Graphical abstract
Keywords: COVID-19, Face mask, Disposable plastic waste, Hydrogen, Carbon dioxide, Pyrolysis
Abstract
It becomes common to wear a disposable face mask to protect from coronavirus disease 19 (COVID-19) amid this pandemic. However, massive generations of contaminated face mask cause environmental concerns because current disposal processes (i.e., incineration and reclamation) for them release toxic chemicals. The disposable mask is made of different compounds, making it hard to be recycled. In this regard, this work suggests an environmentally benign disposal process, simultaneously achieving the production of valuable fuels from the face mask. To this end, CO2-assisted thermo-chemical process was conducted. The first part of this work determined the major chemical constituents of a disposable mask: polypropylene, polyethylene, nylon, and Fe. In the second part, pyrolysis study was employed to produce syngas and C1-2 hydrocarbons (HCs) from the disposable mask. To enhance syngas and C1-2 HCs formations, multi-stage pyrolysis was used for more C–C and C–H bonds scissions of the disposable mask. Catalytic pyrolysis over Ni/SiO2 further expedited H2 and CH4 formations due to its capability for dehydrogenation. In the presence of CO2, catalytic pyrolysis additionally produced CO, while pyrolysis in N2 did not produce it. Therefore, the thermo-chemical conversion of disposable face mask and CO2 could be an environmentally benign way to remove COVID-19 plastic waste, generating value-added products.
1. Introduction
Highly contagious coronavirus disease 19 (COVID-19) was identified/characterized in 2019 [1]. World health organization (WHO) manifested COVID-19 outbreak as pandemic on March 2020 [2] because COVID-19 infects over millions of people in all continents [3]. Under these circumstances, non-pharmaceutical intervention to physically cut off COVID-19 contagion by gearing up a personal protective equipment (PPE) (e.g., mask, glove, protective gown, etc.) is mandated in most countries [4], [5], [6], [7]. Accordingly, the demands for PPE have been increased [8], which has led to the subsequent generation of plastic wastes [9], [10], [11]. Unfortunately, a reliable disposal platform for PPE has not been achieved due to several reasons [12]. First of all, most of the disposable face masks used in highly contaminated areas such as medical centers, public transportations, and public places could have high risks of contaminations with coronavirus. In addition, the heterogeneous nature of PPE makes it difficult to establish a reliable recycle platform [13]. Indeed, PPE is manufactured from various polymers (polystyrene, polypropylene, polyethylene, polyvinyl chloride, polyethylene terephthalate, etc.) and metallic compounds [9], [10], [11]. Despite that recycling is the best disposal option for PPE, such practice is not easily realized due to difficulty in proper separation [14]. Also, upcycling of PPE cannot be made due to a high bio-hazardous potential [15].
In these contexts, thermal destruction through the thermo-chemical process offers a reliable disposal route for PPE [16], [17]. Among the thermo-chemical processes, incinerating PPE is commonly practiced [18], but it suffers from technical incompleteness in terms of air pollution controls (APCs) [19]. At the temperature regimes over the transition state, plastics are melted and volatized [20]. Radical pools created from the thermolysis of polymer, such as polyvinyl chloride (PVC), offer a favorable condition for forming toxic chemicals, such as dioxins [21]. Under these conditions, controlling the stoichiometric ratio of fuel to oxygen is challenging, which leads to a difficult condition for complete oxidation [22], [23]. Given that most air pollutants are combustion byproducts, additional unit operations for APCs are required to meet the stringent air quality standard [24], [25]. Accordingly, finding a reliable disposal platform for PPE beyond incineration is of great importance [17]. It is desirable to recover energy and valuable chemicals from PPE.
