Abstract
Negatively charged tissues are ubiquitous in the human body and are associated with a number of common diseases yet remain an outstanding challenge for targeted drug delivery. While the anionic proteoglycans are critical for tissue structure and function, they make tissue matrix dense, conferring a high negative fixed charge density (FCD) that makes drug penetration through the tissue deep zones and drug delivery to resident cells extremely challenging. The high negative FCD of these tissues is now being utilized by taking advantage of electrostatic interactions to create positively charged multi-stage delivery methods that can sequentially penetrate through the full thickness of tissues, create a drug depot and target cells. After decades of work on attempting delivery using strong binding interactions, significant advances have recently been made using weak and reversible electrostatic interactions, a characteristic now considered essential to drug penetration and retention in negatively charged tissues. Here we discuss these advances using examples of negatively charged tissues (cartilage, meniscus, tendons and ligaments, nucleus pulposus, vitreous of eye, mucin, skin), and delve into how each of their structures, tissue matrix compositions and high negative FCDs create barriers to drug entry and explore how charge interactions are being used to overcome these barriers. We review work on tissue targeting cationic peptide and protein-based drug delivery, compare and contrast drug delivery designs, and also present examples of technologies that are entering clinical trials. We also present strategies on further enhancing drug retention within diseased tissues of lower FCD by using synergistic effects of short-range binding interactions like hydrophobic and H-bonds that stabilize long-range charge interactions. As electrostatic interactions are incorporated into design of drug delivery materials and used as a strategy to create properties that are reversible, tunable and dynamic, bio-electroceuticals are becoming an exciting new direction of research and clinical work.
Keywords: Targeted drug delivery, Negatively charged tissues, Electro-diffusive transport, Electrostatic charge interactions, Cationic drug carriers, Cell penetrating peptides, Cationic protein drug carriers
Introduction
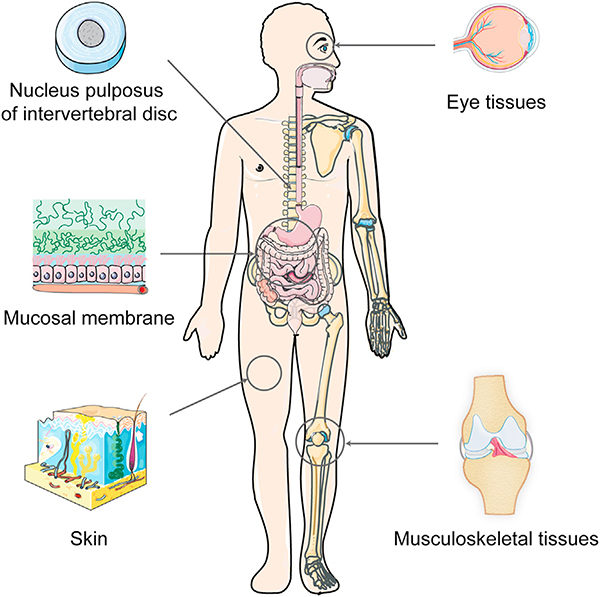
The human body contains a multitude of negatively charged tissues that are dense and avascular and remain an outstanding challenge in the field of targeted drug delivery. While traditional delivery methods can bring drugs close to affected organs, intra-tissue drug penetration to reach cellular target sites is still ineffective. Examples of such tissues include cartilage, meniscus and ligaments of the knee, ankle, shoulder and hip joints etc., intervertebral discs (IVDs) in the spine, mucosal membrane of the gastrointestinal (GI) tract, vitreous humor of the eye, and skin (Fig. 1). Their degeneration is associated with several common diseases such as osteoarthritis (OA) [1] and gout [2] (synovial joints), spondylitis and cervical pain (spine) [3], inflammatory bowel disease (IBD) (GI tract) [4], and macular and retinal degeneration (eye) [5], which affect millions of people worldwide.There exist chemical entities, biologics and gene therapies that can inhibit or reverse tissue degeneration but only if they are able to reach their intra-tissue and intra-cellular sites in sufficient doses; their clinical application is limited by a lack of effective delivery systems that can enable drugs to penetrate through full tissue thickness to reach their cell targets and retain there to provide sustained drug release. Many of these diseases thus remain untreated. The avascular nature of the tissues makes them inaccessible to systemically delivered drugs [6]. Local drug delivery methods like intra-articular (IA) injection for synovial joint tissues, epidural injection for targeting IVDs, sub-acromial injection for shoulder bursa and intra-vitreal injection for back of the eye also remain inadequate; the dense extracellular matrix (ECM) and high density of negatively charged groups hinder entry and transport of drugs which clear out via lymphatics or vasculature before reaching the required intra-tissue therapeutic index. For example, corticosteroid injections used to treat local pain and inflammation have half-lives of only a few hours, necessitating frequent injections of high drug doses [6,7].
Fig. 1.
Negatively charged tissues in the human body remain an outstanding challenge in the field of targeted drug delivery. Examples include musculoskeletal joints(shoulder, elbow, knee, hand and ankle) comprising of tissues like articular cartilage, synovial joint, ligaments, tendons and menisci; nucleus pulposus of the intervertebral disc; eye tissues like the cornea and vitreous humor; mucosal membrane; and skin comprising of multiple layers of negatively charged cells, collagen, proteoglycans and elastin. This high negative FCD can be used to enhance intra-tissue transport, uptake and binding of locally injected drugs or their carriers via electrostatic interactions by modifying them to contain optimally charged cationic domains.
The complex ECM of such avascular tissues consists of a dense meshwork of collagen fibrils, proteoglycans (PGs), containing highly negatively charged glycosaminoglycan (GAG) chains, and cells, which create a barrier to entry for most drugs or drug carriers. For example, cartilage has a complex meshwork of type II collagen, which is densely packed with aggrecans, presenting sub-stantial steric hindrance to penetration of therapeutic molecules. The meniscus has similar ECM structure to cartilage but a lower fixed charge density (FCD) (20 % aggrecan content versus 35 % in cartilage by dry tissue weight) [8]. The nucleus pulposus (NP) of IVDs is mostly composed of negatively charged PG gel held together loosely by a sparse network of type II collagen fibrils. The NP is surrounded by a tough fibrous coating of annulus fibrosus (AF) containing circumferentially aligned collagen making it difficult for drugs injected epidurally to penetrate via passive diffusion [9]. Similar issues plague drug delivery to the back of the eye as passive diffusion of drug in vitreous humor is inefficient, suffering from off-target effects and lacking sustained drug delivery [10]. The negatively charged gastric mucosa also poses a hindrance to solute penetration making oral drug delivery challenging [11]. Similarly, the skin’s stratum corneum (SC) and the viable epidermis-dermis (VED) containing densely packed cells, lipids, collagen, elastin, hyaluronic acid (HA) and GAGs hinder transdermal drug delivery [12].Table 1 presents GAG content and estimated average negative FCD in some tissues.
Table 1.
GAG content of various negatively charged tissues is represented as μg GAG/mg dry tissue weight. Average fixed charge density (FCD) of tissues is calculated using . For sulfated GAGs, MW ∼ 458 g/mol and −2 moles of charge per mole of GAG disaccharide unit is assumed [236]. For HA, MW (of repeating disaccharide unit) ∼ 401 g/mol and −1 mole of charge per mole of repeating unit is assumed. Tissue dry weight was converted to wet weight by using their respective water content. Cartilage, ligament/tendon, meniscus, nucleus pulposus, vitreous humor, mucus and skin water content were assumed 80 %, 80 %, 70 %, 80 %, 99 %, 95 % and 70 %, respectively. Additionally, percentage composition of different types of GAGs (chondroitin sulfate (CS), keratan sulfate (KS), heparan sulfate (HS), dermatan sulfate (DS) and hyaluronic acid (HA)) in each tissue is presented.
| Tissue | GAG content (μg GAG/mg dry tissue) | Average Fixed Charge Density (mM) | Types of GAGs |
|---|---|---|---|
| Cartilage | 100 to 300 [221,222] | −170.0 [223] | CS (80 %) KS (5–20 %) HA (1–10%) [49] |
| Synovial fluid | 28.8 [224] | −8.8 [224] | HA (97%) CS (3 %) [224] |
| Ligaments and tendons | 2 to 35 depending on region [91] | Ligament: −17.9 [82] Tendon: −6.6 [82] |
CS (16–56 %) DS (20 %–66 %) [225] |
| Meniscus | 39 to 9 from inner to outer region [226] | −51.5 [53] | CS (60 %) DS (20 %) KS (15 %) HA (3 %) [227] |
| Nucleus pulposus | 100 to 400 [228] | −138.2 [229] | CS (67 %) KS (21 %) HA (5%) [230] |
| Vitreous humor | 23 [231] | −0.6 [231,232] | HA (96.2 %) CS (3.5 %) HS (0.3%) [231] |
| Gastric mucosal membrane | 19.6–23.4 [233] | −4.4 [233] | DS (45 %) CS (20 %) HS (19 %) HA (16 %) [234] |
| Skin | 3.4 [235] | −2.5 [235] | DS (46.7 %) HA (46 %) HS (3.9 %) CS (3.3 %) [235] |
Electro-diffusive transport of cationic solutes in negatively charged tissues
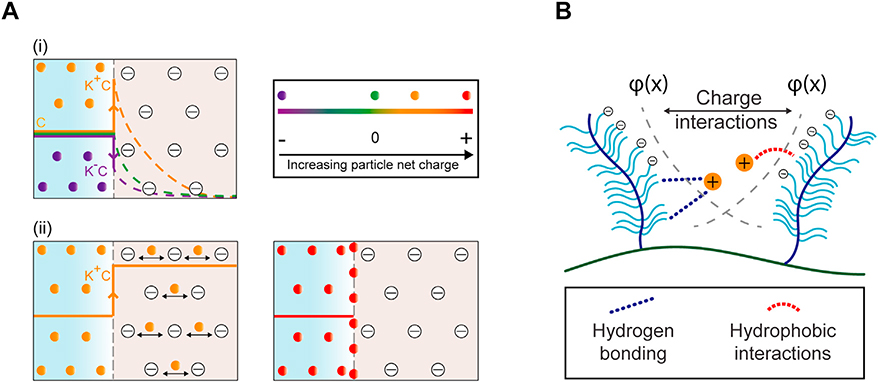
This high negative FCD of tissues can be utilized by modifying therapeutics to add optimally charged cationic domains such that the electrostatic interactions can enhance their transport, uptake and retention rather than hindering them [13,14]. Local administration of cationic carriers to negatively charged tissues results in a sharp increase in their concentration by a Donnan partitioning factor of K+ (K+C versus C at the administration site) [13], due to internal electrical fields exerted by the negative FCD of the tissue. Conversely, negatively charged solutes are repelled from the tissue resulting in a downward partitioning from concentration of C at the administration site to K− C at the tissue interface while neutral solutes that are not sterically hindered can maintain the same concentration at both tissue interface and administration site [13]. Thus, the elevated concentration of positively charged solutes at the tissue interface results in steeper concentration gradients and faster intra-cartilage diffusion rates compared to both neutral and negatively charged counterparts [13] (Fig. 2A (i)). It was recently shown that by using electrostatic interactions, the intra-cartilage uptake of cationic drugs or their carriers increased by 100–400 times as compared to their neutral versions [14–18]. Consequently, the cationic carriers can reach their intra-tissue therapeutic index faster, ensuring an appropriate biological response before getting cleared from the administration site. Recent research efforts have focused on using strong specific binding mechanisms to increase the residence time of drug carriers inside tissues [19–22]. However, the ability of strong binding interactions to promote transport through tissues is paradoxical; while binding enhances retention, it consequently hinders diffusive transport slowing down the penetration of carriers as they would get trapped in the surface of the tissue before reaching deeper zone targets [13,14]. The weak and reversible binding nature of electrostatic interactions is indeed an essential characteristic that enables drug carriers to unbind after their initial binding with negatively charged groups, find another binding site, and continue this process until they diffuse through the full tissue thickness (Fig. 2A (ii)). It is the high negative FCD of such tissues that greatly increases the intra-tissue residence time of cationic solutes despite their weak binding. Several of the diseases affecting negatively charged tissues result in degeneration of tissue matrix, thereby inducing some GAG loss and a reduction in the tissue negative FCD. In these cases, charge-based binding of carriers can be further stabilized with the remaining GAGs by synergistically using short-range hydrophobic interactions and hydrogen bonds [14] (Fig. 2B).
Fig. 2.
Electro-diffusive transport of cationic carriers in negatively charged tissues. A. (i) Concentration of positively charged carriers partitions upward from C to K+C at tissue interface enabling faster diffusion rates compared to their neutral counterpart. Conversely, concentration of negatively charged carriers partitions downward from C to K−C due to electrostatic repulsion resulting in slower intra-tissue diffusion rates. (ii) Optimally charged cationic carriers can penetrate through the full thickness of tissues owing to weak-reversible binding nature of charge interactions while carriers with too high positive charge get stuck within the tissue surface due to strong binding interactions. B. Incorporation of short-range effects such as from hydrophobic or hydrogen bonds can synergistically stabilize long-range charge-based binding of cationic carriers with their intra-tissue negatively charged binding sites. φ(x) denotes the electric potential exerted by fixed negatively charged groups as a function of distance, x inside the tissue.
Cationic peptide and protein-based drug carriers
Proteins have gained attention as safe natural biomolecules that can replace synthetic polymers applied in drug formulations [23]. Protein based drug delivery systems offer several advantages including biodegradation, biocompatibility and possibility of surface modification for targeted drug delivery [23]. Cell penetrating peptides (CPPs), which are commonly known as protein transduction domains, are short-length (5–30 amino acid) peptide sequences with superior cellular internalization capability [24]. CPPs use electrostatic interactions with negatively charged cell membranes for targeting cells and have been investigated for delivering small molecule drugs, peptides, proteins, DNA and plasmids, for treatment of cancer, asthma, ocular diseases, and neurodegenerative diseases [25,26]. While synthetic cationic polymer systems such as poly(ethylene-imine) (PEI) and dendrimers have shown intrinsic cytotoxicity in gene delivery applications [27], cationic peptide carriers generally demonstrate low cytotoxic effects on cells. Cytotoxicity due to high cationic charge can manifest as change in osmotic swelling pressures of tissues and cells potentially causing their disintegration, or by binding with endogenous proteins creating large aggregates causing cell membrane poration eventually leading to reduced cell mitosis and vacuolization of cytoplasm [28,238]. These effects are strongly dependent on the administered concentration [29]. For example, TAT peptide has shown to be relatively non-toxic at concentrations as high as 100 μM [30]. However, model amphipathic peptide (MAP) with KLALKLALKALKAALKLA-amide sequence has shown to be toxic at concentrations above 30 μM [31]. The amphipathic peptides have higher toxicity as they insert into the cellular membrane and form pores which results in collapse of the membrane and cell death [32]. Additionally, the distribution of positively charged amino acids across the helical wheel of peptides can also affect their biosafety. For example, peptides with concentrated charge distribution have shown higher lytic activity while peptides of same composition but disperse charge distribution can effectively transport into the cell without disrupting the cell membrane [33]. This can be achieved by using neutral amino acids as spacers in the sequence or by incorporating heterocyclic ring like imidazolium, guanidinium or pyridinium as a substitution for the linear amine head group in the vector design to help spread the positive charge of the headgroup [34]. PEGylation can also aid in shielding excess charge effects [35]. Other methods using reducible or acid labile linkers to enable easy cellular reduction and minimize toxicity have also been utilized and shown to be successful [36]. The undesirable ‘spreading’ of CPP-conjugated drugs from systemic delivery and the associated off-target effects can be circumvented by delivering them directly into tissues that contain the target cells [37]. Multi-level drug delivery systems are needed that can first penetrate through the full thickness of tissue and reach cell targets and then enable CPPs to interact with cells and deliver the conjugated cargo [38,39]. There is some work on using CPPs to promote absorption of macromolecules across tissues such as the intestine (insulin for diabetes treatment) [40], the skin (cyclosporin A for psoriasis) [41 ], the vitreous humor and sub-retinal space (TAT coupled dextrogyre peptide for ocular inflammation) [42], topical delivery of small interfering RNAs (siRNA) to lungs via intra-nasal, intratracheal or aerosol administration [43] and the nasal mucosa for nose-to-brain delivery [44]. While the reported CPPs have shown effective cell targeting, their net charge may be sub-optimal for tissue penetration. As discussed, depending on the tissue’s net FCD, the charge may be too high and cause excessive binding with intra-tissue negatively charged groups hindering diffusion (as shown in case of mucin) [45,46] or too little resulting in ineffective binding/retention and short intra-tissue residence time (as shown for lysine and arginine-rich peptides with net charge of +7 or +8 in cartilage) ultimately preventing effective cell targeting before drug clearance [14]. With rational design and dose control the benefits of using cationic peptides can outweigh the associated toxicity concerns, which is evident from an increasing number of cationic therapeutics that are entering the clinical trial stage and are high-lighted in this article.
Here we discuss selected negatively charged, avascular tissues, and delve into how each of their structures, tissue matrix compositions, and high negative FCDs create barriers to drug entry. We explore how charge interactions are being used to overcome these barriers using cationic peptide and protein-based drug carrier systems. We review work on tissue targeting charge-based peptide and protein drug delivery, compare and contrast drug delivery designs, and also present examples of technologies that are entering clinical trials.
Musculoskeletal tissues
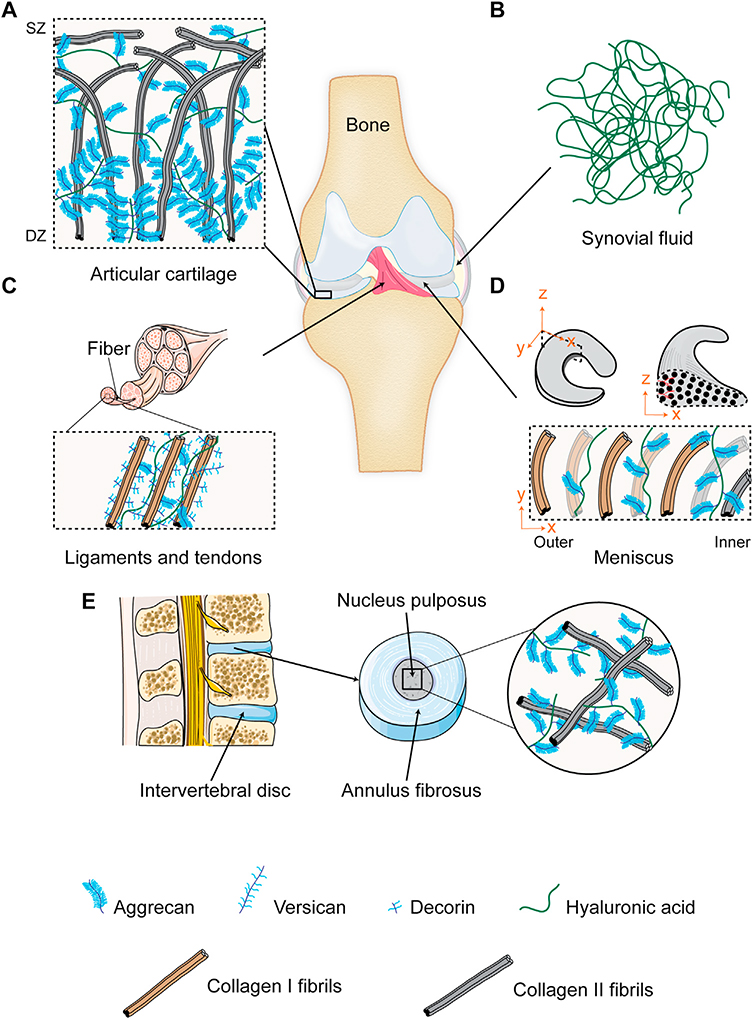
Musculoskeletal disorders impose an ongoing clinical challenge by affecting a wide variety of musculoskeletal tissue systems including articular cartilage, synovial joint and synovium, ligaments/tendons, meniscus and intervertebral discs (Fig. 3A–E).
Fig. 3.
Musculoskeletal joint tissues. A. Articular cartilage is comprised of a network of collagen II and a high density of aggrecans. Collagen fibrils are aligned parallel to the surface in the superficial zone (SZ) while arranged perpendicularly in the cartilage deep zone (DZ); aggrecan density increases from SZ to DZ. B. Synovial fluid is comprised of high molecular weight hyaluronic acid meshwork. C. Ligaments and tendons are comprised of linearly aligned collagen I fibrils containing decorin, aggrecans and versicans. D. Meniscus is comprised of circumferentially aligned collagen fibrils, mainly type I in the outer two-third region and a combination of types I and II in the inner region. Aggrecan density is highest in the inner region and decreases towards the outer region. E. Intervertebral disc is comprised ofgelatinous nucleus pulposus containing a random network of collagen II and proteoglycans.
