Abstract
Lower extremity lymphedema is a chronic, often irreversible condition that affects many patients treated for gynecologic malignancies, with published rates as high as 70% in select populations. It has consistently been shown to affect multiple quality of life metrics. This review focuses on the pathophysiology, incidence, trends and risk factors associated with lower extremity lymphedema secondary to treatment of cervical, endometrial, ovarian and vulvar cancers in the era of sentinel lymph node mapping. We review traditional and contemporary approaches to diagnosis and staging and discuss new technologies and imaging modalities. Finally, we review the data-based treatment of lower extremity lymphedema and discuss experimental treatments currently being developed. This review highlights the need for more prospective studies and objective metrics, so that we may better evaluate and serve these patients.
INTRODUCTION
Lymphedema is a chronic, complex process that affects approximately 20 million people worldwide, causing significant discomfort, morbidity, and financial burden for those affected. Lymphedema is defined as the accumulation of interstitial fluid, leading to soft tissue swelling, chronic inflammation, reactive tissue fibrosis and abnormal adipose deposition.[1] There are two types of lymphedema: primary and secondary. Primary lymphedema is mostly due to an innate defect in the lymphatic system involving either the channels, nodes or both, leading to aplasia, hypoplasia or hyperplasia of these structures.[1, 2] Primary lymphedema is rare, typically occurring early in life, and is further classified based on age of onset.[1] Secondary lymphedema is much more common and occurs when the lymphatics are damaged by underlying medical conditions such as cancer, obesity, surgery, trauma, infection, radiation and other therapies.[1, 3] Secondary lymphedema is the focus of this review.
The most common cause of secondary lymphedema worldwide is infection. In the United States, however, malignancy and cancer-directed treatments account for the majority of cases.[4] The incidence and prevalence of lymphedema after cancer treatments varies based on the surgical procedures performed and use of additional therapies.[3] The most common surgical cause is regional lymphadenectomy, and radiation therapy and chemotherapy also contribute to the risk.[1] Though dangerous side effects are rare, the psychosocial consequences of lymphedema can be debilitating. Ryan et al. showed that, among patients with lower extremity lymphedema, 27% reported a financial burden secondary to the diagnosis and 51% reported alterations in their daily activities.[5] The derangement in aesthetics and functioning that accompany this condition can lead to depression, anxiety and a negative body image. In its severe stages, lymphedema can affect a patient’s ability to perform the functions of daily living.[6]
Pathophysiology
In lymphedema, the buildup of stagnant, protein-rich extracellular fluid impairs normal oxygen and nutrient transport to tissues.[1] The change in hydrostatic pressure in the interstitium also results in cell death and inflammation, CD4+ rich T-cell infiltration, subsequent fibroblast and smooth muscle cell proliferation and, finally, deranged adipose deposition.[7, 8] This impairs baseline skin integrity and elasticity, which may result in skin damage (i.e. fissures and ulcers). The impediment of normal cellular and macromolecular transport results in impaired wound healing of these lesions.[7] In severe lymphedema, chronic ulcers are often the most difficult-to-treat sequelae and act as an obvious source of infection. Furthermore, the damaged lymphatic channels impede the movement of T-cells and Langerhans cells to lymph nodes, where antigen presentation normally allows for immunologic responses to these foreign microbes, resulting in recurrent bouts of cellulitis and lymphangitis.[1] This creates a vicious cycle of infection, worsening skin damage and worsening lymphedema.[7] In rare instances long-standing chronic lymphedema results in secondary cutaneous malignancy, namely lymphangiosarcoma.[9]
DIAGNOSIS AND ASSESSMENT
The diagnosis of lymphedema can be difficult, especially in its early stages. This has resulted in under-diagnosis even in the research setting. Early and accurate diagnosis of lymphedema is key to proper intervention and prevention of the irreversible sequelae of later-stage disease.
It is critical to differentiate true lymphedema from other conditions that lead to swelling of the extremities and are often confused as lymphedema, because the pathophysiology, management and reversibility are quite different. Other causes of peripheral edema which can mimic lymphedema (summarized in Table 1) include chronic venous insufficiency, cardiac/renal failure, hypoalbuminemia and lipedema. It is important to note that some of these comorbid conditions are also risk factors for lymphedema and may occur concomitantly, further muddying the clinical picture.
Table 1.
Disease States that Can Mimic Lymphedema
| Other Disease States |
|---|
| • Morbid obesity |
| • Chronic venous insufficiency |
| • Cardiac/renal failure |
| • Hypoalbuminemia |
| • Complex regional pain syndrome Type 1 |
| • Infection |
| • Musculoskeletal injury |
| • Myedema |
| • May-Thurner syndrome |
| • Obstructive sleep apnea |
| • Medication-induced peripheral edema |
| • Lipedema |
History and physical exam play a large role in the diagnosis of lymphedema; however, other metrics should also be used in diagnosis, grading, and measurement of treatment response (Table 2). Limb volume has long been utilized as a metric for measuring lymphedema as a quantitative adjunct to swelling noted by the patient or on physical exam.[10] These measurements rely on a normal limb as an internal standard, or baseline measurements for comparison.[10] The gold standard for volumetric measurement of lymphedema is use of a water volumeter, which simply measures the true volume of a limb using displacement of water in a standardized container. This method has been shown to detect changes in volume of <1%; however, it does not provide insight into the distribution of edema.[11] Though simple and easily reproducible, the method is inconvenient for patients, and it may be cumbersome to use in a clinic setting, especially with respect to the lower extremities.[12]
Table 2.
