Abstract
Two-pore channels (TPCs) are a ubiquitous class of Ca2+- and Na+-permeable ion channels expressed within the endo-lysosomal system. They have emerged as central regulators of a wide array of physiological processes intimately linked to information processing. In this short review, we highlight how molecular and chemical strategies have uncovered multiple roles for TPCs in regulating various aspects of endo-lysosomal trafficking associated with disease. We summarise advances in the identification of new small molecules to pharmacologically target TPCs for medical benefit. Lastly, we discuss possible underpinning molecular mechanism(s) that translate TPC-mediated ionic fluxes to function.
Current Opinion in Physiology 2020, 17:163–168
This review comes from a themed issue on Calcium signaling
Edited by Indu S Ambudkar and Aldebaran M Hofer
For a complete overview see the Issue and the Editorial
Available online 14th August 2020
https://doi.org/10.1016/j.cophys.2020.08.002
2468-8673/© 2020 Published by Elsevier Ltd.
Introduction
TPCs are evolutionarily ancient cation channels that reside on acidic organelles [1,2]. Humans possess two isoforms - TPC1 and TPC2, that are ubiquitously expressed through the endo-lysosomal system and associated structures such as lysosome-related organelles [3, 4, 5]. The endo-lysosomal system is a ‘super highway’ that connects the cell to its extracellular environment through a highly dynamic system of vesicles that communicate not only with each other [6] but also with other organelles such as the Golgi apparatus and the endoplasmic reticulum (ER) [7]. TPCs are thought to play key roles in regulating aspects of this communication through their ability to modulate cation flux. However, there has been some confusion regarding the activating stimuli and permeability properties of TPCs. On the one hand, TPCs are evidently activated by the Ca2+ mobilising messenger NAADP, which has long been known to mobilise acidic stores of Ca2+ [3,4,8]. On the other hand, evidence suggests TPCs are Na+ channels gated directly by the endo-lysosomal phosphoinositide PI(3,5)P2 [9,10]. A recent study has clarified this issue by showing that TPCs are highly unusual in switching their ion selectivity in an agonist-dependent manner [11••]. Thus, in the presence of NAADP, TPCs behave as non-selective Ca2+-permeable channels, whereas in the presence of PI(3,5)P2, they are Na+-selective – a switch that can be phenocopied by novel cell permeable small molecules [11••]. The fact that both Ca2+ and PI(3,5)P2 are implicated in endo-lysosomal trafficking highlights the potential of TPCs as key regulators of information flow [12]. Here, we summarise recent work implicating TPCs in endo-lysosomal trafficking in both health and disease.
TPCs and endo-lysosomal morphology
Form and function are inextricably linked, thus, changes in endo-lysosomal morphology can signal lysosomal malaise. This is well exemplified by the biomarker potential of an increase in the relative volume of acidic compartments in lysosomal storage diseases [13]. Overexpression of TPC2, but not TPC1, causes enlargement and clustering of endocytic organelles in Xenopus oocytes and is associated with aberrant trafficking of pigment granules [14]. Notably, the effects were reversed by the fast Ca2+ chelator, BAPTA, but not the slower Ca2+ chelator, EGTA, suggesting a role for local Ca2+ fluxes. Here, differences in the location of the channels appears to underlie isoform specificity, that is, the phenotype can be recapitulated by a chimeric channel in which the N-terminus of TPC1 is replaced with the N-terminus of TPC2 that contains a lysosomal targeting sequence [14,15].
Aberrant morphology of the endo-lysosomal system is also a feature of human cells overexpressing TPC2, and fibroblasts derived from people with Parkinson’s disease caused by the G2019S mutation in the Parkinson’s related protein, LRRK2 [16,17]. The latter adds to the growing body of evidence pointing to Parkinson’s being a lysosomal disease [18]. Importantly, this particular phenotype could be reversed by molecular silencing of TPC2 or NAADP antagonism [17]. As with studies in Xenopus, there was a selective dependence on TPC2 and local Ca2+ signals [17].
Collectively, these studies suggest that increased TPC2 activity, be it forced or pathological, has profound effects on the structure of endocytic organelles which, in turn, is likely to impact lysosomal functions.
