Abstract
Atopic dermatitis (AD) is commonly associated with colonization by Staphylococcus aureus in the affected skin. To understand the role of S. aureus in the development of AD, we performed whole genome sequencing of S. aureus strains isolated from the cheek skin of 268 Japanese infants 1 month and 6 months after birth. About 45% of infants were colonized with S. aureus at 1 month regardless of AD outcome. In contrast, skin colonization with S. aureus at 6 months of age increased the risk of developing AD. Acquisition of dysfunctional mutations in the S. aureus Agr quorum-sensing system was observed primarily in strains from 6-month-old infants who did not develop AD. Expression of a functional Agr system in S. aureus was required for epidermal colonization and the induction of AD-like inflammation in mice. Thus, retention of functional S. aureus agr virulence during infancy is associated with pathogen skin colonization and the development of AD.
One Sentence Summary:
Skin colonization at six months of age by S. aureus with functional Agr quorum sensing associates with later atopic dermatitis development in infants.
Introduction
The epidermis, the outermost layer of the skin, plays a critical role in maintaining the barrier function of the skin and protecting the body against invasion by harmful microbes (1). A leading cause of infection originating in the skin is Staphylococcus aureus, a Gram-positive bacterium, which normally resides on the skin surface of 5–10% of healthy individuals (2). Although S. aureus can reside on normal skin, the bacterium is responsible for greater than 70% of all skin and soft tissue infections and is a leading cause of systemic infection (3). The mechanisms by which resident S. aureus become virulent pathogens on the surface of the skin remain poorly understood (4).
S. aureus possesses several gene regulators that control the production of virulence factors (5). One major S. aureus virulence program is the accessory gene regulatory (Agr) quorum-sensing (QS) system that coordinates cell behavior in response to bacterial density (6). Upon binding of the auto-inducing peptide (AIP) to the receptor kinase AgrC, the response regulator AgrA is activated and binds to the P2 and P3 promoters, leading to transcriptional activation of the agrBDCA operon and the regulatory small RNA RNAIII, respectively (6). In turn, RNAIII induces the expression of a broad array of virulent factors including toxins and enzymes that regulate the growth and adaptation of the pathogen at sites of infection (6). In addition, AgrA activates the promoters of genes encoding phenol-soluble modulins (PSMs), a group of cytotoxic peptides that are important for the outcome of community-associated methicillin-resistant S. aureus (MRSA) infections (7). In the skin, Agr-regulated cytotoxic PSM peptides induce keratinocyte damage, leading to the release of alarmins and triggering of skin inflammation after epidermal colonization (8, 9). In addition, epicutaneous administration of purified AIP or inoculation with commensal Staphylococci including S. caprae can inhibit S. aureus skin colonization and intradermal infection by blocking Agr quorum sensing via non-cognate AIP-mediated interference (10). Furthermore, Agr virulence confers increased colonization ability compared to agr mutants in the intradermal skin model of infection (11). However, the role of the Agr system in epidermal colonization of S. aureus remains unclear.
Atopic dermatitis (AD; OMIM 603165) is a chronic or recurrent inflammatory skin disease that affects 15–20% of children and ~ 2–5% of adults in industrialized countries (12). AD usually begin in early childhood with ~ 60% of patients developing the disease in the first 12 months of life (13). The pathogenesis of AD is poorly understood, but both environmental and genetic factors are thought to contribute to the development of disease (12). Notably, ~ 80% of patients with AD are colonized with S. aureus on the epidermis of the lesional skin whereas the great majority of healthy individuals older than 2 years do not harbor the pathogen in the skin (14, 15). Furthermore, increased S. aureus loads in affected skin correlate with disease flares (16). S. aureus virulence factors have been proposed to contribute to AD pathogenesis (17). However, the role of S. aureus in disease pathogenesis and that of virulence genes in skin colonization and AD development remain unclear. To understand the role of S. aureus colonization and the genetic factors of S. aureus that are important for AD, we analyzed the colonization of S. aureus and performed whole-genome sequencing (WGS) of S. aureus strains isolated from the cheek skin of 268 Japanese infants at 1 month and 6 months of age before disease onset and monitored the infants for the development of AD. Analyses of S. aureus strains isolated from infant skin and animal experiments with S. aureus mutants revealed a critical role for Agr virulence in epidermal colonization and an association of the Agr system with the development of AD in Japanese infants.
Results
Increased S. aureus skin colonization at 6 months of age is associated with AD development
To study the role of S. aureus in the development of AD, we first assessed the degree of S. aureus colonization in the skin of the cheek of 268 Japanese infants from the Chiba area (Chiba cohort) at 1 month and 6 months of age before disease onset and monitored the infants for the development of AD until the age of 2 years. Unlike the great majority of healthy adults who are not colonized with S. aureus (18), the skin of 45% of the infants at 1 month and 49% at 6 months of age was colonized with S. aureus (Table 1). Skin colonization with S. aureus at 6 months, but not at 1 month of age, was associated with increased risk for developing AD at 1 and 2 years of age (odds ratio [OR]: 5.433, p<0.001 and OR: 4.670, p<0.001; Table 1). S. aureus skin colonization in infants who did not develop AD was reduced at 6 months compared to that observed at 1 month of age (Fig. 1A). In contrast, S. aureus skin colonization was comparable at 1 and 6 months of age amongst infants who developed AD (Fig. 1A). Furthermore, increased S. aureus colonization in infant skin at 6 months, but not at 1 month, was associated with the presence of transient eczema at 6 months in infants who developed AD by 1 year of age (Fig. 1B). In addition, increased S. aureus colonization at 6 months was associated with the development of AD at 1 and 2 years of age (Fig. 1C). These results indicate that increased S. aureus skin colonization at 6 months of age correlates with AD development.
Table 1.
Skin S. aureus colonization at 1 and 6 months and development of AD in Chiba cohort.
| S. aureus positive frequency | 1-year old | adjusted odds ratio (95%CI) | p value | ||
|---|---|---|---|---|---|
| AD cases | AD frequency | ||||
| 1-month old | 0.883 | ||||
| negative (n=147) | 20 | 13.6% | 1 | ||
| positive (n=121) | 45.1% | 21 | 17.4% | 1.056 (0.514–2.169) | |
| 6-month old | <0.001 | ||||
| negative (n=135) | 8 | 5.9% | 1 | ||
| positive (n=133) | 49.6% | 33 | 24.8% | 5.433 (2.361–12.504) | |
| S. aureus positive frequency | 2-year old | adjusted odds ratio (95%CI) | p value | ||
| AD cases | AD frequency | ||||
| 1-month old | 0.226 | ||||
| negative (n=139) | 23 | 16.5% | 1 | ||
| positive (n=116) | 45.5% | 16 | 13.8% | 1.609 (0.745–3.476) | |
| 6-month old | <0.001 | ||||
| negative (n=130) | 9 | 6.9% | 1 | ||
| positive (n=125) | 49.0% | 30 | 24.0% | 4.670 (2.048–10.648) | |
Fig. 1. Increased S. aureus colonization in infant cheek skin at 6 months is associated with AD development.