Grading Systems for Lymphedema
| ISL Stage | ISL Grading | CTCAE Grade |
|---|---|---|
| 0: Subclinical impaired lymphatic transport without lymphedema | Mild, <20% increase in volume | Grade 1: Trace thickening or faint skin discoloration |
| 1: Relatively high protein edema that reverses with elevation +/− pitting +/− increase in proliferating cells | Moderate, 20–40% increase in volume Grade 2: Limits activities of daily living. Characterized by marked skin discoloration, leathery texture, papillary formation. |
|
| 2: High protein edema with dermal fibrosis that does not easily reverse with elevation. Usually no pitting. | ||
| 3. Trophic skin changes: warty overgrowths, acanthosis, fat deposits, usually without pitting. Also known as lymphatic elephantiasis. | Severe, >40% increase in size | Grade 3: Severe symptoms limiting self-care and activities of daily living. |
Limb circumference as measured by a non-elastic tape measure has also been used as a surrogate for differential limbs volumes. These measurements can be taken at specified anatomical landmarks or at regular intervals along the length of the limb.[12] Though cut-offs may vary by method, a difference of 2 cm between limbs is considered diagnostic of lymphedema.[13, 14] This method is inexpensive and easily taught, which makes it attractive. However, the measurements are often not sensitive enough to detect small changes; furthermore they require a normal contralateral limb, which may not be possible in the setting of bilateral disease.[12] Mathematical formulae have also been used to correlate series of standard tape measurement to volumes. The most commonly used is the Frustum Formula, which calculates volume by assuming that limbs are similar to cones. These methods attempt to maximize the simplicity of tape measurements and the utility of comparing volumes; they are not sets of linear measurements.[15]
A perometer is a more complex tool that utilizes parallel light-emitting diodes to measure corresponding extremity diameter throughout the length of the limb, allowing for volumetric assessment without using water displacement. Perometry has demonstrated higher interobserver reliability compared with tape measurements, especially for clinicians who do not regularly do such assessments.[14] However, the high cost of this diagnostic tool prohibits its widespread use, especially in a clinic setting where many of these assessments are done.[10]
Other diagnostic modalities that examine intrinsic tissue changes include bioimpedance spectroscopy and tissue tonometry. Bioimpedance spectroscopy exploits the fact that edematous tissues have higher water content and a lower tissue resistance. Differential measurement of this resistance allows for an estimation of extracellular water volume as a surrogate marker for lymphedema.[16] Bioimpedance spectroscopy enables the detection of subclinical lymphedema and may facilitate interventions to reduce the likelihood of the disease entering the irreversible stages. Importantly, bioimpedance spectroscopy technology does not require an internal control. This is relevant in the setting of gynecologic malignancy, where bilateral disease is more common.[4] Tissue tonometry objectively measures the resistance of soft tissue to compression, thus acting as a surrogate for edema and tissue fibrosis changes in lymphedema. However, this technology also requires an internal control or baseline measurement. [17]
Imaging studies can also be used in difficult-to-diagnose cases of lymphedema, as well as in staging and surgical treatment planning. The traditional oil contrast lymphography—an x-ray-based imaging modality—has been largely replaced by radionuclide lymphangioscintigraphy, whereby an intradermal injection of a radionuclide such as Technetium-99 is used to provide qualitative information on the lymphatic system as well as quantitative data on lymph transit times.[18] Single-photon emission computed tomography similarly uses a dermally injected radionuclide and gamma rays to visualize the lymphatic system. The extent of dermal backflow is better visualized by this method, compared with lymphoscintigraphy. Similarly, gadolinium-based contrast can be injected dermally to visualize lymphatics and the surrounding soft tissue using magnetic resonance imaging.[11] Near infrared imaging using indocyanine green—a technology best known for its use in the intraoperative identification of sentinel lymph nodes in cancer staging—has also been utilized to visualize lymphatic patterns and active contractility of lymphatic vessels in real time.[12] This can be particularly helpful intraoperatively, and in the diagnosis of lymphatic dysfunction prior to the onset of lymphedema. At the present time, however, the availability of this technology is limited.[18]
It should be noted that not every change in limb circumference, volume or abnormal imaging study is indicative of clinically significant lymphedema. Additionally, lymphedema symptoms may be reported by patients before they become clinically identifiable through circumference or volume changes (i.e. leg heaviness). That is why several studies have focused on patient-reported lymphedema, using validated surveys with good sensitivity and specificity for diagnosing clinically significant lower leg lymphedema. Larger studies comparing patient-reported outcomes to objective metrics are still needed.[13] The Gynecologic Oncology Group study 244 (GOG 244), also known as the Lymphedema and Gynecologic (LEG) cancer study, is a prospective multicenter trial which examines both objective measurement and patient-reported surveys to better our understanding of the true burden of lymphedema in patients with gynecologic malignancy.[19]
Once a diagnosis of lymphedema is established, staging should be done to determine the proper treatment regimen and quantify treatment response. The International Society of Lymphology (ISL) stages of lymphedema are summarized in Table 2 and shown in Figure 1. This system takes into consideration both qualitative stage and quantitative physical assessment (ISL Grade) which allows for streamlined diagnosis and monitoring of treatment response.[10] However, in its 2016 statement on the grading system, the Society states that a “more detailed and inclusive classification needs to be formulated”, one that would ideally take genotypic information, disability grading, assessments of inflammation, and imaging modalities into consideration.[10] The National Cancer Institute’s common terminology criteria for adverse events (CTCAE) is often used to stage secondary lymphedema in both research and clinical settings (Table 2). However, these grades focus on the physical impediments that patients encounter rather than on objective measures, making them unreliable in the diagnosis of true lower extremity lymphedema.[20]
Figure 1.
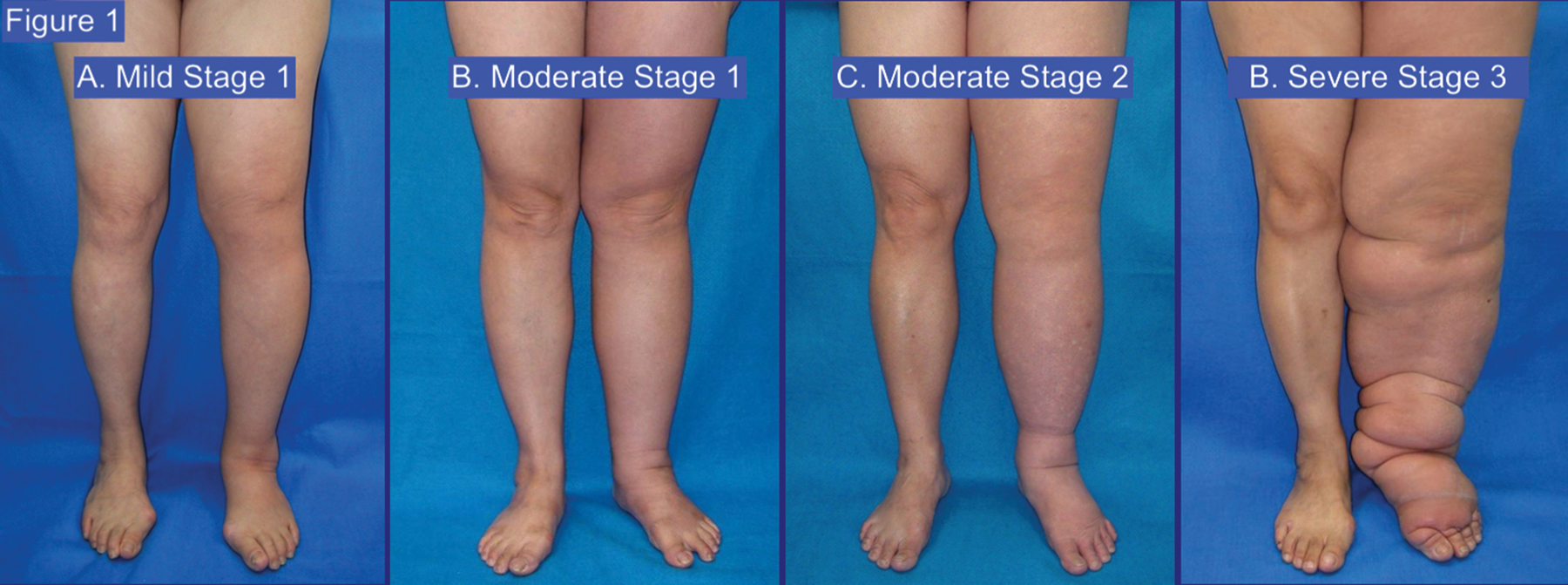
Photographs of the ISL stages/grade of lymphedema. (A) Stage 1 mild lymphedema with <20% difference in limb size. (B) Stage 1 moderate lymphedema with a 20–40% difference in limb size. (C) Stage 2 moderate lymphedema with a 20–40% difference in limb size with associated fibrosis and irreversible edema. (D) Stage 3 severe lymphedema with >40% limb difference, and abnormal fat deposits.
