Abstract
B vitamins involved in one-carbon metabolism have been implicated in the development of inflammation- and angiogenesis-related chronic diseases, such as colorectal cancer (CRC). Yet, the role of one-carbon metabolism in inflammation and angiogenesis among CRC patients remains unclear. The objective of this study was to investigate associations of components of one-carbon metabolism with inflammation and angiogenesis biomarkers among newly diagnosed CRC patients (n 238) in the prospective ColoCare Study, Heidelberg. We cross-sectionally analysed associations between twelve B vitamins and one-carbon metabolites and ten inflammation and angiogenesis biomarkers from pre-surgery serum samples using multivariable linear regression models. We further explored associations among novel biomarkers in these pathways with Spearman partial correlation analyses. We hypothesised that pyridoxal-5’-phosphate (PLP) is inversely associated with inflammatory biomarkers. We observed that PLP was inversely associated with C-reactive protein (CRP) (r −0·33, Plinear < 0·0001), serum amyloid A (SAA) (r −0·23, Plinear = 0·003), IL-6 (r −0·39, Plinear < 0·0001), IL-8 (r −0·20, Plinear = 0·02) and TNFα (r −0·12, Plinear = 0·045). Similar findings were observed for 5-methyl-tetrahydrofolate and CRP (r −0·14), SAA (r −0·14) and TNFα (r −0·15) among CRC patients. Folate catabolite acetyl-para-aminobenzoylglutamic acid (pABG) was positively correlated with IL-6 (r 0·27, Plinear < 0·0001), and pABG was positively correlated with IL-8 (r 0·21, Plinear < 0·0001), indicating higher folate utilisation during inflammation. Our data support the hypothesis of inverse associations between PLP and inflammatory biomarkers among CRC patients. A better understanding of the role and inter-relation of PLP and other one-carbon metabolites with inflammatory processes among colorectal carcinogenesis and prognosis could identify targets for future dietary guidance for CRC patients.
Keywords: One-carbon metabolism, B vitamins, Folate, Vitamin B6, Inflammation, Colorectal cancer, Angiogenesis
Colorectal cancer (CRC) is the third most common cancer among men and the second most common cancer among women worldwide, contributing to >200 000 deaths in 2018 in Europe(1). There is accumulating evidence that up to 50 % of all CRC cases may be preventable through diet, physical activity and being a healthy body weight(2) and that diet could be an important predictor of CRC recurrence and survival(3,4). To date, there are no evidence-based dietary guidelines for CRC survivors and it is recommended to follow the guidelines for cancer prevention(2), including reducing processed meat and alcohol consumption. Evidence is limited for other nutrients and foods(2). Few studies have evaluated dietary intake after CRC diagnosis, whether by dietary recall or dietary biomarkers, nor its association with CRC outcomes. Nonetheless, emerging data suggest that dietary factors implicated in colorectal carcinogenesis may not have the same impact on CRC progression and invasion; thus, further research is needed to support the evidence base for CRC dietary guidance post-diagnosis(5). With the increasing rates of CRC among younger adults <50 years and the increasing prevalence of CRC survivors in Europe(6), the development of dietary recommendations for this population is of major public health relevance.
Evaluating the association of dietary factors with biological pathways implicated in CRC cancer progression among CRC patients may shed light on potential strategies for intervention to improve prognosis. Important pathways in the development, promotion and progression of CRC include inflammation(7–11) and angiogenesis(12,13). Inflammatory bowel diseases and obesity, two strong CRC risk factors, promote inflammation, whereas non-steroidal anti-inflammatory drugs are associated with lower CRC risk(7–9). Inflammatory chemokines are immune modulators that promote angiogenesis, a key feature of tumour progression, supported by vascular endothelial growth factors (VEGF)(12,13). Modulation of one-carbon metabolites that serve many biological functions(14) (e.g. vitamin B6 and folate) has been shown to lower circulating inflammatory biomarkers in non-cancer populations(15), though the role of B vitamins in angiogenesis is less clear.
The interrelationship of inflammation, angiogenesis and components of one-carbon metabolism with colorectal carcinogenesis among CRC patients and survivors remains inconclusive. Indeed, the role of nutrient metabolism in cancer is complex. For example, a U-shaped relationship between micronutrients and cancer is evident where supplementation among those with deficiency is associated with reduced cancer risk, but among those with sufficient or higher micronutrient status may promote cancer growth(16). Considering this complexity, we sought to better characterise the nature of the relationship between one-carbon metabolites and pathways integral to cancer progression. Few studies have evaluated post-diagnosis circulating one-carbon metabolites in relation to cancer prognostic pathways among CRC patients(17).
Our objective was to evaluate associations between inflammation- and angiogenesis-related biomarkers and one-carbon metabolites among CRC survivors. Based on prior findings(15,18–20), we hypothesised that pyridoxal-5’-phosphate (PLP) is inversely associated with pro-inflammatory biomarkers C-reactive protein (CRP), serum amyloid A (SAA), TNFα and IL-6 and IL-8 among CRC patients. We further hypothesised that 5-methyl-tetrahydrofolate (mTHF) inversely associates with inflammatory biomarkers corresponding to higher production of folate catabolites para-aminobenzoylglutamic acid (pABG) and acetyl-pABG (apABG) in this population.
Subjects and methods
Study design
This is a cross-sectional analysis of baseline data from the ColoCare Study, a multicentre, international prospective cohort initiated at the Fred Hutchinson Cancer Research Center (Seattle, WA, USA), described in detail in prior publications(21–23). ColoCare recruits newly diagnosed CRC patients prior to surgery, with the goal to investigate predictors of cancer recurrence, survival, treatment toxicities and health-related QoL.
Patients (eligibility: newly diagnosed primary invasive CRC, aged 18–89 years, German-speaking and able to provide informed consent) from the ColoCare Heidelberg site were included in this study, recruited between October 2010 and December 2014 at the University Hospital of Heidelberg, Germany (ClinicalTrials.gov Identifier: NCT02328677). Participants were staged according to the American Joint Committee on Cancer (AJCC) system based on histopathological findings. Both patients with colon (ICD-10 C18) and rectal or recto-sigmoid junction cancer (ICD-10 C19/C20) were included. The ColoCare Study has been approved by the ethics committee of the Medical Faculty at the University of Heidelberg. All study participants provided written informed consent.
Study population
In total, 356 stage I-IV CRC patients have been recruited into the ColoCare Study in Heidelberg (online Supplementary Fig. S1). For this project, we excluded patients who did not have all measured biomarkers of interest, with 238 CRC patients included in the current analysis. Patients with a history of hereditary nonpolyposis CRC or familial adenomatous polyposis were not excluded from the study. According to estimates(24), approximately 1 % of our participants were likely to have familial adenomatous polyposis and 2–4 % to have hereditary nonpolyposis CRC. The fact that non-steroidal anti-inflammatory drugs intervention prevents tumours among individuals with genetic predisposition to CRC (e.g. in the case of Lynch syndrome)(25,26) suggests that inflammatory pathways remain an important target for this population, and therefore the relationship of inflammation with other cancer-related molecular pathways remains of interest among individuals with or without germline mutations. ColoCare participants were also part of the international FOlate-dependent one-carbon metabolism in Colorectal cancer recUrrence and Survival (FOCUS) Consortium.
Circulating inflammation and angiogenesis biomarkers
Non-fasting blood samples were collected at baseline prior to surgery at the University Hospital Heidelberg. Blood samples were collected 1·3 (sd 1·25) d before surgery, on average. Samples were frozen 6·3 (sd 7·1) h following blood draw and stored for approximately 5·5 years. Serum was extracted within 4 h of blood draw, and aliquots were stored at −80°C until analysis. Aliquots (500 μl) were shipped on dry ice from Heidelberg to the Huntsman Cancer Institute, Salt Lake City, UT, USA. Ten inflammation biomarkers, including CRP, SAA, TNFα, IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), soluble intercellular adhesion molecule 1 (sICAM-1) and soluble vascular cell adhesion molecule 1 (sVCAM-1), and angiogenesis-related biomarkers, including VEGF-A and VEGF-D were measured in serum. Previously established assays on the Mesoscale Discovery Platform (MSD), on the Sector 2400A, were used in the Ulrich laboratory at the Huntsman Cancer Institute(27). Blinded patient samples, intraplate and interplate quality control samples were run on the U-PLEX Proinflammatory Combo 1 for MCP-1, IL-6, IL-8, and TNFα, on the V-PLEX Vascular Injury Plate 2 for CRP, SAA, sICAM-1 and sVCAM-1, and on the V-PLEX Angiogenesis Panel 1 for VEGF-A and VEGF-D. Blinded serum samples were diluted 1:2 (pro-inflammatory panel), 1:1000 (vascular injury plate) and 1:8 (angiogenesis panel). For the vascular injury and angiogenesis panels, the samples were freeze-thawed once and the pro-inflammatory panel was freeze-thawed twice. The plates were read with an MSD sector 2100A, and data analyses were carried out with MSD Discovery Workbench 4.0 software (Meso Scale Diagnostics). The resulting overall interplate CV was 9·9% (range 5·1–15·5%) and intraplate CV was 4·6% (range 1·8–6·4%).
