Abstract
To evaluate the clinical efficacy of concentrated growth factors (CGFs) combined with mineralized collagen (MC) in guided bone regeneration (GBR). A retrospective study involving 29 patients treated with GBR technique, which was performed either CGF and MC complexes or MC alone. Implants were inserted simultaneously and cone-beam computed tomography was taken immediately, at 3 and 6 months postoperation. Questionnaires were completed by all patients so as to evaluate the main symptoms and daily activities during the first week after surgery. The outcomes of the two groups were statistically compared. All implants healed uneventfully. Patients in both groups suffered from different levels of discomfort for the reason of swelling, pain and chewing impairment on 1–2 days. Meanwhile, swelling of the Trial group was weaker than the Control group. When compared with the Control group, pain levels in Trial group were more rapidly reduced and patients took fewer analgesics from Day 3. Furthermore, the reconstitution mean value of the graft was thicker at 3 and 6 months in Trial group. CGFs complex with MC were beneficial to relieve the clinical symptoms, promote the peri-implant bone regeneration and shorten the healing time.
Keywords: concentrated growth factors, mineralized collagen, guided bone regeneration, bone augmentation, osseointegration
Introduction
Alveolar ridge resorption has been considered as an inevitable consequence of tooth extraction for a long time [1, 2]. The deficiency of alveolar bone presents a clinical problem for implant placement. The width of residual bone on buccal and lingual aspects must be at least 1 mm in order to maintain crestal bone levels, which means bone augmentation procedures should be performed when the width of alveolar ridge is <5-mm wide [3]. Hence, guided bone regeneration (GBR) technique, using barrier membranes and bone substitutes such as autografts, allografts, xenografts and alloplasts, has been applied for the reconstruction of defect region [4]. Autografts taken from an adjacent or remote site in the same patient is regarded as the ‘gold standard’ [5]. Due to the shortcomings of limited donor bone grafts, demanding for second surgical procedure and unpredictable resorption, it is imperative to explore alternatives to autografts [6, 7]. Bone substitutes may be applied to avoid these disadvantages without any volume limitations. Allografts such as demineralized freeze-dried bone allograft have properties of osteoconductive and osteoinductive [8]. Xenografts provide scaffolds for new bone regeneration and only possess osteoconductive properties [9]. However, allografts and xenografts have disadvantages of disease transmission, immune rejection and ethical issues.
The biomimetic mineralized collagen (MC), as newly alloplastic graft materials designed by Cui and colleagues [10], consists of orderly arranged nano-hydroxyapatite and Type I collagen. Furthermore, MC has good osteogenic activity, and its composition and microstructure are consistent with natural bone, which has been widely used for bone defect repair in clinic [11, 12].
CGF, first developed by Sacco in 2006, are new generation of platelet concentrated products and become the supplement of bone graft materials [13]. They are produced by centrifuging blood samples with a special centrifuge device. Differential centrifugation results in formatting more growth factors and more rigid fibrin structures than those observed in platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). In addition, it has been sated recently that CGF tend to be more effective in bone regeneration or soft tissue healing [14]. Application of CGF could also significantly increase osteogenesis in sinus augmentation [15].
Research about the compound of MC and CGF applying as bone graft material in GBR has not been reported. We hypothesized that application of CGF and MC in GBR would improve treatment outcomes. Therefore, the purpose of this study was to assess postoperative complications such as pain, swelling and trismus in both groups. Meanwhile, we also wanted to evaluate dimensional changes in bone augmentation between groups.
Materials and methods
Study population and design
Hospital records from January 2016 through June 2018 were retrospectively assessed to identify patients who suffered from with bone deficiency and required GBR protocol. A total of 29 patients from Department of Oral and Maxillofacial Surgery of Shandong Provincial Hospital Affiliated to Shandong University were divided into two groups: the Trial group who were grafted with CGF plus MC (Allgens®, Beijing Allgens Medical Science and Technology Co., Ltd., China) and the Control group who were grafted with MC.
Inclusion/exclusion criteria
The inclusion criteria were as follows:
18 years≤aged≤60 years
No history of systemic disease that not suitable for oral surgery
Periodontal condition with good plaque control
residual bone possesses sufficient width of 2–4 and height >3mm
Exclusion criteria were as follows:
Systemic disease that affect bone healing, such as uncontrolled diabetes, osteoporosis and HIV etc.
Pregnancy and lactation
Previous or current radiation or immunosuppressive therapy
Smoking and excessive drinking
Presurgical treatment
Clinical examination and cone-beam computed tomography (CBCT) were taken for each patient before the operation. Furthermore, all patients received periodontal treatment and oral hygiene instructions to provide a better oral environment.
Gargle with 0.2% chlorhexidine gluconate for 1 min. All patients received systemic antibiotics (Roxithromycin Capsules of 150 mg) prophylactically 1 h prior to surgery.
