Abstract
BACKGROUND
Stereotactic radiosurgery (SRS) is a safe and effective treatment for acromegaly.
OBJECTIVE
To improve understanding of clinical and dosimetric factors predicting biochemical remission.
METHODS
A single-institution cohort study of nonsyndromic, radiation-naïve patients with growth hormone-producing pituitary adenomas (GHA) having single-fraction SRS between 1990 and 2017. Exclusions were treatment with pituitary suppressive medications at the time of SRS, or <24 mo of follow-up. The primary outcome was biochemical remission—defined as normalization of insulin-like growth factor-1 index (IGF-1i) off suppression. Biochemical remission was assessed using Cox proportional hazards. Prior studies reporting IGF-1i were assessed via systematic literature review and meta-analysis using random-effect modeling.
RESULTS
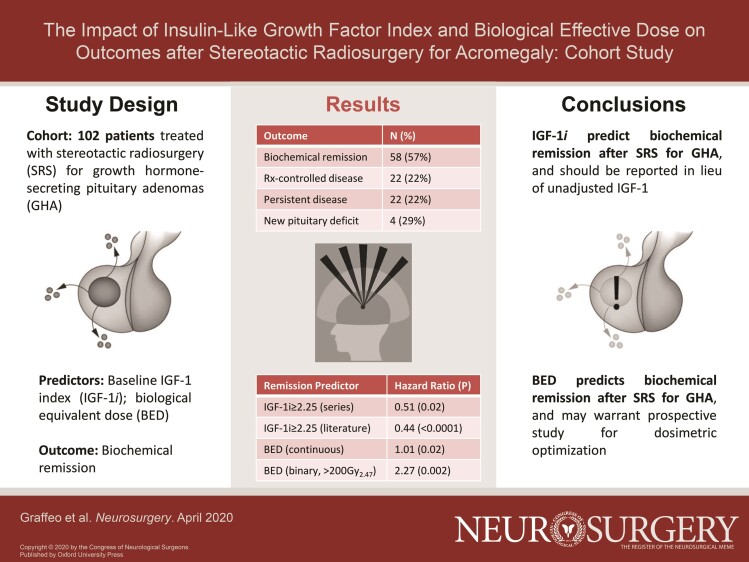
A total of 102 patients met study criteria. Of these, 46 patients (45%) were female. The median age was 49 yr (interquartile range [IQR] = 37-59), and the median follow-up was 63 mo (IQR = 29-100). The median pre-SRS IGF-1i was 1.66 (IQR = 1.37-3.22). The median margin dose was 25 Gy (IQR = 21-25); the median estimated biologically effective dose (BED) was 169.49 Gy (IQR = 124.95-196.00). Biochemical remission was achieved in 58 patients (57%), whereas 22 patients (22%) had medication-controlled disease. Pre-SRS IGF-1i ≥ 2.25 was the strongest predictor of treatment failure, with an unadjusted hazard ratio (HR) of 0.51 (95% CI = 0.26-0.91, P = .02). Number of isocenters, margin dose, and BED predicted remission on univariate analysis, but after adjusting for sex and baseline IGF-1i, only BED remained significant—and was independently associated with outcome in continuous (HR = 1.01, 95% CI = 1.00-1.01, P = .02) and binary models (HR = 2.27, 95% CI = 1.39-5.22, P = .002). A total of 24 patients (29%) developed new post-SRS hypopituitarism. Pooled HR for biochemical remission given subthreshold IGF-1i was 2.25 (95% CI = 1.33-3.16, P < .0001).
CONCLUSION
IGF-1i is a reliable predictor of biochemical remission after SRS. BED appears to predict biochemical outcome more reliably than radiation dose, but confirmatory study is needed.
Keywords: Stereotactic radiosurgery, Acromegaly, Growth hormone, Insulin-like growth factor, Biologically effective dose, Dosimetry, Radiobiology, Evidence-based medicine
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- AICc
corrected Akaike information criterion
- AVP
anterior visual pathway
- BED
biologically effective dose
- CI
confidence interval
- CT
computed tomography
- GH
growth hormone
- GHA
growth hormone-producing pituitary adenoma
- HR
hazard ratio
- IGF-1i
insulin-like growth factor-1 index
- IGKRF
International Gamma Knife Research Foundation
- IQR
interquartile range
- MRI
magnetic resonance imaging
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analysis
- SRS
stereotactic radiosurgery
- ULN
upper limit of normal
Over the past 30 yr, stereotactic radiosurgery (SRS) has been established as a safe and effective treatment for patients with residual or recurrent growth hormone-secreting pituitary adenomas (GHA).1-5 Factors that may predict biochemical remission after SRS include pre-SRS insulin-like growth factor-1 (IGF-1) levels, IGF-1 index (IGF-1i), use of pituitary suppressive medications at the time of SRS, and radiation dose. Biologically effective dose (BED) is a radiobiological parameter that estimates the stochastic risk of injury to the target tissue.6-8 Unlike prescribed radiation dose, BED incorporates a time correction factor that accounts for DNA repair during irradiation. BED is significantly associated with enhanced cell kill in Vitro, as well as normal tissue toxicity in animal models.6,9-14 BED estimation has traditionally been a daunting practice; however, a simplified estimation method has recently been reported, providing a framework to analyze the effect of BED on clinical outcomes after SRS.7 We analyzed clinical and dosimetric variables in a longitudinally followed cohort of patients with GHA having single-fraction SRS, in order to test the study hypothesis that BED better predicts SRS outcomes in GHA, as compared to margin dose. Our analysis constitutes the most comprehensive study of IGF-1i as a predictor of SRS treatment response, and is the first investigation of BED in a pituitary adenoma cohort.
METHODS
Patient Selection and Study Cohort
The current study was approved by our Institutional Review Board, including a minimal-risk waiver of consent. A prospectively maintained registry identified 190 GHA patients having SRS between 1990 and 2017 (Figure 1). Acromegaly was diagnosed by board-certified endocrinologists. Exclusions were patients who declined state-mandated research authorization (n = 7), absent baseline endocrine data (n = 15), history of prior radiotherapy/SRS (n = 12), SRS with computed tomography (CT)-only planning (n = 8), pituitary-suppressive medication treatment at time of SRS (n = 7), or hereditary neuroendocrine syndrome diagnoses (n = 5). Adequate endocrine and radiographic follow-up of ≥24 mo was confirmed in 101 patients; 1 additional patient was identified as an early treatment failure and included (Table 1).
