Abstract
Immune checkpoint inhibitors enhance immune recognition of tumors by interfering with the cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) and programmed death 1 (PD1) pathways. In the past decade, these agents brought significant improvements to the prognostic outlook of patients with metastatic cancers. Recent data from retrospective analyses and a few prospective studies suggest that checkpoint inhibitors have activity against brain metastases from melanoma and nonsmall cell lung cancer, as single agents or in combination with radiotherapy. Some studies reported intracranial response rates that were comparable with systemic ones. In this review, we provide a comprehensive summary of clinical data supporting the use of anti-CTLA4 and anti-PD1 agents in brain metastases. We also touch upon specific considerations on the assessment of intracranial responses in patients and immunotherapy-specific toxicities. We conclude that a subset of patients with brain metastases benefit from the addition of checkpoint inhibitors to standard of care therapeutic modalities, including radiotherapy and surgery.
Keywords: Checkpoint inhibitors, Immunotherapy, PD1, PDL1, CTLA4, Brain metastases, Melanoma, Nonsmall cell lung cancer
ABBREVIATIONS
- BCP
bevacizumab, carboplatin, and paclitaxel
- CNS
central nervous system
- CTL-4
cytotoxic T-lymphocyte-associated antigen
- HR
hazard ratio
- NSCLC
nonsmall cell lung cancer
- OS
overall survival
- PFS
progression-free survival
- SRS
stereotactic radiosurgery
Brain metastases are a common complication of advanced malignancies, occurring most frequently in lung cancer, breast cancer, and melanoma. Patients with these cancers account for 67% to 80% of all diagnoses.1 The prognosis of patients with intracranial metastases is very poor, and therapeutic options other than surgery and radiation are limited. For years, the role of systemic therapies has been underexplored in this patient population because of poor performance status and limited central nervous system (CNS) penetration of many agents. The past decade has seen dramatic improvements in the management of certain metastatic cancers with significant prolongation of patient survival. This was mainly because of the introduction of targeted agents directed against oncogenic drivers and immunotherapeutic agents. With these changes, however, it is thought that the incidence of brain metastases is rising, and more patients are dying from intracranial complications.2 Although individuals with brain metastases were excluded from initial immunotherapy and targeted therapy trials, we are now beginning to understand that some of these agents are effective at treating intracranial disease.
IMMUNE CHECKPOINT INHIBITORS
Immune checkpoints are important physiologic pathways that allow the immune system to distinguish between “self” and “foreign” and thus prevent autoimmunity. Cancers often evade immune recognition by interfering with these checkpoint pathways.
Cytotoxic T-Lymphocyte-Associated Antigen 4
The process of early T-cell activation occurs in lymph nodes and it involves 2 key steps: T-cell receptor binding to an antigen major histocompatibility complex and a co-stimulatory signal in which the receptor CD28 on T cells binds to CD80 or CD86 on antigen-presenting cells. This leads to the activation of proliferation pathways and production of cytokines.3 The co-stimulatory signal can be dampened if the immune checkpoint protein CTLA4 competitively binds to CD80 or CD86 (Figure A).4 Anti-CTLA4 “checkpoint blockade” relies on inhibition of this dampening signal, thus obtaining an overactive T-cell response. CTLA4-directed therapies were the first among the checkpoint inhibitors to show antitumor efficacy and a therapeutic window in Vivo.5 The anti-CTLA4 monoclonal antibody ipilimumab was the first checkpoint blockade agent to show clinical efficacy in patients with metastatic melanoma.6 Ipilimumab and tremelimumab are the 2 clinically available CTLA4 monoclonal antibodies, but only ipilimumab is FDA approved currently.
FIGURE.
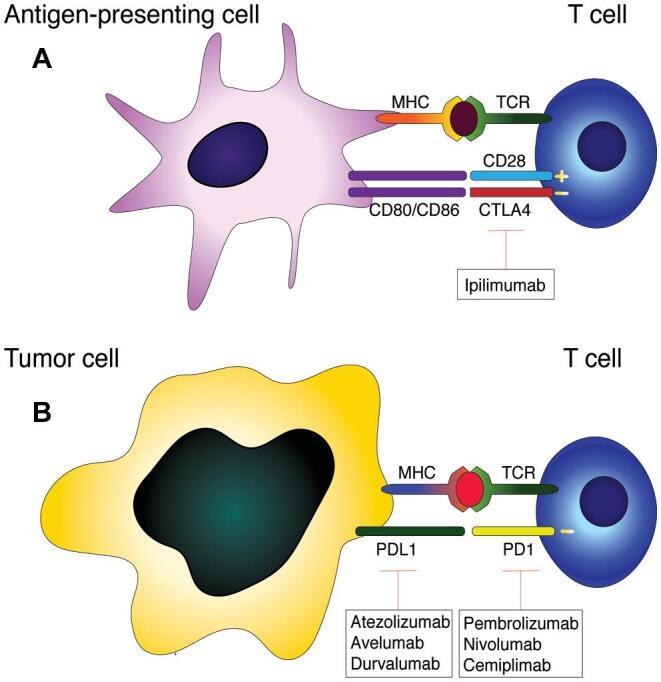
Immune checkpoint pathways. A, CTLA4 on the surface of T cells binds to CD80/CD86 on antigen-presenting cells to dampen early T-cell activation. B, PD1 on activated T cells binds to PDL1 on tumor cells or target organs to dampen the effector phase of T-cell activation. FDA-approved checkpoint blockade agents are shown in boxes. MHC: major histocompatibility complex, TCR: T-cell receptor, CD28: cluster of differentiation 28, CTLA4: cytotoxic T-lymphocyte-associated protein 4, CD80/CD86: cluster of differentiation 80/86, PD1: programmed cell death protein 1, PDL1: programmed death-ligand 1.
