Abstract
Pine rosin (colophony) has been identified as a potentially new adulterant in cannabis oil. Its inhalation toxicity poses a significant health concern to users. For example, pine rosin fumes are released during soldering, and have been cited as a causative agent of occupational asthma. Symptoms also include desquamation of bronchial epithelium, which has also been observed in e-cigarette or vaping product used-associated lung injury (EVALI) patients. The sample analyzed herein was acquired from a cannabis industry source, also contains medium chain triglycerides and oleamide, the latter of which is a hypnotic that is commonly found in the synthetic marijuana product Spice, or K2. A combination of proton nuclear magnetic resonance (1H NMR) and high pressure liquid chromatography-electrospray ionization mass spectrometry (HPLC-ESIMS) was used to unambiguously identify major pine rosin ingredients such as abietic and other resin acids. Comparison to commercial samples of pure pine rosin confirmed the assignment.
Keywords: Cannabis extract, BHO, marijuana, EVALI, rosin, pine rosin, adulterant, cutting agent
Introduction
Since the legalization of medical marijuana in California in 1996, and the legalization of recreational marijuana in Colorado in 2012, 33 states and the District of Colombia have medical cannabis programs, and 10 states and the District of Colombia have fully legalized recreational use as of 2020.[1] Canada first enacted medical marijuana laws in 2001, and now has recreational cannabis as of 2018.[2] With the passage of more lax laws, cannabis extracts (CEs) have surged in popularity as alternative products to cannabis flower, with expenditures on CEs in the legal Washington state cannabis market increasing 145 % between 2014 and 2016.[3] CEs are consumed by inhalation using modified e-cigarettes or via dabbing[4], and increased usage of these among teens and young adults[5] has led to concerns of safety, as up to 11 % of high schooler students[6] report lifetime use of a cannabis vaporizer.
CEs may be consumed via inhalation by three main methods/devices: cartridge vaporizers (CVs), top-loading vaporizers (TLVs), and dabbing.[4] In dabbing, a small amount of CE is placed on a hot surface (i.e. a “nail,” which may be heated with a blow torch or electrically) that is connected to a water pipe.[4, 7] A TLV is an electronic vaporizer device that consists of a battery-powered resistive heating coil in an atomizer, upon which a user manually places small amounts of CE.[4] Disposable CV devices closely mimic nicotine e-cigarettes, and have surged in popularity given their ease of use and discretion, with sales of these increasing more than 10-fold to $224 million in Colorado as of 2018.[8]
The cannabis concentrate hashish, commonly consumed in Europe from illicit manufacturers in North Africa, has an extensive history of containing adulterants.[9] A recent analysis of hashish in Madrid found that 18 % suffers from contamination with glucose, sucrose, and/or abietic acid (a principal component of pine rosin).[10] Pine rosin has also been identified as a hashish adulterant in Italy,[11] Israel, and the Czeck Republic.[12]
CEs available in North America are generally manufactured via solvent extraction (most commonly with butane, though propane or supercritical CO2 have widespread usage) followed by several refinement steps. Butane hash oil (BHO), propane hash oil (PHO) and CO2 oil may all adopt one of several names depending on consistency: shatter, wax, crumble, budder, or pull-nsnap.[7] Recently, applied heat and pressure has been used to press cannabis oils from flower to make a product known as rosin.[13] Despite the similarity in naming, cannabis rosin and pine rosin share few chemical similarities.[13]
Cases of adulteration in North American cannabis products have only recently come into view. The synthetic cannabinoid 5-MDMB-PINACA and the antitussive dextromethorphan have been identified in certain commercially available cannabidiol e-liquids for CV devices.[14] Online reports on Reddit.com and cannabis websites have become grounds where users have aired complaints of BHO adulterated with pine rosin, and have cited specific brands and products as bad actors.[15–17] The timing of these forum posts about pine rosin being used as an adulterant for CEs, or as counterfeit BHO, coincide with the EVALI outbreak. Additionally, several recent patents mention methyl ester of rosin, a pine rosin derivative, as a potential additive to cannabis vaporizers.[18–20]
CEs added to CV devices often require fluidizing agents to ensure better wicking efficiency in the atomizer of a vape pen, given the high viscosity of cannabis extracts.[4] Substances such as terpenes, medium chain triglyceride (MCT) oil, and phytol, among others are commonly used.[21] One CE additive to CV devices, vitamin E acetate (VEA), has been linked with the recent outbreak of e-cigarette, or vaping, product use associated lung injury (EVALI).[22] It’s use as a thickening agent has been suggested, however, the markedly lower viscosity of VEA relative to Δ9-tetrahydrocannabinol (THC), indicates that the former is used to dilute CEs, and that a different additive is the thickening agent, which is introduced to give the appearance of unadulterated CE. Herein is the first report of an adulterant containing pine rosin (a.k.a. rosin colophony or pine resin) for cannabis CV devices. The adulterant was acquired from a formulations consultant that works in the cannabis vaporizer formulations space, which itself acquired the adulterant from cannabis CV device manufacturer.
