Abstract
The whole-plant economic spectrum concept predicts that leaf and root traits evolve in coordination to cope with environmental stresses. However, this hypothesis is difficult to test in many species because their leaves and roots are exposed to different environments, above- and below-ground. In epiphytes, both leaves and roots are exposed to the atmosphere. Thus, we suspect there are consistent water conservation strategies in leaf and root traits of epiphytes due to similar selection pressures. Here, we measured the functional traits of 21 species in the genus Dendrobium, which is one of the largest epiphytic taxa in the family Orchidaceae, and used phylogenetically independent contrasts to test the relationships among traits, and between traits and the environment. Our results demonstrate that species with a thicker velamen tended to have thicker roots, a thicker root cortex and vascular cylinder, and a larger number of vessels in the root. Correspondingly, these species also had higher leaf mass per area, and thicker leaf lower cuticles. Leaf and root traits associated with water conservation showed significantly positive relationships. The number of velamen layers, leaf density and the ratio of vascular cylinder radius to root radius were significantly affected by the species’ differing environments. Thus, traits related to water conservation and transport may play an important role in helping Dendrobium cope with the cool and dry conditions found at high elevations. These findings confirmed the hypothesis that leaf and root traits have evolved in coordination, and also provide insights into trait evolution and ecological adaptation in epiphytic orchids.
Keywords: Co-evolution, elevation, epiphyte, water conservation, water shortage
Our study proposed a model of interaction between leaf and root traits of Dendrobium which is an important epiphytic taxon in Orchidaceae. Both leaves and roots of epiphytes are exposed to the atmosphere; thus, these two organs are under similar selective pressures. To cope with water stress in epiphytic habitats, leaf and root traits associated with water conservation show significantly positive relationships in Dendrobium species, and have evolved in coordination. Our findings provide insights into trait evolution and ecological adaptation in epiphytic orchids.
Introduction
Trade-offs among functional traits reveal the strategies for plants to acquire and conserve resources (Wright and Westoby 2002; Kong et al. 2015), and provide insights into species distribution and ecosystem processes (Fortunel et al. 2012). These functional traits have been described as the ‘spectra’ to separate species with different adaptation strategies (Liese et al. 2017). On one end of the ecological axis are species with an acquisitive strategy. These species with low leaf mass per area (LMA) have higher photosynthetic rates but shorter lifespans (Reich et al. 1998; Westoby et al. 2002). On the other end of this axis are species with a conservative strategy. These species with denser tissue have greater resistance to mechanical damage and pathogen attack, leading to slower growth rates and longer lifespans (Poorter et al. 2008; Liu et al. 2010; Kong et al. 2015). Key traits related to resource acquisition and conservation should be considered as a part of the leaf and root functional coordination (Freschet et al. 2015).
The leaf economic spectrum (LES) concept has been widely applied. This concept hypothesizes that leaf functional traits may co-vary along a distinct spectrum among species (Wright et al. 2004; Somavilla et al. 2013; Poorter et al. 2014). Within the literatures on LES, similar trait spectra have been expanded to stems and roots, thus forming the whole-plant economic spectrum (Freschet et al. 2010; Kong et al. 2015; Díaz et al. 2016; Valverde-Barrantes et al. 2017). Although the root is the main organ for resource acquisition, root traits receive the least attention in plant ecology research (Manschadi et al. 2006; Liese et al. 2017; Valverde-Barrantes et al. 2017; Kong et al. 2019). Research on root traits has been hampered due to constraints in observation and sampling, such that plant roots are labelled ‘the hidden half’ (Eshel and Beeckman 2013). Another reason for the complexity in evaluating the root trait syndrome is the linkage between leaf and root traits (Withington et al. 2006; Mommer and Weemstra 2012). According to the whole-plant economic spectrum hypothesis, leaf and root traits evolve in coordination (Freschet et al. 2010). However, studies on relationships between leaf and root traits across species have showed contrasting results. For example, Craine and Lee (2003) found nitrogen concentration and tissue density of leaves are correlated with those of fine roots. Tjoelker et al. (2005) found a concordance in leaf and root longevity. However, Withington et al. (2006) suggested tissue structure and longevity above-ground (leaves) can contrast markedly with those of below-ground (roots). Thus, more research into root traits is needed to resolve these contrasting findings. The decoupling of leaf and root traits may be caused by the following reasons. Firstly, differences in plant growth form may affect trait correlations (Reich et al. 1998; Withington et al. 2006; Liu et al. 2010). For example, among grass species, the acquisitive strategy is associated with low LMA, low leaf tissue density and low root tissue density (Ryser et al. 1997; Wahl and Ryser 2000), whereas among tree species, acquisitive strategy is associated with higher specific root length and smaller root diameters, but not root tissue density (Comas et al. 2002). This suggests that the trait correlations or plant strategies that have been widely observed in herbaceous plants cannot be directly extrapolated to woody plants (Liu et al. 2010). Secondly, the drivers of morphological variation in leaf and root traits may be different (Kembel and Cahill 2011; Valverde-Barrantes et al. 2017). Previous studies have suggested that phylogeny plays a major role in root trait variation (Kong et al. 2014; Reich 2014), whereas environmental factors may largely account for variations in leaf traits (Baraloto et al. 2012). Thus, when examining species-level responses to environmental changes, phylogeny should be considered (Ackerly and Donoghue 1998; Edwards 2006). Furthermore, leaf and root traits may be decoupled due to the differences in above- and below-ground environments (Freschet et al. 2015; Adair et al. 2019). For example, the availabilities of nutrients and water in soil are significantly higher, and more stable than that in atmosphere or canopy (Zotz et al. 2010). However, it is not clear whether the association between leaf and root traits of epiphytes is stronger than that of terrestrial plants.
The roots of tree- and rock-dwelling epiphytes are exposed to similar environments as their leaves (Zotz and Winkler 2013). Epiphyte habitats supply irregular amounts of water, and the resultant water stress strongly inhibits epiphyte growth and survival (Zotz 2005; Zotz et al. 2010). In response to frequent drought stress, epiphytes have evolved ecophysiological adaptations (Zhang et al. 2018). Specifically, the aerial roots of epiphytes capture water via a special spongy structure called velamen, which absorbs water that flows down the tree trunk or rock surface (Roberts and Dixon 2008). Although velamen is not exclusive to epiphytes (Zotz et al. 2017), its role in epiphytes’ physiology is especially important. Thick velamen significantly delays water loss (Zotz and Winkler 2013), allowing epiphytes to survive in habitats where few other plants can survive, such as habitats with extremely small amounts of water availability (Roberts and Dixon 2008; Zotz and Winkler 2013; Joca et al. 2017). Plants can also respond to water availability by adjusting leaf traits (Wright et al. 2005; Qin et al. 2019). For example, plants can adapt to water shortage by regulating their stomatal area (SA), stomatal density (SD), leaf density (LD) and epidermis or cuticle thickness (Zhang et al. 2012). Although velamen has an important role in water conservation, few researches have tested the coordination between velamen thickness (VT) and leaf traits related to water conservation (Zotz and Winkler 2013). Thus, it would be valuable to explore whether both leaf and root traits follow accordant trends in their water conservative strategies.
To address whether leaf and root traits in epiphytes show coordinated evolution in response to changing environments, we analysed the variations in leaf and root traits in species of the genus Dendrobium. All members within the genus are epiphytic or lithophytic (Zhu et al. 2009), and have roots that are easily observed and sampled. In addition, Dendrobium is one of the largest genera in Orchidaceae, and presents some of the most intricate taxonomic problems in the family (Xiang et al. 2013). Whether Dendrobium is monophyletic have been inconclusive to date (Schuiteman 2011; Takamiya et al. 2014). The phylogenetically independent contrast (PIC) method has been widely used in ecology to detect the evolutionary correlation among traits (Price 1997), because ignoring phylogenetic relationships among species included in a comparative analysis may lead to spurious conclusions due to high type I or type II errors (Morand and Poulin 2003). The correlated evolution between traits has been tested in large taxa by using a PIC method (e.g. angiosperm) or specific clades (Grotkopp and Rejmánek 2007; Fortunel et al. 2012; Zhang et al. 2012). However, previous studies into relationships among traits, and between plant traits and environmental factors in epiphytes mostly focused on above-ground organs, with particular emphasis on leaf traits, but rarely on the roots (Sun et al. 2014; Teixeira da Silva et al. 2016). The leaves and roots of epiphytes may experience similar selection pressures, but no study has been conducted to detect the evolutionary association between leaf and root traits of epiphytes, including Dendrobium.
Here, we determined the patterns of variation for 36 leaf and root traits in 21 species of Dendrobium, and used the PIC method to detect whether species traits co-varied with other traits and/or with the environment, and tried to answer following questions: (i) How do leaf and root traits vary with velamen thickness? (ii) Are there close associations between leaf and root traits in Dendrobium species? (iii) Are leaf and root traits shaped by phylogeny? We suspect leaf and root traits related to water conservation will coordinate along single axes of resource acquisition/conservation in Dendrobium species when their leaves and roots are exposed to similar environments.