TPCs and the vesicular trafficking of receptors and protein toxins
Cell homeostasis is dependent on the ongoing, bidirectional flow of information between the plasma membrane and the cell interior. This includes pathways that mediate the trafficking of internalised cell surface receptors. In this context, EGF receptors and LDL receptors have been shown to accumulate in late endosomes in TPC2-deficient cells [19]. Mis-trafficking of the latter is likely to account for the non-alcoholic fatty liver disease-like symptoms observed in TPC2 knockout mice fed a cholesterol-rich diet [19]. Knockout of TPC2 also modestly delays the trafficking of PDGF receptors [20]. In this study, the effects were isoform-specific as knockout of TPC1 had little effect. Additionally, knockdown of either TPC1 or TPC2 results in accumulation of integrins in early endosomes [21]. This effect likely contributes to the reduced adhesion and migration of cancer cell lines and metastatic tumour formation in vivo [21].
Receptor endocytosis is often hijacked by protein toxins and other pathogens to gain entry into cells. TPC1, but not TPC2, knockout impeded trafficking of cholera toxin to the Golgi apparatus [20]. This effect is reminiscent of overexpression studies of sea urchin TPC1 which also perturbed cholera toxin trafficking [16]. However, in an independent study, knockout of TPC1 had only modest effects on cholera toxin-induced cyclic AMP production [22]. TPC1 knockout reduced the trafficking and downstream effects of a number of so called ‘short trip’ bacterial toxins, such as anthrax and diphtheria toxins, that enter cells through early endocytic compartments [22].
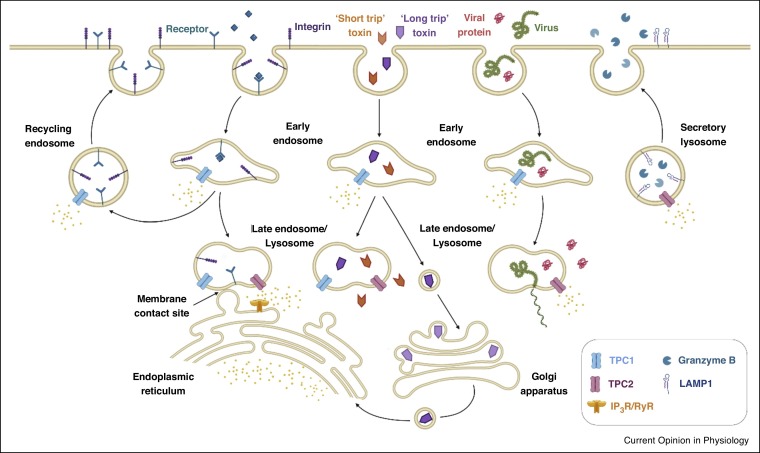
In summary, multiple studies show that TPCs regulate the transit and biological activity of a number of receptors and cargoes that enter cells through receptor-mediated endocytosis, often in an isoform-selective manner (Figure 1 ). It remains to be seen how extensively this applies to other endocytic cargoes.
Figure 1.
Schematic illustrating distinct TPC-dependent membrane trafficking events. TPCs have been implicated in the trafficking of internalised proteins including cell-surface receptors and integrins through early, late and recycling endosomes, and strengthening of membrane contact sites between endosomes and the endoplasmic reticulum (left). They also regulate trafficking of protein toxins, viruses and viral proteins, and the secretion of enzymes and proteins to the extracellular matrix (right).
TPCs and the trafficking of viruses
In recent years, TPCs have emerged as novel host factors supporting infection by clinically relevant viral pathogens. Sakurai et al. demonstrated that disrupting TPC function, molecularly or pharmacologically, prevented the cellular entry of Ebola virus (EBOV) [23••]. Knock down or knockout of either TPC isoform, as well as overexpression of a dominant negative TPC2 pore mutant inhibited EBOV escape from the endosomal network. Similarly, the NAADP antagonist Ned-19 and a range of voltage-gated Ca2+ channel blockers inhibited EBOV infection in vitro. Of the latter, the plant-derived alkaloid tetrandrine appeared to be the most potent inhibitor of EBOV entry with half-maximal inhibitory concentration (IC50) in the nanomolar range. Notably, tetrandrine is a potent blocker of TPCs and showed therapeutic efficacy against EBOV in an in vivo mouse model [23••].