B vitamins and one-carbon metabolites
Twelve B vitamins and one-carbon metabolites were measured in non-fasting blood samples collected at baseline prior to surgery, including PLP, pyridoxal (PL), pyridoxic acid (PA), mTHF, apABG, pABG, folic acid (FA), thiamine, thiamine mono-phosphate, riboflavin, cobalamin and homocysteine. After serum extraction, aliquots were stored at −80°C. Assays were run at the laboratory BEVITAL A/S, Bergen, Norway (www.bevital.no), as part of analyses run for the FOCUS consortium. Biological analyses were performed on seven complementary analytical platforms, using liquid chromatography-tandem MS-based assays (LC-MS/MS) and GC-MS/MS(28). Within-day and between-day CV ranged between 1–9 and 2–16 %, respectively.
Statistical analysis
Descriptive statistics of the study population were calculated for clinical characteristics. Means and standard deviations were computed for normally distributed continuous variables, medians and interquartile ranges for right-skewed variables (inflammation, angiogenesis and one-carbon metabolism biomarkers) and frequencies (n, (%)) for categorical variables. Categorical variables with more than two classes were considered in our analyses as indicator variables. Distributions of variables were analysed using q-q plots and histograms along with measurement of skewness and kurtosis. One-carbon metabolism, inflammation- and angiogenesis-related biomarkers were log2-transformed to meet the normality assumption for parametric statistical tests. No outliers, defined as three times SD above or below the mean value, accounting for multivitamin intake, were found. For data flagged as missing by the laboratory due to biomarker concentrations below and above the detection limit, halfminimum and maximum values were imputed, respectively.
For the primary hypothesis, sequential multivariable linear regression models were used to study associations between prespecified one-carbon metabolites (exposure) and inflammation- and angiogenesis-related biomarkers (outcome). Models were adjusted for (1) sex and age group (<60, 60–70, >70 years); (2) model 1 plus BMI category (<25, 25–30, ≥30 kg/m2), smoking status (current, former, never smoker), regular multivitamin intake in the month prior to surgery weekly (yes, no), CRC site (colon, rectum), CRC stage (I, II, III) and physical activity (min/week). Validity checks (tests for heteroscedasticity and multicollinearity) for the multivariable models were performed. Inclusion of alcohol in the model did not modify effect estimates more than 10%; thus, it was excluded from the final models. We did not adjust for race/ethnicity owing to our homogeneous non-Hispanic white population.
In secondary analyses, regression models were stratified by PLP sufficiency defined as ≥ 20 nmol/l(29), BMI and CRC site (colon v. rectum). Effect modification was evaluated by testing for the significance of an interaction term in the linear regression models. We further calculated the PA:(PLP + PL) ratio (PAr index), an integrated measure of vitamin B6 metabolism that has been proposed as a systemic inflammation indicator(30,31), and evaluated its association with inflammation- and angiogenesis-related biomarkers. Moreover, we calculated the ratio of 3-hydroxykynurenine (HK):xanthurenic acid (XA), both involved in tryptophan metabolism, (HK:XA ratio), as a functional marker of vitamin B6 status(32). Finally, we stratified the analyses of the association of inflammation biomarkers with PLP and mTHF by BMI and by disease stage.
In exploratory analyses, we computed Spearman partial correlation coefficients between inflammation-, angiogenesis-related, B vitamins and one-carbon metabolism biomarkers, adjusting for covariates. A Gaussian graphical model (GGM) was generated to visualise correlated pathways among the analysed biomarkers, conditioned on the presence of all other biomarkers. Conditional correlations ≥ |0·20| are displayed with an edge, connecting biomarker nodes. The GGM is a powerful tool for visualising metabolomics datasets and identifying relationships between biological pathways(33). We further generated a hierarchical clustering heat map to visualise pathway inter-relationships.
All statistical tests were two-sided. Statistical significance was set at P < 0·05 for the primary and secondary hypotheses, and to account for multiple testing in Spearman partial correlation analyses, we considered significance at the Benjamini and Hochberg’s False Discovery Rate (FDR) <0·05(34). With a sample size of 238, eighteen partial variables, and α = 0·0033 (to account for analyses of three one-carbon metabolites with five inflammatory biomarkers for the primary and secondary hypotheses), we estimated about 80 % statistical power to detect partial correlations of r ~ 0·25. The statistical power calculations were conducted using the Proc Power Multreg statement in SAS 9.4 (SAS Institute). Descriptive statistics, multivariable linear regression analyses and Spearman partial correlation analyses were performed using SAS 9.4 (SAS Institute). GGM was created using R (R studio) and visualised using Cytoscape 3.6.1 (Cytoscape Consortium) and the hierarchical clustering heat map with BioVinci 1.1.5 (BioTuring Inc.).
Results
Study population
Baseline clinical and demographic characteristics of the study population are summarised in Table 1. CRC patients in this study (n 238) had a mean age of 64·4 (sd 12·1) years, were predominantly male (65 %), overweight (63 %) and ever smokers (63 %). Only 6 % of participants regularly used multivitamin supplements and 23 % used non-steroidal anti-inflammatory drugs in the month prior to surgery, at least weekly. Approximately 50 % of CRC tumours were in the rectum or recto-sigmoid junction, 24·0 % of individuals were diagnosed with stage I, 40·3 % stage II and 35·7 % stage III disease.
Table 1.
Baseline clinicopathological and demographic characteristics of 238 colorectal cancer patients enrolled in the ColoCare Study*
Mean values and standard deviations; median values and interquartile ranges (IQR); numbers and percentages)
| Characteristics | n | % | |
|---|---|---|---|
| Age (years) | |||
| Mean | 64·4 | ||
| sd | 12·1 | ||
| Age categories (years) | |||
| ≤60 | 82 | 34·4 | |
| 61–70 | 74 | 31·1 | |
| >70 | 82 | 34·5 | |
| BMI (kg/m2) | |||
| Mean | 26·5 | ||
| sd | 3·9 | ||
| WHO BMI categories (kg/m2) | |||
| <25 (Underweight and normal weight) | 25 | 36·7 | |
| 25–<30 (Overweight) | 110 | 46·4 | |
| ≥30 (Obese) | 40 | 16·9 | |
| Sex | |||
| Female | 83 | 34·9 | |
| Male | 155 | 65·1 | |
| Multivitamin intaket†‡ | |||
| Yes | 13 | 5·5 | |
| None | 205 | 86·1 | |
| Smoking status‡ | |||
| Current smoker | 43 | 18·1 | |
| Former smoker§ | 106 | 44·5 | |
| Never smoker | 71 | 29·8 | |
| Cancer stage | |||
| I | 57 | 24·0 | |
| II | 96 | 40·3 | |
| III | 85 | 35–7 | |
| Cancer site | |||
| Colon | 128 | 538 | |
| Rectum and recto-sigmoid junction | 110 | 46·2 | |
| Moderate physical activity¶ (min/week) | |||
| Median | 55 | ||
| IQR | 136·5 | ||
| Vigorous physical activity¶ (min/week) | |||
| Median | 0 | ||
| IQR | 57·0 |
Percentages may not add to 100% due to missing values
Multivitamin intake is defined as 1+times/week of multivitamin use in the past 1month.
Twenty patients had unknown information on multivitamin intake; twenty-threepatients had unknown smoking history.
Former smoker is defined as stopped smoking within the last 2 years.
Moderate physical activity refers to activities, which require moderate effort, such as walkingor bicycling. Vigorous physical activity includes, for example, jogging or fast swimming.
Mean values and standard deviations and medians and interquartile ranges of inflammation- and angiogenesis-related biomarkers, B vitamins and one-carbon metabolites are reported in Table 2. Mean CRP and SAA concentrations were elevated (27·5 (sd 51·7) and 42·5 (sd 73·0) mg/l, respectively) according to clinical reference ranges (<10 mg/l)(35), indicative of active systemic inflammation in our study population. The median concentration of circulating PLP was 26·5 nmol/l (interquartile range 17·1–40·8 nmol/l); 66 % of participants had sufficient PLP concentrations (≥20 nmol/l)(29).
Table 2.