Materials and Methods
Plant materials and study site
Twenty-one (21) species of Dendrobium, including epiphytes and lithophytes, were cultivated in a greenhouse at the Kunming Institute of Botany, Chinese Academy of Sciences (elevation 1990 m, 102°41′E, 25°01′N). Two species, D. kingianum and D. bracteosum, were collected from Australia. Nine species (D. loddigesii, D. nobile, D. longicornu, D. crystallinum, D. crepidatum, D. chrysanthum, D. fimbriatum, D. chrysotoxum and D. thyrsiflorum) were collected from the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, and the remaining 11 species were grown at the Kunming Institute of Botany. Information on the natural habitat, growth form and altitude of the species was sourced from the Flora of China (Zhu et al. 2009; http://www.efloras.org), Teixeira da Silva et al. (2016) and Simpson et al. (2018). To ensure that interspecific differences were not merely the result of plastic responses to variable growth conditions, plants were grown for >1 year in a greenhouse at the Kunming Institute of Botany. Plants were grown on a substrate that consisted of a mixture of 70 % bark (1 cm × 1 cm), 20 % moss and 10 % humus, at 18–27 °C, with a relative humidity of 50–70 %, and 20 % full sunlight. Water and fertilizer were supplied as needed. To avoid changes in root structure due to substrates, only aerial roots were selected as our test material.
Phylogenetic tree
A Phylogram was generated using concatenated data sets of nucleus gene: Internal Transcribed Spacers (ITS) and the chloroplast genes: rbcL, matK–trnK, trnH–psbA regions which were downloaded from GenBank (http://www.ncbi.nlm.nih.gov). Bulbophyllum odoratissimum was chosen as the outgroup because of its close relationship to Dendrobium (Freudenstein and Rasmussen 1999; Xiang et al. 2013). Numbers associated with nodes are maximum-likelihood bootstrap values. Multiple alignments were automatically performed using ClustalX v.2.0.11 and manual corrections through BioEdit v.7.0.9.0, generating a matrix in a NEXUS format for Bayesian analyses in MrBayes v3.2.2 x64. These analyses used the best-fit models selected with model selection criterion AIC by the software jModeltest v.2.1.4. In the Bayesian analyses, trees were generated by running Metropolis-coupled Monte Carlo Markov (MCMC) chains and sampling one tree every 100 generations for 1 000 000 generations, starting with a random tree. The phylogenetic relationships of the studied Dendrobium species and their ecological information are shown in Supporting Information—Fig. S1.
Sampling and measurement
To minimize the confounding effect of plant age, for each species, at least six mature individuals were randomly selected, and three healthy, mature leaves and roots from each individual were collected. Leaves were selected in the middle part of the leaf (avoiding the main vein) and roots were sampled ~2 cm above the apex of new viable roots. After collection, samples were sealed in plastic bags, and anatomical traits were immediately measured. Collection and measurement were conducted during the wet season (from July to September 2018).
After measuring the fresh mass (ML(F)) of leaves, the leaf area (LA) was measured with a Li-Cor 3000A area meter (Li-Cor Inc., Lincoln, NE, USA), and leaves were then oven-dried for 48 h at 70 °C until reaching a constant mass to obtain leaf dry mass (ML(D)). Water content (WC, %) was calculated as (ML(F) − ML(D))/ML(F) × 100 %. Leaf mass per area (LMA) was calculated as ML(D)/LA.
To characterize leaf anatomical traits, we cut 5-mm × 2-mm sections from the middle part of the leaf (avoiding the main vein) with a freezing microtome (CM3050S, Leica, Germany). The sections were observed and photographed under an optical microscope (DM2500, Leica, Germany). Leaf thickness (LT), upper epidermal thickness (UET), lower epidermal thickness (LET), upper cuticle thickness (UCT) and lower cuticle thickness (LCT) were measured with the software ImageJ v.1.43u (National Institutes of Health, Bethesda, MD, USA). Leaf density (LD, kg m−3) was calculated as leaf dry mass per unit volume, which was calculated as LA × LT (Sun et al. 2014).
For stomatal traits, abaxial nail varnish peels were taken centrally, midway between the midrib and margin (Sack et al. 2003), transferred to glass slides after drying and then photographed under an optical microscope. The images were measured using ImageJ. Stomatal density (SD) was measured as the number of stomata per unit area, and was calculated as the mean value of >36 images from each species (6 images per leaf). Stomatal length (SL) and width (SW) were averaged from 60 randomly selected stomata for each species. Stomatal area (SA) was estimated by the formula 1/4 × π × SL × SW (Sun et al. 2014).
To measure vein density (VD), the leaves were boiled for 30 min in 5 % NaOH and washed with distilled water three times, then bleached in 5 % sodium hypochlorite until the mesophyll was transparent. The leaves were then stained for 2 min with 1 % toluidine blue, mounted on glass slides and photographed. Total vein length was measured with ImageJ, and VD was calculated as total vein length per leaf area (LA).
To examine root anatomical traits, we used a freezing microtome to cut 4-mm-thick sections ~2 cm from the root apex and photographed the cross sections with an optical microscope. Velamen thickness (VT) and root radius (r) were measured with ImageJ. The area of velamen (Avel) was calculated as the whole cross-section area minus the area within the epidermis. We measured the length (vcl) and width (vcw) of ~100 randomly selected velamen cells. The area of each velamen cell (Avc) was calculated as vcl × vcw. Exodermic, endodermic and passage cells were counted using ImageJ. The number of vessel (Nves) refers to the number of primary xylem vessels. To determine vessel diameter (Dves) and vessel area (Aves), we measured all the primary xylem vessels.
Data analysis
Before analysis, all data were log10 transformed to improve normality and homoscedasticity. Comparison of traits among different groups was conducted by a one-way ANOVA. A PIC method was used to detect whether species traits co-varied with other traits or with the environment (Price 1997; Purvis and Webster 1999) by employing the ‘ape’ package in R v.3.4.4. Any PIC correlations were evaluated with a ‘Pearson’ correlation in R package.
To evaluate the evolutionary history of leaf and root traits, we first tested for a phylogenetic signal in each trait using the K-statistic, which is based on a ‘Brownian motion model’ of trait evolution (Blomberg et al. 2003). The K metric can be used to assess phylogenetic conservatism. K > 1 indicates that a trait value is more conserved than expected from Brownian motion. K < 1 indicates that a trait value is significantly less conserved than expected from Brownian motion, and instead demonstrates significant lability, while K = 1 shows that a trait value is as expected from a Brownian motion model (Blomberg et al. 2003). The K-statistic was estimated using the ‘picante’ package in R program. We used the ‘Rtsne’ package in R to compute t-SNE dimensional reduction (Van der Maaten and Hinton 2008) and grouped traits and species to distinct clusters. The ‘Rtsne’ was run with ‘perplexity = 5’. A principal component analysis (PCA) was performed with the ‘prcomp’ function of the ‘vegan’ package in R program to analyse the associations among the traits. Multidimensional scaling (MDS) was conducted in SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and was also used to verify the relationships of the traits.
Results
Variations in anatomical traits among species
In total, 22 root traits and 14 leaf traits of 21 Dendrobium species were studied. Coefficient of variation (CV) defined as the ratio of the standard deviation to the mean was used to measure trait variability. Leaf traits varied more than root traits for all Dendrobium species (Table 1). Leaf dry mass (ML(D), CV = 145 %) and fresh mass (ML(F), CV = 117 %) had the largest variation, while leaf area (LA) also varied greatly (CV = 97 %). Leaf water content (WC) had the smallest variation (CV = 15 %). For root traits, the area of vessels in cross section showed the greatest variation (Aves, CV = 88 %), while the area of velamen in cross section (Avel) also varied greatly (CV = 75 %). The ratio of radius of vascular cylinder to root radius (Rvc/r) showed the smallest variation (CV = 17 %).
Table 1.
Variations in leaf and root traits of tested Dendrobium species. SD: standard deviation; CV: coefficient of variation (%).