More recent work by Marchant and colleagues [24] suggested that the intracellular trafficking and subsequent infection of another enveloped RNA virus - MERS-CoV, is also reliant on TPC activity. Loss of function analyses revealed that TPC knock down impairs MERS-CoV pseudovirus translocation through the endocytic system. Furthermore, overexpression of TPC1 or TPC2 also led to significant reduction in translocation events. The observed inhibitory effects were strongly influenced by the correct subcellular distribution of the channels as overexpression of a functionally active TPC2 re-routed to the plasma membrane had no notable effect on MERS-CoV pseudovirus infectivity. Importantly, knock down and overexpression of a distinct lysosomal Ca2+ channel – TRPML1, exerted no inhibitory effects, thus attesting to specificity. A number of bisbenzylisoquinoline alkaloids related to tetrandrine, which were demonstrated to inhibit NAADP-evoked Ca2+ release in intact cells, also potently impaired pseudovirus progression through the endosomal network [24]. Tetrandrine also blocks SARS-CoV-2 pseudo-virus infection [25] pointing to a general requirement for TPCs in Coronavirus infection.
TPCs were also found to regulate the endo-lysosomal escape of the HIV-1 encoded Tat peptide [26]. Following secretion from infected cells, Tat enters uninfected cells via receptor-mediated endocytosis and transits to the nucleus to enhance gene transcription. Khan et al. demonstrated that drug-induced blockade or molecular silencing of TPCs but not TRPML1 prevents Tat from escaping endocytic compartments [26]. Further investigations using more biologically relevant systems are needed to determine whether blockade of TPCs has therapeutic potential against HIV-1.
Collectively, TPCs have emerged as common host factors exploited by a number of non-related viral pathogens (Figure 1).
TPCs and non-vesicular traffic
A hallmark of NAADP-mediated Ca2+ signalling is its global nature, resulting from amplification of local Ca2+ signals deriving from acidic Ca2+ stores by ER Ca2+ channels [27]. It has long been proposed that such inter-organellar communication occurs at membrane contact sites between the acidic organelles and the ER [28]. Both endosome-ER and lysosome-ER contacts are readily identifiable by EM and abundant [29,30]. Importantly, a recent study has provided evidence that local ‘constitutive’ NAADP-mediated Ca2+ signals may act to strengthen contacts between late endosomes and the ER [31••]. Previously, such contacts have been shown to promote de-phosphorylation of endosome-associated internalised EGF receptors by an ER-localised phosphatase [29]. Disruption of contacts, by interfering with NAADP signalling either molecularly (through knock down of TPC1 but not TPC2) or chemically with Ned-19, can delay de-phosphorylation resulting in EGF receptor hyperactivation [31••]. This was again associated with profound changes in endo-lysosomal morphology. As contact sites are formed without membrane fusion, this work links TPCs to non-vesicular traffic and provides scope for investigating Ca2+ fluxes in the control of lipid transfer that is heavily reliant on inter-organelle contact sites [32].
The TPC interactome - towards mechanism
How do TPCs regulate the various aspects of membrane traffic discussed above? The TPC interactome is providing some key insights. To date, three studies have taken unbiased approaches to define proteins that associate with TPC1, TPC2 or both [14,19,22] (reviewed in Ref. [33]). One common feature that emerges is the interaction of TPCs with SNARE proteins that regulate membrane fusion. These include both Q-SNARES and R-SNARES, such as syntaxins and VAMP isoforms. Such associations are consistent with a role for TPCs in vesicular fusion, which could potentially account for TPC2-dependent lysosomal expansion [14,17] and TPC2-dependent lysosomal exocytosis [34]. A notable omission from these data sets is the synaptotagmins that serve as Ca2+ sensors for fusion elsewhere.
A fusogenic role for TPCs could underlie the halted transit of internalised receptors, protein toxins and viruses upon TPC blockade. The identity of the compartment(s) in which cargoes stall is unclear at present. For EBOV, there is evidence both for [35] and against [23••] the virus accumulating in TPC2-positive compartments that are also positive for NPC1, an essential host factor for EBOV fusion. Additionally, we know little of the relationship between this compartment and compartments that accumulate receptors and protein toxins in the absence of TPC activity.