FIGURE 1.
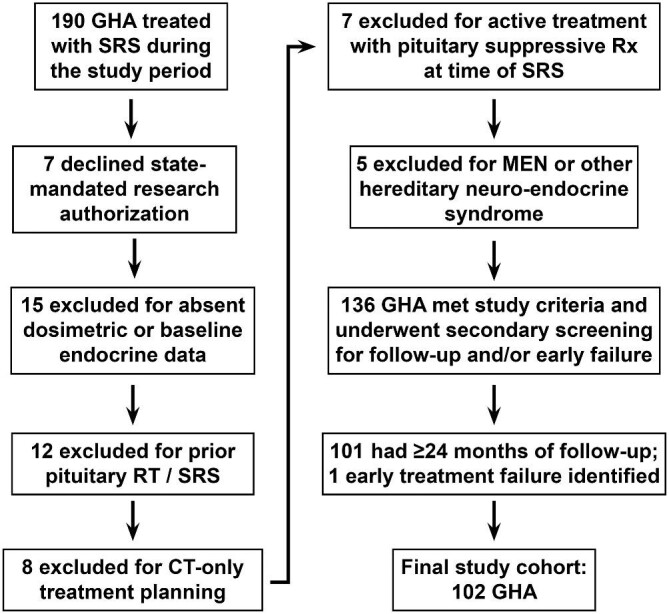
Schematic depiction of study cohort inclusion and exclusion criteria.
TABLE 1.
Patient Characteristics
| Factor | N (%) |
|---|---|
| Female sex | 46 (45%) |
| Median age, yr (IQR) | 49 (37-59) |
| Median maximum tumor diameter, mm (IQR) | 20 (17-28) |
| History of prior resection | 100 (98%) |
| Pre-SRS anterior pituitary deficit | 20 (20%) |
| Baseline acromegaly laboratory studies | |
| GH, ng/mL (IQR) | 4.7 (1.88-9.58) |
| IGF-1, ng/mL (IQR) | 552 (189-1244) |
| IGF-1ia, scale (IQR) | 1.66 (1.37-3.22) |
| IGF-1i ≥ 2.25 | 31 (30%) |
| Total post-SRS endocrine and radiographic | 63 (29-100) |
| follow-up, mo (IQR) |
GH, growth hormone; IGF-1, insulin-like growth factor-1; IQR, interquartile range; SRS, stereotactic radiosurgery.
aIGF-1i is defined as patient laboratory value divided by their age- and sex-adjusted upper limit of normal.
Radiosurgical Technique
SRS was administered in a single fraction for all patients, using the Leksell Gamma Knife® (Elekta Instruments, Elekta AB, Stockholm, Sweden) models U, B, C, and Perfexion. Relative conformality indices were not captured in this analysis, but as we and others have previously reported, treatment efficiency and accuracy were generally superior using the Perfexion system, with all preceding models demonstrating a marked comparative increase in treatment time.15-17 After placement of a stereotactic headframe, magnetic resonance imaging (MRI) alone or co-registered with thin-section head CT was performed. Dose planning was carried out using KULA (Elekta Instruments) until April 1993, or using Leksell GammaPlan® (Elekta Instruments) after April 1993. The pituitary and anterior visual pathways (AVP) were segmented for dose-volume analysis. Dose planning goals were complete tumor coverage and minimized radiation exposure to the gland and AVP (Table 2).18 Margin dose was 25 Gy whenever possible, based on tumor volume and proximity to AVP (achieved in n = 75 treatments, 74%).19 Sector blocking was employed to maintain the maximum AVP point dose <12 Gy.20
TABLE 2.
Dosimetric Variables
| Variable | Median (IQR) |
|---|---|
| Number of isocenters | 8 (6-11) |
| PIV, cm3 | 2.75 (1.5-5.6) |
| Margin dose, Gy | 25 (21-25) |
| Maximum dose, Gy | 50 (45-50) |
| TDR, Gy/m | 2.788 (2.408-3.232) |
| Treatment time, m | 94.7 (73.3-126.0) |
| BED, Gy2.47 | 169.49 (124.95-196.00) |
| Maximum point dose to the anterior | 10 (8.7-11.2) |
| visual pathway, Gy |
BED, biologically effective dose; IQR, interquartile range; PIV, prescription isodose volume; TDR, treatment dose rate.
Follow-up and Study Endpoints
Endocrine assessment was performed at baseline, 6 mo, and 12 mo following SRS, and then yearly. Standard post-SRS radiographic follow-up included MRI at 6, 12, 24, and 48 mo after SRS, and then every 3 to 5 yr thereafter. IGF-1i was calculated for all patients at all available time points. For each observation, assay-specific tables were acquired from the manufacturer/laboratory to confirm the appropriate age- and sex-adjusted upper limit of normal (ULN) for that patient and date; this value provided the denominator, while the patient's test result provided the numerator (eg, a 30-yr-old woman was treated in 2009; at that time, her reference ULN was 321, and her test value was 803, resulting in IGF-1i = 803/321 = 2.50, or highly abnormal. At the last follow-up in 2017, at 38 yr of age and with a different test in use at our institution, her updated reference ULN was 258, and her test value was 85, resulting in IGF-1i = 85/258 = 0.33, or cured [off suppressive medications]). Biochemical remission was defined as IGF-1i ≤ 1, off all pituitary suppressive medications; patients with normalized IGF-1i who either failed or declined a trial off suppression were reported as “medication-controlled disease,” but analyzed as treatment failure equivalents. Estimated BED was calculated as described by Jones and Hopewell7 using the α/β ratio Gy2.47. More specifically, we used the parameter estimates provided for the monoexponential fit equation, along with the equations and BED curves reported to approximate individual BED estimation for each unique combination of treatment time and margin dose (see Jones and Hopewell,7 Table 2, and Figure 7). For patients treated with the Perfexion system, the summative beam on time reported by GammaPlan was used as a treatment time equivalent; for older systems, treatment time was calculated as the sum of the beam on times for each shot, plus 5 min per shot for n–1 shots.
Statistical Analysis
Descriptive statistics included frequency/proportion for categorical and median/interquartile range (IQR) for continuous variables. Comparative analyses between the study groups was completed using Cox proportional hazards and reported as hazard ratios (HR) with 95% CI and P values (likelihood ratio test).
