ± 0.02 nodes (mean ± SE). In fields where refuge plants were found, injury to non-Bt corn was significantly higher than injury to Bt corn (Table 2; T = 2.59, df = 9, P = 0.03; Bt mean ± SE = 0.14 ± 0.044, non-Bt mean ± SE = 0.68 ± 0.23).
Fig. 2.
(A) Mean abundance of adult western corn rootworm in non-problem and problem counties. (B) Mean root injury scores of fields sampled in non-problem and problem counties. Bar heights represent sample means, and error bars are standard error of the mean.
Table 1.
Analysis of variance to test for effects of field type (problem county vs. non-problem county), year of sampling, and their interaction on observed root injury and abundance of western corn rootworm
| Response | Effect | df | F | P |
|---|---|---|---|---|
| Rootworm abundance | Field typea | 1, 31 | 0.87 | 0.34 |
| Yearb | 2, 31 | 0.72 | 0.50 | |
| Year × Type | 2, 31 | 0.65 | 0.53 | |
| Root injury | Field type | 1, 31 | 0 | 0.96 |
| Year | 2, 31 | 0.34 | 0.71 | |
| Year × Type | 2, 31 | 3.02 | 0.06 |
aField type = non-problem county vs. problem county.
bYear = 2015, 2016, and 2017.
Table 2.
Injury to corn roots for Bt corn and non-Bt corn (refuge plants) in fields where refuge plants were collected as part of the sampling transect
| Year | Fielda | Bt root injuryb ± SEM (N) | Non-Bt root injuryb ± SEM (N) |
|---|---|---|---|
| 2015 | PC-2 | 0.046 ± 0.008 (9) | 0.02 ± N/A (1) |
| 2016 | NPC-7 | 0.15 ± 0.05 (7) | 0.08 ± 0.01 (3) |
| PC-7 | 0.05 ± 0.01 (8) | 1.88 ± 0.44 (2) | |
| PC-9 | 0.01 ± 0.003 (9) | 0.1 ± N/A (1) | |
| PC-10 | 0.45 ± 0.13 (9) | 1.7 ± N/A (1) | |
| 2017 | NPC-12 | 0.03 ± 0.009 (8) | 0.02 ± 0.0 (2) |
| NPC-14 | 0.27 ± 0.10 (9) | 1.00 ± N/A (1) | |
| NPC-16 | 0.18 ± 0.14 (8) | 1.03 ± 0.69 (2) | |
| NPC-17 | 0.03 ± 0.005 (8) | 0.04 ± 0.01 (2) | |
| PC-20 | 0.20 ± 0.11 (9) | 0.90 ± N/A (1) | |
| Mean | 0.14 ± 0.04 (84) | 0.68 ± 0.23 (16) |
aPC = problem county field, NPC = non-problem county field. Bt traits present in fields were: Cry3Bb1 single-trait (NPC-7), Cry34/35Ab1 single-trait (NPC-12), Cry34/35Ab1 + Cry3Bb1 (PC-2, PC-7, NPC-14, NPC-16, NPC-17, PC-20), and Cry34/35Ab1 + mCry3A (PC-9, PC-10).