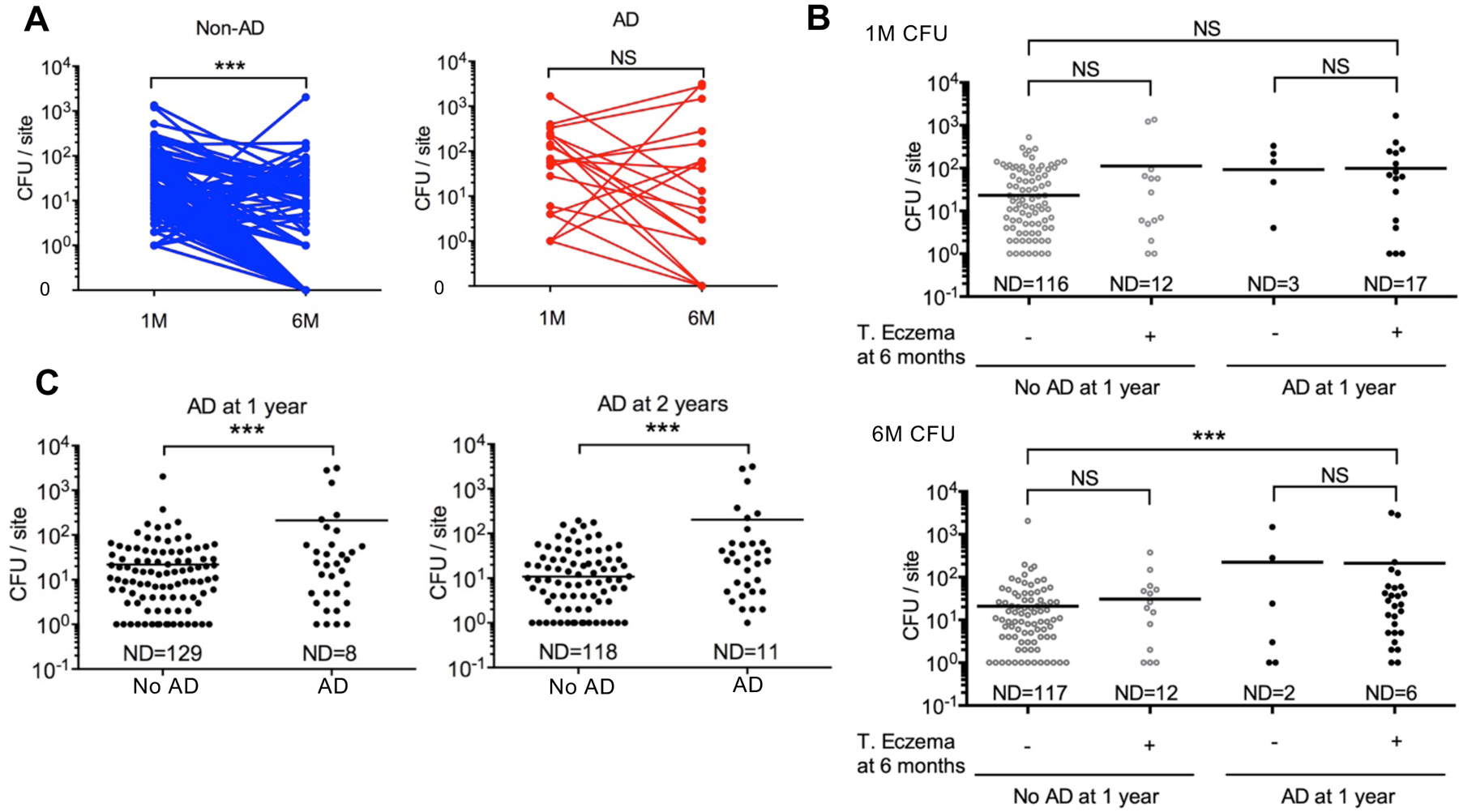
A, S. aureus colony-forming units (CFU) in the cheek skin (CFU per site) of infants at 1-month (1M) and 6-month (6M) of age. Infants were colonized with S. aureus at 1 month. Lines indicate samples from the same infant. Blue indicates infants who did not develop AD (No AD), red indicates infants who developed AD (AD). ***p<0.001, Two-tailed Wilcoxon signed rank test. B, Each dot represents the number of CFU per site (cheek skin) from an infant at 1 month (1M) or 6 months (6M). Infants who developed and did not develop AD at 1 year were subgrouped by the presence of unclassified transient eczema (T. eczema) at 6 months. Transient eczema was defined by the presence of eczema symptoms for less than 2 months. ***p<0.001, Kruskal-Wallis test with Dunn’s post hoc test. . C, S. aureus CFUs in the cheek skin. Each dot represents results from an infant at 6 months. Number of CFUs/per site in infants who did or did not develop AD at 1 year and 2 years of age. ***p<0.001, Mann-Whitney test. ND, not detected; NS, not significant..
Mutations in FLG, the gene encoding profilaggrin/filaggrin, predispose to AD in Western populations and adult Japanese patients (19, 20). In contrast, only 7.9% of children from Ishigaki island and 7.8% of primary school children in the Chiba area of Japan carried FLG mutations that were not associated with AD (21, 22). Consistent with these studies, ~7% of Japanese infants in our Chiba cohort had FLG mutations, but FLG mutations were not associated with either S. aureus skin colonization or AD development (fig. S1).
The phylogenic diversity of S. aureus strains that colonize the skin is comparable in infants who develop and do not develop AD
To understand the genetic factors of S. aureus that are important for skin colonization and development of AD, we performed WGS of S. aureus strains isolated from the cheek skin of 268 Japanese infants from the Chiba cohort (fig. S1). The bacterial genomes of 242 S. aureus strains isolated from infant skin at 1 month and 6 months of age before disease onset were analyzed to identify single nucleotide polymorphisms (SNPs) that may associate with skin colonization and development of AD. We constructed a rooted neighbor-joining tree using SNPs in the core genome of the 242 S. aureus isolates and performed phylogenetic analysis based on traditional multilocus sequence typing (MLST) designations. These analyses revealed S. aureus sequence type (ST) 8 (ST8), ST188 belonging to the Clonal complex (CC), 1 and ST15 as frequent colonizers of the infant skin in our Chiba cohort (Fig. 2, fig. S3A and S3B). However, ST designation was not correlated with either time of S. aureus colonization or AD development (fig. S3A).
Fig. 2. Global phylogeny and clonal relationships of 242 S. aureus strains isolated from infant skin at 1 and 6 months after birth.
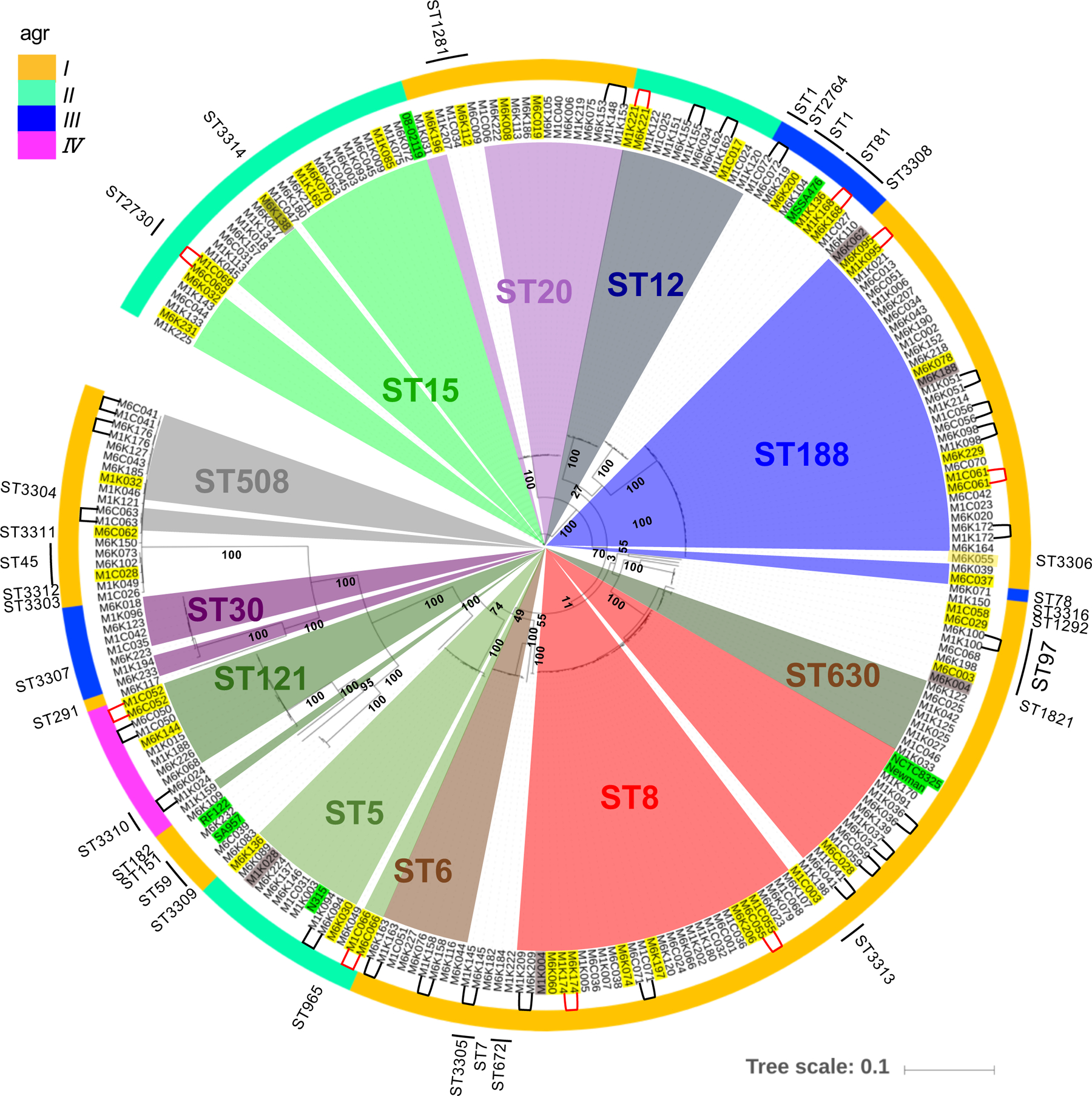
Rooted neighbor-joining tree constructed using SNPs in the core genome of S. aureus isolates. Innermost colored pie shapes and outermost labeling indicate ST types. Outer-colored circle indicates Agr subtypes. The same strain name denotes isolation from the same infant at 1 month (M1) and 6 month (M6). Yellow shaded text: infants who develop AD. Gray shaded text: infants who dropped out from the study. Green shaded text: reference S. aureus strains. Black or red line-connected strain names: continuous colonizer clones in infants who did not develop AD (black) or developed AD (red).
