Abstract
PURPOSE:
Underlying mechanisms of the relationship between body fatness and colorectal cancer remain unclear. This study investigated associations of circulating metabolites with visceral (VFA), abdominal subcutaneous (SFA) and total fat area (TFA) in colorectal cancer patients.
METHODS:
Pre-surgery plasma samples from 212 patients (stage I-IV) from the ColoCare Study were used to perform targeted metabolomics. VFA, SFA and TFA were quantified by computed tomography scans. Partial correlation and linear regression analyses of VFA, SFA and TFA with metabolites were computed and corrected for multiple testing. Cox proportional hazards were used to assess two-year survival.
RESULTS:
In patients with metastatic tumors, SFA and TFA were statistically significantly inversely associated with 16 glycerophospholipids (SFA: pFDR range: 0.017-0.049; TFA: pFDR range: 0.029-0.048), while VFA was not. Doubling of ten of the aforementioned glycerophospholipids was associated with increased risk of death in patients with metastatic tumors, but not in patients with non-metastatic tumors (phet range: 0.00044 – 0.049). Doubling of PC ae C34:0 was associated with nine-fold increased risk of death in metastatic tumors (Hazard Ratio [HR], 9.05; 95% confidence interval [CI], 2.17-37.80); an inverse association was observed in non-metastatic tumors (HR, 0.17; 95% CI, 0.04-0.87; phet=0.00044).
CONCLUSION:
These data provide initial evidence that glycerophospholipids in metastatic colorectal cancer are uniquely associated with subcutaneous adiposity, and may impact overall survival.
Keywords: colorectal cancer, adipose tissue, survival, glycerophospholipids
Introduction
There is strong and consistent evidence that obesity is a major risk factor for colorectal cancer (CRC; as reviewed in (1-3)). Although it is assumed that this relationship extrapolates directly to that after cancer diagnosis, prior studies investigating the association of obesity with colorectal cancer survival yielded inconsistent results and reported a non-linear relationship (4-8). Patients diagnosed with an increased body mass index (BMI; BMI≥25 kg/m2) have a better prognosis compared to patients with normal BMI at diagnosis (BMI<25-≥18.5 kg/m2) (9, 10). Improved survival rates have further been observed among overweight or obese patients as compared to patients with a BMI below 22.5 kg/m2 (11). This conundrum in the association of BMI with CRC is recognized as the obesity paradox (12).
Given that the prevalence of obesity is suggested to increase annually by about 3.5% in colorectal cancer survivors (13), it is critical to understand the complex role of obesity in cancer survivorship, which represents an unmet clinical need.
Although, BMI is the most commonly used measure of body fatness in prospective studies and in the clinical setting (12), it does not allow an accurate assessment of the quantity of different adipose tissue types on cancer survival (14). Adipose tissue is a metabolically active organ (15, 16) with white adipose tissue being the key metabolically active compartment (16). White adipose tissue can further be separated into visceral adipose tissue and subcutaneous adipose tissue (17). Visceral and subcutaneous adipose tissue are two structurally and functionally distinct fat depots (15). These compartments are proposed to play distinct roles in cancer development and progression, independent of overall body composition (18).
Visceral adipose tissue has been associated with higher levels of tumor-promoting metabolites such as inflammation-related lipid metabolites, free arachidonic acid, phospholipases, and prostaglandin synthesis-related enzymes compared to subcutaneous adipose tissue (19-21). These data have mostly been results from studies in healthy individuals (20-22). In cancer patients, visceral adiposity has been associated with poorer clinical outcomes, such as postoperative complications, survival, and recurrence, in the short- and long-term (23, 24).
Subcutaneous adipose tissue has previously been positively associated with circulating leptin in cancer-free participants (25). Using an untargeted metabolomics approach, Kastenmueller and colleagues showed significant associations of SAT with cortisol (inversely) and N1-methyl-2-pyridone-5-carboxamide (positively) in plasma, and 3-sialyllactose (positively) in urine collected from healthy individuals (26). Prior studies investigating the prognostic relevance of subcutaneous adiposity in cancer patients have yielded inconsistent results, which appear to differ by tumor type and stage, possibly consistent with adiposity being a risk factor for some cancers (e.g., breast cancer). In patients diagnosed with hepatocellular carcinoma (27) or bone metastases (28) high SAT was associated with better survival. In a large retrospective Canadian study, including n=1,473 stage I-IV gastrointestinal and lung cancer patients, and n=273 patients diagnosed with metastatic renal cell carcinoma high SAT was an independent prognostic factor, predicting reduction in mortality (29). In the group of patients that were diagnosed with sarcopenia the longest survival was observed in patients with high SAT compared to patients with low SAT. In contrast, among n=3,225 women diagnosed with stage II and III breast cancer higher SAT was associated with increased risk of death (30). Finally, in a retrospective clinical study in non-metastatic colon cancer patients’ (n=167) changes in SATI were not associated with survival (31).
Research is needed that identifies underlying mechanisms of the obesity-colorectal cancer link considering distinct roles of body fat compartments.
To the best of our knowledge, there are no data on the associations between the plasma metabolome and different compartments of adipose tissue with overall survival in prospectively followed colorectal cancer patients. We therefore tested the hypothesis that visceral fat area (VFA) and abdominal subcutaneous fat area (SFA) have distinct metabolomics profiles that are differentially associated with overall survival in colorectal cancer patients. Furthermore, we have investigated the association of metabolites with total fat area (TFA) and compared results to VFA and SFA. We have previously reported differences in the metabolic and transcriptomic profiles of VFA and abdominal SFA and their associations with tumor stage (19). To further our understanding of differences in the plasma metabolic profile of VFA, SFA and TFA in non-metastatic and metastatic colorectal cancer we are leveraging pre-surgery blood samples and computed tomography (CT) scans from n=212 patients diagnosed with primary invasive colorectal cancer within the ColoCare Study (16). We further investigate the associations of metabolites that remain significant after adjustment for multiple testing with overall survival comparing patients with metastatic tumors to patients with non-metastatic tumors.
Methods
Study cohort
This study population includes patients from the international prospective ColoCare Study (Clinicaltrials.gov Identifier: NCT02328677), that has been described in detail in prior publications (19, 23, 32-34). The ColoCare Study includes men and women aged 18-89 years who were diagnosed with a primary invasive colorectal cancer (stages I-IV) undergoing surgery at clinics and sites internationally. The present study used data from n=212 patients recruited at the ColoCare Study site in Heidelberg, Germany, between October 2010 and December 2014.
Patients were recruited after diagnosis of colorectal cancer. Non‐fasting blood samples were collected from patients prior to surgery (baseline time point) at the University Clinic of Heidelberg. The time between surgery and blood draw was on average 1.9 days (Table 1). Electronic medical charts, including pathological reports, were reviewed to collect information on clinical characteristics (e.g., tumor stage and site, treatment regimen). Anthropometric indices (height, weight, waist and hip circumference) were measured at the clinic visit or were obtained from surgical anesthesia records. Data on health behaviors (e.g., smoking status) and medication use (e.g., non-steroidal anti-inflammatory drug (NSAIDs)) were obtained from questionnaires collected at baseline, prior to surgery. BMI was calculated as kg/m2. Patients were eligible for the present study if they had a pre-surgery blood sample available and a CT scan had been performed.
Table 1.