Adapted from Cheng MH, Chang DW, Patel KM (editors): Principles and Practice of Lymphedema Surgery. Elsevier Inc.; Oxford, UK. ISBN: 978-0-323-29897-1. July 2015.
SECONDARY LYMPHEDEMA IN GYNECOLOGIC MALIGNANCIES
To date, the preponderance of research on lymphedema within the field of oncology has focused on upper extremity lymphedema in patients treated for breast cancer. These data have facilitated the development of diagnostic and treatment strategies for lymphedema and have altered the clinical care of breast cancer patients by incorporating sentinel lymph node dissection into the treatment algorithm. Despite these promising strides, lymphedema in other anatomical sites remains under-recognized and under-studied. In addition, there are obvious differences in the upper and lower extremities with respect to tissue composition and mechanical functioning; therefore, extrapolating data based on the upper extremities should be done with caution. In the next part of this review we focus on lower extremity lymphedema and how it affects patients with endometrial cancer, ovarian cancer, vulvar cancer and cervical cancer.
Lymph from the gynecologic organs primarily drains into the three lymph node beds: pelvic, para-aortic, and inguinofemoral. These basins are often sampled or completely excised as part of the surgical management of gynecologic malignancies. Overall, the incidence of treatment-related lymphedema is about 25%, but it may be as high as 70% in some patient populations.[21] There are two consistent contributors to lower extremity lymphedema in these patients: lymphadenectomy and radiation therapy. Lymphadenectomy—defined as complete excision of a lymph node basin—directly disrupts the normal return of lymphatic fluid from the lower extremities. In general, the risk of lymphedema is proportional to the number of lymph nodes sampled, with excision of certain lymph nodes and lymph node basins thought to present a higher risk.[22] Sentinel lymph node mapping alone has been shown to decrease the risk of lower extremity lymphedema to less than 10%, across gynecologic malignancies.[21] Radiation-induced lymphedema is thought to be secondary to lymph node and lymphatic vessel sclerosis, scarring, and subsequent impedance of upstream lymphatic flow. A systematic meta-analysis of all studies examining radiation and risk of lower extremity lymphedema in gynecologic cancer found the risk to be 34% in patients receiving radiation treatment.[21]
Cervical cancer
Worldwide, the median age of diagnosis of cervical cancer is mid- to late 40’s. At the time of diagnosis and treatment 45% of patients have stage 1 disease, with a 5-year overall survival rate of 79–98%.[23] Minimizing the long-term risks of lower extremity lymphedema in this relatively young patient population is particularly important. Management of early-stage cervical cancer typically involves a radical hysterectomy and lymph node assessment, either by pelvic lymphadenectomy with or without para-aortic lymphadenectomy, or by sentinel lymph node mapping. Prior to the introduction of sentinel lymph node mapping, rates of treatment-related lower extremity lymphedema ranged from 10%[24] when assessed retrospectively to 41%[25] when prospectively assessed using objective metrics. Rates of lower extremity lymphedema after fertility-sparing surgery with radical trachelectomy fall within this range at 24%, as lymphadenectomy is also done in these cases.[26]
Radiation contributes to lower extremity lymphedema. This is significant because chemoradiation is the standard of care for locally advanced cervical cancer, and adjuvant radiation is used in intermediate- and high-risk patients after radical hysterectomy and lymphadenectomy or sentinel lymph node mapping. The combination of surgery and radiation appears to be particularly detrimental. Landoni and colleagues reported the results of a randomized trial comparing survival between upfront radical surgery and postoperative radiation in patients with risk factors, compared with upfront radiation therapy for 2008 FIGO (International Federation of Gynecology and Obstetrics) stage IB-IIA patients.[27] Those who underwent both surgery and adjuvant radiation had the highest rates of severe postoperative lower extremity lymphedema: 9% after surgery and postoperative radiation compared with 0.6% after radiation alone and 0% after surgery alone.[27] The modality used in radiation therapy may also affect lymphedema rates. Mohanty et al. prospectively evaluated patient-reported symptoms using a validated quality of life assessment and found that patients undergoing three-dimensional conformational radiation therapy reported higher rates of lymphedema symptoms over time than patients who received intensity modulated radiation therapy.[28]
Togami et al. retrospectively analyzed other risk factors for lymphatic complications after surgery for cervical cancer and found that excision of a large number of lymph nodes and excision of the most distal lymph node in the pelvic lymph node basin (the circumflex iliac node) were associated with increased risk of lymphedema (OR 3.37 and 3.92 respectively). Both of these factors are mitigated by the use of sentinel lymph node mapping alone.[22] Sampling of the sentinel lymph node is considered an acceptable option for surgical staging of early-stage cervical cancers, especially in tumors measuring less than 2 cm (for which detection rates are the highest). Sentinel lymph node mapping has been shown to decrease rates of perioperative morbidity, demonstrating 92% sensitivity and 98% negative predictive value in prospective trials.[29] Mikura et al. found that lower extremity lymphedema rates decreased from 42% to 8.7% using their sentinel lymph node mapping algorithm.[30] SENTICOL III (International Validation Study of Sentinel Node Biopsy in Early Cervical Cancer), a large prospective, multicenter randomized trial examining the validity of sentinel lymph node mapping in cervical cancer, is currently underway.[31]
Endometrial cancer
Lymph node assessment in endometrial cancer is an important part of surgical management. The published risk of lower extremity lymphedema in endometrial cancer ranges widely, from 1.2% in retrospective analyses[32] to 47% in prospective studies utilizing quality of life surveys.[33] Risk factors for development of the most common histology in endometrial cancer (endometroid adenocarcinoma), including obesity and metabolic syndrome, are also risk factors for the development of lower extremity lymphedema due to etiologies such as chronic venous insufficiency and congestive heart failure. Thus, this population may have higher rates of baseline lower extremity lymphedema that can be mistaken for, or exacerbated by, malignancy. In a study by Abu-Rustum et al. assessing rates of postoperative lower extremity lymphedema in endometrial cancer patients prior to the introduction of sentinel lymph node mapping, 5–6% of patients had clinically reported lower extremity lymphedema preoperatively, potentially secondary to another comorbidity.[32] Obesity, a comorbid condition in a large percentage of endometrial cancer patients, makes lymphedema clinically harder to detect in its early stages and can independently contribute to its pathogenesis.[33] Lipedema, the disordered deposition of fat under the skin, can affect an overlapping population as endometrial cancer, i.e. peri/post-menopausal obese women, making the exclusion of this diagnosis in this population particularly important. These patients will have more painful, non-pitting edema which spares the feet and is incited by hormonal shifts (puberty, pregnancy, menopause etc.) and associated with a family history of the disorder. [1]
