Abstract
Importance:
Highly penetrant inherited mutations in the prion protein gene (PRNP) offer a window to study the pathobiology of prion disorders.
Objective:
To characterize the longitudinal clinical features, neuropsychological findings, MRI findings, and FGD-PET scan imaging findings in a subsequent generation of a family with a large 12-octapeptide repeat insertion in the PRNP gene.
Design:
Clinical, neuropsychological, and neuroimaging characterization of a kindred.
Setting:
Mayo Clinic Alzheimer Disease Research Center (Rochester, Minnesota).
Participants:
Four mutation carriers and two non-carriers in the third generation of a kindred with a large octapeptide repeat insertion in the PRNP gene.
Results:
Three of the four mutation carriers have progressed to a frontotemporal dementia phenotype. Declines in neuropsychological function coincided with changes in FDG-PET at the identified onset of cognitive impairment. Processing speed and executive function were abnormal before the identified onset.
Conclusions and relevance:
Gene silencing treatments are on the horizon and when they become available, early detection will be crucial. Longitudinal studies involving familial mutation kindreds can offer important insights into the initial neuropsychological and neuroimaging changes necessary for early detection.
Introduction:
Familial inheritance makes up roughly 15% of known prion disease cases, which is consistent with described inheritance patterns of other disorders such as neurodegenerative diseases (Bertram & Tanzi, 2008; Minikel et al., 2016). Over 20 different octapeptide base pair deletions (OBPD) and octapeptide base pair insertions (OBPI), ranging from 24 to 288 base pairs, are reported in the literature (Schmitz et al., 2016). In addition, allelic prion protein mutations with varying disease penetrance were recently reported (Minikel et al., 2016).
The octapeptide repeat region of the prion protein is an evolutionarily conserved domain among mammals. There are multiple copper motifs present which help regulate superoxide dismutase (SOD) activity and protect against apoptosis. Removal of this region in animal models decreases SOD activity and increases cellular apoptosis (Sakudo et al., 2005). Prion proteins are membrane proteins (PrPC) that are anchored by a glycosylphosphatidylinositol (GPI) moiety within the cell membrane. They are involved in many downstream signals involving transduction pathways, cell proliferation, differentiation, and sensitivity to programmed cell death (Linden et al., 2008). The conversion to misfolded (PrPSc) forms can promote abnormal protein aggregation. This conversion is likely responsible for cellular dysfunction (Mallucci et al., 2007; Verity & Mallucci, 2011) with subsequent intracellular protein aggregation contributing to neuronal death and progressive cognitive decline (Ballatore, Lee, & Trojanowski, 2007; Bucciantini et al., 2002; Chiti & Dobson, 2006).
Prion disorders, similar to other neurodegenerative diseases, are a phenotypically heterogeneous group but with many shared features. The location of abnormal prion protein deposition in the brain can vary depending on host genetic factors and the conformational variants of prion protein present (Chiesa, Restelli, Comerio, Del Gallo, & Imeri, 2016; Head et al., 2004; Parchi et al., 2012). Evidence of protein templating in neurodegenerative diseases has been increasing in recent years (Biasini, Turnbaugh, Unterberger, & Harris, 2012; Cho et al., 2016; Steiner, Angot, & Brundin, 2011), and suggests that phenotypic diversity may be related to unique conformational variants of misfolded proteins (Sanders et al., 2014). Given the parallels to other common neurodegenerative diseases, familial prion disorders serve as a unique model to further our understanding and potential treatment of neurodegenerative disorders.
We originally reported on the second generation of a kindred harboring a novel PRNP mutation with one allele carrying a 288-base pair insertion in 2011 (Kumar et al., 2011). In that previous report, the clinical features, electroencephalographic patterns, genetic analyses, and neuropathologic features were described in the two mothers of these current study participants. In this current study, we present longitudinal clinical findings, neuropsychological data, and neuroimaging findings of four individuals in the third generation of this kindred.
Methods:
Participants
Generation III family members (initial age range 25–37) were consented and enrolled in the Mayo Alzheimer Disease Research Center (ADRC) in Rochester, Minnesota as part of an IRB-approved longitudinal study. The mean follow up time for mutations carriers has been 11.3 years (range 9–13 years). They have undergone neurologic evaluation, neuropsychological testing, and neuroimaging scans every one to two years. The mean follow up time for mutation non-carriers was 4.5 years.
Genetics
All genes (APP, PSEN1, PSEN2, MAPT, and PRNP) were analyzed by Sanger sequencing of genomic DNA. The methionine/valine polymorphism at codon 129 (rs1799990) was determined from sequence data, whereas the APOE genotype status was generated using inventoried Taqman assays (Invitrogen).