S. aureus produce a broad array of virulence factors that are important for pathogen colonization, binding to host proteins, evasion of immune responses, and antibiotic resistance (23). Based on allelic variation in the Agr system, S. aureus strains can be clustered into four Agr types, Agr I–IV, that secrete distinct AIPs (24). Consistent with previous studies (24), strains with the same MLST designation clustered into one Agr type with Agr I being the most commonly observed in strains isolated from the skin of infants at 1 month and 6 months of age (Fig. 2, fig. S3B–C). Notably, Agr type was not associated with AD development (fig. S3C). In addition, the presence of multiple virulence genes in the genome of S. aureus strains isolated at 1 or 6 months was not associated with AD development (fig. S4). Collectively, the results suggest that the phylogenic diversity of S. aureus strains that colonize the skin is not different between infants who do or do not develop AD.
Increased presence of S. aureus harboring agrC mutations in the skin of infants who do not develop AD
To assess the acquisition of mutations in the S. aureus genome during skin colonization, we identified S. aureus strains that were “continuous colonizers”, that is, the same clone was obtained from the same infant at 1 and 6 months of age. To identify continuous colonizer clones, we analyzed infant-isolated S. aureus strains based on pairwise genomic differences in closely related ST clades shown in the rooted neighbor-joining tree for deeply classifying the strains into clones. Based on pairwise genetic distance analysis, we identified continuous colonizer clones by the presence of less than 60 genomic differences in the core genome (fig. S5). These clone pairs collected at 1 month and 6 months clustered on the same clade in a rooted neighbor-joining tree (Fig. 2). We identified 24 infants who did not develop AD and 9 infants who developed AD at 1 year and harbored continuous colonizer clones at 6 months of age (Fig. 3A and fig. S5). We identified a higher percentage of continuous colonizer clone pairs in the skin of infants who developed AD (9 clones from 17 cases, 52.9%) than in infants who did not develop AD (24 clones from 86 cases, 27.9%) (Fig. 3A). To investigate the evolution of the S. aureus genome during skin colonization, we analyzed SNPs in the whole genome of continuous colonizer clones isolated at 6 months compared to those present in their paired clones isolated at 1 month. Most mutations were nonsyonymous, though the ratio of nonsynonmous mutations to synonymous mutations did not significantly correlate with AD development (N/S ratio: non-AD vs AD, p=0.6890, Fisher’s Exact Test) (Fig. 3A). Further analysis of the whole genome of continuous colonizer clones revealed that the agr region was associated with differential acquisition of mutations in the skin of infants who developed or did not develop AD. In continuous colonizer clones, the mutation rate in agr loci per 5 months of skin colonization (4.71 × 10−5 mutations per site) was significantly higher than that observed in the whole genome (~ 2–4 × 10−6 mutations per site) only in non-AD infants (p = 0.0002, Fisher’s Exact Test, Fig. 3B). In continuous colonizer clones, there were more mutations acquired at 6 months of age in the agr operon than in other operons in the genome, in particular mutations that changed the protein sequence or likely altered protein expression (fig. S6 and Data file S1). These included intragenic and frameshift mutations in the agr operon, although we could not perform statistical analysis due to the small number of clones examined. In continuous colonizer clones isolated at 6 months from non-AD infants, two clones (M6K051 and M6K155) acquired frameshift mutations in the cell-surface receptor agrC whereas two other clones (M6C056 and M6C059) acquired mutations within regulatory RNAIII and upstream of agrB, respectively (Fig. 3C).
Fig. 3. Increased frequency of agr mutations in S. aureus strains from infants who do not develop AD.
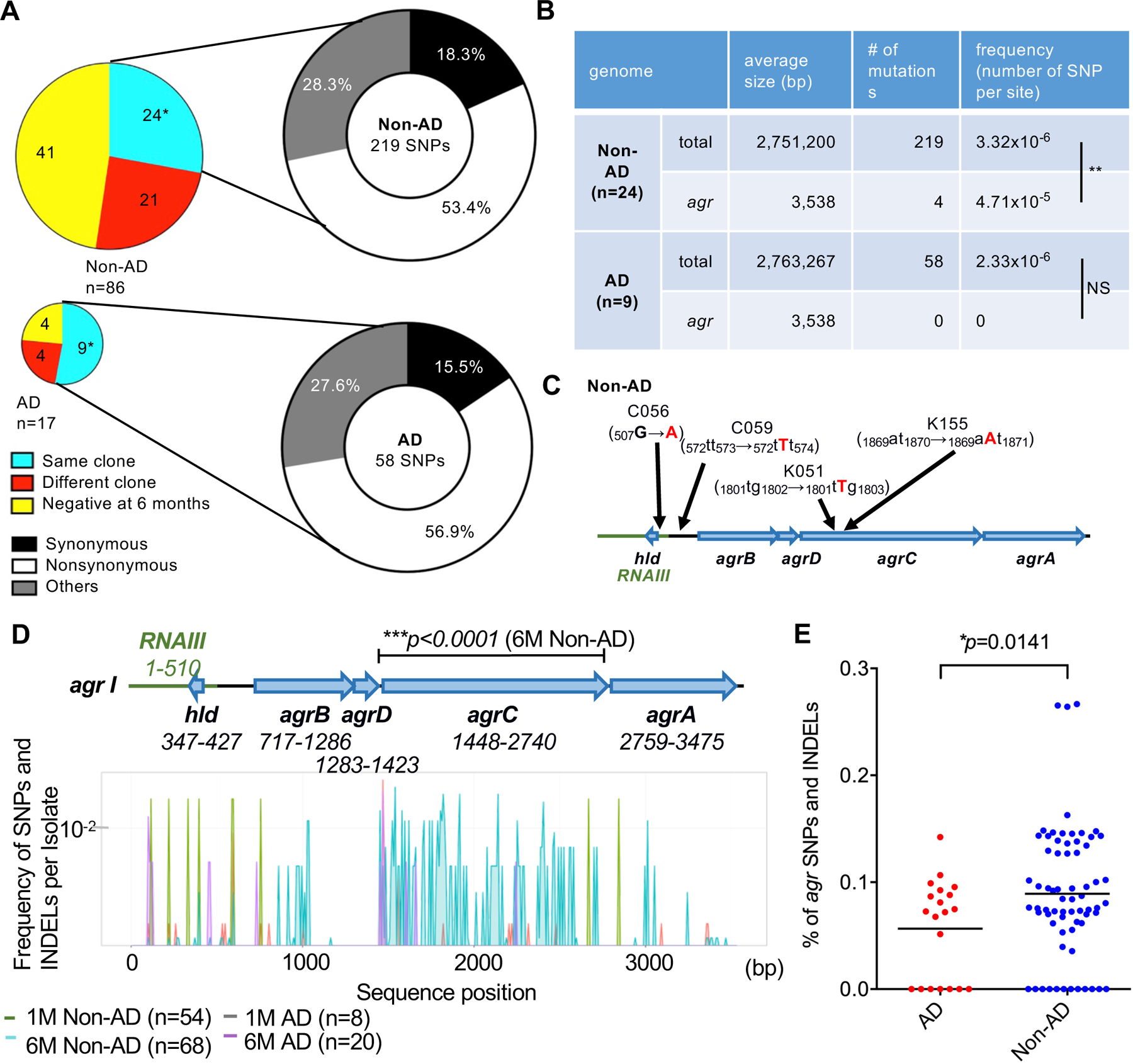
A, Colored pie charts on the left show number of infants colonized with the same S. aureus clone, different S. aureus clones, and no S. aureus clones (negative) at 6 months in infants colonized with S. aureus at 1 month. On the right, pie charts show the percentage of clones with high ratios (>1) of synonymous/nonsynonymous, nonsynonymous/synonymous, and other mutations from infants colonized with the same S. aureus clone at 1 and 6 months. *p<0.05, Chi-squared test. B, Total number and frequency of SNPs per site acquired during 6 months of skin colonization in non-AD and AD infants. NS; not significant, **p<0.01, Fisher’s exact test. C, Location of mutations in agr loci in continuous colonizer clones (C056, C059, K051, and K155) from infants who did not develop AD (non-AD). The positions of mutations are shown in the top panel. D, Frequency of SNPs and INDELs per isolate at indicated positions in agr loci of all non-AD- and AD-associated Agr type I S. aureus strains isolated at 1 month and 6 months. The number of SNPs and INDELs was normalized to the number of strains in the corresponding set and smoothed using 8-bp moving average plots. 1M (1-month old), 6M (6-month old). ***p<0.0001, the asterisk indicates a significant difference only between the 6M non-AD group and the reference Agr type I sequences by Fisher’s exact tests. The SNPs and INDELs in agr loci used for Fig. 3D are shown in Data file S2. E, The relative percentage of agr SNPs and INDELs in isolates from the 6M AD and 6M Non-AD groups taken into account all SNPs and INDELs in the genome. Dots represent the percent of agr SNPs and INDELs in all SNPs and INDELs in the genome per isolate. Data was analyzed for normal distribution and then by two-tailed t-test.