CGF preparation
Autologous CGF was prepared from fresh venous blood of patients. The venous blood samples were taken into 2 sterile 10 ml tubes without anticoagulants. The samples were immediately centrifuged with CGF centrifuge machine (Medifuge, Silfradent, Italy; Fig. 1a) with the following fixed procedures: 30″ acceleration, 2′ 2700 rpm, 4′ 2400 rpm, 4 2700 rpm, 3′ 3000 rpm, 36″ deceleration and stop. Centrifugation divided the blood into four layers: (i) red blood cell layer at the bottom (ii) CGF at the second layer (iii) the buffy coat at the third layer and (iv) the upper supernatant layer (Fig. 1b). The CGF layer was mechanically separated using sterile scissors (Fig. 1c). The CGF layer was then placed in a condensing disc and mixed with MC in 1:1 ratio (Allgens®, Beijing Allgens Medical Science and Technology Co., Ltd., China).
Figure 1.
Preparation of CGF. (a) Blood centrifugation; (b) after centrifugation; (c) separate the CGF layer
Surgical procedure
All patients were operated by Dr S.-Y.H. The procedure was performed under local anesthesia (4% articaine with 1:100 000 epinephrine).
The Nobel implant system (NobelActive®/NobelReplace™, Nobel Biocare, Göteborg, Sweden), the XIVE implant system (XIVE®, Dentsply Friadent, Mannheim, Germany) and the DIO implant system (DIO-SM, Busan, South Korea) were used in this study.
Incisions were made from the one side of alveolar ridge crest to the other, with vertical incisions on either side. Then the periosteum was detached from the bone surface so as to expose both the labial and palatal/lingual aspects of the alveolar ridge. Based on the presurgical CBCT, implants with appropriate dimensions were placed using routine process. The final sitting of implant was achieved primary stability of 30 Ncm or more, and the cover screw was placed. Implant sits were treated for GBR. The membrane (Heal-All® Oral Cavity Repair Membrane, Yantai Zhenghai Bio-tech Co., Ltd., China) was trimmed to a desirable dimension, and carefully covered the bone material. 3/0 Vicryl were used to sutures the flap closely (Vicryl Rapid-Ethicon Johnson, Diegem, Belgium). Then CBCT (ProMax 3D, Planmeca OY, 00880 Helsinki, Finland) scans were performed after surgery.
Patients were instructed to take an analgesic (LOXONIN®, 60 mg) after the surgical intervention and cold compresses also recommended. Postoperative prescriptions included antibiotics (Roxithromycin Capsules of 150 mg) for 3 days, 0.2% chlorhexidine oral rinse twice a day for 6 days (starting the day after surgery) and analgesic medication (LOXONIN®, 60 mg)if necessary. All patients were scheduled for recall at 7–10 days for suture removal.
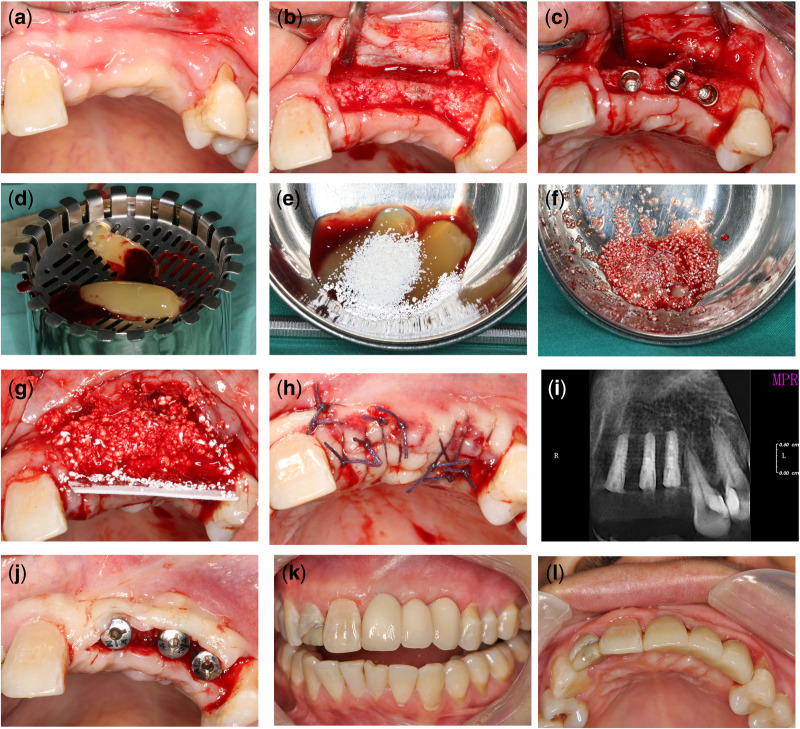
Second stage surgery was performed 3–4 months after implantation and healing abutment was inserted. Then reconstructive treatment protocol was initiated 2 weeks later (Fig. 2a–l).
Figure 2.
(a) Edentulous alveolar ridge in the front maxilla before surgery; (b) incisions from mesial of right maxillary Central incisor to the left maxillary premolars; (c) implant placement; (d) preparation of CGF; (e) the CGF and MC was mixed in 1:1 ratio; (f) complex of CGF and MC (g) GBR procedure; (h) interrupted suture; (i) radiograph after placement; (j) second stage surgery; (k, l) final zirconia crown
Clinical and radiological analysis
CBCT were taken immediately after operation, at 3 and 6 months postsurgery. Method of measurement was similar to that used in previous studies [16, 17]. First, the middle of implant diameter was confirmed in coronal position. Distance from the external surface of the labial bone to the buccal surface of the implant were measured at coronal level (L1), middle level of the implant (L2) and the implant apical (L3; Fig. 3). These parameters were measured at least three times, and the mean values were recorded.