bMean injury was significantly higher for non-Bt plants compared to Bt plants T = 2.59, df = 9, P = 0.03.
Single-Plant Bioassays
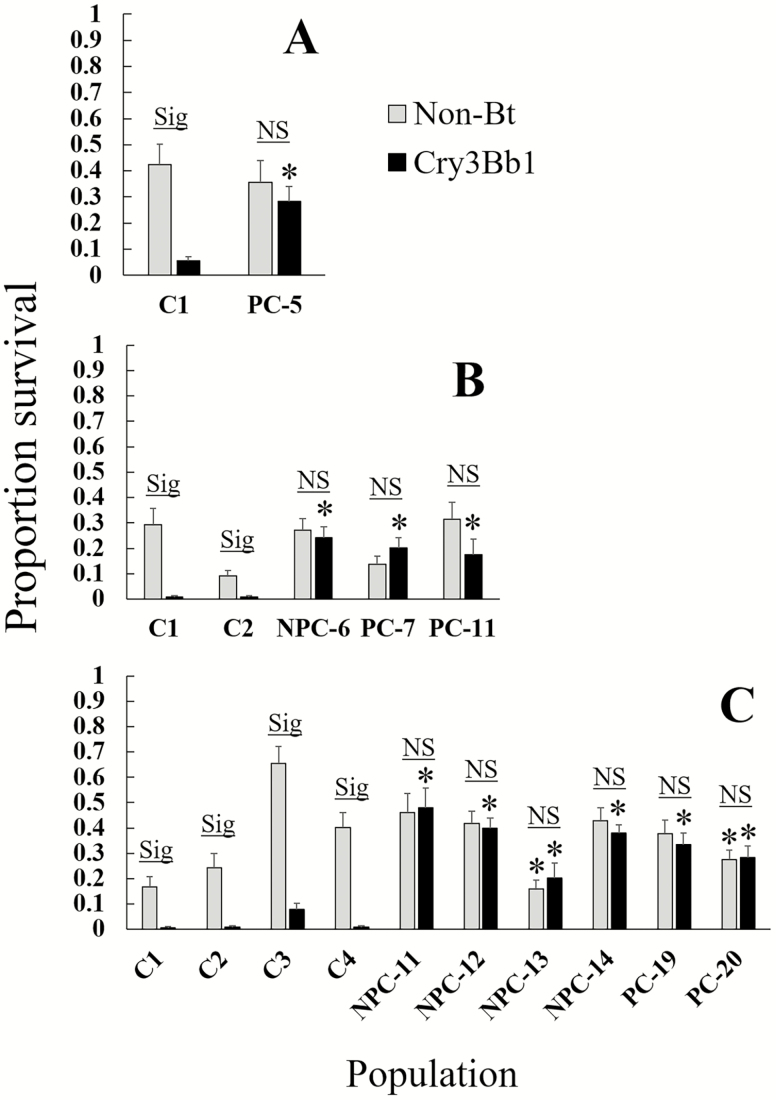
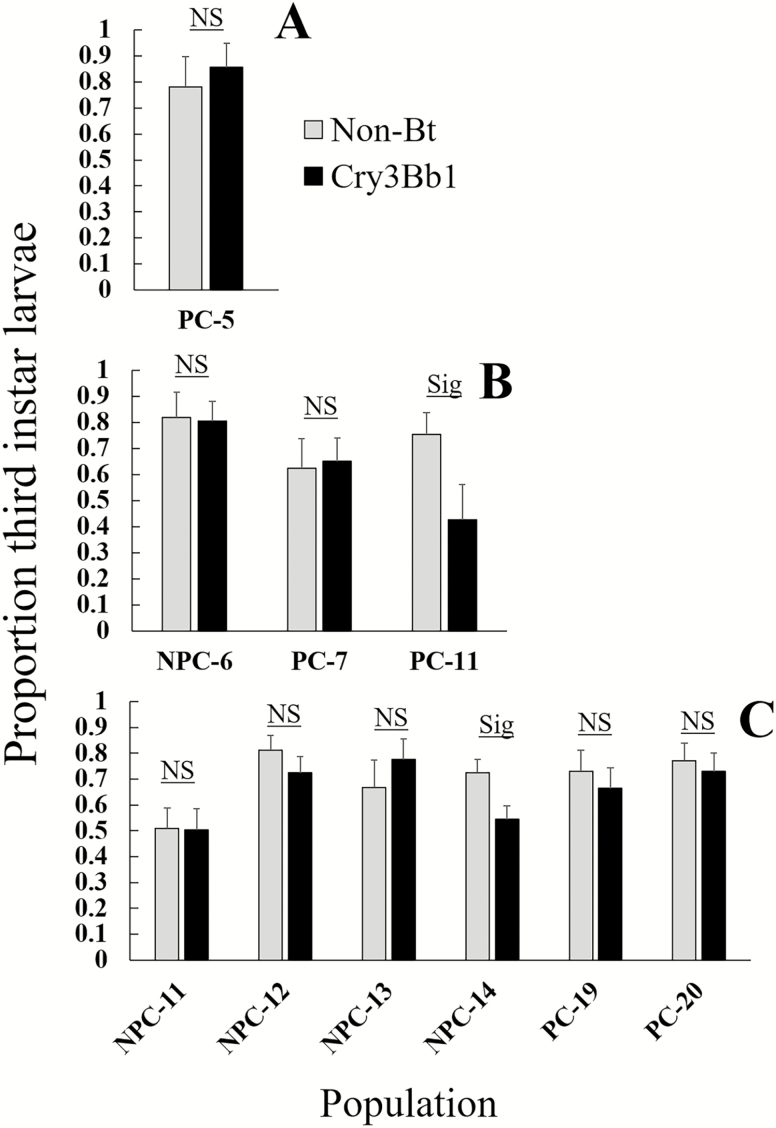
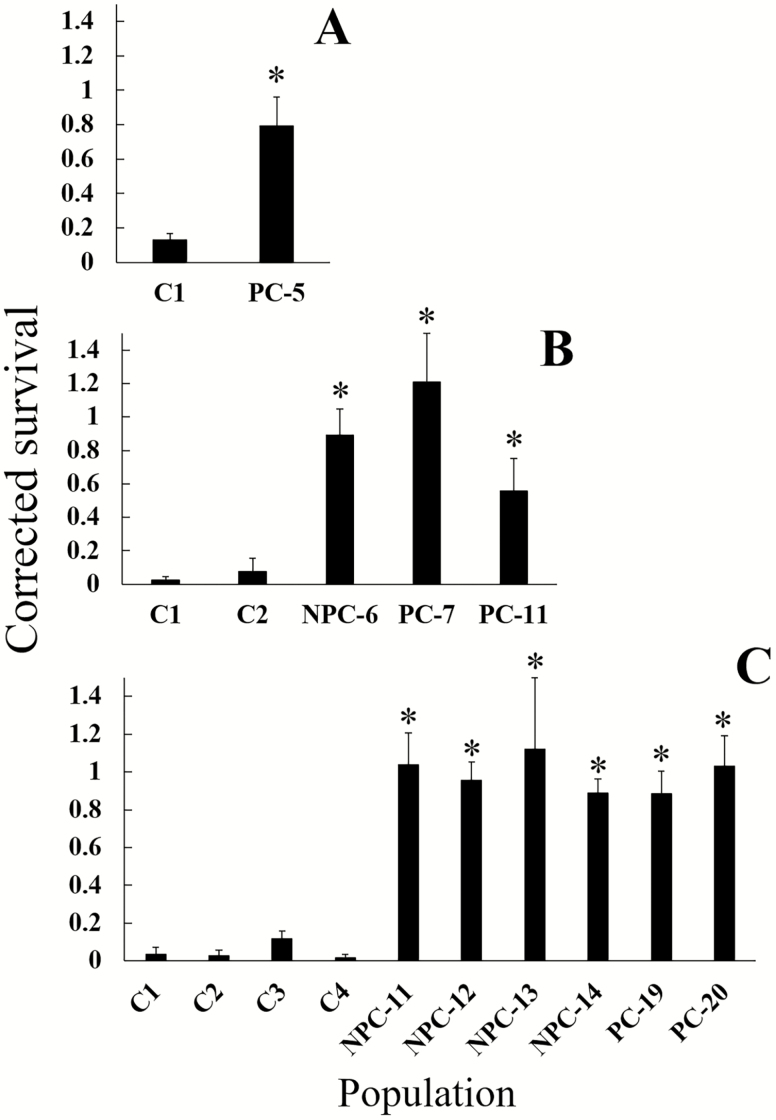
In bioassays to assess resistance to Cry3Bb1, the interaction of population and corn type was significant in all years (Table 3), indicating that survival on Cry3Bb1 corn and non-Bt corn differed among the populations. All field populations from problem and non-problem counties had significantly higher survival on Cry3Bb1 compared to the control replicates of the same year in linear contrasts, indicating the presence of resistance to this Bt trait in both county types (Fig. 3). One non-problem county population from 2017 (NPC-13) had significantly lower survival on non-Bt corn compared to the susceptible control population (Fig. 3). All control populations had significantly lower survival on Cry3Bb1 compared to non-Bt corn, and survival did not differ between Cry3Bb1 corn and non-Bt corn for any field population (Fig. 3). Two field populations had a significantly lower proportion of third instars on Cry3Bb1 corn compared to non-Bt corn, one problem county field from 2016 (PC-11) and one non-problem county field from 2017 (NPC-14) (Fig. 4). Analysis of corrected survival confirmed the presence of resistance to Cry3Bb1 in all fields after accounting for variation in survival on non-Bt corn (Fig. 5). All field populations, regardless of county type, had significantly higher corrected survival compared to the control replicates of the same year in linear contrasts (Table 3; all contrasts P ≤ 0.004). However, corrected survival did not differ between problem and non-problem counties (F1,8 = 0.13, P = 0.72).
Table 3.
Analysis of variance for survival and corrected survival in plant-based bioassays
| Survival | Corrected survival | ||||||
|---|---|---|---|---|---|---|---|
| Year | Effect | df | F | P | df | F | P |
| 2015 | Populationa | 1, 35 | 2.74 | 0.11 | 1, 17 | 29.68 | <0.0001 |
| Corn typed | 1, 35 | 9.54 | 0.004 | - | - | - | |