Biomarkers of inflammation, angiogenesis and one-carbon metabolism among 238 colorectal cancer patients enrolled in the ColoCare Study
(Mean values and standard deviations; median values and interquartile ranges (IQR))
| Biomarkers | Mean | sd | Median | IQR |
|---|---|---|---|---|
| Inflammation and angiogenesis | ||||
| CRP (mg/l) | 275 | 51–7 | 380 | 192 |
| SAA (mg/l) | 425 | 73–0 | 7–05 | 284 |
| IL-6 (pg/ml) | 446 | 202 | 079 | 130 |
| IL-8 (pg/ml) | 31 9 | 102 | 809 | 101 |
| MCP-1 (pg/ml) | 209 | 155 | 175 | 100 |
| sICAM-1 (mg/l) | 046 | 018 | 0–43 | 020 |
| sVCAM-1 (mg/l) | 064 | 030 | 059 | 027 |
| TNFα (pg/ml) | 123 | 089 | 116 | 069 |
| VEGF-A (pg/ml) | 841 | 541 | 749 | 559 |
| VEGF-D (pg/ml) | 836 | 304 | 799 | 314 |
| B vitamins and one-carbon metabolites | ||||
| mTHF (nmol/l) | 224 | 14–4 | 188 | 14–0 |
| Folic acid (nmol/l) | 022 | 210 | 000 | 000 |
| apABG (nmol/l) | 128 | 222 | 085 | 0–54 |
| pABG (nmol/l) | 116 | 126 | 079 | 096 |
| Cobalamin (pmol/l) | 358 | 266 | 315 | 183 |
| Thiamine (nmol/l) | 825 | 702 | 694 | 4–50 |
| TMP (nmol/l) | 291 | 195 | 247 | 228 |
| Riboflavin (nmol/l) | 243 | 236 | 180 | 139 |
| Total homocysteine (μmol/l) | 133 | 5–45 | 123 | 607 |
| PLP (nmol/l) | 352 | 34–4 | 265 | 237 |
| PL (nmol/l) | 208 | 766 | 10–4 | 902 |
| PA (nmol/l) | 358 | 922 | 21 8 | 158 |
CRP, C-reactive protein; SAA, serum amyloid A; MCP-1, monocyte chemoattractant protein 1; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor; mTHF, 5-methyl-tetrahydrofolate; apABG, acetyl-para-aminobenzoylglutamic acid; pABG, para-aminobenzoylglutamic acid; TMP, thiamin monophosphate; PLP, pyridoxal-5’-phosphate; PL, pyridoxal; PA, pyridoxic acid.
Pyridoxal-5′-phosphate and inflammation
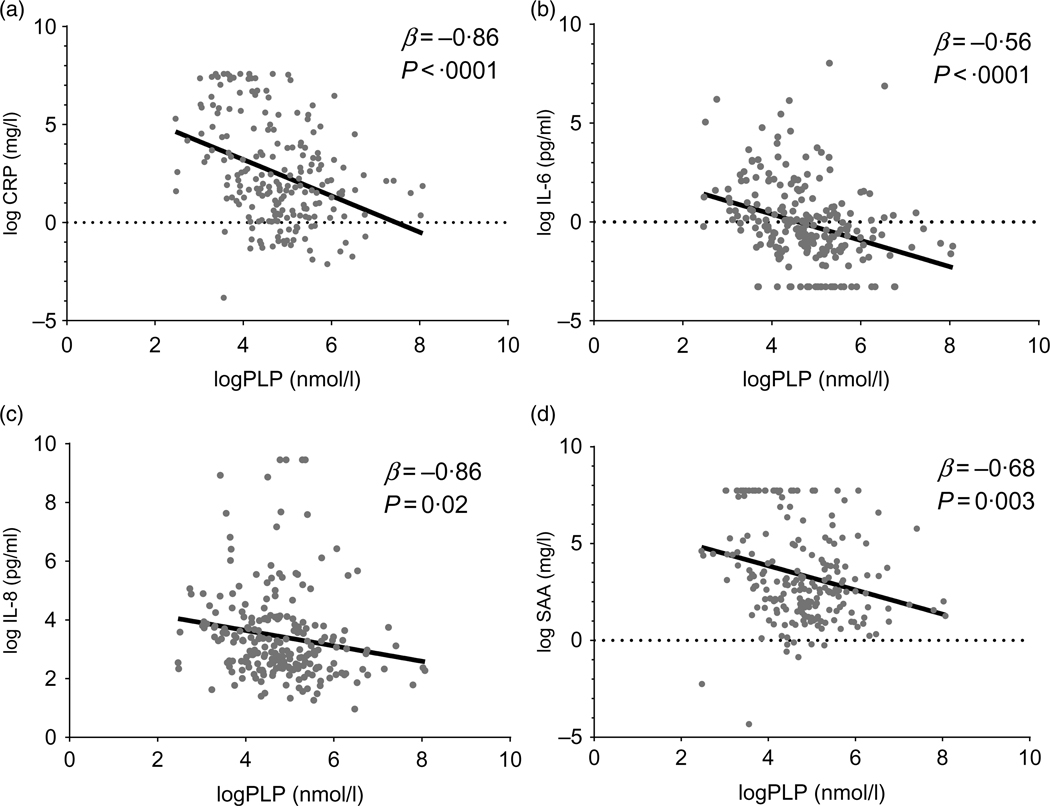
To evaluate our primary and secondary hypotheses, we performed multivariable linear regression analyses (Table 3, Fig. 1). Magnitude of association differed for minimally v. fully adjusted models, but the directions of association were unchanged. In fully adjusted models, PLP was significantly inversely associated with inflammation biomarkers CRP (r −0·33, β = −0·86, Plinear < 0·0001), SAA (r −0·23, β = −0·56, Plinear = 0·003), IL-6 (r −0·39, β = −0·69, Plinear < 0·0001), IL-8 (r −0·20, β = −0·26, Plinear = 0·02) and TNFα (r −0·12, β = −0·15, Plinear = 0·045).
Table 3.
Associations between one-carbon metabolites and inflammation biomarkers among 238 colorectal cancer patients enrolled in the ColoCare Study*
(β-Coefficients and 95 % confidence intervals)
| Inflammation biomarkers | One-carbon metabolites |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLP |
mTHF |
|||||||||||
| Simple† |
Full‡ |
Simple† |
Full‡ |
|||||||||
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| CRP | −098 | −1·33, −0·64 | <0·0001 | −0·86 | −1·24, −0·50 | <0·0001 | −0·58 | −1·00, −0·16 | 0·007 | −0·71 | −1·18, −0·23 | 0·004 |
| SAA | −0·63 | −0·96, −0·30 | 0·0002 | −0·56 | −0·94, −0·19 | 0·003 | −0·52 | −0·91, −0·12 | 0·01 | −0·68 | −1·13, −0·23 | 0·003 |
| IL-8 | −0·25 | −0·45, −0·04 | 0·02 | −026 | −0·48, −0·04 | 0·02 | −0·12 | −0·36, 0·13 | 0·34 | −0·16 | −0·43, 0·11 | 0·24 |
| IL-6 | −0·74 | −1·00, −0·48 | <0·0001 | −0·69 | −0·97, −0·40 | <0·0001 | −0·20 | −0·52, 0·13 | 0·24 | −0·24 | −0·60, 0·11 | 0·17 |
| TNFα | −0·18 | −0·32, −0·05 | 0·009 | −0·15 | −0·30, −0·003 | 0·045 | −0·23 | −0·39, −0·07 | 0·005 | −0·2 | −0·38, −0·03 | 0·02 |
| Inflammation biomarkers | One-carbon metabolites |
|||||||||||
| apABG |
pABG |
|||||||||||
| Simple† |
Full‡ |
Simple† |
Full‡ |
|||||||||
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| CRP | 0·10 | −0·44, 0·64 | 0·71 | 0·05 | −0·52, 0·63 | 0·90 | 0·29 | −0·08, 0·66 | 0·13 | 0·39 | −0·03, 0·80 | 0·07 |
| SAA | −0·14 | −0·65, 0·37 | 0·59 | −0·17 | −0·72, 0·38 | 0·54 | 0·14 | −0·20, 0·49 | 0·42 | 0·19 | −0·20, 0·58 | 0·34 |
| IL-8 | −0·02 | −0·33, 0·29 | 0·89 | −0·04 | −0·36, 0·28 | 0·82 | 0·67 | 0·46, 0·87 | <0·0001 | 0·58 | 0·36, 0·81 | <0·0001 |
| IL-6 | 0·86 | 0·46, 1·25 | <0·0001 | 0·93 | 0·53, 1·33 | <0·0001 | 0·18 | −0·10, 0·47 | 0·21 | 0·11 | −0·20, 0·41 | 0·49 |
| TNFα | −0·07 | −0·28, 0·13 | 0·48 | −0·09 | −0·31, 0·12 | 0·12 | 0·17 | 0·03, 0·32 | 0·02 | 0·12 | −0·04, 0·27 | 0·13 |
PLP, pyridoxal-5’-phosphate; mTHF, 5-methyl-tetrahydrofolate; CRP, C-reactive protein; SAA, serum amyloid A; apABG, acetyl-para-aminobenzoylglutamic acid; pABG, para-aminobenzoylglutamic acid.
Biomarker values were log2-transformed to meet the normality assumptions for linear regression models.
Simple model: multivariable linear regression model, adjusted for: age group (<60, 60 to <70 and ≥70 years) and patient sex.
Full model: multivariable linear regression model, adjusted for: age group, patient sex, BMI category, cancer stage and site, physical activity, multivitamin intake and smoking status.
Fig. 1.
Scatter plots of one-carbon metabolite pyridoxal-5’-phosphate (PLP) and inflammation biomarkers C-reactive protein (CRP) (a), IL-6 (b), IL-8 (c) and serum amyloid A (SAA) (d) based on univariate linear regression. The represented biomarkers were log2-transformed to meet the normality assumption for linear regression. Single biomarker values are visualised in grey and the regression line in black. This figure was created with GraphPad Prism8.