Articular cartilage
Articular cartilage is a connective tissue which acts as a low friction gliding surface for articulation and distribution of mechanical loading across the joint [47]. Its avascular matrix comprises of a collagen II network (50–60 % tissue dry weight), filled with densely packed 300 MDa aggrecans (35 % dry weight) and a low density of chondrocytes (<5 % dry weight). Each aggrecan comprises of a central hyaluronan chain to which hundreds of 2–3 MDa aggrecan monomers are bound and contain negatively charged sulfated GAG chains (mainly chondroitin sulfate (80 %) followed by keratan sulfate (5–20 %)) [48,49] that are spaced only 2–4 nm apart along the monomer core protein. The density of these bottle brush structured aggrecans increases with depth into cartilage, reducing the effective pore size and restricting solute permeability and diffusion [13]. These negatively charged groups create a high FCD inside cartilage (−170 mM, Table 1) that provides the necessary hydration, swelling pressure and compressive stiffness, which are key to tissue function. The collagen II fibrils are aligned parallel to the surface in the articulating superficial zone, randomly oriented in the middle zone enabling load distribution and perpendicular to the subchondral bone in the deep zone (Fig. 3A). Degradation of articular cartilage can lead to a variety of joint degenerative disorders; OA is one of the most common forms affecting over 250 million people worldwide [50,51]. OA onset results in gradual loss of GAGs thus depleting the tissue’s FCD and compromising matrix integrity. While many potential disease modifying OA drugs (DMOADs) have been identified, none of them have passed clinical trials due to poor cartilage targeting and off-target side-effects [6]. Currently, direct IA injections to affected joints are the primary route for delivering pain and inflammation relievers, however, a majority of the drug is rapidly cleared from the joint space through the lymphatic system and capillaries (for example, mean half-lives of NSAIDs in synovial fluid (SF) are only 1–4 h) [6], therefore requiring multiple injections of high drug doses which cause systemic toxicity. This is further complicated by the significant steric hindrance presented by tissue matrix that prevents penetration of therapeutics. Since a majority of chondrocytes reside in the middle and deep zones of cartilage, drug delivery to chondrocytes remains challenging.
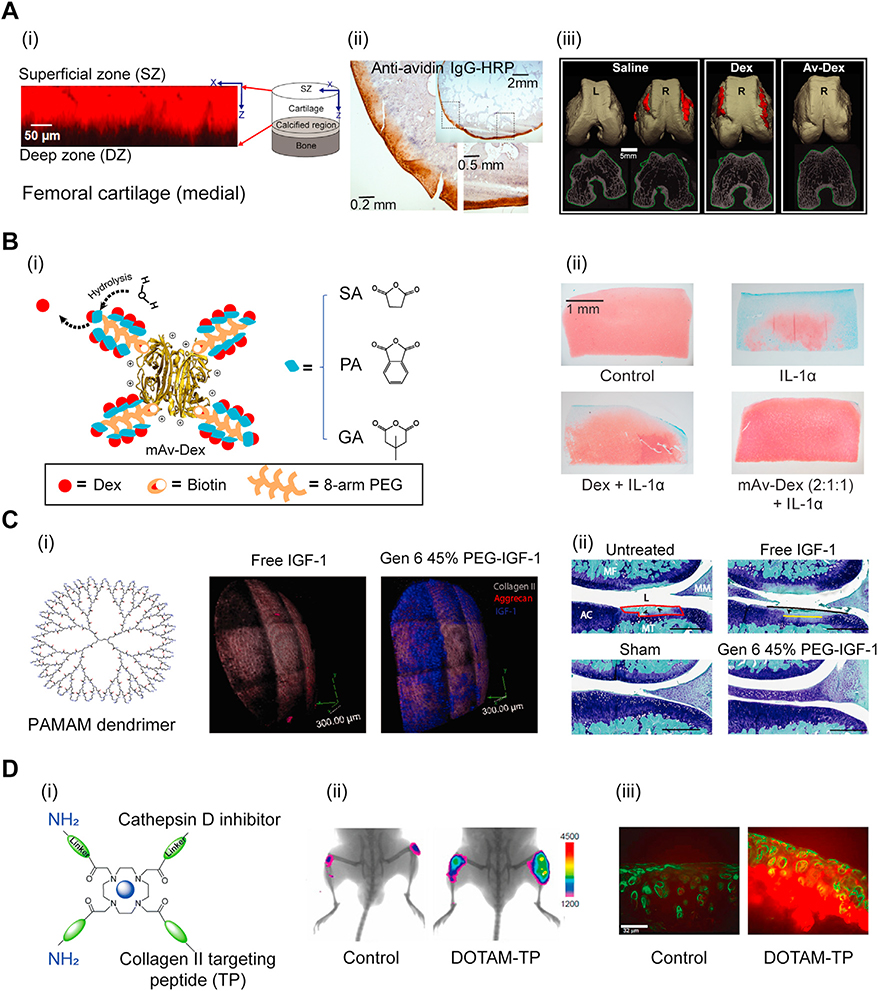
Bajpayee and Grodzinsky showed that the high negative FCD of cartilage can be used for converting cartilage from a barrier to drug transport into a drug depot by modifying drugs or their carriers with optimally charged cationic domains such that the weak-reversible nature of electrostatic interactions can enhance their intra-cartilage transport, uptake, and retention [13,15,35,52]. Using short length cationic peptide carrier motifs, Vedadghavami et al. recently showed that there exists an optimal net positive charge to deliver a drug of given size to a tissue of known FCD that will result in rapid penetration through the full thickness of cartilage before a majority of it is cleared from the joint space, and can enable highest intra-cartilage uptake and long-term retention [14]. Using cationic peptides of varying net charge, they showed that intra-cartilage uptake increased with increasing net charge of peptides to +14 but dropped as charge increased further due to stronger binding interactions that hindered peptide penetrability and uptake [14]. Optimal net positive charge on the carrier is chosen to enable weak and reversible binding with the intra-tissue negatively charged groups, which is criticai to allow for the drug and its carrier to penetrate through full tissue thickness and not get stuck in the tissue’s superficial zones. Despite weak binding, the high negative FCD of aggrecan associated GAGs inside cartilage greatly increases the residence time of optimally charged cationic drug carriers [13,14]. Similarly, the cationic glycoprotein, Avidin, due to its optimal net size (<10 nm hydrodynamic diameter) and charge (between +6 and +20) [15] was shown to penetrate through full thickness of rabbit cartilage following IA injection (Fig. 4A (i)) [53], resulting in a high intra-cartilage uptake ratio of 180 (implying 180x higher concentration of Avidin inside cartilage than surrounding fluid at equilibration) and was still found to be present through the full thickness of cartilage two weeks following its 1A administration in a rabbit anterior cruciate ligament transection (ACLT) model of post-traumatic OA (Fig. 4A (ii)) [16]. Avidin was covalently conjugated with 4 moles of Dexamethasone (Av-Dex) using its four biotin binding sites [54] and administered in a single low dose IA injection one week following ACLT in a rabbit model [16]. Av-Dex suppressed injury induced joint inflammation, synovitis, incidence of osteophyte formation and restored trabecular properties significantly better than free Dex (Fig. 4A(iii)) [16]. Recently, a multi-arm Avidin (mAv) nano-construct comprising of four 8-arm PEGs with high drug loading content (28 sites for covalent conjugation of drugs) was developed that can enable intra-cartilage delivery of a broad array of small molecule OA drugs to chondrocytes [55,237]. mAv was conjugated to Dex (mAv-Dex) using a combination of releasable ester linkers derived from succinic (SA), phthalic (PA) and dimethylglutaric anhydride (GA) in 2:1:1 molar ratio that provided a sustained Dex release over two weeks (Fig. 4B (i)). Consequently, mAv-Dex rescued IL-1 induced GAG loss significantly greater than free Dex (Fig. 4B (ii)).
Fig.4.
A. (i) Full-thickness penetration of positively charged Avidin in rabbit femoral cartilage within 24 h of IA injection. Adapted from [53]. (ii) IHC staining shows presence of Avidin in rabbit cartilage even at 3 weeks after ACLT surgery. (iii) μCT of femoral condyles show significantly suppressed osteophyte volume with Av-Dex compared to free Dex. Adapted from ref [16]. Printed with permission from AO Research Institute Davos. B. (i) Multi-arm Avidin nanoconstruct conjugated to Dex (mAv-Dex) using ester linkers. (ii) Safranin-O/fast green staining of cartilage explants shows mAv-Dex significantly suppressed IL-1 induced GAG loss compared to free Dex over 16 days. Adapted from ref [55]. Printed with permission from Elsevier. C. Cationic PAMAM dendrimer for IGF-1 delivery. (i) 3D reconstruction of multiphoton microscopy of rat cartilage. Gen 6 45 % PEG-IGF-1 PAMAM dendrimer shows superior intra-cartilage penetration and retention compared to free IGF-1 at 6 days post IA injection in rats. Aggrecans, collagen and IGF-1 are marked in red, grey and blue, respectively. (ii) Toluidine blue/fast green staining of rat cartilage show PEG-IGF-1 dendrimer suppressed GAG loss greaterthan free IGF-1 after 4 weeks of ACLT surgery. Adapted from ref [35]. Printed with permission from The American Association for the Advancement of Science. D. (i) DOTAM nanoparticles with collagen II targeting peptide (TP) and amines. (ii) Fluorescent images show increased DOTAM-TP retention in mice knees 48 h post IA injection compared to control probe. (iii) Histological analysis of mice cartilage show intra-cartilage localization and retention of DOTAM-TP. Adapted from ref [63]. Printed with permission from American Chemical Society.
Different classes of cationic carriers such as Poly-beta amino esters [56] and Poly(amidoamine) (PAMAM) dendrimers [35] were later developed for enhancing drug penetration and retention in cartilage. 6th generation PAMAM dendrimer was PEGylated at 45 % of its amine sites to minimize cytotoxicity (Gen 6 45 % PEG). Gen 6 45 % PEG conjugated to insulin like growth factor 1 (IGF-1) (Gen 6 45 % PEG-IGF-1) provided superior intra-cartilage penetration and retention using charge interactions between the remaining cationic amine groups and cartilage GAGs while free 1GF-1 had completely cleared out of the tissue within 6 days post IA injection in rats (Fig. 4C (i)). Gen 6 45 % PEG-IGF-1 reduced cartilage area of degradation from 24 % in the untreated group to only 8 % while free IGF-1 failed to significantly suppress cartilage loss compared to untreated ACLT group 4 weeks post-surgery (Fig. 4C (ii)). An electrostatic based self-assembled complex formed by sequentially mixing 1GF-1 with negatively charged polyglutamic acid and 5.8 kDa positively charged polyarginine, was shown to be retained in the joint space for 4 weeks after IA injection in a rat OA model [57]. Tokunou et al. developed a fusion protein consisting of IGF-1 and a cationic heparin binding (HB) peptide sequence allowing selective binding to cartilage GAGs and chondrocytes [58]. The HB-IGF-1 fusion protein showed cartilage targeting via specific binding with heparan sulfate GAGs and dominant non-specific electrostatic binding with chondroitin sulfate GAGs [59]. HB-IGF-1 was retained within rat articular cartilage for 8 days post IA injection while there was no sign of free IGF-1 present at two days after injection [60]. Additionally, HB-IGF-1 significantly reduced surface cartilage loss compared to unmodified 1GF-1 in meniscus transected rats upon weekly 1A injection for three weeks [60]. Inagawa et al. studied the effect of varying chain lengths of polyarginines on their transport in cartilage [61]. They found that the uptake increased within increasing number of arginine up to 8 (R8) after which intra-cartilage accumulation declined. Also, through enzymatic degradation of different types of cartilage GAGs, they determined that chondroitin sulfates were the main source of binding interaction with R8. Bajpayee and co-workers recently studied the effects of net positive charge, type of cationic residue (arginine or lysine) and hydrophobic moieties on cartilage penetration and retention of cationic peptide carriers of same size [14]. They noted that arginine-rich sequences had higher uptake and retention in cartilage compared to lysine-rich sequences due to stronger binding affinity of arginine to negatively charged GAGs. 1nterestingly, they observed that even after depleting 50 %−90 % of the total intra-cartilage GAGs, arginine-rich sequences still had high intra-cartilage uptake and retention while lysine-rich sequences did not, suggesting the presence of other interactions, likely H-bonds and hydrophobicity that stabilize charge-based binding within cartilage tissue. This can be attributed partly to the chemistry of the guanidinium head-group on arginine residues that form stable bidentate hydrogen bonds with polarizable oxo-anions such as the sulfates in aggrecan GAGs. Additionally, guanidinium cations can form thermodynamically stable (weakly) like-charge pairs in water, where a combination of dispersion and cavitation forces from the medium can overwhelm the coulombic repulsion. Because of this, arginine moieties are capable of binding more strongly (due to synergistic effects of charge, H-bond and hydrophobic interactions) and co-operatively (due to formation of like-charge pair between arginine) with negatively charged GAGs compared to lysine moieties, which instead exhibit mutual coulombic repulsion. They also showed that the addition of hydrophobic moiety to lysine-rich sequence significantly enhanced its intra-cartilage uptake and retention in both healthy and arthritic cartilage, further emphasizing the advantages of combining synergistic effects of short-range hydrophobic interactions and H-bonds in stabilizing intra-tissue charge-based binding of drugs or their carriers, which is especially relevant for targeting mid to late stage arthritic tissues that have lost some GAGs and thus have lower negative FCD [14].
Targeting collagen 11 network has also been explored for enhancing drug specificity and retention within articular cartilage [19,20]. Rothenfluh et al. discovered WYRGRL sequence with specific binding affinity to collagen 11 in cartilage, which was later conjugated to dexamethasone (Dex) through hydrolysable ester linkers [19,20]. This prodrug retained in cartilage deep zones for extended time periods and showed enhanced efficacy compared to free drug, a strategy that can be useful for targeting later stages of OA when the majority of GAGs have been lost [20]. Hu et al. developed 1,4,7,10-tetraacetic acid amide (DOTAM) nanoparticles with improved cartilage affinity by incorporating both WYRGRL for specific collagen 11 targeting (TP) and primary amines for charge-based binding to deliver Cathepsin D inhibitor (pepstatin A) in an acid-stimulated GAG depleted porcine cartilage culture model [62,63] (Fig. 4D (i)). DOTAM nanoparticles decorated with TP (DOTAM-TP) showed enhanced retention in mice knee joints 48 h post 1A injection compared to DOTAM conjugated with a nonbinding control peptide sequence (Fig. 4D (ii)). DOTAM-TP was also highly localized in cartilage 24 h post administration as shown in Fig. 4D (iii). DOTAM comprising of one collagen 11 binding peptide and two amine groups inhibited GAG loss by 41 % within 24 h while the DOTAM with only three collagen 11 binding peptides provided 34 % GAG loss suppression. By 48 h, when a significant extent of GAGs from cartilage was depleted, DOTAM with three collagen 11 binding domains was still effective in suppressing GAG loss by 18 %. The system provides flexibility to adjust the relative extent ofweak charge-based binding and strong specific binding with collagen 11 depending on the degree of GAG loss from cartilage for effective targeting.
Chondrocyte affinity has also been used for targeted drug delivery to cartilage [64,65]. Chondrocyte affinity peptide (CAP) with sequence DWRV11PPRPSA identified using phage display effectively delivered Hif-2a siRNA to chondrocytes when conjugated to PE1 [66]. CAP when conjugated to PEG-PAMAM enhanced penetration into cartilage, joint retention and chondrocyte uptake [67]. Also, VLTTGLPAL1SW1RRRHRRHC (p5RHH), a positively charged peptide derivative of melittin [68] was used to suppress NF-kB pathway using siRNA delivery to chondrocytes in a mechanically induced mouse injury model [69]. Additionally, it was noted that p5RHH-siRNA complexes could penetrate up to 700 μm of cartilage and suppress 1nterleukin 1 beta (1L-1β) activated p65 in cytokine induced human cartilage culture for three weeks with only a single dose treatment [70]. Fusion protein consisting of cationic carrier PEP-1 and Glutaredoxin-1 (GRX-1), an anti-oxidant enzyme in homeostasis, was also shown to significantly suppress inflammatory markers compared to free GRX-1 [71].
Synovial joint and synovium
Since the synovialjoint is subjected to rapid clearance, major research efforts have focused on developing large, non-penetrating, non-binding drug carriers that remain suspended in SF following IA injections and due to their large size, do not exit via the synovium lymphatics or vasculature. These carriers are most relevant when the target sites of the drug are mainly in the SF or synovial membrane, such as with drugs used for relieving pain and inflammation [72]. They are, however, not effective at stimulating disease modifying response in chondrocytes. Examples of such drug delivery systems include drug encapsulating microparticles [73], polymeric micelles [74], liposomes [75], aggregating hydrogels and peptides [76]. The HA in SF is not sulfated but its carboxylic acid groups impart some negative charge (Fig. 3B), which has also been used for enhancing retention of drugs inside the joint by using positively charged chitosan [77,78] orby making nanoparticles cationic [79] so they can ionically crosslink with HA. The negative charge in SF can also hinder transport of cationic carriers into cartilage by competitive binding [80,81], however, the negative FCD of cartilage is significantly higher due to the presence of sulfated GAGs providing greater affinity for charged carriers (20 fold lower FCD, −8.8 mM compared to −170.0 mM, Table 1). Bajpayee et al. showed that Avidin, owing to its optimal net charge, rapidly diffused through the full thickness of rat and rabbit cartilage following IA injection resulting in high uptake and long-term retention despite the presence of SF and dynamic compression induced convective flow in the knee joints [53,82]. Recently, it was reported that the uptake of supercharged cationic green fluorescent proteins (S-GFP) from SF into cartilage reduced by 20–50 % than that from PBS depending on the net charge of S-GFP, yet high intra-cartilage mean uptakes of 5–20 were reported [52].
Strategies to target synovium have also been explored because of its pivotal role in joint inflammation and pain [72]. Mi et al. developed multiple cationic peptide sequences and studied their efficacy in transducing synovial fibroblasts [83]. The net positive charge of peptide was determined as an important factor in their internalization capability. For example, short length peptides with sequences PIRRRKKLRRLK (+8 charge) and RRQRRTSKLMKR (+7 charge) had superior transduction capability compared to KRIIQRILSRNS (+4 charge). However, both peptides showed similar performance compared to their 12-mer polylysine or polyarginine counterparts. Therefore, it was concluded that although cationic charge is crucial, beyond a certain charge limit, factors like peptide conformation also influence peptide transduction efficacy. The same group later identified a specific synovium fibroblast binding sequence, SFHQFARATLAS (HAP-1) with the goal of tar- geted delivery of apoptotic agents to hyperplastic synovium [84]. HAP-1-liposome systems were also used to deliver an immunosuppressive peptide to inflamed joints [85]. Furthermore, HAP-1 liposomes conjugated with NF-KB-blocking peptide were shown to significantly suppress synovial inflammation [86]. Additionally, the unique cell markers present in newly formed blood vessels associated with angiogenesis in inflamed synovium have been used for targeting [87,88]. For example, peptide sequence CKSTHDRLC with binding affinity to synovial vascular endothelium was fused to anti-inflammatory cytokine interleukin 4 (IL-4) that effectively accumulated in the synovium providing enhanced retention and bioactivity [89].
Ligaments, tendons and meniscus
Ligaments and tendons have similar tissue structure, predominately comprising of type I collagen (about 75 % of dry weight) organized linearly to provide load bearing tensile strength [90] (Fig. 3C). Leucine-rich PG, decorin, helps tie adjacent collagen fibrils together, while aggrecans and versicans provide tissue viscoelasticity allowing fibrils to slide smoothly over each other [91] (Fig. 3C). Ligament and tendon injuries constitute 50 % of the total 33 million musculoskeletal injuries reported annually in the United States [92]. These tissues lack self-healing capability and surgical intervention is often required post injury. Meniscus is a fibrocartilaginous tissue located between tibia and femoral condyle, which is responsible for shock absorbance and force transmission [93] (Fig. 3D). Meniscus damage usually occurs as a result of traumatic injury or degenerative diseases. Meniscus dry weight is mainly comprised of collagen (75 %) followed by aggrecan GAGs (17 %) [8]. Its collagen network is predominantly comprised of collagen I (90 %) oriented circumferentially while collagen II fibrils are only found in the inner one-third of meniscus (Fig. 3D) [8]. Aggrecans are the major type of PGs present mostly in the inner two-third of the tissue [8]. Tears in meniscus, especially in the inner two-third avascular zone suffer from poor self-healing capability [94]. Historically, meniscus tissue was considered to be a functionless vestigial structure, which would be excised (or completely removed) after damage [93]. However, the importance of meniscus as a joint stabilizer and shock absorber is now well recognized and its preservation is essential to maintain normal knee biomechanics. Various suturing techniques for repairing torn ligaments [95] and menisci [96], substitution using autologous tissues [97] or allografts [98], biologically inert or cell seeded biomaterials [99,100] and 3D printed tissue engineering strategies [101,102] are being explored.