Image Acquisition
All participants were part of the Mayo ADRC and agreed to undergo brain magnetic resonance imaging (MRI) and fluorodeoxyglucose positron emission tomography (18F-FDG-PET) imaging. All recent MRIs were performed with a 3.0 T with additional 3-D MPRAGE sequences. 18F-FDG-PET images were acquired using a PET/CT scanner (GE Healthcare) operating in 3D mode. Participants were injected in a dimly lit room with 18F-FDG, and after a 30-minute uptake period, an 8-minute 18F-FDG scan was performed, which consisted of four 2-minute dynamic frames following a low dose CT transmission scan. Standard acquisition and vendor reconstruction parameters were used. 18F-FDG-PET scans were processed using CortexID software (GE Healthcare). The activity in each participant’s PET dataset was normalized to the pons and compared with an age-segmented normative database, yielding z-score 3D-stereotactic surface projection (SSP) images.
Neurologic and neuropsychological testing
Each participant underwent a comprehensive neurologic evaluation, including mental status testing (the Short Test of Mental Status or STMS) and neurologic examination by a behavioral neurologist. The STMS is scored out of 38 points (Tang-Wai et al., 2003; Townley et al., 2019). The Clinical Dementia Rating Staging Instrument (CDR®) was completed at all visits for each participant (Hughes, Berg, Danziger, Coben, & Martin, 1982). Detailed neuropsychological tests were performed separately by a trained psychometrist supervised by a neuropsychologist. The neuropsychological battery for each participant was performed at each visit and included combinations of the following tests: Wide Range Achievement Test (WRAT) (Wilkinson & Robertson, 2006), Rey Auditory Verbal Learning Test (AVLT) (Strauss, Sherman, & Spreen, 2006), Wechsler Memory Scale-Revised (WMS-R) Logical Memory (LM) I and II and Visual Reproductions (VR) I and II subtests (Wechsler, 1987), Wechsler Adult Intelligence Scale-Revised (WAIS-R) – Block Design, Picture Completion, Digit Span, Digit Coding (Wechsler, 1981), Rey-Osterrieth Complex Figure copy (Rey-O) (Osterrieth, 1944), Judgement of Line Orientation (JLO) (Benton, Sivan, Hamsher, Varney, & Spreen, 1994), Trail Making Test Part A (Trails A) and Part B (Trails B) (Heaton, Miller, Taylor, & Grant, 2004), Stroop Test: Word-Reading, Color-Naming, and Interference trials (Golden & Freshwater, 1978), Boston Naming Test (BNT) (Heaton et al., 2004), Animal fluency (Heaton et al., 2004), and Controlled Oral Word Association Test (COWAT) (Ruff, Light, Parker, & Levin, 1996).
Due to changes in the ADRC’s neuropsychological test battery in recent years, not every test was administered at each visit. To compare performance across time points, we converted all raw scores to z-scores based on published norms (see above references) and created composites by averaging z-scores within specified cognitive domains. These domains included: immediate memory (LM I, VR I, AVLT sum of learning trials 1–5 [1–5 Sum]), delayed memory (LM II, VR II, AVLT Delay), language (COWAT, Animal Fluency, BNT), executive functioning (Digit Span (combined Forward and Backward), Trails B, Stroop Interference), visuospatial (Picture Completion, Block Design, Rey-O, JLO), and processing speed (Digit Coding, Trails A, Stroop Word-Reading, Stroop Color-Naming).
The clinical diagnosis was made in a consensus meeting with study coordinators, physicians, and neuropsychologists to assign a consensus diagnosis of cognitively normal, mild cognitive impairment (MCI - amnestic or non-amnestic (Petersen, 2004)), or dementia (along with the dementia syndrome). Clinical onset was described as a change in cognition, with cognitive impairment determined by the consensus group. This group considered objective measures (cognitive testing) and subjective measures (changes in daily function reported by the participant and/or an informant). Diagnosis of possible behavioral variant frontotemporal dementia (bvFTD) was based on the most recent consensus criteria (Rascovsky et al., 2011). Of note, participants could not be diagnosed as probable bvFTD due to their prion mutation (exclusionary criteria V.C). The clinical details of each participant are purposefully not described in this report in order to promote the maintenance of confidentiality.
Results:
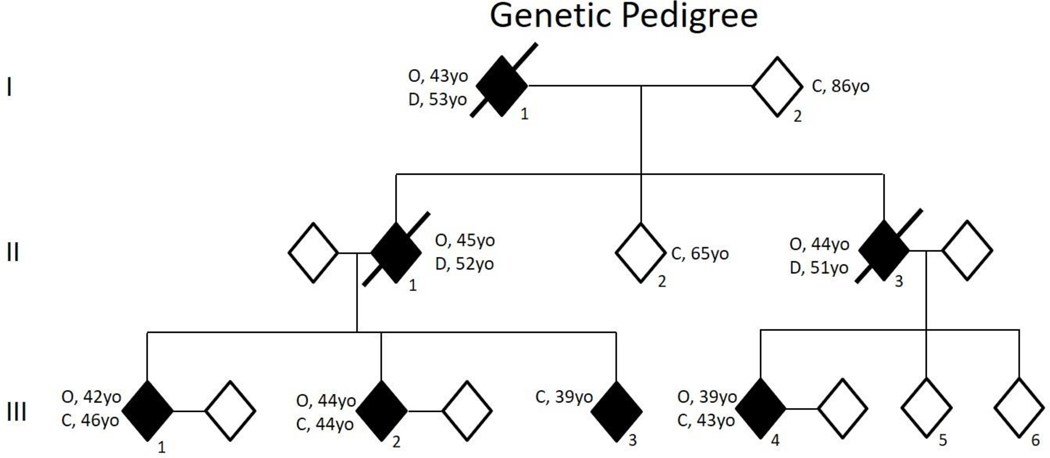
The pedigree of this kindred is shown in Figure 1.