Folate species and inflammation
mTHF showed significantly inverse associations with CRP (r −0·14; β = −0·71, Plinear = 0·004), SAA (r −0·14; β = −0·68, Plinear = 0·003) and TNFα (r −0·15; β = −0·20, Plinear = 0·02). Folate catabolite apABG was positively correlated with IL-6 (r 0·27; β = 0·93, Plinear < 0·0001), and pABG was positively correlated with IL-8 (r 0·21; β = 0·58, Plinear < 0·0001). Folic acid, the synthetic form of folate, was not associated with any inflammation biomarker (P> 0·05 for all associations). In sensitivity analyses, we additionally adjusted the primary analyses for non-steroidal anti-inflammatory drugs use in the month prior to surgery, with similar results.
Secondary analyses
In stratified analyses by serum PLP sufficiency (≥20 nmol/l), PLP remained inversely associated with IL-6 among those with insufficient PLP concentrations (r −0·40; β = −1·14, Plinear = 0·03), while the association was attenuated among those with PLP sufficiency (r −0·22; β = −0·48, Plinear = 0·03). Magnitudes of association were similar for PLP and IL-8, stratified by PLP sufficiency (PLP-insufficient: r −0·23; β = −0·28, Plinear = 0·5; PLP-sufficient: r −0·19; β = −0·37, Plinear = 0·05). Moreover, the results suggested that the associations of PLP with CRP may differ by BMI and CRC stage, but the P values for heterogeneity did not reach statistical significance (Pheterogeneity = 0·07 and 0·09, respectively) (Table 4). For example, there was a stronger inverse association between PLP and CRP among normoweight (r −0·39; β = −1·45, Plinear = 0·0008), compared with overweight participants (r −0·19; β = −0·54, Plinear = 0·03, Phet = 0·02). The magnitude of inverse associations between PLP and CRP was stronger among patients with stage I (r −0·53; β = −2·02, Plinear < 0·0001), compared with stage II (r −0·31; β = −0·72, Plinear = 0·02, Phet = 0·20) and stage III (r −0·20; β = −0·44, Plinear = 0·20, Phet = 0·03) disease. Moreover, there was evidence for effect modification of mTHF associations with TNFα by BMI; the association was stronger for overweight individuals (Phet = 0·009), but the same was not observed for under/normal-weight or obese individuals (Table 5). The inverse association of mTHF with both CRP and SAA appeared to be stronger among those with earlier stage disease (Phet = 0·02).
Table 4.
Associations of active vitamin B6 (pyridoxal-5’-phosphate) with inflammation biomarkers, stratified by BMI and cancer stage among 238 colorectal cancer patients enrolled in the ColoCare Study*
(β-Coefficients and 95 % confidence intervals)
| Inflammation biomarkers | WHO BMI category† |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Underweight/normal weight <25 kg/m2 (n 87) |
Overweight 25 to <30 kg/m2 (n 110) |
Obese 30+ kg/m2 (n 40) |
P for heterogeneity |
|||||||||
| β | 95 % CI | P | β | 95 % CI | P | β | 95 % CI | P | Under-/normal v. overweight | Under-/normal weight v. obese | Overall | |
| CRP | −1·45 | −2·26, −0·63 | 0·0008 | −0·54 | −1·02, −0·06 | 0·03 | −1·03 | −2·89, 0·82 | 0·25 | 0·02 | 0·52 | 0·07 |
| SAA | −1·21 | −1·90, −0·52 | 0·0009 | −0·34 | −0·82, 0·15 | 0·17 | −0·77 | −3·06, 1·53 | 0·48 | 0·03 | 0·29 | 0·0 |
| IL-8 | −0·48 | −0·85, −0·11 | 0·01 | −0·18 | −0·52, −0·16 | 0·29 | −0·33 | −1·17, 0·52 | 0·43 | 0·28 | 0·43 | 0·51 |
| IL-6 | −0·57 | −1·13, −0·02 | 0·04 | −0·65 | −1·07, −0·23 | 0·003 | −0·72 | −1·36, −0·08 | 0·03 | 0·94 | 0·56 | 0·83 |
| TNFα | −0·07 | −0·33, 0·20 | 0·61 | −0·13 | −0·32, 0·06 | 0·18 | −0·49 | −1·01, 0·03 | 0·06 | 0·61 | 0·28 | 0·36 |
| Inflammation biomarkers | Cancer stage† |
|||||||||||
| Stage I (n 57) |
Stage II (n 96) |
Stage III (n 85) |
P for heterogeneity |
|||||||||
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | I v. II | I v. III | Overall | |
| CRP | −2·02 | −2·83, −1·19 | <0·0001 | −0·72 | −1·33, −0·11 | 0·02 | −0·44 | −1·18, 0·29 | 0·23 | 0·22 | 0·03 | 0·09 |
| SAA | −1·42 | −2·32, −0·52 | 0·003 | −0·49 | −1·08, 1·11 | 0·11 | −0·30 | −1·02, 0·42 | 0·41 | 0·39 | 0·32 | 0·58 |
| IL-8 | −0·58 | −1·15, −0·02 | 0·04 | −0·12 | −0·49, 0·25 | 0·52 | −0·32 | 0·71, 0·07 | 0·10 | 0·55 | 0·91 | 0·78 |
| IL-6 | −1·21 | −2·0, −0·44 | 0·003 | −0·54 | −0·92, −0·15 | 0·007 | −0·56 | −1·09, −0·02 | 0·04 | 0·05 | 0·15 | 0·14 |
| TNFα | −0·38 | −0·79, 0·02 | 0·06 | −0·17 | −0·43, 0·09 | 0·19 | −0·07 | −0·30, 0·15 | 0·52 | 0·35 | 0·26 | 0·51 |
CRP, C-reactive protein; SAA, serum amyloid A.
Biomarker values were log2-transformed to meet the normality assumptions for linear regression models.
Multivariable regression analysis adjusted for: age group (<60, 60 to <70 and ≥70 years), sex, BMI category, cancer stage and site, physical activity, multivitamin intake and smoking status.
Table 5.
Associations of active folate species (5-methyl-tetrahydrofolate) with inflammation biomarkers, stratified by BMI and cancer stage among 238 colorectal cancer patients enrolled in the ColoCare Study*
(β-Coefficients and 95 % confidence intervals)
| Inflammation biomarkers | WHO BMI category† |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Underweight/normal weight <25 kg/m2 (n 87) |
Overweight 25 to <30 kg/m2 (n 110) |
Obese 30+ kg/m2 (n 40) |
P for heterogeneity |
|||||||||
| β | 95 % CI | P | β | 95 % CI | P | β | 95 % CI | P | Under-/normal v. overweight | Under-/normal weight v. obese | Overall | |
| CRP | −0·81 | −1·65, 0·02 | 0·06 | −0·22 | −0·95, 0·5 | 0·50 | −0·96 | −2·64, 0·71 | 0·24 | 0·30 | 0·68 | 0·43 |
| SAA | −0·78 | −1·48, −0·08 | 0·03 | −0·26 | −0·99, 0·48 | 0·50 | −1·33 | −3·29, 0·64 | 0·17 | 0·25 | 0·62 | 0·35 |
| IL-8 | −0·32 | −0·7, 0·06 | 0·10 | −0·26 | −0·73, 0·2 | 0·27 | 0·47 | −0·3, 1·24 | 0·22 | 0·86 | 0·08 | 0·20 |
| IL-6 | −0·49 | −1·05, 0·08 | 0·09 | −0·13 | −0·75, 0·48 | 0·70 | 0·47 | −0·16, 1·11 | 0·14 | 0·34 | 0·11 | 0·26 |
| TNFα | −0·03 | −0·29, 0·24 | 0·84 | −0·48 | −0·73, −0·24 | 0·0002 | −0·04 | −0·57, 0·48 | 0·86 | 0·004 | 0·95 | 0·009 |
| Inflammation biomarkers | Cancer stage† |
|||||||||||
| Stage I (n 57) |
Stage II (n 96) |
Stage III (n 85) |
P for heterogeneity |
|||||||||
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | I v. II | I v. III | Overall | |
| CRP | −1·41 | −2·2, −0·62 | 0·0009 | 0·002 | −0·68, 0·68 | 0·99 | −0·22 | −1·48, −1·04 | 0·73 | 0·01 | 0·03 | 0·02 |
| SAA | −1·31 | −2·1, −0·55 | 0·001 | −0·14 | −0·8, 0·5 | 0·67 | −0·42 | −1·64, 0·79 | 0·49 | 0·01 | 0·03 | 0·02 |
| IL-8 | −0·51 | −0·98, −0·03 | 0·04 | −0·002 | −0·41, 0·41 | 0·99 | −0·27 | −0·86, 0·33 | 0·37 | 0·28 | 0·30 | 0·48 |
| IL-6 | −0·76 | −1·45, −0·06 | 0·03 | −0·04 | −0·48, 0·40 | 0·90 | 0·07 | −0·77, 0·90 | 0·88 | 0·04 | 0·03 | 0·05 |
| TNFα | −0·3 | −0·65, 0·04 | 0·08 | −0·18 | −0·47, 0·10 | 0·20 | −0·06 | −0·40, 0·29 | 0·75 | 0·70 | 0·65 | 0·89 |
CRP, C-reactive protein; SAA, serum amyloid A.
Biomarker values were log2-transformed to meet the normality assumptions for linear regression models.