Figure 3.
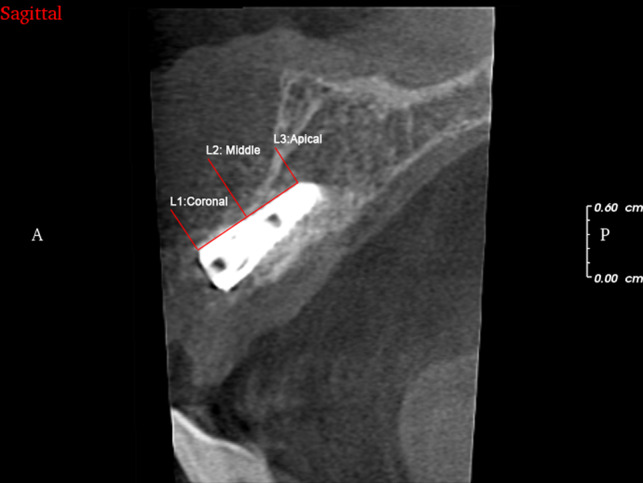
Method of measurement after GBR surgery
A questionnaire followed by Tsesis et al. [18] was filled out by every patient starting the day of surgery for 7 days postoperatively and was applied to assess postoperative patients’ limitations (sleeping, mastication, interincisor distance, phonetics, daily routine and work, pain and other symptoms, such as bleeding, swelling, nausea and bad taste/breath). According to their daily life, patients should answer the 10 questions in the questionnaire with the 5-point Likert-type scale from 1 (‘not at all’) to 5 (‘very much’; Table 1).
Table 1.
Quality of life questionnaire
| None | Little | Some | Quite a bit | Very much | |
|---|---|---|---|---|---|
| Do you experience any difficulties with mouth opening? | 1 | 2 | 3 | 4 | 5 |
| Do you experience any difficulties with chewing? | 1 | 2 | 3 | 4 | 5 |
| Do you experience any difficulties with speaking? | 1 | 2 | 3 | 4 | 5 |
| Do you experience any difficulties with sleeping? | 1 | 2 | 3 | 4 | 5 |
| Have you missed your work/school? | 1 | 2 | 3 | 4 | 5 |
| Do you experience any difficulties with your daily activities? | 1 | 2 | 3 | 4 | 5 |
| Do you have swelling? | 1 | 2 | 3 | 4 | 5 |
| Do you have bleeding? | 1 | 2 | 3 | 4 | 5 |
| Do you feel nausea? | 1 | 2 | 3 | 4 | 5 |
| Do you feel a bad taste or breath? | 1 | 2 | 3 | 4 | 5 |
Did you take any pain-killers today? ______.
Visual analog scales (VASs) scores appeared to be effective tools for assessing dental pain perception, using ‘0 = no pain’ and ‘100 = the most intense pain imaginable’ [19]. The final question involved whether the patient had taken any analgesics on each postoperative day. Questionnaires were returned in 7-day subsequent visit by patients.
Statistical analysis
The two-sample t-test was performed to evaluate statistical differences between the two groups for new buccal plate bone. Fisher exact test was used to evaluate statistically the difference between the groups for analgesics taken as well as differences in any variable related to activities (sleeping, mastication, interincisor distance, phonetics, daily routine and work) and symptoms (bleeding, swelling, nausea and bad taste/breath) on each day after surgery. Patients’ experience of quality of life in pain by VAS scores was assessed using the Shapiro–Wilk test. Statistical significance was considered to be P ≤ 0.05. Statistical analysis was performed by SPSS (SPSS 22.0, SPSS Inc., Chicago, IL, USA).
Results
In total, 29 patients did not have complications and achieved clinically osseointegrated. Although there was no histological analysis described the characteristics of the tissue contacting the implant, the soft tissues surrounding the implants appeared free of inflammation. Thus, there were 15 patients estimated in Control Group (7 male and 8 female, aging from 20 to 58 years and 18 implants) and 14 patients (6 male and 8 female, aging from 26 to 57 years and 17 implants) in Trial Group. There were no statistically significant differences in distribution of patients on the basis of age, gender, smoking history, implant brand and implant site between the two groups(P > 0 .05; Table 2).
Table 2.