Description of baseline demographic and clinical characteristics.
| Overall study population |
Non- metastatic1 |
Metastatic2 | ||
|---|---|---|---|---|
| n=212 | n=167 | n=45 | ||
| Patients deceased, n (%) | 33 (16%) | 18 (11%) | 25 (56%) | |
| Survival time, months mean ± SD* | 21.6 ± 5.94 | 22.8 ± 4.55 | 17.4 ± 8.24 | <0.001 |
| Age at surgery, mean ± SD | 63.3 ± 12.54 | 64.6 ± 11.85 | 58.7 ± 14.02 | 0.005 |
| Age at death, mean ± SD* | 64.2 ± 13.32 | 68.9 ± 10.07 | 61.3 ± 14.44 | 0.11 |
| Age at blood donation, mean ± SD | 62.9 ± 12.49 | 64.1 ± 11.82 | 58.3 ± 13.90 | 0.005 |
| Time between blood draw and death (months) mean ± SD* | 9.7 ± 6.83 | 8.8 ± 7.68 | 10.2 ±6.38 | 0.47 |
| Time between diagnosis and surgery (days), mean ± SD | 61 ± 169 | 41 ± 165 | 133 ± 164 | 0.0014 |
| Time between surgery and blood draw (days) mean ± SD | 1.9 ± 6.51 | 2.1 ± 7.31 | 1.1 ± 0.79 | 0.098 |
| Sex, n (%) | ||||
| Female | 66 (31%) | 53 (32%) | 13 (29%) | 0.71 |
| Male | 146 (69%) | 114 (68%) | 32 (71%) | |
| BMI (kg/m2), n (%) | 0.021 | |||
| Underweight, <18.5 | 6 (3%) | 2 (1%) | 4 (9%) | |
| Normoweight, 18.5-24.9 | 69 (36%) | 51 (34%) | 18 (42%) | |
| Overweight, 25-29.9 | 86 (44%) | 69 (46%) | 17 (40%) | |
| Obese, ≥30 | 33 (17%) | 29 (19%) | 4 (9%) | |
| BMI (kg/m2), mean ± SD | 26.2 ± 4.26 | 26.6 ± 4.15 | 24.8 ± 4.39 | 0.012 |
| Tumor Site, n (%) | ||||
| Colon | 118 (56%) | 95 (57%) | 23 (51%) | 0.49 |
| Rectum | 94 (44%) | 72 (43%) | 22 (49%) | |
| Adjuvant treatment, n (%)* | 0.01 | |||
| No | 122 (60%) | 103 (65%) | 19 (43%) | |
| Yes | 81 (40%) | 56 (35%) | 25 (57%) | |
| Missing | 9 | 8 | 1 | |
| Neo-adjuvant treatment, n (%) | ||||
| No | 138 (65%) | 117 (70%) | 21 (47%) | 0.004 |
| Yes | 74 (35%) | 50 (30%) | 24 (53%) | |
| Fat Area (cm2), mean ± SD | ||||
| VFA, L3/4 | 178.30 ± 104.50 | 192.5 ± 105.46 | 140.9 ± 93.18 | 0.007 |
| SFA, L3/4 | 201.80 ± 94.55 | 211.9 ± 91.37 | 176.8 ± 98.83 | 0.053 |
| TFA, L3/4 | 376.92 ± 162.44 | 401.47 ± 153.00 | 316.20 ± 171.08 | 0.006 |
| VFA, L4/5 | 152.20 ± 83.56 | 165.8 ± 84.49 | 116.5 ± 70.26 | 0.001 |
| SFA, L4/5 | 233.80 ± 96.22 | 246.7 ± 91.98 | 201.8 ± 100.19 | 0.015 |
| TFA, L4/5 | 383.78 ± 149.11 | 410.54 ± 139.30 | 317.56 ± 153.67 | 0.001 |
Abbreviations: SD=Standard Deviation; BMI=Body Mass Index, VFA=Visceral Fat Area, SAT=Subcutaneous Fat Area.
Non-metastatic: colorectal cancer stages I-III
Metastatic: colorectal cancer stage IV
only deceased patients
Vital status was obtained through review of local medical records, follow-up mailings, requests for medical records from outside providers, and state or national cancer and death registries. Primary medical records were reviewed for any signs that a patient is deceased, followed by request of outside medical records, and any information received from follow-up mailings. Any informal reports such as from next-of-kin were confirmed through other data sources. Patient information was used to search national and local data sources for vital status. In Germany, every person is registered and vital status information including date of death can be reliably obtained at no cost from the Registration Office. The study was approved by the ethics committee of the medical faculty at the University of Heidelberg. All study participants provided written informed consent.
Area-based computed tomography (CT) quantification of abdominal adipose tissue
Abdominal CT scans conducted between August 2010 and December 2014 were assessed retrospectively using Centricity RIS 4.1i and GE PACS (GE Medical Systems, Buckinghamshire, UK) at the Department of Diagnostic and Interventional Radiology, University Hospital Heidelberg. CT scans were predominantly performed before surgery (mean time before: 42 days, after: 41 days). A prior study that used data from the present study population showed that pre- and post-surgical CT scans were similar and, thus, could be combined for statistical analyses (35). The quantification of VFA and abdominal SFA based on diagnostic CT scan data was performed using a dedicated post-processing software (Syngo Volume tool, MMPW, Siemens Healthineers, Erlangen, Germany).
Area-based quantification of adipose tissue compartments was performed on two spinal levels most representative of the abdominal adipose tissue distribution (L3/L4, L4/L5). The quantity of adipose tissue measured on levels L3/L4 has been reported (e.g., in the Framingham Heart Study) to best reflect the volume-based quantification of abdominal adipose tissue compartments including age- and sex-specific subgroups (36). Spinal level L4/L5 has been observed to be strongly correlated with diabetes and hypertension (37). By manually tracing specific regions of interest at L3/L4 and L4/L5, total fat area (TFA, whole circumference), VFA (along the fascial plane tracing the abdominal wall) were measured (volumetric quantification of selected slice, divided by slice thickness) (35). Adipose tissue was selected by limiting the measurements to a lower attenuation limit of −190 Hounsfield units (HU) and an upper attenuation limit of −30 HU (38). Abdominal SFA was determined by subtracting VFA from TFA.
Laboratory analysis, sample preparation, and quality control
Blood samples were collected and processed within four hours after sample blood draw, according to a standardized processing protocol, and stored at −80°C. Samples were shipped on dry ice to the International Agency for Research on Cancer (IARC) in Lyon, France for laboratory analysis using the AbsoluteIDQ p180 Kit (Biocrates Life Sciences AG, Innsbruck, Austria) following the procedure recommended by the vendor. The kit quantifies up to 188 metabolites from six compound classes (amino acids, biogenic amines, glycerophospholipids, sum of hexoses, sphingomyelins, acylcarnitines). Metabolites were selected based upon clinical and epidemiological relevance in colorectal carcinogenesis and progression, as well as direct links to body fatness. The instrumentation consisted of an AB Sciex Triple Quad 4500 mass spectrometer (MS/MS) equipped with an electrospray ion source and coupled with an Agilent Infinity 1290 ultra-high performance liquid chromatography (UHPLC) system. The amino acids and biogenic amines were quantified by UHPLC-MS/MS whereas lipids, sugar and acylcarnitines were analyzed by flow injection analysis on the same mass spectrometer (FIA-MS/MS). Chromatographic peaks (UPLC-MS/MS analyses) were integrated with the MultiQuant Software (AB Sciex, Framingham, MA, USA) and exported into the MetIDQ software (Biocrates Life Sciences AG, Innsbruck, Austria). For FIA-MS/MS analyses, files were directly exported to MetIDQ software to be parsed.
Each plate from the kit included three wells with phosphate buffer saline (PBS), used as a zero sample, seven wells with increasing concentration levels of standard mixes of amino acids and biogenic amines for calibration, as well as three quality control samples (QCs) supplied by Biocrates kit. All samples were analyzed once. QCs were lyophilized human plasma samples, to which 59 metabolites had been spiked at three concentration levels. In addition, two IARC QC samples (QC1 and QC2) were analyzed in duplicate in each 96-well plate. These QCs were two citrate plasma samples. To assess the quality of the data, intra- and inter-batch variabilities were calculated as coefficients of variation (CV) for all metabolites based on results obtained for the QC1 and QC2 samples. Metabolites were excluded if CVs (intra- and inter-batch) were above 20% (7 biogenic amines and 12 glycerophospholipids). In case a CV was above 20% for one of the two calculated values, we examined the inter-batch variability of the Biocrates QC samples to evaluate the validity of the data.