In case of partial tears, the presence of negatively charged GAGs in these tissues can be utilized for delivering anti-catabolic drugs and growth factors via IA injected charged carriers (average FCD of −51.5 mM for meniscus, −6.6 mM for tendon and −17.9 mM for ligament, Table 1). Distribution of positively charged Avidin post IA injection in rat knee joints revealed that both uptake and retention of Avidin in joint tissues correlated strongly with tissue GAG content [82], which decreased in the order of cartilage, meniscus, ligament and patellar tendon. Charge interactions can be coupled with motifs with binding affinity to specific matrix constituents within the tissue to further enhance tissue specificity.
Intervertebral disc (IVD)
The IVD is avascular and functions as a ligament holding the vertebrae together, a shock absorber mitigating stresses on the body, and a pivot point allowing for spine flexibility. The IVD is composed of the NP, the gelatinous center region of the IVD, the AF, the tensile connective tissue that restricts the NP using circumferential (hoop) stresses, and the cartilaginous endplate which connects the NP and AF to the vertebra. The NP is composed of negatively charged PG gel held together loosely by a sparse network of type II collagen fibrils [103] (Fig. 3E). Degeneration of the IVD, particularly in the NP is often characterized by inflammation and a loss of GAGs resulting in decreased disc height and function. This leads to lower back pain, which is one of the leading causes of disability worldwide [104]. For IVD regeneration, a wide range of therapeutics including anti-catabolic, pro-anabolic factors and chemo-attractants that can stimulate the resident cells and recruit endogenous progenitors are under consideration [105–107]. The avascular nature of this tissue makes it challenging for systemically administered drugs to reach their target cells inside the NP.Therefore, local intra-discal injection to deliver drugs directly into the NP is a suitable approach especially since the tissue is well encapsulated by endplates and AF. Drugs injected in this manner, however, suffer from rapid and wide diffusion outside the injection site resulting in short lived benefits while causing systemic toxicity [105,106]. Toxicity concerns are further aggravated when potent biologics, gene editing/silencing vectors, or proteolytic enzymes are used. Current in vitro and in vivo work relies on frequent drug dosing, which is clinically infeasible. It is, therefore, criticai to develop effective approaches for enhancing intra-NP drug residence time and celi targeting.
Drug delivery systems under investigation include both nano- and micron-sized polymeric particles such as injectable nanoparticles [108], microspheres [9], hydrogels [109], and tissue engineering scaffolds [110] but have shown short intradiscal residence time of few hours requiring the need for multiple injections. Other systems consist of synthetic biodegradable polymers like poly(lactide) (PLA), poly(glycolide) (PGA), poly(ε-caprolactone) (PCL) for their tunable properties and minimal immunogenicity [111]. The high negative FCD of the NP (Average FCD of −138.2 mM, Table 1) also presents an opportunity for enhancing the residence time of cationic drug depots enabling sustained therapeutic benefits [13]. Recently, chitosan functionalized Pluronic-based nanoparticles were used for intra-discal delivery of chymopapain; electrostatic binding within the NP kept the delivery of encapsulated enzyme localized to the injection site at least over 24 h [108]. Wagner et al. showed that cationic Avidin grafted dextran can enable a month long intra-discal residence time [112].
Clinical translation and future direction
Musculoskeletal tissues remain an outstanding challenge in the field of drug delivery due to their avascularity and complex architecture comprising of densely packed negatively charged groups (Table 1). There is a critical need for targeted drug delivery systems to pave way for treating burdensome diseases like OA. Recently, intra-joint sustained release formulations of triamcinolone encapsulated within micron sized poly (lactic-co-glycolic acid) (PLGA) particles (Flexion Therapeutics, Burlington, MA, USA) were approved by the FDA for OA pain and inflammation [113,114], but such systems fail to target cells inside cartilage and to elicit long-term disease modifying effects to restore joint function. Use of optimally charged cationic protein and peptide carriers is a promising direction that can enable safe, effective delivery of drugs to cell and matrix targets residing deep within tissues, thereby eliciting disease modifying effects as well as facilitating long-term symptomatic pain and inflammation relief. Significant success has been demonstrated using pre-clinical models in recent years and these approaches can now be considered for moving to clinical trial stage to translate newly discovered drugs or to repurpose those that previously failed OA trials due to lack of cartilage targeting and associated systemic toxicity. Finally, while the vast amount of data in this field has been generated using cartilage, more work is foreseen in using these approaches for other tissues like meniscus, ligaments, tendons and IVD.
Eye
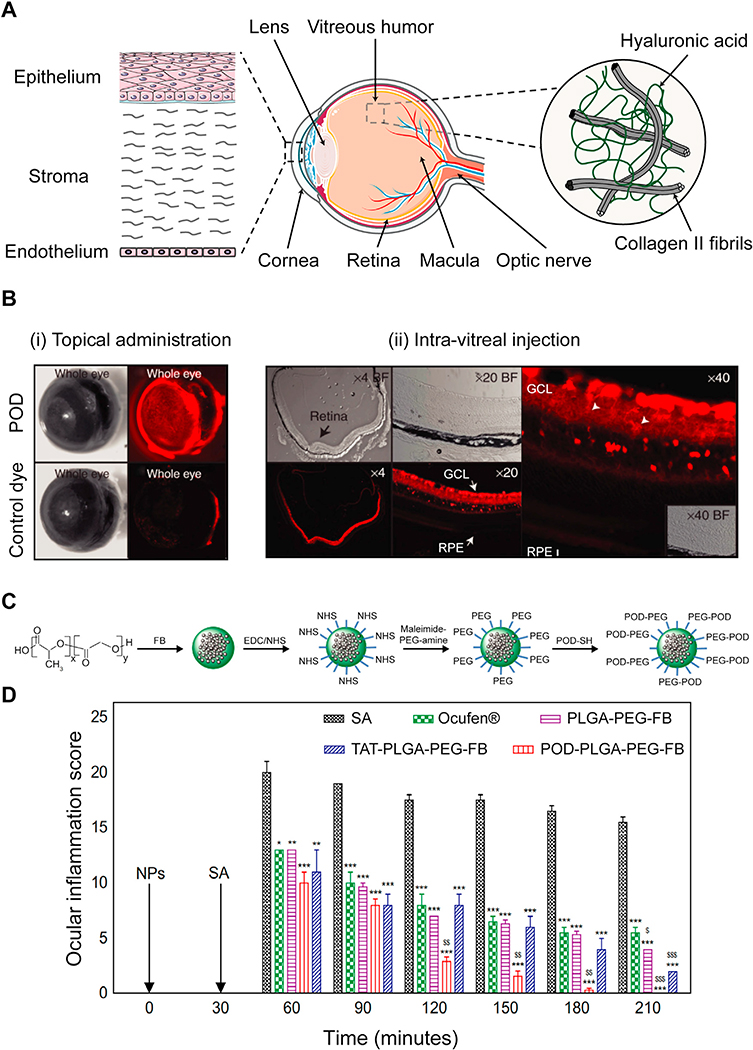
Over 250 million people are affected by vision impairment worldwide, mostly prevalent among population over 50 years old [115]. The anterior part ofthe eye is composed of cornea, pupil and the lens, which function to collect and focus the light while the posterior segment is comprised of the retina, vitreous, macula and optical nerve which together function to detect light [116] (Fig. 5A). Dry eye, glaucoma and cataract are common diseases affecting the anterior tissues. In contrast, the posterior chamber is threatened by age-related macular degeneration (AMD), retinitis pigmentosa and diabetic retinopathy [117]. If left untreated, most of these diseases can ultimately lead to vision loss and blindness. Despite the need for treatment of ocular diseases, there is currently no cure available for AMD [118]. Ocular drug delivery remains challenging due to the presence of several physiological and anatomical barriers that are normally responsible for protecting the eye against foreign materials [119].
Fig. 5.
A. Structure of ocular tissue in the anterior (cornea and the lens) and posterior (vitreous humor, retina and macula) regions. Barriers to local drug delivery include negatively charged corneal epithelium layer and vitreous humor comprising of collagen II and hyaluronic acid that prevent diffusion of drugs and their carriers to the posterior regions. B. Transport of POD in ocular tissues upon (i) topical administration and (ii) intra-vitreal injection. (i) POD was uptaken by external ocular tissues within 45 minutes upon topical administration in mice eyes while the control free dye only weakly stained the eye. (ii) Intra-vitreal injection of POD transduced 85 % of neural retina within 2 h. RPE and GCL represent retinal pigment epithelium and ganglion cell layer, respectively. Adapted from ref [137]. Printed with permission from Elsevier. C. Synthesis of POD-PLGA-PEG-FB nanoparticles. D. Effectiveness of POD-PLGA-PEG-FB in prevention of sodium arachidonate (SA) induced ocular inflammation in rabbit eyes (*p < 0.05, **p < 0.01, and ***p < 0.001 vs inflammation induced by SA.$p< 0.05, $ $p< 0.01, and $ $ $p < 0.001 vs anti-inflammatory effect of Ocufen®). Adapted from ref [140]. Printed with permission under Creative Commons Attribution License.
95 % of the total drug administered topically is lost through nasolacrimal drainage, tear turnover and conjunctival blood circulation [119]. Consequently, corneal penetration of the remaining dose of drug is impaired by the epithelium [120]. The vitreous humor accounts for 80 % of eye volume, stretching from lens to retina, also acts as a major barrier to diffusion of drug delivery systems. It is predominanti comprised of hydrophilic negatively charged 2–4 MDa HA chains (Fig. 5A) and to a lesser degree chondroitin and heparin sulfate (average FCD of −0.6 mM, Table 1). While cationic carriers have been shown to effectively penetrate through the negatively charged pores of the corneal epithelium, transport of positively charged carriers could be hindered in the vitreous as they become immobilized through charge interactions [121,122]. Käsdorf et al. studied the effects of net positive charge on diffusion of cationic drug carriers in the vitreous humor using oligopeptides of same size but varying charge (between +7 and +23) and showed that there exists a threshold of positive charge on a carrier for effective diffusion through the vitreous humor (up to +15) [46]. Further increase in net charge to +19 and +23 resulted in significant sup-pression and complete blocking of transport of cationic peptides in the vitreous humor. Various types of cationic carriers including naturally derived peptides such as penetratin [123,124], TAT [125,126] and PEP-1 [127,128] as well as synthetic peptides like lysine [129] or arginine-rich [130] sequences have been used for enhancing drug bioavailability and ocular penetration of drugs or their carriers. For example. penetratin, TAT and polyarginine (R8) have shown 87.5, 31.8 and 16.3 times higher corneal permeation respectively compared to negatively charged control, poly(serine)8 in vitro [131]. Additionally, in vivo studies showed that topically administered penetratin rapidly diffused to the posterior section of the eye within 10 min and was retained for more than 6 h while the negatively charged polyserine peptide completely cleared out of the eyeball within 2 h. The superior performance of penetratin compared to other cationic peptides was mainly attributed to its amphipathic nature. Similar results were observed byJiang et al., who showed that penetratin’s permeability through rabbit cornea increased significantly when hydrophilic residues in its sequence were replaced with hydrophobic tryptophan [123]. Penetratin-PAMAM systems for DNA plasmid delivery were later developed that retained for more than 8 h in rat retina following topical administration and reached the posterior segment of the eye through the sclera [124]. Tai et al. developed penetratin-PAMAM antisense oligonucleotide (ASO) for ocular tumor gene silencing for topical use that reduced expression of target gene by 2-fold compared to PAMAM-ASO with no penetratin [132]. Following this, topical application of penetratin functionalized nanoparticles were shown to effectively deliver siRNA and suppress target protein expression in retinoblastoma mouse models [133].
TAT is another naturally derived peptide used for enhancing ocular penetration of nanoparticles. Zhang et al. [134] formed anti-angiogenesis delivery systems through a fusion protein system comprising of TAT and endostatin that could target retina and elicit anti-angiogenesis response. Similarly, TAT has been coupled with human acidic fibroblast growth factor (TAT-aFGF) and with inhibitory peptide sequence against rat mitochondrial μ-calpain (responsible for photoreceptor cell death) and shown to rapidly target retina and elicit therapeutic response for at least 6–8 h when topically administered in rats [135,136].
The Kumar-Singh group designed a peptide for ocular delivery (POD), GGG(ARKKAAKA)4, which is based on the heparin binding sequence (XBBBXXBX)n as the concentration of heparin in retina increases with disease progression [137,138]. POD showed high uptake in human embryonic retinal cells, strongly bound with cornea and sclera within 45 minutes of topical administration (Fig. 5B (i)) and transduced 85 % of neural retina within 2 h post intra-vitreal injection (Fig. 5B (ii)) in mice [137]. Subretinal injection of PEGylated POD nanoparticles for DNA delivery were shown to increase gene expression by 215 times compared to native DNA. An updated version of PEG-POD with a reducible disulfide linkage (PEG-SS-POD) conjugated with vascular endothelial growth factor (VEGF) receptor 1 transgene was later developed which allowed dissociation of PEG in the tissue matrix for enhanced intra-cellular uptake of the POD modified nanoparticle [139]. Subretinal injection of this reducible nanoparticle showed 50 % reduction of neovascular growth in a choroidal neovascularization (CNV) mouse model compared to the control group. Vasconcelos et al. modified PLGA-PEG nanoparticles with POD for corneal delivery of flurbiprofen (FB) with the goal of suppressing inflammatory response in surgical intervention of cataract [140]. Briefly, FB encapsulated PLGA particles were conjugated to bifunctional Maleimide-PEG-amine through EDC/NHS chemistry. Then POD-sulfhydryl (POD-SH) groups were incorporated on PLGA-PEG particles through reaction with Maleimide groups to form POD modified PLGA-PEG-FB nanoparticles (POD-PLGA-PEG-FB) (Fig. 5C). POD-PLGA-PEG-FB formulation significantly prevented inflammation compared to commercial eye drops (Ocufen® ), PLGA-PEG-FB and TAT-PLGA-PEG-FB (Fig. 5D) in a sodium arachidonate (SA) inflammation induced rabbit model due to its higher retention and uptake in corneal epithelium. Furthermore, de Cogan et al. examined ocular penetration of synthetic arginine-rich peptide with sequence RRRRRR forming complexes with anti-VEGF drugs [141]. Upon topical administration, the complexes diffused to anterior segment of rat eyes within 6 min, which were as efficient as the intra-vitreal injection of the anti-VEGF agent in CNV mouse models.
Clinical translation and future direction
As discussed, a significant body of research exists in using charge-based peptides for enhancing drug targeting to cornea, the vitreous humor and retina of eye thereby improving their residence times and therapeutic efficacy even via the topical route. XG-102 or Brimapitide, (Xigen, Epalinges, Switzerland), a TAT coupled dextrogyre peptide that selectively inhibits the c-Jun N-terminal kinase previously shown to effectively suppress ocular inflammation in rat uveitis model [42] became the first peptide to be subconjunctivally administered clinically to bypass the epithelium for treating post-operative ocular inflammation [142]. In a phase II randomized, double blind, controlled, multi-center trial of 145 patients in Europe, a single subconjunctival injection of Brimapitide immediately following an ocular surgery was shown to be non-inferior to Dex eye drops given 4 times daily for 21 days [143]. An outstanding challenge, however, is reaching the target sites in the posterior region of the eye, including macula at the far end of the back of the eye, which is the site for a number of diseases such as AMD, retinitis pigmentosa and diabetic retinopathy. Currently intra-vitreal injections are required to target the macula since topical delivery of drugs/drug carriers suffer from low bioavailability and getting stuck in the vitreous humor before reaching the macula, likely due to strong electrostatic binding within the vitreous. It should be possible to design optimally charged drug carriers with weak and reversible binding properties as discussed above in the example of musculoskeletal tissues. These carriers would have net charge that is weak enough so as not to hinder their diffusion through the vitreous yet strong enough to have long residence times and not exit quickly through lymphatics.
Mucosal membrane of the GI tract
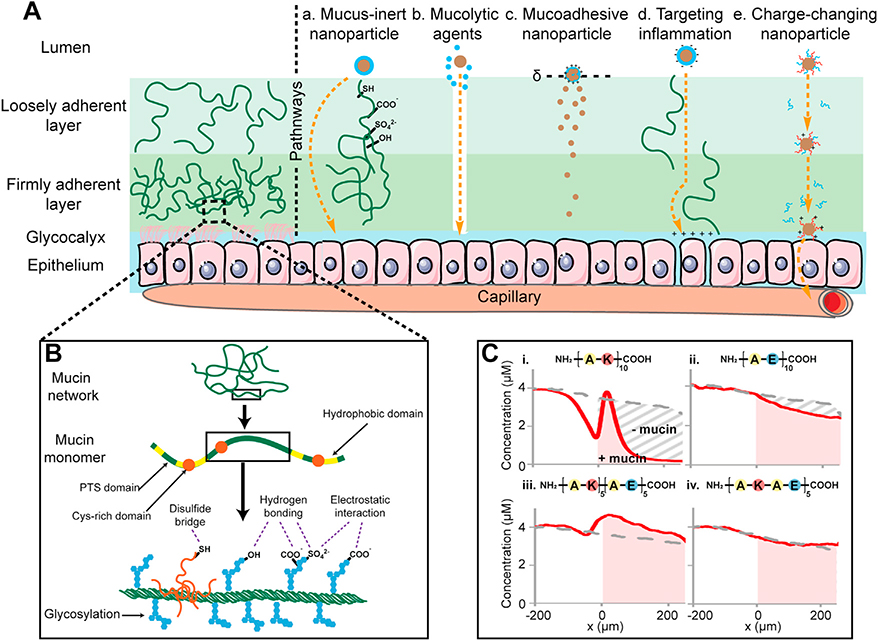
Oral drug delivery is the most desired method for drug administration due to its convenient, non-invasive and patient-friendly merits. However, oral delivery of biologics, proteins, peptides and other macromolecular drugs is limited due to their lack of stability from enzymatic degradation in the acidic environment of Gl tract and their inability to transport across the mucus-epithelial barrier [144]. Mucus is a viscoelastic and dynamic gel layer consisting primarily of 90–98 % water, 2–5 % (w/v) crosslinked and entangled mucin fibers, and small amounts of lipids and proteins [144]. Mucins are high molecular weight glycoproteins consisting of two domains: PTS (proline, threonine, serine) domain and a naked hydrophobic region [145]. As Fig. 6A and B show, the heavily glycosylated (e.g. sialic acid and fucose) repeats in the PTS domain make mucin highly negatively charged (typically 20–30 carbohydrates per 100 amino acids) contributing to the majority of non-specific hydrogen bonding and electrostatic interactions (Average FCD of −4.4 mM, Table 1) [145–147]. The non-glycosylated (naked) protein domains are hydrophobic and rich in cysteine containing disulfide bonds that enable crosslinking of monomers to form mucin gel. The mucin gel network provides a 500–700 μm protective barrier with a heterogeneous mesh pore size ranging from 50–1800 nm depending on its location along the Gl tract and the state of disease, providing steric hindrance to solute diffusive transport [148,149]. The thickness of mucus layer in the intestinal lumen of adolescents with IBD was found to be about three times thinner than the healthy mucin layer [150]. Typically, Gl mucus consists of an outer loosely adherent luminal layer and a firmly adherent layer (Fig. 6A) that attaches to a dense array of negatively charged glycoproteins, termed glycocalyx that are present on the apical surface of the tightly connected epithelial cells, which form the next barrier layer after the mucin gel network [145].
Fig. 6.
A. Characteristics of the mucus barrier coating epithelium and strategies used for promoting mucus penetration (a. Mucus-inert nanoparticle b. Mucolytic agents c. Mucoadhesive nanoparticle d. Targeting inflammation e. Charge-changing nanoparticle). B. Architecture of the mucus network and structure of the heavily glycosylated mucin monomer. C. Charge-based transport behavior of short length peptides of varying net charge and spatial distribution in mucin gel, i. Cationic peptide, ii. Anionic peptide, iii. Block peptide and iv. Alternate peptide. Adapted from ref [45]. Printed with permission from Elsevier.
Electro-diffusive transport of solutes across mucosal barrier
Transport rates of solutes across the porcine Gl mucus have been shown to significantly decrease with increasing particle size from 20 to 500 nm [151]. In addition, mucus permeability highly depends on charge-based binding interactions between the solute of given net charge and the negatively charged mucus. Positively charged nanoparticles are immobilized within the mucus due to electrostatic interaction but negatively charged or neutral nanoparticles can penetrate through mucus easily. Crater et al. demonstrated that high degree of interaction between amine-modified particles and negatively charged mucin fibers significantly reduced particle transport rates whereas carboxylate-modified particles showed five-fold higher transport rates [152]. A recent study, however, showed that the penetration ability depends on binding strengths of solutes with mucus constituents; cationic dextrans that weakly adhered to mucin gels with low to intermediate binding affinities were shown to penetrate through the native mucus at equally high efficiencies as uncharged, non-mucoadhesive molecules [153]. Li et al. demonstrated that the transport behavior through the mucosal barrier cannot be predicted by solute net charge alone and that its spatial distribution is also a critical parameter that modulates its transport [45]. A zwitterionic block peptide (AKAKAKAKAKAEAEAEAEAE) containing cationic and anionic segments arranged in separate blocks exhibited a discernable concentration peak at the entry of mucin barrier due to Donnan’s effect resulting in a high transport rate, which was greater than that in the mucin-free (buffer control) group. ln contrast, a peptide with similar amino acids as the block peptide but arranged in an alternating sequence, as opposed to separate blocks (AKAEAKAEAKAEAKAEAKAE) showed a monotonically descending concentration profile at the entry of mucin barrier (Fig. 6C) [45]. Additionally, unlike the block peptide, the alternate peptide had similar transport rates in mucin and buffer control groups [45]. These differences between transport profiles of the block and alternate peptides show that the mucin barrier can distinguish the spatial configuration of charged residue on a nanometer length scale [45]. The study also noted that the transport rate was higher for zwitterionic peptides (block or alternating) compared to purely anionic or cationic peptides. This is important as viruses and native proteins also contain combinations of positive and negative surface charges that may modulate their transport across the mucosal barrier. While the positive charge on the particle can lead to enhanced transport rate owing to Donnan partitioning, it can also cause binding to mucins, which can slow down solute diffusive transport through the mucin layer. Therefore, both these mechanisms may interact in complex ways to produce the selective permeability effect observed with cationic, anionic, block and alternating peptides in this study. These concepts can be used for multi-functionalizing drug and gene carriers with positive and negative charges to modulate their transport and targeting in the mucus barrier [45,154].