Programmed Death 1
Contrary to CTLA4, the PD1 pathway takes place in target organs during the effector phase of T-cell activation, and it serves to dampen the duration of immune responses and to promote self-tolerance. PD1 is located on the surface of activated T cells as well as on B cells and myeloid cells.3 PD1 can bind to either PDL1 or PDL2; PDL1 is expressed in a wide range of cell types and tissues, whereas PDL2 is expressed by dendritic cells and monocytes. PDL1 is also widely expressed by tumors as a means of immune evasion. As with CTLA4, PD1 binding to its ligands causes inhibition of T-cell proliferation and reduction in cytokine production (Figure B).3 Prolonged antigen exposure can also lead to PD1 expression and T-cell exhaustion,4 a phenomenon that is thought to occur in tumor infiltrating lymphocytes. Blocking the PD1 pathway can enhance immune recognition of tumors and restore the function of exhausted T cells. Additionally, reports have shown that tumor-intrinsic PD1 signaling can itself promote growth in melanoma models.7 At present, clinically available PD1 pathway inhibitors include the anti-PD1 monoclonal antibodies, such as pembrolizumab, nivolumab, cemiplimab, and pidilizumab, and the anti-PDL1 monoclonal antibodies, such as durvalumab and atezolizumab.
TABLE 1.
Immunotherapy Brain Metastases-Specific Clinical Trials
| Melanoma | |||
|---|---|---|---|
| Trial | Phase | Drug | Results |
| Margolin et al (2012)8 | II | Ipilimumab | RR 24% for asymptomatic patients, RR10% for symptomatic patients |
| Goldberg et al (2016)12 | II | Pembrolizumab | RR 22%, all responses ongoing at 24 mo |
| Long et al (2018)15 | II | Ipilimumab + nivolumab, nivolumab alone | RR 46% for ipilimumab + nivolumab, RR 20% for nivolumab alone |
| Tawbi et al (2018)14 | II | Ipilimumab + nivolumab | RR 57%, 26% complete responses; 55% grade 3-4 adverse events |
| Nonsmall cell lung cancer | |||
| Goldberg et al (2016)12 | II | Pembrolizumab | RR 33% |
Published prospective clinical trials looking at checkpoint inhibitors in melanoma and nonsmall cell lung cancer brain metastases. All trials showed intracranial response rates comparable to what was seen in the systemic setting. In melanoma, combination of ipilimumab and nivolumab yielded high response rates but with a large number of severe adverse events.
RR, response rate.
IMMUNE CHECKPOINT INHIBITORS IN BRAIN METASTASES
Melanoma
Immune checkpoint inhibitors were first investigated in metastatic melanoma and have revolutionized the treatment of this disease. The first landmark study was of ipilimumab against the gp100 vaccine,6 showing improvement in overall survival (OS) of 10 vs 6.4 mo. A total of 82 patients with brain metastases were included (out of 676), most of whom had received prior treatment. There was a trend towards improved survival for this population (hazard ratio [HR] for death 0.70, 95% CI 0.41-1.20 for ipilimumab + gp100 vaccine, HR 0.76, 95% CI 0.38-1.54 for ipilimumab alone). These results prompted the development of a phase II clinical trial looking specifically at ipilimumab in brain metastases.8 Patients were divided into 2 cohorts based on neurological symptoms and corticosteroid use and were given 4 doses of ipilimumab at 10 mg/kg. Patients who were initially asymptomatic from a neurological standpoint had a 24% intracranial response rate, whereas symptomatic patients on corticosteroids had a 10% response rate.8 More studies are needed to ascertain the reason why patients taking corticosteroids had worse outcomes. It is possible that these patients’ tumors had unfavorable prognostic features at baseline, or that corticosteroid use counteracted the effectiveness of checkpoint inhibitors. In other tumors, these effects seem to be driven by poor prognostic factors rather than corticosteroid use itself.9 However, studies also suggest that corticosteroids can have a direct effect in therapeutic efficacy of antineoplastic agents and even dampen immunotherapy response.10-12 This study did not show unexpected intracranial adverse events.