Concentrations below the calibration curve ranges were replaced by the median between zero and the lower limit of quantification (if not more than 5% of metabolite data were missing). Concentrations above the calibration curve were replaced by upper limits of quantification (ULOQ). For compounds semi-quantified (FIA-MS/MS; with one point calibration), the limit for reporting concentration values was the limit of detection (LOD), set to three times the median intensity value of the three PBS zero samples. Some compounds measured by FIA had concentration values close to the LOD, and were thus, often detected in a small fraction of the samples. Therefore, compounds detected in <10% of the samples were excluded.
After quality control, a total of 126 metabolites were retained for further analysis. These included n=76 phospholipids, n=20 amino acids, n=7 biogenic amines, n=14 sphingolipids and n=9 acylcarnitines. Data was acquired using Analyst 1.6.2 Software (AB Sciex). For LC-MS/MS analyses, MultiQuant 3.0.1 Software (AB Sciex) was used to integrate chromatographic peaks. A .txt file was generated, and exported into the MetIDQ software (version 5.5.4-DB100-2623 Boron, Biocrates). For FIA-MS/MS analyses, files were directly exported to MetIDQ software to be parsed.
Statistical analysis
Patients’ demographical and clinical characteristics were compared between non-metastatic (stage I/II/III) and metastatic (stage IV) tumors. Chi-squared tests and t-tests were used to test differences of patient characteristics with categorical and continuous variables by presence of metastasis, respectively.
Plasma metabolite concentrations were log-2 transformed, as the distribution is generally right-skewed and used as continuous variables in statistical analyses. Each unit increase corresponds to a doubling in concentration. Pearson’s partial correlations and linear regression models were applied to investigate the associations between different fat areas (VFA, SFA, and TFA) and plasma metabolites. In the regression model, metabolite concentrations are the outcomes and the fat area compartments are the predictors. Models were adjusted for age, sex, analytical batch and tumor stage in non-metastatic tumors (I/II/III). We used the Benjamini-Hochberg procedure to control the false discovery rate (FDR) and to account for multiple testing (39).
Cox proportional hazard models were computed to assess overall survival (OS) after 24 months of follow-up. Hazard ratios (HRs) and 95% confidence intervals (CIs) were computed and adjusted for age, sex, tumor stage, and analytical batch. Survival analyses were conducted for all patients (data not shown) and additionally stratified by the presence of metastasis (non-metastatic [stages I/II/III] versus metastatic [stage IV]). Metabolites that were emerging from the previous fat analysis and remained statistically significant after FDR adjustment were targeted in the survival analyses.
Heterogeneity in associations between metabolites and OS comparing patients with non-metastatic and metastatic tumors was assessed using likelihood‐ratio tests for the comparison of the model fit for logistic regression models with and without corresponding interaction terms (40). For each metabolite, model fit of Cox regressions was compared between the model with and without the interaction terms of non-metastatic (stages I/II/III) versus metastatic tumors (stage IV) × metabolite (continuous), given age, sex, tumor stage and analytical batch were included in the model. All analyses were conducted using SAS (version 9.4), and two-sided P values < 0.05 were considered as statistically significant. Forest plots were prepared using the R software (package ‘rmeta’, function ‘forestplot’) version 2.15.2 (R Core Team 2014).
Results
A total of n=212 colorectal cancer patients were included in the present study (Table 1). Seventy-nine percent of patients were diagnosed with non-metastatic tumors (n=167 out of n=212 patients) and 21% (n=45) were diagnosed with metastatic disease. Patients were followed for 24 months. After a median follow-up time of 10.98 months, a total of n=43 (20%) patients were deceased, including n=18 (11%) non-metastatic colorectal cancer patients (median follow-up of 12.61 months) and n=25 patients (56%) metastatic colorectal cancer patients (median-follow-up time of 10.69 months). Mean age at surgery differed statistically significantly by presence of metastasis; patients with metastatic disease were younger compared to patients diagnosed with non-metastatic disease (58.7 years vs. 64.6 years, p=0.005; [Table 1]).
The exact date of diagnosis was available for 94% of the patients. The median time between date of diagnosis and date of surgery was 34 days (mean=69.7 days, ± 104.0). The median time for patients diagnosed with metastatic tumors was 64 days (mean=132.7 days, ± 164.0) and was significantly longer compared to the median time of 29 days for patients diagnosed with non-metastatic tumors (mean=52.4 ± SD 164.0 days; p=0.0014). Patients diagnosed with metastatic tumors were more likely to receive neo-adjuvant treatment (p=0.004) and adjuvant treatment (p=0.01) compared to patients diagnosed with non-metastatic tumors.
BMI on a continuous scale was statistically significantly lower in patients diagnosed with metastatic tumors compared to patients diagnosed with non-metastatic tumors, both on a continuous scale (mean BMI: 26.6 kg/m2 vs BMI: 24.8 kg/m2, p=0.012, respectively) and using BMI categories as defined by the World Health Organization (p=0.021; Table 1). No significant differences were observed for tumor site (p=0.49) or sex (p=0.71).
Visceral, subcutaneous and total fat areas differed significantly by presence of metastasis. These differences were observed on both lumbar spine levels, although statistical significance was marginal for abdominal SFA at L3/L4. Patients diagnosed with metastatic tumors had on average a lower amount of VFA and abdominal SFA compared to patients with non-metastatic tumors (e.g., VFA: L3/L4: p=0.007, L4/L5: VFA: p=0.001; SFA: L3/L4: p=0.053, L4/L5: p=0.015; Table 1). A total of 126 plasma metabolites from five different compound classes (acylcarnitines, amino acids, biogenic amines, sphingolipids and glycerophospholipids) were used for the present analyses.
Correlations of visceral fat area with subcutaneous fat area
We observed significant, but modest, correlation of VFA with SFA in patients with non-metastatic tumors: level L3/4: r=0.27, p=0.008 and L4/5: r=0.29, p=0.0046. In patients diagnosed with metastatic tumors we observed significant and higher correlations of VFA with SFA: level L3/4: r=0.68, p<0.0001 and L4/5: r=0.71, p<0.0001.
Correlations of visceral, subcutaneous and total fat area with metabolites in patients diagnosed with non-metastatic CRC
We observed statistically significant inverse correlations between VFA and two metabolites in patients with non-metastatic tumors (Table 2): asparagine (r=−0.34, pFDR=0.04) and serine (r=−0.38, pFDR=0.02) after adjustment for multiple testing. Similarly, robust associations of VFA with these two metabolites were observed in linear regression models (asparagine, pFDR=0.017; serine pFDR=0.017; Table 2) TFA was inversely associated with PC aa C42:2 (r=−0.39, pFDR=0.03). No significant correlations were observed for abdominal SFA on both lumbar spine levels and plasma metabolites in patients with non-metastatic tumors with pFDR>0.64. All results are presented in Supplementary Tables 1 and 2.
Table 2.
Correlation coefficients and linear regression of fat areas and metabolites in patients diagnosed with non-metastatic colorectal cancer patients stages I-III adjusted for age, sex, tumor stage and analytical batch.*
| Fat Area | |||||
|---|---|---|---|---|---|
| Correlation coefficients | Metabolite* | Cases | r | pValue | pFDR |
| Level L3/L4 | |||||
| VFA | Asparagin | 105 | −0.37 | 0.0002 | 0.017 |
| VFA | Serine | 105 | −0.36 | 0.0003 | 0.017 |
| Level L4/L5 | |||||
| VFA | Asparagine | 105 | −0.34 | 0.0006 | 0.040 |
| VFA | Serine | 105 | −0.38 | 0.0001 | 0.016 |
| Linear regression models | |||||
| Level L3/L4 | Metabolite* | Cases |
Standardized ß-coefficent |
pValue | pFDR |
| VFA | Asparagin | 105 | −0.43 | 0.0002 | 0.017 |
| VFA | Serine | 105 | −0.43 | 0.0003 | 0.017 |
| TFA | PC aa C42:2 | 105 | −0.41 | 0.0003 | 0.03 |
| Level L4/L5 | |||||
| VFA | Asparagine | 105 | −0.37 | 0.0006 | 0.041 |
| VFA | Serine | 105 | −0.43 | 0.0001 | 0.016 |
Abbreviations: VFA=Visceral Fat Area, TFA=Total Fat Area, FDR=false discovery rate
Presented are metabolites that were significant after FDR-adjustment.