Demographic information of the Control/Trial group
| Patient no. | Sex | Age | Smokers | Implant brand | Implant site | Implant dimension (mm) | Insertion torque (Ncm) |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| 1 | F | 33 | No | XIVE® | 11 21 | 3.8 × 9.5 | 30 |
| 2 | M | 20 | Light | NobelActive® | 43 | 4.3 × 13 | 35 |
| 3 | M | 45 | No | XIVE® | 35 | 3.8× 8 | 30 |
| 4 | F | 58 | Light | DIO-SM | 37 | 4.1 × 8 | 35 |
| 5 | M | 43 | No | NobelReplaceTM | 46 | 4.3 × 11.5 | 30 |
| 6 | F | 28 | Light | DIO-SM | 37 | 4.5 × 10 | 35 |
| 7 | M | 37 | No | DIO-SM | 11 | 3.8 × 10 | 40 |
| 8 | F | 52 | No | XIVE® | 14 | 3.8 × 11 | 30 |
| 9 | F | 45 | Light | DIO-SM | 24 | 3.8 × 12 | 30 |
| 10 | M | 24 | No | NobelActive® | 31 32 42 | 3.5 × 10 | 35 |
| 11 | M | 31 | No | XIVE® | 21 | 3.8 × 13 | 35 |
| 12 | F | 29 | No | XIVE® | 46 | 4.5 ×9.5 | 25 |
| 13 | M | 49 | No | NobelReplaceTM | 37 | 4.3 × 11.5 | 30 |
| 14 | F | 31 | No | XIVE® | 12 | 3.4 × 11 | 30 |
| 15 | M | 38 | No | NobelActive® | 46 | 4.3 × 10 | 35 |
| Trial | |||||||
| 1 | M | 29 | No | DIO-SM | 47 | 4.1 × 8 | 35 |
| 2 | M | 43 | No | XIVE® | 45 | 3.8 × 9.5 | 30 |
| 3 | F | 57 | No | XIVE® | 46 | 3.8 × 9.5 | 40 |
| 4 | F | 52 | Light | NobelReplaceTM | 37 | 4.3 × 11.5 | 35 |
| 5 | M | 50 | No | NobelActive® | 11 | 3.5 × 11.5 | 30 |
| 6 | F | 28 | Light | XIVE® | 15 | 3.8 × 9.5 | 35 |
| 7 | M | 45 | No | DIO-SM | 46 | 4.5 × 10 | 25 |
| 8 | F | 26 | No | XIVE® | 12 | 3.8 × 13 | 30 |
| 9 | F | 40 | No | DIO-SM | 21 22 23 | 3.8 × 10 | 30 |
| 10 | M | 27 | No | NobelActive® | 13 | 3.5 × 11.5 | 35 |
| 11 | F | 31 | No | XIVE® | 36 37 | 4.5 × 9.5 | 35 |
| 12 | F | 36 | Light | DIO-SM | 25 | 3.8 × 10 | 30 |
| 13 | M | 30 | No | NobelReplaceTM | 25 | 3.5× 11.5 | 30 |
| 14 | F | 54 | No | XIVE® | 21 | 3.4 × 11 | 30 |
Clinical outcomes
All patients suffered from different levels of discomfort for the reason of swelling, pain and chewing impairment on the first day after surgery. The items of daily routine life and missed work were reported in either group, especially in the first 2 days. Whereas, these uncomfortable experiences were relieved more rapidly in Trial Group than that in Control group from the second day. The recovery of sleeping and phonetics was similar in both groups. When compared with the Trial group, swelling was more serious in Control group from Days 2 to 5.There was no difference in bleeding, nausea and bad taste/breath between the two groups (Tables 3 and 4).
Table 3.
The outcomes of the evaluation for functional symptoms
| Day 1 (%) |
Day 2 (%) |
Day 3 (%) |
Day 4 (%) |
Day 5 (%) |
Day 6 (%) |
Day 7 (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | C | T | C | T | C | T | C | T | C | T | C | T | C | T |
| Bleeding | ||||||||||||||
| Very much | ||||||||||||||
| Quite a bit | ||||||||||||||
| Some | 86.7 | 78.6 | 13.3 | 7.1 | ||||||||||
| Little/None | 13.3 | 21.4 | 86.7 | 92.9 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Swelling | ||||||||||||||
| Very much | 13.3 | 14.3 | 40.0 | 21.4 | 53.3 | 26.7 | 1 | |||||||
| Quite a bit | 53.3 | 57.1 | 40.0 | 28.6 | 33.3 | 21.4 | 40.0 | 26.7 | ||||||
| Some | 33.3 | 28.6 | 20.0 | 50.0 | 6.7 | 50.0 | 20.0 | 28.6 | 33.3 | 13.3 | ||||
| Little/none | 6.7 | 28.6 | 13.3 | 71.4 | 40.0 | 100 | 86.7 | 100 | 100 | 100 | ||||
| Nausea | ||||||||||||||
| Very much | ||||||||||||||
| Quite a bit | ||||||||||||||
| Some | 20.0 | 7.1 | ||||||||||||
| Little/none | 80.0 | 92.9 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Bad taste/breath | ||||||||||||||
| Very much | 6.7 | |||||||||||||
| Quite a bit | 20.0 | 21.4 | 6.7 | |||||||||||
| Some | 40.0 | 35.7 | 40.0 | 42.9 | 20.0 | 14.3 | 6.7 | |||||||
| Little/none | 33.3 | 42.9 | 53.3 | 57.1 | 80.0 | 85.7 | 93.3 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 4.