Mucoadhesive nanoparticles
Since the hydrophobic regions in mucin can trap hydrophobic particles, use of hydrophilic ‘mucus-inert’ coating has been proven to be a successful strategy to minimize particle entrapment within the mucus layer [155]. For example, PEGylation of tobramycin [156] and lipid polymer nanoparticles [157] shield their positive charge and hydrophobicity respectively, thereby enhancing their permeability across mucus. Multiple studies have suggested using mucolytic agents to break disulfide bridges and cleave the mucin glycoprotein cross-linked network as a strategy to overcome the mucus barrier, but this disruption is not immediately reversible raising serious concerns [158,159]. A few reports have reported the issue of back-diffusion following elimination of particles in the loosely adherent mucus layer due to its rapid turnover and clearance by peristaltic forces, which results in a drug concentration gradient in the opposite direction of penetration [158]. Therefore, contrary to ‘mucus-inert’ particles, mucoadhesive strategy has also been utilized by using positively charged and thiolated chitosan-modified particles [21,160] or by co-administering drugs with polyarginine [161].Jain et al. designed cationic ‘layersomes’, containing alternate layers of anionic poly (acrylic acid) and cationic poly-amine hydrochloride synthesized using layer by layer method, which increased oral bioavailability of encapsulated doxorubicin by 5.9 folds compared to free drug significantly inhibiting tumor growth [162]. Mucoadhesive nanoparticles, however, have the tendency to disrupt mucosal barriers, which can increase the risk of exposure to bacteria and foreign particles [163].
Researchers have also developed approaches for localized targeting of mucus at the site of inflammation [164–167]. Zhang et al. developed anionic self-assembling hydrogels comprising of amphiphilic ascorbyl palmitate (AP) capable of cleaving under inflammatory environment for local targeting of the inflamed intestine to treat IBD [164]. They noted that the negative charge of the hydrogel allowed selective binding to the inflamed colon epithelium which had a lower FCD due to loss of mucin layer and was retained up to 12 h post a single enema in a colitis mouse model. The hydrogel was used to deliver Dex which provided higher therapeutic efficacy than the free drug.
Epithelium targeting mucus penetrating nanoparticles
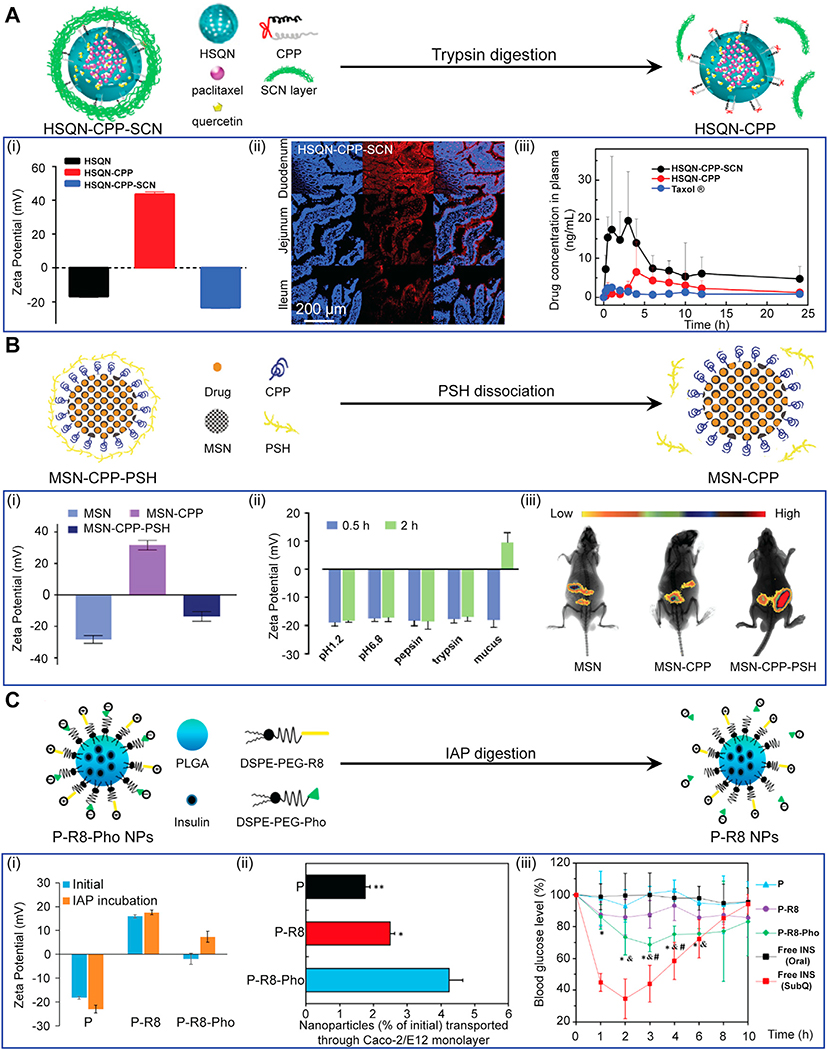
While negatively charged or net neutral nanoparticles could rapidly penetrate through the mucus with minimal binding interactions and back-diffusion, their uptake by epithelial cells is expected to be much lower compared to positively charged particles. For example, insulin when conjugated with cationic CPP resulted in 6–8 times higher intestinal absorption compared to normal insulin in Caco-2 cell monolayer [40]. Although the mechanism of CPP mediated cell uptake remains unclear, one of the well-accepted hypotheses is that the initial contact and binding between the negatively charged cell membrane and positively charged CPPs triggers both endocytosis and micropinocytosis pathways [168]. PEGylation can help shield some cationic charge to minimize binding with mucus while using the residual charge for enhancing targeting and uptake in the epithelial cells. For example, CPP functionalized PEG-modified mesostructured silica nanoparticles were shown to penetrate through the mucus, enhancing cellular uptake of encapsulated recombinant growth hormone (RGH) by 4.9 fold compared to free RGH in vivo [169]. Recently, a novel nano-delivery system capable of changing its zeta potential from neutral or negative charge to positive charge while penetrating through the mucus barrier was developed to overcome the issue of binding with mucus while enabling epithelial cell targeting and uptake [158,170,171]. Wang et al. modified quantum dots doped hollow mesoporous silica nanocarriers (HSQN) with cationic CPPs and concealed these peptides using a hydrophilic succinylated casein (SCN) layer (Fig. 7A) [172]. SCN layer digested by trypsin enabled zeta potential of nanoparticles to markedly change from around −20 mV to +40 mV. In vivo oral administration of this dual-modified charge-changing nanoparticle HSQN@CPP@SCN encapsulating paclitaxel and quercetin revealed that SCN coating enabled nanocarriers to easily penetrate through the rat mucus; presence of trypsin in gastric juices degraded SCN exposing CPPs enabling 8-fold higher plasma concentration of encapsulated paclitaxel compared to oral delivery of free drug, Taxol ®. A similar approach was used by coating dissociable thiolated polymer (PSH) on CPP functionalized mesoporous silica nanoparticles (MSN) [173]. PSH worked as a mucoadhesive agent and gradually dissociated from nanoparticles as it penetrated through the mucus leaving a strong positive surface charge (+31.9 mV) on nanoparticles [173]. Ex vivo imaging of whole-body distribution exhibited higher concentration of MSN-CPP-PSH nanoparticles than that of MSN or MSN-CPP nanoparticles remaining in isolated intestines (Fig. 7B). As such, MSN coated particles resulted in 6.8-fold increase of relative bioavailability compared to unfunctionalized silica particles. Huang and colleagues utilized the charge-reversal strategy by adding negatively charged phosphoserine (Pho) to their octa-arginine (R8) functionalized PLGA nanoparticles (P-R8-Pho), imparting virus like neutral charged surface (−2.39 mV) which rapidly penetrated through the mucus (Fig. 7C) [174]. The surface anchored anionic Pho was hydrolyzed by intestinal alkaline phosphatase (IAP), which reversed its zeta potential to positive (+7.37 mV), exposing cationic R8 peptide that enabled effective CPP-mediated cellular uptake and transepithelial transport in a Caco-2/E12 coculture epithelium model [174]. P-R8-Pho also effectively induced a maximal reduction in blood glucose by 32 % in fasted diabetic rats, while oral administration of insulin showed no hypoglycemic effect.
Fig. 7.
Charge-reversal nanoparticle systems developed for both effective mucus penetration and GI epithelium targeting. A. Quantum dots doped hollow silica nanoparticles(HSQN) modified by CPPs with a cleavable outermost shell of hydrophilic succinylated casein (SCN). (i) Zeta potential, (ii) Confocal laser scanning microscopy images of rat intestine 2 h post oral administration (blue showing cell nuclei and red showing presence of nanoparticles). (iii) Drug levels in plasma after oral administration of HSQN-CPP-SCN. Adapted from ref [172]. Printed with permission from Elsevier. B. Mesoporous silica nanoparticles (MSN) with an interlayer of CPP and an outermost shell of thiolated polymer (PSH). (i) Zeta potential, (ii) Enzymatic and pH stability of MSN-CPP-PSH showing specific degradation of PSH shell in presence of mucus that reverses its net charge. (iii) In vivo bio-distribution of MSN-CPP-PSH after oral administration in mice. Adapted from ref [173]. Printed with permission from Elsevier. C. PLGA nanoparticles coated with R8 and anionic phosphoserine (Pho), which dissociates upon digestion with intestinal alkaline phosphatase (IAP) leading to a net positive surface charge. (i) Zeta potential, (ii) Enhanced in-vitro transepithelial transport of P-R8-Pho (iii) Suppression of blood glucose levels after oral administration of insulin-loaded P-R8-Phonanoparticles in diabetic rats. Adapted from ref [174]. Printed with permission from American Chemical Society.
Clinical translation and future direction
Recently, orally ingestible macro gastro-retentive devices and microneedles have gained attention to improve oral drug bioavailability. Traverso and colleagues designed an oral capsule that unfolds into a star shape after reaching the stomach that due to its large expandable size, cannot pass through the stomach resulting in long residence time of 3–4 weeks during which desired drugs loaded in polymeric arms of the star are released into the stomach [175–177]. Overtime, the star breaks down into small pieces due to presence of dissolvable elements made of enteric polymers that remain stable in the acidic gastric environment but dissolve in near neutral or basic pH (as is in the duodenum) enabling easy passage out of the body [175–178]. The team’s orally ingestible microneedles demonstrated significant improvements in insulin bioavailability compared to the subcutaneous route, and is especially promising for the delivery of large sized protein and other biomolecule drugs [179]. In summary, mucosal administration of biologics and protein drugs is desirable especially because it can bypass the hepatic first-pass metabolism thereby increasing the bioavailability of drugs. Its use, however, is limited by poor drug absorption and drug degradation in the acidic GI tract, which is particularly concerning for biologics including peptides, proteins, anti-bodies and nucleic acids. As a result, biologics are still administered using injections. In recent years, a few of mucus penetrating formulations have translated to clinical trials. For example, Rybelsus (Novo Nordisk, Bagsværd, Denmark) became the first FDA approved oral tablet, a co-formulation of Glucagon like peptide 1 receptor antagonist (GLP-1 RA) with sodium N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC), for treatment of Type 2 diabetes in the United States [180]. SNAC protects the peptide against enzymatic degradation by exerting buffering action to neutralize local acidic environment near the site of tablet erosion, thereby decreasing the efficacy of digestive enzymes and protecting the peptide from pepsin degradation. SNAC also efficiently inserts into the plasma membrane of gastric epithelium affecting membrane fluidity but not affecting the tight junctions. This enhances transcellular absorption of the non-covalently associated GLP-1 RA allowing it to pass through the gastric epithelium into the systemic circulation [181]. An inhibitor of P-glycoprotein (P-gp), Encequidar (Athenex Inc., Buffalo, NY, USA), has been shown to enhance oral absorption of a wide range of anticancer drugs and is in clinical trials [182]. P-gp is an active transport protein expressed in intestinal epithelium that can pump back orally administered anti-cancer drugs that are P-gp substrates. Oraxol, an oral tablet which is a combination of oral paclitaxel with Encequidar was shown to achieve similar paclitaxel exposures compared to widely used intravenous weekly dosing regimen [183,184]. This formulation is now completing a phase 3 clinical trial (NCT02594371) in metastatic breast tumors [185].
Skin
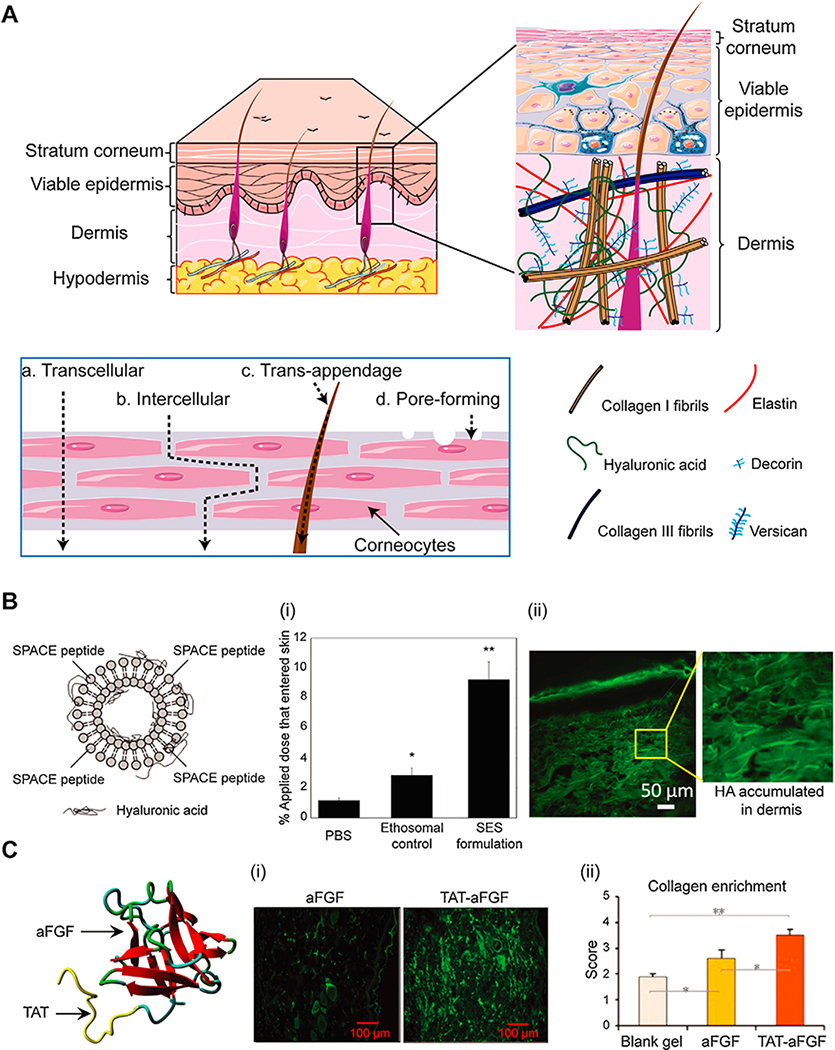
The transdermal route offers another non-invasive and convenient method for drug delivery. Abundant vasculature and capillary beds present in the dermis layer of skin enable direct transport of drugs or drug carriers into the circulation escaping the enterohepatic circulation, thereby providing a more reliable clinical response. However, effective methods to transport across the SC and VED barriers are needed to make transdermal route viable for drug delivery [186]. The outermost layer of skin is the SC composed of 40 % water by weight, 40 % protein (mostly keratin) and 15–20 % lipid predominately triglycerides, cholesterol, fatty acids and phospholipids [186].The tightly packed corneocytes (keratin enriched dead keratinocytes) in SC are embedded in a lipid matrix giving it a “bricks in mortar” like structure [187] (Fig. 8A). The intracellular space is packed with semi-crystalline α- and β-keratin. Human SC is 10~15 μm thick containing 10–25 corneocyte layers that are oriented approximately parallel to the skin surface [186]. The intercellular lipids are arranged orderly in layers (lamellae) making the SC barrier tight and dense enough to prevent penetration of macromolecules over 500 Da [186]. The next layer below SC is viable epidermis (50~100 μm thick) which is a stratified, squamous and keratinizing epithelium containing melanocytes, Langerhans cells and Merkel cells [188]. Dermis (1~2 mm thick) is composed of ample levels of collagens, elastin, HA and other GAGs (Fig. 8A). HA is one of the most abundant components of the ECM of dermis (~0.5 mg/g wet weight) and more than 50 % of total HA in the body is found in skin (average FCD of −2.5 mM, Table 1) [189]. In addition to vasculature and capillary beds, dermis contains nerve tissues and appendages such as hair follicle, hair shaft, sebaceous glands and sweat glands. VED are relatively hydrophilic compared to SC and serve as an important permeation barrier for transdermal delivery [190]. Hypodermis is a fatty connective tissue layer carrying sensory nerves and blood vessels [188]. A wide range of methods have been designed to permeate the skin by physically disrupting the epidermis (iontophoresis, ultrasound, jet injection, microneedles, thermal ablation) or by using chemical permeating enhancers [186].
Fig. 8.
A. Different layers of skin (stratum corneum, viable epidermis, dermis and hypodermis). Sublayers of viable epidermis and dermis are comprised of a collagen network, elastin, versican and HA. Different routes of transdermal drug delivery (a. Transcellular, b. Intercellular, c. Trans-appendage and d. Pore-forming). B. SPACE peptide conjugated to phospholipids designed as an ethosomal carrier system (SES) to deliver hyaluronic acid (HA). (i) Percent penetration oftopically applied dose into total skin. (ii) Confocal image of dermis showing SES can penetrate through the full skin to deliver HA. Adapted from ref[196]. Printed with permission from Elsevier. C. Conjugation of TAT to aFGF to improve its transdermal delivery efficiency. (i) Immunofluorescence of aFGF or TAT-aFGF accumulated in dermal and subcutaneous tissues. (ii) Semi-quantitative scoring showing greater collagen enrichment in rat skin injury model with TAT-aFGF. Adapted from ref[199]. Printed with permission under Creative Commons Attribution License.
Peptide based transdermal delivery systems
Recently, CPPs have sparked considerable interest due to their ability to penetrate through the skin SC, which is the first barrier for transdermal drug delivery. The cationic charge from arginine and lysine residues in CPPs or CPP-drug conjugates enhances their binding to negatively charged cell membrane and ECM components like heparin sulfate, phospholipids and keratin (pI = 4.0–6.5) in the SC barrier [191–193]. This creates an electrical potential gradient generating inward pointing electric fields providing a driving force that plays a significant role in the translocation and penetration of CPPs into different layers of the skin [194].
Chang et al. showed that higher positively charged cyclopeptide TD-34 (ACSSKKSKHCG) with bis-substituted lysine in N-5 and N-6 locations of TD-1 (ACSSSPSKHCG) significantly enhanced transdermal permeability of insulin lowering blood glucose levels to about 26 % of initial levels in diabetic rats in vivo [195]. A skin penetrating and cell entering (SPACE) peptide (AC-TGSTQHQ-CG) ethosome nanoparticle system was shown to enhance HA delivery to dermal layer by 5-fold compared to vehicle free HA delivery in PBS in vivo (Fig. 8B) [196]. The HA concentration was 1000-fold higher in the dermal layer under the local application site than that tested in the blood [196]. The same system was also shown to enhance transdermal siRNA delivery [197]. Kumar et al. demonstrated that SPACE, TD-1 and polyarginine (R7) significantly enhanced cyclosporine A (CsA) penetration into skin via the transcellular pathway mediated by their enhanced partitioning into keratin-rich corneocytes due to charge-based binding with keratin and CsA [198]. The three skin penetrating peptides did not alter skin lipid barrier and showed negligible effect on skin integrity and cytotoxicity. SPACE peptide was reported to be least toxic to keratinocytes and among the most effective at delivering CsA into the skin. Recently, TAT conjugated with aFGF showed enhanced epidermis penetration and enabled superior recovery of skin ulcers compared to free aFGF by depositing more organized collagen rich dermis in rat skin injury models (Fig. 8C) [199]. Catalase (CAT), an anti-oxidant enzyme, was fused with TAT and arginine-rich peptide (R9) to form TAT-CAT and R9-CAT fusion proteins that efficiently penetrated the epidermis as well as the dermis when sprayed on the animal skin [200]. The HIV-TAT peptide was also shown to enhance the transdermal delivery of NSAIDs [201].