Figure 1:
Genetic pedigree of three affected generations. Participant III-3 has an MRI abnormality, but FDG-PET is normal and clinical symptoms do not indicate clear onset at last evaluation. Two unaffected individuals in the third generation had normal findings on neuropsychological examination, brain MRI, and FDG-PET. C – current age; O – symptom onset; D – age of death. *Ages of generation III participants range from the late thirties to early forties but exact ages have been removed for confidentiality purposes.
There were no mutations in PSEN1, PSEN2, APP or MAPT. Sequence analysis of the PRNP gene showed a mutated allele carrying a 288-BPI consisting of 12 octapeptide repeats in the four participants presented. The methionine/valine polymorphism at codon 129 was homozygous for methionine and the genetic APOE status was ε3/ε4. The STMS was abnormal at the initial visit in all four participants but improved to the normal range within the study period in three out of four participants (Table 1). Participant III-4 never had a normal STMS but also had an abnormal WRAT Reading score, suggesting moderately low premorbid functioning.
Table 1.
WRAT and STMS scores for each participant
| WRATa z-score | Initial STMS | Peak STMS | Most Recent STMS | |
|---|---|---|---|---|
| Participant III-1 | −.13 | 29 | 34 | 11 |
| Participant III-2 | −.52 | 31 | 35 | 33 |
| Participant III-3 | .08 | 32 | 37 | 34 |
| Participant III-4 | −2.33 | 25 | 33 | 25 |
WRAT - Wide Range Achievement Test – Reading subtest
STMS – Short Test of Mental Status is based out of 38 points where a score below 34 is consistent with possible mild cognitive impairment, and a score below 30 is consistent with possible dementia in the correct clinical setting
Participant III-1:
Participant III-1 was the oldest sibling and has been followed over 8 visits. Executive functioning (e.g. organizing and completing complex tasks at work) was a premorbid weakness based on participant and family reports and work tasks were kept simple. Most domains were grossly normal at visit 1, except for executive functioning and processing speed, which were mildly abnormal (< −1.5 z-score). The trajectory of cognitive performance remained relatively stable until onset. There was a gradual, but not clinically meaningful, reduction in executive functioning and an improvement in memory performance that was suggestive of practice effects. At visit 5 the informant described changes in cognition, but the functional abilities were unchanged from baseline. Executive functioning and processing remained abnormal, language declined (though not to the impaired range), and practice effects were no longer sustained on memory tests. These cognitive changes resulted in a diagnosis of non-amnestic, multi-domain, mild cognitive impairment (Figure 2A). A diagnosis of possible bvFTD was made at visit 6 based on prominent changes in behavior and executive functioning associated with a decline in functional abilities at work and at home. Most cognitive domains were below −1.5 z-score, CDR® sum of boxes was 4.5, and there were more difficulties with independently carrying out instrumental activities of daily living. The MRI at visit 7 showed moderate cerebral and cerebellar atrophy, with more severe atrophy in the left anterior temporal lobe (Figure 2B). The FDG-PET scan became abnormal between visits 4 and 6, with hypometabolism progressing in the left > right frontal, parietal, and temporal lobes at visit 7 (Figure 2C). Neuropsychological testing at visit 7 was limited to four domains – all of which were impaired, and no testing could be performed at visit 8 due to the severity of global cognitive impairment and aphasia (CDR® sum of boxes was 12). On the most recent neurologic examination, there were saccadic intrusions, increased reflexes, and a mildly spastic gait with an inability to tandem gait walk. Severe nonfluent aphasia with little spontaneous speech was present, with profound deficits naming objects, moderate to severe comprehension and fluency deficits, and moderately impaired repetition.
Figure 2:
Participant III-1 with evolving mild cognitive impairment by visit 5 and behavioral variant frontotemporal dementia by visit 7. (A) Neuropsychological domain z-scores (y-axis range: −3.5 to 3.5) across 7 visits are shown. Grey area represents the average range. Of note, this participant was unable to complete any neuropsychological testing at visit 8 due to global aphasia. (B) MRI MP-RAGE sequences demonstrated cerebellar and midline atrophy on sagittal view, frontal and parietal atrophy on axial view, and L > R temporal and frontal lobe atrophy on coronal view. (C) FDG-PET (using Cortex ID, GE Healthcare with z-score from 0 to −7 on the left side) went from normal at visit 4 to abnormal by visit 6 with worsening hypometabolism in the L>R frontal, parietal, temporal, precuneus regions by visit 7.