Variables included age, sex, IGF-1i, number of isocenters, treatment dose rate, treatment time, margin dose, and BED. IGF-1i, number of isocenters, margin dose, and BED were assessed by univariate analysis using continuous and binary data modeling. Binary models were dichotomized using thresholds derived from received operating characteristic tables and the maximum (sensitivity–(1–specificity)). Factors significant in univariate analysis were evaluated using adjusted Cox models, with a maximum of 4 covariates (eg, ≤1 variable/10 events). Co-linear or derived variables were considered in violation of the test assumptions and prohibited from co-inclusion in single models. Models were optimized using backwards stepwise regression, with final models compared using corrected Akaike information criterion (AICc) and Harrell C statistic. Following final model selection, the failure function was calculated for each observation and regressed on BED with an exponential transformation, generating the predicted probability of biochemical remission as a function of BED, adjusted for age and IGF-1i. Statistical testing was performed using STATA 14 (StataCorp, College Station, Texas) or JMP 14 Pro (SAS Institute, Cary, North Carolina). Statistical tests were 2-sided; significance was defined as P < .05.
Systematic Literature Review and Meta-analysis
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were used to query Medline and Embase from inception through 4/2019 (Supplemental Digital Content 1).21 Inclusion criteria were as follows: (1) case series, cohort studies, or clinical trials, (2) including ≥10 GHA treated with SRS, (3) reporting IGF-1i or equivalent, normalized, IGF-1-derived parameter as a predictor of (4) the biochemical remission, which was (5) defined objectively using laboratory parameters, (6) assessed at a median follow-up ≥ 12 mo, and (7) published in English during the study period, 1990-2018.
Primary search identified 118 unique abstracts; all titles and abstracts underwent review by 2 independent investigators (C.S.G., A.P.) and any citations potentially meeting criteria underwent full-text assessment (n = 38; Figure, Supplemental Digital Content 2). Data abstraction was completed in duplicate and included sample size, IGF-1i medians and thresholds, and effect sizes (HR, 95% CI); HRs were pooled using meta-analysis with random-effect modeling. Heterogeneity was assessed using the I2 statistic. Included studies were graded using the modified Newcastle-Ottawa Scale (Supplemental Digital Content 3).22
RESULTS
Biochemical Remission
Biochemical remission off all pituitary suppressive medications was achieved in 58 patients (57%) at a median 19 mo (IQR = 12-41) (Figure 2). Additional 22 patients achieved medication-controlled disease, for an overall control rate of 78% (not shown). Persistent IGF-1i elevation was noted in 22 patients (22%). One early failure was observed was treated with repeat SRS at 18 mo; 4 additional patients underwent repeat SRS for persistent growth hormone (GH) hypersecretion at >24 mo (median 38; range 28-204).
FIGURE 2.
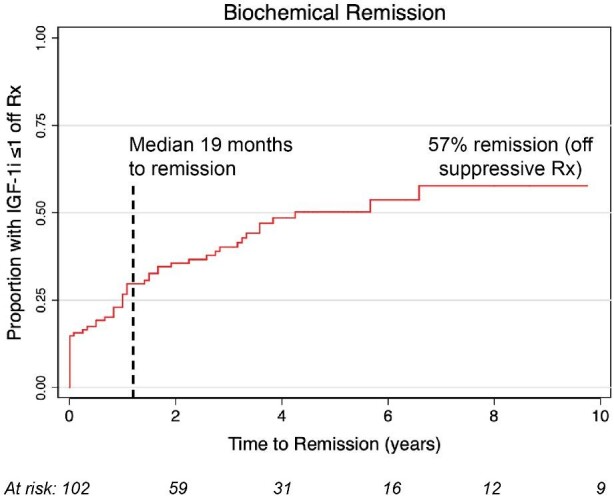
Rate of biochemical remission after radiosurgery.
On univariate analysis, IGF-1i, number of isocenters, margin dose, and BED were significantly associated with biochemical remission (Table 3). IGF-1i modeled as a categorical variable was considered the strongest independent predictor of biochemical remission, as it demonstrated the most extreme statistically significant effect size (HR = 0.51, 95% CI = 0.26-0.91, P < .02) (Figure 3). Based on the univariable analysis, 4 multivariable models were developed and tested (Table, Supplemental Digital Content 4). Models 1 and 2 incorporated sex, IGF-1i, and BED using either continuous (model 1) or categorical (model 2) data modeling; number of isocenters and margin dose were excluded from models 1 and 2, due to co-linearity with BED. Models 3 and 4 incorporated sex, IGF-1i, number of isocenters, and margin dose, again using either continuous (model 3) or categorical (model 4) data modeling; BED was excluded from models 3 and 4, due to co-linearity with margin dose. Models 1 to 4 were compared using likelihood ratio tests for variable HRs, as well as AICc and Harrell C statistic for overall fit, which collectively demonstrated that model 1 was preferred (BED-based, continuous modeling), followed by model 2 (BED-based, categorical modeling).
TABLE 3.