Full-thickness skin penetrating peptides
The exact mechanisms of CPP transport across the transdermal barrier remain unknown but can happen through one of the following pathways, a. transcellular, b. intercellular, c. trans-appendage and d. pore-forming (Fig. 8A) [202,203]. While some research has ruled out transcellular route and endocytosis mechanism as the corneocytes in the SC of skin are non-viable [203,204], Rothbard et al. suggested that SC is still a metabolically active environment despite no viable cells [41]. This was corroborated by Hou et al. efforts that showed that macropinocytosis inhibitor, 5-(N-ethyl-N-isopropyl)-amiloride and the F-actin polymerization inhibitor, cytochalasin D, reduced transdermal delivery of arginine-rich CPPs and proteins conjugated to them [205]. CPPs can interact with lipids triggering the intercellular delivery pathway [204,206]. For example, polyarginine was shown to enhance transdermal permeability of FITC-labeled dextran by opening tight junctions in rabbit nasal epithelium [207,208]. A T2 peptide (LVGVFH) enhanced penetration of bacteriophage across porcine and mouse skin which was mediated by interactions with intercellular lipids of SC owing to the presence of histidine and lysine in the sequence [209]. A significant level of accumulation of CPP IMT-P8 (RRWRRWNRFNRRRCR), TAT and their peptide-protein fusion constructs inside the hair follicles after 24 h transdermal delivery have also indicated that trans-appendage mechanisms are at play [210]. Magainin (GIGKFLHSAKKFGKAFVGEIMNS), a peptide known to form pores in bacterial cell membranes has been shown to increase skin permeability by disrupting the SC lipid structure [211]. Magainin’s charge has been modulated to electrostatically bind the molecule of interest for its effective delivery across the skin. For example, Magainin with a +2 charge at pH 7.4 enhanced transdermal penetration of negatively charged fluoresceinby 35 fold; however, when its charge was neutralized by increasing the pH to 10, effects of electrostatic interactions between magainin and fluorescein were removed which eliminated the enhanced permeability effect from Magainin [212].
For a long time, VED barrier was not considered in designing transdermal delivery systems; transporting across the SC was considered sufficient. Thus, the transdermal delivery of compounds was overestimated in experiments using epidermal membrane and underestimated in those using full thickness skin or in animal models [190,213]. Matrix properties of SC and VED barriers vary in composition, thickness, fixed charge distribution and hydrophobicity. For example, since the dermis has high water content, hydrophilic compounds can penetrate through the full thickness of skin and distribute throughout the dermis, while a lipophilic propoxur was found to get stuck in the SC and only partially penetrate the dermis [214,215]. Pepe et al. compared the transport behavior of microemulsions modified with primary amphipathic CPP (transportan), secondary amphipathic CPP (penetratin) and TAT in SC and VED, all of which rapidly penetrated through the SC but transportan demonstrated the highest penetration rate through the VED while TAT displayed the slowest, showing that amphiphilic properties also play a strong role in addition to positive charge [216]. Although microemulsion TAT showed the lowest penetration rate in VED barrier in Pepe et al.’s study [216], Zheng et al. showed that TAT enhanced the accumulation of aFGF in both dermis and subcutaneous tissues facilitating healing of deep tissue injury in skin [199]. Peptides that can transport across the VED can create drug depots in the dermis that can potentially find applications in wound healing of deep skin injuries and for stem cell therapies [217,218].
Clinical translation and future direction
A CPP based formulation that successfully entered phase II clinical trials is PsorBan, a cyclosporine – hepta-arginine conjugate (Cell Gate Inc., Redwood City, CA, USA) for topical treatment of psoriasis by transdermal delivery of CsA [37]. While phase IIa trials showed potential benefit in treating mild-to-moderate psoriasis without the adverse effects associated with systemic use of cyclosporine, subsequent trials were interrupted in 2003 as the release of the free drug was not rapid enough to compete with clearance [37]. Revance Therapeutics Inc., Newark, CA, USA, developed a topical gel, RT001 for treatment of crow’s feet lines (wrinkles around the eyes) comprising of 150 kDa botulinum toxin type A (BoNTA) and a novel CPP which is a reverse sequence of basic residues of TAT, suggested to have improved transdermal penetrability [219,220]. The peptide electrostatically binds with BoNTA such that its PTDs (protein transduction domains) are directed outward to attach with cell membrane [219,220]. While phase II studies were promising, the topical gel preparation of RT001 did not achieve its co-primary endpoints in phase III clinical trials. An injectable formulation was later developed which is now in clinical trials [220].
Conclusión
Negatively charged PGs are critical for structure and function by providing hydration, swelling pressures, compressive stiffness and signal transduction. Large PGs like aggrecans in cartilage have numerous highly sulfated GAG side chains, which hold water and resist deformation owing to increased electrostatic repulsion between the negative charges as they come closer to each other during compressive loading. Small PGs like decorin, biglycan, fibromodulin, lumican are closely associated with collagen fibrils and are believed to regulate collagen fibril diameter. At the cell membrane, PGs stabilize ligand-receptor binding triggering signaling complexes that regulate cell proliferation, migration, matrix adhesion and endocytosis. While critical for tissue function, these PGs make the tissue extremely dense conferring a high negative FCD (Table 1) and making intra-tissue drug delivery extremely challenging.
This high negative FCD, however, can be converted from being a challenge to an opportunity by utilizing the weak, reversible nature of electrostatic interactions by making drugs optimally positively charged. Mechanistic transport studies using peptides and proteins with different net charges across tissues of varying FCD (cartilage, vitreous of eye, mucin, skin) (Table 2) have shown that an optimal charge can be determined for a peptide carrier such to take advantage of Donnan partitioning induced enhanced transport such that the electrostatic interactions are weak enough for the carriers to rapidly get past the tissue superficial zones but strong enough to bind within the deep zones for long term retention. Other short-range binding interactions like hydrophobic and H-bonds can synergistically stabilize long-range charge interactions and thus can enhance drug retention within diseased tissues that have lost PGs and have lower negative FCD. A vast body of literature exists on charge mediated CPP based drug delivery to cells some of which are now in clinical trials. Multi-stage delivery systems that can sequentially penetrate the tissue and then target cells are under development.
Table 2.
Transport studies using peptides and proteins with different net charges (z) across tissues of varying FCD.
| Peptide/Protein | Sequence | z | Cargo | Tissue | Ref. |
|---|---|---|---|---|---|
|
TAT/TAT derivative |
GRKKRRQRRRPQ or RKKRRORRRGC |
+8 |
β-galactosidase, Endostatin, aFGF, μ-calpain inhibitory peptide, c-Jun N-Terminal kinase inhibitor | Ocular | [42,125,126,131,134,135,136,142] |
| aFGF, Catalase, sodium diclofenac, celecoxib | Skin, Hair follicle | [199,200,201,210] | |||
| CGGGYGRKKRRQRRR | +8 | Insulin | Caco-2 monolayer | [40] | |
| Arginine-rich peptides | (RRAAAA)3RR | +8 | – | Cartilage | [14] |
| RRRR(AARRR)3R | +14 | – | Cartilage | [14] | |
| R8GC | +8 | – | Cartilage | [61] | |
| R8K | +9 | – | Ocular | [131] | |
| R6 | +6 | Bevacizumab, Ranibizumab | Ocular | [141] | |
| (D)-R8 | +8 | Insulin | Mucus | [161] | |
| CR8 | +8 | Insulin | Mucus | [174] | |
| R7 | +7 | Cyclosporine A | Skin | [198] | |
| Collagen II-binding peptide | WYRGRL | +2 | Dexamethasone, Cathepsin D inhibitor | Cartilage | [20,62,63] |
| Heparin binding peptide | KKKRKGKGLGKKRDPCLKKYK | +11 | Insulin like growth factor 1 | Cartilage | [58,59,60] |
| Avidin | – | +6–+20 | Dexamethasone | Cartilage | [15,16,53,54,55,82] |
| Chondrocyte affinity peptide | DWRVIIPPRPSAC | +1 | siRNA | Cartilage | [66] |
| p5RHH | VLTTGLPALISWIRRRHRRHC | +7 | siRNA | Cartilage | [68,70] |
| PEP-1 | KETWWETWWTEWSQPKKKRKV | +3 | GRX-1 | Cartilage | [71] |
| FK506 binding proteins | Ocular | [127,128] | |||
| PTD-4 | PIRRRKKLRRLK | +8 | – | Synovium | [83] |
| PTD-5 | RRQRRTSKLMKR | +7 | – | Synovium | [83] |
| PTD-2 | KRIIQRILSRNS | +4 | – | Synovium | [83] |
| HAP-1 | SFHQFARATLAS | +2 | NF-κB-blocking peptide | Synovium | [84,85,86] |
| SyETP | CKSTHDRLC | +2 | Interleukin-4 | Synovium | [89] |
| Penetratin | RQIKIWFQNRRMKWKKK | +8 | Red fluorescent protein plasmid, Antisense oligonucleotide | Ocular | [123,124,131,132] |
| Peptide for ocular delivery | GGG(ARKKAAKA)4 | +16 | siRNA, plasmid DNA, Flurbiprofen | Ocular | [137,138,139,140] |
| Lysine-rich peptides | AKAKAKAKAKAEAEAEAEAE | Neutral | – | Mucus | [45] |
| AKAEAKAEAKAEAKAEAKAE | Neutral | – | Mucus | [45] | |
| (AK)7ANANAN | +7 | – | Cartilage | [14] | |
| (AK)7AFAFAF | +7 | – | Cartilage | [14] | |
| Cell penetrating peptide | CRQIKIWFQNRRMKWKK | +7 | Paclitaxel, Lopinavir | Mucus | [172,173] |
| Cyclopeptide TD-34 | ACSSKKSKHCG | +4 | Insulin, Cyclosporine A | Skin | [195,198] |
| SPACE | ACTGSTQHQCG | +1 | Hyaluronic acid, Cyclosporine A | Skin | [196,198] |
| T2 | LVGVFH | +1 | 5-fluorouracil | Skin | [209] |
| IMT-P8 | RRWRRWNRFNRRRCR | +9 | Pro-apoptotic peptide | Skin | [210] |
| Magainin | GIGKFLHSAKKFGKAFVGEIMNS | +4 | – | Skin | [211] |
| Transportan | GWTLNSAGYLLGKINLKALAALAKKIL | +4 | Paclitaxel | Skin | [216] |
These concepts provide rational methods of designing drug carriers that are tunable based on the net tissue FCD, state of disease, where target sites reside and the physico-chemical properties ofthe drug. As electrostatic interactions are incorporated into design of drug delivery materials and used as a strategy to create properties that are reversible, tunable and dynamic, bio-electroceuticals are becoming an exciting new direction of research and clinical work. Such smart materials promise tremendous impact by addressing the needs of an entire set of negatively charged tissues which are ubiquitous in the body, are associated with a large number of common diseases but have been largely overlooked primarily due to drug delivery challenges.
Acknowledgment
This work was supported by the United States Department of Defense through the Congressionally Directed Medical Research Programs (CDMRP) under contract W81XWH-17-1-0085, National Institutes of Health (NIH)R03 EB025903-1 and NIH TrailblazerR21 EB028385-01.
Biography
 Armin Vedadghavami received his B.S. degree in Chemical Engineering from Sharif University of Technology, Iran in 2016. He is currently a Ph.D. candidate in Bioengineering at Prof. Ambika Bajpayee’s lab at Northeastern University, Boston, MA, USA. His research is focused on targeted drug delivery to cartilage using cationic peptide carriers.
Armin Vedadghavami received his B.S. degree in Chemical Engineering from Sharif University of Technology, Iran in 2016. He is currently a Ph.D. candidate in Bioengineering at Prof. Ambika Bajpayee’s lab at Northeastern University, Boston, MA, USA. His research is focused on targeted drug delivery to cartilage using cationic peptide carriers.
 Chenzhen Zhang is pursuing his Ph.D. degree under the supervision of Prof. Ambika Bajpayee in Bioengineering, Northeastern University, Boston, MA, USA. He focused on transdermal drug delivery system and received his B.S. degree in 2015 from Zhejiang University, China. Now his research interests include cationic drug delivery to cartilage and exosomes as carriers for mucus penetration.
Chenzhen Zhang is pursuing his Ph.D. degree under the supervision of Prof. Ambika Bajpayee in Bioengineering, Northeastern University, Boston, MA, USA. He focused on transdermal drug delivery system and received his B.S. degree in 2015 from Zhejiang University, China. Now his research interests include cationic drug delivery to cartilage and exosomes as carriers for mucus penetration.
 Ambika Bajpayee is an Assistant Professor in the Department of Bioengineering at Northeastern University. Her interests include targeted drug delivery, bio-electrostatics, peptide and protein-based nanocarriers, and modeling of bio-transport and biomechanics with a specific focus on degenerative musculoskeletal diseases. She received her Masters and PhD in Mechanical Engineering at Massachusetts Institute of Technology (MIT) and also completed her post-doctoral work focused on developing devices for oral drug delivery to gastrointestinal tract with Prof. Robert Langer. Previously, she worked as a medical device engineer on development and FDA approval of orthopedic and dental implants. Ambika is a recipient of the US Department of Defense Discovery Award, National Institute of Health Trailblazer Award, MIT Women of Excellence and MIT Meredith Kamm Award in Mechanical Engineering.
Ambika Bajpayee is an Assistant Professor in the Department of Bioengineering at Northeastern University. Her interests include targeted drug delivery, bio-electrostatics, peptide and protein-based nanocarriers, and modeling of bio-transport and biomechanics with a specific focus on degenerative musculoskeletal diseases. She received her Masters and PhD in Mechanical Engineering at Massachusetts Institute of Technology (MIT) and also completed her post-doctoral work focused on developing devices for oral drug delivery to gastrointestinal tract with Prof. Robert Langer. Previously, she worked as a medical device engineer on development and FDA approval of orthopedic and dental implants. Ambika is a recipient of the US Department of Defense Discovery Award, National Institute of Health Trailblazer Award, MIT Women of Excellence and MIT Meredith Kamm Award in Mechanical Engineering.
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- [1].Zhang Y,Jordan JM, Epidemiology of osteoarthritis, Clin. Geriatr. Med. 26 (2010) 355–369, 10.1016/jxger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roddy E, Choi HK, Epidemiology of gout, Rheum. Dis. Clin. North Am. 40 (2014) 155–175, 10.1016/j.rdc.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Freburger JK, Holmes GM, Agans RP, Jackman AM,Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS, The rising prevalence of chronic low back pain, Arch. Intern. Med. 169 (2009) 251–258, 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ananthakrishnan AN, Epidemiology and risk factors for IBD, Nat. Rev. Gastroenterol. Hepatol. 12(2015) 205–217, 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- [5].Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng CY, Wong TY, Global prevalence ofage-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis, Lancet Glob. Heal. 2 (2014), 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- [6].Evans CH, Kraus VB, Setton LA, Progress in intra-articulartherapy, Nat. Rev. Rheumatol. 10 (2014) 11–22, 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grodzinsky AJ, Wang Y, Kakar S, Vrahas MS, Evans CH, Intra-articular dexamethasone to inhibit the development ofpost-traumatic osteoarthritis, J. Orthop. Res. 35 (2017) 406–411, 10.1002/jor.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Makris EA, Hadidi P, Athanasiou KA, The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration, Biomaterials.32 (2011) 7411–7431, 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gorth DJ, Mauck RL, Chiaro JA, Mohanraj B, Hebela NM, Dodge GR, Elliott DM, Smith LJ, IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1p-mediated degradation of nucleus pulposus in vitro, Arthritis Res. Ther. 14 (2012), 10.1186/ar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].del Amo EM, Rimpela AK, Heikkinen E, Kari OK, Ramsay E, Lajunen T, Schmitt M, Pelkonen L, Bhattacharya M, Richardson D, Subrizi A, Turunen T, Reinisalo M,Itkonen J, Toropainen E, Casteleijn M, Kidron H, Antopolsky M, Vellonen KS, Ruponen M, Urtti A, Pharmacokinetic aspects of retinal drug delivery, Prog. Retin. Eye Res. 57(2017) 134–185, 10.1016/j.preteyeres.2016.12.001. [DOI] [PubMed] [Google Scholar]
- [11].Ensign LM, Cone R, Hanes J, Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers, Adv. Drug Deliv. Rev. 64 (2012) 557–570, http://dx.doi.org/10.1016Zj.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ita KB, Transdermal drug delivery: progress and challenges, J. Drug Deliv. Sci. Technol. 24 (2014) 245–250, 10.1016/S1773-2247(14)50041-X. [DOI] [Google Scholar]
- [13].Bajpayee AG, Grodzinsky AJ, Cartilage-targeting drug delivery: can electrostatic interactions help? Nat. Rev. Rheumatol. 13(2017) 183–193, 10.1038/nrrheum.2016.210. [DOI] [PubMed] [Google Scholar]
- [14].Vedadghavami A, Wagner EK, Mehta S, He T, Zhang C, Bajpayee AG, Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues, Acta Biomater. 93 (2019) 258–269, 10.1016/j.actbio.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bajpayee AG, Wong CR, Bawendi MG, Frank EH, Grodzinsky AJ, Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis, Biomaterials 35 (2014) 538–549, 10.1016/J.BIOMATERIALS.2013.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bajpayee AG, De laVega RE, Scheu M, Varady NH, Yannatos IA, Brown LA, Krishnan Y, Fitzsimons TJ, Bhattacharya P, Frank EH, Grodzinsky AJ, Porter RM, Sustained intra-cartilage delivery oflow dose dexamethasone using a cationic carrier fortreatment of post traumatic osteoarthritis, Eur. Cell. Mater.34 (2017) 341–364, 10.22203/eCM.v034a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].US10226427B2- Surfacebindingof nanoparticle baseddrugdeliveryto tissue - Google Patents, (n.d.). https://patents.google.com/patent/US10226427B2/en (Accessed 25 November 2019).
- [18].US9289506B2- Surface binding of nanoparticle based drugdeliveryto tissue - Google Patents, (n.d.). https://patents.google.com/patent/US9289506B2/en (accessed 25 November 2019).