The analysis of continuous colonizer clones revealed a higher mutation rate in agr loci in S. aureus clones isolated from infants who did not develop AD, but the limited number of paired clones did not allow us to assess the association between agr mutations and AD development. To further investigate the role of agr mutations in the development of AD, we next assessed the presence of mutations in agr loci (agrA, agrB, agrC, agrD and hld) in all S. aureus strains isolated from infant skin. Because the sequences of agr loci differ in different Agr types (6), we analyzed the frequency of SNPs and insertions or deletions (INDELs) in agr loci of all S. aureus strains belonging to Agr I, the most common Agr type detected in infant skin, and compared the frequency of agr mutations in each group to agr I reference sequences (Fig. 2 and fig. S3B). Consistent with the analysis of continuous colonizer clones, there was a higher number of SNPs and INDELs in agrC from S. aureus strains isolated from the skin of 6-month infants who did not develop AD compared to strains isolated from 1-month old infants regardless of AD development as well as strains from 6-month old infants who developed AD (p < 0.0001 by Fisher’s exact test, Fig. 3D, Data file S2 and S3). We next assessed the relative percentage of agr mutations in S. aureus isolates belonging to Agr I from 6-month infants in the non-AD and AD groups taking into account all SNPs and INDELs in the genome. The analysis showed a higher percentage of agr SNPs and INDELs per genome in the non-AD than in the AD group (Fig. 3E). These results indicate that there is an increased presence of S. aureus harboring agr mutations in the skin of infants who do not develop AD.
Retention of a functional S. aureus Agr system is associated with AD development
To investigate the functional importance of agr mutations identified in non-AD S. aureus isolates, we assessed the expression of RNAIII in continuous colonizer clones that had acquired mutations in agr loci during skin colonization. Consistent with previous studies (25), the highest expression of Agr-regulated RNAIII was observed during the late exponential growth of S. aureus cultures (fig. S7). Expression of RNAIII in the 4 continuous colonizer clones with agr mutations from the Chiba cohort shown in Figures 3B and 3C was greatly reduced compared with the expression in the same clone isolated at 1 month of age compared to the agr-unmutated continuous colonizer clones in non-AD and AD groups (Fig. 4A and fig. S8). We next assessed the expression of RNAIII in all S. aureus isolates from infant skin in the primary Chiba cohort. RNAIII expression in strains isolated at 6 months from infants who did not develop AD was reduced compared to strains isolated at 1 month; in contrast, the expression of RNAIII in S. aureus isolated at 1 and 6 months from infants who developed AD was comparable (Fig. 4B). agr mutations in clinical S. aureus strains are known to cluster in agrC and agrA loci (26). Consistent with these previous studies, sequence analysis of strains with impaired RNAIII expression revealed that 10 out of 13 of the strains isolated from non-AD-associated skin from 6-month old infants harbored agrA and agrC mutations, resulting in either large amino acid truncations due to frameshift mutations, sequence deletions, or single amino acid substitutions that clustered in the AgrC receptor (Data file S4). Notably, 7 of 8 of the strains from 1-month old infants and one strain from 6-month old infant who developed AD exhibited impaired RNAIII expression, but lacked detectable agr mutations (Data file S4). Genetic complementation with wild-type type I agrC plasmid, but not control plasmid, restored the ability of non-AD associated strains (K051 and K232) with mutated type I agrC to induce RNAIII expression in culture (Fig. 4C). In contrast, expression of wild-type type I agrC plasmid neither restored RNAIII expression in an Agr type I strain with defective RNAIII expression but lacking agrC mutations nor in an Agr type II S. aureus strain with mutant agrC (Fig. 4C). Collectively, these results indicate that retention of Agr-regulated RNAIII expression in S. aureus is associated with AD development.
Fig. 4. Impaired Agr function in S. aureus strains isolated at 6 months from infants who did not develop AD.
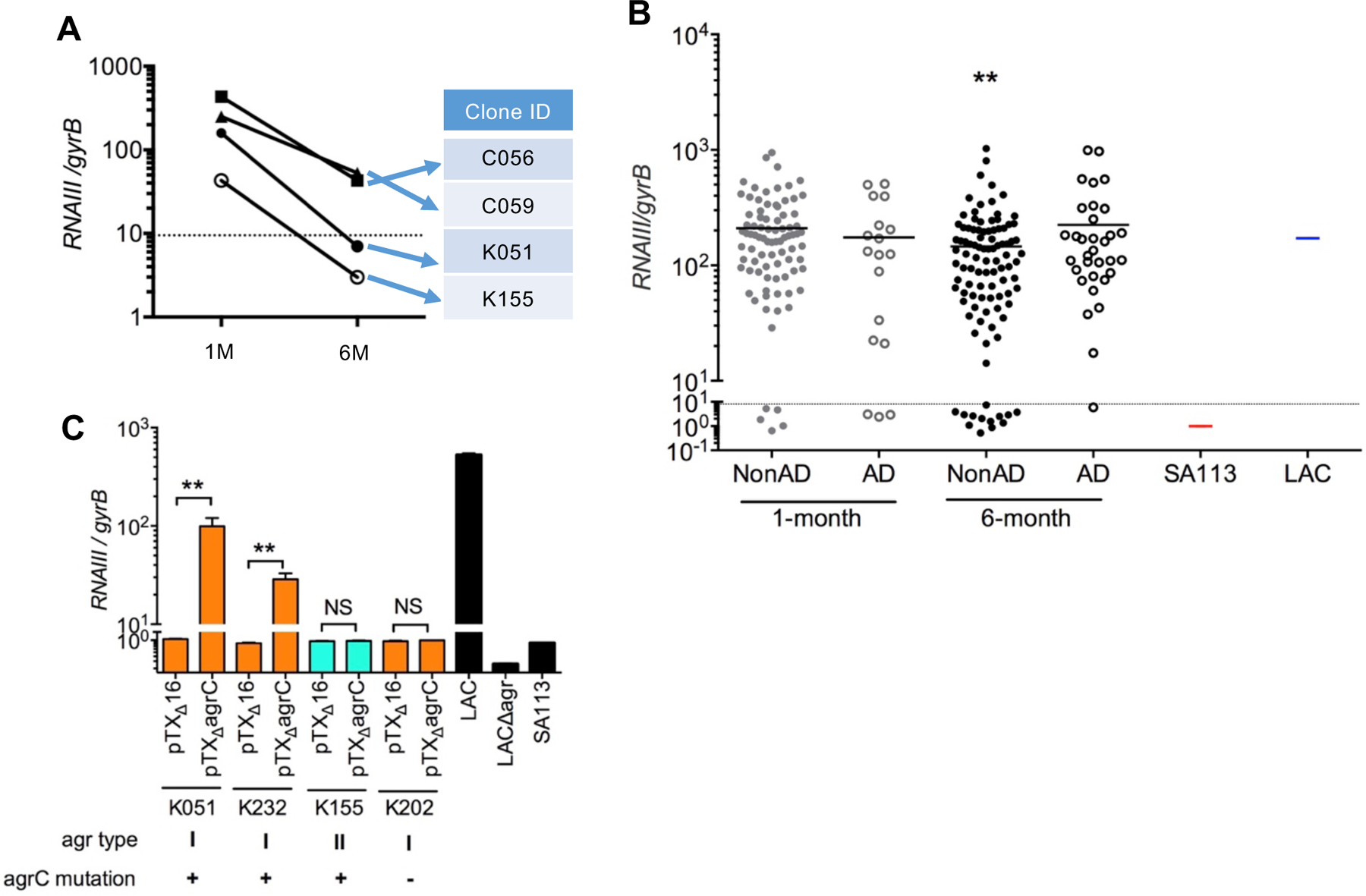
A, Normalized RNAIII expression in four non-AD-associated continuous colonizers that developed mutations in agr loci during skin colonization. Straight lines indicate that S. aureus strains were isolated from the same infant. B, Normalized RNAIII expression in all S. aureus strains isolated from infant skin. Strains below the dotted line were considered agr-deficient based on qPCR and immunoblotting analyses shown in fig. S7. SA113 and LAC S. aureus strains were used as negative and positive controls, respectively. Further details about the Agr-defective strains are shown in Data file S4. ** p<0.01; the asterisk indicates a significant difference from the 1-month non-AD group, as determined by a Steel-Dwass test. C, Normalized RNAIII expression was analyzed in Agr type I strains with mutated agrC (K051 and K232), an Agr type II strain with mutated agrC (K155), an Agr-defective strain without agr mutations (K202) transformed with vector alone (K051pTXΔ16, K232 pTXΔ16, K155 pTXΔ16 and K202 pTXΔ16), or Agr type I wild-type AgrC plasmid (K051pTXΔagrC, K232 pTXΔagrC, K155 pTXΔagrC and K202 pTXΔagrC). LAC is shown as positive control and LACΔagr and SA113 as negative controls. *p<0.05, NS; not significant, data was analyzed for normal distribution and then by two-tailed t-test.