Participant III-2:
Participant III-2 has been followed over 10 visits. At visit 1, processing speed was moderately abnormal and executive functioning was mildly abnormal, while memory and language were normal (there was no measure of visuospatial abilities). Most cognitive domains remained relatively stable and within the broadly normal range (except for processing speed, which was variable and mildly abnormal) until onset, but there were less clear practice effects on memory testing across visits. By visit 9 (onset), memory and judgment had declined by history. At this point, there were clear declines in executive functioning, visuospatial abilities, and delayed memory (all below −1.5 z-score), and processing speed continued to be slow. In addition to notable cognitive decline at visit 9, CDR® sum of boxes was 3.5 and a diagnosis of mild dementia was made based on impairment of instrumental activities of daily living (including losing driving privileges and being let go from work) and the neuropsychological profile. Delayed memory performance and executive functioning improved at visit 10, but deficits in processing speed and visuospatial abilities remained (Figure 3A). The most recent MRI showed mild cerebellar and cerebral atrophy (Figure 3B). There was evidence of mild hydrocephalus and brainstem sag, with low-lying cerebellar tonsils and dural thickening. There were no reported orthostatic headaches or daytime somnolence symptoms consistent with brainstem sag. Between visits 8 and 9, the FDG-PET scan showed progressing hypometabolism in the anterior and posterior cingulate gyrus, caudate nucleus, and patchy hypometabolism throughout the entire cerebral cortex. On neurologic examination, their mood was described as happy and gregarious, but with very tangential thought processes. There was mild aphasia, but it was not classifiable. Saccadic intrusions were present on smooth pursuit. There were increased reflexes in the upper extremities, mild irregularity with rapid alternating movements of fingers, and difficulty with tandem gait walking.
Figure 3:
Participant III-2 with evolving mild dementia by visit 9. (A) Neuropsychological domain z-scores (y-axis range: −3.5 to 3.5) across visits 1–10 are shown. Grey area represents the average range. (B) MRI MP-RAGE sequences demonstrated mild cerebellar atrophy and brainstem sag on sagittal view and minimal cortical atrophy on axial and coronal views. (C) FDG-PET (using Cortex ID, GE Healthcare with z-score from 0 to −7 on the left side) became most abnormal by visit 9. There was thalamus, caudate, anterior cingulate and mild posterior cingulate hypometabolism along with patchy hypometabolism throughout the cortex.
Participant III-3:
Participant III-3, the youngest sibling, has been followed over 12 visits. From the initial evaluation, personality was described as reserved and quiet, but a few years into the study personality was described as more outgoing, attentive, and engaged with others. Over this time, bedside cognitive screenings improved from abnormal to normal. At the first visit, their visuospatial performance was moderately abnormal while memory and language were normal (there were no executive functioning and processing speed data available). Processing speed was mildly slow on visits 2 and 3 but subsequently improved and remained relatively normal throughout the following assessments. Otherwise, performance across cognitive domains was stable and normal across visits, with evidence of benefit from practice effects on memory testing. At visits 11 and 12, visuospatial abilities became mildly abnormal again (Figure 4A). The informant described the participant as different from some of the siblings/cousins since childhood and reported mild and variable memory and judgment difficulties across all visits. CDR® sum of boxes was consistently 1. MRI showed mild cerebellar atrophy (Figure 4B) and there were mild cerebellar signs on exam, with saccadic interruptions of smooth pursuit, saccadic latency with overshooting, increased reflexes, irregularities in rapid alternating motions of hands and fingers, and mild difficulties with tandem gait walking. FDG-PET scan imaging was stable over three separate visits and is read as normal (Figure 4C).
Figure 4:
Participant III-3 with variable degrees of memory and judgment problems through all 12 visits. (A) Neuropsychological domain z-scores (y-axis range: −3.5 to 3.5) across visits 1–12 are shown. Grey area represents the average range. (B) MRI MP-RAGE sequences demonstrated mild to moderate cerebellar atrophy on sagittal view and minimal cortical atrophy on axial and coronal views. (C) FDG-PET (using Cortex ID, GE Healthcare with z-score from 0 to −7 on the left side) was normal across all time points.