| Pop. × Corn type | 1, 35 | 6.61 | 0.01 | - | - | - | |
| 2016 | Populationb | 5, 153 | 8.59 | <0.0001 | 4, 74 | 11.95 | <0.0001 |
| Corn type | 1, 153 | 13.74 | 0.0003 | - | - | - | |
| Pop. × Corn type | 5, 153 | 5.21 | 0.0002 | - | - | - | |
| 2017 | Populationc | 9, 271 | 17.90 | <0.0001 | 9, 136 | 25.97 | <0.0001 |
| Corn type | 1, 271 | 60.10 | <0.0001 | - | - | - | |
| Pop. × Corn type | 9, 271 | 10.89 | <0.0001 | - | - | - |
a2015 populations: one control, one problem county field population.
b2016 populations: two control replicates, one non-problem county field populations, two problem county field populations.
c2017 populations: four control replicates, four non-problem county field populations, two problem county field populations.
dCorn type = Cry3Bb1 corn versus non-Bt corn.
Fig. 3.
Proportion survival of susceptible controls and field populations on non-Bt and Cry3Bb1 corn. A = 2015, B = 2016, C = 2017. For field labels, ‘C’ indicates a control replicate, while ‘PC’ and ‘NPC’ prefixes denote problem- and non-problem county fields. Bar heights represent sample means, and error bars are standard error of the mean. Sig. indicates that the population experienced a significant difference in survival between non-Bt and Cry3Bb1 corn, while NS indicates that there was not a difference in survival on the two corn types. Bars with an asterisk were significantly different from controls on the same corn type (e.g., an asterisk above a black bar indicates a difference between survival on Cry3Bb1 corn in the field population compared to the controls).
Fig. 4.
Proportion of third instar larvae of field populations on non-Bt and Cry3Bb1 corn. A = 2015, B = 2016, C = 2017. For field labels, ‘PC’ and ‘NPC’ prefixes denote problem- and non-problem county fields. Bar heights represent sample means, and error bars are standard error of the mean. Sig. indicates that a significantly greater proportion of third instar larvae were recovered on non-Bt corn compared to Cry3Bb1 corn. NS indicates that there was no difference in the proportion of third instar larvae between the two corn types.
Fig. 5.