Correlations of visceral, subcutaneous and total fat area with metabolites in patients diagnosed with metastatic CRC
Statistically significant inverse associations of SFA with 15 glycerophospholipids were observed in patients diagnosed with metastatic tumors (pFDR range 0.017- 0.049). The strongest correlation coefficients were observed for: PC ae C34:0: r=−0.59, pFDR=0.020 and PC ae C36:1: r=−0.52, pFDR=0.04; level L3/L4; Table 3). Similarly, linear regression models revealed significant associations of SFA with these 15 glycerophospholipids after FDR adjustment (pFDR range=0.017-0.049), Table 4). Comparably, TFA was statistically significantly inversely associ ated with 12 glycerophospholipids in patients diagnosed with metastatic tumors (pFDR range 0.029- 0.049). The strongest correlation coefficient was observed for PC ae C40: 2: r=−0.61, pFDR=0.028; level L4/5 (Table 4). Linear regression models revealed similar associations of TFA with those glycerophospholipids after FDR adjustment (pFDR range=0.017-0.049), Table 4). We did not observe significant associations of VFA with any of the investigated metabolites (all pFDR>0.25). All results are presented in Supplementary Tables 3 and 4.
Table 3.
Correlation coefficients of subcutaneous fat areas (at level L3/L4 and L4/L5) and metabolites in metastatic colorectal cancer patients (stage IV) adjusted for age, sex and analytical batch.
| Fat Area | |||||
|---|---|---|---|---|---|
| Level L3/L4 | Metabolite * | Cases | r | pValue | pFDR2 |
| SFA | PC ae C40:2 | 38 | −0.61 | 0.0003 | 0.017 |
| SFA | PC ae C40:6 | 38 | −0.63 | 0.0002 | 0.017 |
| SFA | PC ae C34:0 | 38 | −0.59 | 0.0005 | 0.020 |
| SFA | PC aa C42:0 | 38 | −0.57 | 0.0007 | 0.024 |
| SFA | PC aa C42:5 | 38 | −0.54 | 0.0017 | 0.037 |
| SFA | PC ae C42:2 | 38 | −0.55 | 0.0015 | 0.037 |
| SFA | PC ae C30:0 | 38 | −0.52 | 0.0025 | 0.041 |
| SFA | PC ae C36:1 | 38 | −0.52 | 0.0026 | 0.041 |
| SFA | PC aa C40:6 | 38 | −0.50 | 0.0043 | 0.045 |
| SFA | PC aa C42:1 | 38 | −0.50 | 0.0046 | 0.045 |
| SFA | PC aa C42:4 | 38 | −0.50 | 0.0039 | 0.045 |
| SFA | PC ae C42:3 | 38 | −0.50 | 0.0042 | 0.045 |
| SFA | PC ae C44:6 | 38 | −0.50 | 0.0039 | 0.045 |
| SFA | PC ae C34:1 | 38 | −0.49 | 0.0053 | 0.048 |
| SFA | PC aa C38:0 | 38 | −0.48 | 0.0059 | 0.049 |
| TFA | PC aa C42:0 | 38 | −0.57 | 0.0008 | 0.048 |
| TFA | PC ae C40:6 | 38 | −0.59 | 0.0005 | 0.048 |
| Level L4/L5 | |||||
| SFA | PC ae C40:2 | 38 | −0.61 | 0.0003 | 0.034 |
| SFA | PC aa C42:4 | 38 | −0.53 | 0.0021 | 0.045 |
| SFA | PC aa C42:5 | 38 | −0.55 | 0.0015 | 0.045 |
| SFA | PC ae C30:0 | 38 | −0.53 | 0.0021 | 0.045 |
| SFA | PC ae C34:0 | 38 | −0.57 | 0.0009 | 0.045 |
| SFA | PC ae C40:6 | 38 | −0.54 | 0.0016 | 0.045 |
| SFA | PC aa C42:0 | 38 | −0.52 | 0.0029 | 0.046 |
| SFA | PC ae C34:1 | 38 | −0.51 | 0.0036 | 0.046 |
| SFA | PC ae C36:1 | 38 | −0.52 | 0.0027 | 0.046 |
| SFA | PC ae C42:2 | 38 | −0.51 | 0.0036 | 0.046 |
| TFA | PC aa C42:0 | 38 | −0.54 | 0.0019 | 0.030 |
| TFA | PC aa C42:4 | 38 | −0.54 | 0.0018 | 0.030 |
| TFA | PC aa C42:5 | 38 | −0.53 | 0.0019 | 0.030 |
| TFA | PC ae C30:0 | 38 | −0.53 | 0.0023 | 0.030 |
| TFA | PC ae C34:0 | 38 | −0.56 | 0.0009 | 0.030 |
| TFA | PC ae C34:1 | 38 | −0.57 | 0.0008 | 0.030 |
| TFA | PC ae C36:1 | 38 | −0.53 | 0.0023 | 0.030 |
| TFA | PC ae C36:2 | 38 | −0.50 | 0.0041 | 0.044 |
| TFA | PC ae C40:2 | 38 | −0.62 | 0.0002 | 0.028 |
| TFA | PC ae C40:6 | 38 | −0.58 | 0.0006 | 0.030 |
| TFA | PC ae C42:2 | 38 | −0.48 | 0.0061 | 0.048 |
| TFA | PC ae C44:6 | 38 | −0.50 | 0.004 | 0.044 |
| TFA | SM C26:1 | 38 | −0.55 | 0.001 | 0.030 |
| TFA | C16 | 38 | 0.48 | 0.006 | 0.048 |
| TFA | C18:1 | 38 | 0.48 | 0.005 | 0.048 |
| TFA | C18:2 | 38 | 0.48 | 0.0060 | 0.048 |
Abbreviations: SFA=Subcutaneous Fat Area, TFA=Total Fat Area, FDR=false discovery rate.
Presented are metabolites that were significant after FDR-adjustment.
Table 4.