In endometrial cancer, as in cervical cancer, the number of lymph nodes removed at the time of lymphadenectomy is associated with risk of lymphedema, though an exact threshold has not been established.[33, 34] In a published series from our institution, all cases of lower extremity lymphedema were in patients with greater than 10 lymph nodes sampled.[32] However, the presence of lower extremity lymphedema was determined by reviewing medical records, which underestimates the true rate of this condition, making it difficult to draw definitive conclusions. The presence of metastatic disease in lymph nodes is also associated with development of lower extremity lymphedema even after authors controlled for the performance of lymphadenectomy, suggesting that lymphatic metastasis may independently contribute to later lymphatic dysfunction.[35]
The acceptance of sentinel lymph node mapping as a standard in the staging of endometrial cancer provides a method for reducing morbidity secondary to lymphadenectomy. Prospective multicenter trials have demonstrated a sensitivity of 97.3% and negative predictive value of 99.6% using sentinel lymph node mapping.[36] This method has already been shown to decrease rates of lower extremity lymphedema. Gerpert et al. noted that, in patients who underwent sentinel lymph node mapping compared with patients undergoing full lymphadenectomy, the rates of lymphedema (as prospectively diagnosed by a physiotherapist) were significantly lower: 1.3% versus 18.1% (P=0.0003).[37] However, the exact method of diagnosis was unclear. We have presented the results of a study conducted at our institution assessing the prevalence of patient-reported lower extremity lymphedema after surgery for gynecologic cancer. The patient-reported rate was significantly lower in those who had undergone a sentinel lymph node mapping procedure per our institutional algorithm, compared with those who had undergone bilateral lymphadenectomy (27 vs 41% respectively).
Ovarian Cancer
The lowest rate of lower extremity lymphedema is reported in ovarian cancer patients, ranging from 4.7% in a retrospective study[38] to 30.4% in prospective patient-reported surveys.[39] Again, lymphadenectomy is the most important prognostic factor.[38, 39] In ovarian cancer, surgical evaluation of the pelvic nodes and para-aortics up to the renal vessels is considered the standard of care for early-stage disease confined to the ovary and/or pelvis.[40] Lim et al. examined a cohort of ovarian cancer patients with early-stage disease, 97.2% of whom underwent lymphadenectomy, and reported that 55% had lymphedema. It should be noted that 57.4% had greater than 35 lymph nodes excised.[40] The majority of patients with newly diagnosed ovarian cancer actually present with advanced stage disease, in which case such a systematic lymphadenectomy is not routinely performed except in the setting of grossly enlarged nodes.[41]
The lymphatic drainage from the ovaries has been shown to follow at least two major pathways and one minor pathway. Sentinel lymph node mapping currently has no role in the treatment of ovarian cancer, due to lack of understanding of the common lymphatic pathways. Furthermore, sentinel lymph node mapping would likely require injection of radiotracer into the ovarian cortex or the ligaments, presenting a risk of tumor spread or vascular damage.[42] When lymph node assessment is indicated for patients with ovarian cancer clinically confined to the ovary and/or pelvis, complete regional lymphadenectomy is warranted.[42]
Vulvar Cancer
Vulvar cancers, compared with other gynecologic malignancies, have a more reliable and predictable lymphatic drainage to the inguinofemoral nodal basins. Rates of lower extremity lymphedema after inguinofemoral lymphadenectomy range from 10% in retrospective reports[43] to 73% in studies assessing patient-reported symptoms.[8] In studies using uniform methodology to diagnose lower extremity lymphedema across types of malignancy, the rates are consistently highest in vulvar cancer; based on a recent meta-analysis, the pooled incidence is 28.8%.[5, 39, 44] This is mostly, but not entirely, related to inguinofemoral lymphadenectomy.[43] Berger and colleagues reported that 6.7% of patients with vulvar cancer treated with radiation alone developed chronic lower extremity lymphedema.[43] Other risk factors include infection, extensive lymph node dissection, and postoperative adjuvant radiation therapy.[44] Small series have suggested possible ways to prevent lower extremity lymphedema after lymphadenectomy. Dardrian et al. reported that sparing the saphenous vein decreased rates of clinically identified lower extremity lymphedema from 38% to 11% (P<0.05).[45] Other surgical techniques associated with decreased rates include omental flaps, prophylactic diverting lymphatic microsurgery, and fascia-preserving dissections.[46] Sentinel lymph node mapping is now considered an acceptable method for nodal assessment in vulvar cancer. It has been prospectively assessed by the Groningen International Study on Sentinel nodes in Vulvar cancer (GROINNS) study group as well as the GOG, and has demonstrated acceptable reliability in determining nodal status[47, 48] In a large single-arm prospective study, the rate of lower extremity lymphedema after sentinel lymph node mapping was 1.9%, compared with 25.3% after lymphadenectomy (P<0.05).[49] It should be noted that patients undergoing treatment for gynecologic malignancies can also suffer from lymphedema isolated to the pelvis or the perineum (particularly with vulvar cancers). Pelvic/genital lymphedema may present with similar heaviness and pressure in the pelvic floor with minimal or no leg manifestations. Treatment of this patient populations is particularly difficult and centers around pelvic harnesses and surgical management.[50]
PREVENTION AND TREATMENT OF LOWER EXTREMITY LYMPHEDEMA
The first step in preventing lower extremity lymphedema is to identify at-risk patients. Low-risk treatment modalities should be employed when possible. Careful operative planning, use of sentinel lymph node mapping if available, and appropriate use of adjuvant therapies will significantly reduce the risk. Unfortunately, there are very limited data that would enable us to determine truly effective, preventative postoperative measures. A randomized trial in patients undergoing surgery for breast cancer demonstrated that increased upper extremity mobility and prophylactic physiotherapy significantly decreased the risk of chronic lymphedema from 25% to 7% (p-0.010).[51] However, similar randomized studies have not been done in patients undergoing surgery for other malignancies. Prophylactic compressive garments can be helpful in preventing upper extremity lymphedema after breast cancer surgery, but appear to offer limited benefit in lower extremity lymphedema.[52] Hnin et al randomized a pilot cohort of 56 patients to the use of customized compression garments versus usual care and found that the prophylactic use of these garments decreased the incidence of clinically and objectively measured lower extremity lymphedema (13.3% versus 7.7%) however this did not reach statistically significance (P=0.496).[53]
In a small study of highly selected patients undergoing lymphadenectomy, prophylactic lymphovenous anastomosis and shunts have demonstrated efficacy, though its applicability on a larger scale is unknown at this time.[54] Omental (gastroepiploic) free flaps containing lymph node bundles have been described for the management of patients with secondary lymphedema in the upper and lower extremities.[55] The procedure might prove useful as a preventative measure at the time of inguinofemoral lymphadenectomy. We have been considering this option at our institution. However, there is currently no data that would provide recommendations.