Participant III-4:
Participant III-4 has been followed over 11 visits. Baseline premorbid functioning was moderately low and all cognitive domain z-scores were < −1.5, except for language which was −1.49. In the context of suspected moderately low premorbid functioning, abnormal cognitive performances likely reflect this individual’s baseline rather than decline. Language remained relatively stable across visits. Like other participants, practice effects for memory tasks were evident across visits until identified onset. By visit 8 rather prominent changes in behavior and cognition had evolved, and employment had been terminated. There was a clear indication of decline across cognitive domains (except for language) by visit 7 or 8. Executive functioning and processing speed domains were the most affected, but there was also a mild decline in immediate memory at visit 8 when a diagnosis of possible bvFTD was documented. At the time of possible bvFTD diagnosis, emotional lability, increased irritability, and decreased social interactions were evident. CDR® sum of boxes was 4.5. The MRI showed moderate cerebral and cerebellar atrophy (Figure 5B). Between visits 7 and 9, the FDG-PET scan showed interval progression of hypometabolism in the frontal cortex (Figure 5C). By visit 11, progressive left greater than right hypometabolism was present in the medial and lateral frontal cortex, superior parietal cortex, and the precuneus. Neurologic examination revealed a flattened affect and tangential thought process. Dysarthria was described as mildly ataxic with additional ideomotor apraxia, irregularity with rapid alternating movements of fingers, mild right upper extremity rigidity, and an abnormal Luria motor sequence test. Oculomotor findings showed mild conjugate up gaze restriction, oculomotor apraxia, and saccadic pursuit intrusions. Reflexes were increased throughout with a spastic gait and significant tandem gait difficulties.
Figure 5:
Participant III-4 (who had moderately low baseline functioning) with evolving behavioral variant frontotemporal dementia diagnosed at visit 8. (A) Neuropsychological domain z-scores (y-axis range: −3.5 to 3.5) across 11 visits are shown. Grey area represents the average range. (B) MRI MP-RAGE sequences demonstrated moderate cerebellar and midline atrophy on sagittal view, frontal and parietal atrophy on axial view, and temporal atrophy on coronal view. (C) FDG-PET (using Cortex ID, GE Healthcare with z-score from 0 to −7 on the left side) had abnormalities from the first scan which progressed to involve more L > R frontal lobe by visit 9 and then further progression of L > R frontal, medial frontal, superior parietal, and precuneus regions by visit 11.
Other family members:
The two non-carriers within generation III, participant III-5 and participant III-6, also underwent multiple clinical evaluations between ages 27–36 and were neurologically normal. Their neuropsychological profiles were also normal over their four and five visits, respectively. Their MRI scans of the brain and FDG-PET scans of the brain were normal. Neither participant carries the octapeptide mutation in PRNP and they have not returned for subsequent evaluations due to no reported concerns and not wishing to continue evaluations.
Discussion:
We present an update on a PRNP kindred family now with three generations of early-onset neurodegenerative disease due to a 12-octapeptide repeat insertion in the PRNP gene spanning a decade of clinical, neuropsychological, MRI, and FDG-PET scan data which has illuminated the progression of this neurodegenerative prion disorder. Clinical cases with an FTD-phenotype, cerebellar signs on exam, and strong family history should be considered for OBPI mutations. Of note, standard genetic sequencing for OBPI PRNP mutations can miss inversions, deletions, and insertions and the laboratory performing genetic testing should be made aware of the mutations being considered.
One participant is in the age range of symptom onset for generation I and II, and this individual is the most severely affected, with global cognitive impairment and aphasia (participant III-1). The other two with identified symptom onset are younger than when generation I and II were identified. The increased surveillance among family members, and yearly neurology visits likely account for the detection of the earlier age of symptom onset in our kindred.
Mild cerebellar findings are present on a detailed neurological exam in all mutation carrying participants, which are consistent with the cerebellar atrophy findings on MRI. These abnormalities were present before the onset of cognitive decline but did not interfere with daily activities early in the disease. The cerebellar atrophy and exam findings progressively worsened at later stages, as evidenced by comparing participants III-1 and III-3 sagittal MRI (Figure 2B and 4B). Previous OBPI and Gerstmann‐Straüssler–Scheinker cases have also described cerebellar abnormalities on exam and autopsy examination (Vital et al., 1998). Cerebellar hypometabolism on FDG-PET was not well visualized with the imaging techniques used in our institution’s standard reporting protocol. The normative database for z-score reporting on Cortex ID at the time these scans were performed starts at age 50 and since metabolism declines with age, mild hypometabolism in cerebellar regions may be missed in younger individuals. Individual voxel-wise analysis of the cerebellar region was not performed in this study.
The initial evaluation with each mutation carrier revealed mild to moderate difficulties on STMS (Table 1) and detailed neuropsychological testing. Domains most likely affected even before identified onset were processing speed and executive functioning, a pattern which may represent an early indicator of disruption in frontocerebellar circuitry, and there were notable declines after the identified onset. In contrast, bedside screening, immediate memory, and delayed memory tests all showed practice effects until identified onset (Machulda et al., 2013). The STMS, while a good screening tool for many common neurodegenerative diseases, lacks specific processing speed and executive function testing.