Materials and Methods
Two adulterants were donated by Vialpando LLC. Initial analysis by nuclear magnetic resonance spectroscopy (NMR) identified one of them to be pure VEA, while the other (Figure 1, dubbed cannabis extra adulterant [CEA]) required further analysis for identification. The CEA was initially assayed by GC-MS, which first suggested the presence of substituted abietanes and pimaranes. Analysis of the NMR spectrum showed peaks in the alkenyl region that are known to be characteristic of the resin acids in question,[23] and the characteristic glycolic methylene peaks from a triglyceride (Figure S1). 2D NMR techniques COSY and NOESY aided the confirmation of the identity of different isomeric resin acids, as well as the identification of communic acid, which was aided by semi-preparative HPLC. An HPLC-ESIMS chromatogram of CEA provided confirmation of the abietane and pimarane molecules and oleamide (Figure S2). Oleamide is not directly visible in the NMR spectrum of CEA, but the amide N-H protons are visible in the semi-preparative HPLC fraction that contains it when this is dissolved in DMSO-d6 (Cambridge Isotope Laboratories), which was spiked with a pure standard of oleamide (TCI America) to confirmed its presence (Figure S3). Commercially available medium chain triglyceride (MCT) oil (Nature’s Way) was spiked in a CEA NMR sample (Figure S4). An approximate %mass of each identified component was determined by quantitative NMR (Q-NMR).[24] See the supplementary appendix for further experimental details.
Figure 1:
Cannabis extract thickener provided in a glass syringe.
Results and Discussion
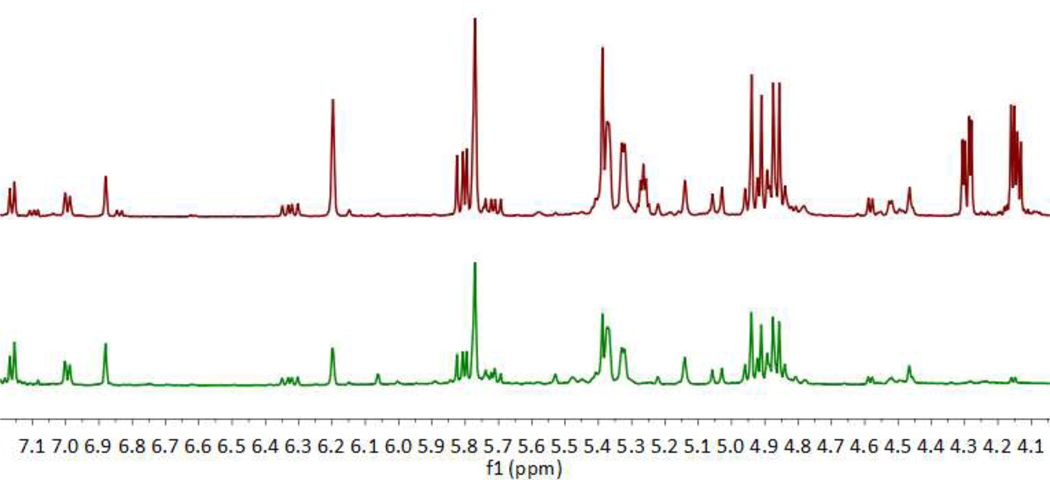
The analytical methods used discovered that the unknown CEA contains resin acids consistent with pine rosin (68 %), MCT oil (15 %), and small amounts of oleamide (Table 1). An overlay of a commercially available sample of gum rosin (Sigma Aldrich) and CEA demonstrates the similarity of these two substances (Figure 2), with the major visible difference being the presence of the triglyceride peaks from MCT oil in the CEA. Rosin, a solid at room temperature, appears to have been amended with MCT oil to thin its consistency to allow extrusion from a syringe, making its final appearance very similar to pure THC or clarified cannabis extract. For the purposes of this study, only approximate quantification was necessary to determine the composition of the sample. Given that this adulterant is destined for use in cannabis e-cigarettes, it is unknown how the final matrix will affect identification and quantification of resin acids in a black market sample. The analytical methods presented herein may serve as a guide for identifying resin acids in a cannabis sample, but a more comprehensive quantitative method will need to be developed for cannabis extracts adulterated with pine rosin and/or oleamide.