Corrected survival for all populations assayed on non-Bt and Cry3Bb1 corn. A = 2015; B = 2016; C = 2017. Corrected survival was calculated as proportion of larvae surviving on Cry3Bb1 corn in a replicate ÷ mean proportion surviving larvae on non-Bt corn in all replicates of a population. For field labels, ‘PC’ and ‘NPC’ prefixes denote problem- and non-problem county fields. Bar heights are means for corrected survival for a population, and error bars are standard error of the mean. Asterisks indicate that corrected survival in the population was significantly higher than the controls in the year the assay was conducted.
Field Management History
More non-problem county fields were planted with non-Bt corn in the year of sampling compared to problem county fields (Table 4). Four field management metrics differed between county types (Table 5). Problem county fields had a significantly higher number of years when corn had been grown continuously, which was approximately two additional years on average. Problem counties also had a significantly higher proportion of corn grown in the last 6 yr (i.e., had rotated less frequently), had used more years of soil insecticide use than non-problem counties, and had grown Cry34/35Ab1 corn approximately twice as often as non-problem counties.
Table 4.
Differences in management of fields in non-problem and problem counties in the year the field was sampled
| Field history metrica | Non-problem countyb | Problem countyb | df | χ 2 | P |
|---|---|---|---|---|---|
| Non-Bt corn planted | 0.18 (3) | 0.05 (1) | 1 | 1.52 | 0.22 |
| Cry3 corn planted | 0.29 (5) | 0.00 (0) | 1 | 6.80 | 0.01 |
| Cry34/35Ab1 corn planted | 0.06 (1) | 0.30 (6) | 1 | 3.48 | 0.06 |
| Cry34/35Ab1 + Cry3 corn planted | 0.47 (8) | 0.65 (13) | 1 | 1.20 | 0.27 |
| Soil insecticide | 0.17 (2) | 0.20 (4) | 1 | 0.05 | 0.82 |
aMetrics are present or absent for the year the field was sampled. Absent = 0, Present = 1. For field categories, Non-problem county = 0, Problem county = 1.
bProportion of fields in the field category using the management tactic (N).
Table 5.
Differences in management approaches for 6 yr in non-problem and problem counties
| Field history metrica | Non-problem countyb | Problem countyb | df | T | P |
|---|---|---|---|---|---|
| Years cont. cornc | 5.24 ± 1.35 (17) | 7.20 ± 1.03 (20) | 35 | 2.28 | 0.01 |
| Proportion cornc | 0.74 ± 0.06 (17) | 0.88 ± 0.05 (20) | 35 | 1.83 | 0.03 |
| Proportion S.I. usec,e | 0.04 ± 0.03 (12) | 0.27 ± 0.09 (20) | 25.23 | 2.24 | 0.02 |
| Proportion non-Btc,e | 0.32 ± 0.11 (10) | 0.11 ± 0.03 (20) | 11.53 | 1.67 | 0.06 |
| Proportion Cry3d,e | 0.21 ± 0.11 (10) | 0.05 ± 0.03 (20) | 10.71 | 1.31 | 0.22 |
| Proportion Cry34/35Ab1d,e | 0.05 ± 0.05 (10) | 0.42 ± 0.08 (20) | 27.95 | 3.87 | 0.0006 |
| Proportion Cry34/35Ab1 + Cry3d,e | 0.42 ± 0.12 (10) | 0.42 ± 0.06 (20) | 28 | 0.05 | 0.96 |
| Proportion S.I. on Btd,e | 0.05 ± 0.04 (10) | 0.24 ± 0.09 (20) | 26.94 | 1.82 | 0.08 |
| Proportion S.I. on non-Btf | 0.00 ± N/A (10) | 0.03 ± 0.02 (20) | - | - | - |
aField history metrics are proportions of years the management tactics was used out of the most recent 6 yr, except years of continuous corn, which is the total number of years the field had been planted to corn since 2003. S.I. = soil insecticide.
bMean ± SE (N).
cOne-tailed t-test was conducted.
dTwo-tailed t-test was conducted.
eSatterthwaite method was used due to unequal variances.
fNo test was conducted.
In multiple regression, pyramided corn (Cry34/35Ab1 + Cry3Bb1 or Cry34/35Ab1 + mCry3A) planted in the year of sampling had a positive correlation with rootworm abundance. The proportion of years corn had been grown in the last six years was also positively correlated with rootworm abundance, and this correlation was significant at the P ≤ 0.05 level. The regression model for rootworm abundance explained approximately 23% of the observed variation in abundance (Table 6). For root injury, the number of years of continuous corn growth was positively correlated with injury, and proportion of soil insecticide use over the past 6 yr and proportion Cry3 corn grown in the past 6 yr were both negatively correlated with injury. Only the proportion of soil insecticide use was significant at the P ≤ 0.05 level. The regression model for root injury explained approximately 25% of the observed variation in root injury.
Table 6.
