Sir,
We thank Sperber and Karnath (2020) for their stimulating comments in response to our recent article (Toba et al., 2019) in which we reappraised and extended the notion of ‘brain modes’ proposed by Godefroy et al. (1998) two decades ago. The authors ask two fundamental and important questions: do inhibitory interactions in the human brain really exist, and can such interactions be reliably revealed with the help of multivariate lesion inferences? Here, we answer both questions strongly in the affirmative.
First, do inhibitory interactions exist in the human brain? Strong evidence for such interactions comes from so-called ‘paradoxical’ lesion effects (Kapur, 1996, 2011), which are classically perceived of as unexpected behavioural improvements caused by focal lesions. However, such ‘paradoxical’ effects, while initially appearing surprising and counter-intuitive, are in fact the predictable result of inhibitory interactions (single or multiple) in the brain, such as those driven by the fibres of the corpus callosum or the midbrain’s intercollicular commissure, or by multiple inhibitory interactions among basal ganglia regions (see Valero-Cabré et al., 2020, for review). Thus, such interactions are an essential aspect of the fifth brain mode (Toba et al., 2019), and may reveal inhibitory contributions of a single brain region or several regions in the emergence of a brain function, evaluated via behavioural performance measures (Toba et al., 2017, 2020). While classical studies conducted in the cat particularly argue in favour of inhibitory interactions driven by the midbrain’s collicular commissure (Sprague, 1966; Payne and Rushmore, 2004), single case studies (Vuilleumier et al., 1996; Weddell, 2004) and experimental neuromodulation experiments (Nyffeler et al., 2019, to cite a recent example) also attest in favour of the biological plausibility of inhibitory interactions in human brain systems, particularly those linking homotopic regions of both hemispheres via transcallosal commissural projections (for a review see Valero-Cabré et al., 2020). Indeed, interhemispheric interactions have thus far provided the most solid examples of the fifth brain mode of ‘mutual inhibition’ included in our review (Toba et al., 2019). Hence, mutually inhibitory transcallosal interactions appear to be wired into the human connectome and appear to be of functional relevance (see indirect evidence in Lunven et al., 2015; Nyffeler et al., 2019; Bartolomeo, 2019). However, the extent to which this brain mode substantiates other inhibitory or competitive interactions, particularly within the same lobe or the same hemisphere, remains to be illustrated via clinical cases, which to date, remain rare (for evidence from experimental perturbation approaches see Walsh et al., 1998; Maniglia et al., 2019).
Second, can inhibitory interactions be reliably revealed by lesion inferences? In this context, Sperber and Karnath raise two issues and illustrate them with ad hoc simulations.
In their first example, they suggest that inhibitory interactions may be misinferred because of lesion pattern correlations even when inhibitory interactions are not a priori present in a ground truth model (Fig. 1 in Sperber and Karnath, 2020). For this demonstration, they implemented the four brain modes of Toba et al. (2019) that do not explicitly involve inhibitory interactions (namely ‘unicity’, ‘equivalence’, ‘association’ and ‘summation’); however, choosing an implementation that differs from the one suggested by the definitions in Toba et al. (2019). We reanalysed their example with the help of estimated Multiperturbation Shapley value Analysis (MSA; Keinan et al., 2004, 2006), a multivariate lesion inference approach based on coalitional game theory, and find that the analysis substantially reduces the misinference of negative contributions found by Sperber and Karnath (Supplementary Fig. 1A). Moreover, in their simulations, Sperber and Karnath added a significant amount of noise to the actual signal (70% signal and 30% noise). We find that this added noise is responsible for some part of the negative contributions they report and, when removed, MSA infers the actual positive contributions built into the model largely correctly (Supplementary Fig. 1B). We conclude that misinferences of negative contributions in clinical lesion data can be largely avoided when using a reliable multivariate inference approach in conjunction with lesion data that are processed to be as free from noise as possible. A more systematic quantitative investigation of these factors in the context of comparing different lesion inference approaches should be the subject of upcoming studies.
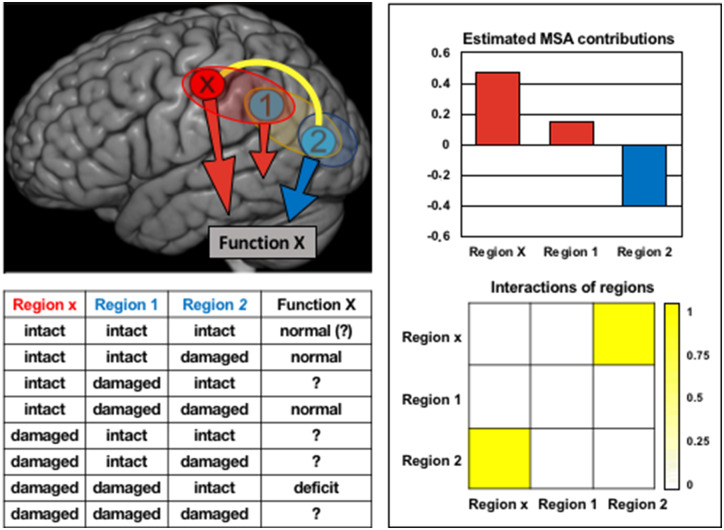
Figure 1.