| Traits | Abbr. | Function | Unit | Range | Mean | SD | CV (%) |
|---|---|---|---|---|---|---|---|
| Leaf fresh mass | M L(F) | Growth performance | g | 0.05–2.42 | 0.54 | 0.63 | 117.49 |
| Leaf dry mass | M L(D) | Growth performance | g | 0.0072–0.49 | 0.08 | 0.12 | 145.04 |
| Water content | WC | Water status | % | 52.28–98.13 | 82.31 | 12.17 | 14.79 |
| Leaf area | LA | Water loss | cm2 | 2.02–40.96 | 12.32 | 11.90 | 96.58 |
| Leaf mass per area | LMA | Water conservation | g m−2 | 18.50–139.18 | 57.34 | 27.16 | 47.36 |
| Leaf density | LD | Water conservation | kg m−3 | 57.62–210.33 | 138.70 | 46.63 | 33.62 |
| Vein density | VD | Water transport | mm mm−2 | 1.60–5.64 | 2.80 | 1.08 | 38.39 |
| Leaf thickness | LT | Water conservation | µm | 157.31–899.75 | 446.11 | 217.42 | 48.74 |
| Upper epidermal thickness | UET | Water conservation | µm | 19.26–70.93 | 38.32 | 11.24 | 29.32 |
| Upper cuticle thickness | UCT | Water conservation | µm | 2.36–18.10 | 7.26 | 3.31 | 45.64 |
| Lower epidermal thickness | LET | Water conservation | µm | 9.55–56.69 | 24.44 | 9.93 | 40.65 |
| Lower cuticle thickness | LCT | Water conservation | µm | 1.01–12.75 | 5.92 | 3.25 | 54.89 |
| Stomatal density | SD | Water loss | No. per mm2 | 36.72–108.16 | 67.44 | 22.22 | 32.95 |
| Stomatal area | SA | Water loss | µm2 | 261.9–1160.0 | 623.39 | 194.38 | 31.18 |
| Layer of velamen | LV | Water conservation | No. | 3–10 | 5.76 | 2.05 | 35.53 |
| Velamen thickness in cross section | VT | Water conservation | µm2 | 87.22–589.31 | 305.32 | 140.96 | 46.17 |
| Root radius in cross section | r | Water absorbability | µm | 413.02–1550.99 | 843.18 | 280.79 | 33.30 |
| Velamen thickness/radius | VT/r | Water conservation | % | 18.59–46.96 | 34.83 | 7.06 | 20.26 |
| Velamen area in cross section | A vel | Water conservation and storage | mm2 | 0.23–4.65 | 1.49 | 1.12 | 75.09 |
| Unit velamen cell length | vcl | Water storage | µm | 20.45–72.71 | 43.14 | 13.92 | 32.28 |
| Unit velamen cell width | vcw | Water storage | µm | 13.46–45.59 | 27.67 | 7.67 | 27.73 |
| Velamen cell length/width | vcl/vcw | Water storage | 0.85–2.49 | 1.58 | 0.41 | 25.86 | |
| Area of velamen cell | A vc | Water storage | µm2 | 769.3–4472.1 | 1849.88 | 984.59 | 53.22 |
| Number of exodermis cell | N exo | Water transport | No. | 70–196 | 115.76 | 31.28 | 27.02 |
| Number of exodermis passage cell | N exopc | Water transport | No. | 1–13 | 6.85 | 3.05 | 44.49 |
| Ratio of passage cell to exodermis cell | exopc% | Water transport | % | 1.28–11.25 | 6.31 | 2.92 | 46.27 |
| Number of endodermis cell | N en | Water transport | No. | 32–100 | 54.95 | 17.94 | 32.65 |
| Number of endodermis passage cell | N enpc | Water transport | No. | 3.33–14 | 8.8 | 2.77 | 31.52 |
| Ratio of passage cell to endodermis cell | enpc% | Water transport | % | 4.76–20.00 | 16.6 | 3.96 | 23.88 |
| Number of vessel | N ves | Water transport | No. | 7–20 | 11.71 | 3.95 | 33.73 |
| Diameter of vessel | D ves | Water transport | µm | 13.74–65.43 | 27.62 | 10.61 | 38.40 |
| Area of vessel in cross section | A ves | Water transport | µm2 | 152.12–2782.72 | 614.09 | 538.15 | 87.63 |
| Root cortex thickness | RCT | Water storage | µm | 157.25–624.20 | 291.48 | 104.54 | 35.87 |
| Root cortex thickness/radius | RCT/r | Water storage | % | 21.53–44.26 | 35.10 | 6.09 | 17.35 |
| Radius of vascular cylinder | R vc | Water transport | µm | 121.37–462.87 | 246.96 | 85.52 | 34.63 |
| Radius of vascular cylinder/radius | R vc/r | Water transport | % | 21.22–41.04 | 29.61 | 4.98 | 16.83 |
| Elevation | EL | m | 700–2500 | 29.74 |
In terms of the function that the leaf and root traits reflected, the traits related to water conservation showed relatively large variation. Among leaf traits, the CV values for leaf mass per area (LMA), leaf thickness (LT), upper cuticle thickness (UCT), lower cuticle thickness (LCT) were 47 %, 49 %, 46 % and 55 %, respectively. Among root traits, the CV values for velamen thickness (VT) and Avel were 46 % and 75 %, respectively.
Correlations between leaf and root traits in Dendrobium
For leaf traits, significant positive correlations were observed between LMA and LT (r = 0.85), UCT and LCT (r = 0.72 and 0.79, respectively) and lower epidermal thickness (LET, r = 0.68) [seeSupporting Information—Table S1]. Leaf thickness (LT) was positively correlated with ML(D) (r = 0.56), LMA (r = 0.85), UCT and LCT (r = 0.62 and 0.73, respectively), UET and LET (r = 0.53 and 0.74, respectively). Stomatal density (SD) was positively correlated with leaf density and vein density (LD and VD, r = 0.56 and 0.45, respectively), but negatively correlated with LET (r = −0.52). The LET was also positively correlated with UET, UCT and stomatal area (SA, r = 0.86, 0.58 and 0.46, respectively).
Traits related to root velamen were strongly correlated with root radius (r), whether or not phylogenetic effects were considered [seeSupporting Information—Table S2]. For instance, VT and Avel were positively correlated with root radius (r = 0.96 and 0.99, respectively). Velamen thickness (VT) was also positively correlated with the number of exodermis cells (Nexo, r = 0.69) and endodermis cells (Nen, r = 0.67), and the number of vessels (Nves, r = 0.62). Meanwhile, VT was positively correlated with root cortex thickness (RCT) and radius of vascular cylinder (Rvc, r = 0.78 and 0.83, respectively), but negatively correlated with Rvc/r (r = −0.52). The Nves was not only positively correlated with the variables associated with velamen including LV, VT and Avel (r = 0.59, 0.62 and 0.67, respectively), but also positively correlated with Nexo, Nen and Nenpc (r = 0.87, 0.98 and 0.48, respectively).
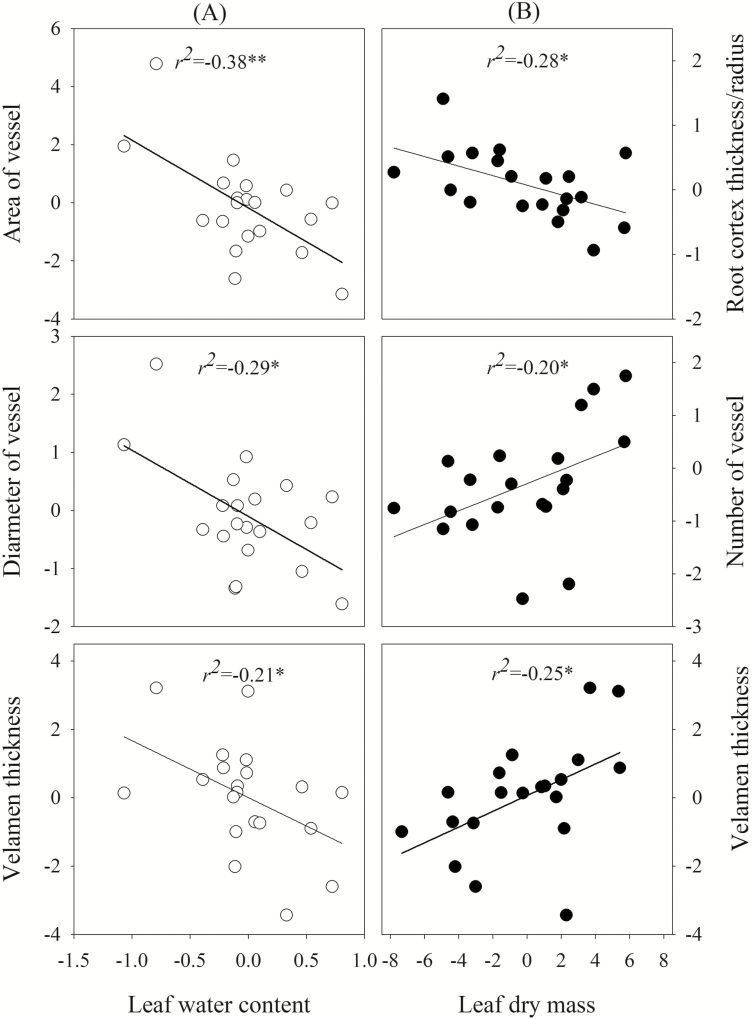
Several leaf and root traits were positively correlated (Table 2). ML(D) was positively correlated with LV (r = 0.47), VT (r = 0.50), the ratio of velamen thickness to radius (VT/r, r = 0.58), Avel (r = 0.45), Nen (r = 0.48) and Nves (r = 0.45), but negatively correlated with the ratio of root cortex thickness to root radius (RCT/r, r = −0.53; Fig. 1). Leaf water content (WC) was negatively correlated with Aves, Dves and VT (r = −0.62, −0.54 and −0.46, respectively; Fig. 1). Leaf area (LA) was positively correlated with VT/r (r = 0.47) and negatively correlated with RCT/r (r = −0.55). Leaf density (LD) was positively correlated with Nen and Nves (r = 0.45 and 0.49, respectively). There were also positive correlations between LCT and VT (r = 0.53), VT/r (r = 0.46), Avel (r = 0.53) and root radius (r = 0.51).
Table 2.