Multiple regression analysis for rootworm abundance and root injury, with field type and management metrics as possible independent variables
| Dependent variable | Parameters | Slope | SE | F | P | Model r2 |
|---|---|---|---|---|---|---|
| Rootworm abundance (square root) | Cry34/35Ab1 + Cry3 (year of sampling)a | 0.56 | 0.31 | 3.25 | 0.08 | 0.23 |
| Proportion years corn (6 yr)b | 1.47 | 0.69 | 4.58 | 0.04 | ||
| Intercept | −0.69 | 0.60 | 1.30 | 0.26 | ||
| Root injury (square root) | Year cont. corn (log)c | 0.06 | 0.04 | 2.60 | 0.12 | 0.25 |
| Soil insecticide used | −0.20 | 0.09 | 4.64 | 0.04 | ||
| Cry3 corn growne | −0.20 | 0.12 | 2.71 | 0.11 | ||
| Intercept | 0.32 | 0.06 | 32.96 | <0.0001 |
aUse of Cry34/35Ab1 + Cry3Bb1 or Cry34/35Ab1 + mCry3A corn in the year the field was sampled (0 = no, 1 = yes).
bProportion of years corn had been grown out of the previous 6 yr.
cNumber of years fields have been planted to corn continuously since 2003.
dVariable represents variation in response due to soil insecticide use in the year of sampling, proportion of years soil insecticide was used in 6 yr, and proportion of years soil insecticide was used on Bt corn due to collinearity among variables.
eVariable represents variation in response due to Cry3 corn grown in the year of sampling and proportion of years Cry3 corn had been grown in the last 6 yr due to collinearity among variables.
Discussion
Our study revealed rootworm abundance and injury to corn did not differ between non-problem and problem counties (Table 1; Fig. 2). All rootworm populations assayed in this experiment were resistant to Cry3Bb1, and resistance did not differ between county types (Figs. 3–5). Thus, our first hypothesis, that problem counties would have higher abundance, root injury, and resistance to Cry3Bb1 compared to non-problem counties was not supported. Other studies have found similarly that resistance to Cry3Bb1 is present among fields with various management histories (Shrestha et al. 2018b). We also found that problem county fields had grown more continuous corn, rotated less frequently, used more soil insecticide, and planted more Cry34/35Ab1 corn than fields in non-problem counties (Table 5). These differences in management indicate that our second hypothesis was supported; growers in problem counties managed rootworm differently compared to non-problem counties. Such differences are expected, given that problem counties have experienced severe rootworm issues in the past, and farmers have likely responded with additional management for rootworm (Gassmann et al. 2011, 2014; Dunbar et al. 2016).
Resistance to Cry3Bb1 was present in two non-problem counties (Jasper and Greene), located in central Iowa (Fig. 1). We cannot confirm that resistance to Cry3Bb1 was present in Taylor County, as we were unable to assay any populations from that county. However, resistance was similar in northeastern problem counties and centrally-located non-problem counties in this study, despite some differences in management approaches (Figs. 3–5; Tables 4 and 5). While several field management metrics differed between the county types, the proportion of years in which Cry3 corn had been grown, either singly or as a pyramid with Cry34/35Ab1, did not (Table 5). It is also noteworthy that both county types were characterized by multiple years of consecutive corn growth (Table 5). Although problem counties had grown corn for significantly longer, non-problem county fields still averaged over five consecutive years of corn per field. This indicates that selection pressure for resistance to Cry3 proteins was similar in both county types. Continuous planting of Cry3Bb1 corn has been directly correlated with resistance to that trait (Gassmann et al. 2011). For our study Cry3 corn was defined as either Cry3Bb1 or mCry3A corn (no fields had grown eCry3.1Ab corn). These two proteins are structurally similar, and cross-resistance occurs where resistance to one trait confers a degree of resistance to the other (Gassmann et al. 2014, Zukoff et al. 2016). Additionally, Reinders et al. (2018), found that selection for Bt resistance within a field was associated with the level of Bt resistance within that field. Thus, growth of Cry3 corn was the key component to selecting for resistance in the populations of this study, despite differences in other management tactics.
Rootworm abundance and root injury to corn did not differ between non-problem counties and problem counties (Fig. 2; Table 1). One explanation for this result is that the baseline rootworm presence was higher in the problem counties of our study, but differences in management resulted in similar observations in both county types. Problem counties had experienced rootworm issues in the past (e.g., Gassmann et al. 2011, 2014; Dunbar et al. 2016). We observed similar levels of abundance and root injury because the active rootworm management tactics employed in these counties kept populations lower than they would have been without intervention. Problem counties were more likely to contain fields that received soil insecticide or were planted with Cry34/35Ab1 corn in the last 6 yr (Table 5). Soil insecticide can reduce adult emergence, which can lead to lower population numbers overall (Shrestha et al. 2018a). While field-evolved resistance to Cry34/35Ab1 does exist, resistant populations were relatively rare in Iowa at the time of this study, so this Bt toxin was likely an effective method of managing rootworm (Gassmann et al. 2016, 2020). Thus, differences in management can explain why rootworm abundance and root injury did not differ between county types, even though a higher rootworm prevalence would be expected in counties where fields had previously experienced injury to corn.
In light of the results of our study, it is interesting that greater-than-expected injury was reported in problem counties but not non-problem counties, when we observed similar rootworm populations in the two county types. The first cases of field failure and resistance to Cry3Bb1 occurred in northeastern Iowa in 2009 and 2010, and were more broadly geographically distributed thereafter (Gassmann et al. 2011, 2012, 2014; Dunbar et al. 2016; Jakka et al. 2016). When Cry3Bb1 was introduced in 2003, intense selection pressure likely led to initial cases of resistance in northeastern Iowa (Gassmann 2016). Selection pressure in non-problem counties was probably less than in problem counties during this time, as evidenced by the fact that resistance evolved in the northeast first. Corn producing a combination of Cry34/35Ab1 and Cry3Bb1 was marketed in 2009 (U.S. EPA 2019), giving farmers had an effective second Bt option for rootworm management. By the time resistance to Cry3Bb1 evolved in non-problem counties, this second Bt option was available for use, preventing field failures and resistance issues that would be reported in the more corn-intensive northeast area of the state. This would explain why Cry3Bb1 resistance was found in counties that had not previously reported rootworm injury to Cry3Bb1 corn.