Each affected family member in the third generation has an APOE ε3/ε4 genotype, raising the possibility of AD interaction effects. There are no cerebrospinal fluid (CSF) AD biomarkers in this generation, but the original proband participant had low Aβ−42 and elevated tau in the CSF, levels typical for AD (Kumar et al., 2011). Neuropathologic data in the proband participant and the sibling were similar. Each had bilateral frontal atrophy with sparing of the medial temporal cortex. There were dense multicentric plaques in the cerebellar and frontal cortex, which were negative for Aβ40 and Aβ42 staining and were consistent with prion-associated beta-pleated sheet plaques. There were numerous tau-positive neurofibrillary tangles and neuropil threads in the neocortex as well (Kumar et al., 2011). Similar AD-like tau neurofibrillary tangle pathologic findings have been reported in other prion disorders (Giaccone et al., 1990; Jayadev et al., 2011; Poulter et al., 1992; Tranchant et al., 1997). We previously reported significant neocortical uptake of AV-1451 tau-PET tracer in all four of these participants, but PiB-PET did not demonstrate abnormally elevated signal (Jones et al., 2018). The pattern of tau uptake on participant III-I overlaps with a young-onset Alzheimer’s disease pattern, raising the question of similar brain network involvement (Dickerson, Wolk, & Initiative, 2011; Mendez, Lee, Joshi, & Shapira, 2012). Similarly, precuneus and posterior cingulate hypometabolism on FDG-PET in cases 1, 2, and 4 raise the possibility of an APOE4 effect on network-level changes in these regions (Machulda et al., 2011). APOE ε4 contributes to decreased amyloid clearance in AD and protein instability in other neurodegenerative diseases (Agosta et al., 2009; Mahley & Huang, 2012). The prion protein receptor has a high affinity for amyloid-beta (Kudo, Petersen, & Lee, 2013) and can trigger increased tau gene expression and tau hyperphosphorylation (Lauren, Gimbel, Nygaard, Gilbert, & Strittmatter, 2009; Um & Strittmatter, 2013). Other reports have suggested APOE ε4 and PRNP codon 129 polymorphisms increase the risk of both AD and Creutzfeldt-Jakob disease (Calero et al., 2011; Wei et al., 2014), but we are not aware of studies analyzing PRNP OBPIs and APOE ε4 interaction.
There are no known treatments for familial prion diseases currently. A gain of function mechanism is thought to drive disease in PRNP mutations and PrP knockout models in mice (Büeler et al., 1992), cows (Richt et al., 2007), and goats (Yu et al., 2009) prevented neurodegeneration without cognitive or behavioral deficits. From a large population-based study, four healthy elderly individuals harbored mutations causing truncated PRNP (Minikel et al., 2016) but detailed PrP levels in those individuals are unknown. Genetic editing and gene silencing are potential future treatment options for PRNP mutation carriers. Clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 is also a promising treatment for PRNP mutations (Kaczmarczyk, Mende, Zevnik, & Jackson, 2016; Mehrabian et al., 2014). Based on prior mouse model data, early intervention to potentially rescue neurons is important (Verity & Mallucci, 2011). Following future family members and similar patient cohorts with clinical and neuroimaging studies will be invaluable when genome editing and silencing becomes a treatment-based reality.
Acknowledgments
We thank the staff of the Mayo Clinic ADRC program for recruiting and following the participants longitudinally. We specifically thank the participants and their families for their involvement in aging and dementia research.
Funding Statement
This work was supported by the National Institutes of Health through our Mayo ADRC grant P50 AG016574.
Disclosure statement:
Mary M. Machulda, Ph.D. - receives funding from the National Institutes of Health.
Jonathan Graff-Radford, MD - receives funding from the National Institutes of Health
Val J. Lowe, MD - Dr. Lowe serves as a consultant for Bayer Schering Pharma, Philips Molecular Imaging, Piramal Imaging, and GE Healthcare and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the NIH (NIA, NCI), and the MN Partnership for Biotechnology and Medical Genomics.
Bradley F. Boeve, MD - has served as an investigator for clinical trials sponsored by Biogen and Alector. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from the NIH, the Mayo Clinic Dorothy, and Harry T. Mangurian Jr. Lewy Body Dementia Program and the Little Family Foundation.