The outcomes of the evaluation for functional activity
| Day 1 (%) |
Day 2 (%) |
Day 3 (%) |
Day 4 (%) |
Day5 (%) |
Day 6 (%) |
Day 7 (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity | C | T | C | T | C | T | C | T | C | T | C | T | C | T |
| Sleeping | ||||||||||||||
| Very much | 33.3 | 28.6 | 20.0 | 14.3 | 6.7 | |||||||||
| Quite a bit | 46.7 | 35.7 | 26.7 | 28.6 | 20.0 | 14.3 | ||||||||
| Some | 20.0 | 35.7 | 40.0 | 35.7 | 26.7 | 35.7 | 26.7 | 21.4 | 6.7 | |||||
| Little/none | 13.3 | 21.4 | 46.7 | 50.0 | 73.3 | 78.6 | 93.3 | 100 | 100 | 100 | 100 | 100 | ||
| Mastication | ||||||||||||||
| Very much | 66.7 | 64.3 | 53.3 | 50.0 | 26.7 | 14.3 | 13.3 | 7.1 | 13.3 | 7.1 | ||||
| Quite a bit | 20.0 | 21.4 | 20.0 | 14.3 | 40.0 | 21.4 | 6.7 | 14.3 | 6.7 | 7.1 | 6.7 | |||
| Some | 13.3 | 14.3 | 20.0 | 21.4 | 20.0 | 21.4 | 53.3 | 21.4 | 13.3 | 14.3 | 13.3 | |||
| Little/none | 6.7 | 14.3 | 13.3 | 42.9 | 20.0 | 57.1 | 66.7 | 71.4 | 80.0 | 100 | 100 | 100 | ||
| Interincisor distance | ||||||||||||||
| Very much | 53.3 | 50.0 | 33.3 | 35.7 | 26.7 | 7.1 | 13.3 | |||||||
| Quite a bit | 20.0 | 28.6 | 20.0 | 21.4 | 20.0 | 7.1 | 6.7 | |||||||
| Some | 26.7 | 21.4 | 20.0 | 14.3 | 13.3 | 14.3 | 13.3 | 7.1 | 13.3 | |||||
| Little/None | 26.7 | 28.6 | 40.0 | 71.4 | 66.7 | 92.9 | 86.7 | 100 | 100 | 100 | 100 | 100 | ||
| Phonetics | ||||||||||||||
| Very much | 7.1 | |||||||||||||
| Quite a bit | 66.7 | 64.3 | 53.3 | 50.0 | 26.7 | 21.4 | ||||||||
| Some | 33.3 | 28.6 | 33.3 | 35.7 | 13.3 | 7.1 | 6.7 | |||||||
| Little/none | 13.3 | 14.3 | 60.0 | 71.4 | 93.3 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Daily routine | ||||||||||||||
| Very much | 6.7 | |||||||||||||
| Quite a bit | 26.7 | 28.6 | 20.0 | 14.3 | 6.7 | |||||||||
| Some | 53.3 | 35.7 | 26.7 | 21.4 | 13.3 | |||||||||
| Little/none | 13.3 | 35.7 | 53.3 | 64.3 | 80.0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Missed work | ||||||||||||||
| Yes | 86.7 | 92.9 | 80.0 | 85.7 | 66.7 | 50.0 | 20.0 | |||||||
| No | 13.3 | 7.1 | 20.0 | 14.3 | 33.3 | 50.0 | 80.0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
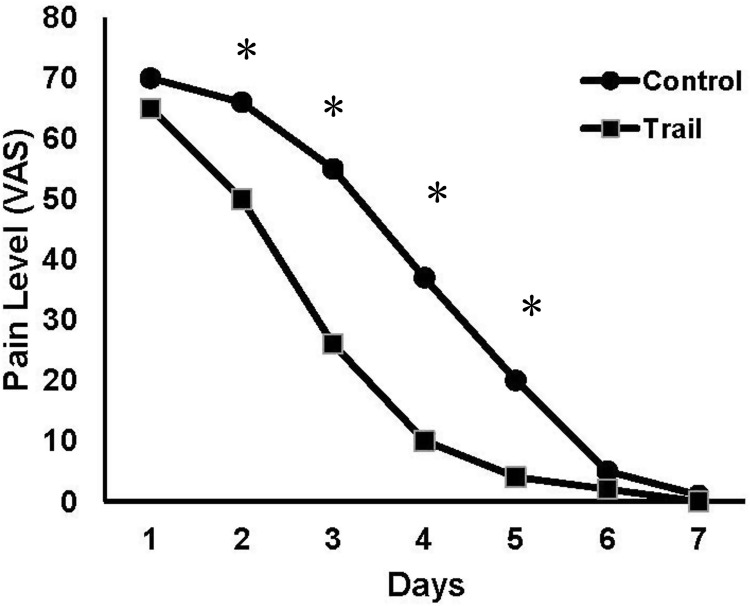
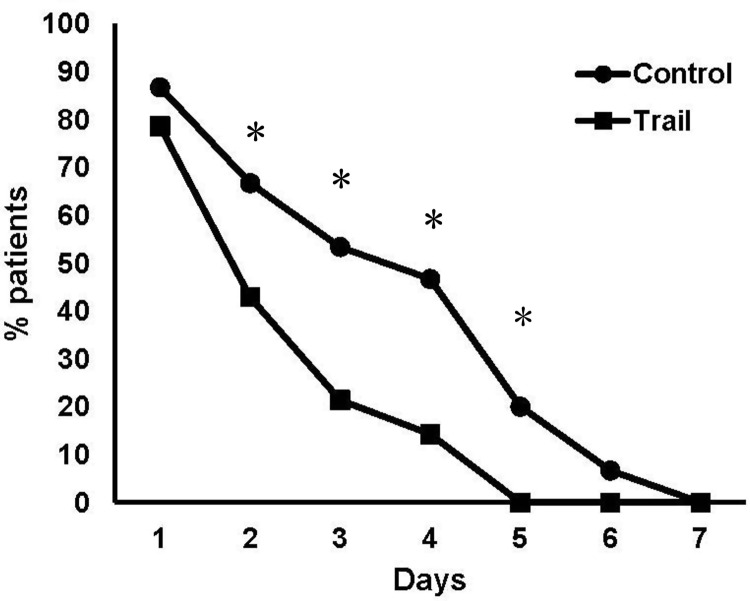
Furthermore, pain levels were rapidly reduced from Day 2 (Fig. 4) and patients took dramatically fewer analgesics on Day 2 in Trial Group compared with the Control group (Fig. 5; P < 0.05). The differences turned into negligible after Day 6.