TPC2 also associates with a number of Rab proteins, including the endo-lysosomal Rab - Rab7, through an identified binding site in its N-terminus [14]. Rab proteins are key regulators of both vesicular and non-vesicular traffic [36]. Consequently, TPC2 might act as a Rab effector. Consistent with this, mutant channels unable to bind Rabs are less able to support NAADP-evoked Ca2+ signals [14]. Furthermore, morphology defects associated with TPC2 overexpression and mutant LRRK2 are reversed by Rab7 inhibition [14,17]. Rab7 and several isoforms of the annexin family of Ca2+ binding proteins associate with TPCs and regulate contact site formation between endocytic compartments and the ER [37,38]. These interactors could thus act upstream and downstream of TPCs, respectively to direct non-vesicular traffic.
Interestingly, Gunaratne et al. provided several lines of evidence indicating that interfering with TPC action impairs the activity of furin, a Ca2+- dependent endoprotease, which may mediate the proteolytic priming of MERS virus spike protein [24]. This could underpin the decrease in MERS pseudovirus entry upon TPC blockade. However, furin is reportedly dispensable for the activation of MERS-CoV envelope protein [39]. Moreover, this mechanism is unlikely to apply to EBOV fusion/entry since EBOV glycoprotein (GP) is processed by endogenous cathepsins rather than furin [40]. Additionally, tetrandrine and Ned-19 inhited the entry of EBOV virus-like particles (VLPs) bearing cleaved GP, suggesting that TPCs regulate a step distal to proteolysis [23••].
In summary, TPCs likely regulate both vesicular and non-vesicular trafficking through physical association with regulatory proteins but further work is required to fully define these interactions and relate them to Ca2+ fluxes.
Chemical targeting of TPCs
Despite the increasingly recognised pathophysiological significance of TPCs [41], their pharmacology is poorly defined. The fact that TPCs are essential host factors required by EBOV and MERS-CoV to enter target cells is significant. This is because the available literature on viral entry blockers offers a rich source of potential TPC modulators since some of the compounds with unknown mechanisms of action may elicit their antiviral effects through inhibition of TPCs.
In this context, Penny et al. screened a library of FDA-approved drugs against an in silico model of TPC2 to identify lead compounds targeting the pore region of the channel [42•]. To further refine the hits from the virtual screen, the highest-ranking compounds were compared with the hits from two independent high-throughput screens for EBOV antivirals [43,44]. This cross-referencing returned drugs targeting oestrogen and dopamine receptors as common hits [42•]. The selected hits inhibited endogenous NAADP-evoked Ca2+ release and recombinant TPC2 channel activity. Mechanistically, the drugs behaved as TPC2 pore blockers, reducing the mean open time of the channel. Notably, the relative potency of the drugs in electrophysiological studies directly correlated with their potencies for inhibition of EBOV VLP entry [42•]. Gunaratne et al. undertook an independent screening campaign using sea urchin egg homogenates and identified several novel inhibitors of NAADP-dependent Ca2+ signalling that were active in mammalian cells [45]. Importantly, the compounds blocked NAADP-evoked Ca2+ signals and MERS-CoV pseudovirus translocation with similar potencies [24]. Whether these compounds act directly on TPCs remains to be established.
Collectively, these findings underscore the importance of NAADP signalling through TPCs in viral entry and uncover novel blocking drugs for manipulating TPC-dependent trafficking events.
Outlook
To date most of the effects of TPCs have been ascribed to local Ca2+ fluxes. However, the downstream effectors that translate these fluxes to function are largely unknown. Further mining of TPC interactomes and functional validation is thus warranted not least due to their potential in combatting disease. Moreover, the permeability of the channels to Na+, associated with activation by PI(3,5)P2, in a functional context is still largely unexplored. Early work suggested that TPCs are tonically inhibited by the nutrient sensing protein kinase mTOR under nutrient replete conditions but become activated during starvation to regulate the lysosome membrane potential through Na+ permeability [46]. This in turn was proposed to maintain lysosomal H+, amino acid flux and exercise endurance in vivo. Recent work suggests an additional role for TPC-associated Na+ flux in regulating the volume of macropinosomes [47••]. This osmoregulatory role may apply more generally to the endocytic system and associated trafficking functions. Recent work also suggests that Na+ flux through TPCs may selectively couple to lysosomal exocytosis [11••], even though this process is thought to be Ca2+-dependent [12]. Understanding and maintaining endocytic well-being necessitate teasing apart fluxes of Ca2+ and Na+ in endocytic trafficking, defining molecularly the sequelae of events that these fluxes initiate, and developing strategies for their selective manipulation.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
Acknowledgements
This work was funded by a BBSRC PhD studentship, BBSRC grant BB/N01524X/1 and Medical Research Council funding to the MRC-UCL LMCB University Unit (MC_UU_12018/1).