Additional strategies to prevent lower extremity lymphedema focus on early identification of stage 0 and 1 lymphedema, in which skin changes are absent and edema is reversible. Educating patients about the symptoms may facilitate early diagnosis. Beesley and colleagues reported that in 802 gynecologic oncology patients without diagnosis of lower extremity lymphedema, 15% had some symptoms that warranted further evaluation.[38]
The International Society of Lymphology recommends specific interventions to reduce progression and limit long-term sequelae for patients with early or subclinical lymphedema.[10] These measures are not all evidence-based; rather, they are based on the biology of lymphedema and the reduction of potential risk factors for progression. Early conservative management includes encouraging lymphatic flow into the venous system and avoiding lymphatic stasis that causes fibrosis and further damage. This can be achieved by elastic hosiery or non-elastic compression leggings in patients with stage 0 or mild stage 1 lower extremity lymphedema.[10] In patients with more clinically significant edema, multiple layers of short stretch compression bandages has been prospectively shown (by Badger et al.) to decrease leg volume by an additional 15.3% compared with compressive hosiery alone (p=0.001).[56] The next phase of treatment for persistent edema that does not respond to simple compression involves mechanical and targeted displacement of lymphatic fluid from tissues. Traditionally this has been done by manual lymphatic draining and sequentially intermittent pneumatic compression. Manual lymphatic drainage is specialized physiotherapy utilizing targeted massage and limb movements that stimulate the flow of lymphatic fluid out of damaged tissues to tissues with intact lymphatic drainage.[11] Both of these measures have proven to be good adjuncts to compressive garments and bandaging in mild to moderate breast cancer-related lymphedema, and this has been recapitulated in smaller observation studies focusing on lower extremity lymphedema.[57]
More significant lymphedema must often be managed by more intensive, multimodal treatment, collectively described as complete decongestive treatment. This includes a combination of intensive regular physiotherapy, manual lymphatic drainage and multilayer short stretch compression bandaging. Once a plateau of response is identified, maintenance of treatment response is obtained using daily limb compression with compression garments and continued skin and nail hygiene.[11] Prospective data shows about a 60% reduction in limb volume using this method in moderate to severe lymphedema.[58] Kim et al. demonstrated that complete decongestive treatment not only improved lower limb volume but also improved quality of life metrics.[59] However, given the variation in complete decongestive treatment regimens and the heterogeneity of the patient populations in these studies, it is difficult to precisely determine which part of the therapy is most efficacious. Some small randomized, controlled trials suggest that manual lymphatic drainage may not provide a significant amount of volume reduction beyond wrapping alone.[60] Furthermore, the continued benefits of limb volume reduction depends on patient compliance with maintenance therapy.
There are some surgical and invasive interventions that can be considered to prevent or treat lower extremity lymphedema. Multiple microsurgical techniques have been investigated.[54, 61] The core concept is restoration of normal lymphatic drainage, whether by anastomosing lymphatics to each other (lymphatic-lymphatic bypass), anastomosing afferent lymphatics to the venous circulation (lymphovenous bypass), creating anastomoses between subdermal lymphatic and venules (lymphaticovenular anastomosis) or transplanting vascularized lymph node bundles.[61] These methods all have varying degrees of success. Campisi and colleagues reported on 1,800 cases of lower and upper primary and secondary edema (>90% stage II and III) managed with various lymphatic/venous bypass, using native tissues to the lymph node bed or autologous venous grafting.[62] Corrective procedures were most commonly performed in the sub-inguinal region. In this patient population, 87% had subjective improvement of symptoms, 83% had objective reduction in limb volume, and 85% were able to discontinue other conservative treatments.[62] Allographic vascularized nodal tissue transplantation has also been utilized. Theoretically, after anastomosis with blood vessel in the receipt lymphatic bed, the vital nodal tissue will form new lymphatic connections via lymphangiogenic mechanism. This should lead to improved afferent lymphatic drainage. In patients with lower extremity lymphedema, limb circumference reductions of 46–64% are reported, with documented improvement in lymphatic flow as assessed with indocyanine green lymphography and/or lymphoscintigraphy.[61, 63] A potential drawback of this method is risk of lymphedema in the afferent limb or tissue of the donor site, which in lower limbs often includes the inguinal or supraclavicular lymph nodes.[63] A meta-analysis of 27 studies focusing on lymphovenous shunting and vascularized lymph node transplantation reported an average reduction in lower limb circumference of 57%, which is greater than that reported for the upper extremities (46%). It is important to note that this meta-analysis was not based on randomized trials and, as always, proper patient selection is critical.[64]
Other surgical methods focus on removal of abnormal tissues. Early lymphedema results in excessive adipose tissue propagation, which cannot be treated with fluid decompression alone. Some small studies have shown that, in the upper extremities, adjunctive excision of adipose tissues using liposuction alongside compressive techniques reduced limb volume by up to 70%.[61] The fat composition in the lower extremities has made adoption of this technique in the leg slightly more difficult. However, a reduction of 43% in limb circumference was described in a small series of 6 patients.[65] Other ablative methods involve the surgical excision and removal of significantly affected subcutaneous extra-fascial tissues and/or overlying skin. Though effective, these procedures are highly invasive and morbid, and are sometimes quite disfiguring. They are best reserved for later stage disease, in which irreversible fibrosis and lipodystrophy have already taken place and fluid decompression alone will not address limb issues.[61]
CONCLUSION
Secondary lower extremity lymphedema causes significant morbidity for survivors of gynecologic cancers. Lack of uniform assessment and diagnosis has led to difficulty in identifying the true rates of lymphedema. However, data has shown that patients with vulvar cancers, those undergoing lymphadenectomy, and those treated with radiation are at highest risk. Early identification of at-risk populations and patient education regarding early symptoms may aid in prevention, early diagnosis and treatment. Standardized methods for identifying at-risk patients (predictive risk factor model and symptom assessment) and improved provider education (accurate incidence and risk factor data) are greatly needed to address these issues. Newer, more objective measures, including patient-reported outcomes, can aid in the diagnosis and monitoring of treatment response. It is essential that we continue to introduce surgical techniques that place patients at the lowest possible risk and avoid high-risk procedures whenever possible. Prevention, early diagnosis, and timely interventions are key, but more research is needed to help us better understand lower extremity lymphedema. Patients who appear to be developing this condition should be referred in the early stages, when intervention has a greater chance of success. With risk mitigation, early diagnosis, and appropriate treatment, we can improve the quality of life for patients burdened by lower extremity lymphedema secondary to treatment of gynecologic malignancies.