Linear regression of subcutaneous and total fat areas (at level L3/L4 and L4/L5) and metabolites in metastatic colorectal cancer patients (stage IV) adjusted for age, sex and analytical batch.*
| Fat area | |||||
|---|---|---|---|---|---|
| Level L3/L4 | Metabolite * | Cases |
Standardized ß-coefficient |
pValue | pFDR2 |
| SFA | PC ae C40:2 | 38 | −0.65 | 0.0003 | 0.017 |
| SFA | PC ae C40:6 | 38 | −0.66 | 0.0002 | 0.017 |
| SFA | PC ae C34:0 | 38 | −0.59 | 0.0005 | 0.020 |
| SFA | PC aa C42:0 | 38 | −0.62 | 0.0007 | 0.024 |
| SFA | PC aa C42:5 | 38 | −0.59 | 0.0017 | 0.037 |
| SFA | PC ae C42:2 | 38 | −0.58 | 0.0015 | 0.037 |
| SFA | PC ae C30:0 | 38 | −0.56 | 0.0025 | 0.041 |
| SFA | PC ae C36:1 | 38 | −0.54 | 0.0026 | 0.041 |
| SFA | PC aa C40:6 | 38 | −0.54 | 0.0043 | 0.045 |
| SFA | PC aa C42:1 | 38 | −0.52 | 0.0046 | 0.045 |
| SFA | PC aa C42:4 | 38 | −0.53 | 0.0039 | 0.045 |
| SFA | PC ae C42:3 | 38 | −0.53 | 0.0042 | 0.045 |
| SFA | PC ae C44:6 | 38 | −0.50 | 0.0039 | 0.045 |
| SFA | PC ae C34:1 | 38 | −0.50 | 0.0053 | 0.048 |
| SFA | PC aa C38:0 | 38 | −0.49 | 0.0059 | 0.049 |
| TFA | PC aa C42:0 | 38 | −0.68 | 0.0008 | 0.048 |
| TFA | PC ae C40:6 | 38 | −0.68 | 0.0005 | 0.048 |
| Level L4/L5 | |||||
| SFA | PC ae C40:2 | 38 | −0.65 | 0.0003 | 0.034 |
| SFA | PC aa C42:4 | 38 | −0.56 | 0.0021 | 0.046 |
| SFA | PC aa C42:5 | 38 | −0.59 | 0.0015 | 0.046 |
| SFA | PC ae C30:0 | 38 | −0.57 | 0.0021 | 0.046 |
| SFA | PC ae C34:0 | 38 | −0.57 | 0.0009 | 0.046 |
| SFA | PC ae C40:6 | 38 | −0.57 | 0.0016 | 0.046 |
| SFA | PC aa C42:0 | 38 | −0.56 | 0.0029 | 0.046 |
| SFA | PC ae C34:1 | 38 | −0.52 | 0.0036 | 0.046 |
| SFA | PC ae C36:1 | 38 | −0.54 | 0.0027 | 0.046 |
| SFA | PC ae C42:2 | 38 | −0.53 | 0.0036 | 0.046 |
| TFA | PC aa C42_0 | 38 | −0.61 | 0.0019 | 0.030 |
| TFA | PC aa C42_4 | 38 | −0.60 | 0.0018 | 0.030 |
| TFA | PC aa C42_5 | 38 | −0.62 | 0.0019 | 0.030 |
| TFA | PC ae C30_0 | 38 | −0.60 | 0.0023 | 0.030 |
| TFA | PC ae C34_0 | 38 | −0.60 | 0.0010 | 0.030 |
| TFA | PC ae C34_1 | 38 | −0.62 | 0.0008 | 0.030 |
| TFA | PC ae C36_1 | 38 | −0.58 | 0.0023 | 0.030 |
| TFA | PC ae C36_2 | 38 | −0.53 | 0.0041 | 0.044 |
| TFA | PC ae C40_2 | 38 | −0.69 | 0.0002 | 0.028 |
| TFA | PC ae C40_6 | 38 | −0.65 | 0.0006 | 0.030 |
| TFA | PC ae C42_2 | 38 | −0.54 | 0.0058 | 0.048 |
| TFA | PC ae C44_6 | 38 | −0.53 | 0.0042 | 0.044 |
| TFA | SM C26:1 | 38 | −0.61 | 0.0014 | 0.030 |
| TFA | C16 | 38 | 0.55 | 0.0061 | 0.048 |
| TFA | C18:1 | 38 | 0.54 | 0.0058 | 0.048 |
| TFA | C18:2 | 38 | 0.56 | 0.0060 | 0.048 |
Abbreviations: SFA=Subcutaneous Fat Area, TFA: Total Fat Area, FDR=false discovery rate.
Presented are metabolites that were significant after FDR-adjustment.
Associations of metabolites significant after FDR adjustment with overall survival in patients diagnosed with non-metastatic and metastatic CRC
A doubling of serine was associated with a 90% reduced risk of death in patients with non-metastatic tumors (HR, 0.09; 95% CI, 0.01-0.85) and similarly a reduced risk in patients with metastatic tumors, although not statistically significant (HR, 0.44; 95% CI, 0.09-2.23).
We did not observe a significant association of asparagine with risk of death in patients with either non-metastatic [HR, 3.46; 95% CI, 0.38-31.55] or metastatic colorectal cancer patients (HR, 0.29; 95% CI, 0.04-2.24). Although not statistically significant, doubling of asparagine was associated with reduced risk of death in patients diagnosed with non-metastatic tumors, while doubling of asparagine in patients with metastatic tumors was associated with an increase in risk of death.
In patients with non-metastatic tumors we observed statistically significant inverse associations between four glycerophospholipids and overall survival, with up to 82% risk reduction of death (e.g., PC aa C36:1 [HR, 0.17; 95% CI, 0.03-0.87], PC ae C40:6 [HR, 0.18; 95% CI, 0.04-0.89], respectively; Figure 1). Among patients diagnosed with metastatic disease, a doubling of glycerophospholipid concentrations was associated with increased risk of death for seven glycerophospholipids, including a five-fold increase in risk of death for glycerophospholipid PC ae C40:2 (HR, 5.58; 95% CI, 1.16-26.74), and a seven-fold increased hazard of death for PC ae C30:0 (HR, 7.96; 95% CI, 1.84-34.48; Figure 1). Statistically significant heterogeneity in associations between patients with non-metastatic and metastatic disease was observed for ten glycerophospholipids: e.g., PC ae C36:1 (phet=0.00044) and PC ae C34:1 (phet=0.016; Figure 1).
Figure 1. Adjusted hazard of overall death for colorectal cancer patients (at two-year follow-up) by presence of metastasis.
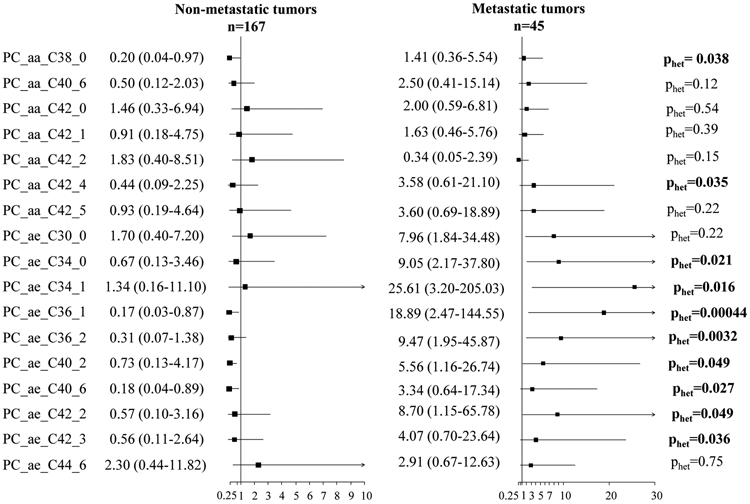
Analyses were adjusted for age, sex, stage (non-metastatic tumors) and analytical batch. The black box indicates the hazard ratio (HR), with horizontal grey lines representing the bounds of the 95% confidence interval (95% CI). Non-metastatic includes stage I – III tumors and metastatic includes stage IV tumors.
Associations of visceral, subcutaneous and total fat area with overall survival in patients diagnosed with non-metastatic and metastatic CRC
We performed survival analyses for all body compartments on both lumbar levels. Since results were comparable on level L3/L4 and level L4/L5, we present only data on level L3/L4. We did not observe significant associations of SFA, VFA or TFA with survival in patients diagnosed with non-metastatic tumors: SFA: HR, 0.99; 95% CI, 0.99-1.06, p=0.81; VFA: HR, 1.00; 95% CI, 0.99-1.01, p=0.97; TFA: HR, 1.00; 95% CI, 0.99-1.00, 0.89.
In patients diagnosed with metastatic tumors we observed statistically significant inverse associations for SFA and TFA but not VFA: SFA: HR, 0.99; 95% CI, 0.98-1.00, p=0.04; VFA: HR, 0.99; 95% CI, 0.99-1.00, p=0.05; TFA: HR, 0.99; 95% CI, 0.99-1.00, p=0.04. Notably the HR of all results was close to 1.00.
Sensitivity analyses
Adjustment for neo-adjuvant treatment and adjuvant treatment did not alter results (data not shown), with one exception. The association of PC ae C36:1 that was inversely, but not statistically significantly, associated with risk of death in patients diagnosed with stage I-III cancer became statistically significant after adjustment for adjuvant treatment: e.g. prior to adjustment: HR, 0.26; 95% CI, 0.05-1.23 and after adjustment for adjuvant treatment: HR, 0.14; 95% CI, 0.02-0.96.