Table 1:
Components identified in CEA by nuclear magnetic resonance (NMR) spectroscopy and HPLC-ESIMS, and approximate %masses in the sample were determined by Q-NMR.
| Common Name | CAS Number | RT in LC/MS (min.) | NMR Shift (ppm) | Mass Accuracy (ppm) | % in Sample |
|---|---|---|---|---|---|
| Dehydroabietic acid | 1740-19-8 | 16.5 | 6.88 | 0.03 | 3 |
| Communic acid | 2761-77-5 | 21.8 | 6.32 | 0.03 | 4 |
| Pimarol | 1686-59-5 | 23.9 | NA | 0.52 | NA |
| Pimaric acid | 127-27-5 | 23.9 | 5.71 | 1.25 | 3.2 |
| Sandaracopimaric acid | 471-74-9 | 23.9 | 5.22 | 1.25 | 1.5 |
| Palustric acid | 1945-53-5 | 23.9 | 5.39 | 1.25 | 14 |
| Abietic acid | 514-10-3 | 25.1 | 5.77 | 1.25 | 17 |
| Oleamide | 301-02-0 | 25.1 | 6.65-7.19 | 0.64 | NA |
| Neoabietic acid | 471-77-2 | 25.1 | 6.2 | 1.25 | 12 |
| Isopimaric acid | 5835-26-7 | 25.1 | 5.81 | 1.25 | 13 |
| Sandaracopimarinal | 3855-14-9 | 30.3 | 5.22 | 0 | NA |
| MCT oil | 438544-49-1 | NA | 4.3 | NA | 15 |
Figure 2:
Overlaid 1H NMR spectra of CEA (top, maroon) and commercially-available gum rosin (bottom, green) from Sigma Aldrich (CAS no. 8050-09-7).
Rosin is a known respiratory tract irritant and a significant contributor to occupational asthma due to its use in soldering.[25] Occupational exposure to pine rosin vapor from solder flux at levels of 50 μg/m3, the 8-h Time Weighted Average (TWA) exposure limit, has not been known to produce sever acute lung injuries.[25] However, CEA added to CE at a level of just 1 % will produce nearly 0.6 g/m3 of pine rosin in the aerosol from a cannabis vaporizer pen with each puff, or ~3,500 times the 15-min TWA exposure limit.[25] In vivo exposure of abietic acid to rat lungs produced desquamation of bronchial epithelium,[26] which has also been reported in EVALI cases.[27] We are unaware of efforts to date to test for pine rosin compounds in samples from patients with vaping-induced lung injuries. Oleamide appears to have been added to increase the psychoactivity of resulting adulterated CE, as this compound is a cannabinoid receptor agonist and sleep-inducing agent.[28] Interestingly, oleamide is a common additive to synthetic cannabinoid “Spice” mixtures.[29] It is unknown what, if any, are the health effects of inhaling oleamide. Oleamide is also mentioned as a potential additive to vaping formulations in a patent registered to a cannabis vaporizer formulations company.[30]
Conclusion
The use of pine rosin as an adulterant in cannabis oil has not been previously reported in the scientific literature. It is available through online vendors, typically used as an ingredient in industrial products such as varnishes, adhesives, soldering fluxes and sealing wax. It has significant inhalation toxicity. To date, there are no reports of testing for this substance in cannabis oil samples from patients with lung injury. Due to the significant toxicity and prevalence based on social media posts, regulators and laboratory personnel should be aware of its use in adulterated cannabis oil.