The Agr system is critical for epidermal S. aureus colonization and induction of inflammation in the mouse skin
We next performed studies to assess the importance of agr virulence in epidermal colonization using an epicutaneous mouse model that induces activation of the Agr system and inflammation on the skin surface (27). In these experiments, the skin of C57BL/6 mice was colonized with wild-type or isogenic agr-deficient LAC S. aureus without physical disruption of the skin barrier (27). In TSB medium, wild-type and agr-deficient (Δagr) S. aureus strains increased in number similarly in vitro (Fig. 5A). In contrast, the ability of the LAC Δagr mutant to colonize the skin on day 7 after epidermal inoculation was impaired compared to the isogenic wild-type LAC strain, and the wild-type bacterium triggered robust skin inflammation associated with skin disease score (Fig. 5B) as well as hyperkeratosis, epidermal thickening, and dermal inflammatory cell infiltrates in histology (Fig. 5C and 5D), whereas the Δagr mutant did not. Consistently, wild-type LAC S. aureus was detected on the skin surface whereas only few LAC Δagr mutant bacteria were observed on day 7 after colonization in the histological sections (Fig. 5D). Consistent with these findings, infant Agr-deficient clone M6K051 isolated at 6 months exhibited impaired skin colonization compared to its paired Agr-sufficient M1K051 clone isolated from the same infant at 1 month using the same epicutaneous mouse model (Fig. 5E). Unlike the S. aureus LAC strain, the clinical Agr-sufficient M1K051 clone colonized the mouse skin poorly, with no or minimal induction of skin inflammation (Fig. 5E). We verified these results using another model of epicutaneous colonization that is associated with minimal or no skin inflammation (28). As observed with the epicutaneous model that triggers marked skin inflammation after colonization with the LAC strain, the ability of Δagr S. aureus LAC strain to colonize the skin was impaired compared to the isogenic wild-type strain (Fig. 5F). Likewise, the Agr-deficient M6K051 clone that acquired an agrC mutation by 6 months of colonization in infant skin exhibited impaired epidermal colonization when compared to the paired agr-expressing M1K051 clone isolated at 1 month from the same infant (Fig. 5G). These results indicate that the Agr system is important for epidermal S. aureus colonization in the presence and absence of inflammation.
Fig. 5. A functional S. aureus Agr system is required for epidermal colonization and induction of inflammation in mice.
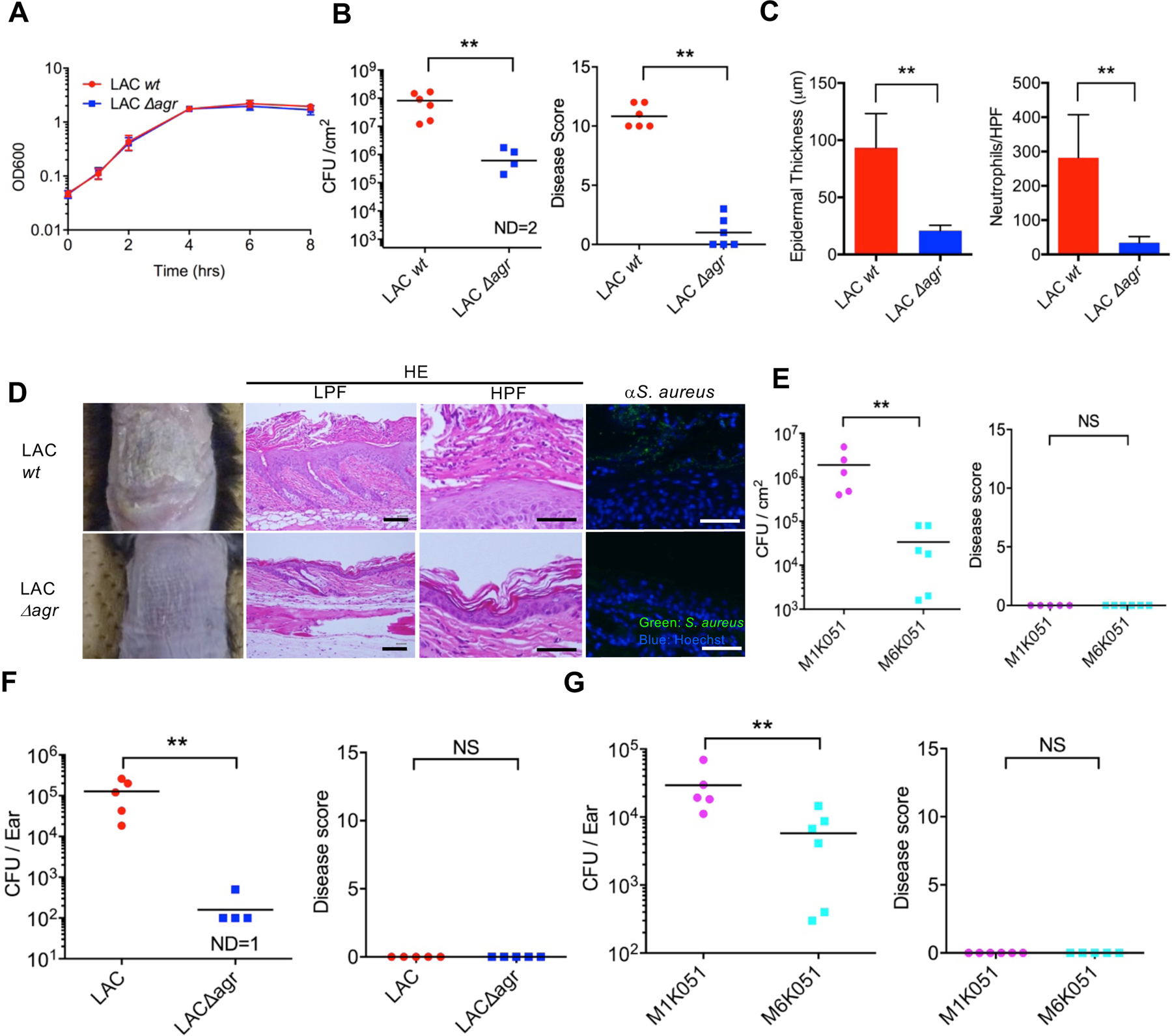
A, Growth curves of wild-type and agr-deficient (Δagr) LAC S. aureus strains cultured in TSB medium in vitro. B, C57BL/6 mice colonized epicutaneously with wild-type (LAC) or agr-deficient (LACΔagr) S. aureus. S. aureus loads (left) and disease score (right) were assessed on day 7 after inoculation. C, Epidermal thickness (left) and the number of neutrophils in skin tissue (right) on day 7 after pathogen colonization. D, representative skin phenotype (left panels), representative low power fields (LPF) and high power fields (HPF) of skin tissue (middle panels) of mice colonized with wild-type and agr-deficient S. aureus strains. Skin sections were stained with haematoxylin and eosin (HE, middle panels) or stained with anti-S. aureus antibody (αS. aureus) and Hoechst 33258 (right panels). Scale bar = 50 μm. E, C57BL/6 mice colonized epicutaneously with wild-type (M1K051) or agr-deficient (M6K051) S. aureus. S. aureus loads (left) and disease score (right) were assessed on day 7 after inoculation. F, C57BL/6 mice were colonized on the skin of the ear with wild-type or agr-deficient (Δagr) S. aureus using an established protocol (28). S. aureus loads (left panel) and disease score (right panel) were assessed on day 7 after inoculation. G, C57BL/6 mice were colonized on the skin of the ear with M1K051 (wild-type agr) and M6K051 (agrC mutation) S. aureus paired clones isolated from the skin of the same infant who did not develop AD at 1 and 6 months of age, respectively. S. aureus loads (left panel) and disease score (right panel) were assessed on day 7 after inoculation. ND; not detected. Dots represent individual mice. **p<0.01, NS, not significant, data was analyzed for normal distribution and then by two-tailed t-test.
Discussion
Collectively these studies show that retention of agr virulence is associated with increased S. aureus skin colonization and development of AD in Japanese infants. The reduced colonization of S. aureus in the skin of infants who did not develop AD was correlated with the acquisition of dysfunctional mutations in agr loci. Although QS is typically associated with pathogen survival and growth, QS-dysfuntional mutants in pathogens including S. aureus commonly arise in vitro and in vivo, particularly during biofilm-associated infections (11, 16). In our studies, we found that dysfunctional QS mutants were acquired under normal conditions in infant skin. The underlying mechanism to account for the emergence of S. aureus QS dysfuntional mutants during infection is not well understood, but has been associated with both social cheating behavior and host immune evasion in different infection models (11, 29). Although the association of increased S. aureus colonization in lesional skin is well established, few studies have assessed the association of skin S. aureus colonization prior to the development of AD. A large cohort study found that S. aureus colonization detected in nasal swabs at 6 months by culture was associated with higher incidence of AD development (30–33). However, these findings were not reproduced in another cohort study (33). Likewise, another study analyzed 50 infants longitudinally and did not detect increases in S. aureus colonization on the 10 infants who developed AD (30). In contrast, a large prospective study of 149 infants found that S. aureus colonization at 3 months of age was more prevalent on the skin of infants who develop AD (32), consistent with our studies. The reason for the differences in results is unclear, but it may reflect the use of different methodologies to detect S. aureus, or differences in swab sites or infant populations.