The field management tactics of farmers in this study partially explained the observed rootworm abundance and root injury (Table 6). The proportion of the past 6 yr that the field had been planted to corn was positively associated with rootworm abundance, which reflects this pest’s requirement of multiple years of corn for reproduction and population growth. The proportion of the past 6 yr soil insecticide had been used in the field was negatively correlated with root injury. This is to be expected, as soil insecticide has been shown to reduce root feeding by western corn rootworm (Shrestha et al. 2018a). Both of these results are in keeping with current knowledge of rootworm biology.
We found lower root injury on Bt plants compared to non-Bt plants in fields where refuge plants were sampled (Table 2). As each node of injury to corn roots can result in a 15 to 17% loss in yield, protection of roots is important for ensuring profitability (Dun et al. 2010, Tinsley et al. 2013). The mean difference in injury between Bt and non-Bt corn in this analysis was 0.54 nodes, which would result in an estimated 8–10% loss in yield. Wechsler and Smith (2018) found that the average price premium for an 80,000-seed bag of single-trait rootworm-active Bt corn was approximately $33.35. With an average seeding rate of 30,000 seeds/acre, the price for managing rootworm using Bt corn would be approximately $12.50/acre. Between 2015 and 2017, when our study was conducted, the average yield in Iowa was 199 bushels/acre, with an average selling price of $3.38/bushel (U.S. Department of Agriculture 2020). If a farmer were to lose 8% of the yield based on the statewide average (15.92 bushels), the monetary loss would have been approximately $53.81/acre. Thus, the difference between the cost of management ($12.50/acre) and minimum projected economic loss in the absence of Bt corn ($53.81/acre) is $41.31/acre. This means that the farmers in our study derived an economic benefit from planting Bt corn. Notably, all fields used in the comparison between Bt and refuge plants included Cry34/35Ab1, with one exception (Table 2). Across the entire study, 53 and 95% of fields in non-problem and problem counties, respectively, used a corn hybrid expressing Cry34/35Ab1, either singly or as a pyramid with a Cry3 protein (Table 4). While it is evidently providing an economic benefit to farmers, such widespread use of this trait is selecting for resistance to Cry34/35Ab1, cases of which have already been documented (Gassmann et al. 2016, 2020).
Our study demonstrates that resistance to Cry3Bb1 corn by western corn rootworm is now nearly ubiquitous in northeastern and central Iowa. Other studies have also shown that resistance to this toxin is widespread and present in fields of differing management histories (Shrestha et al. 2018b). Farmers in the northeast and central Iowa managed rootworm differently. In addition to growing more continuous corn, a practice that is expected to increase rootworm abundance and root injury, farmers in northeastern Iowa also used more soil insecticide and Cry34/35Ab1 corn, practices that are expected to decrease rootworm abundance and root injury (Table 5). These management tactics may have counter-balanced one another such that these metrics did not differ from non-problem counties. Additionally, Cry34/35Ab1 was effective in protecting roots in general, as has been shown elsewhere (Table 2) (Johnson et al. 2017). However, overreliance on this trait is beginning to result in resistance issues (Gassmann et al. 2016, 2020). The next generation of transgenic technology, which uses RNA interference, is currently in the process of commercialization (Head 2017, U.S. EPA 2019). However, because this new technology is pyramided with Cry34/35Ab1, selection pressure for resistance to Cry34/35Ab1 will persist. Pyramids can act to delay resistance but do so most effectively when resistance alleles are rare for both traits in the pyramid (Roush 1998). Thus, if resistance to Cry34/35Ab1 is present, the efficacy of the pyramid will diminish quickly. More traditional integrated pest management methods, such as crop rotation and soil insecticide use on non-Bt corn, will be needed to limit the intensity of selection pressure for resistance to this trait.
Supplementary Material
Acknowledgments
We thank Erin Hodgson, John Miranowski, Richard Hellmich, and Steven Bradbury for input on the study design and comments on the manuscript. We thank Taylor Best, Christine Cate, Wesley Graham, and Ben Brenizer for help in collection of field and bioassay data. This study was conducted with funding from Bayer Crop Science.
Data Availability Statement
Data from this study are available from the Iowa State University DataShare Digital Repository: https://doi.org/10.25380/iastate.12146172.v1.