Multivariable regression analysis adjusted for: age group (<60, 60 to <70 and ≥70 years), sex, BMI category, cancer stage and site, physical activity, multivitamin intake and smoking status.
The PAr index showed positive associations with IL-6 (r 0·18; β = 0·57, Plinear = 0·004), IL-8 (r 0·18; β = 0·36, Plinear = 0·02) and TNFα (r 0·15; β = 0·20, Plinear = 0·04); associations with CRP were positive but not statistically significant (r 0·11; β = 0·36, Plinear = 0·20) or SAA (r 0·08; β = 0·26, Plinear = 0·30). The HK: XA ratio was positively associated with CRP (r 0·46; β = 1·16, Plinear < 0·0001), SAA (r 0·38; β = 0·83, Plinear < 0·0001) and IL-6 (r 0·43; β = 0·86, Plinear < 0·0001); associations with IL-8 were also positive but not statistically significant (r 0·21; β = 0·13, Plinear = 0·20) or TNFα (r 0·13; β = 0·06, Plinear = 0·40).
Exploratory analyses
In exploratory analyses, we evaluated partial correlations between the full panel of circulating biomarkers (online Supplementary Table S1). Forty-two partial correlations reached the FDR significance threshold of <0·05. For example, PL was inversely correlated with CRP (r −0·29, P < 0·001), IL-6 (r −0·30, P < 0·001) and VEGF-D (r 0·23, P = 0·002). We also noted positive correlations between pABG and VEGF-D (r 0·27, P = 0·0003) and between total homocysteine and VEGF-D (r 0·31, P < 0·001). Thiamine and thiamine monophosphate were inversely correlated with CRP (r −0·20, P < 0·001 and r −0·35, P < 0·001) and IL-6 (r −0·38, P < 0·001 and r −0·33, P < 0·001), while thiamine monophosphate was inversely correlated with SAA and monocyte chemoattractant protein 1 (r −0·27, P < 0·001 and r −0·18, P = 0·01, respectively). In contrast, correlations between cobalamin (vitamin B12) and biomarkers of inflammation were positive, but not statistically significant at FDR < 0·05.
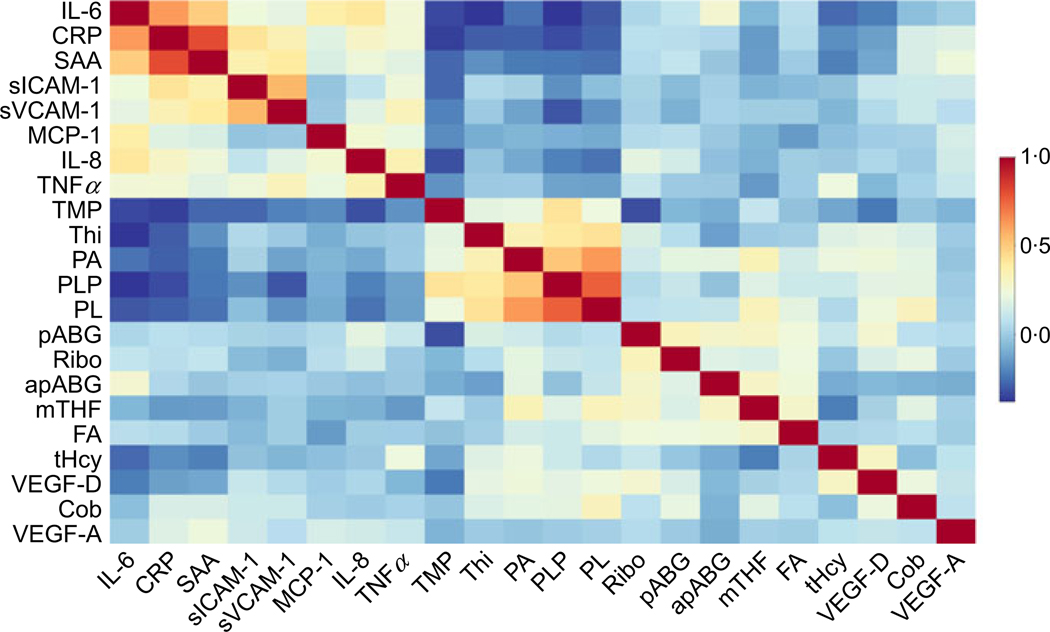
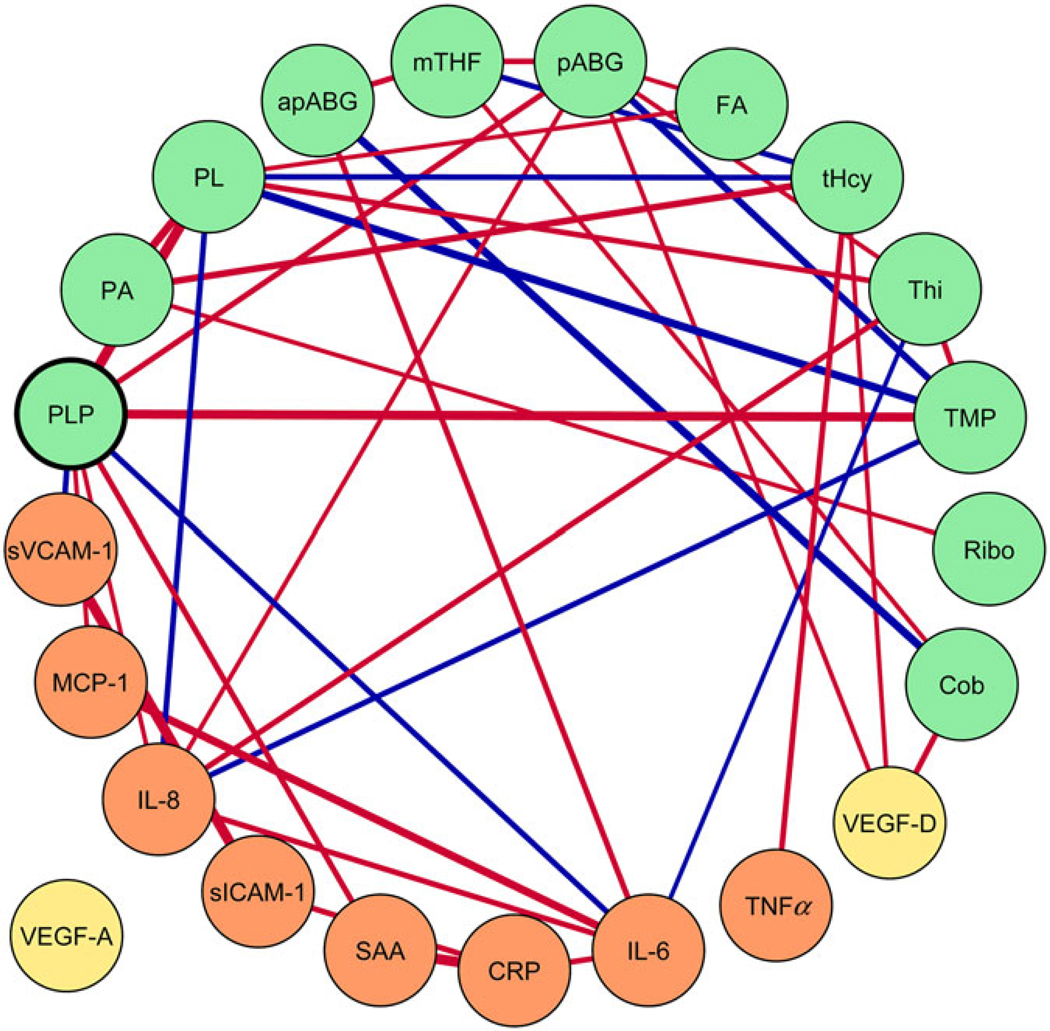
Biomarker intercorrelations were visualised using a hierarchical clustering heat map (Fig. 2) and GGM (Fig. 3). The GGM showed a single interconnected network between B vitamins, one-carbon metabolites and angiogenesis- and inflammation- related biomarkers, including inverse conditional correlations of PLP with IL-6, PL and SAA. The GGM visualises correlations conditional on the presence of all other biomarkers, with a threshold (r |0·20|) for connecting nodes with edges.
Fig. 2.
Hierarchical clustering heat map, based on Spearman partial correlations of B vitamins and one-carbon metabolites with inflammation and angiogenesis biomarkers, adjusted for age group, patient sex, BMI category, cancer stage and site, physical activity, multivitamin intake and smoking status. Biomarkers involved in the same or similar pathways (such as pyridoxal (PL), pyridoxic acid (PA) and pyridoxal-5’-phosphate (PLP)) cluster together in the heat map. Blue indicates inverse correlations, for example, between PLP and C-reactive protein (CRP). Orange and red represent positive correlations, for example, between CRP and serum amyloid A (SAA). This heat map was created with BioVinci 1.1.5 (BioTuring Inc.). sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1; TMP, thiamin monophosphate; Thi, thiamin; pABG, para-aminobenzoylglutamic acid; Ribo, riboflavin; apABG, acetyl-pABG; mTHF, 5-methyl-tetrahydrofolate; FA, folic acid; tHcy, total homocysteine; VEGF, vascular endothelial growth factor; Cob, cobalamin.
Fig. 3.