Pearson’s correlation coefficients among leaf and root traits across 21 Dendrobium species. Data were corrected by PICs. Significant correlations are showed in boldface. See Table 1 for definitions of abbreviations. Asterisks denote significant levels: **P ≤ 0.01; *P ≤ 0.05.
| Variables | LV | VT | r | VT/r | A vel | vcl | vcw | A vc | N exo | N exopc | N en | N enpc | N ves | D ves | A ves | RCT | RCT/r | R vc | R vc/r |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M L(F) | 0.21 | 0.11 | 0.02 | 0.24 | 0.07 | −0.12 | −0.23 | −0.08 | 0.19 | 0.03 | 0.30 | 0.11 | 0.30 | 0.05 | 0.03 | −0.11 | −0.29 | 0.11 | 0.20 |
| M L(D) | 0.47* | 0.50* | 0.39 | 0.58** | 0.45* | 0.21 | 0.14 | 0.29 | 0.40 | 0.10 | 0.48* | 0.15 | 0.45* | 0.38 | 0.41 | 0.15 | −0.53* | 0.44 | 0.02 |
| WC | −0.21 | −0.46* | −0.41 | −0.44 | −0.44 | −0.47* | −0.38 | −0.41 | −0.10 | −0.18 | −0.08 | −0.07 | −0.02 | −0.54* | −0.62** | −0.25 | 0.37 | −0.36 | 0.21 |
| LA | 0.31 | 0.31 | 0.18 | 0.47* | 0.24 | 0.10 | −0.06 | 0.08 | 0.20 | 0.04 | 0.31 | 0.03 | 0.27 | 0.35 | 0.38 | −0.07 | −0.55* | 0.27 | 0.17 |
| LMA | 0.51* | 0.59** | 0.57** | 0.48* | 0.58** | 0.31 | 0.42 | 0.50* | 0.54* | 0.15 | 0.54* | 0.28 | 0.54* | 0.25 | 0.27 | 0.47* | −0.23 | 0.51* | −0.26 |
| LD | 0.30 | 0.31 | 0.34 | 0.17 | 0.33 | 0.00 | 0.16 | 0.15 | 0.37 | 0.02 | 0.45* | 0.20 | 0.49* | −0.04 | 0.04 | 0.26 | −0.18 | 0.41 | 0.08 |
| VD | −0.15 | 0.01 | 0.09 | −0.14 | 0.05 | 0.26 | 0.40 | 0.32 | −0.08 | −0.08 | −0.16 | 0.12 | −0.16 | 0.14 | 0.12 | 0.13 | 0.09 | 0.03 | −0.15 |
| LT | 0.31 | 0.38 | 0.34 | 0.35 | 0.37 | 0.28 | 0.30 | 0.38 | 0.30 | 0.12 | 0.26 | 0.16 | 0.24 | 0.25 | 0.23 | 0.29 | −0.12 | 0.25 | −0.28 |
| UET | 0.20 | 0.14 | 0.06 | 0.24 | 0.10 | 0.20 | 0.22 | 0.14 | 0.10 | 0.05 | −0.06 | −0.19 | −0.02 | 0.27 | 0.15 | −0.07 | −0.28 | −0.03 | −0.23 |
| UCT | 0.30 | 0.43 | 0.43 | 0.33 | 0.43 | 0.25 | 0.48* | 0.32 | 0.29 | 0.29 | 0.24 | 0.13 | 0.26 | 0.25 | 0.24 | 0.36 | −0.16 | 0.34 | −0.31 |
| LET | 0.41 | 0.35 | 0.26 | 0.44 | 0.31 | 0.29 | 0.25 | 0.26 | 0.29 | 0.20 | 0.16 | 0.03 | 0.18 | 0.31 | 0.20 | 0.12 | −0.31 | 0.16 | −0.29 |
| LCT | 0.42 | 0.53* | 0.51* | 0.46* | 0.53* | 0.32 | 0.46* | 0.39 | 0.43 | 0.29 | 0.35 | 0.11 | 0.35 | 0.41 | 0.36 | 0.35 | −0.35 | 0.43 | −0.30 |
| SD | −0.04 | 0.22 | 0.32 | −0.02 | 0.27 | 0.15 | 0.22 | 0.31 | 0.21 | 0.03 | 0.31 | 0.24 | 0.34 | −0.20 | −0.10 | 0.39 | 0.13 | 0.31 | −0.11 |
| SA | 0.24 | 0.03 | 0.01 | 0.06 | 0.02 | −0.10 | 0.18 | −0.12 | 0.11 | 0.18 | −0.03 | 0.08 | 0.00 | 0.14 | −0.04 | −0.01 | −0.06 | −0.01 | −0.06 |
| EL | 0.24 | 0.25 | 0.30 | 0.08 | 0.28 | −0.07 | −0.24 | 0.11 | 0.44 | 0.08 | 0.47* | 0.31 | 0.57** | −0.14 | −0.13 | 0.25 | −0.12 | 0.30 | −0.08 |
Figure 1.
(A) Leaf water content was negatively correlated with root traits: cross-section area of vessel, diameter of vessel and velamen thickness (white circle); (B) leaf dry mass was positively correlated with root traits: velamen thickness and number of vessel, but negatively with the ratio of root cortex thickness to radius (black circle). Significance levels are expressed as follows: *P ≤ 0.05; **P ≤ 0.01. Data were corrected by PICs.
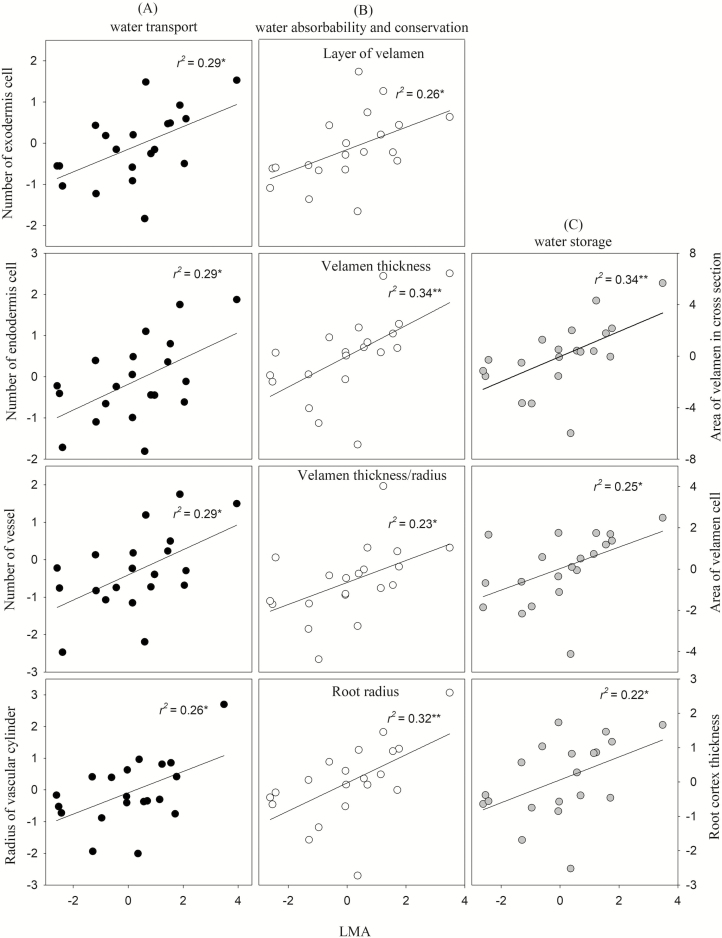
Interestingly, LMA was positively correlated with root traits related to water absorbability (root radius, r = 0.57), water storage (Avel, Avc and RCT, r = 0.58, 0.50 and 0.47, respectively), water transport (Nexo, Nen, Nves, Rvc, r = 0.54, 0.54, 0.54 and 0.51, respectively) and water conservation (LV, VT, VT/r, r = 0.51, 0.59 and 0.48, respectively; Fig. 2).
Figure 2.
(A) Correlations between leaf mass per area (LMA) and root traits related to water transport (black circle), (B) water absorption and conservation (white circle), (C) water storage (grey circle). Significance levels are expressed as follows: *P ≤ 0.05; **P ≤ 0.01. Data were corrected by PICs.
Influence of phylogeny and elevation on leaf and root traits in Dendrobium
To test whether variations observed in leaf and root traits were shaped by phylogeny or environmental factors, we tested traits in 21 Dendrobium species for phylogenetic signals using the K-statistic (Table 3). Almost all the traits showed a weak phylogenetic signal, except UET. This finding indicated that the effect of ecological variation on these traits overshadowed evolutionary constraints, especially LD (K = 0.721, P = 0.004), LV (K = 0.805, P = 0.035) and Rvc/r (K = 0.606, P = 0.041).
Table 3.