References
- 1.Rahman T., Cai X., Brailoiu G.C., Abood M.E., Brailoiu E., Patel S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci Signal. 2014;7:ra109. doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel S. Function and dysfunction of two-pore channels. Sci Signal. 2015;8:re7. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- 3.Calcraft P.J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K.T., et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brailoiu E., Churamani D., Cai X., Schrlau M.G., Brailoiu G.C., Gao X., Hooper R., Boulware M.J., Dun N.J., Marchant J.S., Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai X., Patel S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol Biol Evol. 2010;27:2352–2359. doi: 10.1093/molbev/msq122. [DOI] [PubMed] [Google Scholar]
- 6.Luzio J.P., Pryor P.R., Bright N.A. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 7.Wu H., Carvalho P., Voeltz G.K. Here, there, and everywhere: the importance of ER membrane contact sites. Science. 2018:361. doi: 10.1126/science.aan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churchill G.C., Okada Y., Thomas J.M., Genazzani A.A., Patel S., Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Zhang X., Dong X.P., Samie M., Li X., Cheng X., Goschka A., Shen D., Zhou Y., Harlow J., et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.She J., Guo J., Chen Q., Zeng W., Jiang Y., Bai X.C. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature. 2018;556:130–134. doi: 10.1038/nature26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Gerndt S., Chen C.-C., Chao Y.-K., Yuan Y., Burgstaller S., Scotto Rosato A., Krogsaeter E., Urban N., Jacob K., Nguyen O.N.P., et al. Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function. eLife. 2020;9 doi: 10.7554/eLife.54712. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identified an unusual property of the TPCs whereby TPC2 exhibits an agonist-dependent switch in its ion selectivity. The authors demonstrated that TPC2 is a non-selective Ca2+--permeable channel in the presence of NAADP but Na+-selective in the presence of PI(3,5)P2. Notably, a high throughput screen yielded two cell permeable small molecules that effectively mirrored each agonist in switching ion selectivity. These findings resolve opposing views on the activation and permeability properties of these abundant cation channels, and provide new molecular tools to strategically manipulate them.
- 12.Xu H., Ren D. Lysosomal physiology. Annu Rev Physiol. 2015;77:57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.te Vruchte D., Speak A.O., Wallom K.L., Al Eisa N., Smith D.A., Hendriksz C.J., Simmons L., Lachmann R.H., Cousins A., Hartung R., et al. Relative acidic compartment volume as a lysosomal storage disorder-associated biomarker. J Clin Investig. 2014;124:1320–1328. doi: 10.1172/JCI72835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin-Moshier Y., Keebler M.V., Hooper R., Boulware M.J., Liu X., Churamani D., Abood M.E., Walseth T.F., Brailoiu E., Patel S., Marchant J.S. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc Natl Acad Sci U S A. 2014;111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brailoiu E., Rahman T., Churamani D., Prole D.L., Brailoiu G.C., Hooper R., Taylor C.W., Patel S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J Biol Chem. 2010;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruas M., Rietdorf K., Arredouani A., Davis L.C., Lloyd-Evans E., Koegel H., Funnell T.M., Morgan A.J., Ward J.A., Watanabe K., et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hockey L.N., Kilpatrick B.S., Eden E.R., Lin-Moshier Y., Brailoiu G.C., Brailoiu E., Futter C., Schapira A.H., Marchant J.S., Patel S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci. 2015;128:232–238. doi: 10.1242/jcs.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein A.D., Mazzulli J.R. Is Parkinson’s disease a lysosomal disorder? Brain. 2018;141:2255–2262. doi: 10.1093/brain/awy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimm C., Holdt L.M., Chen C.C., Hassan S., Muller C., Jors S., Cuny H., Kissing S., Schroder B., Butz E., et al. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat Commun. 2014;5 doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- 20.Ruas M., Chuang K.T., Davis L.C., Al-Douri A., Tynan P.W., Tunn R., Teboul L., Galione A., Parrington J. TPC1 has two variant isoforms and their removal has different effects on endo-lysosomal functions compared to loss of TPC2. Mol Cell Biol. 2014;34:3981–3992. doi: 10.1128/MCB.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen O.N., Grimm C., Schneider L.S., Chao Y.K., Atzberger C., Bartel K., Watermann A., Ulrich M., Mayr D., Wahl-Schott C., et al. Two-pore channel function is crucial for the migration of invasive cancer cells. Cancer Res. 2017;77:1427–1438. doi: 10.1158/0008-5472.CAN-16-0852. [DOI] [PubMed] [Google Scholar]
- 22.Castonguay J., Orth J.H.C., Muller T., Sleman F., Grimm C., Wahl-Schott C., Biel M., Mallmann R.T., Bildl W., Schulte U., Klugbauer N. The two-pore channel TPC1 is required for efficient protein processing through early and recycling endosomes. Sci Rep. 2017;7 doi: 10.1038/s41598-017-10607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Sakurai Y., Kolokolstov A.A., Chen C.C., Tidwell M.W., Bauta W.E., Klugbauer N., Grimm C., Wahl-Schott C., Biel M., Davey R.A. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper first identified a link between TPCs and Ebola virus (EBOV) infection. Following macropinocytosis and proteolytic cleavage of the EBOV envelope glycoprotein, EBOV requires the Niemann-Pick disease type C protein 1 for fusion and entry into cells. Using gene knock outs, siRNA and small molecule inhibitors, this work shows that TPCs are also required for EBOV entry. One particular inhibitor of TPCs, tetandrine, efficiently inhibited EBOV infection in tissue culture and in a mouse model, indicating the potential that drugs against TPCs might be used to inhibit EBOV infection.
- 24.Gunaratne G.S., Yang Y., Li F., Walseth T.F., Marchant J.S. NAADP-dependent Ca(2+) signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium. 2018;75:30–41. doi: 10.1016/j.ceca.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11 doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan N., Halcrow P.W., Lakpa K.L., Afghah Z., Miller N.M., Dowdy S.F., Geiger J.D., Chen X. Two-pore channels regulate Tat endolysosome escape and Tat-mediated HIV-1 LTR transactivation. FASEB J. 2020;34:4147–4162. doi: 10.1096/fj.201902534R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galione A., Receptors N.A.A.D.P. Cold Spring Harb Perspect Biol. 2019;11 doi: 10.1101/cshperspect.a035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S., Brailoiu E. Triggering of Ca2+ signals by NAADP-gated two-pore channels: a role for membrane contact sites? Biochem Soc Trans. 2012;40:153–157. doi: 10.1042/BST20110693. [DOI] [PubMed] [Google Scholar]
- 29.Eden E.R., White I.J., Tsapara A., Futter C.E. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat Cell Biol. 2010;12:267–272. doi: 10.1038/ncb2026. [DOI] [PubMed] [Google Scholar]
- 30.Kilpatrick B.S., Eden E.R., Schapira A.H., Futter C.E., Patel S. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J Cell Sci. 2013;126:60–66. doi: 10.1242/jcs.118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Kilpatrick B.S., Eden E.R., Hockey L.N., Yates E., Futter C.E., Patel S. An endosomal NAADP-sensitive two-pore Ca2+ channel regulates ER-endosome membrane contact sites to control growth factor signaling. Cell Rep. 2017;18:1636–1645. doi: 10.1016/j.celrep.2017.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper revealed an essential role for TPC1 in strengthening membrane contact sites between endosomes and the ER. The authors demonstrated that molecular or chemical interference with TPC1 or NAADP signalling reduced contact site formation and delayed de-phosphorylation of endocytosed EGF receptors leading to their hyperactivation. The study highlights the importance of endo-lysosomal Ca2+ in the structural and functional wellbeing of the endocytic system through regulating non-vesicular traffic.