Anti-PD1 agents are also known to be effective at treating metastatic melanoma. The Keynote-006 study compared pembrolizumab with ipilimumab and showed improvement in progression-free (PFS) and OS with pembrolizumab.13 The Checkmate 066 study showed prolongation in PFS and a higher response rate with nivolumab compared to dacarbazine.14 In both studies, having active brain metastases was part of the exclusion criteria. The first study included 8.2% to 10.1% of patients with treated brain metastases and the second study 7% to 15%. A prospective phase II study was performed to investigate the effectiveness of pembrolizumab in the brain metastases population (Table 1).15 This included 18 patients with melanoma and 34 with nonsmall cell lung cancer (NSCLC). Four (22%) patients with melanoma had responses, 2 complete and 2 partial and all of them durable. Long-term follow-up of these patients showed that median PFS for this cohort was 2 mo and median OS was 17 mo.16 All responses were ongoing at 24 mo. Additionally, a phase II study was conducted looking at the combination of ipilimumab and nivolumab in patients with untreated melanoma brain metastases.17 A total of 94 patients were enrolled in this study, and the intracranial response rate was 57% with 26% complete responses, results that were comparable to extracranial response rates. These striking results came with the caveat that there was a 55% rate of grade 3-4 adverse events, including 7% in the CNS. These severe CNS adverse events included headaches in 7 patients, paresthesias in 3 patients, cerebral edema in 2 patients, intracranial hemorrhage in 1 patient, and syncope in 1 patient. A separate multicenter randomized phase II study comparing nivolumab with ipilimumab + nivolumab in patients with untreated melanoma brain metastases showed that both regimens had activity, but response rates were higher for the ipilimumab + nivolumab cohort (46% vs 20%).18
Nonsmall Cell Lung Cancer
The first checkpoint blockade agents to show promise in metastatic NSCLC were PD1-directed therapies.19,20 The Keynote-001 study showed that pembrolizumab had an overall response rate of 19.4% for all patients and 45.2% for patients with PDL1 expression in >50% of tumor cells.19 The subsequent Keynote-010 study showed a significant increase in median OS for patients with tumor PDL1 expression ≥1% who received pembrolizumab vs docetaxel (12.7 mo with pembrolizumab 10 mg/kg, 10.4 mo with pembrolizumab 2 mg/kg, and 8.5 mo with docetaxel).20 The Keynote-21 study then looked at the combination of carboplatin, pemetrexed, and pembrolizumab in chemotherapy-naïve patients with stage IIIB or IV NSCLC without targetable alterations. This trial showed that combination therapy improved response rate (33% vs 18%) and PFS (13 vs 8.9 mo) compared to chemotherapy alone.21 The phase III trial Keynote-189 showed that chemoimmunotherapy improved OS across different levels of PDL-1 expression.22 Similarly, the anti-PDL1 agent atezolizumab was found to improve PFS in patients with metastatic nonsquamous NSCLC when administered in combination with bevacizumab, carboplatin, and paclitaxel(BCP) when compared to BCP alone (median PFS 8.3 vs 6.8 mo).23 This agent was previously found to be superior to docetaxel in the phase III OAK study.24 Additionally, nivolumab showed superior OS compared to docetaxel with a difference of 9.2 vs 6.0 mo25 (squamous histology, Checkmate 017) and 12.2 vs 9.4 mo 26 (nonsquamous histology, Checkmate 057). The Checkmate 026 study, which investigated the role of first-line nivolumab in patients with PDL-1 expression >1%, did not show a survival benefit. Most recently, the Checkmate 227 study looked at the combination of ipilimumab and nivolumab compared with chemotherapy in patients with a high tumor mutational burden and found that there was a significant difference in response rate of 42.6% vs 13.2%.27 Lastly, the anti-PDL1 agent durvalumab showed a significant improvement in PFS compared to placebo when administered after chemoradiotherapy in patients with stage III NSCLC.28
Based on these landmark studies, the current first-line standard of care regimen to treat metastatic NSCLC without targetable genetic alterations is to administer either single-agent pembrolizumab if PDL-1 positive (≥50% of tumor cells) or chemoimmunotherapy if PDL-1 negative.