Figure 2.
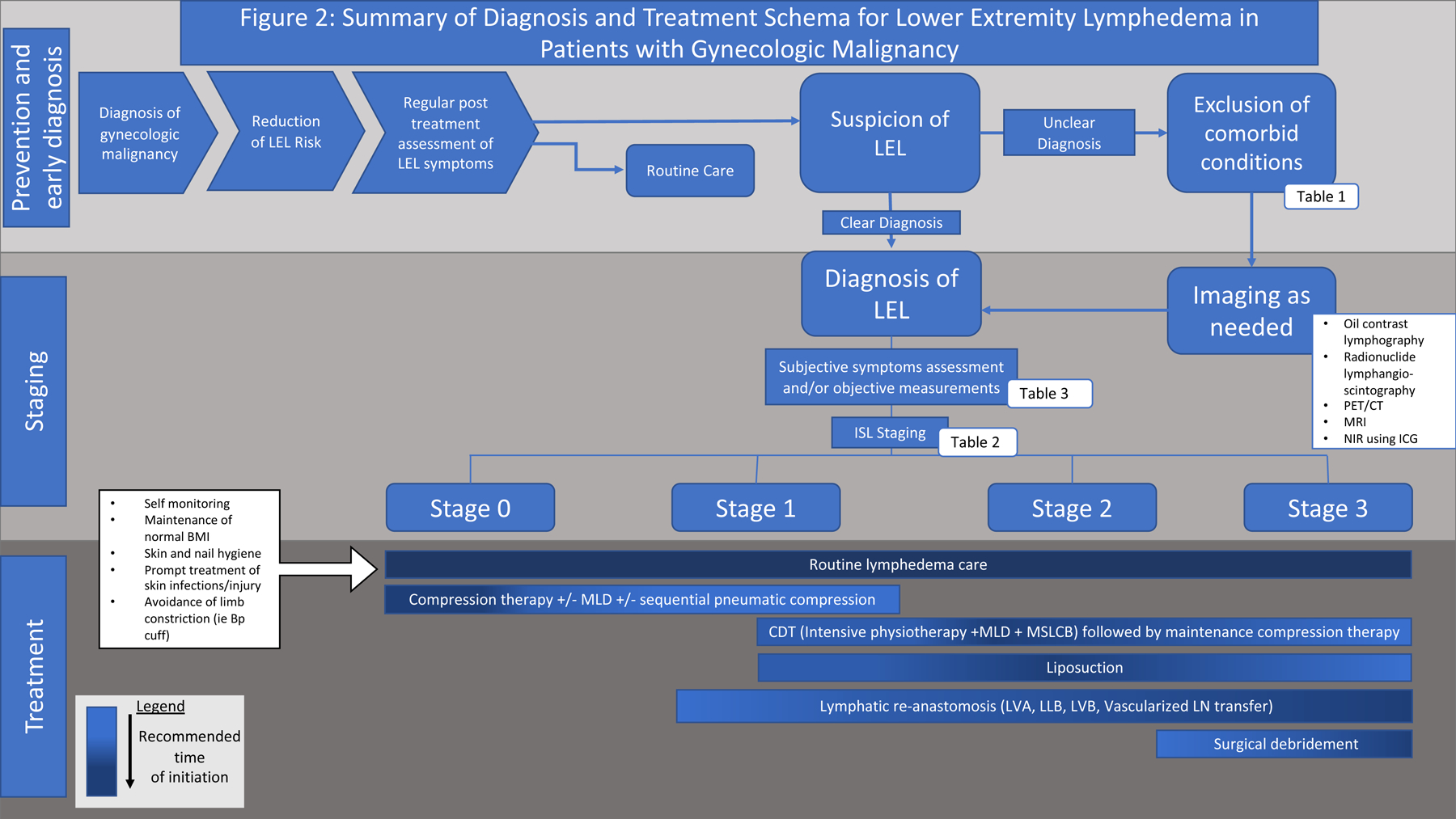
Summary algorithm for the treatment of lymphedema. All patients should be evaluated for risk of lymphedema prior to treatment for gynecologic malignancies and appropriate steps taken during treatment to reduce their risk, if possible. Patients should then be evaluated for lymphedema post-treatment. If clinically suspicious limb edema is present, other etiologies should be ruled out (Table 1), with the help of imaging or diagnostic modalities described in Table 3 as needed. Once lymphedema is diagnosed and appropriately staged, treatment is tailored to the patient stage, with more aggressive measures usually taken at higher stages.
LEL, lower extremity lymphedema; MLD, manual leg decompression; LVA, lymphaticovenous anastomosis; LLB, lymphatic-lymphatic bypass; LVB, lymphaticovenous anastomosis; LN, lymph node; CTD, complete decongestive therapy.
Table 3.
Methods for Diagnosis and Quantification of Lymphedema
| Advantages | Disadvantages | ||
|---|---|---|---|
| Linear measurements | Circumference |
|
|
| Volume measurements | Water displacement |
|
|
| Calculated |
|
|
|
| Perometry |
|
|
|
| Objective tissue evaluation | Bioimpedance spectroscopy |
|
|
| Tonometry |
|
|
|
ACKNOWLEDGEMENTS
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Footnotes
Conflict of Interest Statement: None declared.
Disclosures: None.