Statistical Analysis of Biologically Remission
| Factor | Univariate analysis HR (95% CI), P value | Model 1: BED, continuous modeling HR (95% CI), P value | Model 2: BED, binary modelinga HR (95% CI), P value | Model 3: margin, continuous modeling HR (95% CI), P value | Model 4: margin, binary modelinga HR (95% CI), P value |
|---|---|---|---|---|---|
| Age | 0.99 (0.97-1.01), 0.35 | NT | NT | NT | NT |
| Sex | 1.87 (1.11-3.19), .02 | 1.92 (1.12-3.30), .02 | 2.22 (1.30-3.84), .004 | 1.94 (1.14-3.33), .02 | 2.16 (1.27-3.70), .005 |
| IGF-1i, scale | 0.53 (0.36-0.75), < .0001 | 0.53 (0.35-0.74), <.0001) | – | 0.54 (0.37-0.77), .0002 | – |
| IGF-1i ≥ 2.25a,b | 0.51 (0.26-0.91), .02 | – | 0.45 (0.23-0.82), .01 | – | – |
| Isocenters | 0.92 (0.85-0.99), .03 | (co-linear w BED) | (co-linear w BED) | 0.97 (0.89-1.04), .40 | 0.95 (0.87-1.03), .22 |
| Isocenters ≥ 11a | 0.75 (0.40-1.32), .32 | NT | NT | NT | NT |
| TDR, Gy/m | 0.91 (0.56-1.47), .69 | NT | NT | NT | NT |
| Treatment time, m | 1.00 (0.99-1.00), .28 | NT | NT | NT | NT |
| Margin dose, Gy | 1.11 (1.01-1.22), .03 | (co-linear w BED) | (co-linear w BED) | 1.09 (0.98-1.24), .10 | – |
| Margin ≥ 18Gyc | 2.36 (0.73-14.51), .18 | (co-linear w BED) | (co-linear w BED) | NT | NT |
| Margin ≥ 25Gya | 1.97 (1.11-3.67), .03 | (co-linear w BED) | (co-linear w BED) | (see model 4) | 1.68 (0.90-3.30), .11 |
| BED, Gy2.47 | 1.01 (1.00-1.01), .05 | 1.01 (1.00-1.01), .02 | – | (co-linear w margin) | (co-linear w margin) |
| BED ≥ 200a | 2.44 (1.24-4.48), .01 | – | 2.72 (1.36-5.13), .006 | (co-linear w margin) | (co-linear w margin) |
BED, biologically effective dose; HR, hazard ratio; NT, not tested; TDR, treatment dose rate; IGF-1i, insulin-like growth factor-1 index.
aVariable dichotomization for binary modeling was defined using received operating characteristic curve analysis, or canticipated clinical utility.
bModel 2 tested with each IGF-1i threshold independently, not simultaneously.
FIGURE 3.
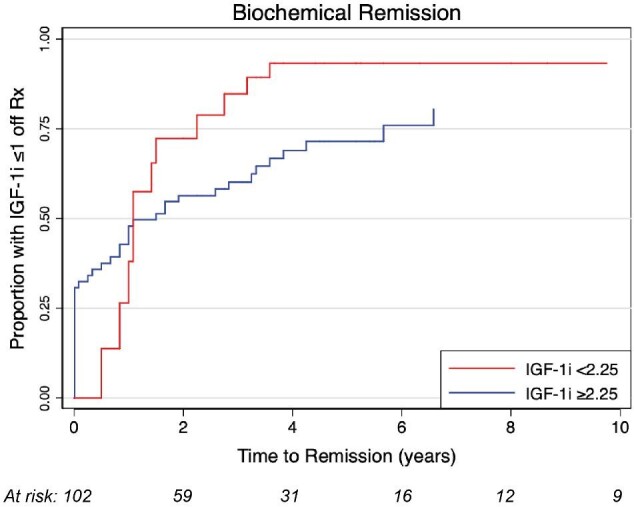
Rate of biochemical remission after radiosurgery based on pre-SRS IGF-1i. Graph showing incidence curves based on IGF-1i < 2.25 vs IGF-1i ≥ 2.25 (HR = 0.51; 95% CI 0.26-0.91; P = .02).
In model 1, continuous BED was associated with an HR of 1.01 (95% CI = 1.00-1.01, P = .02), whereas in model 2, BED dichotomized around the threshold 200Gy2.47 was associated an HR = 2.27 (95% CI = 1.39-5.22, P = .002) (Figure 4A). Estimated failure functions were regressed on BED, which demonstrated a statistically significant linear association (β = 0.002, P < .0001, r = 0.4) (Figure 4B). Thus, for 2 treatment plans that differ in BED by 50, we anticipate a 10% difference in predicted probability of biochemical remission, adjusting for sex and IGF-1i. The model was then exponentially transformed and stratified by margin dose to estimate the probability of biochemical remission as a function of BED for a given prescription isodose (P = .0005) (Figure 4C).
FIGURE 4.
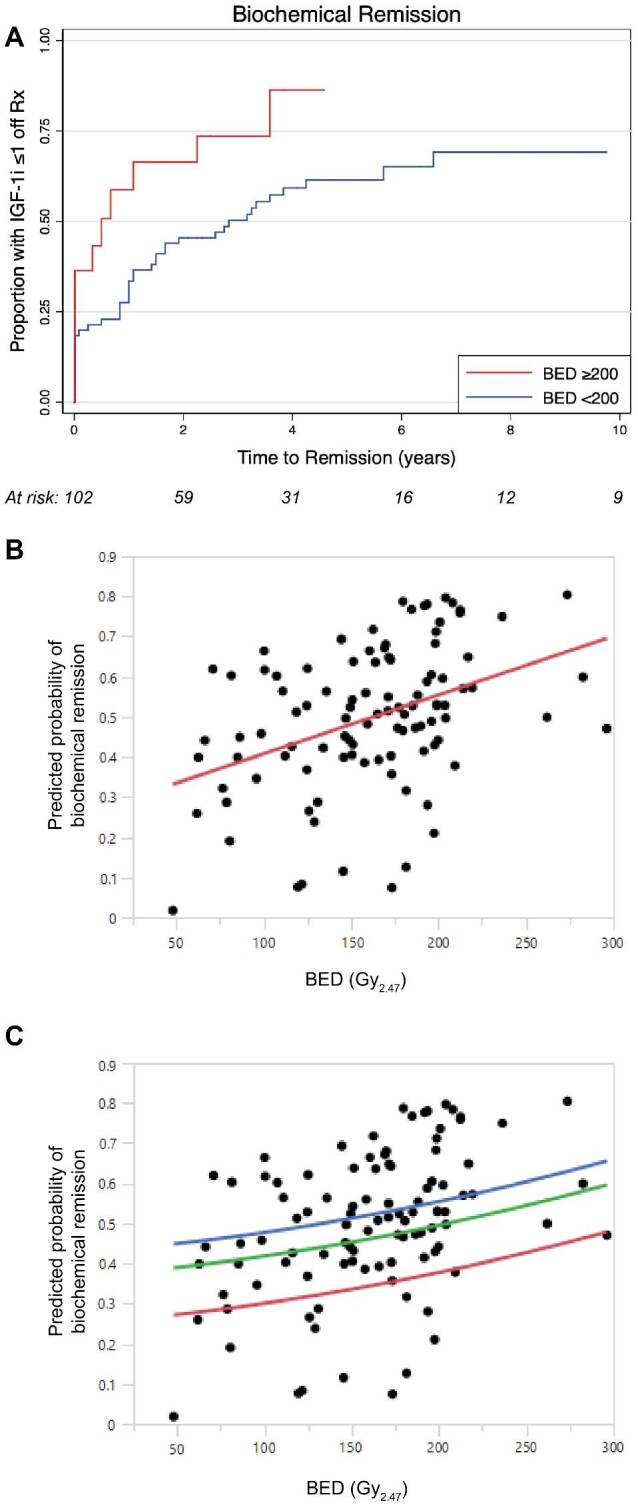
Cox proportional hazard models plotted as the proportion achieving biochemical remission over time since radiosurgery, for A, subgroup analysis stratified by BED ≥ 200. B, Simple linear regression demonstrates a significant (P < .0001) positive association between BED and predicted probability of biochemical remission, as derived from the Cox failure function. C, Exponential transformation.