Gaussian graphical model (GGM) of biomarker correlations, conditioned on the presence of all other biomarkers (conditional r ≥ |0·20|). Biomarkers are represented by nodes (circles), and conditional correlations are connected by edges (lines). Orange nodes represent inflammation, yellow nodes represent angiogenesis biomarkers and green nodes represent B vitamins and one-carbon metabolites. The line width represents the strength of conditional correlation. Red lines indicate positive correlations, and blue lines represent inverse correlations. Vascular endothelial growth factor A (VEGF-A) conditional r < 0·20. The GGM was created with Cytoscape 3.6.1 (Cytoscape Consortium). sICAM-1, soluble intercellular adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1; sVCAM-1, soluble vascular cell adhesion molecule 1; PLP, pyridoxal-5’-phosphate; PA, pyridoxic acid; PL, pyridoxal; apABG, acetyl-para-aminobenzoylglutamic acid; mTHF, 5-methyl-tetrahydrofolate; pABG, para-aminobenzoylglutamic acid; FA, folic acid; tHcy, total homocysteine; Thi, thiamin; TMP, thiamin monophosphate; Ribo, riboflavin; Cob, cobalamin; CRP, C-reactive protein; SAA, serum amyloid A.
Discussion
In this study, we measured associations between one-carbon metabolites, inflammation- and angiogenesis-related biomarkers in a cross-sectional analysis among CRC patients participating in the ColoCare Study. We found that vitamin B6 species, PLP, PL and PA, were inversely associated with inflammatory biomarkers CRP, SAA, IL-6 and IL-8. Thiamine and active folate were also inversely associated with inflammatory biomarkers, and folate catabolites were positively associated with inflammation, indicating higher folate utilisation.
PLP is the bioactive form of vitamin B6, an essential vitamin that may be obtained from many sources including fortified cereals, milk products, beans, nuts and vitamin supplements(29,30). PL represents the dephosphorylated form of PLP, and PA derives from PLP in an oxidation reaction with aldehyde oxidase I(31). PLP serves as a coenzyme in over 4% of human metabolic reactions(30), including preventing the formation of reactive oxygen species(30). Inverse associations of vitamin B6 intake and circulating PLP with CRC risk have been reported(18,36,37). The relationship of PLP with CRC outcomes is less established(38).
An inverse association between PLP and inflammatory biomarkers was previously reported among postmenopausal women in the Women’s Health Initiative Observational Study(15), among healthy adults in the Boston Puerto Rican Health Study(20) and Framingham Heart Study(19), and in patients with chronic inflammatory conditions, such as depression and inflammatory bowel disease(39,40). In a prior intervention trial among rheumatoid arthritis patients, vitamin B6 supplementation suppressed IL-6 and TNFα concentrations(41). In our study, we also noted that participants with increased CRP concentrations (>10mg/l) were less PLP-sufficient than those within the physiological range of CRP. Similar findings were described among 2686 adults in the US National Health & Nutrition Examination Survey(42). Our results corroborate prior findings and show that these processes additionally apply to CRC patients. Furthermore, we provide evidence of an inverse association between other one-carbon metabolites and inflammation among CRC patients.
Our observations may reflect a modified distribution and utilisation of PLP in serum during inflammatory processes, rather than hypovitaminosis(30,43). PLP is stored predominantly in muscle and liver. During inflammation, PLP is preferentially mobilised from the liver. Due to the high demand of PLP by enzymes involved in the inflammatory response, circulating concentration may decrease(43). Alternately, a recent study suggested that vitamin B6 species could inhibit the activation of NF-κB, which is involved in the inflammatory response, and nucleotide-binding oligomerisation domain, Leucine-rich Repeat and Pyrin domain containing-mediated caspase-1 activation, which suppresses cytokine production(44). Postulated anti-inflammatory mechanisms relevant for CRC patients include activation of p53 and p21 tumour suppressor gene expression in the colon(45,46). Moreover, human colon carcinoma cell line studies demonstrated that vitamin B6 induced a reduction in inflammatory cytokine (including IL-6 and TNFα) secretion from peripheral blood mononuclear cells(47).
Our findings do not support strong effect modification of the cross-sectional relationship between PLP and inflammation by BMI or CRC stage, but it is possible that our study was underpowered to observe effect modification. Nonetheless, a trend was apparent suggesting that advancing stage of CRC and increasing BMI weakened the association between markers of inflammation and B vitamins/one-carbon metabolites. Specifically, the inverse relationship of PLP with inflammation biomarkers appeared stronger among normal-weight compared with overweight or obese participants. Similarly, it appeared stronger among those with earlier stage disease, although results were not statistically significant. The same heterogeneity according to disease stage was apparent for the association of mTHF with CRP and SAA. This might suggest that normal associations become increasingly perturbed with advancing disease and adiposity. Indeed, both obesity and cancer stage have been shown to promote systemic inflammation(4,48–50).
We observed positive associations between the PAr index (PA/(PL + PLP)) and inflammation biomarkers, supporting prior findings(30,31,51,52). As a systemic inflammation indicator(31), the PAr index is an independent predictor of stroke(51) and cardiovascular mortality(53). Furthermore, we observed strong positive associations between HK:XA ratio, a marker of functional vitamin B6 status(32), and several inflammation biomarkers (in particular the acute-phase proteins CRP and SAA). PAr index and HK:XA ratio may represent alternative biomarkers for identifying those who might benefit from anti-inflammatory dietary intervention.
Our findings suggest increased folate catabolism during inflammation among CRC patients given that elevated inflammatory state was associated with higher folate catabolite concentrations in parallel with lower mTHF concentration. Prior interventions of mTHF infusion among haemodialysis patients demonstrated reduced circulating CRP(54). As excretion products, pABG and apABG are indicators of folate catabolism and status(55) and would be expected to increase in response to higher folate utilisation during inflammatory processes. This observation of increased folate metabolism in relation to inflammation may be important for informing future studies designed to understand folate metabolism in the context of CRC. A meta-analysis of nine randomised controlled FA intervention trials (n 1179 adults) reported decreased serum CRP post-intervention(56). These data were partially supported by an analysis within the Women’s Health Initiative Observational Study cohort that showed a positive association of folate status with SAA, but not with CRP(15). However, the Aspirin/Folate Polyp Prevention Trial did not observe a significant decrease of CRP, IL-6 or TNFα among participants receiving FA(57). Whether folate’s influence on inflammation plays a causal role in CRC is unclear. Rapidly proliferating cancer cells have higher folate requirements to accommodate accelerated DNA synthesis(58), though a dual modulatory effect on cancer risk has been observed according to dosage and timing(59–62). Folate may prevent CRC among subjects with a healthy colon, but increase risk among those with colon premalignant lesions(59–63), although these findings have been inconsistent(17).
Unlike in other countries, food products in Germany are not fortified with FA. Moreover, the prevalence of supplement use in our population was very low. We did not observe synthetic FA associations with inflammation biomarkers. In contrast to dietary folate, FA is not metabolised immediately to active mTHF. The role of circulating unmetabolised FA in health remains under debate(64). Future studies among CRC populations should consider the roles of synthetic FA in comparison with the active form mTHF in inflammatory processes.
We observed positive correlations of PA, PL and PLP with angiogenesis biomarker VEGF-D, a cytokine involved in blood vessel formation(13). Prior studies have found an inverse association between these metabolites and angiogenesis biomarkers(65,66). Hypothesised mechanisms include inhibition of DNA topoisomerases and DNA polymerase by PLP(65,66). In a study measuring mRNA expression levels of VEGF-A, VEGF-C and VEGF-D and their receptors VEGFR-2 and VEGFR-3 in seventy CRC (thirty-five with paired mucosae) and twenty adenomatous polyps, it was found that VEGF-D mRNA expression was significantly lower in both polyps and CRC compared with normal mucosa but VEGF-A and VEGF-C were significantly raised in CRC. It was postulated that decreased VEGF-D may enable higher levels of VEGF-A and VEGF-C to bind readily to the VEGF receptors, promoting tumour growth(67). Therefore, our observation that vitamin B metabolites were associated with VEGF-D but not VEGF-A might suggest that PLP associates with an anti-angiogenic microenvironment.
We observed inverse correlations between thiamine (vitamin B1) and its metabolite thiamine monophosphate with inflammation and angiogenesis biomarkers. Vitamin B1 plays a key role in supporting enzymes involved in cellular and carbohydrate metabolism and has been shown to inhibit cell growth(68). Thiamine supplementation trials have shown mixed results with respect to inflammation biomarkers(69,70), possibly due to the short intervention duration (1 month)(70), or thiamine deficiency at baseline(71). Preclinical studies have demonstrated elevated TNFα in thiamine-deficient mice(72) and reduced TNFα and IL-6 after thiamine administration(73). Hypothesised mechanisms for thiamine’s anti-inflammatory potential include inhibition of mitogen-activated protein kinase (MAPK) signalling, a key inflammatory pathway(74).
Total homocysteine was positively correlated with TNFα and VEGF-D. These findings support prior observations of homocysteine associations with inflammation(75). The inverse association of total homocysteine with IL-6 is not in line with prior studies(76,77) and warrants investigation. However, IL-6 has been suggested to play both anti- and pro-inflammatory roles(78,79).