REFERENCES
- 1.Grada AA, Phillips TJ. Lymphedema: Pathophysiology and clinical manifestations. J Am Acad Dermatol 2017;77:1009–20. [DOI] [PubMed] [Google Scholar]
- 2.Lee BB, Villavicencio JL. Primary lymphoedema and lymphatic malformation: are they the two sides of the same coin? Eur J Vasc Endovasc Surg 2010;39:646–53. [DOI] [PubMed] [Google Scholar]
- 3.Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann N Y Acad Sci 2008;1131:147–54. [DOI] [PubMed] [Google Scholar]
- 4.Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann Plast Surg 2007;59:464–72. [DOI] [PubMed] [Google Scholar]
- 5.Ryan M, Stainton MC, Slaytor EK, Jaconelli C, Watts S, Mackenzie P. Aetiology and prevalence of lower limb lymphoedema following treatment for gynaecological cancer. Aust N Z J Obstet Gynaecol 2003;43:148–51. [DOI] [PubMed] [Google Scholar]
- 6.Fu SHR Mei R., Hu Sophia H., Stewart Bob R., Cormier Janice N. and Armer Jane M.. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psycho-Oncology 2013:1466–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallon EC, Ryan TJ. Lymphedema and wound healing. Clin Dermatol. 1994;12:89–93. [DOI] [PubMed] [Google Scholar]
- 8.Farrell R, Gebski V, Hacker NF. Quality of life after complete lymphadenectomy for vulvar cancer: do women prefer sentinel lymph node biopsy? Int J Gynecol Cancer 2014;24:813–9. [DOI] [PubMed] [Google Scholar]
- 9.Felmerer G, Dowlatshahi AS, Stark GB, Foldi E, Foldi M, Ahls MG, et al. Lymphangiosarcoma: Is Stewart-Treves Syndrome a Preventable Condition? Lymphat Res Biol 2016;14:35–9. [DOI] [PubMed] [Google Scholar]
- 10.Executive C The Diagnosis and Treatment of Peripheral Lymphedema: 2016 Consensus Document of the International Society of Lymphology. Lymphology 2016;49:170–84. [PubMed] [Google Scholar]
- 11.Shaitelman SF, Cromwell KD, Rasmussen JC, Stout NL, Armer JM, Lasinski BB, et al. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J Clin 2015;65:55–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mihara M, Hara H, Araki J, Kikuchi K, Narushima M, Yamamoto T, et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS One 2012;7:e38182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter J, Raviv L, Appollo K, Baser RE, Iasonos A, Barakat RR. A pilot study using the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) as a clinical care tool to identify lower extremity lymphedema in gynecologic cancer survivors. Gynecol Oncol 2010;117:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharkey AR, King SW, Kuo RY, Bickerton SB, Ramsden AJ, Furniss D. Measuring Limb Volume: Accuracy and Reliability of Tape Measurement Versus Perometer Measurement. Lymphat Res Biol 2018;16:182–6. [DOI] [PubMed] [Google Scholar]
- 15.Karges JR, Mark BE, Strikeleather SJ, Worrell TW. Concurrent validity of upper-extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther 2003;83:134–45. [PubMed] [Google Scholar]
- 16.Warren AG, Janz BA, Slavin SA, Borud LJ. The use of bioimpedance analysis to evaluate lymphedema. Ann Plast Surg 2007;58:541–3. [DOI] [PubMed] [Google Scholar]
- 17.Thorne CH, Gurtner GC, Chung KC, Gosain A, Mehrara B, Rubin P, Spear SL, editors. Grabb and Smith’s Plastic Surgery, 7th Edition Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2014. [Google Scholar]
- 18.O’Donnell TF Jr, Rasmussen JC, Sevick-Muraca EM. New diagnostic modalities in the evaluation of lymphedema. J Vasc Surg Venous Lymphat Disord 2017;5:261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson JW, Kauderer J, Hutson A, Carter J, Armer JA, Lockwood S, et al. GOG 244, the lymphedema and gynecologic cancer (LEG) study: Incidence and risk factors in newly diagnosed patients. Gynecol Oncol 2018;149:6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institutes of Health (NIH). The National Cancer Institute Common Terminology Criteria for Adverse Events 2019. [Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf
- 21.Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010;116:5138–49. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Rustum NR, Barakat RR. Observations on the role of circumflex iliac node resection and the etiology of lower extremity lymphedema following pelvic lymphadenectomy for gynecologic malignancy. Gynecol Oncol 2007;106:4–5. [DOI] [PubMed] [Google Scholar]
- 23.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev 2017;26:444–57. [DOI] [PubMed] [Google Scholar]
- 24.Snijders-Keilholz A, Hellebrekers BW, Zwinderman AH, van de Vijver MJ, Trimbos JB. Adjuvant radiotherapy following radical hysterectomy for patients with early-stage cervical carcinoma (1984–1996). Radiother Oncol 1999;51:161–7. [DOI] [PubMed] [Google Scholar]
- 25.Werngren-Elgstrom M, Lidman D. Lymphoedema of the lower extremities after surgery and radiotherapy for cancer of the cervix. Scand J Plast Reconstr Surg Hand Surg 1994;28:289–93. [DOI] [PubMed] [Google Scholar]
- 26.Froding LP, Ottosen C, Mosgaard BJ, Jensen PT. Quality of life, urogynecological morbidity, and lymphedema after radical vaginal trachelectomy for early-stage cervical cancer. Int J Gynecol Cancer 2015;25:699–706. [DOI] [PubMed] [Google Scholar]
- 27.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997;350:535–40. [DOI] [PubMed] [Google Scholar]
- 28.Mohanty SK, Chopra S, Mudaliar A, Kannan S, Mahantshetty U, Engineer R, et al. A comparative analysis of quality of life after postoperative intensity-modulated radiotherapy or three-dimensional conformal radiotherapy for cervical cancer. Indian J Cancer 2018;55:327–35. [DOI] [PubMed] [Google Scholar]
- 29.Bats AS, Mathevet P, Buenerd A, Orliaguet I, Mery E, Zerdoud S, et al. The sentinel node technique detects unexpected drainage pathways and allows nodal ultrastaging in early cervical cancer: insights from the multicenter prospective SENTICOL study. Ann Surg Oncol 2013;20:413–22. [DOI] [PubMed] [Google Scholar]
- 30.Niikura H, Okamoto S, Otsuki T, Yoshinaga K, Utsunomiya H, Nagase S, et al. Prospective study of sentinel lymph node biopsy without further pelvic lymphadenectomy in patients with sentinel lymph node-negative cervical cancer. Int J Gynecol Cancer 2012;22:1244–50. [DOI] [PubMed] [Google Scholar]
- 31.Lecuru FR, McCormack M, Hillemanns P, Anota A, Leitao M, Mathevet P, et al. SENTICOL III: an international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int J Gynecol Cancer 2019;29:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Rustum NR, Alektiar K, Iasonos A, Lev G, Sonoda Y, Aghajanian C, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol 2006;103:714–8. [DOI] [PubMed] [Google Scholar]
- 33.Yost KJ, Cheville AL, Al-Hilli MM, Mariani A, Barrette BA, McGree ME, et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol 2014;124(2 Pt 1):307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hareyama H, Ito K, Hada K, Uchida A, Hayakashi Y, Hirayama E, et al. Reduction/prevention of lower extremity lymphedema after pelvic and para-aortic lymphadenectomy for patients with gynecologic malignancies. Ann Surg Oncol 2012;19:268–73. [DOI] [PubMed] [Google Scholar]