References:
- Agosta F, Vossel KA, Miller BL, Migliaccio R, Bonasera SJ, Filippi M, … Gorno-Tempini ML (2009). Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer’s disease and frontotemporal dementia. Proc Natl Acad Sci U S A, 106(6), 2018–2022. doi: 10.1073/pnas.0812697106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C, Lee VM-Y, & Trojanowski JQ (2007). Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nature Reviews Neuroscience, 8(9), 663. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher K. d., Varney NR, & Spreen O (1994). Contributions to neuropsychological assessment: A clinical manual: Oxford University Press, USA. [Google Scholar]
- Bertram L, & Tanzi RE (2008). Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci, 9(10), 768–778. doi: 10.1038/nrn2494 [DOI] [PubMed] [Google Scholar]
- Biasini E, Turnbaugh JA, Unterberger U, & Harris DA (2012). Prion protein at the crossroads of physiology and disease. Trends Neurosci, 35(2), 92–103. doi: 10.1016/j.tins.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, … Stefani M. (2002). Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature, 416(6880), 507. [DOI] [PubMed] [Google Scholar]
- Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H-P, DeArmond SJ, … Weissmann C. (1992). Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature, 356(6370), 577–582. [DOI] [PubMed] [Google Scholar]
- Calero O, Bullido MJ, Clarimon J, Frank-Garcia A, Martinez-Martin P, Lleo A, … Calero M. (2011). Genetic cross-interaction between APOE and PRNP in sporadic Alzheimer’s and Creutzfeldt-Jakob diseases. PLoS One, 6(7), e22090. doi: 10.1371/journal.pone.0022090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R, Restelli E, Comerio L, Del Gallo F, & Imeri L. (2016). Transgenic mice recapitulate the phenotypic heterogeneity of genetic prion diseases without developing prion infectivity: Role of intracellular PrP retention in neurotoxicity. Prion, 10(2), 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, & Dobson CM (2006). Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem, 75, 333–366. [DOI] [PubMed] [Google Scholar]
- Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, … Lyoo CH (2016). In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol, 80(2), 247–258. doi: 10.1002/ana.24711 [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Wolk DA, & Initiative, A. s. D. N. (2011). Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. Journal of Neurology, Neurosurgery & Psychiatry, 82(1), 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccone G, Tagliavini F, Verga L, Frangione B, Farlow MR, Bugiani O, & Ghetti B. (1990). Neurofibrillary tangles of the Indiana kindred of Gerstmann-Strä ussler-Scheinker disease share antigenic determinants with those of Alzheimer disease. Brain research, 530(2), 325–329. [DOI] [PubMed] [Google Scholar]
- Golden CJ, & Freshwater SM (1978). Stroop color and word test. [Google Scholar]
- Head MW, Bunn TJ, Bishop MT, McLoughlin V, Lowrie S, McKimmie CS, … Knight R. (2004). Prion protein heterogeneity in sporadic but not variant Creutzfeldt–Jakob disease: UK cases 1991–2002. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 55(6), 851–859. [DOI] [PubMed] [Google Scholar]
- Heaton R, Miller S, Taylor MJ, & Grant I. (2004). Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Hughes CP, Berg L, Danziger W, Coben LA, & Martin RL (1982). A new clinical scale for the staging of dementia. The British journal of psychiatry, 140(6), 566–572. [DOI] [PubMed] [Google Scholar]
- Jayadev S, Nochlin D, Poorkaj P, Steinbart EJ, Mastrianni JA, Montine TJ, … Leverenz JB (2011). Familial prion disease with Alzheimer disease‐like tau pathology and clinical phenotype. Annals of neurology, 69(4), 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Townley RA, Graff-Radford J, Botha H, Knopman DS, Petersen RC, … Boeve BF (2018). Amyloid-and tau-PET imaging in a familial prion kindred. Neurology Genetics, 4(6), e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarczyk L, Mende Y, Zevnik B, & Jackson WS (2016). Manipulating the prion protein gene sequence and expression levels with CRISPR/Cas9. PloS one, 11(4), e0154604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo W, Petersen RB, & Lee HG (2013). Cellular prion protein and Alzheimer disease: link to oligomeric amyloid-beta and neuronal cell death. Prion, 7(2), 114–116. doi: 10.4161/pri.22848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Boeve BF, Boot BP, Orr CF, Duffy J, Woodruff BK, … Dickson DW (2011). Clinical characterization of a kindred with a novel 12-octapeptide repeat insertion in the prion protein gene. Arch Neurol, 68(9), 1165–1170. doi: 10.1001/archneurol.2011.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, & Strittmatter SM (2009). Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature, 457(7233), 1128–1132. doi: 10.1038/nature07761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, & Brentani RR (2008). Physiology of the prion protein. Physiol Rev, 88(2), 673–728. doi: 10.1152/physrev.00007.2007 [DOI] [PubMed] [Google Scholar]
- Machulda MM, Jones DT, Vemuri P, McDade E, Avula R, Przybelski S, … Jack CR (2011). Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Archives of neurology, 68(9), 1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Pankratz VS, Christianson TJ, Ivnik RJ, Mielke MM, Roberts RO, … Petersen RC (2013). Practice effects and longitudinal cognitive change in normal aging vs. incident mild cognitive impairment and dementia in the Mayo Clinic Study of Aging. The Clinical Neuropsychologist, 27(8), 1247–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, & Huang Y. (2012). Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron, 76(5), 871–885. doi: 10.1016/j.neuron.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallucci GR, White MD, Farmer M, Dickinson A, Khatun H, Powell AD, … Collinge J. (2007). Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron, 53(3), 325–335. [DOI] [PubMed] [Google Scholar]