Patients with previously untreated brain metastases were excluded from the majority of these trials. A pooled analysis of Checkmate studies 017, 057, and 063 showed that a total of 46 patients with treated intracranial disease received nivolumab; of these, one third did not have intracranial progression at the time of systemic disease progression or last tumor assessment. There appeared to be no difference in OS between brain metastases patients treated with nivolumab and docetaxel.29 Exploratory analyses of the OAK study revealed that patients with asymptomatic brain metastases had longer median OS when treated with atezolizumab than with docetaxel (OS 16 vs 11.9 mo)30; these patients also had lower probability of developing new symptomatic lesions with atezolizumab. To date, clinical data on immunotherapy in untreated NSCLC intracranial metastases mainly consist of case reports31,32 and retrospective studies. The only prospective trial available is a single-institution phase II study looking at pembrolizumab in melanoma and NSCLC brain metastases.15 In this study, patients had to be free of neurological symptoms and NSCLC tumors needed to be PDL-1 positive (≥1%). A total of 18 patients with NSCLC were enrolled, and 6 (33%) had responses, 4 of which were complete and 2 partial. Response rates were similar to what was seen in the systemic setting. An interim analysis of this study showed that PFS in the CNS was 10.7 mo and median OS was 8.9 mo, with 31% of patients living at least 2 yr.33
Other Tumor Types
Although most clinical data on the use of immunotherapy in brain metastases are currently focused on melanoma and NSCLC, many other cancers that frequently give rise to intracranial disease have shown responses to checkpoint inhibitors. For example, atezolizumab was recently FDA approved for the treatment of metastatic triple negative breast cancer in combination with protein-bound paclitaxel for tumors expressing PDL1 in >1% of cells. This was based on a phase III study showing that combination therapy improved PFS and OS when compared to placebo plus protein-bound paclitaxel (median OS 25.0 vs 15.5 mo, HR 0.62 95% CI 0.45-0.86).34 Brain metastases are a very common complication of triple negative breast cancer, occurring in up to 25% of patients.35 Immunotherapy should be tested as a therapeutic modality in this population, especially given the promising results of atezolizumab in NSCLC brain metastases. Additionally, immunotherapy is widely used to treat metastatic renal cell carcinoma, which can spread to the brain in 10% to 20% of cases.36 Possible approved regimens include nivolumab plus ipilimumab37 and pembrolizumab plus axitinib,38 both of which were found to be superior to tyrosine kinase inhibitors alone. Other regimens under investigation that have shown promise are avelumab plus axitinib,39 single-agent pembrolizumab,40 or atezolizumab plus bevacizumab.41 Although the brain metastases population has not been extensively studied, a phase II trial of single-agent nivolumab in metastatic renal cell carcinoma showed that intracranial responses were only 12%, and 50% of patients progressed through therapy.42
Synergistic Effects With Other Treatment Modalities
Investigators have attempted to address the question of whether brain metastases patients would benefit from receiving a combination of immunotherapy and other treatment modalities. In melanoma, multiple retrospective reviews have been conducted looking at concurrent stereotactic radiosurgery (SRS) with ipilimumab. A single-institution study of 77 patients who received SRS between 2002 and 2010 showed that individuals who received ipilimumab before or after SRS had a higher 2-yr survival rate than those who did not receive it (47.2% vs 19.7%); ipilimumab treatment was found to be an independent predictor of longer survival (HR 0.48, 95% CI 0.24-0.93).43 Another study of 91 patients showed similar results; OS was higher in the ipilimumab-treated cohort (15.1 vs 7.8 mo, P = .02), and ipilimumab was a predictor of improved survival in multivariate analysis.44 However, a study comparing patients who received SRS with ipilimumab and those who received SRS alone did not show a benefit in intracranial response rate and 1-yr OS with the addition of ipilimumab.45 Given the retrospective nature of these analyses, it is not surprising that results differ between reports. Anti-PD1 agents have also been explored in this setting. A study comparing 3 cohorts of patients treated with SRS and ipilimumab, SRS and pembrolizumab, and SRS alone showed that the pembrolizumab cohort had the highest response rate (62% for pembrolizumab vs 32% for ipilimumab vs 23% for control).46 Additionally, a study of 198 patients with BRAF-mutant tumors who received SRS showed that the use of PD1 inhibitors was associated with improved survival from primary diagnosis, from brain metastases diagnosis, and from SRS.47
In addition to melanoma, different groups have investigated the role of concomitant immunotherapy and SRS in NSCLC. One of these studies compared outcomes of patients who received SRS with concurrent or prior immunotherapy with those with chemotherapy and did not detect differences in survival.48 Another study of 150 patients with brain metastases, 80% of whom at NSCLC, showed that patients on combination therapy had higher response rates and more durable responses when compared to SRS alone.49 Two subsequent studies analyzed the effect of timing of PD-1-directed immunotherapy in relation to the administration of SRS. One study of 27 patients found that individuals who received SRS at the same time as immunotherapy had longer OS than people who received it before or after (1-yr OS 87.3% vs 0% vs 70%).50 Another study of 17 patients found that the rate of distant brain control was 57% for patients who received SRS during or prior to immunotherapy and 0% for those who received it after.51
The encouraging data described above prompted the initiation of multiple prospective clinical trials dedicated to answering this question (Table 2).
TABLE 2.
Ongoing Clinical Trials of Checkpoint Inhibitors in Brain Metastases
| Melanoma | ||
|---|---|---|
| Trial number | Phase | Interventions |
| NCT02858869 | I | Pembrolizumab ± SRS |
| NCT02716948 | I | SRS + nivolumab in newly diagnosed patients |
| NCT02097732 | II | Ipilimumab induction in patients receiving SRS |
| NCT02374242 | II | Nivolumab ± ipilimumab |
| NCT03728465 | II | Nivolumab + ipilimumab in patients with >4 symptomatic brain metastases |
| NCT03175432 | II | Bevacizumab + atezolizumab in untreated patients |
| NCT02460068 | II | Fotemustine vs fotemustine + ipilimumab or ipilimumab + nivolumab |
| NCT02681549 | II | Bevacizumab + pembrolizumab in untreated patients |
| NCT03563729 | II | Pembrolizumab or ipilimumab + nivolumab in patients in need of steroid treatment |
| NCT03340129 | II | Ipilimumab + nivolumab + salvage radiotherapy |
| Nonsmall cell lung cancer | ||
| NCT02858869 | I | Pembrolizumab ± SRS |
| NCT02681549 | II | Bevacizumab + pembrolizumab in untreated patients |
| NCT02978404 | II | Nivolumab + SRS |
| NCT03325166 | II | Pembrolizumab + MRI with ferumoxytol |
| NCT02696993 | I/II | Ipilimumab + SRS |
Ongoing prospective clinical trials looking at immunotherapy in melanoma and nonsmall cell lung cancer brain metastases. A number of trials are investigating the role of combining checkpoint inhibitors with stereotactic radiosurgery.