Pyrolysis offers an effective means for recovering energy and chemicals through carbon rearrangement [26]. Specifically, carbonaceous materials made for PPE can turn into different phases of pyrogenic products, including syngas, gaseous/liquid hydrocarbons, and solid residue (char) [27]. Carbon distributions into the three phase pyrolysates are controllable by altering the operational parameters (heating rate, temperature, duration, etc.) [28], [29] and adopting catalysts during the process [30]. As compared to the case of lignocellulosic biomass, the formation of char from pyrolysis of plastic waste (e.g., PPE) is negligible [31]. Accordingly, carbon distribution of PPE likely designated into gaseous and liquid pyrolysates [32]. It is readily inferred that valorization of PPE through the pyrolysis process is advantageous over that of lignocellulosic biomass in that the more gaseous/liquid pyrolysates can be obtained. To offer more environmentally benign means to valorize PPE, this study particularly choose carbon dioxide (CO2) as a reaction medium. In short, the mechanistic functionality of CO2 as soft oxidant was reported in our previous study [33]. Given that CO2 serves a role to alter the compositional modification of gaseous/liquid pyrolysates [34], this study laid great stress on pyrolysis of PPE. More specifically, pyrolysis of a disposable mask from the CO2 environment was mainly conducted as a case study. To reinforce the mechanistic functionality of CO2 during pyrolysis of a disposable mask, earth abundant Ni catalyst was adopted, and all pyrolysates through catalytic pyrolysis were characterized. This catalyst was utilized due to its known catalytic capability for dehydrogenation for H2 production [35], [36]. For fundamental study, the main constituents of a disposable mask were determined through analyses of functional groups, organic/inorganic contents, and thermal stability.
2. Materials and methods
2.1. Chemical reagents
Disposable face masks (KF94 grade: the first-class Korean masks that must meet ≥ 94% filtration efficiency of air pollutants) were purchased from a pharmacy store in Seoul, Korea. Prior to pyrolysis studies, the disposable face mask was cut into small pieces and dried at 100 ˚C overnight to remove moisture. Dichloromethane (>99.95%), silica support (high purity grade, pore size: 60 Å, particle size: 53–210 µm), low density polyethylene (average MW: 4,000), and isotactic polypropylene (average Mw: 12,000) were obtained from Sigma-Aldrich. Nickel nitrate hexahydrate (>97.0%) and nitric acid (69.0%) were acquired from Daejung Chemical (Korea). ICP multi-element standard solutions (IV and XVI) were purchased from Merck (Germany). Ultra-high purity (99.999%) N2 and CO2 gases, and N2 balanced H2 (20 vol% H2) gas were provided from Rigas (Korea).
2.2. Characterization of disposable face mask
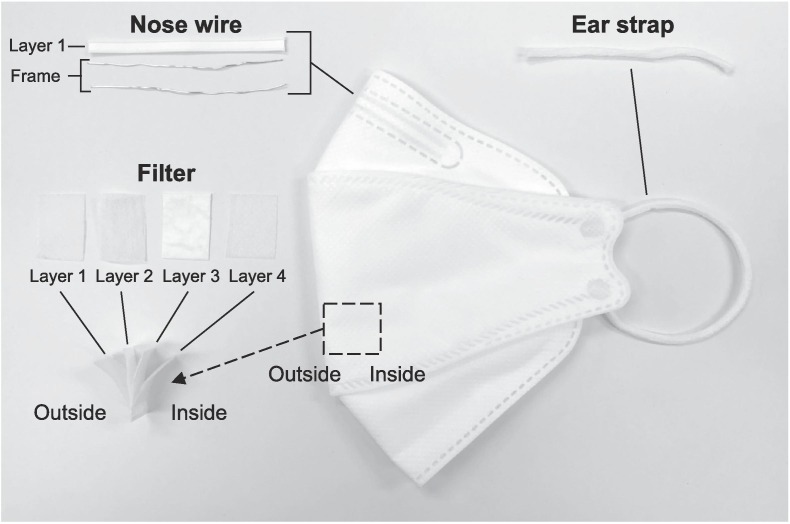
Prior to pyrolysis study of the disposable face mask, its chemical compositions were identified. As shown in Fig. 1 , the face mask was disassembled into filter (layers 1 – 4), nose wire (layer 1 cover and metal frame), and ear strap parts. Each plastic part was analyzed with FT-IR spectroscopy (Thermo Scientific Nicolet 380) to identify its functional groups, while metal frame in the nose wire was quantified with an ICP-OES (Perkin-Elmer Optima 5300 DV). The detailed procedure for ICP-OES is describe elsewhere [37]. To scrutinize the thermolytic profiles of different parts (filter, nose wire, and ear strap) of the disposable face mask, thermo-gravimetric analyses (TGA: Netzsch STA 499) were done under the N2 and CO2 environments, varying thermolysis temperature from 35 to 900 ˚C with a constant heating rate (10 ˚C min−1).