Discussion
Our study of 212 patients diagnosed with primary invasive colorectal cancer showed statistically significant correlations of abdominal SFA and TFA with glycerophospholipids. These correlations differed when comparing patients with non-metastatic (stages I-III) and metastatic (stage IV) tumors. A strong inverse correlation between abdominal SFA and TFA and glycerophospholipids was observed in patients diagnosed with metastatic tumors, while no correlation was observed in patients diagnosed with non-metastatic tumors. We further investigated the association of metabolites emerging from these analyses and their association with overall two-year survival among colorectal cancer patients. Significant heterogeneity in the associations of glycerophospholipids with risk of death was observed comparing patients with non-metastatic and metastatic tumors. Doubling of glycerophospholipids was associated with reduced risk of death in patients diagnosed with non-metastatic tumors. On the contrary, doubling of glycerophospholipids in patients diagnosed with metastatic tumors was associated with an increased risk of death. While visceral fat area has been previously associated with increased risk and worse prognosis in colorectal cancer (19, 41, 42), the present study did not observe an association between VFA and circulating glycerophospholipids in either patients with non-metastatic or metastatic tumors.
To our knowledge, this is the first study that revealed differences in the correlations of abdominal SFA with glycerophospholipids comparing patients with non-metastatic and metastatic colorectal tumors. This is intriguing since previous studies, including ours, predominantly focused on VAT as a metabolically active organ that is critical in cancer development and progression (43-46). For example, we have previously shown that VFA, but not SFA, is associated with increased levels of pro-angiogenic cytokines (such as vascular endothelial growth factor [VEGF]) (47). Using state-of-the art metabolomics and transcriptomics we have reported that VAT displayed elevated markers of inflammatory lipid metabolism, free arachidonic acid, phospholipases, and prostaglandin synthesis-related enzymes compared to SAT in colorectal cancer patients.(19) There is further evidence that VAT adipocytes are more metabolically active compared to SAT adipocytes. (48) Based on this understanding, VAT has been the focus of prior research on the obesity-cancer link; however, a comprehensive understanding of the contribution of lipid metabolism in carcinogenic processes needs to consider the role of SFA as well.
Aerobic glycolysis, lipid and glutamine metabolism have been shown to be essential drivers of metastasis-promoting processes (49, 50). Metabolically active cancer cells require growth of new blood vessels, for the supply with essential nutrients, such as glycerophospholipids (51). Signaling pathways are stimulated to drive pro-angiogenic processes and direct interactions with cells and tissues in the tumor microenvironment, which play a critical role in the formation of new blood vessels and tumor progression (52). Tumor cells depend particular on reprogrammed lipid metabolic function for survival and growth to fulfill cholesterol needs for membrane biosynthesis and to complete de novo lipid synthesis. (53, 54)
Glycerophospholipids such as phosphatidylcholines and phosphatidylethanolamines (55) are ubiquitous metabolites and key components of cell lipid bilayers (55), and have a direct impact on membrane structure and signaling pathways. Changes in the composition of plasma glycerophospholipids may lead to improper membrane function and signaling, altered cell viability and proliferation, as documented by in vitro and in vivo studies (56, 57).
Prior research has revealed specific differences in lipid metabolism comparing visceral and subcutaneous adipose tissue. For example the basal lipolytic rate is higher in subcutaneous compared to omental adipocytes (58), and we have previously described differences in metabolic as well as transcriptomic pathways between visceral and subcutaneous adipose tissue (19).There is clinical evidence that lower subcutaneous adiposity in cancer patients is associated with increased overall mortality (HR: 1.26; p<0.01) compared to cancer patients with high subcutaneous adiposity (29).
These studies are in line with the presented findings of strong inverse correlations between SAT and glycerophospholipids in patients with newly diagnosed metastatic CRC. One possible meachanism underlying these results may be increasing subcutaneous adipocyte lipolysis that results in increased glycerophospholipid concentrations. Indeed, subcutaneous adipocyte lipolysis has been identified as independent contributor to circulating lipid concentrations (59), particularly circulating phosphatidylcholines (60).
There is in vitro evidence that exosomes derived from pancreatic cancer cell lines can initiate lipolysis in subcutaneous adipose tissue (61). Tumor cells of metastatic CRC are known to release exosomes into the circulation that mediate communication between cells and affect tumor-related and other metabolic processes in target cells, such as adipocytes (62). Although there are no data in colorectal cancer yet, a similar mechanism to that described in pancreatic cancer cells may be underlying the inverse association of SAT with glycerophospholipids in metastatic tumors.
Alterations in metabolic pathways related to lipolysis and apoptosis have been repeatedly linked to cachexia. Cachexia is a multifactorial induced energy balance disorder, where energy intake and expenditure are imbalanced (63). This disorder affects over 50% of cancer patients (mostly advanced cancer patients) and is suggested to indirectly cause about 20% of deaths in cancer patients (63). Cachexia is characterized by substantial weight loss mainly from muscle mass and body fat loss (63). While the molecular underpinnings of cachexia remain unclear, changes in several metabolic pathways including carbohydrate, lipid, and nitrogen metabolisms are key drivers of the drastic involuntary weight loss (63). Studies on cachexia and lipid metabolism suggest that the loss of body fat, particularly white adipose tissue is associated with increased lipolysis rather than a dysregulation of lipid synthesis (64). The observed significant inverse association of SFA with circulating glycerophospholipids limited to patients with metastatic disease is an indicator for an increased lipolysis of subcutaneous adipose tissue in advanced disease and disease-related symptoms, such as cachexia.
This observation further lends support to prior retrospective clinical studies that have observed increased concentrations of circulating glycerophospholipids in non-small cell lung cancer (65) and breast cancer patients (66). While these retrospective studies described changes in the glycerophospholipid metabolism in cancer patients free of metastasis (65, 66), recent in vitro data support the results from our prospective cohort study (57). Proliferating tumor cells are in need of increased aerobic glycolysis to ensure nutrient supply that is essential for highly proliferating cells (57). There is mechanistic evidence that increased glycosylation impacts cell-cell adhesion, and therefore, stimulates cancer invasiveness and development of metastasis (67, 68). Halama et al. have shown that a metabolic switch in numerous pathways including glycerophospholipid metabolism causes the progression and transition towards more aggressive phenotypes of cancer (57). This is in agreement with the present findings that higher concentrations of glycerophospholipids are associated with poorer overall survival in colorectal cancer patients with metastatic disease, but not in earlier stages of colorectal cancer.
Our study has several strengths and limitations. To date, this is the largest study to evaluate associations between different fat areas and plasma metabolic profiles in non-metastatic and metastatic colorectal cancer patients. The presented results have to be interpreted with caution given the potential reverse causation, as the associations of metabolites with fat areas in patients with metastatic disease may be a consequence of pre-existing cachexia, which can distort the true relationship between adipose tissue and metabolomics.
Yet, with regards to the association of plasma metabolites with overall survival, sample size was limited and results need to be replicated in additional studies. A limitation of this study is that we have no additional information on whether the cause of death is colorectal cancer or other causes. An advantage of this study is the use of data from a well-characterized cohort of prospectively followed colorectal cancer patients. (16).
The stability of metabolite measurements over a two-year period has previously been shown (69). Repeated intra-individual measurements for the identified glycerophospholipids in plasma have demonstrated reasonable intra-class correlation coefficients: PC aa C42:2 r=0.55, PC ae C34:0: r=0.78, PC ae C36:0: r=0.70 and PC ae C36:1: r=0.87(69).
Determination of adipose tissue areas using CT imaging is another strength of this study, as it provides a reliable and non-invasive method to quantify body composition as compared to BMI (70) and dual-energy x-ray absorptiometry (DXA). Prior research has shown that DXA is likely to underestimate VAT mass at low VAT levels and overestimate it at high VAT levels. (71, 72). Considering the advantages of CT scans compared to BMI and DXA and the general availability of CT scans as part of clinical routine, it was decided to use this approach to quantify VAT, SAT and TAT in this prospectively followed cohort of cancer patients. Given the relative size of the SFA in comparison to VFA, it is plausible that SFA is more broadly involved in cancer metabolism.