Complications
Of 82 patients, 24 (29%) with normal pre-SRS anterior pituitary function developed new hypopituitarism at a median 29.5 mo (range 6-143; Figure, Supplemental Digital Content 5). Based on univariate analysis, no statistically significant association between baseline characteristics or dosimetric variables and new deficits was observed (Table, Supplemental Digital Content 6). BED was not associated with increased risk of hypopituitarism. No patient developed new visual loss, cranial nerve deficit, internal carotid artery stenosis, or radiation-induced neoplasm. Visual field assessments incorporated formal testing pre- and post-SRS in 62%; 18% had either pre- or post-SRS testing (but not both). Patients lacking formal testing at either or both time points were assessed based on clinical documentation (eg, confrontational field testing).
Systematic Literature Review and Meta-Analysis
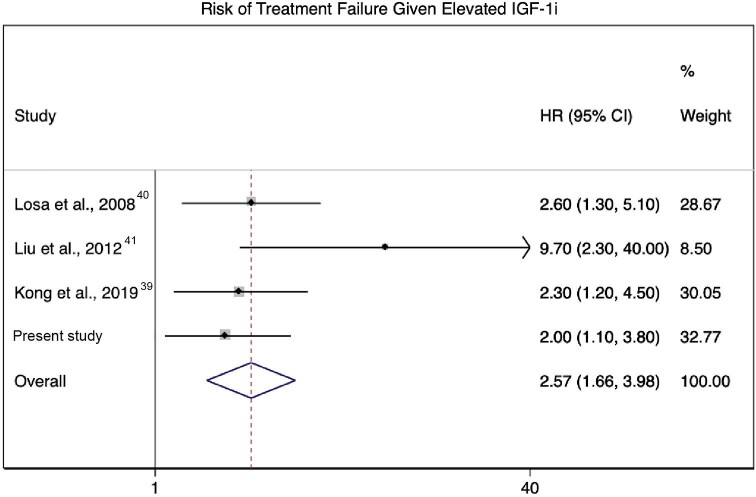
Four retrospective observational studies met criteria, with a median sample size of 65 (range 40-138). Observed median IGF-1i values in the study samples ranged from 1.83 to 2.25. Tested IGF1i thresholds were 1.5, 2.0, 1.83, and 2.25; these resulted in HRs for treatment failure of 9.7, 2.3, 2.6, and 2.9, respectively, with the patients whose IGF-1i levels were above the tested threshold demonstrating an increased risk of treatment failure in all 4 studies (Table 4). Evidence quality using the modified Newcastle-Ottawa Scale was graded as 5 (“high”) for all included studies (Supplemental Digital Content 3). The current study was pooled with 3 of the 4 preceding studies for meta-analysis, with the results reported by Pollock et al5excluded due to overlap with the study cohort. Meta-analysis evaluating the effect measure of HRs for IGF-1i via random-effects modeling with a logarithmic transformation demonstrated a pooled effect size of HR = 2.57 (95% CI = 1.66-3.98, P < .0001) (Figure 5). Heterogeneity assessment demonstrated I2 = 25.6% (P-heterogeneity = 0.26).
TABLE 4.
Summary of Studies Reporting IGF-1 Index As a Predictor of Treatment Failure
| Study | N | Median IGF-1 index (range) | IGF-1i threshold assessed | HR (95% CI), P value |
|---|---|---|---|---|
| Pollock et al, 200737 | 46 | 2.25 (1.05-7.0) | 2.25 | 2.9 (1.2-6.9), .02 |
| Losa et al, 200841 | 83 | 1.83 (1.35-2.56)b | 1.83 | 2.6 (1.3-5.1), .01 |
| Liu et al, 201242 | 40 | 1.9 (0.8-4.6) | 1.5 | 9.7 (2.3-40), .002 |
| Kong et al, 201940 | 138 | NR | 2.0 | 2.3 (1.2-4.5), .01 |
| Present study (IGF-1i ≥ 2.25)a | 102 | 1.66 (1.37-3.22)b | 2.25 | 2.0 (1.1-3.8), .02 |
FIGURE 5.
Forest plot derived from meta-analysis of proportions for studies reporting treatment outcome hazard ratios as a function of elevated IGF-1i(HR = 2.25, 95% CI = 1.33-3.16, P < .0001).
DISCUSSION
IGF-1i As a Prognostic Factor for SRS
There has been considerable debate regarding the predictive value of pre-SRS IGF-1 and GH levels with respect to biochemical remission after SRS for acromegaly. At least 10 single-institution series, 1 multi-national consortium, and 2 systematic reviews have assessed unadjusted baseline IGF-1 and/or GH, with equivocal results.4,23-34 This inconsistency likely reflects confounding due to sample heterogeneity, and the use of unadjusted, inconsistent IGF-1 measurement strategies—within and between studies. Considerable systematic error has been introduced by the failure to normalize IGF-1 values to assay-specific ranges, as these thresholds vary by laboratory/manufacturer. For example, within our institution, at least 5 assays were used during the study period, with the ULN for a hypothetical 49-yr-old woman—the “most representative” patient in our cohort—ranging from 194 to 360.
The recently published results from the multicenter, retrospective study from the International Gamma Knife Research Foundation (IGKRF) benefit from an increase in sample size compared to previous single institution reports, yet this comes at the cost of increased vulnerability to bias.35 Unadjusted IGF-1 was pooled across 10 centers over a 26-yr study period, and reported as simple mean pretreatment IGF-1. Correspondingly, the finding that unadjusted baseline IGF-1 was significant in univariate but not multivariate analysis is unsurprising, and not necessarily indicative of the true biologically relationship between degree of pretreatment hypersecretion and SRS response.