Figure 4.
The levels of pain reported in the first week after surgery
Figure 5.
The proportion of patients taking analgesics in the first week after surgery
Radiographic outcomes
The radiographic evaluation confirmed that all the implants were healed uneventfully. The results (Table 5) indicated that the reconstitution mean value of the graft thickness was significantly decreased in the first 3 months. Moreover, the tendency was gradually stable between 3 and 6 months in both groups (P < 0.05). New bone formation in both groups was usually satisfactory. Importantly, the width of new buccal plate bone was thicker in Trial group at 3 and 6 months (P < 0.05).
Table 5.
Distance from the external surface of the labial bone to the buccal surface of the implant
| Postsurgery |
3 months |
6 months |
||||
|---|---|---|---|---|---|---|
| C | T | C | T | C | T | |
| L1 | 2.50 ± 0.85 | 2.6 ± 0.55 | 2.13 ± 0.21 | 2.45 ± 0.57 | 2.09 ± 0.46 | 2.37 ± 0.77 |
| L2 | 1.53 ± 0.49 | 1.49 ± 0.76 | 1.28 ± 0.70 | 1.39 ± 0.54 | 1.22 ± 0.42 | 1.35 ± 0.85 |
| L3 | 1.48 ± 0.78 | 1.45 ± 0.31 | 1.30 ± 0.50 | 1.38 ± 0.32 | 1.25 ± 0.35 | 1.29 ± 0.36 |
Discussion
In our study, CGF combined with MC achieved satisfactory effects in improving the quality of Life and bone reconstruction. To the best of our knowledge, this retrospective study first evaluated the clinical effects of CGF and MC as the grafting materials in GBR.
Platelet concentrated products such as PRP, plasma-rich in growth factors, PRF and concentrated growth factor (CGF) have been shown to be an efficient biomaterial for tissue regeneration [20]. As the third new generation of platelet concentrated products, CGF contains a variety of growth factors, such as platelet-derive growth factors (PDGFs), transforming growth factors β (TGFs-β), vascular endothelial growth factors (VEGFs), insulin-like growth factors, epidermal growth factor, fibroblast growth factor, as well as bone morphogenic protein. These factors can significantly promote healing of hard and soft tissue [13, 21]. Furthermore, a large number of CD34+ cells in CGF having been proved to play an important role in vascular maintenance, angiogenesis and neovascularization [13, 22]. Previous studies [23, 24] have demonstrated that CGF could validly accelerate the proliferation and differentiation of cells, promote wound healing processes and new bone formation. Reports also have indicated that CGF accelerated new bone formation in GBR and sinus grafting for many years [15, 25]. What’s more, CGF is widely applied for regeneration of alveolar ridge bone in combination with various biological materials or used alone [26, 27]. Ozveri Koyuncu et al. [28] reported that using CGF after third molar extraction significantly accelerated soft tissue healing and relieved the postoperative symptoms, particularly pain, swelling and trismus.
Based on the recognition of MC and its formation process, experts have focused on the preparation of biomimetic MC materials to imitate natural bone [29, 30]. MC is an artificial biomimetic with characteristics of osteogenic activity. Orderly arrangement of Type I collagen and nano-hydroxyapatite are the components of MC [10]. The Type I collagen was extracted from bovine tendon, and used as a template to form nano-sized hydroxyapatite through in vitro biomineralization [10, 12]. Feng et al. [11] reported that MC showed better effect on new bone formation in alveolar ridge preservation.