Another important question in this field is whether craniotomy to resect symptomatic lesions can improve outcomes in patients receiving checkpoint inhibitors. Surgery could lead to an improvement in patients’ performance status and reduce the use of corticosteroids. A single-institution study identified 12 melanoma patients who received ipilimumab in close proximity to craniotomy.52 Nine of these patients had improvement in their performance status after surgery, and 3 out of 6 patients using corticosteroids were able to taper them. Ipilimumab did not lead to unexpected surgical complications. Given the small sample size of this study, no conclusions could be made regarding disease-specific outcomes. Another study of 142 patients with melanoma brain metastases treated with checkpoint inhibitors found that immunotherapy-naïve patients who received surgery prior to immunotherapy had longer median OS than patients treated with immunotherapy alone or immunotherapy followed by surgery (22.7 mo, 95% CI 12.6-39.2 vs 10.8 mo, 95% CI 7.8-16.3 vs 9.4 mo, 95% CI 4.1-∞).53 The timing of immunotherapy administration in relation to craniotomy may also have a significant effect on outcomes. In gliomas, studies have suggested that neoadjuvant immunotherapy can modify the tumor microenvironment and improve survival in patients with resectable disease.54,55 As with SRS, controlled prospective studies are necessary to determine whether craniotomy can lead to better outcomes in patients receiving checkpoint inhibitors.
Toxicity
Immune checkpoint inhibitor therapy can lead to a spectrum of adverse events. Increased T-cell activation can cause autoimmune reactions against host organs, a phenomenon that is seen more frequently in patients with underlying autoimmune disease.56 The incidence of severe immune-related adverse events is higher with anti-CTLA4 therapy than anti-PD1 therapy (27% vs 16%), and it can be as high as 55% when these agents are combined.57 Although autoimmune adverse reactions can affect every organ, they more commonly occur in the skin, gastrointestinal tract, endocrine system, and lungs. Rashes tend to arise in the first weeks after initiation of therapy and they frequently manifest as an eczema-like rash or vitiligo. However, in rare cases, severe reactions including blistering disorder, Steven-Johnson syndrome, and toxic epidermal necrolysis can occur. Gastrointestinal manifestations include colitis and hepatitis; both are more common with anti-CTLA4 therapy than anti-PD1 agents. Colitis can range from mild abdominal discomfort to severe diarrhea with peritoneal signs and even intestinal perforation. Endocrine abnormalities are also more common with anti-CTLA4 agents and include thyroid dysfunction (both hyper- and hypothyroidism), hypophysitis, and adrenal insufficiency. Adrenal insufficiency can be severe and present as adrenal crisis.57 Lung toxicity (pneumonitis) occurs more frequently with anti-PD1 agents and can be severe (even fatal) despite treatment with immunosuppressive agents.57 The management of immune-related adverse events can be limited to interruption of therapy and supportive care in mild cases; however, it often requires immunosuppression with corticosteroids. In severe cases, patients are hospitalized and treated with other agents, including infliximab, mycophenolate mofetil, or cyclophosphamide.57 Grade 3 or 4 adverse events occur in only 1% of cases or less.
Patients with brain metastases receiving checkpoint inhibitors are susceptible to all the adverse events mentioned above with the addition of potential CNS-specific toxicity. A few studies have reported increased rates of radiation necrosis in individuals receiving concomitant SRS and immunotherapy. A retrospective study of 180 patients with brain metastases from melanoma, lung, breast, renal, and colorectal cancers who received Gamma Knife (Elekta) together with other forms of therapy showed that the rate of radiation necrosis was 37.5% with immunotherapy vs 25% and 16% with targeted therapy and chemotherapy, respectively.58 The odds ratio to develop radiation necrosis with immunotherapy was 2.4 (95% CI 1.06-5.44) in univariate analysis and 2.71 (95% CI 0.94-7.76) in multivariate analysis. In this study, patients who developed radiation necrosis had significantly longer median OS (23.7 vs 9.9 mo).58 Other studies looking at melanoma patients treated with anti-CTLA4 therapy and SRS found the rates of radiation necrosis to be 30% and 21%45,59; this is significantly higher than the historical control rate of 6% to 9%.60 It is still unclear whether immunotherapy increases the risk of clinically significant or severe radiation necrosis and whether this is a marker of response. Prospective studies are needed to further address this question.