Importantly, the IGKRF paper did show a negative correlation between the use of pituitary suppressive medications at the time of SRS and durable remission. The concept of pituitary suppressive medications reducing the efficacy of SRS was originally described by Landolt et al36 in 2000, who noted that octreotide treatment reduced the likelihood of an endocrine cure from 60% to 11% in acromegalic patients having SRS. A later study from the Mayo Clinic37 found that acromegalic patients not receiving pituitary suppressive medications were greater than 4 times more likely to achieve biochemical remission, as compared to patients receiving these medications at the time of SRS. As a result, most centers have employed a strategy of discontinuing pituitary suppressive medications for patients with acromegaly prior to SRS, despite a lack of class I evidence.38
To correct for the variability in IGF-1 levels over time and from different centers, Gutt et al39 first described IGF-1i in a 44-patient cohort. At a median follow-up of 1.9 yr, they reported a significant decline in median IGF-1i from 1.9 to 1.1. A total of 21 patients (48%) had normal sex- and age-adjusted IGF‐1 at last follow-up. Pollock et al37 reviewed 46 patients with GHA having SRS between 1991 and 2004. The reported actuarial remission rate at 5 yr after SRS was 60%. Patients with an IGF-Ii ≤ 2.25 had a remission rate almost 3 times higher than patients with an IGF-Ii > 2.25.
The association between pre-SRS IGF-Iiwas confirmed in our series, and has previously been reproduced in 3 independent samples from other institutions.37,40-42 In spite of these consistent findings, most studies assessing SRS in GHA have not provided normalized baseline IGF-1 levels, limiting their interpretation.
BED As a Predictor of Radiosurgery Outcomes
However, tumors and normal tissue activate both fast and slow mechanisms of cellular DNA repair, resulting in variable bioeffectiveness when a prescribed physical dose is delivered over varying time intervals.6-8 This is particularly relevant for SRS, in which dose rate, number of isocenters, and setup time all contribute to overall treatment time.
The longer the treatment time, the greater the opportunity for DNA repair, and so the biologically effectiveness of a given radiation dose is decreased, as compared to shorter treatment times.43,44 Preceding in Vitro and animal studies of BED have demonstrated that optimization enhances cell kill and increases survival time, while analyses of SRS treatment plans for patients having single-fraction SRS for vestibular schwannomas have demonstrated variation in BED on the order of 15% for a fixed prescription dose.6,9-14 Taken together, these findings form the foundational arguments for prioritizing BED over physical dose in SRS treatment planning.
Although this concept is intellectually compelling, it is also clear from the mathematical derivation that BED is directly correlated with prescription dose.7 This invites speculation as to whether the observed differences in BED result in clinically meaningful differences in outcomes beyond those attributable to differences in physical dose, which was the core question underlying our analysis. In our sample, we observed a significant relationship between BED and biochemical remission, after adjusting for sex and IGF-1i. Parallel models based on margin dose did not retain statistical significance after adjustment—even when adjusted for number of isocenters, a crude surrogate for time. We interpret these results to mean that BED appears to provide a more reliable predictor of biochemical remission than prescribed dose.
Strengths and Limitations
The selection criteria defining the cohort eliminated major sources of confounding, such as prior irradiation, or medical pituitary suppression at the time of SRS. We also included early treatment failures, while otherwise restricting inclusion to patients with ≥24 mo of follow-up. With respect to IGF-1 levels, we normalized all assessments pre- and post-treatment, and reinforced the study findings with a systematic review and meta-analysis.
In our clinical practice, there is variability among the staff endocrinologists regarding the management of patients who eventually reach medication-controlled disease. Some physicians prefer to maintain patients who are tolerating the medications on suppression after reaching IGF-1i ≤ 1.0, whereas others present patients with a relatively unbiased choice, rather than a recommendation. Among the 22 patients with medication-controlled disease, approximately half elected or were recommended to continue the same pituitary suppressive medication, whereas the remainder failed a trial off suppression and either resumed their preceding treatment or were transitioned to an alternative agent or formulation (eg, depot). As we did not discriminate between patients maintained on suppression by whether a medication holiday had been failed or simply never attempted, our results may include a number of patients that would meet the criteria of biochemical remission if pituitary medications were stopped (false negative assessment). Although we report complete endocrine and radiographic follow-up for all our patients, when taken together with our exclusion criteria, these rigorous criteria restricted the cohort to a modest sample size of 102 patients—an acceptable trade-off for minimized confounding and strengthened results, in our view.
A similar limitation tied to the inherent restrictions of our practice environment is the lack of reliable data on GH. We attempted to abstract and analyze GH with respect to IGF-1i and biochemical remission in the study cohort; however, the test is infrequently and inconsistently administered at our institution, and we ultimately deferred including these data, as they were deemed highly susceptible to bias and confounding. Although we hold that IGF-1i is likely the preferable metric, explicit comparison between these parameters may be of interest in future studies—particularly among patients with normalized IGF-1i, but without resolution of symptoms.
In addition to the routine restrictions applicable to any cohort study (selection bias, residual confounding), as well as the limitations of a cohort study conducted on a small sample, there are limitations specific to the current study. Most importantly, although the derived estimation technique reported by Jones and Hopewell7 is robust, the BED values used in all of our models are by definition approximate values for the entire treatment volume, rather than by-voxel calculations of the actual treatment BED.7 Further, their approximation technique relies on numerous assumptions, and BED estimations can be conducted using several other approaches relying on different assumptions, which have not been explicitly compared to the approach preferred by Jones and Hopewell.7 Correspondingly, the models we report are potentially influenced by a major source of systematic error, which is essentially impossible to estimate or adjust for, given the available data and analytic techniques.
Several additional statistical considerations merit commentary. In the meta-analysis, only 4 studies were included—fewer than the preferred 5 to 10 minimum studies typically reported in similar studies. Although this emphasizes the need for cautious interpretation of the results, the core conclusion remains unambiguous: IGF-1i is a consistent predictor of biochemical remission, and should be considered the standard for reporting practices in future studies of SRS or GHA. Finally, in spite of reporting numerous models, we did not apply the Bonferroni or other correction to the alpha threshold. Our underlying rationale is that we have approached the analysis of BED as an exercise in hypothesis generation, more so than hypothesis testing. Correspondingly, we justify a more liberal interpretation of the statistical tests when considering the fact that our primary recommendation is to further evaluate the usefulness of BED in future studies.