In our study, we assessed the effectiveness of CGF with MC on soft tissue healing and bone formation. As previously described in the Results section, patients in Trial group were tended to obtain more excellent quality of life after operation. With regard to soft tissue healing, our findings accorded closely with Ozveri Koyuncu et al.’s study [28], demonstrating that CGF is of great value for soft tissue healing and postoperative symptoms alleviation. The primary outcomes of our study showed that all patients suffered from discomforts such as swelling, pain and chewing impairment on the first day after surgery, while swelling was more serious in Control group from Days 2 to 5. When compared with the Control group, we found that the total amount of analgesic consumption in CGF group seemed to be lower than the Control group. Al-Hamed et al. [31] and Uyanık et al. [32] reported that fewer analgesic tablets were taken after PRF application, which was similar to our study.
CGF provides a powerful biological scaffold with and acts as an integrated reservoir to emit growth factors for accelerating tissue regeneration [22, 33–36]. What’s more, increased levels of transforming growth factor β-1 (TGF-β1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in CGF contribute to promote soft tissue healing [37] and reduce postoperative complications. The secondary outcomes in the radiographic evaluation demonstrated that the reconstitution of new buccal plate bone in the Trial group was found to be more efficient than the Control group. Durmuslar et al. [26] evaluated CGF on the healing of peri-implant bone defects and restoration was achieved applied by autogenous bone and CGF. Wang et al. [27] found that Bio-Oss combined with CGF was more effective in increasing new bone formation than using Bio-Oss alone in a canine model. Honda et al. [38] implemented bone regeneration experiments on rat calvaria defects using CGF + BMSC and signaled remarkable healing of a critical-size bone defect in vivo in 2013. What’s more, Bonazza et al. [39] also showed that the combination of CGF and sodium orthosilicate stimulated cell proliferation and osteogenic differentiation, which could be effective in tissue regeneration. In agreement with the results of these previous studies, the application of CGF, our results showed better bone augmentation in Trial group with GBR protocol.
It is necessary to acknowledge some limitations in our study. One of the shortcomings was that the effect of CGF on bone regeneration was not evaluated. The other was that patients who participated in this study was limited to a short-term time-span and demanded for further observation to assess the success rate of implants.
Conclusion
In conclusion, according to our results, application of CGF and MC has a positive impact on reducing postoperative discomforts and new bone formation. The complex of CGF and MC seems to be appropriate and efficient as a biomaterial for bone augmentation.
Funding
This study was funded by China Postdoctoral Science Foundation Grant (No. 2019M652380).
Conflict of interest statement. None declared.
References
- 1. Esposito M, Grusovin MG, Felice P. et al. Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev 2009; Issue 4. Art. No.:CD003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tan WL, Wong TL, Wong MC. et al. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res 2012;23(Suppl 5):1–21. [DOI] [PubMed] [Google Scholar]
- 3. Kheur M, Gokhale S, Sumanth S. et al. Staged ridge splitting technique for horizontal expansion in mandible: a case report. J Oral Implantol 2014;40:479–83. [DOI] [PubMed] [Google Scholar]
- 4. Al Yafi F, Alchawaf B, Nelson K.. What is the optimum for alveolar ridge preservation? Dent Clin North Am 2019;63:399–418. [DOI] [PubMed] [Google Scholar]
- 5. Block MS. Treatment of the single tooth extraction site. Oral Maxillofac Surg Clin North Am 2004;16:41–63.vi. [DOI] [PubMed] [Google Scholar]
- 6. Clavero J, Lundgren S.. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res 2003;5:154–60. [DOI] [PubMed] [Google Scholar]
- 7. Giannoudis PV, Dinopoulos H, Tsiridis E.. Bone substitutes: an update. Injury 2005;36(Suppl 3):S20–7. [DOI] [PubMed] [Google Scholar]
- 8. Yukna RA. Synthetic bone grafts in periodontics. Periodontol 2000 1993;1:92–9. [PubMed] [Google Scholar]
- 9. Gross JS. Bone grafting materials for dental applications: a practical guide. Compend Contin Educ Dent 1997;18:1013–8, 1020–2, 1024, passim; quiz. [PubMed] [Google Scholar]
- 10. Liao SS, Cui FZ, Zhang W. et al. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater 2004;69:158–65. [DOI] [PubMed] [Google Scholar]
- 11. Feng L, Zhang L, Cui Y. et al. Clinical evaluations of mineralized collagen in the extraction sites preservation. Regen Biomater 2016;3:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao SS, Guan K, Cui FZ. et al. Lumbar spinal fusion with a mineralized collagen matrix and rhBMP-2 in a rabbit model. Spine (Phila Pa 1976) 2003;28:1954–60. [DOI] [PubMed] [Google Scholar]
- 13. Rodella LF, Favero G, Boninsegna R. et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech 2011;74:772–7. [DOI] [PubMed] [Google Scholar]