Assessment of Intracranial Response and Limitations
Until recently, there were no standardized criteria to assess for therapeutic response in brain metastases. This was primarily because of the scarcity of brain metastases-specific trials and the small numbers of patients included in large cancer studies. Assessments were based on established methods, including RECIST, WHO, and Macdonald.61 These were limited in the evaluation of intracranial disease because of the variation in imaging modalities and time intervals, choice of unidimensional vs bidimensional measurements, and the exclusion of important factors such as neurological symptoms and corticosteroid use. In 2015, the RANO brain metastases group proposed response criteria specifically designed for this population (Table 3).61,62 These criteria established that response assessment should be made using unidimensional measurements and that measurable disease should have a minimum size of 10 mm. The same method should be used for all measurements, and gadolinium-enhanced MRI is encouraged. Measurements should be made in target lesions at 6- to 12-wk intervals. Response is determined based on a combination of tumor measurements, neurological symptom, and corticosteroid use.
TABLE 3.
RANO Brain Metastases61
| Complete | Partial | Progressive | Stable | |
|---|---|---|---|---|
| response | response | disease | disease | |
| Target lesion size | Disappearance of all lesions | 30% or more decrease in the sum longest diameter | 20% or more increase in the sum longest diameter; at least one lesion increased by 5 mm | 30% or more decrease from baseline but 20% in sum longest diameter from nadir |
| New lesions | None | None | Yes | None |
| Corticosteroid use | No use | Stable to decreased | N/A | Stable to decreased |
| Neurological symptoms | Stable or improved | Stable or improved | Worse | Stable or improved |
Criteria to assess for intracranial response in brain metastases and with the use of immunotherapy. Reprinted from Lin et al61 with permission from Elsevier.
The use of immunotherapy in intracranial disease added a new challenge. Investigators in early trials of CTLA4 and PD1 inhibitors in metastatic melanoma noticed an unusual pattern of response that was not previously seen with other agents. In the first months of therapy, some patients developed larger and more numerous lesions that met criteria for progression based on standard methods. After a few months, however, these patients had significant and durable responses.63 This phenomenon, termed “pseudoprogression,” is thought to be related to inflammatory infiltrates, making tumors appear larger in the initial phases of therapy. Pseudoprogression was observed in metastatic brain tumors treated with immunotherapy,64,65 leading to modifications in the RANO criteria for these patients (iRANO).62,66 The iRANO dictates that if a scan performed less than 6 mo from the start of immunotherapy shows progressive disease, more treatment is allowed if the patient is clinically stable. Additionally, new lesions detected in this time window do not indicate progression. In order to confirm progressive disease, a repeat scan is required at least 3 mo from the initial one (Table 4).
TABLE 4.
RANO Immunotherapy66
| 6 mo or less from | More than 6 mo from | |
|---|---|---|
| start of immunotherapy | start of immunotherapy | |
| New lesions indicate progression | No | Yes |
| Treatment continuation allowed if scans show progression (and clinically stable) | Yes | No |
| Repeat scan required to confirm progression | Yes, at least 3 mo from initial one | No |
Criteria to assess for intracranial response in brain metastases and with the use of immunotherapy. Reprinted from Okada et al,66 Copyright (2015), with permission from Elsevier.
CONCLUSION
Brain metastases are a dire complication of cancer that may be increasing in incidence because of improved therapies for systemic disease. In the past decade, the treatment of metastatic melanoma and NSCLC has been revolutionized by the introduction of checkpoint inhibitors, prolonging survival and leading to long-term responses that, in some cases, lasted for many years. Although brain metastases patients were not included in many of the original immunotherapy studies, more and more data are pointing to the fact that these agents may be effective at treating intracranial disease. Here, we reviewed clinical studies looking at checkpoint inhibitors in melanoma and NSCLC brain metastases. We also addressed methods to determine intracranial response in patients on immunotherapy and immunotherapy-specific toxicities. Available data for melanoma are more comprehensive, and they include a larger number of prospective studies, albeit with a small number of patients. It appears that intracranial response rates for brain metastases are similar to systemic ones for both anti-CTLA4 and anti-PD1 therapy; however, many patients do progress intracranially even after initial responses. Combination therapy (anti-CTLA4 and anti-PD1) yielded a striking 57% response rate, but with a large number of serious adverse events. Data for NSCLC are more limited, and it is yet to be determined whether checkpoint inhibitors as single agents are effective at treating this disease. Lastly, a number of other tumor types that often spread to the brain were found to be responsive to checkpoint inhibitors, but results in the brain metastasis population have not been extensively investigated. A number of retrospective studies suggest that combining SRS with immunotherapy can improve locoregional control and even prolong survival in both melanoma and NSCLC. However, this may come with an increased risk of radiation necrosis. The exact biological mechanism of action of checkpoint inhibitors in the CNS is yet to be determined, but it is likely that the clinical effect derives from activated lymphocytes infiltrating the CNS.67 The synergistic effect with radiation is supported by preclinical data demonstrating that radiotherapy can lead to the local release of tumor antigens and inflammatory signals, causing activation of tumor-specific T cells.68 Many prospective trials are currently underway to determine whether checkpoint inhibitors with or without SRS are a valuable first-line therapeutic approach for brain metastases. This may be a stepping stone to improving standard of care regimens for a patient population with very limited therapeutic options.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Brastianos receives research funding from Merck, Lilly, Pfizer, and Bristol-Myers Squibb; has consulted for AngioChem, ElevateBio, Tesaro, Lilly, and Genentech-Roche; received speaker honoraria from Genentech and Merck; and receives grant support from Damon Runyon Cancer Research Foundation, Breast Cancer Research Foundation, and NIH (1R01CA227156-01, 5R21CA220253-02, and 1R01CA244975-01).