References Cited
- Abbott W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267. [Google Scholar]
- Abendroth L. J., Elmore R. W., Boyer M. J., and Marlay S. K.. 2011. Corn growth and development (PMR 1009). Iowa State University, Ames, IA. [Google Scholar]
- Carrière Y., and Tabashnik B. E.. 2001. Reversing insect adaptation to transgenic insecticidal plants. Proc. Biol. Sci. 268: 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière Y., Crowder D. W., and Tabashnik B. E.. 2010. Evolutionary ecology of insect adaptation to Bt crops. Evol. Appl. 3: 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H. C. 1973. Bionomics of the northern and western corn rootworms. Annu. Rev. Entomol. 18: 47–72. [Google Scholar]
- Dun Z., Mitchell P. D., and Agosti M.. 2010. Estimating Diabrotica virgifera virgifera damage functions with field trial data: applying an unbalanced nested error component model. J. Appl. Entomol. 134: 409–419. [Google Scholar]
- Dunbar M. W., O’Neal M. E., and Gassmann A. J.. 2016. Effects of field history on corn root injury and adult abundance of northern and western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 109: 2096–2104. [DOI] [PubMed] [Google Scholar]
- Gassmann A. J. 2016. Resistance to Bt maize by western corn rootworm: insights from the laboratory and the field. Curr. Opin. Insect Sci. 15: 111–115. [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Carrière Y., and Tabashnik B. E.. 2009. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 54: 147–163. [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Petzold-Maxwell J. L., Keweshan R. S., and Dunbar M. W.. 2011. Field-evolved resistance to Bt maize by western corn rootworm. PLoS One 6: e22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann A. J., Petzold-Maxwell J. L., Keweshan R. S., and Dunbar M. W.. 2012. Western corn rootworm and Bt maize: challenges of pest resistance in the field. GM Crops Food. 3: 235–244. [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Petzold-Maxwell J. L., Clifton E. H., Dunbar M. W., Hoffmann A. M., Ingber D. A., and Keweshan R. S.. 2014. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proc. Natl. Acad. Sci. U. S. A. 111: 5141–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann A. J., Shrestha R. B., Jakka S. R., Dunbar M. W., Clifton E. H., Paolino A. R., Ingber D. A., French B. W., Masloski K. E., Dounda J. W., . et al. 2016. Evidence of resistance to Cry34/35Ab1 corn by western corn rootworm (Coleoptera: Chrysomelidae): root injury in the field and larval survival in plant-based bioassays. J. Econ. Entomol. 109: 1872–1880. [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Shrestha R. B., Kropf A. L., St Clair C. R., and Brenizer B. D.. 2020. Field-evolved resistance by western corn rootworm to Cry34/35Ab1 and other Bacillus thuringiensis traits in transgenic maize. Pest Manag. Sci. 76: 268–276. [DOI] [PubMed] [Google Scholar]
- Godfrey L. D., Meinke L. J., and Wright R. J.. 1993. Vegetative and reproductive biomass accumulation in field corn: response to root injury by western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 86: 1557–1573. [Google Scholar]
- Gould F. 1998. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu. Rev. Entomol. 43: 701–726. [DOI] [PubMed] [Google Scholar]
- Hammack L., Ellsbury M. M., Roehrdanz R. L., and Pikul J. L. Jr. 2003. Larval sampling and instar determination in field populations of northern and western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 96: 1153–1159. [DOI] [PubMed] [Google Scholar]
- Head G. P., Carroll M. W., Evans S. P., Rule D. M., Willse A. R., Clark T. L., Storer N. P., Flannagan R. D., Samuel L. W., and Meinke L. J.. 2017. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: efficacy and resistance management. Pest Manag. Sci. 73: 1883–1899. [DOI] [PubMed] [Google Scholar]
- Hoffmann A. M., French B. W., Hellmich R. L., Lauter N., and Gassmann A. J.. 2015. Fitness costs of resistance to Cry3Bb1 maize by western corn rootworm. J. Appl. Entomol. 139: 403–415. [Google Scholar]
- Ingber D. A., and Gassmann A. J.. 2015. Inheritance and fitness costs of resistance to Cry3Bb1 corn by western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 108: 2421–2432. [DOI] [PubMed] [Google Scholar]
- Jakka S. R., Shrestha R. B., and Gassmann A. J.. 2016. Broad-spectrum resistance to Bacillus thuringiensis toxins by western corn rootworm (Diabrotica virgifera virgifera). Sci. Rep. 6: 27860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Campbell L. A., Lepping M. D., and Rule D. M.. 2017. Field trial performance of herculex XTRA (Cry34Ab1/Cry35Ab1) and SmartStax (Cry34Ab1/Cry35Ab1 + Cry3Bb1) hybrids and soil insecticides against western and northern corn rootworms (Coleoptera: Chrysomelidae). J. Econ. Entomol. 110: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler A. L., Olness A. E., Sutter G. R., Dybing C. D., and Devine O. J.. 1985. Root damage by western corn rootworm and nutrient content in maize. Agron. J. 77: 769–774. [Google Scholar]
- Martinez J. C., and Caprio M. A.. 2016. IPM Use with the deployment of a non-high dose Bt pyramid and mitigation of resistance for western corn rootworm (Diabrotica virgifera virgifera). Environ. Entomol. 45: 747–761. [DOI] [PubMed] [Google Scholar]