The follow-up time of 24 months may have been inadequate time for events to occur in patients with non-metastatic disease. Since the five-year follow-up has not been completed for all patients yet, we decided to use two-year overall survival as primary outcome for the present study. Our data provide initial evidence that metabolic profiles are different between patients with metastatic and non-metastatic tumors and are uniquely linked to abdominal subcutaneous but not visceral adiposity. The results regarding associations of glycerophospholipids with survival in patients with metastatic disease are intriguing. Further clinical as well as mechanistic studies are needed to improve our understanding of the role of glycerophospholipid metabolism in cancer progression. Together, the present findings yield promising new avenues to enhance our understanding of processes that are linked to the development of metastasis.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the contribution of all ColoCare participants. ColoCare samples were stored by the liquid biobank of the National Center for Tumor Diseases according to the SOPs of the Biomaterialbank Heidelberg. Lin Zielske, Anett Brendel, Renate Skatula, Marita Wenzel supported biobanking at the National Center for Tumor Diseases. In addition, we would like to thank Rifraz Farook and Werner Diehl for their data management support and the ColoCare team, specifically Torsten Kölsch, Clare Abbenhardt-Martin, Susanne Jakob, and Judith Kammer for patient recruitment and follow-up.
Financial Support: Researchers involved in this work were funded by the ERA-NET, TRANSCAN project 01KT1503, the National Cancer Institute project R01 CA189184, R01 CA207371, U01 CA206110, and P30 CA042014, the Huntsman Cancer Foundation, as well as the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the National Human Genome Research Institute. This study was further supported by the Austrian Science Fund (FWF, Austria; project no. 1578-B19), the Federal Ministry of Education and Research (BMBF, Germany; project no. 01KT1512), the National Cancer Institute (INCa, France; project no. 2014-007), The Research Council of Norway (RCN, Norway; project no. 236564/H10), the Dutch Cancer Society (KWF Kankerbestrijding), and the Netherlands Organization for Health Research and Development (ZonMw, the Netherlands; project no. UW2013-6397) coordinated by the ERA-NET, JTC 2012 call on Translational Cancer Research (TRANSCAN).
Abbreviations
- BMI
Body Mass Index
- CALS
Concentration levels of standard mixes for amino acids and biogenic amines calibration
- CI
Confidence interval
- CRC
Colorectal cancer
- CT
Computed tomography
- CV
Coefficient of variation
- DXA
Dual-energy x-ray absorptiometry
- FDR
False discovery rate
- FIA
Flow injection analysis
- HR
Hazard ratio
- HU
Hounsfield units
- IARC
International Agency for Research on Cancer
- LC
Liquid chromatography
- LLOQ
Lower limit of quantification
- LOD
Limit of detection
- MS
Mass spectrometry
- NSAID
Non-steroidal anti-inflammatory drugs
- OS
Overall survival
- PBS
Phosphate buffer saline
- QC
Quality control
- SAT
Subcutaneous adipose tissue
- SFA
Subcutaneous fat area
- SATI
Subcutaneous adiposity index
- TAT
Total adipose tissue
- TFA
Total fat area
- UHPLC
Ultra-high performance liquid chromatography
- ULOQ
Upper limit of quantification
- VAT
Visceral adipose tissue
- VEGF
Vascular endothelial growth factor
- VFA
Visceral fat area
Footnotes
Disclosure: C.M.U. has as cancer center director oversight over research funded by several pharmaceutical companies, but has not received funding directly herself. The remaining authors declare no conflict of interest.
References
- 1.Murphy N, Jenab M, Gunter MJ. (2018) Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nature reviews. Gastroenterology & hepatology 15: 659–70. [DOI] [PubMed] [Google Scholar]
- 2.Moghaddam AA, Woodward M, Huxley R. (2007) Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 16: 2533–47. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, Zhou J, Zhu Y, et al. (2017) Abdominal obesity and colorectal cancer risk: systematic review and meta-analysis of prospective studies. Bioscience reports. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caan BJ, Meyerhardt JA, Kroenke CH, et al. (2017) Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 26: 1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. (2019) The Impact of Muscle and Adipose Tissue on Long-term Survival in Patients With Stage I to III Colorectal Cancer. Diseases of the colon and rectum. 62: 549–60. [DOI] [PubMed] [Google Scholar]
- 6.Charette N, Vandeputte C, Ameye L, et al. (2019) Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: a post hoc analysis of two non-randomized phase II trials. BMC Cancer. 19: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renehan AG, Sperrin M. (2016) The Obesity Paradox and Mortality After Colorectal Cancer: A Causal Conundrum. JAMA Oncol. 2: 1127–9. [DOI] [PubMed] [Google Scholar]
- 8.Kroenke CH, Neugebauer R, Meyerhardt J, et al. (2016) Analysis of Body Mass Index and Mortality in Patients With Colorectal Cancer Using Causal Diagrams. JAMA Oncol. 2: 1137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. (2005) Life After Cancer Epidemiology (LACE) Study: a cohort of early stage breast cancer survivors (United States). Cancer causes & control : CCC. 16: 545–56. [DOI] [PubMed] [Google Scholar]
- 10.Renehan AG, Zwahlen M, Egger M. (2015) Adiposity and cancer risk: new mechanistic insights from epidemiology. Nature reviews. Cancer. 15: 484–98. [DOI] [PubMed] [Google Scholar]
- 11.Lennon H, Sperrin M, Badrick E, Renehan AG. (2016) The Obesity Paradox in Cancer: a Review. Current oncology reports. 18: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. (2014) Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nature reviews. Endocrinology. 10: 455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. (2016) Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 34: 3133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JC, Caan BJ, Prado CM, et al. (2020) The Association of Abdominal Adiposity With Mortality in Patients With Stage I-III Colorectal Cancer. Journal of the National Cancer Institute. 112: 377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaitkus JA, Celi FS. (2017) The role of adipose tissue in cancer-associated cachexia. Exp Biol Med (Maywood). 242: 473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulrich CM, Himbert C, Holowatyj AN, Hursting SD. (2018) Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nature reviews. Gastroenterology & hepatology. 15: 683–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Himbert C, Delphan M, Scherer D, Bowers LW, Hursting S, Ulrich CM. (2017) Signals from the Adipose Microenvironment and the Obesity-Cancer Link-A Systematic Review. Cancer prevention research (Philadelphia, Pa.). 10: 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. (2017) Gut microbiota modulation of chemotherapy efficacy and toxicity. Nature reviews. Gastroenterology & hepatology. [DOI] [PubMed] [Google Scholar]
- 19.Liesenfeld DB, Grapov D, Fahrmann JF, et al. (2015) Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare study. The American journal of clinical nutrition. 102: 433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neamat-Allah J, Johnson T, Nabers D, et al. (2015) Can the use of blood-based biomarkers in addition to anthropometric indices substantially improve the prediction of visceral fat volume as measured by magnetic resonance imaging? Eur J Nutr. 54: 701–8. [DOI] [PubMed] [Google Scholar]
- 21.Boulet MM, Chevrier G, Grenier-Larouche T, et al. (2015) Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. American journal of physiology. Endocrinology and metabolism. 309: E736–46. [DOI] [PubMed] [Google Scholar]
- 22.Szymanska E, Bouwman J, Strassburg K, et al. (2012) Gender-dependent associations of metabolite profiles and body fat distribution in a healthy population with central obesity: towards metabolomics diagnostics. Omics. 16: 652–67. [DOI] [PubMed] [Google Scholar]