CONCLUSION
Our results confirm that SRS is an effective treatment for patients with persistent or recurrent acromegaly. IGF-1i is a reliable predictor of biochemical remission—a consistent finding across all preceding reports, which has not been previously reported with unadjusted IGF-1. BED appears better correlated with outcome than prescribed dose. Future studies of SRS for GHA are strongly encouraged to report IGF-1i and BED in order to validate these findings, and consideration should be given to prospective study of BED optimization at time of treatment.
Disclosures
This project was supported in part by CTSA Grant Number TL1 TR002380 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
The authors are grateful to Ian Paddick, M.Sc., and Prof. John Hopewell, D.Sc, for their thoughtful guidance and support as we applied their momentous work on BED to our clinical study.
Notes
The preliminary results of this study were presented as an oral abstract at the Congress of Neurological Surgeons 2019 annual meeting in San Francisco, California, on October 21, 2019.
Contributor Information
Christopher S Graffeo, Department of Neurological Surgery, Mayo Clinic, Rochester, Minnesota.
Diane Donegan, Division of Endocrinology, Indiana University, Indianapolis, Indiana.
Dana Erickson, Department of Endocrinology, Mayo Clinic, Rochester, Minnesota.
Paul D Brown, Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota.
Avital Perry, Department of Neurological Surgery, Mayo Clinic, Rochester, Minnesota.
Michael J Link, Department of Neurological Surgery, Mayo Clinic, Rochester, Minnesota; Department of Otorhinolaryngology, Mayo Clinic, Rochester, Minnesota.
William F Young, Department of Endocrinology, Mayo Clinic, Rochester, Minnesota.
Bruce E Pollock, Department of Neurological Surgery, Mayo Clinic, Rochester, Minnesota; Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota.
Supplemental Digital Content 1. Search strategy for systematic literature review.
Supplemental Digital Content 2. Figure. PRISMA diagram depicting studies screened, included, and excluded via systematic review.
Supplemental Digital Content 3. Quality assessment by Modified Newcastle-Ottawa Scale.
Supplemental Digital Content 4. Table. Comparative analysis of multivariable models for biochemical remission.
Supplement 5: Kaplan-Meier curve demonstrating post-SRS preservation of normal pituitary function.
Supplemental Digital Content 6. Table. Analysis of new post-SRS anterior pituitary deficits.
REFERENCES
- 1.Marquez Y, Tuchman A, Zada G. Surgery and radiosurgery for acromegaly: a review of indications, operative techniques, outcomes, and complications. Int J Endocrinol. 2012;2012:386401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naeem K, Darbar A, Shamim MS. Role of stereotactic radiosurgery in the treatment of acromegaly. J Pak Med Assoc. 2018;68(12):1843-1845. [PubMed] [Google Scholar]
- 3.Mahmoud-Ahmed AS, Suh JH, Mayberg MR. Gamma Knife radiosurgery in the management of patients with acromegaly: a review. Pituitary. 2001;4(4):223-230. [DOI] [PubMed] [Google Scholar]
- 4.Rolston JD, Blevins LS Jr. Gamma Knife radiosurgery for acromegaly. Int J Endocrinol. 2012;2012:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollock BE, Nippoldt TB, Stafford SL, Foote RL, Abboud CF. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas: factors associated with endocrine normalization. J Neurosurg. 2002;97(3):525-530. [DOI] [PubMed] [Google Scholar]
- 6.Millar WT, Hopewell JW, Paddick Iet al.. The role of the concept of biologically effective dose (BED) in treatment planning in radiosurgery. Physica Med. 2015;31(6):627-633. [DOI] [PubMed] [Google Scholar]
- 7.Jones B, Hopewell JW. Modelling the influence of treatment time on the biological effectiveness of single radiosurgery treatments: derivation of “protective” dose modification factors. Br J Radiol. 2018;92(1093):20180111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopewell JW, Millar WT, Lindquist C, Nordstrom H, Lidberg P, Garding J. Application of the concept of biologically effective dose (BED) to patients with vestibular schwannomas treated by radiosurgery. J Radiosurg SBRT. 2013;2(4):257-271. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Xiong X-P, Lu Jet al.. The in vivo study on the radiobiologic effect of prolonged delivery time to tumor control in C57BL mice implanted with Lewis lung cancer. Radiat Oncol. 2011;6(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones B, Dale R. The evolution of practical radiobiological modelling. Br J Radiol. 2018;92(1093):20180097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88(2):254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song CW, Kim M-S, Cho LC, Dusenbery K, Sperduto PW. Radiobiological basis of SBRT and SRS. Int J Clin Oncol. 2014;19(4):570-578. [DOI] [PubMed] [Google Scholar]
- 13.Pop LA, Millar WT, van der Plas M, van der Kogel AJ. Radiation tolerance of rat spinal cord to pulsed dose rate (PDR-) brachytherapy: the impact of differences in temporal dose distribution. Radiother Oncol. 2000;55(3):301-315. [DOI] [PubMed] [Google Scholar]
- 14.Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DA. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol (R Coll Radiol). 2001;13(2):71-81. [DOI] [PubMed] [Google Scholar]
- 15.Graffeo CS, Link MJ, Brown PD, Young WF Jr, Pollock BE. Hypopituitarism after single-fraction pituitary adenoma radiosurgery: dosimetric analysis based on patients treated using contemporary techniques. Int J Radiat Oncol Biol Phys. 2018;101(3):618-623. [DOI] [PubMed] [Google Scholar]
- 16.Régis J, Tamura M, Guillot Cet al.. Radiosurgery with the world's first fully robotized Leksell Gamma Knife PerfeXion in clinical use: a 200-patient prospective, randomized, controlled comparison with the Gamma Knife 4C. Neurosurgery. 2009;64(2):346-356. [DOI] [PubMed] [Google Scholar]