Alternative explanations for functional deficits revealed by lesion inference. The figure summarizes the findings of a formal reanalysis of the example proposed by Fig. 2 in Sperber and Karnath (2020) with the help of the estimated MSA inference approach (Keinan et al., 2006). The findings are consistent with the two explanatory scenarios outlined by Sperber and Karnath; a functional deficit may arise either from damage of region x, which makes a positive functional contribution, or from disinhibiting and thus boosting region 2, which has a negative functional contribution (i.e. hampers function). Moreover, regions x and 2 show a positive, ‘synergistic’ interaction (i.e. the joint functional contribution of these regions is larger than the sum of their individual contributions; Keinan et al., 2004). Specifically, this interaction can be interpreted as a competition between the contributions of the regions. Of note, on the limited basis of the provided lesion states and associated functional performances (summarized in the table), the formal analysis cannot decide which of the two scenarios is more plausible. Further data or additional context information on the underlying anatomical-physiological interactions of these regions are required in order to explain the observations on functional performance in terms of neural mechanisms (cf. Zavaglia and Hilgetag, 2016).
In their second example, Sperber and Karnath suggest that inhibitory interactions may be deduced even though positive functional contributions of brain regions may provide a more parsimonious explanation. They set up a two-way scenario where a hypothetical brain function X may be affected either by lesion of a region x, or by disinhibition of a second region (Fig. 2 in Sperber and Karnath, 2020). Indeed, our reanalysis of the example with MSA delivers results that are consistent with either scenario (Fig. 1). Particularly, region x makes a large positive contribution to the putative function, such that a lesion of x would induce functional deficit. Conversely, region 2 makes a negative contribution to the function, such that releasing this region from inhibition would increase a functional deficit. Moreover, the analysis reveals that regions x and 2 have a ‘synergistic’ interaction, specifically indicating functional competition, as a contribution of region x may suppress the contribution of region 2 and vice versa. Thus, even with the limited information given in the example of Sperber and Karnath, the analysis correctly infers the contributions and interactions consistent with the two scenarios, including the potential presence of negative functional contributions. Naturally, however, the analysis cannot decide which scenario the authors intended to be the actual causal explanation, as both are equally possible.
This finding highlights the fact that lesion inferences operate in the space of behavioural data and therefore cannot directly reveal physiological-anatomical interactions without further context (Zavaglia and Hilgetag, 2016). Thus, we agree with Sperber and Karnath that a hypothesis-free interpretation of paradoxical lesion-behaviour relations captured by MSA or other inference approaches can be misleading and may pose a risk of spurious findings. Consequently, the accurate identification of relationships between brain function and specific neuroarchitectures (i.e. ‘brain modes’) must rely on a priori hypotheses (Sperber, 2020). In this context, more realistic computational models of brain function and systematic ground truth simulations of functional perturbations will be a powerful strategy for elucidating the validity of lesion inference approaches and revealing the fundamental brain modes underlying brain function.
Data availability
The simulation was based on a lesion sample included in LESYMAP (Pustina et al., 2018; https://github.com/dorianps/LESYMAP); the AAL atlas is included in MRIcron (https://www.nitrc.org/projects/mricron).
Funding
Groundwork leading to this paper has been funded in part by past EU project eraNET-NEURON 2009 “BEYONDVIS” to C.C.H. and A.V-C., as well as grants from the German research Council DGF HI 1286/3-1, SFB 936/A1, Z3 and TRR 169/A2 to C.C.H. and SFB 963/C2 to C.M. It is also supported by research grants by the IHU-A-ICM-Translationnel, ANR projet Générique “OSCILOSCOPUS”, ANR project Flag Era-JTC-HBP “CAUSALTOMICS”, PHRC Regional “NEGLECT”, PHRC National “STIM-SD” to A.V-C., and travel funds by the Naturalia & Biologia Foundation; grants from Amiens University Hospital (France) and the French Department of Health (France; reference: DGOS R1/2013/144) to O.G; and NIH R01MH112748.
Competing interests
The authors report no competing interests.