Phylogenetic signals of leaf and root traits in 21 Dendrobium species. Significant correlations are shown in boldface. Asterisks denote significant levels: **P ≤ 0.01; *P ≤ 0.05, respectively.
| Trait | Phylogenetic signal | |
|---|---|---|
| K | P | |
| Leaf fresh mass (ML(F)) | 0.425 | 0.664 |
| Leaf dry mass (ML(D)) | 0.439 | 0.416 |
| Water content (WC) | 0.584 | 0.082 |
| Leaf area (LA) | 0.487 | 0.230 |
| Leaf mass per area (LMA) | 0.402 | 0.723 |
| Leaf density (LD) | 0.721 | 0.004** |
| Vein density (VD) | 0.385 | 0.742 |
| Leaf thickness (LT) | 0.366 | 0.854 |
| Upper epidermal thickness (UET) | 1.223 | 0.253 |
| Upper cuticle thickness (UCT) | 0.64 | 0.749 |
| Lower epidermal thickness (LET) | 0.861 | 0.531 |
| Lower cuticle thickness (LCT) | 0.554 | 0.803 |
| Stomatal density (SD) | 0.723 | 0.475 |
| Stomatal area (SA) | 0.92 | 0.363 |
| Layer of velamen (LV) | 0.805 | 0.035* |
| Velamen thickness (VT) | 0.48 | 0.558 |
| Root radius (r) | 0.392 | 0.889 |
| Velamen thickness/radius (VT/r) | 0.639 | 0.116 |
| Velamen area in cross section (Avel) | 0.433 | 0.755 |
| Unit velamen cell length (vcl) | 0.6 | 0.321 |
| Unit velamen cell width (vcw) | 0.594 | 0.046 |
| Velamen cell length/width (vcl/vcw) | 0.579 | 0.343 |
| Area of velamen cell (Avc) | 0.382 | 0.828 |
| Number of exodermis cell (Nexo) | 0.446 | 0.605 |
| Number of exodermis passage cell (Nexopc) | 0.361 | 0.831 |
| Ratio of passage cell to exodermis cell (exopc%) | 0.367 | 0.809 |
| Number of endodermis cell (Nen) | 0.547 | 0.494 |
| Number of endodermis passage cell (Nenpc) | 0.37 | 0.802 |
| Passage cell/endodermis cell (enpc%) | 0.404 | 0.769 |
| Number of vessel (Nves) | 0.454 | 0.843 |
| Diameter of vessel (Dves) | 0.527 | 0.244 |
| Area of vessel in cross section (Aves) | 0.56 | 0.145 |
| Root cortex thickness (RCT) | 0.338 | 0.913 |
| Root cortex thickness/radius (RCT/r) | 0.641 | 0.162 |
| Radius of vascular cylinder (Rvc) | 0.533 | 0.414 |
| Vascular cylinder radius/radius (Rvc/r) | 0.606 | 0.041* |
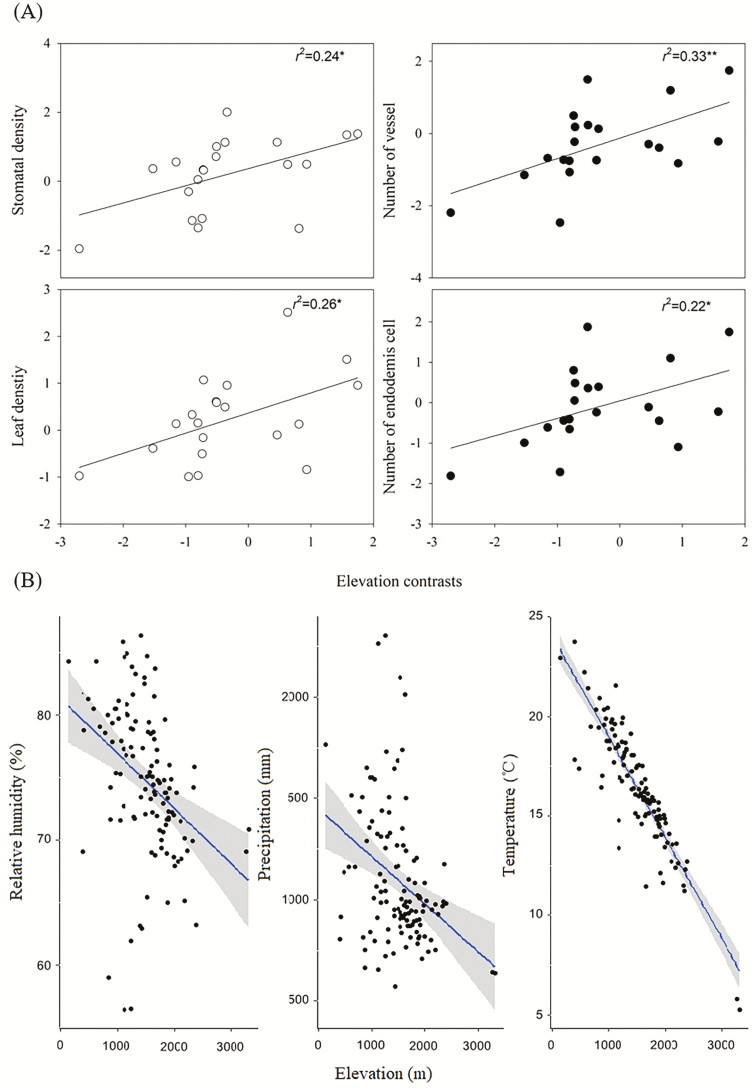
We found that leaf traits such as LD and SD, root traits such as Nen and Nves were positively correlated with elevation (Fig. 3A). In Yunnan Province, increase in elevation is accompanied by decreasing temperature, relative humidity and precipitation (Fig. 3B). These findings indicated that leaf and root traits in Dendrobium were affected by temperature and moisture level.
Figure 3.
Elevation is positively correlated with (A) root traits (black circle): number of vessel and endodermis cell, and leaf traits (white circle): leaf density and stomatal density. Significance levels are expressed as follows: *P ≤ 0.05; **P ≤ 0.01. Data were corrected by PICs. (B) Variations of relative humidity, precipitation and temperature with elevation in Yunnan Province. Each scatterplot represents a meteorological station (n = 119).
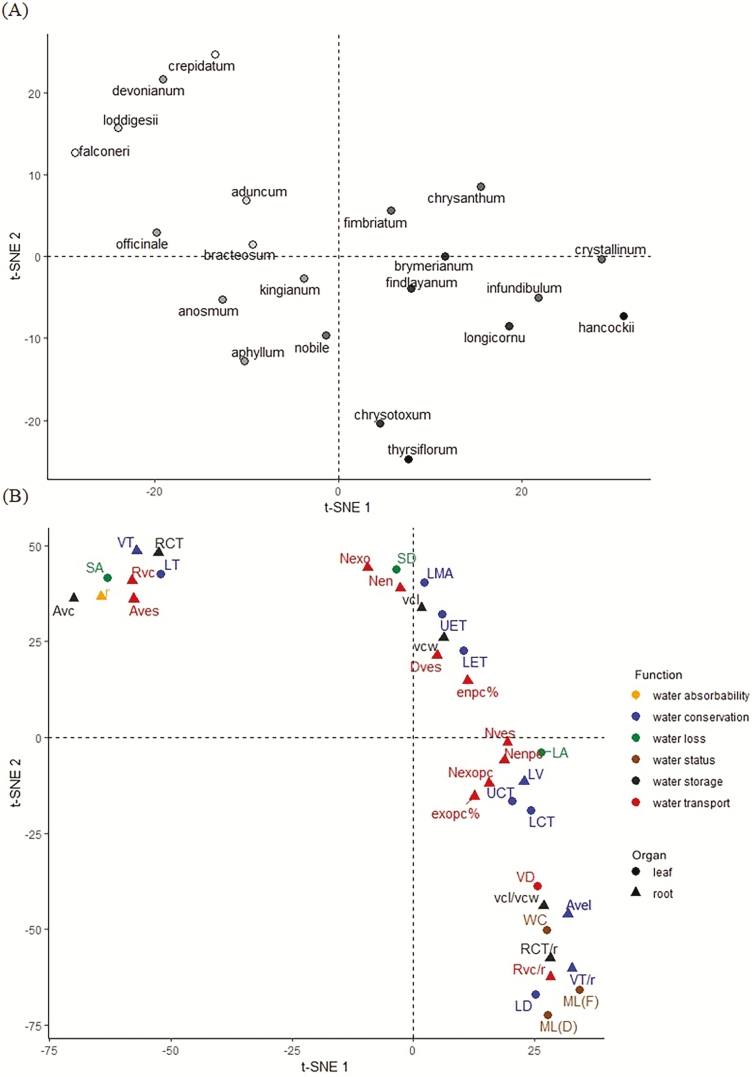
The analysis based on the t-distributed stochastic neighbourhood embedding (t-SNE) showed the clustering results of the species and traits among Dendrobium (Fig. 4). The species were separated by the zero axis vertical to t-SNE 1. One group of species was those with thick roots and the other was those with thin roots (Fig. 4A). The leaf and root traits were gathered into four parts with different functions, and both leaf and root traits were included in each part (Fig. 4B). We also used the PCA and MDS to compare the leaf and root traits among Dendrobium species [seeSupporting Information—Fig. S2], and obtained results consistent with the t-SNE. This indicated that the functional traits tended to coupling between leaves and roots.
Figure 4.
t-SNE (t-distributed stochastic neighbourhood embedding) visualization to compare the species and traits of Dendrobium. (A) Species were separated along zero axis of t-SNE 1. Dot colour was used to denote the relative size of the species root radius, and darker colours were used to denote thicker roots. (B) Traits were gathered in several parts with different function and belonging to different organs. Each dot denotes a trait. Colours denote corresponding function. The circle and triangle represent leaf and root traits, respectively.