Our study utilised rigorously collected data from the well-established ColoCare Study, including state-of-the-art and highly reproducible biomarker measurements. Our results support prior findings of associations between some B vitamins and inflammation biomarkers but apply these findings to a CRC patient population. The current study also expands on prior work by measuring a more comprehensive panel of one-carbon and inflammatory biomarkers, in addition to angiogenesis biomarkers. Although cross-sectional, our observations of a relationship between B vitamins and angiogenesis biomarkers are novel and warrant further investigation in future prospective studies. The dietary intake of CRC patients might change after diagnosis depending on cancer treatment and on lifestyle modification. Considering that blood was drawn in our study within days of surgery and that B-vitamin half-lives are relatively long (e.g. 25 d for PLP(80)), it is unlikely that dietary intake changed substantially in this period to affect circulating vitamin status. We note some study limitations. Study participants were predominantly non-Hispanic white; thus, studies in diverse populations are warranted to enhance generalisability. Our inflammatory biomarker panel did not include potentially anti-inflammatory cytokines like IL-10. IL-10 has been shown to both associate with CRC tumour progression and to inhibit tumour growth depending on host immunity(81). Further investigation of the relationship between both IL-10 and CRC progression, and between IL-10 and B vitamins like PLP, may shed further light on this relationship. Finally, the cross-sectional study design precludes causal inference; therefore, results should be interpreted with caution and further studies are needed to determine the relevance of our observations to CRC outcomes.
In conclusion, our study showed that PLP and folate species within the one-carbon metabolism pathway are associated with both inflammation and angiogenesis pathways among CRC patients. These findings reinforce the notion that B vitamins involved in the one-carbon metabolism may be correlated with carcinogenic processes. Prospective studies are needed to determine whether modulation of B-vitamin status leads to changes in inflammation or angiogenesis pathways among CRC patients, and the influence on CRC prognosis to further reinforce B-vitamin status as a target for future dietary guidance in this population.
Supplementary Material
Acknowledgements
The authors thank all the study participants for their commitment and time to the ColoCare Study and the entire Heidelberg ColoCare team.
This work was supported by grants from the National Institutes of Health/National Cancer Institute (U01CA206110, R01CA189184 and R01 CA207371 to C. M. U.), the German Consortium of Translational Cancer Research (DKTK) and the German Cancer Research Center, the Matthias Lackas Foundation, Stiftung LebensBlicke, and Claussen-Simon Stiftung (Germany), and the Huntsman Cancer Foundation. This work was also supported by the ERA-NET, JTC 2012 call on Translational Cancer Research (TRANSCAN), JTC2013-F0CUS, project FOCUS I2104-B26 and the Federal Ministry of Education and Research, Germany (01KT1503). A. N. H. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the National Human Genome Research Institute. E. H. R. was financially supported by Wereld Kanker Onderzoek Fonds (WKOF), as part of the World Cancer Research Fund International grant program (grant number 2016/1620). J. L. K. was supported by a grant from Kankeronderzoekfonds Limburg as part of Health Foundation Limburg (Grant No. 00005739). The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA042014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the funding institutions had any role in the design, analysis or writing of this article.
R. K., C. M. U. and M. P. conceived and designed the study, wrote the manuscript and had primary responsibility for the final content; C. A. W., C. H. and R. K. conducted research; R. K., T. L. and M. P. analysed data; R. K., A. N. H., B. G., J. O., M. P. and C. M. U. participated in interpreting the data. All authors provided critical intellectual content to review the manuscript and read and approved the final manuscript.
C. M. U. has Cancer Center Director oversight over research funded by several pharmaceutical companies, but has not received funding directly herself. The other authors declare that there are no conflicts of interest.
Abbreviations:
- apABG
acetyl-para-aminobenzoylglutamic acid
- CRC
colorectal cancer
- CRP
C-reactive protein
- FA
folic acid
- GGM
Gaussian graphical model
- HK:XA ratio
3-hydroxykynurenine:xanthurenic acid ratio
- mTHF
5-methyl-tetrahydrofolate
- PA
pyridoxic acid
- pABG
para-aminobenzoylglutamic acid
- PL
pyridoxal
- PLP
pyridoxal-5’-phosphate
- SAA
serum amyloid A
- VEGF
vascular endothelial growth factor
Footnotes
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520000422
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund/American Institute for Cancer Research (2018) Continuous Update Project Expert Report. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. London: World Cancer Research Fund International. [Google Scholar]
- 3.Rock CL, Doyle C, Demark-Wahnefried W, et al. (2012) Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62, 243–274. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich CM, Himbert C, Holowatyj AN, et al. (2018) Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nat Rev Gastroenterol Hepatol 15, 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Zutphen M, Kampman E, Giovannucci EL, et al. (2017) Lifestyle after colorectal cancer diagnosis in relation to survival and recurrence: a review of the literature. Curr Colorectal Cancer Rep 13, 370–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ait Ouakrim D, Pizot C, Boniol M, et al. (2015) Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ 351, h4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drew DA, Cao Y & Chan AT (2016) Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 16, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ullman TA & Itzkowitz SH (2011) Intestinal inflammation and cancer. Gastroenterology 140, 1807–1816. [DOI] [PubMed] [Google Scholar]
- 9.Ulrich CM, Bigler J & Potter JD (2006) Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 6, 130–140. [DOI] [PubMed] [Google Scholar]
- 10.Toriola AT & Ulrich CM (2011) Is there a potential use for C-reactive protein as a diagnostic and prognostic marker for colorectal cancer? Future Oncol 7, 1125–1128. [DOI] [PubMed] [Google Scholar]
- 11.Landskron G, De la Fuente M, Thuwajit P, et al. (2014) Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albini A, Bruno A, Noonan DM, et al. (2018) Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: implications for immunotherapy. Front Immunol 9, 527.29675018 [Google Scholar]
- 13.Tonini T, Rossi F & Claudio PP (2003) Molecular basis of angiogenesis and cancer. Oncogene 22, 6549. [DOI] [PubMed] [Google Scholar]
- 14.Locasale JW (2013) Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13, 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbenhardt C, Miller JW, Song X, et al. (2014) Biomarkers of one-carbon metabolism are associated with biomarkers of inflammation in women. J Nutr 144, 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayne ST, Ferrucci LM & Cartmel B (2012) Lessons learned from randomized clinical trials of micronutrient supplementation for cancer prevention. Annu Rev Nutr 32, 369–390. [DOI] [PubMed] [Google Scholar]
- 17.Lochhead P, Nishihara R, Qian ZR, et al. (2015) Postdiagnostic intake of one-carbon nutrients and alcohol in relation to colorectal cancer survival. Am J Clin Nutr 102, 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zschabitz S, Cheng TY, Neuhouser ML, et al. (2013) B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr 97, 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friso S, Jacques PF, Wilson PW, et al. (2001) Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation 103, 2788–2791. [DOI] [PubMed] [Google Scholar]
- 20.Shen J, Lai CQ, Mattei J, et al. (2010) Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr 91, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gigic B, Boeing H, Toth R, et al. (2018) Associations between dietary patterns and longitudinal quality of life changes in colorectal cancer patients: the ColoCare Study. Nutr Cancer 70, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liesenfeld DB, Grapov D, Fahrmann JF, et al. (2015) Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal 43. cancer patients: the ColoCare study. Am J Clin Nutr 102,433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulrich CM, Gigic B, Böhm J, et al. (2018) The ColoCare Study - A paradigm of transdisciplinary science in colorectal cancer outcomes. Cancer Epidemiol Biomarkers Prev 28, 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gryfe R (2009) Inherited colorectal cancer syndromes. Clin Colon Rectal Surg 22, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labayle D, Fischer D, Vielh P, et al. (1991) Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 101, 635–639. [DOI] [PubMed] [Google Scholar]
- 26.Burn J, Gerdes AM, Macrae F, et al. (2011) Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 378, 2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Himbert C, Ose J, Nattenmüller J, et al. (2019) Body fatness, adipose tissue compartments, and biomarkers of inflammation and angiogenesis in colorectal cancer: the ColoCare Study. Cancer Epidemiol Biomarkers Prev 28, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Midttun O, Kvalheim G & Ueland PM (2013) High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal Bioanal Chem 405, 2009–2017. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine (2006) Dietary Reference Intakes, The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press. [Google Scholar]