Supplementary Material
Highlights.
Pine rosin was identified as component in a sample of cannabis extract adulterant.
The inhalation toxicology of pine rosin is well-studied.
Current analytical methods may misidentify pine rosin as an additive.
The prevalence of this additive is unknown.
Acknowledgments
We thank the NIH and the FDA for their support via award R01ES025257. Content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA. We would like to acknowledge NSF grant #0741993 for the HPLC-ESIMS data collection. We would like to thank the laboratory of Dr. James Pankow for assistance with acquiring GCMS data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].State Info. <https://norml.org/states>, (accessed January, 8.2020). [Google Scholar]
- [2].Cannabis laws and regulations. <https://www.canada.ca/en/health-canada/services/drugsmedication/cannabis/laws-regulations.html>, (accessed January, 8.2020). [Google Scholar]
- [3].Smart R, Caulkins JP, Kilmer B, Davenport S, Midgette G, Variation in cannabis potency and prices in a newly legal market: evidence from 30 million cannabis sales in Washington state, Addiction 112(12) (2017) 2167–2177. 10.1111/add.13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Meehan-Atrash J, Luo W, McWhirter KJ, Strongin RM, Aerosol Gas-Phase Components from Cannabis E-Cigarettes and Dabbing: Mechanistic Insight and Quantitative Risk Analysis, ACS Omega 4(14) (2019) 16111–16120. 10.1021/acsomega.9b02301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].A.A.L. Knapp DC; Borodovsky JT; Auty SG; Gabrielli J; Budney AJ, Emerging Trends in Cannabis Administration Among Adolescent Cannabis Users, J Adolesc Health 64 (2019) 487493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Morean ME, Kong G, Camenga DR, Cavallo DA, Krishnan-Sarin S, High School Students’ Use of Electronic Cigarettes to Vaporize Cannabis, Pediatrics 136(4) (2015) 611–616. 10.1542/peds.2015-1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meehan-Atrash J, Luo W, Strongin RM, Toxicant formation in dabbing: the terpene story, ACS Omega 2 (2017) 6112–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cannabis Vaping: Opportunities in an Uncertain Future, Executive Summary, Arcview Market Research in partnership with BDS Analytics, bdsanalytics.com, 2019. [Google Scholar]
- [9].Chouvy PA, The Supply of Hashish to Europe, European Monitoring Center for Drugs and Drug Addiction, Lisbon, Portugal, 2015. [Google Scholar]
- [10].Perez-Moreno M, Perez-Lloret P, Gonzalez-Soriano J, Santos-Alvarez I, Cannabis resin in the region of Madrid: Adulteration and contamination, Forensic Sci Int 298 (2019) 34–38. 10.1016/j.forsciint.2019.02.049 [DOI] [PubMed] [Google Scholar]
- [11].Caligiani A, Palla G, Bernardelli B, GC-MS analysis of hashish samples: a case of adulteration with colophony, J Forensic Sci 51(5) (2006) 1096–100. 10.1111/j.1556-4029.2006.00202.x [DOI] [PubMed] [Google Scholar]
- [12].Hanuš LO, De La Vega D, Roman M, Tomíček P, False hashish without cannabis resin, Isr. J. Plant Sci. 62(4) (2015) 277–282. 10.1080/07929978.2015.1053202 [DOI] [Google Scholar]