- 32.Wong L.H., Gatta A.T., Levine T.P. Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes. Nat Rev Mol Cell Biol. 2019;20:85–101. doi: 10.1038/s41580-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 33.Krogsaeter E.K., Biel M., Wahl-Schott C., Grimm C. The protein interaction networks of mucolipins and two-pore channels. Biochim Biophys Acta Mol Cell Res. 2019;1866:1111–1123. doi: 10.1016/j.bbamcr.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis L.C., Morgan A.J., Chen J.L., Snead C.M., Bloor-Young D., Shenderov E., Stanton-Humphreys M.N., Conway S.J., Churchill G.C., Parrington J., et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr Biol. 2012;22:2331–2337. doi: 10.1016/j.cub.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons J.A., D’Souza R.S., Ruas M., Galione A., Casanova J.E., White J.M. Ebolavirus glycoprotein directs fusion through NPC1+ endolysosomes. J Virol. 2016;90:605–610. doi: 10.1128/JVI.01828-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zerial M., McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 37.Rocha N., Kuijl C., van der Kant R., Janssen L., Houben D., Janssen H., Zwart W., Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eden E.R., Sanchez-Heras E., Tsapara A., Sobota A., Levine T.P., Futter C.E. Annexin A1 tethers membrane contact sites that mediate ER to endosome cholesterol transport. Dev Cell. 2016;37:473–483. doi: 10.1016/j.devcel.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuyama S., Shirato K., Kawase M., Terada Y., Kawachi K., Fukushi S., Kamitani W. Middle east respiratory syndrome coronavirus spike protein is not activated directly by cellular Furin during viral entry into target cells. J Virol. 2018:92. doi: 10.1128/JVI.00683-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandran K., Sullivan N.J., Felbor U., Whelan S.P., Cunningham J.M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel S., Kilpatrick B.S. Two-pore channels and disease. Biochim Biophys Acta Mol Cell Res. 2018;1865:1678–1686. doi: 10.1016/j.bbamcr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Penny C.J., Vassileva K., Jha A., Yuan Y., Chee X., Yates E., Mazzon M., Kilpatrick B.S., Muallem S., Marsh M., Rahman T., Patel S. Mining of Ebola virus entry inhibitors identifies approved drugs as two-pore channel pore blockers, Biochimica et biophysica acta. Mol Cell Res. 2019;1866:1151–1161. doi: 10.1016/j.bbamcr.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper builds on the work of Sakurai et al. [23]. Using in silico screening of a library of FDA approved drugs against a model of the human TPC2 pore, and cross-referencing the ‘hits’ against two high through put screens for inhibitors of Ebola virus entry, the authors identified a number of compounds targeting dopamine and oestrogen receptors as common leads. These compounds inhibited channel activity of TPC2 as well as the entry of Ebola virus-like particles, providing further support for a key role for TPCs in virus entry.
- 43.Kouznetsova J., Sun W., Martinez-Romero C., Tawa G., Shinn P., Chen C.Z., Schimmer A., Sanderson P., McKew J.C., Zheng W., Garcia-Sastre A. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect. 2014;3 doi: 10.1038/emi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansen L.M., DeWald L.E., Shoemaker C.J., Hoffstrom B.G., Lear-Rooney C.M., Stossel A., Nelson E., Delos S.E., Simmons J.A., Grenier J.M., et al. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa5597. [DOI] [PubMed] [Google Scholar]
- 45.Gunaratne G.S., Johns M.E., Hintz H.M., Walseth T.F., Marchant J.S. A screening campaign in sea urchin egg homogenate as a platform for discovering modulators of NAADP-dependent Ca(2+) signaling in human cells. Cell Calcium. 2018;75:42–52. doi: 10.1016/j.ceca.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cang C., Zhou Y., Navarro B., Seo Y.J., Aranda K., Shi L., Battaglia-Hsu S., Nissim I., Clapham D.E., Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Freeman S.A., Uderhardt S., Saric A., Collins R.F., Buckley C.M., Mylvaganam S., Boroumand P., Plumb J., Germain R.N., Ren D., Grinstein S. Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance. Science. 2020;367:301–305. doi: 10.1126/science.aaw9544. [DOI] [PMC free article] [PubMed] [Google Scholar]; This very interesting paper shows that TPCs contribute to maintaining the volume of endocytic organelles. The authors find that in macrophages, removal of extracellular Na+ or chemical inhibition, or knockout, of TPCs prevents the shrinkage of macropinosomes and associated tubules formed during macropinocytosis. The authors conclude that Na+ flux through TPCs and associated osmotic movement of water across the organellar membrane is important for volume ‘resolution’.