The mechanism that promotes the acquisition of agr mutations in the skin of infants who do not develop AD remains unclear. One possibility is that the development of S. aureus clones with dysfuntional QS mutations is driven by selective pressure. Individuals with AD display barrier skin defects which are associated with enhanced production of TH2 and TH17 cytokines in the skin (34, 35). Thus, unlike AD skin, the immune response in the skin of healthy infants is more restrained and the selective pressure to produce agr-regulated immune evasion factors such as toxins may be reduced, leading to accumulation of clones with dysfunctional QS mutations. Analysis of skin samples from patients with AD showed abnormal composition of stratum corneum lipids in both lesional and nonlesional skin (36, 37). Thus, differences in the strength or quality of immune responses or the skin metabolic environment between AD and healthy infant skin may lead to differences in the selective pressure to maintain a functional Agr system in S. aureus clones. Because we have shown that the Agr system is important for epidermal colonization, the frequency of S. aureus clones with agr mutations in the skin of infants who develop AD is likely to be underestimated. We detected the presence of clones with agr mutations in the skin of infants who did not develop AD. Such clones may represent bacteria that exhibit reduced ability to colonize the skin. Because the skin microbiome in infants is different from that of adults and older children (38, 39), it is also possible the skin environment of infants is more suitable for S. aureus colonization, including by those with agr mutations. We found an association between S. aureus colonization and the development of transient eczema at 6 months. Therefore, early skin pathology may affect the colonization of S. aureus strains with functional Agr system in infants who develop AD.
A limitation of our study is that we only have available one S. aureus isolate at 1 or 6 months of age for each infant and we could not assess the genetic diversity of the S. aureus population in the skin samples. Another limitation is that the data are not able to determine whether agr functionality is required for development and progression of AD. However, studies of S. aureus populations isolated from patients with AD have revealed that the skin of the great majority of patients are colonized by a single clone (40). Further studies are needed to assess and understand the genetic diversity of S. aureus in the skin of healthy infants and in infants prior and after AD development.
We found that retention of Agr virulence was associated with development of AD. The acquisition of dysfunctional QS mutants correlated with reduced S. aureus colonization at 6 months in the skin of infants who did not develop AD. The simplest explanation for these findings is that dysfunctional QS mutant clones exhibited reduced fitness to colonize the epidermis. This explanation is supported by the observation that agr mutants exhibited greatly impaired ability to colonize the epidermis of mice. In contrast, S. aureus colonization was not reduced in the epidermis of infants who developed AD associated with retention of Agr virulence. Agr-dependent virulence factors are expressed in lesional AD skin colonized with S. aureus (27). Furthermore, Agr-dependent virulence factors and specifically PSM peptides are critical for the induction of cutaneous inflammation with features of skin flares in an epicutaneous S. aureus infection model of new-onset pediatric AD (9, 27). Furthermore, S. aureus blooms in the lesional AD skin microbiota are associated with a decrease in coagulase-negative staphylococci species that produce AIPs with inhibitory activity against the S. aureus Agr system (41). Collectively, these studies indicate a critical role for agr virulence in S. aureus colonization in the mouse skin and suggest an important role for agr virulence in the development of AD in humans. Further studies are needed to establish a causal role for agr virulence in the development of AD.
Materials and Methods
Study design
The study cohort included 306 infants born at the Chiba University Hospital and Chiba Medical Center Hospital recruited for the study. The eligibility criteria of the study were the following; Japanese, infants of any sex whose mother, father, or siblings did not have or have any allergic disease, who themselves did not have any severe congenital abnormalities (such as congenital heart disease), and who had written informed consent from parent(s) or guardian(s). From April 2010 to March 2014, S. aureus isolates were collected from the cheek skin of 268 infants at 1 month and 6 months of age at their regular health checkups at Seikei-kai Chiba Medical Center or Chiba University Hospital. The study protocol was approved by the Biomedical Research Ethics Committee of the Graduate School of Medicine, Chiba University (ID: 559). AD was diagnosed upon later checkups at 1 and 2 years of age according to the Definition and Diagnostic Criteria for Atopic Dermatitis by the Japanese Dermatological Association. Unclassified transient ezcema was defined by the presence of eczema symptoms for less than 2 months. The presence of unclassified eczema at 6 months was based on clinical examination of infants at 1 month and 6 months after birth. To assess S. aureus colonization, the cheek of the infant was touched lightly with a S. aureus-selective agar plate (Foodstamp X-SA, Nissui Pharmaceutical). The plate was marked with the date and the side from which the sample was taken, incubated at 37°C for 24 hours and then refrigerated at 5°C. Light blue and blue colonies were identified as S. aureus and counted for CFU analysis.
Bacteria strains and culture conditions
All S. aureus strains were stored in tryptic soy broth with 40% glycerol at −80°C. S. aureus strains were grown in tryptic soy broth (TSB) overnight at 37°C with shaking, and then used for experiments. LAC wild-type and LAC Δagr strains were described previously (42). Plasmid pTXΔagrC was constructed by cloning the agrC coding sequence containing the ribosomal binding site region in the BamHI/MluI sites of plasmid pTXΔ (42).
Bacterial DNA isolation and whole genome sequencing and analysis
Bacterial DNA was isolated using NucleoSpin Tissue (Macherey-Nagel, 790452). KAPA HyperPlus Kit (Kapa Biosystems) was used to prepare the multiplexed shotgun libraries of DNA samples. The quality of all libraries was determined by Agilent 2100 Bioanalyzer (Agilent Technologies), and Qubit (Life Technologies). DNA sequencing was performed with HiSeq (125 bp paired-end) and MiSeq (300 bp paired-end) (Illumina) platforms according to the manufacturer’s instructions. All raw data were deposited to the Sequence Read Archive (DDBJ BioProject ID: PRJDB5246, detailed IDs are described in Data file S5. All Illumina data sets were cleaned using Trimmomatic v.0.33 (43). Details of reads and depth of coverage/N50 are provided in Data file S5. Trimmed reads were used to generate de novo assemblies of the draft genomes using SPAdes v3.11.1 with default parameters other than ‘--cov-cutoff auto’ and ‘--careful’ (44). Annotations of all predicted open reading frames of the draft genomes were performed using PROKKA v.1.12 (45). For genome-wide phylogenetic analysis, a total of 249 S. aureus strains isolated in the current study and seven reference strains downloaded from GenBank were used to assemble the core genome (Data file S6). 1,815 core genes were defined using Roary 3.12.0 with default parameters, ‘-i 95’ (46). A maximum likelihood tree of 249 S. aureus isolates was constructed based on 79,416 SNPs in the core genes using RAxML v. 8.2.11 with 1,000 bootstraps, and visualized using iTOL (47). The core genes were used for the assembly of the rooted neighbor-joining tree shown in Fig. 2. Then, pairwise genetic distances between isolates of the closely related ST in the rooted neighbor-joining tree were calculated within the core genome (excluded mobile genetic elements, phage, and repetitive regions) using an in-house script, following a method previously described (3). The reference genomes used for pairwise genetic distances were MW2 (ST1, Accession ID: NC_003923.1) for ST1, 12, 1281, 15, 188, 20, 2730, 2764, 3305, 3306, 3308, 3314, 3316, 6, 672, 81 (core size: 2,465,640 bp), CA-347(ST45, Accession ID: NC_021554.1) for ST45, 508, 30, 3303, 3304. 3307, 3311, 3312, 291 (core size: 2,481,614 bp), N315 (ST5, Accession ID: NC_002745.2) for ST5, 1292, 97, 965 (core size: 2,480,839 bp), M013 (ST59, Accession ID: NC_016928.1) for ST59, 121, 3310, 182, 3309 (core size: 2,465,753 bp), USA300_FPR3757 (ST8, Accession ID: NC_007793.1) for ST8, 3313, 630, 78, 1821 (core size: 2,457,543 bp). Continous colonizer clones isolated from an infant at the different time points were defined based on less than 60 nucleotide differences in pairwise genetic distance analysis (3). The other genomic analyses in continuous colonizer clones were performed using the entire genome sequences (Fig. 3A–C). For analysis of the agr region, trimmed reads were mapped to four different types of agr sequences using SMALT v.0.7.6 (http://www.sanger.ac.uk/science/tools/smalt-0). SNPs were identified using SAMtools v.0.1.19–44428cd (48), and filtered with ≥10-fold coverage, ≥30 mapping quality, and 75% consensus using in-house scripts. For nucleotide differences between continuous colonizers, for example two strains obtained from 1-month and 6-month infants, SNPs and small INDELs were identified using samtools, filtered (small INDEL 70 % consensus) (49, 50), and visually checked using IGV v.2.3.91 (51) and Mauve v.snapshot_2015-02-13 (52). For each INDEL detected, sequence reads covered at the site were extracted and aligned using T-coffee v.10.00 (53). All INDELs were manually inspected with a visual output. The functional effect of SNPs and small INDELs was annotated with SnpEff v.4.1l (54). For agr sequence analysis, all SNPs and INDELs were visually checked using IGV v.2.3.91 and GENETIX software. SNPs and INDELs in Agr type I isolates that were identified using the Newman genome as a reference to be present in >5% of isolates were considered as common variants and removed from the analysis in Fig. 3D. The nucleotide sequences of seven genes (arc, aro, glp, gmk, pta, tpi, and yqi) were utilized for MLST typing and compared to the S. aureus MLST database (55). STs were derived from assemblies, and CCs were assigned. The details of ST, CC, and agr classifications are shown in fig. S3. Sequence data were submitted to the DNA Data Bank of Japan (DDBJ: https://www.ddbj.nig.ac.jp/index-e.html) under the accession numbers listed in Data file S5.