Discussion
Coordinated evolution of leaf and root traits within Dendrobium
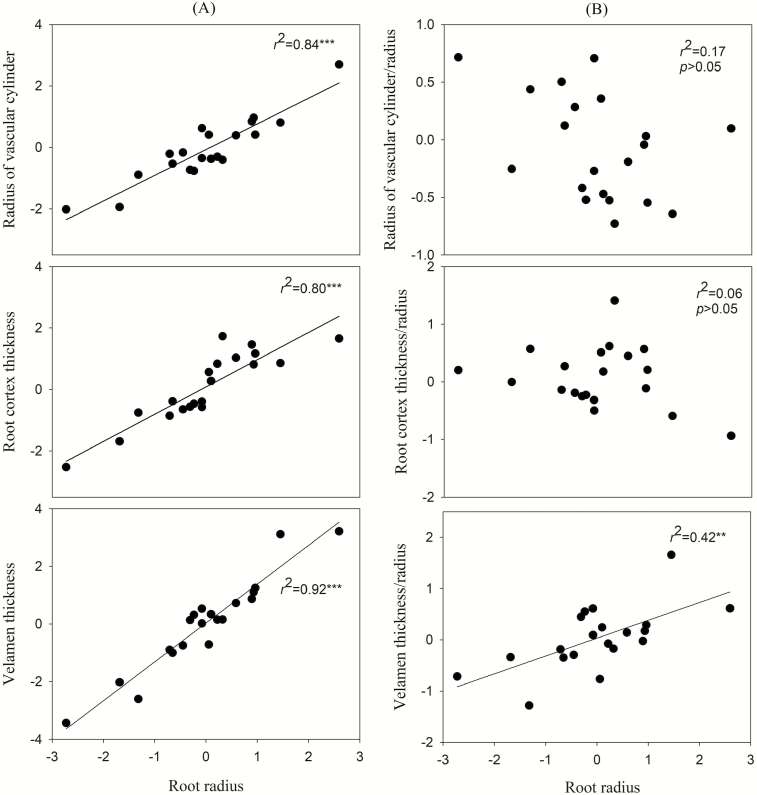
Our study suggests that leaf and root traits in Dendrobium have evolved in coordination to cope with water stress, which is consistent with our hypothesis. Roots are the major organ for absorbing water and nutrients (Pregitzer et al. 2002; Guo et al. 2008; Liese et al. 2017). Most researches have focused on ‘fine roots’, which are defined as those <2 mm in diameter (Mommer and Weemstra 2012; Kong et al. 2014). For absorptive roots, the radius is a key trait because thicker roots have greater dependence on mycorrhizal fungi and may lead to a different absorptive strategy compared to thinner roots (Guo et al. 2008; Kong et al. 2015; Ma et al. 2018). In our study, the radius of the thickest root (1551 µm) was nearly 4-fold greater than the thinnest root (413 µm). Even the thinnest root exceeds the standard for thick roots (diameter > 470 µm) in a previous study (Kong et al. 2014). This indicates that the root traits of Dendrobium in this study may be different to the thin root traits of other plants. Meanwhile, root radius had significant positive relationships with velamen thickness, root cortex thickness and radius of vascular cylinder (Fig. 5A). This finding indicates that the variation in root radius may arise from the combined thickening of the velamen, cortex and vascular cylinder. We also found that the ratio of velamen thickness to root radius (VT/r) was positively correlated with root radius, but there were no relationships between root radius and the ratio of root cortex thickness to root radius (RCT/r), and the ratio of radius of vascular cylinder to root radius (Rvc/r) (Fig. 5B). This suggests that the thicker roots of Dendrobium may be caused by a higher proportion of velamen. Thus, velamen thickness was a proxy for the root radius. This result conflicts with some previous researches on tree species that find a negative relationship between root density and root diameter, because the lower density of thick roots is caused by a larger proportion of root cortex (Chen et al. 2013; Kong et al. 2015). This may be because the roots of Dendrobium plants are exposed to the atmosphere. Velamen plays a crucial role in arboreal habitats (Joca et al. 2017). Although thicker velamen (due to greater numbers of cell layers) incurs greater construction costs (Enquist et al. 1999), it confers greater resistance to water loss and mechanical damage (Zotz and Hietz 2001). Thus, velamen is an important regulator to enhance the adaptability of Dendrobium plants to the environment.
Figure 5.
Correlations of root radius with velamen thickness, root cortex thickness and radius of vascular cylinder (A), and with the ratio of velamen thickness to radius, ratio of root cortex thickness to radius and ratio of vascular cylinder radius to root radius (B). Significance levels are expressed as follows: ***P ≤ 0.001; **P ≤ 0.01. Data were corrected by PICs.
Species with thicker root velamen had a higher leaf mass per area (LMA) and thicker leaf lower cuticle thickness (LCT). Meanwhile, in the species with higher leaf water content, the velamen thickness and area tended to be thinner in roots. Leaf dry mass is commonly used to measure the leaf strength and durability (Portillo-Estrada et al. 2015). The leaves with higher dry mass always have thicker laminas and higher tissue density because of their greater concentration of fibres and cell walls (Shipley and Vu 2002). Leaf mass per area (LMA) is used as an indicator of water and nutrient retention in plants (Witkowski and Lamont 1991), and a higher LMA represents a more conservative strategy. Greater LMA also brings a greater cost to the plant (Westoby et al. 2002). The average LMA is well known to be higher in low rainfall environments, owing to thicker leaves, denser tissue or both (Cunningham et al. 1999; Niinemets 2001). Likewise, leaf lower cuticle thickness is also related to water conservation (Zhang et al. 2012; Sun et al. 2014). All of the leaf traits mentioned above were correlated with root traits. This greatly supported our hypothesis that leaf and root traits were coordinated in terms of water conservation.
Leaf area was positively correlated with the ratio of velamen thickness to root radius (VT/r), but negatively correlated with the ratio of root cortex thickness to root radius (RCT/r). Leaf surfaces are the primary border of energy and mass exchange. Some important processes such as evapotranspiration and photosynthesis are directly proportional to leaf area (Agrawal et al. 2009). Previous studies have shown that lower leaf area helps plants prevent water loss in xeric conditions (Qin et al. 2019). The roots with larger proportions of velamen have a higher capacity for water conservation, but a larger leaf area means greater water loss. This may be because species with a conservative water use strategy tends to generate a larger total leaf area to offset the costs of construction in water conservation tissue (Reich et al. 1992; Westoby et al. 2002).
The results of the t-SNE showed that leaf and root traits were gathered, and not separated by functional category (Fig. 4B). This result is consistent with PCA and MDS. This provided further evidence that leaf and root traits coordinate to improve water utilization. Improvement of water utilization depends on the coupling of functional trait categories, supporting the idea of a whole-plant-based strategy. We also found the species were separated into two axes (Fig. 4A). This suggests that root radius may have an effect in driving leaf and root trait spectra, which is consistent with the findings in a previous study (Kong et al. 2015).
The environment drives variation in water conservation traits within Dendrobium
Most leaf and root traits, especially the number of velamen layers, leaf density and the ratio of vascular cylinder radius to root radius, varied in response to the environments. This variation helps Dendrobium plants adapt to water stress. Two pieces of evidence support this finding: the patterns of leaf and root trait variations were consistent with the responses to environmental conditions in the arboreal habitats of Dendrobium (as discussed above), and leaf and root traits were correlated with elevational distribution.
No strong phylogenetic signal was detected in all leaf and root traits. This indicated that the effect of ecological factors on these traits overshadowed evolutionary constraints. Leaf density, layer of velamen and the ratio of vascular cylinder radius to root radius showed high adaptability to the environments (Table 3). This suggested that the environment, not phylogeny, was the main driver of leaf and root traits variation in Dendrobium. Leaf density responds generally to the changes in moisture (Xu and Zhou 2008). High leaf density can help plants cope with water stress (Witkowski and Lamont 1991). The increase in velamen layer numbers confers greater resistance to water loss and mechanical damage (Zotz and Hietz 2001). The vascular cylinder is responsible for the transport of water and nutrients to the shoot (Mellor et al. 2016). Ribeiro et al. (2019) reported that the increase in vascular cylinder diameter of Glycine max seedlings alleviates the effect aroused by water deficits. All these traits showed a strong relationship with environmental factors, and indicated that Dendrobium have a great capacity to withstand drought stress. But somewhat contradictory to our result, a study on leaf functional traits in Dendrobium found that phylogeny has a significant effect on leaf density and leaf upper cuticle thickness, although most traits measured also have weak signals (Sun et al. 2014). The discrepancy was probably caused by different materials, a wider diversity of species and cultivation conditions than in our study.
We found that elevational distribution was positively correlated with root traits such as the number of endodermis cell and vessel, and with leaf traits such as leaf density and stomatal density. All these traits are related to water use efficiency. The endodermis not only separates the vascular cylinder and provides a diffusion barrier (Roppolo et al. 2011), but also functions as a protective layer during drought (Ranathunge et al. 2003). When plants are deprived of water, the endodermis resists water movement from the stele to the outside, allowing internal layers to survive (Stasovski and Peterson 2011). A previous study has shown that water transport efficiency is promoted by increased number of vessels with a larger diameter (Dickison 2000). In contrast, drought can lead to a higher proportion of narrower (less efficient) vessels and decreased vessel numbers (Durante et al. 2011; Jupa et al. 2016). Moreover, some studies have shown that a water deficit leads to an increase in stomatal density, which is positively correlated with water use efficiency (Martínez et al. 2007; Xu and Zhou 2008).