Fig. 1.
A photograph of disposal face mask disassembled into different parts.
2.3. Catalyst preparation and characterizations
Ni/SiO2 (5 wt% of Ni) was employed as a pyrolysis catalyst in this study. To impregnate Ni metal onto SiO2 support, wetness incipient impregnation method was adapted. Ni precursor was fully dissolved in deionized water, and the precursor solution was added to the SiO2 support dropwise. When the impregnation was done, it was placed in a convection oven overnight at 105 ˚C, followed by calcination in the air at 600 ˚C for 5 h. To obtain metal catalyst, the oxidized sample was reduced under 20 vol% of H2 (N2 balanced) at 510 ˚C for 4 hr with a heating rate of 2 ˚C min−1. ICP-OES analysis was done to measure the Ni metal loading (4.98 ± 0.02 wt%)
2.4. Experimental setups for disposable face mask pyrolysis
A tubular reactor was assembled with cylindrical furnaces for various pyrolysis experiments (one-stage, two-stage, and catalytic pyrolysis). In the experimental setup for one-stage pyrolysis, 1.00 g (±0.02) of face mask was inserted into the center of tubular reactor, which operated from 35 to 600 ˚C with a constant heating rate (10 ˚C min−1). In the two-stage pyrolysis, additional furnace (the second heating zone) was placed right next to the first heating zone (furnace operating from 200 to 600 ˚C at 10 ˚C min−1). The second heating zone was operated at a constant temperature (600 ˚C). For catalytic pyrolysis, 5 wt% Ni/SiO2 (1.00 ± 0.01 g) was added to the second heating zone in the two-stage pyrolysis setup. During pyrolysis in all the experimental setups, either N2 or CO2 (100 mL min−1) was flowed through the tubular reactor as a purge gas.
2.5. Product analysis
Gaseous products from face mask pyrolysis were monitored using an on-line micro-GC (3000A, Inficon), directly connected to the tubular reactor. Identification of gaseous products and their quantification were done using a calibration gas mixture (Lot#: 160–401257255-1) made from Inficon. For analysis of condensable hydrocarbons (HCs), a cold solvent trap (-1 ˚C), filled with dichloromethane, was used to condense them. The compositional matrixes of condensed products were analyzed using GC/MS (Agilent 7820A GC and Agilent 5977E MS) and GC/TOF-MS (ALMSCO TOF-MS).