- [19].Rothenfluh DA, Bermudez H, O’Neil CP,Hubbell JA, Biofunctional polymer nanoparticles for intra-articulartargeting and retention in cartilage, Nat. Mater. 7 (2008) 248–254, 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- [20].Formica FA, Barreto G, Zenobi-Wong M, Cartilage-targeting dexamethasone prodrugs increase the efficacy of dexamethasone,J. Control. Release 295 (2019) 118–129, 10.1016/JJCONREL.2018.12.025. [DOI] [PubMed] [Google Scholar]
- [21].Liu Y, Yang T, Wei S, Zhou C, Lan Y, Cao A, Yang J, Wang W, Mucus adhesion- and penetration-enhanced liposomes for paclitaxel oral delivery, Int.J. Pharm. 537 (2018) 245–256, 10.1016/j.ijpharm.2017.12.044. [DOI] [PubMed] [Google Scholar]
- [22].Liu S, Dozois MD, Chang CN, Ahmad A, Ng DLT, Hileeto D, Liang H, Reyad MM, Boyd S, Jones LW, Gu FX, Prolonged ocular retention of mucoadhesive nanoparticle eye drop formulation enables treatment of eye diseases using significantly reduced dosage, Mol. Pharm. 13 (2016) 2897–2905, 10.1021/acs.molpharmaceut.6b00445. [DOI] [PubMed] [Google Scholar]
- [23].Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y, Protein nanoparticles as drug delivery carriers for cancertherapy, Biomed Res. Int. 2014. (2014), 10.1155/2014/180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guo Z, Peng H, Kang J, Sun D, Cell-penetrating peptides: possible transduction mechanisms and therapeutic applications (review), Biomed. Reports. 4 (2016) 528–534, 10.3892/br.2016.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Derakhshankhah H, Jafari S, Cell penetrating peptides: a concise review with emphasis on biomedical applications, Biomed. Pharmacother. 108 (2018) 1090–1096, 10.1016/J.BIOPHA.2018.09.097. [DOI] [PubMed] [Google Scholar]
- [26].Guidotti G, Brambilla L, Rossi D, Cell-penetrating peptides: from basic research to clinics, Trends Pharmacol. Sci. 38 (2017) 406–424, 10.1016/j.tips.2017.01.003. [DOI] [PubMed] [Google Scholar]
- [27].Rai R, Alwani S, Badea I, Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications, Polymers (Basel) 11 (2019) 745, 10.3390/polym11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Frohlich E, The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles, Int. J. Nanomed. 7 (2012) 5577–5591, 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Foged C, Nielsen HM, Cell-penetrating peptides for drug delivery across membrane barriers, Expert Opin. Drug Deliv. 5 (2008) 105–117, 10.1517/17425247.5.1.105. [DOI] [PubMed] [Google Scholar]
- [30].Jones SW, Christison R, Bundell K, Voyce CJ, Brockbank SMV, Newham P, Lindsay MA, Characterisation ofcell-penetrating peptide-mediated peptide delivery, Br.J. Pharmacol. 145 (2005) 1093–1102, 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bocsik A, Gróf I, Kiss L, Otvos F, Zsíros O, Daruka L, Fülop L, Vastag M, Kittei Á, Imre N, Martinek TA, Pál C, Szabó-Révész P, Deli MA, Dual action ofthe PN159/KLAL/MAP peptide: increase of drug penetration across caco-2 intestinal barrier model by modulation oftightjunctions and plasma membrane permeability, Pharmaceutics 11 (2019), 10.3390/pharmaceutics11020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Derakhshankhah H, Jafari S, Cell penetrating peptides: a concise review with emphasis on biomedical applications, Biomed. Pharmacother. 108 (2018) 1090–1096, 10.1016/j.biopha.2018.09.097. [DOI] [PubMed] [Google Scholar]
- [33].Chen L, Zhang Q, Yuan X, Cao Y, Yuan Y, Yin H, Ding X, Zhu Z, Luo SZ, How charge distribution influences the function of membrane-active peptides: lytic orcell-penetrating? Int.J. Biochem. Cell Biol. 83 (2017) 71–75, 10.1016/jJbiocel.2016.12.011. [DOI] [PubMed] [Google Scholar]
- [34].Lv H, Zhang S, Wang B, Cui S, Yan J, Toxicity of cationic lipids and cationic polymers in gene delivery,J. Control. Release 114 (2006) 100–109, 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- [35].Geiger BC, Wang S, Padera RF, Grodzinsky AJ, Hammond PT, Cartilage-penetrating nanocarriers improve delivery and efficacy ofgrowth factortreatment ofosteoarthritis, Sci.Transl. Med. 10 (2018), 10.1126/scitranslmed.aat8800, eaat8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Read ML, Bremner KH, Oupicky D, Green NK, Searle PF, Seymour LW, Vectors based on reducible polycations facilitate intracellular release of nucleic acids, J. Gene Med. 5 (2003) 232–245, 10.1002/jgm.331. [DOI] [PubMed] [Google Scholar]
- [37].Vivès E,Schmidt J, Pèlegrin A, Cell-penetrating and cell-targeting peptides in drug delivery, Biochim. Biophys. Acta - Rev. Cancer 1786 (2008) 126–138, 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- [38].Wong C, Stylianopoulos T,Cui J,Martin J, Chauhan VP, Jiang W, Popovíc Z, Jain RK, Bawendi MG, Fukumura D, Multistage nanoparticle delivery system for deep penetration into tumor tissue, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 2426–2431, 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen B, Dai W, He B, Zhang H, Wang X, Wang Y, Zhang Q, Current multistage drug delivery systems based on the tumor microenvironment, Theranostics 7 (2017) 538–558, 10.7150/thno.16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liang JF, Yang VC, Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency, Biochem. Biophys. Res. Commun. 335 (2005) 734–738, 10.1016/j.bbrc.2005.07.142. [DOI] [PubMed] [Google Scholar]
- [41].Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, Wender PA, Khavari PA, Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition ofinflammation, Nat. Med. 6 (2000) 1253–1257, 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- [42].El Zaoui I, Touchard E, Berdugo M, Abadie C, Kowalczuk L, Deloche C, Zhao M, Naud M-C, Combette J-M, Behar-Cohen F, Subconjunctival injection ofXG-102, a c-jun N-terminal kinase inhibitor peptide, in the treatment of endotoxin-induced uveitis in rats,J. Ocul. Pharmacol. Ther. 31 (2015) 17–24, 10.1089/jop.2014.0019. [DOI] [PubMed] [Google Scholar]
- [43].Moschos SA, Jones SW, Perry MM, Williams AE,Erjefalt JS,Turner JJ, Barnes PJ, Sproat BS, Gait MJ, Lindsay MA, Lung delivery studies using siRNA conjugated toTAT(48–60) and penetratin reveal peptide induced reduction in gene expression and induction ofinnate immunity, Bioconjug. Chem. 18 (2007) 1450–1459, 10.1021/bc070077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lin T, Liu E, He H, Shin MC, Moon C, Yang VC, Huang Y, Nose-to-brain delivery ofmacromolecules mediated by cell-penetrating peptides, Acta Pharm. Sin. B 6 (2016) 352–358, 10.1016/j.apsb.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li LD, Crouzier T, Sarkar A, Dunphy L, Han J, Ribbeck K, Spatial configuration and composition ofcharge modulates transport into a mucin hydrogel barrier, Biophys.J. 105 (2013) 1357–1365, 10.1016/j.bpj.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Käsdorf BT, Arends F, Lieleg O, Diffusion regulation inthe vitreous humor, Biophys.J. 109(2015) 2171–2181, 10.1016/J.BPJ.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bhosale AM, Richardson JB, Articular cartilage: structure, injuries and review of management, Br. Med. Bull. 87 (2008) 77–95, 10.1093/bmb/ldn025. [DOI] [PubMed] [Google Scholar]
- [48].Kuettner KE, Biochemistry of articular cartilage in health and disease, Clin. Biochem. 25 (1992) 155–163, 10.1016/0009-9120(92)90224-G. [DOI] [PubMed] [Google Scholar]
- [49].Kuiper NJ, Sharma A, A detailed quantitative outcome measure of glycosaminoglycans in human articular cartilage for cell therapy and tissue engineering strategies, Osteoarthr. Cartil. 23 (2015) 2233–2241, 10.1016/J.JOCA.2015.07.011. [DOI] [PubMed] [Google Scholar]
- [50].Mora JC, Przkora R, Cruz-Almeida Y, Knee osteoarthritis: pathophysiology and current treatment modalities,J. Pain Res. 11 (2018) 2189–2196, 10.2147/JPR.S154002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chen D,Shen J, Zhao W, Wang T, Han L,Hamilton JL, Im H-J, Osteoarthritis: toward a comprehensive understanding ofpathological mechanism, Bone Res. 5 (2017) 16044, 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Krishnan Y, Rees HA, Rossitto CP, Kim SE, Hung HHK, Frank EH, Olsen BD, Liu DR, Hammond PT, Grodzinsky AJ, Green fluorescent proteins engineered forcartilage-targeted drug delivery: insights fortransport into highly charged avasculartissues, Biomaterials 183 (2018) 218–233, 10.1016/j.biomaterials.2018.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM, A rabbit model demonstrates the influence ofcartilage thickness on intra-articular drug delivery and retention within cartilage, J. Orthop. Res. 33 (2015) 660–667, 10.1002/jor.22841. [DOI] [PubMed] [Google Scholar]
- [54].Bajpayee AG, Quadir MA, Hammond PT, Grodzinsky AJ, Charge based intra-cartilage delivery ofsingle dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term, Osteoarthr. Cartil. 24 (2016) 71–81, 10.1016/j.joca.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].He T, Zhang C, Vedadghavami A, Mehta S, Clark HA, Porter RM, Bajpayee AG, Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs,J. Control. Release 318(2020) 109–123, 10.1016/j.jconrel.2019.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Perni S, Prokopovich P, Poly-beta-amino-esters nano-vehicles based drug deliverysystem forcartilage, Nanomedicine 13 (2017) 539–548, 10.1016/j.nano.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shah NJ, Geiger BC, Quadir MA, Hyder N, Krishnan Y, Grodzinsky AJ, Hammond PT, Synthetic nanoscale electrostatic particles as growth factor carriers forcartilage repair, Bioeng. Transl. Med. 1 (2016)347–356, 10.1002/btm2.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tokunou T, Miller R, Patwari P, Davis ME, Segers VFM, Grodzinsky AJ, Lee RT, Engineering insulin-like growth factor-1 for local delivery, FASEBJ. 22 (2008) 1886–1893, 10.1096/fj.07-100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Miller RE, Grodzinsky AJ, Cummings K, Plaas AHK, Cole AA, Lee RT, Patwari P, Intraarticular injection ofheparin-binding insulin-like growth factor 1 sustains delivery of insulin-like growth factor 1 to cartilage through binding to chondroitin sulfate, Arthritis Rheum. 62 (2010) 3686–3694, 10.1002/art.27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Loffredo FS, Pancoast JR, Cai L, Vannelli T, Dong JZ, Lee RT, Patwari P, Targeted delivery to cartilage is critical for in vivo efficacy of insulin-like growth factor 1ina rat model of osteoarthritis, Arthritis Rheumatol. 66 (2014) 1247–1255, 10.1002/art.38357. [DOI] [PubMed] [Google Scholar]
- [61].Inagawa K, Oohashi T, Nishida K, Minaguchi J, Tsubakishita T, Yaykasli KO, Ohtsuka A, Ozaki T, Moriguchi T, Ninomiya Y, Optical imaging of mouse articular cartilage using the glycosaminoglycans binding property of fluorescent-labeled octaarginine, Osteoarthr. Cartil. 17 (2009) 1209–1218, 10.1016/J.JOCA.2009.03.010. [DOI] [PubMed] [Google Scholar]
- [62].Hu HY, Lim NH, Juretschke HP, Ding-Pfennigdorff D, Florian P, Kohlmann M, Kandira A, Peter Von Kries J, Saas J, Rudolphi KA, Wendt KU, Nagase H, Plettenburg O, Nazare M, Schultz C, In vivo visualization of osteoarthritic hypertrophic lesions, Chem. Sci. 6 (2015) 6256–6261, 10.1039/c5sc01301a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hu H-Y, Lim N-H, Ding-Pfennigdorff D,Saas J, Wendt KU, Ritzeler O, Nagase H, Plettenburg O, Schultz C, Nazare M, DOTAM derivatives as active cartilage-targeting drug carriers forthe treatment of osteoarthritis, Bioconjug. Chem. 26 (2015) 383–388, 10.1021/bc500557s. [DOI] [PubMed] [Google Scholar]
- [64].Pi Y, Zhang X, Shi J, Zhu J, Chen W, Zhang C, Gao W, Zhou C, Ao Y, Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display, Biomaterials 32 (2011)6324–6332, 10.1016/J.BIOMATERIALS.2011.05.017. [DOI] [PubMed] [Google Scholar]
- [65].Cheung CSF, Lui JC, Baron J, Identification ofchondrocyte-binding peptides by phage display,J. Orthop. Res.31 (2013) 1053–1058, 10.1002/jor.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pi Y, Zhang X, Shao Z, Zhao F, Hu X, Ao Y, Intra-articular delivery of anti-hif-2a siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice, Gene Ther. 22 (2015) 439–448, 10.1038/gt.2015.16. [DOI] [PubMed] [Google Scholar]
- [67].Hu Q, Chen Q, Yan X, Ding B, Chen D, Cheng L, Chondrocyte affinity peptide modified PAMAM conjugate as a nanoplatform for targeting and retention in cartilage, Nanomedicine 13 (2018) 749–767, 10.2217/nnm-2017-0335. [DOI] [PubMed] [Google Scholar]
- [68].Hou KK, Pan H, Lanza GM, Wickline SA, Melittin derived peptides for nanoparticle based siRNA transfection, Biomaterials 34 (2013) 3110–3119, 10.1016/J.BIOMATERIALS.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yan H, Duan X, Pan H, Holguin N, Rai MF, Akk A, Springer LE, Wickline SA, Sandell LJ, Pham CTN, Suppression ofNF-KB activityvia nanoparticle-based siRNA delivery alters early cartilage responses to injury, Proc. Natl. Acad. Sci. U. S. A. 113 (2016) E6199–E6208, 10.1073/pnas.1608245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yan H, Duan X, Pan H, Akk A, Sandell LJ, Wickline SA, Rai MF, Pham CTN, Development of a peptide-siRNA nanocomplex targeting NF- KB for efficient cartilage delivery, Sci. Rep. 9 (2019) 442, 10.1038/s41598-018-37018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hwang HS, Park IY, Kim HA, Choi SY, PEP-1-GRX-1 modulates matrix metalloproteinase-13 and nitric oxide expression ofhuman articular chondrocytes, Cell. Physiol. Biochem. 41 (2017) 252–264, 10.1159/000456090. [DOI] [PubMed] [Google Scholar]
- [72].Mehta S, Akhtar S, Porter RM, Onnerfjord P, Bajpayee AG, Interleukin-1 receptorantagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture, Arthritis Res. Ther. 21 (2019) 238, 10.1186/s13075-019-2003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hsu Y-H, Chen D, Li M-J, Yu Y-H, Chou Y-C, Liu S-J, Sustained delivery of analgesic and antimicrobial agents to knee joint by direct injections of electrosprayed multipharmaceutical-loaded nano/microparticles, Polymers (Basel) 10 (2018) 890, 10.3390/polym10080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Crielaard BJ, Rijcken CJF, Quan L, Van DerWal S, Altintas I, Van Der Pot M,Kruijtzer JAW, Liskamp RMJ, Schiffelers RM, Van Nostrum CF, E Hennink W, Wang D, Lammers T, Storm G, Glucocorticoid-loaded core-cross-linked polymeric micelles with tailorable release kinetics for targeted therapy of rheumatoid arthritis, Angew. Chem. - Int. Ed. 51 (2012) 7254–7258, 10.1002/anie.201202713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Elron-Gross I, Glucksam Y, Margalit R, Liposomal dexamethasone-diclofenac combinations for local osteoarthritis treatment, Int. J. Pharm. 376 (2009) 84–91, 10.1016/j.ijpharm.2009.04.025. [DOI] [PubMed] [Google Scholar]
- [76].Kimmerling KA, Furman BD, Mangiapani DS, Moverman MA, Sinclair SM,Huebner JL, Chilkoti A, Kraus VB, Setton LA, Guilak F, Olson SA, Sustained intra-articular delivery ofIL-1Ra from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis, Eur. Cells Mater. 29 (2015) 124–140, 10.22203/eCM.v029a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhou P, Qiu B, Deng R, Li H, Xu X, Shang X, Chondroprotective effects of hyaluronic acid-chitosan nanoparticles containing plasmid DNA encoding cytokine response modifierain a rat knee osteoarthritis model, Cell. Physiol. Biochem. 47(2018) 1207–1216, 10.1159/000490217. [DOI] [PubMed] [Google Scholar]
- [78].Wang J, Wang X, Cao Y, Huang T, Song DX, Tao HR, Therapeutic potential of hyaluronic acid/chitosan nanoparticles for the delivery of curcuminoid in knee osteoarthritis and an in vitro evaluation in chondrocytes, Int. J. Mol. Med. 42 (2018) 2604–2614, 10.3892/ijmm.2018.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Morgen M, Tung D, Boras B, Miller W, Malfait AM, Tortorella M, Nanoparticles for improved local retention after intra-articular injection intothe kneejoint, Pharm. Res.30 (2013) 257–268, 10.1007/s11095-012-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pouran B, Arbabi V, Bajpayee AG,van Tiel J, Toyras J,Jurvelin JS,Malda J, Zadpoor AA, Weinans H, Multi-scale imaging techniques to investigate solutetransport across articularcartilage,J. Biomech. 78 (2018) 10–20, 10.1016/j.jbiomech.2018.06.012. [DOI] [PubMed] [Google Scholar]
- [81].Brown S,Pistiner J, Adjei IM, Sharma B, Nanoparticle properties for deliveryto cartilage: the implications of disease state, synovial fluid, and off-target uptake, Mol. Pharm. 16 (2019) 469–479, 10.1021/acs.molpharmaceut.7b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM, Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints, J. Orthop. Res. 32 (2014) 1044–1051, 10.1002/jor.22630. [DOI] [PubMed] [Google Scholar]
- [83].Mi Z,Mai J, Lu X, Robbins PD, Characterization of a class of cationic peptides able to facilitate efficient protein transduction in vitro and in vivo, Mol. Ther. 2 (2000) 339–347, 10.1006/mthe.2000.0137. [DOI] [PubMed] [Google Scholar]
- [84].Mi Z, Lu X,Mai JC, Ng BG, Wang G, Lechman ER, Watkins SC, Rabinowich H, Robbins PD, Identification of a synovial fibroblast-specific protein transduction domain for delivery of apoptotic agents to hyperplastic synovium, Mol. Ther. 8 (2003) 295–305, 10.1016/S1525-0016(03)00181-3. [DOI] [PubMed] [Google Scholar]
- [85].Vanniasinghe AS, Manolios N, Schibeci S, Lakhiani C, Kamali-Sarvestani E, Sharma R, Kumar V, Moghaddam M, Ali M, Bender V, Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition ofexperimental arthritis, Clin. Immunol. 151 (2014) 43–54, 10.1016/j.clim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- [86].You C, Zu J, Liu X, Kong P, Song C, Wei R, Zhou C, Wang Y, Yan J, Synovial fibroblast-targeting liposomes encapsulating an NF-KB-blocking peptide ameliorates zymosan-induced synovial inflammation, J. Cell. Mol. Med. 22 (2018) 2449–2457, 10.1111/jcmm.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Meka RR, Venkatesha SH, Moudgil KD, Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis, J. Control. Release 286 (2018) 279–288, 10.1016/j.jconrel.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yang YH, Rajaiah R, Ruoslahti E, Moudgil KD, Peptides targeting inflamed synovial vasculature attenuate autoimmune arthritis, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 12857–12862, 10.1073/pnas.1103569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wythe SE, DiCara D, Taher TEI, Finucane CM, Jones R, Bombardieri M, Man YKS, Nissim A, Mather SJ, Chernajovsky Y, Pitzalis C, Targeted delivery ofcytokine therapy to rheumatoid tissue by a synovial targeting peptide, Ann. Rheum. Dis. 72 (2013) 129–135, 10.1136/annrheumdis-2012-201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Rees SG, Dent CM, Caterson B, Metabolism of proteoglycans intendon, Scand.J. Med. Sci. Sports 19 (2009) 470–478, 10.1111/j.1600-0838.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- [91].Yoon JH,Halper J, Tendon proteoglycans: biochemistry and function,J. Musculoskelet. Neuronal Interact. 5 (2005) 22–34. [PubMed] [Google Scholar]
- [92].Wu F, Nerlich M, Docheva D, Tendon injuries: basic science and new repair proposals, EFORT Open Rev. 2 (2017) 332–342, 10.1302/2058-5241.2.160075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shimomura K, Hamamoto S, Hart DA, Yoshikawa H, Nakamura N, Meniscal repairand regeneration: current strategies and future perspectives, J. Clin. Orthop. Trauma 9 (2018) 247–253, 10.1016/j.jcot.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mauck RL, Burdick JA, From repair to regeneration: biomaterials to reprogram the meniscus wound microenvironment, Ann. Biomed. Eng. 43 (2015) 529–542, 10.1007/s10439-015-1249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rawson S, Cartmell S,Wong J, Suture techniques fortendon repair; A comparative review, Muscles Ligaments Tendons J. 3 (2013) 220–228, 10.11138/mltj/2013.3.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Çetinkaya E, Kuyucu E, Gül M, Lapçin O, Albayrak K, Gürsu S, A suture technique foreasierreductionand repairof bucket-handle meniscal tears while using the all-inside devices, SICOT-J. 2 (2016) 42, 10.1051/sicotj/2016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gorschewsky O, Klakow A, Pütz A, Mahn H, Neumann W, Clinical comparison of the autologous quadriceps tendon (BQT) and the autologous patellatendon (BPTB) forthe reconstruction ofthe anterior cruciate ligament, knee surgery, Sport. Traumatol. Arthrosc. 15 (2007) 1284–1292, 10.1007/s00167-007-0371-3. [DOI] [PubMed] [Google Scholar]
- [98].Lubowitz JH, Verdonk PCM, Reid JB, Verdonk R, Meniscus allograft transplantation: a current concepts review, knee surgery, Sport. Traumatol. Arthrosc. 15 (2007) 476–492, 10.1007/s00167-006-0216-5. [DOI] [PubMed] [Google Scholar]
- [99].Niu W, Guo W, Han S, Zhu Y, Liu S, Guo Q, Cell-based strategies for meniscus tissue engineering, Stem Cells Int. 2016 (2016), 10.1155/2016/4717184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yilgor C, Yilgor Huri P, Huri G, Tissue engineering strategies in ligament regeneration, Stem Cells Int. 2012(2012) 9, 10.1155/2012/374676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Castilho M, Dias M, Vorndran E, Gbureck U, Fernandes P, Pires I, Gouveia B, Armés H, Armés A, Pires E, Rodrigues J, Application of a 3D printed customized implant for canine cruciate ligament treatment by tibial tuberosity advancement, Biofabrication 6 (2014) 13, 10.1088/1758-5082/6/2/025005. [DOI] [PubMed] [Google Scholar]
- [102].Filardo G, Petretta M, Cavallo C, Roseti L, Durante S, Albisinni U, Grigolo B, Patient-specific meniscus prototype based on 3D bioprinting of human cell-laden scaffold, BoneJt. Res. 8 (2019) 101–106, 10.1302/2046-3758.82.BJR-2018-0134.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Whatley BR, Wen X, Intervertebral disc (IVD): structure, degeneration, repairand regeneration, Mater. Sci. Eng. C32 (2012) 61–77, 10.1016/j.msec.2011.10.011. [DOI] [Google Scholar]