Contributor Information
Elisa Aquilanti, Division of Hematology/Oncology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts; Division of Neuro-Oncology, Department of Neurology, Stephen E. Catherine Pappas Center for Neuro Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts; Department of Medical Oncology, Dana Farber Cancer Institute, Boston, Massachusetts; Cancer Center, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts; Cancer Program, Broad Institute, Boston, Massachusetts.
Priscilla K Brastianos, Division of Hematology/Oncology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts; Division of Neuro-Oncology, Department of Neurology, Stephen E. Catherine Pappas Center for Neuro Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts; Cancer Center, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts; Cancer Program, Broad Institute, Boston, Massachusetts.
REFERENCES
- 1. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54. [DOI] [PubMed] [Google Scholar]
- 2. Arvold ND, Lee EQ, Mehta MP et al.. Updates in the management of brain metastases. Neuro Oncol. 2016;18(8):1043-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways. Am J Clin Oncol. 2016;39(1):98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734-1736. [DOI] [PubMed] [Google Scholar]
- 6. Hodi FS, O’Day SJ, McDermott DF et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kleffel S, Posch C, Barthel SR et al.. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162(6):1242-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margolin K, Ernstoff MS, Hamid O et al.. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465. [DOI] [PubMed] [Google Scholar]
- 9. Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. 2019;37(22):1927-1934. [DOI] [PubMed] [Google Scholar]
- 10. Moran TJ, Gray S, Mikosz CA, Conzen SD. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res. 2000;60(4):867-872. [PubMed] [Google Scholar]
- 11. Stringer-Reasor EM, Baker GM, Skor MN et al.. Glucocorticoid receptor activation inhibits chemotherapy-induced cell death in high-grade serous ovarian carcinoma. Gynecol Oncol. 2015;138(3):656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiocca EA, Yu JS, Lukas RV et al.. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med. 2019;11(505):eaaw5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schachter J, Ribas A, Long GV et al.. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet North Am Ed. 2017;390(10105):1853-1862. [DOI] [PubMed] [Google Scholar]
- 14. Robert C, Long GV, Brady B et al.. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330. [DOI] [PubMed] [Google Scholar]
- 15. Goldberg SB, Gettinger SN, Mahajan A et al.. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kluger HM, Chiang V, Mahajan A et al.. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol. 2019;37(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tawbi HA, Forsyth PA, Algazi A et al.. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Long GV, Atkinson V, Lo S et al.. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672-681. [DOI] [PubMed] [Google Scholar]
- 19. Garon EB, Rizvi NA, Hui R et al.. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. [DOI] [PubMed] [Google Scholar]
- 20. Herbst RS, Baas P, Kim DW et al.. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet North Am Ed. 2016;387(10027):1540-1550. [DOI] [PubMed] [Google Scholar]
- 21. Langer CJ, Gadgeel SM, Borghaei H et al.. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gandhi L, Rodriguez-Abreu D, Gadgeel S et al.. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. [DOI] [PubMed] [Google Scholar]
- 23. Socinski MA, Jotte RM, Cappuzzo F et al.. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. [DOI] [PubMed] [Google Scholar]
- 24. Rittmeyer A, Barlesi F, Waterkamp D et al.. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet North Am Ed. 2017;389(10066):255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brahmer J, Reckamp KL, Baas P et al.. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hellmann MD, Ciuleanu TE, Pluzanski A et al.. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antonia SJ, Villegas A, Daniel D et al.. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. [DOI] [PubMed] [Google Scholar]
- 29. Jonathan Wade Goldman LC, Everett EV, Esther H et al.. Nivolumab (nivo) in patients (pts) with advanced (adv) NSCLC and central nervous system (CNS) metastases (mets). J Clin Oncol. 2016;34(15_suppl):9038-9038. [Google Scholar]
- 30. Gadgeel SM, Lukas RV, Goldschmidt J et al.. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: exploratory analyses of the phase III OAK study. Lung Cancer. 2019;128:105-112. [DOI] [PubMed] [Google Scholar]
- 31. Di M, Zhang L. Pembrolizumab for non-small cell lung cancer with central nervous system metastases: a two-case report. Thorac Cancer. 2019;10(2):381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pluchart H, Pinsolle J, Cohen J et al.. Partial response of pulmonary adenocarcinoma with symptomatic brain metastasis to nivolumab plus high-dose oral corticosteroid: a case report. J Med Case Rep. 2017;11(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldberg S, Gettinger S, Mahajan A et al.. Durability of brain metastasis response and overall survival in patients with non-small cell lung cancer (NSCLC) treated with pembrolizumab. J Clin Oncol 2018;36(15_suppl):2009-2009.29787359 [Google Scholar]
- 34. Schmid P, Adams S, Rugo HS et al.. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108-2121. [DOI] [PubMed] [Google Scholar]