- Mehrabian M, Brethour D, MacIsaac S, Kim JK, Gunawardana CG, Wang H, & Schmitt-Ulms G. (2014). CRISPR-Cas9-based knockout of the prion protein and its effect on the proteome. PLoS One, 9(12), e114594. doi: 10.1371/journal.pone.0114594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Lee AS, Joshi A, & Shapira JS (2012). Nonamnestic presentations of early-onset Alzheimer’s disease. American Journal of Alzheimer’s Disease & Other Dementias®, 27(6), 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minikel EV, Vallabh SM, Lek M, Estrada K, Samocha KE, Sathirapongsasuti JF, … MacArthur DG (2016). Quantifying prion disease penetrance using large population control cohorts. Sci Transl Med, 8(322), 322ra329. doi: 10.1126/scitranslmed.aad5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth P. (1944). Filetest de copie d’une figure complexe.[The test of a complex copied figure]. Archives de Psychologie, 30, 206–256. [Google Scholar]
- Parchi P, De Boni L, Saverioni D, Cohen ML, Ferrer I, Gambetti P, … Höftberger R. (2012). Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: an inter-rater study among surveillance centres in Europe and USA. Acta neuropathologica, 124(4), 517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC (2004). Mild cognitive impairment as a diagnostic entity. Journal of internal medicine, 256(3), 183–194. [DOI] [PubMed] [Google Scholar]
- Poulter M, Baker HF, Frith CD, Leach M, Lofthouse R, Ridley RM, … Crow TJ (1992). INHERITED PRION DISEASE WITH 144 BASE PAIR GENE INSERTION.1. GENEALOGICAL AND MOLECULAR STUDIES. Brain, 115, 675–685. doi: 10.1093/brain/115.3.675 [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, … Onyike CU (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richt JA, Kasinathan P, Hamir AN, Castilla J, Sathiyaseelan T, Vargas F, … Koster J. (2007). Production of cattle lacking prion protein. Nature biotechnology, 25(1), 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R, Light R, Parker S, & Levin H. (1996). Benton controlled oral word association test: Reliability and updated norms. Archives of clinical neuropsychology, 11(4), 329–338. [PubMed] [Google Scholar]
- Sakudo A, Lee DC, Nishimura T, Li S, Tsuji S, Nakamura T, … Onodera T. (2005). Octapeptide repeat region and N-terminal half of hydrophobic region of prion protein (PrP) mediate PrP-dependent activation of superoxide dismutase. Biochem Biophys Res Commun, 326(3), 600–606. doi: 10.1016/j.bbrc.2004.11.092 [DOI] [PubMed] [Google Scholar]
- Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A, … Serpell LC (2014). Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron, 82(6), 1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz M, Dittmar K, Llorens F, Gelpi E, Ferrer I, Schulz-Schaeffer WJ, & Zerr I. (2016). Hereditary human prion diseases: An update. Molecular Neurobiology, 1–12. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Angot E, & Brundin P. (2011). A deadly spread: cellular mechanisms of α-synuclein transfer. Cell Death & Differentiation, 18(9), 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EM, & Spreen O. (2006). A compendium of neuropsychological tests: Administration, norms, and commentary. In: New York: Oxford University Press. [Google Scholar]
- Tang-Wai DF, Knopman DS, Geda YE, Edland SD, Smith GE, Ivnik RJ, … Petersen RC (2003). Comparison of the short test of mental status and the mini-mental state examination in mild cognitive impairment. Archives of neurology, 60(12), 1777–1781. [DOI] [PubMed] [Google Scholar]
- Townley RA, Syrjanen JA, Botha H, Kremers WK, Aakre JA, Fields JA, … Jones DT (2019). Comparison of the Short Test of Mental Status and the Montreal Cognitive Assessment across the cognitive spectrum. Mayo Clinic Proceedings, 94(8), 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranchant C, Sergeant N, Wattez A, Mohr M, Warter J, & Delacourte A. (1997). Neurofibrillary tangles in Gerstmann-Sträussler-Scheinker syndrome with the A117V prion gene mutation. Journal of Neurology, Neurosurgery & Psychiatry, 63(2), 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, & Strittmatter SM (2013). Amyloid-beta induced signaling by cellular prion protein and Fyn kinase in Alzheimer disease. Prion, 7(1), 37–41. doi: 10.4161/pri.22212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity NC, & Mallucci GR (2011). Rescuing neurons in prion disease. Biochemical Journal, 433(1), 19–29. [DOI] [PubMed] [Google Scholar]
- Vital C, Gray F, Vital A, Parchi P, Capellari S, Petersen R, … Gambetti P. (1998). Prion encephalopathy with insertion of octapeptide repeats: the number of repeats determines the type of cerebellar deposits. Neuropathology and applied neurobiology, 24(2), 125–130. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1981). The Wechsler Adult Intelligence Scale-Revised (Manual) Psychological Corp. New York, NY. [Google Scholar]
- Wechsler D. (1987). Wechsler Memory Scale-Revised. San Antonio: Harcourt Brace Jovanovich. [Google Scholar]
- Wei Y, Tang Y, He W, Qu Z, Zeng J, & Qin C. (2014). APOE gene polymorphisms and susceptibility to Creutzfeldt–Jakob disease. Journal of Clinical Neuroscience, 21(3), 390–394. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, & Robertson GJ (2006). Wide range achievement test (WRAT4). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Yu G, Chen J, Xu Y, Zhu C, Yu H, Liu S, … Wu Y. (2009). Generation of goats lacking prion protein. Molecular Reproduction and Development: Incorporating Gamete Research, 76(1), 3–3. [DOI] [PubMed] [Google Scholar]