Quantitative real-time PCR with reverse transcription
Complementary DNA was synthesized using a High Capacity RNA-to-cDNA Kit (Applied Biosystems), according to the manufacturer’s instructions. Quantitative real time RT–PCR (qPCR) was performed using a SYBR green PCR master mix (Applied Biosystems) and StepOne Real-time PCR system (Applied Biosystems). The primers to amplify mouse bacterial genes (RNAIII, gyrB) have been described (56). RNAIII expression was normalized to that of S. aureus gyrB and relative expression calculated by the 2-ΔΔCt method. LAC wild type and SA113 cultured for 7 h were used as reference controls.
Epicutaneous S. aureus inoculation models
The superficial skin of mice was colonized with S. aureus without prior skin disruption as described previously (27). Briefly, the dorsal skin of 6- to 8-week-old female mice was shaved 2–3 days before experiments. Ten million CFU of S. aureus strains were placed on a patch of sterile gauze and attached to the shaved skin with a transparent bio-occlusive dressing (Tegaderm; 3M). Each mouse was exposed to S. aureus for 1 week through the patch, and the animals were then sacrificed for analyses. In the S. aureus topical model, S. aureus suspension (one hundred million CFU per ml) was applied across the entire ear pina skin using a sterile cotton swab on day 0. Mice were euthanized for analysis on day 7. Experimental protocols were approved by the Chiba University Institutional Animal Care and Use Committee or the University of Michigan Review Board for Animal Care.
Histology and immunofluorescence staining
Skin tissue was formalin fixed, paraffin embedded, and sectioned for haematoxylin and eosin. For immunofluorescence staining, sections were subjected to labeling with anti-S. aureus antibody (mouse monoclonal ab37644; Abcam) followed by fluorescein isothiocyanate-conjugated secondary antibody and Hoechst 33258 (H3569; ThermoFisher).
Immunoblotting
Overnight-cultured supernatants from S. aureus strains were filtrated by 0.2 μm filter mesh. Clarified supernatants were resolved by SDS-PAGE and proteins transferred to polyvinylidene fluoride membranes by electroblotting. The polyclonal anti-δ-toxin antibody produced in rabbits by immunization with a synthetic multiple antigenic peptide showing an 18 amino-acid peptide (IGDLVKWIIDTVNKFTKK) (Sigma-Genosys) from the full-length δ-toxin sequence was described previously (27).
Real-time PCR-based genotyping of FLG mutations
For comprehensive screening of FLG mutation carriers, we studied 10 Japanese-specific FLG mutations as previously reported (57). Real-time PCR-based genotyping of the FLG mutations was performed with a TaqMan probe genotyping assay. Genomic DNA was extracted from buffy coat conserved at −80 °C using BioRobot EZ1 (Qiagen). To detect an allele carrying each mutation, a pair of TaqMan probes labeled with a fluorescent dye (FAM or CAL Fluor Orange 560), a quencher dye (BHQ-1), and sequence-specific forward and reverse primers were synthesized by Biosearch Technologies. The sequences of assay probes and primers have been described (57). Real-time PCR was performed with a LightCycler 480 system II 384 plate (Roche Diagnostics). Endpoint fluorescence was measured for each sample. Genotyping results were obtained using endpoint genotyping analysis and LightCycler 480 software.
Statistical analysis
All data were analyzed using GraphPad Prism and R statistical language (58) except Table 1, which was analyzed using JMP Pro software (versions 12.0: SAS Institute). Lilliefors test was performed to verify that the data were normally distributed prior to parametric tests including Studen’ts t-test, Pearson’s correlation or Fisher’s exact test. If the data distribution was not normal, the data was analyzed using nonparametric Kruskal-Wallis or Steel-Dwass test. For clinical data analysis of Table 1, logistic regression was used to calculate odds ratios and corresponding 95% confidence intervals (95% CI) to describe the association between the selected variables and AD. Independent variables included S. aureus colonization at 1 month and 6 months, gender, gestational age, birth weight, breast-feeding, and maternal history of allergy. For all analyses, p < 0.05 was considered statistically significant.
Supplementary Material
Materials and Methods
Fig. S1. Scheme of S. aureus sampling for whole genome sequence-based analysis.
Fig. S2. FLG gene mutations are not associated with AD development or S. aureus skin colonization in Japanese infants.
Fig. S3. Phylogenetic relationships by multi locus sequence typing (MLST) and Agr typing of infant isolated strains.
Fig. S4. Prevalence of major virulence genes among non-AD- and AD-associated S. aureus strains isolated from infant skin.
Fig. S5. Pairwise distance comparisons between S. aureus isolates belonging to the same ST.
Fig S6. Genomic mutations detected in continuous colonizer clones isolated from non-AD associated infants.
Fig. S7. Representative growth curves, RNAIII gene and δ-toxin expression in S. aureus infant isolates.
Fig. S8. RNAIII expression in S. aureus continuous colonizer clones.
Data file S1. SNP and INDEL information of continuous colonizer clones.
Data file S2. SNP and INDEL positions in Agr I isolates.
Data file S3. Statistical analysis of Figure 3D.
Data file S4. Clustering of agr mutations and RNAIII expression in agr-deficient strains.
Data file S5. ID and sequence depth of S. aureus isolates.
Data file S6. Reference genomes used in this study.
Acknowledgements
The authors thank M. Shozu, M. Kawada, T. Ohwada, H. Kojima, and R. Kobayasi for recruitment and follow-up of participants in the birth cohort; A. Oikawa, N. Saito, A. Mizuno and M. Omori for technical assistance; F. Nomura, A. Miyabe, S. Murata, M. Watanabe and staff for the Chiba Cohort study for cheek skin culture; and M. Zeng for review of the manuscript. We also thank J. Oscherwitz and K.B. Cease for delta toxin antibody.
Funding
This work was supported by JSPS KAKENHI grants 26713038 (Y.N.), 16H06252 (Y.N.), 16K15272 (A.T.), 18H02832 (M.A.), MEXT KAKENHI grants 16K18671 (H.T.) and 16H06279 (H.T.), the Naito Foundation (Y.N.), the Takeda Science Foundation (H.T.), AMED grants JP16ek0410029h0001 (Y.N., A.T., H.T. and N.Shimojo), JP18gm6010016h0002 (Y.N.), and 19gm0910002h0105 (M.A.). M.O. was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health (grant number ZIA AI000904-16). G. N. was supported by NIH grant AR069303 and a grant from the University of Michigan Host Microbiome Initiative (G. N). N. Shimojo was supported by a grant from the Environmental Restoration and Conservation Agency of Japan in fiscal years 2012–2016. Y.N., H.T., A.T., S.N. and N. Shimojo were supported by the Institute for Global Prominent Research, Chiba University.
Footnotes
Competing interests
G. N. is a scientific consultant of Boehringer Ingelheim. The other authors declare that they have no competing interests.
Data and materials availability
Sequence data were submitted to to DNA Data Bank of Japan (DDBJ: https://www.ddbj.nig.ac.jp/index-e.html) under the accession numbers listed in Data file S4.