- 14. Kim TH, Kim SH, Sandor GK. et al. Comparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF) in rabbit-skull defect healing. Arch Oral Biol 2014;59:550–8. [DOI] [PubMed] [Google Scholar]
- 15. Sohn DS, Heo JU, Kwak DH. et al. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone. Implant Dent 2011;20:389–95. [DOI] [PubMed] [Google Scholar]
- 16. Lee EA, Gonzalez-Martin O, Fiorellini J.. Lingualized flapless implant placement into fresh extraction sockets preserves buccal alveolar bone: a cone beam computed tomography study. Int J Periodontics Restorative Dent 2014;34:61–8. [DOI] [PubMed] [Google Scholar]
- 17. Sarnachiaro GO, Chu SJ, Sarnachiaro E. et al. Immediate implant placement into extraction sockets with labial plate dehiscence defects: a clinical case series. Clin Implant Dent Relat Res 2016;18:821–9. [DOI] [PubMed] [Google Scholar]
- 18. Tsesis I, Shoshani Y, Givol N. et al. Comparison of quality of life after surgical endodontic treatment using two techniques: a prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:367–71. [DOI] [PubMed] [Google Scholar]
- 19. Seymour RA, Charlton JE, Phillips ME.. An evaluation of dental pain using visual analogue scales and the Mcgill Pain Questionnaire. J Oral Maxillofac Surg 1983;41:643–8. [DOI] [PubMed] [Google Scholar]
- 20. Kumar N, Prasad K, Ramanujam L. et al. Evaluation of treatment outcome after impacted mandibular third molar surgery with the use of autologous platelet-rich fibrin: a randomized controlled clinical study. J Oral Maxillofac Surg 2015;73:1042–9. [DOI] [PubMed] [Google Scholar]
- 21. Dohan Ehrenfest DM, de Peppo GM, Doglioli P. et al. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009;27:63–9. [DOI] [PubMed] [Google Scholar]
- 22. Qiao J, An N.. Effect of concentrated growth factors on function and Wnt3a expression of human periodontal ligament cells in vitro. Platelets 2017;28:281–6. [DOI] [PubMed] [Google Scholar]
- 23. Sahin IO, Gokmenoglu C, Kara C.. Effect of concentrated growth factor on osteoblast cell response. J Stomatol Oral Maxillofac Surg 2018;119:477–81. [DOI] [PubMed] [Google Scholar]
- 24. Pirpir C, Yilmaz O, Candirli C. et al. Evaluation of effectiveness of concentrated growth factor on osseointegration. Int J Implant Dent 2017;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim JM, Sohn DS, Bae MS. et al. Flapless transcrestal sinus augmentation using hydrodynamic piezoelectric internal sinus elevation with autologous concentrated growth factors alone. Implant Dent 2014;23:168–74. [DOI] [PubMed] [Google Scholar]
- 26. Durmuslar MC, Balli U, Dede FO. et al. Histological evaluation of the effect of concentrated growth factor on bone healing. J Craniofac Surg 2016;27:1494–7. [DOI] [PubMed] [Google Scholar]
- 27. Wang F, Li Q, Wang Z.. A comparative study of the effect of Bio-Oss((R)) in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting. J Oral Pathol Med 2017;46:528–36. [DOI] [PubMed] [Google Scholar]
- 28. Ozveri Koyuncu B, Isik G, Ozden Yuce M. et al. Effect of concentrated growth factor (CGF) on short-term clinical outcomes after partially impacted mandibular third molar surgery: a split-mouth randomized clinical study. J Stomatol Oral Maxillofac Surg 2019. [DOI] [PubMed] [Google Scholar]
- 29. Palmer LC, Newcomb CJ, Kaltz SR. et al. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev 2008;108:4754–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nudelman F, Lausch AJ, Sommerdijk NA. et al. In vitro models of collagen biomineralization. J Struct Biol 2013;183:258–69. [DOI] [PubMed] [Google Scholar]
- 31. Al-Hamed FS, Tawfik A-M, Abdelfadil E. et al. Efficacy of platelet-rich fibrin after mandibular third molar extraction: a systematic review and meta-analysis. Journal of Oral and Maxillofacial Surgery 2017;75:1124–35. [DOI] [PubMed] [Google Scholar]
- 32. Uyanık LO, Bilginaylar K, Etikan İ.. Effects of platelet-rich fibrin and piezosurgery on impacted mandibular third molar surgery outcomes. Head Face Med 2015;11: 10.1186/s13005-015-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choukroun J, Diss A, Simonpieri A. et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e56–60. [DOI] [PubMed] [Google Scholar]
- 34. Dohan DM, Choukroun J, Diss A. et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e51–5. [DOI] [PubMed] [Google Scholar]
- 35. Dohan DM, Choukroun J, Diss A. et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e45–50. [DOI] [PubMed] [Google Scholar]
- 36. Dohan DM, Choukroun J, Diss A. et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e37–44. [DOI] [PubMed] [Google Scholar]
- 37. Masuki H, Okudera T, Watanebe T. et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int J Implant Dent 2016;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Honda H, Tamai N, Naka N. et al. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J Artif Organs 2013;16:305–15. [DOI] [PubMed] [Google Scholar]
- 39. Bonazza V, Borsani E, Buffoli B. et al. In vitro treatment with concentrated growth factors (CGF) and sodium orthosilicate positively affects cell renewal in three different human cell lines. Cell Biol Int 2018;42:353–64. [DOI] [PubMed] [Google Scholar]