Supplementary Material
References
- Bartolomeo P. Visual neglect: getting the hemispheres to talk to each other. Brain 2019; 142: 840–2. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Duhamel A, Leclerc X, Saint MT, Henon H, Leys D.. Brain behaviour relationships. Some models and related statistical procedures for the study of brain-damaged patients. Brain 1998; 121: 1545–56. [DOI] [PubMed] [Google Scholar]
- Kapur N. Paradoxical functional facilitation in brain-behaviour research: a critical review. Brain 1996; 119: 1775–90. [DOI] [PubMed] [Google Scholar]
- Kapur N, editor. The paradoxical brain. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- Keinan A, Sandbank B, Hilgetag CC, Meilijson I, Ruppin E.. Fair attribution of functional contribution in artificial and biological networks. Neural Comput 2004; 16: 1887. [DOI] [PubMed] [Google Scholar]
- Keinan A, Sandbank B, Hilgetag CC, Meilijson I, Ruppin E.. Axiomatic scalable neurocontroller analysis via the shapley value. Artif Life 2006; 12: 333–52. [DOI] [PubMed] [Google Scholar]
- Lunven M, Thiebaut de Schotten M, Bourlon C, Duret C, Migliaccio R, Rode G, et al. White matter lesional predictors of chronic visual neglect: a longitudinal study. Brain 2015; 138: 746–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniglia M, Trotter Y, Aedo-Jury F.. TMS reveals inhibitory extrastriate cortico-cortical feedback modulation of V1 activity in humans. Brain Struct Funct 2019; 224: 3399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyffeler T, Vanbellingen T, Kaufmann BC, Pflugshaupt T, Bauer D, Frey J, et al. Theta burst stimulation in neglect after stroke: functional outcome and response variability origins. Brain 2019; 142: 992–1008. [DOI] [PubMed] [Google Scholar]
- Payne BR, Rushmore RJ.. Functional circuitry underlying natural and interventional cancellation of visual neglect. Exp Brain Res 2004; 154: 127–53. [DOI] [PubMed] [Google Scholar]
- Pustina D, Avants B, Faseyitan OK, Medaglia JD, Coslett HB. . Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia 2018; 115: 154–66. [DOI] [PubMed] [Google Scholar]
- Sperber C. Rethinking causality and data complexity in brain lesion-behaviour inference and its implications for lesion-behaviour modelling. Cortex, 2020; 126: 49–62. [DOI] [PubMed] [Google Scholar]
- Sperber C, Karnath H-O.. Inhibition between human brain areas or methodological artefact? Brain 2020; 143: e38. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 1966; 153: 1544–7. [DOI] [PubMed] [Google Scholar]
- Toba MN, Godefroy O, Rushmore RJ, Zavaglia M, Maatoug R, Hilgetag CC, et al. Revisiting ‘brain modes’ in a new computational era: approaches for the characterization of brain-behavioural associations. Brain 2019; 10.1093/brain/awz343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba MN, Zavaglia M, Malherbe C, Moreau T, Rastelli F, Kaglik A, et al. Game theoretical mapping of white matter contributions to visuospatial attention in stroke patients with hemineglect. Human Brain Mapping 2020. doi: 10.1002/hbm.24987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba MN, Zavaglia M, Rastelli F, Valabrégue R, Pradat‐Diehl P, Valero‐Cabré A, et al. Game theoretical mapping of causal interactions underlying visuo‐spatial attention in the human brain based on stroke lesions. Hum Brain Mapp 2017; 38: 3454–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cabré A, Toba MN, Hilgetag CC, Rushmore RJ. Perturbation-driven paradoxical facilitation of visuo-spatial function: Revisiting the ‘Sprague effect’. Cortex 2020; 122: 10–39. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Hester D, Assal G, Regli F.. Unilateral spatial neglect recovery after sequential strokes. Neurology 1996; 46: 184–9. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Battelli L, Cowey A.. Task–specific impairments and enhancements induced by magnetic stimulation of human visual area V5. Proc R Soc Lond B 1998; 265: 537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weddell RA. Subcortical modulation of spatial attention including evidence that the Sprague effect extends to man. Brain Cogn 2004; 55: 497–506. [DOI] [PubMed] [Google Scholar]
- Zavaglia M, Hilgetag CC.. Causal functional contributions and interactions in the attention network of the brain: an objective multiperturbation analysis. Brain Struct Funct 2016; 221: 2553–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The simulation was based on a lesion sample included in LESYMAP (Pustina et al., 2018; https://github.com/dorianps/LESYMAP); the AAL atlas is included in MRIcron (https://www.nitrc.org/projects/mricron).