- 35. Jin J, Gao Y, Zhang J et al.. Incidence, pattern and prognosis of brain metastases in patients with metastatic triple negative breast cancer. BMC Cancer. 2018;18(1):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowman A, Le T, Christie A, Brugarolas J. Incidence of brain metastases in metastatic renal cell carcinoma in the era of targeted therapies. J Clin Oncol. 2016;34(15_suppl):e16103. [Google Scholar]
- 37. Motzer RJ, Tannir NM, McDermott DF et al.. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rini BI, Plimack ER, Stus V et al.. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. [DOI] [PubMed] [Google Scholar]
- 39. Motzer RJ, Penkov K, Haanen J et al.. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McDermott D, Lee J, Ziobro M et al.. First-line pembrolizumab (pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): results from KEYNOTE-427 cohort B. J Clin Oncol. 2019;37(7_suppl.):546. [Google Scholar]
- 41. Rini BI, Powles T, Atkins MB et al.. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet North Am Ed. 2019;393(10189):2404-2415. [DOI] [PubMed] [Google Scholar]
- 42. Reed J, Posadas E, Figlin R. Brain metastases in renal cell carcinoma: immunotherapy responsiveness is multifactorial and heterogeneous. J Clin Oncol. 2019;37(23):1987-1989. [DOI] [PubMed] [Google Scholar]
- 43. Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117(2):227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Diao K, Bian SX, Routman DM et al.. Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. J Neurooncol. 2018;139(2):421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patel KR, Shoukat S, Oliver DE et al.. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol. 2017;40(5):444-450. [DOI] [PubMed] [Google Scholar]
- 46. Anderson ES, Postow MA, Young R, Chan TA, Yamada Y, Beal K. Initial report on safety and lesion response of melanoma brain metastases after stereotactic radiosurgery or hypofractionated radiation therapy in patients receiving concurrent pembrolizumab. Int J Radiat Oncol Biol Phys. 2016;96(2):E132. [DOI] [PubMed] [Google Scholar]
- 47. Mastorakos P, Xu Z, Yu J et al.. BRAF V600 mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases: a multicenter retrospective study. Neurosurgery. 2019;84(4):868-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh C, Qian JM, Yu JB, Chiang VL. Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in non-small cell lung cancer with brain metastases. J Neurosurg. 2019;132(2):333-679. [DOI] [PubMed] [Google Scholar]
- 49. Kotecha R, Kim JM, Miller JA et al.. The impact of sequencing PD-1/PD-L1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol 2019;21(8):1060-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schapira E, Hubbeling H, Yeap BY et al.. Improved overall survival and locoregional disease control with concurrent pd-1 pathway inhibitors and stereotactic radiosurgery for lung cancer patients with brain metastases. Int J Radiat Oncol Biol Phys. 2018;101(3):624-629. [DOI] [PubMed] [Google Scholar]
- 51. Ahmed KA, Kim S, Arrington J et al.. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J Neurooncol. 2017;133(2):331-338. [DOI] [PubMed] [Google Scholar]
- 52. Jones PS, Cahill DP, Brastianos PK, Flaherty KT, Curry WT. Ipilimumab and craniotomy in patients with melanoma and brain metastases: a case series. Neurosurg Focus. 2015;38(3):E5. [DOI] [PubMed] [Google Scholar]
- 53. Alvarez-Breckenridge C, Giobbie-Hurder A, Gill CM et al.. Upfront surgical resection of melanoma brain metastases provides a bridge toward immunotherapy-mediated systemic control. Oncologist. 2019;24(5):671-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cloughesy TF, Mochizuki AY, Orpilla JR et al.. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R et al.. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470-476. [DOI] [PubMed] [Google Scholar]
- 56. Johnson DB, Chandra S, Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320(16):1702-1703. [DOI] [PubMed] [Google Scholar]
- 57. Marin-Acevedo JA, Chirila RM, Dronca RS. Immune checkpoint inhibitor toxicities. Mayo Clin Proc. 2019;94(7):1321-1329. [DOI] [PubMed] [Google Scholar]
- 58. Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016;125(1):17-23. [DOI] [PubMed] [Google Scholar]
- 59. Kiess AP, Wolchok JD, Barker CA et al.. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sneed PK, Mendez J, Fogh SE, Barani IJ, Ma L, McDermott MW. Risk factors for radiation necrosis after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2012;84(3):S118-S119. [Google Scholar]
- 61. Lin NU, Lee EQ, Aoyama H et al.. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270-e278. [DOI] [PubMed] [Google Scholar]
- 62. Chukwueke U, Wen P. Use of the response assessment in neuro-oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019;8(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seymour L, Bogaerts J, Perrone A et al.. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cohen JV, Alomari AK, Vortmeyer AO et al.. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res. 2016;4(3):179-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Simard JL, Smith M, Chandra S. Pseudoprogression of melanoma brain metastases. Curr Oncol Rep. 2018;20(11):91. [DOI] [PubMed] [Google Scholar]
- 66. Okada H, Weller M, Huang R et al.. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534-e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol. 2017;13(12):755-763. [DOI] [PubMed] [Google Scholar]
- 68. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325-1332. [DOI] [PubMed] [Google Scholar]