Taken together, the significant correlations between elevation with endodermis and vessels number, leaf density and stomatal density indicated that a higher elevation tended to select traits that increased water use efficiency in Dendrobium. In Yunnan Province, high elevation is often accompanied by lower temperature and humidity (Fig. 3B). The number of epiphytic orchid species decreases with increasing elevation (Zhang et al. 2015). This indicates that a low moisture level is an important factor limiting the distribution of epiphytic orchids in high-altitude areas. We speculated that the species with thicker velamen may be more adapted to higher elevations as the velamen has the function of retaining moisture and warmth in roots. Although endodermis and vessels number were positively correlated with velamen thickness, elevation was not correlated with velamen thickness. It would be helpful to investigate the role of temperature and moisture levels in measuring the capacity for plants to adapt to the potential changing environmental conditions in the future.
Conclusions
We proposed a model of interaction between leaf and root traits of Dendrobium which is an important epiphytic taxon. The majority of leaf and root traits were shaped by the environment rather than evolutionary constraints. To maintain water balance and improve water use efficiency, leaf and root traits showed close coordination in Dendrobium. The traits related to water uptake and conservation might play an important role in helping Dendrobium species to adapt to cold and dry conditions at high elevations. The results of this study confirmed the plant economic hypothesis, which states that plant populations adapt to the environment through coordinated leaf and root trait evolution. These findings improve our understanding of the interactive pattern of leaf and root traits in epiphytes.
Supporting Information
The following additional information is available in the online version of this article—
Figure S1. Phylogenetic relationships and ecological information across 21 Dendrobium species.
Figure S2. (A) Principal component analysis (PCA) and (B) multidimensional scaling (MDS) are used to compare leaf and root traits among 21 Dendrobium species.
Table S1. Coefficients of Pearson’s correlations and phylogenetically independent contrast correlations among leaf traits, and between leaf traits and elevation across 21 Dendrobium species.
Table S2. Coefficients of Pearson’s correlations and phylogenetically independent contrast correlations among root traits across 21 Dendrobium species.
Data Availability
All data used in this study are available at https://osf.io/8dkur.
Acknowledgements
We thank Dr Wei Zhang for his assistance in species identification, and Dr John Meadows for proofreading and editing.
Sources of Funding
This work was financially supported by the National Natural Science Foundation of China (31670342), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB31000000) and the Science and Technology Plan of Yunnan (2018BB010).
Contributions by the Authors
All authors conceived and designed the experiments. Y.Q. performed the experiments, collected and analysed the data before wrote the first draft, J.H. collected and identified the species. S.Z. revised the manuscript and gave final approval for its publication.
Conflict of Interest
None declared.
Compliance with Ethical Standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Literature Cited
- Ackerly DD, Donoghue MJ. 1998. Leaf size, sapling allometry, and Corner’s rules: phylogeny and correlated evolution in maples (Acer). The American Naturalist 152:767–791. [DOI] [PubMed] [Google Scholar]
- Adair KL, Lindgreen S, Poole AM, Young LM, Bernard-Verdier M, Wardle DA, Tylianakis JM. 2019. Above and belowground community strategies respond to different global change drivers. Scientific Reports 9:2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal AA, Fishbein M, Jetter R, Salminen JP, Goldstein JB, Freitag AE, Sparks JP. 2009. Phylogenetic ecology of leaf surface traits in the milkweeds (Asclepias spp.): chemistry, ecophysiology, and insect behavior. The New Phytologist 183:848–867. [DOI] [PubMed] [Google Scholar]
- Baraloto C, Hardy OJ, Paine CET, Dexter KG, Cruaud C, Dunning LT, Gonzalez MA, Molino JF, Sabatier D, Savolainen V, Chave J. 2012. Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities. Journal of Ecology 100:690–701. [Google Scholar]
- Blomberg S, Garland T, Ives A. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745. [DOI] [PubMed] [Google Scholar]
- Chen WL, Zeng H, Eissenstat DM, Guo DL. 2013. Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Global Ecology and Biogeography 22:846–856. [Google Scholar]
- Comas L, Bouma T, Eissenstat D. 2002. Linking root traits to potential growth rate in six temperate tree species. Oecologia 132:34–43. [DOI] [PubMed] [Google Scholar]
- Craine JM, Lee WG. 2003. Covariation in leaf and root traits for native and non-native grasses along an altitudinal gradient in New Zealand. Oecologia 134:471–478. [DOI] [PubMed] [Google Scholar]
- Cunningham SA, Summerhayes B, Westoby M. 1999. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecological Monographs 69:569–588. [Google Scholar]
- Díaz S, Kattge J, Cornelissen JH, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Prentice IC, Garnier E, Bönisch G, Westoby M, Poorter H, Reich PB, Moles AT, Dickie J, Gillison AN, Zanne AE, Chave J, Wright SJ, Sheremet’ev SN, Jactel H, Baraloto C, Cerabolini B, Pierce S, Shipley B, Kirkup D, Casanoves F, Joswig JS, Günther A, Falczuk V, Rüger N, Mahecha MD, Gorné LD. 2016. The global spectrum of plant form and function. Nature 529:167–171. [DOI] [PubMed] [Google Scholar]
- Dickison WC. 2000. Evolutionary, physiological, and ecological plant anatomy. In: Dickison, ed. Integrative plant anatomy. San Diego, CA: Harcourt Academic Press, 320–322. [Google Scholar]
- Durante M, Maseda PH, Fernández RJ. 2011. Xylem efficiency vs. safety: acclimation to drought of seedling root anatomy for six Patagonian shrub species. Journal of Arid Environments 75:397–402. [Google Scholar]
- Edwards EJ. 2006. Correlated evolution of stem and leaf hydraulic traits in Pereskia (Cactaceae). The New Phytologist 172:479–789. [DOI] [PubMed] [Google Scholar]
- Enquist BJ, West GB, Charnov EL, Brown JH. 1999. Allometric scaling of production and life-history variation in vascular plants. Nature 401:907–911. [Google Scholar]
- Eshel A, Beeckman T. 2013. Plant roots: the hidden half, 4th edn. New York: CRC Press. [Google Scholar]
- Fortunel C, Fine PVA, Baraloto C. 2012. Leaf, stem and root tissue strategies across 758 Neotropical tree species. Functional Ecology 26:1153–1161. [Google Scholar]
- Freschet GT, Cornelissen JHC, Van-Logtestijn RSP, Aerts R. 2010. Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology 98:362–373. [Google Scholar]
- Freschet GT, Kichenin E, Wardle DA. 2015. Explaining within-community variation in plant biomass allocation: a balance between organ biomass and morphology above vs below ground? Journal of Vegetation Science 26:431–440. [Google Scholar]
- Freudenstein JV, Rasmussen FN. 1999. What does morphology tell us about orchid relationships? – a cladistic analysis. American Journal of Botany 86:225–248. [PubMed] [Google Scholar]
- Grotkopp E, Rejmánek M. 2007. High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperms. American Journal of Botany 94:526–532. [DOI] [PubMed] [Google Scholar]
- Guo D, Xia M, Wei X, Chang W, Liu Y, Wang Z. 2008. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. The New Phytologist 180:673–683. [DOI] [PubMed] [Google Scholar]
- Joca TAC, Oliveira DC, Zotz G, Winkler U, Moreira ASFP. 2017. The velamen of epiphytic orchids: variation in structure and correlations with nutrient absorption. Flora 230:66–74. [Google Scholar]
- Jupa R, Plavcová L, Flamiková B, Gloser V. 2016. Effects of limited water availability on xylem transport in liana Humulus lupulus L. Environmental and Experimental Botany 130:22–32. [Google Scholar]
- Kembel SW, Cahill JF Jr. 2011. Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS One 6:e19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Ma C, Zhang Q, Li L, Chen X, Zeng H, Guo D. 2014. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. The New Phytologist 203:863–872. [DOI] [PubMed] [Google Scholar]
- Kong DL, Wang JJ, Kardol P, Wu H, Zeng H, Deng X, Deng Y. 2015. The root economics spectrum: divergence of absorptive root strategies with root diameter. Biogeosciences Discussions 12:13041–13067. [Google Scholar]
- Kong DL, Wang JJ, Wu HF, Valverde-Barrantes OJ, Wang RL, Zeng H, Kardol P. Zhang HY, Feng YL. 2019. Nonlinearity of root trait relationships and the root economics spectrum. Nature Communications 10:2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese R, Alings K, Meier IC. 2017. Root branching is a leading root trait of the plant economics spectrum in temperate trees. Frontiers in Plant Science 8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Freschet GT, Pan X, Cornelissen JH, Li Y, Dong M. 2010. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. The New Phytologist 188:543–553. [DOI] [PubMed] [Google Scholar]
- Ma Z, Guo D, Xu X, Lu M, Bardgett RD, Eissenstat DM, McCormack ML, Hedin LO. 2018. Evolutionary history resolves global organization of root functional traits. Nature 555:94–97. [DOI] [PubMed] [Google Scholar]
- Manschadi AM, Christopher J, De-Voil P, Hammer GL. 2006. The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33:823–837. [DOI] [PubMed] [Google Scholar]