LACΔagr and plasmid pTXagrC are available from M. Otto (motto@niaid.nih.gov) under a material transfer agreement with the National Institute of Allergy and Infectious Diseases. Requests for all other materials should be addressed to ymatsuoka@derma.med.osaka-u.ac.jp
References and notes
- 1.Segre JA, Epidermal barrier formation and recovery in skin disorders. J Clin Invest 116, 1150–1158 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowy FD, Staphylococcus aureus infections. N Engl J Med 339, 520–532 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Tong SY, Holden MT, Nickerson EK, Cooper BS, Koser CU, Cori A, Jombart T, Cauchemez S, Fraser C, Wuthiekanun V, Thaipadungpanit J, Hongsuwan M, Day NP, Limmathurotsakul D, Parkhill J, Peacock SJ, Genome sequencing defines phylogeny and spread of methicillin-resistant Staphylococcus aureus in a high transmission setting. Genome Res 25, 111–118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boguniewicz M, Leung DY, Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev 242, 233–246 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronner S, Monteil H, Prevost G, Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Rev 28, 183–200 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Novick RP, Geisinger E, Quorum sensing in staphylococci. Annu Rev Genet 42, 541–564 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Peschel A, Otto M, Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11, 667–673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Archer NK, Dillen CA, Wang Y, Ashbaugh AG, Ortines RV, Kao T, Lee SK, Cai SS, Miller RJ, Marchitto MC, Zhang E, Riggins DP, Plaut RD, Stibitz S, Geha RS, Miller LS, Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe 22, 653–666 e655 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa S, Matsumoto M, Katayama Y, Oguma R, Wakabayashi S, Nygaard T, Saijo S, Inohara N, Otto M, Matsue H, Nunez G, Nakamura Y, Staphylococcus aureus Virulent PSMalpha Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe 22, 667–677 e665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paharik AE, Parlet CP, Chung N, Todd DA, Rodriguez EI, Van Dyke MJ, Cech NB, Horswill AR, Coagulase-Negative Staphylococcal Strain Prevents Staphylococcus aureus Colonization and Skin Infection by Blocking Quorum Sensing. Cell Host Microbe 22, 746–756 e745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Le KY, Khan BA, Nguyen TH, Hunt RL, Bae JS, Kabat J, Zheng Y, Cheung GYC, Li M, Otto M, Resistance to leukocytes ties benefits of quorum sensing dysfunctionality to biofilm infection. Nat Microbiol 4, 1114–1119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams H, Flohr C, How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J Allergy Clin Immunol 118, 209–213 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG, The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol 30, 35–39 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Program NCS, Murray PR, Turner ML, Segre JA, Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22, 850–859 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh J, Conlan S, Polley EC, Segre JA, Kong HH, Shifts in human skin and nares microbiota of healthy children and adults. Genome Med 4, 77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D, Viode C, Schmitt AM, Serre G, Simon M, Paul CF, Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol 137, 1272–1274 e1273 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Geoghegan JA, Irvine AD, Foster TJ, Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol, (2017). [DOI] [PubMed] [Google Scholar]
- 18.Dahl MV, Staphylococcus aureus and atopic dermatitis. Arch Dermatol 119, 840–846 (1983). [PubMed] [Google Scholar]
- 19.Osawa R, Konno S, Akiyama M, Nemoto-Hasebe I, Nomura T, Nomura Y, Abe R, Sandilands A, McLean WH, Hizawa N, Nishimura M, Shimizu H, Japanese-specific filaggrin gene mutations in Japanese patients suffering from atopic eczema and asthma. J Invest Dermatol 130, 2834–2836 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, Irvine AD, Weidinger S, Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol 123, 1361–1370 e1367 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Kono M, Akiyama M, Inoue Y, Nomura T, Hata A, Okamoto Y, Takeichi T, Muro Y, McLean WHI, Shimizu H, Sugiura K, Suzuki Y, Shimojo N, Filaggrin gene mutations may influence the persistence of food allergies in Japanese primary school children. Br J Dermatol, (2018). [DOI] [PubMed] [Google Scholar]
- 22.Sasaki T, Furusyo N, Shiohama A, Takeuchi S, Nakahara T, Uchi H, Hirota T, Tamari M, Shimizu N, Ebihara T, Amagai M, Furue M, Hayashi J, Kudoh J, Filaggrin loss-of-function mutations are not a predisposing factor for atopic dermatitis in an Ishigaki Island under subtropical climate. J Dermatol Sci 76, 10–15 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Thammavongsa V, Kim HK, Missiakas D, Schneewind O, Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 13, 529–543 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright JS 3rd, Traber KE, Corrigan R, Benson SA, Musser JM, Novick RP, The agr radiation: an early event in the evolution of staphylococci. J Bacteriol 187, 5585–5594 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balaban N, Novick RP, Translation of RNAIII, the Staphylococcus aureus agr regulatory RNA molecule, can be activated by a 3’-end deletion. FEMS Microbiol Lett 133, 155–161 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Shopsin B, Eaton C, Wasserman GA, Mathema B, Adhikari RP, Agolory S, Altman DR, Holzman RS, Kreiswirth BN, Novick RP, Mutations in agr do not persist in natural populations of methicillin-resistant Staphylococcus aureus. J Infect Dis 202, 1593–1599 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, Otto M, Inohara N, Nunez G, Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature 503, 397–401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, Quinones M, Brenchley JM, Kong HH, Tussiwand R, Murphy KM, Merad M, Segre JA, Belkaid Y, Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollitt EJ, West SA, Crusz SA, Burton-Chellew MN, Diggle SP, Cooperation, quorum sensing, and evolution of virulence in Staphylococcus aureus. Infect Immun 82, 1045–1051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WHI, Murray D, Jo JH, Segre JA, Kong HH, Irvine AD, Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol 139, 166–172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebon A, Labout JA, Verbrugh HA, Jaddoe VW, Hofman A, van Wamel WJ, van Belkum A, Moll HA, Role of Staphylococcus aureus nasal colonization in atopic dermatitis in infants: the Generation R Study. Arch Pediatr Adolesc Med 163, 745–749 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Meylan P, Lang C, Mermoud S, Johannsen A, Norrenberg S, Hohl D, Vial Y, Prod’hom G, Greub G, Kypriotou M, Christen-Zaech S, Skin Colonization by Staphylococcus aureus Precedes the Clinical Diagnosis of Atopic Dermatitis in Infancy. J Invest Dermatol 137, 2497–2504 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Skov L, Halkjaer LB, Agner T, Frimodt-Moller N, Jarlov JO, Bisgaard H, Neonatal colonization with Staphylococcus aureus is not associated with development of atopic dermatitis. Br J Dermatol 160, 1286–1291 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Guttman-Yassky E, Krueger JG, Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol 48, 68–73 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Han H, Roan F, Ziegler SF, The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev 278, 116–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, Vreeken RJ, Hankemeier T, Kezic S, Wolterbeek R, Lavrijsen AP, Bouwstra JA, Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res 53, 2755–2766 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Smeden J, Janssens M, Boiten WA, van Drongelen V, Furio L, Vreeken RJ, Hovnanian A, Bouwstra JA, Intercellular skin barrier lipid composition and organization in Netherton syndrome patients. J Invest Dermatol 134, 1238–1245 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Capone KA, Dowd SE, Stamatas GN, Nikolovski J, Diversity of the human skin microbiome early in life. J Invest Dermatol 131, 2026–2032 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R, Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 107, 11971–11975 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harkins CP, Pettigrew KA, Oravcova K, Gardner J, Hearn RMR, Rice D, Mather AE, Parkhill J, Brown SJ, Proby CM, Holden MTG, The Microevolution and Epidemiology of Staphylococcus aureus Colonization during Atopic Eczema Disease Flare. J Invest Dermatol 138, 336–343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams MR, Costa SK, Zaramela LS, Khalil S, Todd DA, Winter HL, Sanford JA, O’Neill AM, Liggins MC, Nakatsuji T, Cech NB, Cheung AL, Zengler K, Horswill AR, Gallo RL, Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M, Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13, 1510–1514 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Bolger AM, Lohse M, Usadel B, Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA, SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19, 455–477 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seemann T, Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, Parkhill J, Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31, 3691–3693 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Letunic I, Bork P, Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44, W242–W245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome S Project Data Processing, The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki S, Horinouchi T, Furusawa C, Prediction of antibiotic resistance by gene expression profiles. Nat Commun 5, 5792 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenaillon O, Rodriguez-Verdugo A, Gaut RL, McDonald P, Bennett AF, Long AD, Gaut BS, The molecular diversity of adaptive convergence. Science 335, 457–461 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP, Integrative genomics viewer. Nat Biotechnol 29, 24–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darling AC, Mau B, Blattner FR, Perna NT, Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14, 1394–1403 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Notredame C, Higgins DG, Heringa J, T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol 302, 205–217 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM, A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6, 80–92 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aanensen DM, Spratt BG, The multilocus sequence typing network: mlst.net. Nucleic Acids Res 33, W728–733 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seidl K, Chen L, Bayer AS, Hady WA, Kreiswirth BN, Xiong YQ, Relationship of agr expression and function with virulence and vancomycin treatment outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 55, 5631–5639 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kono M, Nomura T, Ohguchi Y, Mizuno O, Suzuki S, Tsujiuchi H, Hamajima N, McLean WH, Shimizu H, Akiyama M, Comprehensive screening for a complete set of Japanese-population-specific filaggrin gene mutations. Allergy 69, 537–540 (2014). [DOI] [PubMed] [Google Scholar]
- 58.R. D. C. Team, R: A language and environment for statistical computing R Foundation for Statistical Computing.Vienna, Austria: ISBN 3-900051-07-0, URL http://www.R-project.org. (2005). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Fig. S1. Scheme of S. aureus sampling for whole genome sequence-based analysis.
Fig. S2. FLG gene mutations are not associated with AD development or S. aureus skin colonization in Japanese infants.
Fig. S3. Phylogenetic relationships by multi locus sequence typing (MLST) and Agr typing of infant isolated strains.
Fig. S4. Prevalence of major virulence genes among non-AD- and AD-associated S. aureus strains isolated from infant skin.
Fig. S5. Pairwise distance comparisons between S. aureus isolates belonging to the same ST.
Fig S6. Genomic mutations detected in continuous colonizer clones isolated from non-AD associated infants.
Fig. S7. Representative growth curves, RNAIII gene and δ-toxin expression in S. aureus infant isolates.
Fig. S8. RNAIII expression in S. aureus continuous colonizer clones.
Data file S1. SNP and INDEL information of continuous colonizer clones.
Data file S2. SNP and INDEL positions in Agr I isolates.
Data file S3. Statistical analysis of Figure 3D.
Data file S4. Clustering of agr mutations and RNAIII expression in agr-deficient strains.
Data file S5. ID and sequence depth of S. aureus isolates.
Data file S6. Reference genomes used in this study.