- [104].Freemont AJ, The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain, Rheumatology 48 (2009) 5–10, 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- [105].Blanquer SBG, Grijpma DW, Poot AA, Delivery systems forthe treatment ofdegenerated intervertebral discs, Adv. Drug Deliv. Rev. 84 (2015) 172–187, http://dx.doi.org/10.1016Zj.addr.2014.10.024. [DOI] [PubMed] [Google Scholar]
- [106].Poynton AR, Lane JM, Safety profile for the clinical use of bone morphogenetic proteins inthe spine, Spine (Phila. Pa. 1976) 27 (2002), 10.1097/00007632-200208151-00010. [DOI] [PubMed] [Google Scholar]
- [107].Jacobsen T, Chahine N, Anti-inflammatory agents prevent intervertebral disc cell mechanobiological alterations, Glob. Spine J. 6 (2016), 10.1055/s-0036-1582599, s-0036-1582599-s-0036-1582599. [DOI] [Google Scholar]
- [108].Choi W Il, Sahu A, Vilos C,Lee JH, Kim S, Hong YK, Sul D, Hwang SW, Lee SH, Tae G, Chitosan functionalized thermosponge nano-carriers for prolonged retention and local delivery ofchymopapain at the nucleus pulposus in porcine discs ex vivo, RSCAdv. 6 (2016) 90967–90972, 10.1039/c6ra17848k. [DOI] [Google Scholar]
- [109].Sawamura K, Ikeda T, Nagae M, Okamoto S, Mikami Y, Hase H, Ikoma K, Yamada T, Sakamoto H, Matsuda K, Tabata Y, Kawata M, Kubo T, Characterization ofin vivo effects ofplatelet-rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs, Tissue Eng. Part A 15 (2009) 3719–3727, 10.1089/ten.TEA.2008.0697. [DOI] [PubMed] [Google Scholar]
- [110].Vadala G, Mozetic P,Rainer A, Centola M, Loppini M, Trombetta M, Denaro V, Bioactive electrospun scaffold forannulus fibrosus repairand regeneration, Eur. Spine J. 21 (2012), 10.1007/s00586-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Vert M, Li SM, Spenlehauer G, Guerin P, Bioresorbability and biocompatibility of aliphatic polyesters,J. Mater. Sci. Mater. Med. 3 (1992) 432–446, 10.1007/BF00701240. [DOI] [Google Scholar]
- [112].Wagner EK, Vedadghavami A, Jacobsen TD, Chahine NO, Bajpayee AG, Avidin nanocarriers forsustained intra-discal deliveryofdrugs fortreatment ofdisc degeneration, in: Orthop. Res. Soc. Annu. Meet., Austin, Texas, 2019. [Google Scholar]
- [113].Langworthy MJ, Conaghan PG, Ruane JJ, Kivitz AJ, Lufkin J, Cinar A, Kelley SD, Efficacy oftriamcinolone acetonide extended-release in participants with unilateral knee osteoarthritis: a post hoc analysis, Adv. Ther. 36 (2019) 1398–1411, 10.1007/s12325-019-00944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Conaghan PG, Hunter DJ, Cohen SB, Kraus VB, Berenbaum F,Lieberman JR, Jones DG, Spitzer AI, Jevsevar DS, Katz NP, Burgess DJ, Lufkin J,Johnson JR, Bodick N, Effects of a single intra-articular injection of a microsphere formulation oftriamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study, J. BoneJt. Surg. - Am. Vol. 100 (2018) 666–677, 10.2106/JBJS.17.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Tsai CH, Wang PY, Lin IC, Huang H, Liu GS, Tseng CL, Ocular drug delivery: role ofdegradable polymeric nanocarriers forophthalmic application, Int. J. Mol. Sci. 19 (2018), 10.3390/ijms19092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kels BD, Grant-Kels JM, Human ocular anatomy, Clin. Dermatol. 33 (2015) 140–146, 10.1016/J.CLINDERMATOL.2014.10.006. [DOI] [PubMed] [Google Scholar]
- [117].Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health, Understanding the epidemiology ofvision loss and impairment in the United States, in: Mak. Eye Heal. a Popul. Heal. Imp., 2016, pp. 55–114. [Google Scholar]
- [118].Moschos MM, Physiology and psychology ofvision and its disorders: a review, Med. Hypothesis Discov. Innov. Ophthalmol. J. 3 (2014) 83–90. [PMC free article] [PubMed] [Google Scholar]
- [119].Bachu RD, Chowdhury P, Al-Saedi ZHF, Karla PK, Boddu SHS, Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment oculardiseases, Pharmaceutics 10 (2018) 1–31, 10.3390/pharmaceutics10010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mun EA, Morrison PWJ,Williams AC,Khutoryanskiy VV, Onthe barrier properties of the cornea: a microscopy study of the penetration of fluorescently labeled nanoparticles, polymers, and sodium fluorescein, Mol. Pharm. 11 (2014)3556–3564, 10.1021/mp500332m. [DOI] [PubMed] [Google Scholar]
- [121].Xu Q, Boylan NJ,Suk JS, Wang Y-Y, Nance EA,Yang J-C, McDonnell PJ, Cone RA, Duh EJ,Hanes J, Nanoparticle diffusion in, and microrheology of, the bovine vitreous exvivo,J. Control. Release 167 (2013) 76–84, 10.1016/jjconrel.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Agarwal R, Iezhitsa I, Agarwal P, Abdul Nasir NA, Razali N, Alyautdin R, Ismail NM, Liposomes in topical ophthalmic drug delivery: an update, Drug Deliv. 23 (2016) 1075–1091, 10.3109/10717544.2014.943336. [DOI] [PubMed] [Google Scholar]
- [123].Jiang K, Gao X, Shen Q, Zhan C, Zhang Y, Xie C, Wei G, Lu W, Discerning the composition of penetratin forsafe penetration from corneato retina, Acta Biomater. 63 (2017) 123–134, 10.1016/j.actbio.2017.09.023. [DOI] [PubMed] [Google Scholar]
- [124].Liu C, Jiang K, Tai L, Liu Y, Wei G, Lu W, Pan W, Facile noninvasive retinal gene delivery enabled by penetratin, ACS Appl. Mater. Interfaces 8 (2016) 19256–19267, 10.1021/acsami.6b04551. [DOI] [PubMed] [Google Scholar]
- [125].Guo X, Hutcheon AE,Zieske JD, Transduction of functionally active TAT fusion proteins into cornea, Exp. Eye Res. 78 (2004) 997–1005, 10.1016/j.exer.2003.12.010. [DOI] [PubMed] [Google Scholar]
- [126].Barnett EM, Elangovan B, Bullok KE, Piwnica-Worms D, Selective cell uptake of modified Tat peptide-fluorophore conjugates in rat retina in ex vivo and in vivo models, Investig. Ophthalmol. Vis. Sci. 47 (2006) 2589–2595, 10.1167/iovs.05-1470. [DOI] [PubMed] [Google Scholar]
- [127].Kim DW, Lee SH, Ku SK, Lee JE, Cha HJ, Youn JK, Kwon HY, Park JH, Park EY, Cho SW, Han KH, Park J, Eum WS, Choi SY, The effects of PEP-1-FK506BP on dry eye disease in a rat model, BMB Rep. 48 (2015) 153–158, 10.5483/BMBRep.2015.483.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Pescina S, Sala M, Padula C, Scala MC, Spensiero A, Belletti S, Gatti R, Novellino E, Campiglia P, Santi P, Nicoli S, Ostacolo C, Design and synthesis of new cell penetrating peptides: diffusion and distribution inside the cornea, Mol. Pharm. 13 (2016) 3876–3883, 10.1021/acs.molpharmaceut.6b00658. [DOI] [PubMed] [Google Scholar]
- [129].Sasaki H, Karasawa K, Hironaka K, Tahara K, Tozuka Y, Takeuchi H, Retinal drug delivery using eyedrop preparations ofpoly-l-lysine-modified liposomes, Eur. J. Pharm. Biopharm. 83 (2013) 364–369, 10.1016/j.ejpb.2012.10.014. [DOI] [PubMed] [Google Scholar]
- [130].Melgar-Asensio I, Kandela I, Aird F, Darjatmoko SR, de los Rios C, Sorenson CM, Albert DM, Sheibani N, Henkin J, Extended intravitreal rabbit eye residence of nanoparticles conjugated with cationic arginine peptides for intraocular drug delivery: in vivo imaging, Investig. Opthalmol. Vis. Sci. 59 (2018) 4071, 10.1167/iovs.18-24087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Liu C, Tai L,Zhang W, Wei G,Pan W,Lu W, Penetratin, a potentially powerful absorption enhancer for noninvasive intraocular drug delivery, Mol. Pharm. 11 (2014) 1218–1227, 10.1021/mp400681n. [DOI] [PubMed] [Google Scholar]
- [132].Tai L, Liu C, Jiang K, Chen X, Wei G, Lu W, Pan W, Noninvasive delivery of oligonucleotide by penetratin-modified polyplexes to inhibit protein expression ofintraocular tumor, Nanomed. Nanotechnol. Biol. Med. 13 (2017) 2091–2100, http://dx.doi.org/10.1016Zj.nano.2017.04.011. [DOI] [PubMed] [Google Scholar]
- [133].Jiang K, Hu Y, Gao X, Zhan C, Zhang Y, Yao S, Xie C, Wei G, Lu W, Octopus-like flexible vector for noninvasive intraocular delivery of short interfering nucleic acids, Nano Lett. 19 (9) (2019) 6410–6417, 10.1021/acs.nanolett.9b02596, acs.nanolett.9b02596. [DOI] [PubMed] [Google Scholar]
- [134].Zhang X, Li Y, Cheng Y, Tan H, Li Z, Qu Y, Mu G, Wang F, Tat PTD-endostatin: a novel anti-angiogenesis protein with ocular barrier permeabilityvia eye-drops, Biochim. Biophys. Acta - Gen. Subj. 1850 (2015) 1140–1149, 10.1016/J.BBAGEN.2015.01.019. [DOI] [PubMed] [Google Scholar]
- [135].Wang Y, Lin H, Lin S, Qu J, Xiao J, Huang Y, Xiao Y, Fu X, Yang Y, Li X, Cell-penetrating peptide TAT-mediated delivery of acidic FGF to retina and protection against ischemia-reperfusion injury in rats, J. Cell. Mol. Med. 14 (2010) 1998–2005, 10.1111/j.1582-4934.2009.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ozaki T, Nakazawa M, Yamashita T, Ishiguro S, Delivery of topically applied calpain inhibitory peptide to the posterior segment ofthe rat eye, PLoS One 10 (2015), e0130986, 10.1371/journal.pone.0130986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Johnson LN, Cashman SM, Kumar-Singh R, Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retinaand cornea, Mol.Ther. 16 (2008) 107–114, 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Johnson LN, Cashman SM, Read SP, Kumar-Singh R, Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin, Vision Res. 50 (2010) 686–697, 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Dasari BC, Cashman SM, Kumar-Singh R, Reducible PEG-POD/DNA nanoparticles for gene transfer in vitro and in vivo: application in a mouse model of age-related macular degeneration, Mol. Ther. - Nucleic Acids 8 (2017) 77–89, 10.1016/j.omtn.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Vasconcelos A, Vega E, Pérez Y, Gómara MJ, García ML, Haro I, Conjugation ofcell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery, Int. J. Nanomed. 10 (2015) 609–631, 10.2147/IJN.S71198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].de Cogan F, Hill LJ, Lynch A, Morgan-Warren PJ, Lechner J, Berwick MR, Peacock AFA, Chen M, Scott RAH, Xu H, Logan A, Topical delivery of anti-VEGF drugs to the ocular posterior segment using cell-penetrating peptides, Investig. Ophthalmol. Vis. Sci. 58 (2017) 2578–2590, 10.1167/iovs.16-20072. [DOI] [PubMed] [Google Scholar]
- [142].Beydoun T, Deloche C, Perino J, Kirwan B-A, Combette J-M, Behar-Cohen F, Subconjunctival injection ofXG-102, a JNK inhibitor peptide, in patients with intraocular inflammation: a safety and tolerabitily study, J. Ocul. Pharmacol. Ther. 31 (2014) 93–99, 10.1089/jop.2013.0247. [DOI] [PubMed] [Google Scholar]
- [143].Chiquet C, Aptel F, Creuzot-Garcher C,Berrod JP, Kodjikian L, Massin P, Deloche C, Perino J, Kirwan BA, de Brouwer S, Combette JM, Behar-Cohen F, Postoperative ocular inflammation: a single subconjunctival injection of XG-102 compared to dexamethasone drops in a randomized trial, Am. J. Ophthalmol. 174 (2017) 76–84, 10.1016/j.ajo.2016.10.012. [DOI] [PubMed] [Google Scholar]
- [144].Lai SK, Wang YY,Hanes J, Mucus-penetrating nanoparticles fordrug and gene delivery to mucosal tissues, Adv. Drug Deliv. Rev. 61 (2009) 158–171, 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Frohlich E, Roblegg E, Mucus as barrierfordrugdeliveryby nanoparticles,J. Nanosci. Nanotechnol. 14 (2014) 126–136, 10.1166/jnn.2014.9015. [DOI] [PubMed] [Google Scholar]
- [146].Boegh M, Foged C, Müllertz A, H. M0rck Nielsen, Mucosal drug delivery: barriers, in vitro models and formulation strategies, J. Drug Deliv. Sci. Technol. 23 (2013) 383–391, 10.1016/S1773-2247(13)50055–4. [DOI] [Google Scholar]
- [147].Zhang X, Cheng H, Dong W, Zhang M, Liu Q, Wang X, Guan J, Wu H, Mao S, Design and intestinal mucus penetration mechanism ofcore-shell nanocomplex, J. Control. Release 272 (2018) 29–38, 10.1016/j.jconrel.2017.12.034. [DOI] [PubMed] [Google Scholar]
- [148].Araújo F, Martins C, Azevedo C, Sarmento B, Chemical modification of drug molecules as strategy to reduce interactions with mucus, Adv. Drug Deliv. Rev. 124 (2018) 98–106, 10.1016/j.addr.2017.09.020. [DOI] [PubMed] [Google Scholar]
- [149].Lai SK, Wang YY, Hida K, Cone R, Hanes J, Erratum: nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses (Journal of Biological Chemistry (2010) 107, 2 (598–603) DOI: 10.1073/pnas.0911748107), [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Pullan RD, Thomas GAO, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J, Thickness of adherent mucus gel on colonic mucosa in humans and its relevance tocolitis, Gut 35 (1994)353–359, 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Yildiz HM, McKelvey CA, Marsac PJ, Carrier RL, Size selectivity of intestinal mucus to diffusing particulates is dependent on surface chemistry and exposure to lipids, J. Drug Target. 23 (2015) 768–774, 10.3109/1061186X.2015.1086359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Crater JS, Carrier RL, Barrier properties ofgastrointestinal mucus to nanoparticle transport, Macromol. Biosci. 10 (2010) 1473–1483, 10.1002/mabi.201000137. [DOI] [PubMed] [Google Scholar]
- [153].Marczynski M, Kasdorf BT, Altaner B, Wenzler A, Gerland U, Lieleg O, Transient binding promotes molecule penetration into mucin hydrogels by enhancing molecular partitioning, Biomater. Sci. 6 (2018) 3373–3387, 10.1039/c8bm00664d. [DOI] [PubMed] [Google Scholar]
- [154].Cone RA, Barrier properties of mucus, Adv. Drug Deliv. Rev. 61 (2009) 75–85, 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- [155].Liu M, Wu L, Zhu X, Shan W, Li L, Cui Y, Huang Y, Core-shell stability of nanoparticles plays an important role forovercomingthe intestinal mucus and epithelium barrier,J. Mater. Chem. B4(2016) 5831–5841, 10.1039/c6tb01199c. [DOI] [PubMed] [Google Scholar]
- [156].Bahamondez-Canas TF, Zhang H, Tewes F,Leal J, Smyth HDC, PEGylation of tobramycin improves mucus penetration and antimicrobial activity against pseudomonas aeruginosa biofilms in vitro, Mol. Pharm. 15 (2018) 1643–1652, 10.1021/acs.molpharmaceut.8b00011. [DOI] [PubMed] [Google Scholar]
- [157].Li P, Chen X, Shen Y, Li H, Zou Y, Yuan G, Hu P, Hu H, Mucus penetration enhanced lipid polymer nanoparticles improve the eradication rate of helicobacterpylori biofilm,J. Control. Release 300 (2019) 52–63, 10.1016/j.jconrel.2019.02.039. [DOI] [PubMed] [Google Scholar]
- [158].Dünnhaupt S, Kammona O, Waldner C, Kiparissides C, Bernkop-Schnürch A, Nano-carrier systems: strategies to overcome the mucus gel barrier, Eur.J. Pharm. Biopharm. 96 (2015) 447–453, 10.1016/j.ejpb.2015.01.022. [DOI] [PubMed] [Google Scholar]
- [159].Sheffner AL, The reduction in vitro in viscosity ofmucoprotein solutions by a new mucolytic agent, n-acetyl-l-cysteine, Ann. N. Y. Acad. Sci. 106 (1963) 298–310, 10.1111/j.1749-6632.1963.tb16647.x. [DOI] [PubMed] [Google Scholar]
- [160].Han HK, Shin HJ, Ha DH, Improved oral bioavailability of alendronate via the mucoadhesive liposomal delivery system, Eur. J. Pharm. Sci. 46 (2012) 500–507, 10.1016/j.ejps.2012.04.002. [DOI] [PubMed] [Google Scholar]
- [161].Morishita M, Kamei N, Ehara J, Isowa K, Takayama K, A novel approach using functional peptides forefficient intestinal absorption ofinsulin, J. Control. Release 118 (2007) 177–184, 10.1016/jjconrel.2006.12.022. [DOI] [PubMed] [Google Scholar]
- [162].Jain S, Patil SR, Swarnakar NK, Agrawal AK, Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes), Mol. Pharm. 9 (2012) 2626–2635, 10.1021/mp300202c. [DOI] [PubMed] [Google Scholar]
- [163].Wang YY, Lai SK, So C, Schneider C, Cone R,Hanes J, Mucoadhesive nanoparticles may disrupt the protective human mucus barrier by altering its microstructure, PLoS One 6 (2011) 1–7, 10.1371/journal.pone.0021547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Zhang S, Langer R, Traverso G, Nanoparticulate drug delivery systems targeting inflammation fortreatment ofinflammatory bowel disease, Nano Today 16 (2017) 82–96, http://dx.doi.org/10.1016Zj.nantod.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Lamprecht A, Ubrich N, Yamamoto H, Schàfer U, Takeuchi H, Maincent P, Kawashima Y, Lehr CM, Biodegradable nanoparticles fortargeted drug delivery intreatment ofinflammatory bowel disease,J. Pharmacol. Exp. Ther. 299(2001)775–781. [PubMed] [Google Scholar]
- [166].Jubeh TT, Barenholz Y, Rubinstein A, Differential adhesion of normal and inflamed rat colonic mucosa by charged liposomes, Pharm. Res. 21 (2004) 447–453, 10.1023/B:PHAM.0000019298.29561.cd. [DOI] [PubMed] [Google Scholar]
- [167].Zhang S,Ermann J, Succi MD, Zhou A, Hamilton MJ, Cao B,Korzenik JR, Glickman JN, Vemula PK, Glimcher LH, Traverso G, Langer R,Karp JM, An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease, Sci. Transl. Med. 7 (2015) 1–11, 10.1126/scitranslmed.aaa5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Foged C, Nielsen HM, Cell-penetrating peptides for drug delivery across, Expert Opin. Drug Deliv. (2008) 105–118. [DOI] [PubMed] [Google Scholar]
- [169].Tan X, Zhang Y, Wang Q, Ren T,Gou J, Guo W, Yin T, He H, Zhang Y, Tang X, Cell-penetrating peptide togetherwith PEG-modified mesostructured silica nanoparticles promotes mucous permeation and oral delivery of therapeutic proteins and peptides, Biomater. Sci. 7 (2019) 2934–2950, 10.1039/c9bm00274j. [DOI] [PubMed] [Google Scholar]
- [170].Bonengel S, Prüfert F, Perera G,Schauer J, Bernkop-Schnürch A, Polyethylene imine-6-phosphogluconic acid nanoparticles - a novel zeta potential changing system, Int.J. Pharm. 483 (2015) 19–25, 10.1016/j.ijpharm.2015.01.041. [DOI] [PubMed] [Google Scholar]
- [171].Jeong YJ, Lee DY, Choe K, Ahn H, Kim P, Park JH, Kim YC, Polypeptide-based polyelectrolyte complexes overcoming the biological barriers oforal insulin delivery, J. Ind. Eng. Chem. 48 (2017) 79–87, 10.1016/j.jiec.2016.12.022. [DOI] [Google Scholar]
- [172].Wang Y, Zhao Y, Cui Y, Zhao Q, Zhang Q, Musetti S, Kinghorn KA, Wang S, Overcoming multiple gastrointestinal barriers by bilayer modified hollow mesoporous silica nanocarriers, Acta Biomater. 65 (2018) 405–416, 10.1016/j.actbio.2017.10.025. [DOI] [PubMed] [Google Scholar]
- [173].Mao Y, Feng S, Zhang X, Zhao Q, Fang Y, Wang S, Thiolated polymer and cell-penetrating peptide dual-surface functionalization of mesoporous silicon nanoparticles to overcome intestinal barriers, J. Drug Deliv. Sci. Technol. 53 (2019), 101184, 10.1016/jjddst.2019.101184. [DOI] [Google Scholar]
- [174].Wu J, Zheng Y, Liu M, Shan W, Zhang Z, Huang Y, Biomimetic viruslike and charge reversible nanoparticles to sequentially overcome mucus and epithelial barriers fororal insulin delivery, ACS Appl. Mater. Interfaces 10 (2018) 9916–9928, 10.1021/acsami.7b16524. [DOI] [PubMed] [Google Scholar]
- [175].Kirtane AR, Abouzid O, Minahan D, Bensel T, Hill AL, Selinger C, Bershteyn A, Craig M, Mo SS, Mazdiyasni H, Cleveland C, Rogner J, Lee YAL, Booth L, Javid F, Wu SJ, Grant T, Bellinger AM, Nikolic B, Hayward A, Wood L, Eckhoff PA, Nowak MA, Langer R, Traverso G, Development of an oral once-weekly drug delivery system forHIV antiretroviral therapy, Nat. Commun. 9 (2018), 10.1038/s41467-017-02294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Hayward A, Bensel T, Mazdiyasni H,Rogner J, Kirtane AR, Lee YAL, Hua T, Bajpayee A,Collins J, McDonnell S, Cleveland C, Lopes A, Wahane A, Langer R, Traverso G, Scalable gastric resident systems forveterinary application, Sci. Rep. 8 (2018), 10.1038/s41598-018-30212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bellinger AM, Jafari M, Grant TM, Zhang S, Slater HC, Wenger EA, Mo S, Lee YAL, Mazdiyasni H, Kogan L, Barman R, Cleveland C, Booth L, Bensel T, Minahan D, Hurowitz HM, Tai T,Daily J, Nikolic B, Wood L, Eckhoff PA, Langer R, Traverso G, Oral, ultra-long-lasting drug delivery: applicationtoward malaria eliminationgoals, Sci. Transl. Med. 8 (2016), 10.1126/scitranslmed.aag2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kirtane AR, Hua T, Hayward A, Bajpayee A, Wahane A, Lopes A, Bensel T, Ma L, Stanczyk FZ, Brooks S, Gwynne D, Wainer J, Collins J, Tamang SM, Langer R, Traverso G, A once-a-month oral contraceptive, Sci. Transl. Med. 11 (2019), 10.1126/scitranslmed.aay2602, eaay2602. [DOI] [PubMed] [Google Scholar]
- [179].Traverso G, Schoellhammer CM, Schroeder A, Maa R, Lauwers GY, Polat BE, Anderson DG, Blankschtein D, Langer R, Microneedles fordrug deliveryviathe gastrointestinaltract,J. Pharm. Sci. 104 (2015)362–367, 10.1002/jps.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Bucheit JD, Pamulapati LG, Carter N, Malloy K, Dixon DL, Sisson EM, Oral semaglutide: a review ofthe first Oral glucagon-like peptide-1 receptor agonist, Diabetes Technol. Ther. 22 (1) (2019), 10.1089/dia.2019.0185/dia.2019.0185. [DOI] [PubMed] [Google Scholar]
- [181].Buckley ST, B$kdal TA, Vegge A, Maarbjerg SJ, Pyke C,Ahnfelt-Ronne J, Madsen KG, Schéele SG, Alanentalo T, Kirk RK, Pedersen BL, Skyggebjerg RB, Benie AJ, Strauss HM, Wahlund PO, Bjerregaard S, Farkas E, Fekete C, Sondergaard FL,Borregaard J, Hartoft-Nielsen ML, Knudsen LB, Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptoragonist, Sci. Transl. Med. 10 (2018), 10.1126/scitranslmed.aar7047. [DOI] [PubMed] [Google Scholar]
- [182].Graham L, Carter, Krebs MG, Jodrell D, Armstrong A, Kilgour E, Illidge T, Clarke J, Chown R, Williams K, Dive C, Yorke J, Dickinson C, Hughes A, Thistlethwaite F, Bristow R, Cook N, Meeting proceedings ofthe “Phase I: where science becomes medicine” Conference, Manchester, UK: Meeting Overview, J. Immunother. Precis. Oncol. 2 (2019) 156, 10.4103/2666-2345.269514. [DOI] [Google Scholar]
- [183].Jackson CGCA, Bayston KF, McLaren BR, Bremer L, Eden K, Kwan M-FR, Kramer D, Chan WK, Hung NA, Hung T, An open label, randomised cross-over bioavailability study of oral paclitaxel (oraxol) compared to intravenous paclitaxel 80mg/m 2, J. Clin. Oncol. 34 (2016) 2569, 10.1200/jco.2016.34.15_suppl.2569. [DOI] [Google Scholar]
- [184].Lee K-W, Lee KH, Zang DY, Park YI, Shin DB,Kim JW, Im S-A, Koh SA, Yu K-S,Cho J-Y, Jung J-A, Bang Y-J, Phase I/II study of weekly oraxol for the second-line treatment of patients with metastatic or recurrent gastric cancer, Oncologist 20 (2015) 896–897, 10.1634/theoncologist.2015-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Ph3 StudyTo Determine Safety, Tolerability&TumorResponse OfOraxol Compared To Taxol In Metastatic Breast Cancer - Full Text View - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT02594371 (accessed November 24, 2019).