- [13].Lamy FR, Daniulaitye R, Zathred M, Nahhas RW, Sheth A, Martins SS, Boyer EW, Carlson RG, “You got to love rosin: Solventless dabs, pure, clean, natural medicine.” Exploring Twitter data on emerging trends in Rosin Tech marijuana concentrates, Drug Alcohol Depend 183 (2018) 248–252. 10.1016/j.drugalcdep.2017.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Poklis JL, Mulder HA, Peace MR, The unexpected identification of the cannabimimetic, 5F-ADB, and dextromethorphan in commercially available cannabidiol e-liquids, Forensic Sci Int 294 (2019) e25–e27. 10.1016/j.forsciint.2018.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fake shatter - Pine Resin cut hit Canada? <https://future4200.com/t/fake-shatter-pineresin-cut-hit-canada/44648>, 2019. (accessed November 29, 2019.). [Google Scholar]
- [16].The dabs of death aka pine resin. <https://www.reddit.com/r/CanadianMOMs/comments/dd9frt/the_dabs_of_death_aka_pine_resin_aka_that_shit/>, 2019. (accessed November 29, 2019.). [Google Scholar]
- [17].Pine resin and vitamin e acetate scare. <https://www.reddit.com/r/CanadianMOMs/comments/df3n2k/pine_resin_and_vitamin_e_acetate_scare/>, 2019. (accessed November 29, 2019.). [Google Scholar]
- [18].Cameron JD, Natural-based liquid composition and electronic vaporizing devices for using such composition, LunaTech, LLC, USA, 2017. [Google Scholar]
- [19].Cameron JD, Water-based vaporizable liquids, methods and systems for vaporizing same, Lunatch, LLC, United States, 2017. [Google Scholar]
- [20].Cameron JD, Becker D, Fein G, Liquid composition containing nicotine from non-tobacco source for use with electronic vaporizing devices, Lunatech, LLC, United States, 2017. [Google Scholar]
- [21].Erickson BE, Cannabis industry gets crafty with terpenes, Chemical and Engineering News, American Chemical Society, cen.acs.org, 2019. [Google Scholar]
- [22].CDC, Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. <https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html>, 2019. (accessed November 27, 2019.). [Google Scholar]
- [23].Ioannidis K, Melliou E, Magiatis P, High-Throughput (1)H-Nuclear Magnetic Resonance-Based Screening for the Identification and Quantification of Heartwood Diterpenic Acids in Four Black Pine (Pinus nigra Arn.) Marginal Provenances in Greece, Molecules 24(19) (2019). 10.3390/molecules24193603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bharti SK, Roy R, Quantitative 1H NMR spectroscopy, TrAC Trends in Analytical Chemistry 35 (2012) 5–26. 10.1016/j.trac.2012.02.007 [DOI] [Google Scholar]
- [25].Baldwin PEJ, Cain JR, Fletcher R, Jones K, Warren N, Dehydroabietic acid as a biomarker for exposure to colophony, Occup. Med. 57 (2007) 362–66. [DOI] [PubMed] [Google Scholar]
- [26].Ayars GH, Altman LC, Frazier CE, Chi EY, The toxicity of constituents of cedar and pine woods to pulmonary epithelium, J. Allergy Clin. Immunol 83(3) (1989) 610–18. [DOI] [PubMed] [Google Scholar]
- [27].Butt YM, Smith ML, Tazelaar HD, Laslo TV, Swanson KL, Cecchini MJ, Boland JM, Bois MC, Boyum JH, Froemming AT, Khoor A, Mira-Avendano I, Patel A, Larsen BT, Pathology of Vaping-Associated Lung Injury, NEJM 381(18) (2019). 10.1056/NEJMc1913069 [DOI] [PubMed] [Google Scholar]
- [28].Leggett JD, Aspley S, Beckett SRG, D’Antona AM, Kendall DA, Kendall DA, Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors, Brit J Pharmacol 141 (2004) 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fattore L, Fratta W, Beyond THC: The New Generation of Cannabinoid Designer Drugs, Front Behav Neurosci 5 (2011) 60 10.3389/fnbeh.2011.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Elzinga S, Raber JC, Terpene-based compositions, processes, methodologies for creation and products thereby, The Werc Shop LLC, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.