- Meinke L. J., Sappington T. W., Onstad D. W., Guillemaud T., Miller N. J., Komáromi J., Levay N., Furlan L., Kiss J., and Toth F.. 2009. Western corn rootworm (Diabrotica virgifera virgifera LeConte) population dynamics. Agric. For. Entomol. 11: 29–46. [Google Scholar]
- Oleson J. D., Park Y. L., Nowatzki T. M., and Tollefson J. J.. 2005. Node-injury scale to evaluate root injury by corn rootworms (Coleoptera: Chrysomelidae). J. Econ. Entomol. 98: 1–8. [DOI] [PubMed] [Google Scholar]
- O’Rourke M. E., Rienzo-Stack K., and Power A. G.. 2011. A multi-scale, landscape approach to predicting insect populations in agroecosystems. Ecol. Appl. 21: 1782–1791. [DOI] [PubMed] [Google Scholar]
- Paolino A. R., and Gassmann A. J.. 2017. Assessment of inheritance and fitness costs associated with field-evolved resistance to Cry3Bb1 maize by western corn rootworm. Toxins 9: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders, J. D., B. D. Hitt, W. W. Stroup, B. Wade French, and L. J. Meinke. 2018. Spatial variation in western corn rootworm (Coleoptera: Crysomelidae) susceptibility to Cry3 toxins in Nebraska. PLoS One 13: e0208266. doi: 10.1371/journal.pone.0208266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedell W. E. 1990. Rootworm and mechanical damage effects on root morphology and water relations in maize. Crop Sci. 30: 628–631. [Google Scholar]
- Roush R. T. 1998. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philos. Trans. R. Soc.B: Biol. Sci. 353: 1777–1786. [Google Scholar]
- Scandizzo P. L., and Savastano S.. 2010. The adoption and diffusion of GM crops in United States: a real option approach. AgBioForum. 13: 142–157. [Google Scholar]
- Shrestha R. B., Jakka S. R. K., and Gassmann A. J.. 2018a. Response of Cry3Bb1-resistant western corn rootworm (Coleoptera: Chrysomelidae) to Bt maize and soil insecticide. J. Appl. Entomol. 142: 937–946. [Google Scholar]
- Shrestha R. B., Dunbar M. W., French B. W., and Gassmann A. J.. 2018b. Effects of field history on resistance to Bt maize by western corn rootworm, Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae). PLoS One 13: e0200156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R. R., and Rohlf F. J.. 1995. Biometry. W.H. Freeman and Company, New York, NY. [Google Scholar]
- Spencer J. L., Hibbard B. E., Moeser J., and Onstad D. W.. 2009. Behaviour and ecology of the western corn rootworm (Diabrotica virgifera virgifera LeConte). Agric. For. Entomol. 11: 9–27. [Google Scholar]
- Spike B. P., and Tollefson J. J.. 1991. Yield response of corn subjected to western corn rootworm (Coleoptera: Chrysomelidae) infestation and lodging. J. Econ. Entomol. 84: 1585–1590. [Google Scholar]
- St Clair C., Head G., and Gassmann A.. 2020. Population comparison of western corn rootworm (Coleoptera: Chrysomelidae) in regions with and without a history of injury to Cry3 corn. Iowa State University DataShare Repository. doi: 10.25380/iastate.12146172.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik B. E., Gassmann A. J., Crowder D. W., and Carriére Y.. 2008. Insect resistance to Bt crops: evidence versus theory. Nat. Biotechnol. 26: 199–202. [DOI] [PubMed] [Google Scholar]
- Tinsley N. A., Estes R. E., and Gray M. E.. 2013. Validation of a nested error component model to estimate damage caused by corn rootworm larvae. J. Appl. Entomol. 137: 161–169. [Google Scholar]
- U.S. Department of Agriculture 2020. National Agricultural Statistics Service. Available from https://quickstats.nass.usda.gov
- U.S. Environmental Protection Agency. 2003. Biopesticides registration action document: event MON838 Bacillus thuringiensis Cry3Bb1 corn. Available from https://www3.epa.gov/pesticides/chem_search/reg_actions/pip/cry3bb1-brad.pdf [PubMed]
- U.S. Environmental Protection Agency. 2005. Biopesticides registration action document: Bacillus thuringiensis Cry34Ab1 and Cry35Ab1 Proteins and the Genetic Material Necessary for Their Production (PHP17662 T-DNA) in Event DAS-59122–7 Corn (OECD Unique Identifier: DAS-59122–7). Available from https://www3.epa.gov/pesticides/chem_search/reg_ac [PubMed]
- U.S. Environmental Protection Agency. 2011. 2009 resistance monitoring review for Cry3Bb1. Available from https://www.regulations.gov/document?D=EPA-HQ-OPP-2011-0922-0003 [PubMed]
- U.S. Environmental Protection Agency 2019. Current and previously registered section 3 plant-incorporated protectant (PIP) registrations. Available from https://www.epa.gov/ingredients-used-pesticide-products/current-and-previously-registered-section-3-plant-incorporated [PubMed]
- Wechsler S., and Smith D.. 2018. Has resistance taken root in U.S. corn fields? Demand for insect control. Amer. J. Agr. Econ. 100: 1136–1150. [Google Scholar]
- Zukoff S. N., Ostlie K. R., Potter B., Meihls L. N., Zukoff A. L., French L., Ellersieck M. R., Wade French B., and Hibbard B. E.. 2016. Multiple assays indicate varying levels of cross resistance in Cry3Bb1-selected field populations of the western corn rootworm to mCry3A, eCry3.1Ab, and Cry34/35Ab1. J. Econ. Entomol. 109: 1387–1398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are available from the Iowa State University DataShare Digital Repository: https://doi.org/10.25380/iastate.12146172.v1.