- 35.Hopp EE, Osborne JL, Schneider DK, Bojar CJ, Uyar DS. A prospective pilot study on the incidence of post-operative lymphedema in women with endometrial cancer. Gynecol Oncol Rep 2016;15:25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 2017;18:384–92. [DOI] [PubMed] [Google Scholar]
- 37.Geppert B, Lonnerfors C, Bollino M, Persson J. Sentinel lymph node biopsy in endometrial cancer-Feasibility, safety and lymphatic complications. Gynecol Oncol 2018;148:491–8. [DOI] [PubMed] [Google Scholar]
- 38.Beesley V, Janda M, Eakin E, Obermair A, Battistutta D. Lymphedema after gynecological cancer treatment : prevalence, correlates, and supportive care needs. Cancer 2007;109:2607–14. [DOI] [PubMed] [Google Scholar]
- 39.Biglia N, Librino A, Ottino MC, Panuccio E, Daniele A, Chahin A. Lower limb lymphedema and neurological complications after lymphadenectomy for gynecological cancer. Int J Gynecol Cancer 2015;25:521–5. [DOI] [PubMed] [Google Scholar]
- 40.Lim MC, Lee JS, Nam BH, Seo SS, Kang S, Park SY. Lower extremity edema in patients with early ovarian cancer. J Ovarian Res 2014;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N Engl J Med 2019;380:822–32. [DOI] [PubMed] [Google Scholar]
- 42.Kleppe M, Kraima AC, Kruitwagen RF, Van Gorp T, Smit NN, van Munsteren JC, et al. Understanding Lymphatic Drainage Pathways of the Ovaries to Predict Sites for Sentinel Nodes in Ovarian Cancer. Int J Gynecol Cancer 2015;25:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berger J, Scott E, Sukumvanich P, Smith A, Olawaiye A, Comerci J, et al. The effect of groin treatment modality and sequence on clinically significant chronic lymphedema in patients with vulvar carcinoma. Int J Gynecol Cancer 2015;25:119–24. [DOI] [PubMed] [Google Scholar]
- 44.Huang J, Yu N, Wang X, Long X. Incidence of lower limb lymphedema after vulvar cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dardarian TS, Gray HJ, Morgan MA, Rubin SC, Randall TC. Saphenous vein sparing during inguinal lymphadenectomy to reduce morbidity in patients with vulvar carcinoma. Gynecol Oncol 2006;101:140–2. [DOI] [PubMed] [Google Scholar]
- 46.Abbas S, Seitz M. Systematic review and meta-analysis of the used surgical techniques to reduce leg lymphedema following radical inguinal nodes dissection. Surg Oncol 2011;20:88–96. [DOI] [PubMed] [Google Scholar]
- 47.Levenback CF, Ali S, Coleman RL, Gold MA, Fowler JM, Judson PL, et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a gynecologic oncology group study. J Clin Oncol 2012;30:3786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oonk MH, van Hemel BM, Hollema H, de Hullu JA, Ansink AC, Vergote I, et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol 2010;11:646–52. [DOI] [PubMed] [Google Scholar]
- 49.Van der Zee AG, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol 2008;26:884–9. [DOI] [PubMed] [Google Scholar]
- 50.Ogunbiyi SO, Modarai B, Smith A, Burnand KG; London Lymphoedema Consortium. Quality of life after surgical reduction for severe primary lymphoedema of the limbs and genitalia. Br J Surg 2009;96:1274–9. [DOI] [PubMed] [Google Scholar]
- 51.Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, Prieto Merino D, Mayoral del Moral O, Cerezo Tellez E, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ. 2010;340:b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuiver MM, de Rooij JD, Lucas C, Nieweg OE, Horenblas S, van Geel AN, et al. No evidence of benefit from class-II compression stockings in the prevention of lower-limb lymphedema after inguinal lymph node dissection: results of a randomized controlled trial. Lymphology. 2013;46(3):120–31. [PubMed] [Google Scholar]
- 53.Hnin YK, Ong LX, Tsai CC, Ong SS, Yee SG, Choo BA, et al. Does initial routine use of a compression garment reduce the risk of lower limb lymphedema after gynecological cancer treatment? A randomized pilot study in an Asian institution and review of the literature. Lymphology 2018;51:174–83. [PubMed] [Google Scholar]
- 54.Jorgensen MG, Toyserkani NM, Sorensen JA. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: a systematic review and meta-analysis. Microsurgery 2018;38:576–85. [DOI] [PubMed] [Google Scholar]
- 55.Kenworthy EO, Nelson JA, Verma R, Mbabuike J, Mehrara BJ, Dayan JH. Double vascularized omentum lymphatic transplant (VOLT) for the treatment of lymphedema. J Surg Oncol 2018;117:1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Badger CM, Peacock JL, Mortimer PS. A randomized, controlled, parallel-group clinical trial comparing multilayer bandaging followed by hosiery versus hosiery alone in the treatment of patients with lymphedema of the limb. Cancer 2000;88:2832–7. [PubMed] [Google Scholar]
- 57.Zaleska M, Olszewski WL, Durlik M. The effectiveness of intermittent pneumatic compression in long-term therapy of lymphedema of lower limbs. Lymphat Res Biol 2014;12:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lasinski BB, McKillip Thrift K, Squire D, Austin MK, Smith KM, Wanchai A, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R. 2012;4:580–601. [DOI] [PubMed] [Google Scholar]
- 59.Kim SJ, Park YD. Effects of complex decongestive physiotherapy on the oedema and the quality of life of lower unilateral lymphoedema following treatment for gynecological cancer. Eur J Cancer Care (Engl) 2008;17:463–8. [DOI] [PubMed] [Google Scholar]
- 60.Tambour M, Holt M, Speyer A, Christensen R, Gram B. Manual lymphatic drainage adds no further volume reduction to Complete Decongestive Therapy on breast cancer-related lymphoedema: a multicentre, randomised, single-blind trial. Br J Cancer 2018;119:1215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garza R 3rd, Skoracki R, Hock K, Povoski SP. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer 2017;17:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campisi C, Bellini C, Campisi C, Accogli S, Bonioli E, Boccardo F. Microsurgery for lymphedema: clinical research and long-term results. Microsurgery 2010;30:256–60. [DOI] [PubMed] [Google Scholar]
- 63.Ozturk CN, Ozturk C, Glasgow M, Platek M, Ashary Z, Kuhn J, et al. Free vascularized lymph node transfer for treatment of lymphedema: A systematic evidence based review. J Plast Reconstr Aesthet Surg 2016;69:1234–47. [DOI] [PubMed] [Google Scholar]
- 64.Basta MN, Gao LL, Wu LC. Operative treatment of peripheral lymphedema: a systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplantation. Plast Reconstr Surg 2014;133:905–13. [DOI] [PubMed] [Google Scholar]
- 65.Boyages J, Kastanias K, Koelmeyer LA, Winch CJ, Lam TC, Sherman KA, et al. Liposuction for Advanced Lymphedema: A Multidisciplinary Approach for Complete Reduction of Arm and Leg Swelling. Ann Surg Oncol 2015;22 Suppl 3:S1263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