- 30.Ueland PM, McCann A, Midttun Ø, et al. (2017) Inflammation, vitamin B6 and related pathways. Mol Aspects Med 53, 10–27. [DOI] [PubMed] [Google Scholar]
- 31.Ulvik A, Midttun O, Pedersen ER, et al. (2014) Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am J Clin Nutr 100, 250–255. [DOI] [PubMed] [Google Scholar]
- 32.Ulvik A, Theofylaktopoulou D, Midttun O, et al. (2013) Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr 98, 934–940. [DOI] [PubMed] [Google Scholar]
- 33.Krumsiek J, Suhre K, Illig T, et al. (2011) Gaussian graphical modeling reconstructs pathway reactions from high-throughput metabolomics data. BMC Syst Biol 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y & Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57, 289–300. [Google Scholar]
- 35.Kushner I, Rzewnicki D & Samols D (2006) What does minor elevation of C-reactive protein signify? Am J Med 119, 166.e117–166.e128. [DOI] [PubMed] [Google Scholar]
- 36.Gylling B, Myte R, Schneede J, et al. (2017) Vitamin B-6 and colorectal cancer risk: a prospective population-based study using 3 distinct plasma markers of vitamin B-6 status. Am J Clin Nutr 105, 897–904. [DOI] [PubMed] [Google Scholar]
- 37.Larsson SC, Orsini N & Wolk A (2010) Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA 303, 1077–1083. [DOI] [PubMed] [Google Scholar]
- 38.Je Y, Lee JE, Ma J, et al. (2013) Prediagnostic plasma vitamin B6 (pyridoxal 5’-phosphate) and survival in patients with colorectal cancer. Cancer Causes Control 24, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hvas AM, Juul S, Bech P, et al. (2004) Vitamin B6 level is associated with symptoms of depression. Psychother Psychosom 73, 340–33. [DOI] [PubMed] [Google Scholar]
- 40.Saibeni S, Cattaneo M, Vecchi M, et al. (2003) Low vitamin B(6) plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol 98, 112–117. [DOI] [PubMed] [Google Scholar]
- 41.Huang SC, Wei JC, Wu DJ, et al. (2010) Vitamin B(6) supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur J Clin Nutr 64, 1007–1013. [DOI] [PubMed] [Google Scholar]
- 42.Morris MS, Sakakeeny L, Jacques PF, et al. (2010) Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr 140, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul L, Ueland PM & Selhub J (2013) Mechanistic perspective on the relationship between pyridoxal 5’-phosphate and inflammation. Nutr Rev 71, 239–244. [DOI] [PubMed] [Google Scholar]
- 44.Zhang P, Tsuchiya K, Kinoshita T, et al. (2016) Vitamin B6 prevents IL-1beta protein production by inhibiting NLRP3 inflammasome activation. J Biol Chem 291, 24517–24527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9, 400–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Suidasari S, Hasegawa T, et al. (2014) Vitamin B(6) activates p53 and elevates p21 gene expression in cancer cells and the mouse colon. Oncol Rep 31, 2371–2376. [DOI] [PubMed] [Google Scholar]
- 47.Bessler H & Djaldetti M (2016) Vitamin B6 modifies the immune cross-talk between mononuclear and colon carcinoma cells. Folia Biol 62, 47–52. [PubMed] [Google Scholar]
- 48.Park J, Morley TS, Kim M, et al. (2014) Obesity and cancer – mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 10, 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoelson SE, Herrero L & Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132, 2169–2180. [DOI] [PubMed] [Google Scholar]
- 50.Nicklas BJ, Ambrosius W, Messier SP, et al. (2004) Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr 79, 544–551. [DOI] [PubMed] [Google Scholar]
- 51.Zuo H, Tell GS, Ueland PM, et al. (2018) The PAr index, an indicator reflecting altered vitamin B-6 homeostasis, is associated with long-term risk of stroke in the general population: the Hordaland Health Study (HUSK). Am J Clin Nutr 107, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuo H, Ueland PM, Midttun Ø, et al. (2018) Results from the European prospective investigation into cancer and nutrition link vitamin B6 catabolism and lung cancer risk. Cancer Res 78, 302. [DOI] [PubMed] [Google Scholar]
- 53.Ulvik A, Pedersen ER, Svingen GFT, et al. (2016) Vitamin B-6 catabolism and long-term mortality risk in patients with coronary artery disease. Am J Clin Nutr 103, 1417–1425. [DOI] [PubMed] [Google Scholar]
- 54.Cianciolo G, La Manna G, Coli L, et al. (2008) 5-methyltetrahydrofolate administration is associated with prolonged survival and reduced inflammation in ESRD patients. Am J Nephrol 28, 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hannisdal R, Svardal A & Ueland PM (2008) Measurement of folate in fresh and archival serum samples as p-amobenzoylglutamate equivalents. Clin Chem 54, 665–672. [DOI] [PubMed] [Google Scholar]
- 56.Fatahi S, Pezeshki M, Mousavi SM, et al. (2018) The effects of folic acid supplementation on C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 29, 432–439. [DOI] [PubMed] [Google Scholar]
- 57.Ho GYF, Xue X, Cushman M, et al. (2009) Antagonistic effects of aspirin and folic acid on inflammation markers and subsequent risk of recurrent colorectal adenomas. J Natl Cancer Inst 101, 1650–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suh JR, Herbig AK & Stover PJ (2001) New perspectives on folate catabolism. Annu Rev Nutr 21, 255–282. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y-I (2008) Folic acid supplementation and cancer risk: point. Cancer Epidemiol Biomarkers Prev 17, 2220–2225. [DOI] [PubMed] [Google Scholar]
- 60.Luebeck EG, Moolgavkar SH, Liu AY, et al. (2008) Does folic acid supplementation prevent or promote colorectal cancer? Results from model-based predictions. Cancer Epidemiol Biomarkers Prev 17, 1360–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller JW & Ulrich CM (2013) Folic acid and cancer – where are we today? Lancet 381, 974–976. [DOI] [PubMed] [Google Scholar]
- 62.Ulrich CM (2007) Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr 86, 271–273. [DOI] [PubMed] [Google Scholar]
- 63.Ulrich CM & Potter JD (2007) Folate and cancer – timing is 73. everything. JAMA 297, 2408–2409. [DOI] [PubMed] [Google Scholar]
- 64.Ebbing M, Bonaa KH, Nygard O, et al. (2009) Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 302, 2119–2126. [DOI] [PubMed] [Google Scholar]
- 65.Matsubara K, Mori M, Matsuura Y, et al. (2001) Pyridoxal 5’-phosphate and pyridoxal inhibit angiogenesis in serum-free rat aortic ring assay. Int J Mol Med 8, 505–508. [DOI] [PubMed] [Google Scholar]
- 66.Matsubara K, Mori M, Akagi R, et al. (2004) Anti-angiogenic effect of pyridoxal 5’-phosphate, pyridoxal and pyridoxamine on embryoid bodies derived from mouse embryonic stem cells. Int J Mol Med 14, 819–823. [PubMed] [Google Scholar]
- 67.George ML, Tutton MG, Janssen F, et al. (2001) VEGF-A, VEGF-C, and VEGF-D in colorectal cancer progression. Neoplasia 3, 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zastre JA, Sweet RL, Hanberry BS, et al. (2013) Linking vitamin B1 with cancer cell metabolism. Cancer Metab 1, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alaei-Shahmiri F, Soares MJ, Zhao Y, et al. (2015) The impact of thiamine supplementation on blood pressure, serum lipids and C-reactive protein in individuals with hyperglycemia: a randomised, double-blind cross-over trial. Diabetes Metab Syndr 9, 79. 213–217. [DOI] [PubMed] [Google Scholar]
- 70.González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, et al. (2011) Effect of thiamine administration on metabolic profile, cytokines and inflammatory markers in drug-naïve patients with type 2 diabetes. Eur J Nutr 50, 145–149. [DOI] [PubMed] [Google Scholar]
- 71.Page GL, Laight D & Cummings MH (2011) Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract 65, 81. 684–690. [DOI] [PubMed] [Google Scholar]
- 72.de Andrade JAA, Gayer CRM, NPdA Nogueira, et al. (2014) The effect of thiamine deficiency on inflammation, oxidative stress and cellular migration in an experimental model of sepsis. J Inflamm 11, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menezes RR, Godin AM, Rodrigues FF, et al. (2017) Thiamine and riboflavin inhibit production of cytokines and increase the anti-inflammatory activity of a corticosteroid in a chronic model of inflammation induced by complete Freund’s adjuvant. Pharmacol Rep 69, 1036–1043. [DOI] [PubMed] [Google Scholar]
- 74.Yadav UCS, Kalariya NM, Srivastava SK, et al. (2010) Protective role of benfotiamine, a fat-soluble vitamin B1 analogue, in lipopolysaccharide-induced cytotoxic signals in murine macrophages. Free Radical Biol Med 48, 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ross R (1999) Atherosclerosis – an inflammatory disease. N Engl J Med 340, 115–126. [DOI] [PubMed] [Google Scholar]
- 76.Araki A, Hosoi T, Orimo H, et al. (2005) Association of plasma homocysteine with serum interleukin-6 and C-peptide levels in patients with type 2 diabetes. Metabolism 54, 809–814. [DOI] [PubMed] [Google Scholar]
- 77.Gori AM, Corsi AM, Fedi S, et al. (2005) A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr 82, 335–341. [DOI] [PubMed] [Google Scholar]
- 78.Scheller J, Chalaris A, Schmidt-Arras D, et al. (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813, 878–888. [DOI] [PubMed] [Google Scholar]
- 79.Schett G (2018) Physiological effects of modulating the interleukin-6 axis. Rheumatology 57, ii43–ii50. [DOI] [PubMed] [Google Scholar]
- 80.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate OBV, and Choline (1998) Vitamin B6. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 81.Evans C, Morrison I, Heriot AG, et al. (2006) The correlation between colorectal cancer rates of proliferation and apoptosis and systemic cytokine levels; plus their influence upon survival. Br J Cancer 94, 1412–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.