- [186].Higaki K, Amnuaikit C, Kimura T, Strategies forovercoming the stratum corneum, Am. J. Drug Deliv. 1 (2003) 187–214, 10.2165/00137696-200301030-00004. [DOI] [Google Scholar]
- [187].Van Smeden J, Janssens M, Gooris GS, Bouwstra JA, The important role of stratum corneum lipids forthe cutaneous barrier function, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1841 (2014) 295–313, 10.1016/j.bbalip.2013.11.00. [DOI] [PubMed] [Google Scholar]
- [188].Kamboj S, Jhawat V, Saini V, Bala S, Recent advances in permeation enhancement techniques fortransdermal drug delivery systems: a review, Curr. Drug Ther. 8 (2014) 181–188, 10.2174/15748855113086660012. [DOI] [Google Scholar]
- [189].Lee DH,Oh JH,Chung JH, Glycosaminoglycan and proteoglycan in skin aging, J. Dermatol. Sci. 83 (2016) 174–181, 10.1016/j.jdermsci.2016.05.016. [DOI] [PubMed] [Google Scholar]
- [190].Yamaguchi K, Mitsui T, Aso Y, Sugibayashi K, Structure-permeability relationship analysis of the permeation barrier properties of the stratum corneum and viable epidermis/dermis of rat skin,J. Pharm. Sci. 97 (2008) 4391–4403, 10.1002/jps.21330. [DOI] [PubMed] [Google Scholar]
- [191].Nasrollahi SA, Taghibiglou C, Azizi E, Farboud ES, Cell-penetrating peptides as a novel transdermal drug delivery system, Chem. Biol. Drug Des. 80(2012) 639–646, 10.1111/cbdd.12008. [DOI] [PubMed] [Google Scholar]
- [192].Manosroi J, Lohcharoenkal W, Gotz F, Werner RG, Manosroi W, Manosroi A, Transdermal absorption and stability enhancement of salmon calcitonin byTat peptide, Drug Dev. Ind. Pharm. 39 (2013) 520–525, 10.3109/03639045.2012.684388. [DOI] [PubMed] [Google Scholar]
- [193].Menegatti S, Zakrewsky M, Kumar S,De Oliveira JS,Muraski JA, Mitragotri S, De Novo design of skin-penetrating peptides forenhanced transdermal delivery of peptide drugs, Adv. Healthc. Mater. 5 (2016) 602–609, 10.1002/adhm.201500634. [DOI] [PubMed] [Google Scholar]
- [194].Zorko M, Langel Ü, Cell-penetrating peptides: mechanism and kinetics of cargo delivery, Adv. Drug Deliv. Rev. 57 (2005) 529–545, 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- [195].Chang M, Li X, Sun Y, Cheng F, Wang Q, Xie X, Zhao W, Tian X, Effect of cationic cyclopeptides on transdermal and transmembrane delivery of insulin, Mol. Pharm. 10 (2013) 951–957, 10.1021/mp300667p. [DOI] [PubMed] [Google Scholar]
- [196].Chen M, Gupta V, Anselmo AC, Muraski JA, Mitragotri S, Topical delivery ofhyaluronic acid into skin using SPACE-peptide carriers,J. Control. Release 173 (2014) 67–74, 10.1016/jjconrel.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hsu T, Mitragotri S, Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 15816–15821, 10.1073/pnas.1016152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Kumar S, Zakrewsky M, Chen M, Menegatti S, Muraski JA, Mitragotri S, Peptides as skin penetration enhancers: mechanisms ofaction, J. Control. Release 199(2015) 168–178, 10.1016/jjconrel.2014.12.006. [DOI] [PubMed] [Google Scholar]
- [199].Zheng L, Hui Q, Tang L, Zheng L, Jin Z, Yu B, Wang Z, Lin P, Yu W, Li H, Li X, Wang X, Dettman RW, TAT-mediated acidic fibroblast growth factor delivery to the dermis improves wound healing of deep skin tissue in rat, PLoS One 10 (2015) 1–13, 10.1371/journal.pone.0135291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Jin LH,Bahn JH, Eum WS, Kwon HY, Jang SH, Han KH, Kang TC, Won MH, Kang JH, Cho SW, Park J, Choi SY, Transduction ofhuman catalase mediated by an HIV-1 Tat protein basic domain and arginine-rich peptides into mammalian cells, Free Radic. Biol. Med.31 (2001) 1509–1519, 10.1016/S0891-5849(01)00734-1. [DOI] [PubMed] [Google Scholar]
- [201].Cohen-Avrahami M, Shames AI, Ottaviani MF, Aserin A, Garti N, HIV-TAT enhances the transdermal delivery ofNSAID drugs from liquid crystalline mesophases,J. Phys. Chem. B 118 (2014) 6277–6287, 10.1021/jp412739p. [DOI] [PubMed] [Google Scholar]
- [202].Desai P, Patlolla RR, Singh M, Interaction of nanoparticles and cell-penetrating peptides with skin fortransdermal drug delivery, Mol. Membr. Biol. 27 (2010) 247–259, 10.3109/09687688.2010.522203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Chaulagain B, Jain A, Tiwari A, Verma A, Jain SK, Passive deliveryof protein drugs through transdermal route, Artif. Cells Nanomed. Biotechnol. 46 (2018) 472–487, 10.1080/21691401.2018.1430695. [DOI] [PubMed] [Google Scholar]
- [204].Lopes LB, Brophy CM, Furnish E, Flynn CR, Sparks O, Komalavilas P, Joshi L, Panitch A, Bentley MVLB, Comparative study of the skin penetration ofprotein transduction domains and a conjugated peptide, Pharm. Res. 22 (2005) 750–757, 10.1007/s11095-005-2591-x. [DOI] [PubMed] [Google Scholar]
- [205].Hou YW, Chan MH, Hsu HR, Liu BR, Chen CP, Chen HH, Lee HJ, Transdermal delivery ofproteins mediated by non-covalently associated arginine-rich intracellular delivery peptides, Exp. Dermatol. 16 (2007) 999–1006, http://dx.doi.Org/10.1111/j.1600-0625.2007.00622.x. [DOI] [PubMed] [Google Scholar]
- [206].Thorén PEG, Persson D, Esbjorner EK, Goksor M, Lincoln P, Nordén B, Membrane binding and translocation of cell-penetrating peptides, Biochemistry 43 (2004) 3471–3489, 10.1021/bi0360049. [DOI] [PubMed] [Google Scholar]
- [207].Ohtake K, Maeno T, Ueda H, Natsume H, Morimoto Y, Poly-L-arginine predominantly increases the paracellular permeability ofhydrophilic macromolecules across rabbit nasal epithelium in vitro, Pharm. Res. 20 (2003) 153–160, 10.1023/A:1022485816755. [DOI] [PubMed] [Google Scholar]
- [208].Ohtake K, Maeno T, Ueda H, Ogihara M, Natsume H, Morimoto Y, Poly-L-arginine enhances paracellular permeability via serine/threonine phosphorylation ofZO-1 and tyrosine dephosphorylation of occludin in rabbit nasal epithelium, Pharm. Res. 20 (2003) 1838–1845, 10.1023/B:PHAM.0000003383.86238.d1. [DOI] [PubMed] [Google Scholar]
- [209].Kumar S, Sahdev P, Perumal O, Tummala H, Identification of a novel skin penetration enhancement peptide by phage display peptide library screening, Mol. Pharm. 9 (2012) 1320–1330, 10.1021/mp200594z. [DOI] [PubMed] [Google Scholar]
- [210].Gautam A,Nanda JS,Samuel JS, Kumari M, Priyanka P, Bedi G, Nath SK, Mittal G, Khatri N, Raghava GPS, Topical delivery ofprotein and peptide using novel cell penetrating peptide IMT-P8, Sci. Rep. 6 (2016) 1–13, 10.1038/srep26278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Kim YC, Ludovice PJ, Prausnitz MR, Transdermal delivery enhanced by magainin pore-forming peptide,J. Control. Release 122 (2007)375–383, 10.1016/j.jconrel.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Kim YC, Late S, Banga AK, Ludovice PJ, Prausnitz MR, Biochemical enhancement of transdermal delivery with magainin peptide: modification of electrostatic interactions by changing pH, Int.J. Pharm. 362 (2008) 20–28, 10.1016/j.ijpharm.2008.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Cnubben NHP, Elliott GR, Hakkert BC, Meuling WJA,Van de Sandt JJM, Comparative in vitro-in vivo percutaneous penetration of the fungicide ortho-phenylphenol, Regul. Toxicol. Pharmacol. 35 (2002) 198–208, 10.1006/rtph.2001.1530. [DOI] [PubMed] [Google Scholar]
- [214].van de Sandt JJM, Comparative in vitro-in vivo percutaneous absorption of the pesticide propoxur, Toxicol. Sci. 58 (2000) 15–22, 10.1093/toxsci/58.1.15. [DOI] [PubMed] [Google Scholar]
- [215].Clowes HM, Scott RC,Heylings JR, Skin absorption: flow-through orstatic diffusion cells, Toxicol. Vitr. 8 (1994) 827–830, 10.1016/0887-2333(94)90078-7. [DOI] [PubMed] [Google Scholar]
- [216].Pepe D, Carvalho VFM, McCall M, De Lemos DP, Lopes LB, Transportan in nanocarriers improves skin localization and antitumor activity of paclitaxel, Int.J. Nanomed. 11 (2016) 2009–2019, 10.2147/IJN.S97331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Niu J, Chu Y, Huang YF, Chong YS, Jiang ZH, Mao ZW, Peng LH,Gao JQ, Transdermal gene delivery by functional peptide-conjugated cationic gold nanoparticle reverses the progression and metastasis ofcutaneous melanoma, ACS Appl. Mater. Interfaces 9 (2017) 9388–9401, 10.1021/acsami.6b16378. [DOI] [PubMed] [Google Scholar]
- [218].Chunmeng S, Tianmin C, Skin: a promising reservoir foradult stem cell populations, Med. Hypotheses 62 (2004) 683–688, 10.1016/j.mehy.2003.12.022. [DOI] [PubMed] [Google Scholar]
- [219].Johnson RM, Harrison SD, Maclean D, Therapeutic applications of cell-penetrating peptides, Methods Mol. Biol. 683 (2011)535–551, 10.1007/978-1-60761-919-2_38. [DOI] [PubMed] [Google Scholar]
- [220].Fonfria E, Maignel J, Lezmi S, Martin V, Splevins A, Shubber S, Kalinichev M, Foster K, Picaut P, Krupp J, The expanding therapeutic utility of botulinum neurotoxins, Toxins (Basel) 10 (2018), 10.3390/toxins10050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Plaas AHK, West LA, Wong-Palms S, Nelson FRT, Glycosaminoglycan sulfation in human osteoarthritis: disease-related alterations at the non-reducing termini ofchondroitin and dermatan sulfate, J. Biol. Chem. 273 (1998) 12642–12649, 10.1074/jbc.273.20.12642. [DOI] [PubMed] [Google Scholar]
- [222].Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA, Quantitative analysis and comparative regional investigation of the extracellularmatrix ofthe porcinetemporomandibularjoint disc, Matrix Biol. 24 (2005) 45–57, 10.1016/j.matbio.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Shapiro EM,Borthakur A, Gougoutas A, Reddy R, 23NA MRI accurately measures fixed charge density in articular cartilage, Magn. Reson. Med. 47 (2002) 284–291, 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Price FM, Levick JR, Mason RM, Glycosaminoglycan concentration in synovium and othertissues of rabbit knee in relation to synovial hydraulic resistance,J. Physiol. 495(1996) 803–820, 10.1113/jphysiol.1996.sp021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Hazleman BL, Chard MD, Riley GP, Glycosaminoglycans ofhuman rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis, Ann. Rheum. Dis. 53 (1994)367–376, 10.1136/ard.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Sanchez-Adams J, Willard VP, Athanasiou KA, Regional variation in the mechanical role of knee meniscus glycosaminoglycans, J. Appl. Physiol. 111 (2011) 1590–1596, 10.1152/japplphysiol.00848.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Herwig J, Egner E, Buddecke E, Chemical changes ofhuman kneejoint menisci in various stages of degeneration, Ann. Rheum. Dis. 43 (1984) 635–640, 10.1136/ard.43.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Iatridis JC, MacLean JJ, O’Brien M, Stokes IAF, Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs, Spine (Phila. Pa. 1976) 32 (2007) 1493–1497, 10.1097/BRS.0b013e318067dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Wang C, Witschey W, Elliott MA, Borthakur A, Reddy R, Measurement of intervertebral disc pressure with T1p MRI, Magn. Reson. Med. 64 (2010) 1721–1727, 10.1002/mrm.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Lohmander S, Antonopoulos CA, Friberg U, Chemical and metabolic heterogeneity ofchondroitin sulfate and keratan sulfate in guinea pig cartilage and nucleus pulposus, BBA - Gen. Subj. 304 (1973) 430–448, 10.1016/0304-4165(73)90263-8. [DOI] [PubMed] [Google Scholar]
- [231].Peng Y, Yu Y, Lin L, Liu X, Zhang X, Wang P, Hoffman P, Kim SY, Zhang F, Linhardt RJ, Glycosaminoglycans from bovine eye vitreous humour and interactionwithcollagentype II, Glycoconj.J.35 (2018) 119–128, 10.1007/s10719-017-9808-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Cowman MK, Lee HG, Schwertfeger KL,McCarthy JB, Turley EA, The content and size ofhyaluronan in biological fluids and tissues, Front. Immunol. 6 (2015), 10.3389/fimmu.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Yu Y, Chen Y, Mikael P, Zhang F, Stalcup AM, German R, Gould F,Ohlemacher J, Zhang H, Linhardt RJ, Surprising absence ofheparin inthe intestinal mucosa of baby pigs, Glycobiology. 27 (2017) 57–63, 10.1093/glycob/cww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Theocharis AD, Vynios DH, Papageorgakopoulou N, Skandalis SS, Theocharis DA, Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma, Int. J. Biochem. Cell Biol. 35 (2003) 376–390, 10.1016/S1357-2725(02)00264-9. [DOI] [PubMed] [Google Scholar]
- [235].Veiga DF, Bussolaro RA, Kobayashi EY, Medeiros VP, Martins JRM, Garcia EB, Novo NF, Nader HB,Ferreira LM, Glycosaminoglycans of abdominal skin after massive weight loss in post-bariatric female patients, Obes. Surg. 21 (2011) 774–782, 10.1007/s11695-011-0405-2. [DOI] [PubMed] [Google Scholar]
- [236].Byun S, Tortorella MD, Malfait AM, Fok K, Frank EH, Grodzinsky AJ, Transport and equilibrium uptake of a peptide inhibitor of PACE4 into articular cartilage is dominated by electrostatic interactions, Arch. Biochem. Biophys. 499 (2010) 32–39, http://dx.doi.org/10.1016Zj.abb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Zhang C, He T, Vedadghavami A, Bajpayee AG, Avidin-biotin technology to synthesize multi-arm nano-construct for drug delivery, MethodsX. 7 (2020), 10.1016/j.mex.2020.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Young CC, Vedadghavami A, Bajpayee AG, Bioelectricity for Drug Delivery: The Promise of Cationic Therapeutics, Bioelectricity. (2020), 10.1089/bioe.2020.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]