- 23.Ozoya OO, Siegel EM, Srikumar T, Bloomer AM, DeRenzis A, Shibata D. (2017) Quantitative Assessment of Visceral Obesity and Postoperative Colon Cancer Outcomes. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 21: 534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark W, Siegel EM, Chen YA, et al. (2013) Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg. 216: 1070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JJ, Britton KA, Pedley A, et al. (2016) Adipose Tissue Depots and Their Cross-Sectional Associations With Circulating Biomarkers of Metabolic Regulation. J Am Heart Assoc. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otto L, Budde K, Kastenmuller G, et al. (2020) Associations between adipose tissue volume and small molecules in plasma and urine among asymptomatic subjects from the general population. Sci Rep. 10: 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi T, Kawai H, Nakano O, et al. (2018) Prognostic value of subcutaneous adipose tissue volume in hepatocellular carcinoma treated with transcatheter intra-arterial therapy. Cancer Manag Res. 10: 2231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang WC, Tsang NM, Chuang CC, et al. (2020) Association of subcutaneous and visceral adipose tissue with overall survival in Taiwanese patients with bone metastases - results from a retrospective analysis of consecutively collected data. PLoS One. 15: e0228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebadi M, Martin L, Ghosh S, et al. (2017) Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer. 117: 148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradshaw PT, Cespedes Feliciano EM, Prado CM, et al. (2019) Adipose Tissue Distribution and Survival Among Women with Nonmetastatic Breast Cancer. Obesity (Silver Spring). 27: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung E, Lee HS, Cho ES, et al. (2019) Changes in Body Composition During Adjuvant FOLFOX Chemotherapy and Overall Survival in Non-Metastatic Colon Cancer. Cancers. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gigic B, Boeing H, Toth R, et al. (2018) Associations Between Dietary Patterns and Longitudinal Quality of Life Changes in Colorectal Cancer Patients: The ColoCare Study. Nutr Cancer. 70: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liesenfeld D, Habermann N, Toth R, et al. (2015) Changes in urinary metabolic profiles of colorectal cancer patients enrolled in a prospective cohort study (ColoCare). Metabolomics : Official journal of the Metabolomic Society. 11: 998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skender S, Bohm J, Schrotz-King P, et al. (2017) Plasma 25-Hydroxyvitamin D3 Levels in Colorectal Cancer Patients and Associations with Physical Activity. Nutr Cancer. 69: 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nattenmueller J, Hoegenauer H, Boehm J, et al. (2016) CT-based compartmental quantification of adipose tissue versus body metrics in colorectal cancer patients. European radiology. 26: 4131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. (2010) Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond). 34: 781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balentine CJ, Marshall C, Robinson C, et al. (2010) Validating quantitative obesity measurements in colorectal cancer patients. J Surg Res. 164: 18–22. [DOI] [PubMed] [Google Scholar]
- 38.Yoshizumi T, Nakamura T, Yamane M, et al. (1999) Abdominal fat: standardized technique for measurement at CT. Radiology. 211: 283–6. [DOI] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological). 57: 289–300. [Google Scholar]
- 40.DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials. 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 41.Choe EK, Kim D, Kim HJ, Park KJ. (2013) Association of visceral obesity and early colorectal neoplasia. World J Gastroenterol. 19: 8349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CS, Murphy DJ, McMahon C, et al. (2015) Visceral Adiposity is a Risk Factor for Poor Prognosis in Colorectal Cancer Patients Receiving Adjuvant Chemotherapy. J Gastrointest Cancer. 46: 243–50. [DOI] [PubMed] [Google Scholar]
- 43.Petrangeli E, Coroniti G, Brini AT, et al. (2016) Hypoxia Promotes the Inflammatory Response and Stemness Features in Visceral Fat Stem Cells From Obese Subjects. J Cell Physiol. 231: 668–79. [DOI] [PubMed] [Google Scholar]
- 44.Lee JY, Lee HS, Lee DC, et al. (2014) Visceral fat accumulation is associated with colorectal cancer in postmenopausal women. PLoS One. 9: e110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto N, Fujii S, Sato T, et al. (2012) Impact of body mass index and visceral adiposity on outcomes in colorectal cancer. Asia Pac J Clin Oncol. 8: 337–45. [DOI] [PubMed] [Google Scholar]
- 46.Donohoe CL, Doyle SL, Reynolds JV. (2011) Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Himbert C, Ose J, Nattenmuller J, et al. (2019) Body Fatness, Adipose Tissue Compartments, and Biomarkers of Inflammation and Angiogenesis in Colorectal Cancer: The ColoCare Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 28: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim MM. (2010) Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 11: 11–8. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Han J. (2017) Branched-chain amino acid transaminase 1 (BCAT1) promotes the growth of breast cancer cells through improving mTOR-mediated mitochondrial biogenesis and function. Biochem Biophys Res Commun. 486: 224–31. [DOI] [PubMed] [Google Scholar]
- 50.Palmieri EM, Menga A, Martin-Perez R, et al. (2017) Pharmacologic or Genetic Targeting of Glutamine Synthetase Skews Macrophages toward an M1-like Phenotype and Inhibits Tumor Metastasis. Cell Rep. 20: 1654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanahan D, Weinberg RA. (2011) Hallmarks of cancer: the next generation. Cell. 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 52.Quail DF, Joyce JA. (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med. 19: 1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward PS, Thompson CB. (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 21: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. (2016) Lipid metabolic reprogramming in cancer cells. Oncogenesis. 5: e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dolce V, Cappello AR, Lappano R, Maggiolini M. (2011) Glycerophospholipid synthesis as a novel drug target against cancer. Curr Mol Pharmacol. 4: 167–75. [DOI] [PubMed] [Google Scholar]
- 56.Kurabe N, Hayasaka T, Ogawa M, et al. (2013) Accumulated phosphatidylcholine (16:0/16:1) in human colorectal cancer; possible involvement of LPCAT4. Cancer Sci. 104: 1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halama A, Guerrouahen BS, Pasquier J, Satheesh NJ, Suhre K, Rafii A. (2017) Nesting of colon and ovarian cancer cells in the endothelial niche is associated with alterations in glycan and lipid metabolism. Sci Rep. 7: 39999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Virtanen KA, Lonnroth P, Parkkola R, et al. (2002) Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab. 87: 3902–10. [DOI] [PubMed] [Google Scholar]
- 59.Ryden M, Arner P. (2017) Subcutaneous Adipocyte Lipolysis Contributes to Circulating Lipid Levels. Arterioscler Thromb Vasc Biol. 37: 1782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jung TW, Kim ST, Lee JH, et al. (2017) Phosphatidylcholine Causes Lipolysis and Apoptosis in Adipocytes through the Tumor Necrosis Factor Alpha-Dependent Pathway. Pharmacology. 101: 111–9. [DOI] [PubMed] [Google Scholar]
- 61.Sagar G, Sah RP, Javeed N, et al. (2016) Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut. 65: 1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, Xie Y, Xu L, et al. (2017) Protein content and functional characteristics of serum-purified exosomes from patients with colorectal cancer revealed by quantitative proteomics. Int J Cancer. 140: 900–13. [DOI] [PubMed] [Google Scholar]
- 63.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. (2014) Cancer cachexia: understanding the molecular basis. Nature reviews. Cancer. 14: 754–62. [DOI] [PubMed] [Google Scholar]
- 64.Dahlman I, Mejhert N, Linder K, et al. (2010) Adipose tissue pathways involved in weight loss of cancer cachexia. Br J Cancer. 102: 1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marien E, Meister M, Muley T, et al. (2015) Non-small cell lung cancer is characterized by dramatic changes in phospholipid profiles. Int J Cancer. 137: 1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mistry DA, French PW. (2016) Circulating Phospholipids as Biomarkers of Breast Cancer: A Review. Breast Cancer (Auckl). 10: 191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinho SS, Figueiredo J, Cabral J, et al. (2013) E-cadherin and adherens-junctions stability in gastric carcinoma: functional implications of glycosyltransferases involving N-glycan branching biosynthesis, N-acetylglucosaminyltransferases III and V. Biochim Biophys Acta. 1830: 2690–700. [DOI] [PubMed] [Google Scholar]
- 68.Liwosz A, Lei T, Kukuruzinska MA. (2006) N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J Biol Chem. 281: 23138–49. [DOI] [PubMed] [Google Scholar]
- 69.Carayol M, Licaj I, Achaintre D, et al. (2015) Reliability of Serum Metabolites over a Two-Year Period: A Targeted Metabolomic Approach in Fasting and Non-Fasting Samples from EPIC. PLoS One. 10: e0135437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goodpaster BH, Thaete FL, Kelley DE. (2000) Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 904: 18–24. [DOI] [PubMed] [Google Scholar]
- 71.Fang H, Berg E, Cheng X, Shen W. (2018) How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care. 21: 360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheung AS, de Rooy C, Hoermann R, et al. (2016) Correlation of visceral adipose tissue measured by Lunar Prodigy dual X-ray absorptiometry with MRI and CT in older men. Int J Obes (Lond). 40: 1325–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