- Martínez JP, Silva H, Ledent JF, Pinto M. 2007. Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). European Journal of Agronomy 26:30–38. [Google Scholar]
- Mellor N, Adibi M, El-Showk S, De Rybel B, King J, Mähönen AP, Weijers D, Bishopp A. 2016. Theoretical approaches to understanding root vascular patterning: a consensus between recent models. Journal of Experimental Botany 68:5–16. [DOI] [PubMed] [Google Scholar]
- Mommer L, Weemstra M. 2012. The role of roots in the resource economics spectrum. The New Phytologist 195:725–727. [DOI] [PubMed] [Google Scholar]
- Morand S, Poulin R. 2003. Phylogenies, the comparative method and parasite evolutionary ecology. Advances in Parasitology 54:281–302. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. 2001. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469. [Google Scholar]
- Poorter H, Lambers H, Evans JR. 2014. Trait correlation networks: a whole-plant perspective on the recently criticized leaf economic spectrum. The New Phytologist 201:378–382. [DOI] [PubMed] [Google Scholar]
- Poorter L, Wright SJ, Paz H, Ackerly DD, Condit R, Ibarra-Manríquez G, Harms KE, Licona JC, Martínez-Ramos M, Mazer SJ, Muller-Landau HC, Peña-Claros M, Webb CO, Wright IJ. 2008. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89:1908–1920. [DOI] [PubMed] [Google Scholar]
- Portillo-Estrada M, Copolovici L, Niinemets Ü. 2015. Bias in leaf dry mass estimation after oven-drying isoprenoid-storing leaves. Trees 29:1805–1816. [Google Scholar]
- Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL. 2002. Fine root architecture of nine North American trees. Ecological Monographs 72:293–309. [Google Scholar]
- Price T. 1997. Correlated evolution and independent contrasts. Philosophical Transactions of the Royal Society of London 352:519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A, Webster AJ. 1999. Phylogenetically independent contrasts and primate phylogeny. In: Lee PC, ed. Comparative primate socioecology. Cambridge: Cambridge University Press, 44–70. [Google Scholar]
- Qin J, Shang-Guan ZP, Xi WM. 2019. Seasonal variations of leaf traits and drought adaptation strategies of four common woody species in South Texas, USA. Journal of Forestry Research 30:1715–1725. [Google Scholar]
- Ranathunge K, Steudle E, Lafitte R. 2003. Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta 217:193–205. [DOI] [PubMed] [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102:275–301. [Google Scholar]
- Reich PB, Tjoelker MG, Walters MB, Vanderklein DW, Buschena C. 1998. Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Functional Ecology 12:327–338. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1992. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecological Monographs 62:365–392. [Google Scholar]
- Ribeiro DGd, Silva BRS, Lobato AKd. 2019. Brassinosteroids induce tolerance to water deficit in soybean seedlings: contributions linked to root anatomy and antioxidant enzymes. Acta Physiologiae Plantarum 41:82. [Google Scholar]
- Roberts DL, Dixon KW. 2008. Orchids. Current Biology 18:R325–R329. [DOI] [PubMed] [Google Scholar]
- Roppolo D, De Rybel B, Dénervaud Tendon V, Pfister A, Alassimone J, Vermeer JE, Yamazaki M, Stierhof YD, Beeckman T, Geldner N. 2011. A novel protein family mediates Casparian strip formation in the endodermis. Nature 473:380–383. [DOI] [PubMed] [Google Scholar]
- Ryser P, Verduyn B, Lambers H. 1997. Phosphorus allocation and utilization in three grass species with contrasting response to N and P supply. The New Phytologist 137:293–302. [DOI] [PubMed] [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. 2003. The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell & Environment 26:1343–1356. [Google Scholar]
- Schuiteman A. 2011. Dendrobium (Orchidaceae): to split or not to split? Gardens’ Bulletin Singapore 63:245–257. [Google Scholar]
- Shipley B, Vu TT. 2002. Dry matter content as a measure of dry matter concentration in plants and their parts. The New Phytologist 153:359–364. [Google Scholar]
- Simpson L, Clements MA, Crayn DM, Nargar K. 2018. Evolution in Australia’s mesic biome under past and future climates: insights from a phylogenetic study of the Australian Rock Orchids (Dendrobium speciosum complex, Orchidaceae). Molecular Phylogenetics and Evolution 118:32–46. [DOI] [PubMed] [Google Scholar]
- Somavilla NS, Kolb RM, Rossatto DR. 2013. Leaf anatomical traits corroborate the leaf economic spectrum: a case study with deciduous forest tree species. Brazilian Journal of Botany 37:69–82. [Google Scholar]
- Stasovski E, Peterson C. 2011. Effects of drought and subsequent rehydration on the structure, vitality, and permeability of Allium cepa adventitious roots. Canadian Journal of Botany 71:700–707. [Google Scholar]
- Sun M, Yang SJ, Zhang JL, Bartlett M, Zhang SB. 2014. Correlated evolution in traits influencing leaf water balance in Dendrobium (Orchidaceae). Plant Ecology 215:1255–1267. [Google Scholar]
- Takamiya T, Wongsawad P, Sathapattayanon A, Tajima N, Suzuki S, Kitamura S, Shioda N, Handa T, Kitanaka S, Iijima H, Yukawa T. 2014. Molecular phylogenetics and character evolution of morphologically diverse groups, Dendrobium section Dendrobium and allies. AoB Plants 6:plu045; doi: 10.1093/aobpla/plu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira da Silva JA, Jin XH, Dobránszki J, Lu JJ, Wang HZ, Zotz G, Cardoso JC, Zeng SJ. 2016. Advances in Dendrobium molecular research: applications in genetic variation, identification and breeding. Molecular Phylogenetics and Evolution 95:196–216. [DOI] [PubMed] [Google Scholar]
- Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D. 2005. Linking leaf and root trait syndromes among 39 grassland and savannah species. The New Phytologist 167:493–508. [DOI] [PubMed] [Google Scholar]
- Valverde-Barrantes OJ, Freschet GT, Roumet C, Blackwood CB. 2017. A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. The New Phytologist 215:1562–1573. [DOI] [PubMed] [Google Scholar]
- Van der Maaten L, Hinton G. 2008. Visualizing data using t-SNE. Journal of Machine Learning Research 9:2579–2605. [Google Scholar]
- Wahl S, Ryser P. 2000. Root tissue structure is linked to ecological strategies of grasses. The New Phytologist 148:459–471. [DOI] [PubMed] [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33:125–159. [Google Scholar]
- Withington JM, Reich PB, Oleksyn J, Eissenstat DM. 2006. Comparisons of structure and life span in roots and leaves among temperate trees. Ecological Monographs 76:381–397. [Google Scholar]
- Witkowski ET, Lamont BB. 1991. Leaf specific mass confounds leaf density and thickness. Oecologia 88:486–493. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Groom PK, Hikosaka K, Lee W, Lusk CH, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Warton DI, Westoby M. 2005. Modulation of leaf economic traits and trait relationships by climate. Global Ecology and Biogeography 14:411–421. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R. 2004. The worldwide leaf economics spectrum. Nature 428:821–827. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Westoby M. 2002. Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. The New Phytologist 155:403–416. [DOI] [PubMed] [Google Scholar]
- Xiang XG, Schuiteman A, Li DZ, Huang WC, Chung SW, Li JW, Zhou HL, Jin WT, Lai YJ, Li ZY, Jin XH. 2013. Molecular systematics of Dendrobium (Orchidaceae, Dendrobieae) from mainland Asia based on plastid and nuclear sequences. Molecular Phylogenetics and Evolution 69:950–960. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhou G. 2008. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany 59:3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SB, Guan ZJ, Sun M, Zhang JJ, Cao KF, Hu H. 2012. Evolutionary association of stomatal traits with leaf vein density in Paphiopedilum, Orchidaceae. PLoS One 7:e40080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Yan YJ, Tian Y, Li JS, He JS, Tang ZY. 2015. Distribution and conservation of orchid species richness in China. Biological Conservation 181:64–72. [Google Scholar]
- Zhang S, Yang Y, Li J, Qin J, Zhang W, Huang W, Hu H. 2018. Physiological diversity of orchids. Plant Diversity 40:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu GH, Ji ZH, Wood JJ, Wood HP. 2009. Dendrobium. In: Chen XQ, Liu ZJ, Zhu GH, Lang KY, Ji ZH, Luo YB, Jin XH, Phillip JC, Jeffrey JW, Stephan WG, Paul O, Jaap JV, Howard PW, Dudley C, Alexandra B, eds. Flora of China, Orchidaceae. Beijing and St. Louis: Science Press and Missouri Botanical Garden, 367–397. [Google Scholar]
- Zotz G. 2005. Vascular epiphytes in the temperate zones–a review. Plant Ecology 176:173–183. [Google Scholar]
- Zotz G, Bogusch W, Hietz P, Ketteler N. 2010. Growth of epiphytic bromeliads in a changing world: the effects of CO2, water and nutrient supply. Acta Oecologica 36:659–665. [Google Scholar]
- Zotz G, Hietz P. 2001. The physiological ecology of vascular epiphytes: current knowledge, open questions. Journal of Experimental Botany 52:2067–2078. [DOI] [PubMed] [Google Scholar]
- Zotz G, Schickenberg N, Albach D. 2017. The velamen radicum is common among terrestrial monocotyledons. Annals of Botany 120:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz G, Winkler U. 2013. Aerial roots of epiphytic orchids: the velamen radicum and its role in water and nutrient uptake. Oecologia 171:733–741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are available at https://osf.io/8dkur.