- 17.Pollock BE, Link MJ, Stafford SL, Garces YI, Foote RL. Stereotactic radiosurgery for arteriovenous malformations: the effect of treatment period on patient outcomes. Neurosurgery. 2016;78(4):499-509. [DOI] [PubMed] [Google Scholar]
- 18.Leber KA, Berglöff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88(1):43-50. [DOI] [PubMed] [Google Scholar]
- 19.Ding D, Starke RM, Sheehan JP. Treatment paradigms for pituitary adenomas: defining the roles of radiosurgery and radiation therapy. J Neurooncol. 2014;117(3):445-457. [DOI] [PubMed] [Google Scholar]
- 20.Pollock BE, Link MJ, Leavitt JA, Stafford SL. Dose-volume analysis of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Neurosurgery. 2014;75(4):456-460; discussion 460. [DOI] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff Jet al.. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 23.Attanasio R, Epaminonda P, Motti Eet al.. Gamma-Knife radiosurgery in acromegaly: a 4-year follow-up study. J Clin Endocrinol Metab. 2003;88(7):3105-3112. [DOI] [PubMed] [Google Scholar]
- 24.Castinetti F, Taieb D, Kuhn JMet al.. Outcome of Gamma Knife radiosurgery in 82 patients with acromegaly: correlation with initial hypersecretion. J Clin Endocrinol Metab. 2005;90(8):4483-4488. [DOI] [PubMed] [Google Scholar]
- 25.Cohen-Inbar O, Ramesh A, Xu Z, Vance ML, Schlesinger D, Sheehan JP. Gamma knife radiosurgery in patients with persistent acromegaly or Cushing's disease: long-term risk of hypopituitarism. Clin Endocrinol. 2016;84(4):524-531. [DOI] [PubMed] [Google Scholar]
- 26.Franzin A, Spatola G, Losa M, Picozzi P, Mortini P. Results of Gamma Knife radiosurgery in acromegaly. Int J Endocrinol. 2012;2012:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagannathan J, Sheehan JP, Pouratian N, Laws ER Jr, Steiner L, Vance ML. Gamma Knife radiosurgery for acromegaly: outcomes after failed transsphenoidal surgery. Neurosurgery. 2008;62(6):1262-1270; discussion 1269-1270. [DOI] [PubMed] [Google Scholar]
- 28.Jezkova J, Marek J, Hana Vet al.. Gamma Knife radiosurgery for acromegaly—long-term experience. Clin Endocrinol. 2006;64(5):588-595. [DOI] [PubMed] [Google Scholar]
- 29.Ronchi CL, Attanasio R, Verrua Eet al.. Efficacy and tolerability of Gamma Knife radiosurgery in acromegaly: a 10-year follow-up study. Clin Endocrinol (Oxf). 2009;71(6):846-852. [DOI] [PubMed] [Google Scholar]
- 30.Vik-Mo EO, Oksnes M, Pedersen PHet al.. Gamma Knife stereotactic radiosurgery for acromegaly. Eur J Endocrinol. 2007;157(3):255-263. [DOI] [PubMed] [Google Scholar]
- 31.Wilson PJ, De-Loyde KJ, Williams JR, Smee RI. Acromegaly: a single centre's experience of stereotactic radiosurgery and radiotherapy for growth hormone secreting pituitary tumours with the linear accelerator. J Clin Neurosci. 2013;20(11):1506-1513. [DOI] [PubMed] [Google Scholar]
- 32.Abu Dabrh AM, Asi N, Farah WHet al.. Radiotherapy versus radiosurgery in treating patients with acromegaly: a systematic review and meta-analysis. Endocr Pract. 2015;21(8):943-956. [DOI] [PubMed] [Google Scholar]
- 33.Yang I, Kim W, De Salles A, Bergsneider M. A systematic analysis of disease control in acromegaly treated with radiosurgery. Neurosurg Focus. 2010;29(4):E13. [DOI] [PubMed] [Google Scholar]
- 34.Trifiletti DM, Xu Z, Dutta SWet al.. Endocrine remission after pituitary stereotactic radiosurgery: differences in rates of response for matched cohorts of Cushing disease and acromegaly patients. Int J Radiat Oncol Biol Phys. 2018;101(3):610-617. [DOI] [PubMed] [Google Scholar]
- 35.Ding D, Mehta GU, Patibandla MRet al.. Stereotactic radiosurgery for acromegaly: an international multicenter retrospective cohort study. Neurosurgery. 2019;84(3):717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landolt AM, Haller D, Lomax Net al.. Stereotactic radiosurgery for recurrent surgically treated acromegaly: comparison with fractionated radiotherapy. J Neurosurg. 1998;88(6):1002-1008. [DOI] [PubMed] [Google Scholar]
- 37.Pollock BE, Jacob JT, Brown PD, Nippoldt TB. Radiosurgery of growth hormone-producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg. 2007;106(5):833-838. [DOI] [PubMed] [Google Scholar]
- 38.Jagannathan J, Yen CP, Pouratian N, Laws ER, Sheehan JP. Stereotactic radiosurgery for pituitary adenomas: a comprehensive review of indications, techniques and long-term results using the Gamma Knife. J Neurooncol. 2009;92(3):345-356. [DOI] [PubMed] [Google Scholar]
- 39.Gutt B, Wowra B, Alexandrov Ret al.. Gamma-Knife surgery is effective in normalising plasma insulin-like growth factor I in patients with acromegaly. Exp Clin Endocrinol Diabetes. 2005;113(4):219-224. [DOI] [PubMed] [Google Scholar]
- 40.Kong DS, Kim YH, Kim YHet al.. Long-Term efficacy and tolerability of Gamma Knife radiosurgery for growth hormone-secreting adenoma: a retrospective multicenter study (MERGE-001). World Neurosurg. 2019;122:e1291-e1299. [DOI] [PubMed] [Google Scholar]
- 41.Losa M, Gioia L, Picozzi Pet al.. The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J Clin Endocrinol Metab. 2008;93(7):2546-2552. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Kano H, Kondziolka Det al.. Gamma Knife radiosurgery for clinically persistent acromegaly. J Neurooncol. 2012;109(1):71-79. [DOI] [PubMed] [Google Scholar]
- 43.Dale R, Huczkowski J, Trott K. Possible dose rate dependence of recovery kinetics as deduced from a preliminary analysis of the effects of fractionated irradiations at varying dose rates. Br J Radiol. 1988;61(722):153-157. [DOI] [PubMed] [Google Scholar]
- 44.Canney PA, Millar WT. Biphasic cellular repair and implications for multiple field radiotherapy treatments. Br J Radiol. 1997;70(836):817-822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.