3. Results and discussion
3.1. Identification of the main constituents of face mask
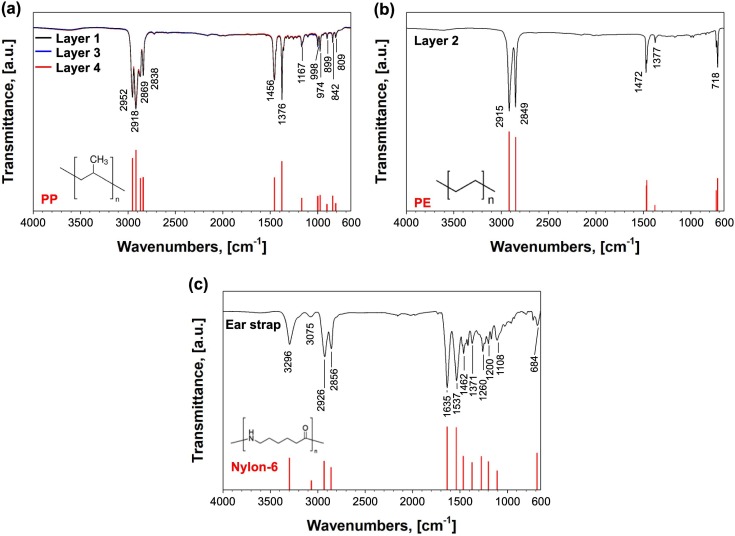
Disposable face masks are manufactured with various plastics (polymers) and inorganic compounds. Given that the disposed face masks into the environment have no available detailed information on their major constituents, it is required to analyze the types of materials made for them before further treatments. As depicted in the Fig. 1, the disposable face mask was disintegrated into several parts: filter layers 1 – 4, nose wire metal frame, and ear strap. At a glance, it can be realized that filter layers and ear strap are composed of plastics, while nose wire frame is made of metallic compound. Note that plastics are long chain organic carbon-based molecules. Each plastic has their own repeating units, which refer to monomers [38]. To obtain the information on the major repeating units from plastic parts of the face mask, FT-IR was used to characterize functional groups of each part. The resulting FT-IR spectra of plastic parts are shown in the Fig. 2 (a) – (c).
Fig. 2.
FT-IR spectra of different plastic parts of a disposable face mask: (a) filter layers 1, 3, and 4, (b) filter layer 2, and (c) ear strap with relevant reference peaks (red bars) of (a) PP [39], (b) PE [40], and (c) nylon-6 [41].
The filter layers 1, 3, and 4 showed identical peaks in the two broad ranges from 2838 to 2952 and from 809 to 1456 cm−1, which are characteristic FT-IR peaks of polypropylene (PP) [39]. In contrast, the filter layer 2 had identical peaks at 2915, 2849, 1472, 1377 and 718 with polyethylene (PE) [40]. Ear strap exhibited various peaks with nylon-6 [41]. Nose wire is composed of metal frame and covered by the filter layer 1. The major constituent of the metal frame was Fe (4.58 wt% of total mass of face mask), and the trace amount of other metal species (Zn, Ti, Ca, and Mn) were detected. In Table 1 , relative mass portion of plastic and metallic parts of the face mask are summarized with identification of their chemical constituents. The mass portion of different parts of the face mask was measured gravimetrically. In overall, PP (73.33 wt%) and PE (13.77 wt%) are major constituents of the face mask, because they are made for mask filter. Also, nylon (8.27 wt%) and Fe (4.58 wt%) are other main components for ear strap and nose wire frame.
Table 1.
Chemical compositions of disposable face mask identified by FT-IR and ICP-OES.
| Parts | Chemical compositions | Weight percentage (wt.%) |
|---|---|---|
| Filter layers 1, 3, 4 | PP | 73.33 |
| Filter layer 2 | PE | 13.77 |
| Ear strap | Nylon | 8.27 |
| Nose frame | Metals | 4.63 (Fe: 4.58, Zn: 0.02, Ti: 0.01, Ca: 0.01, and Mn: 0.01) |
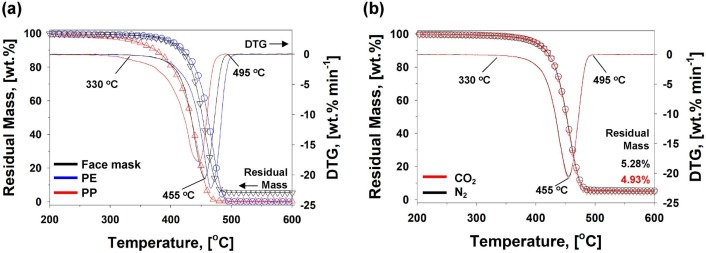
To fundamentally observe the thermolytic profiles of the disposal face mask, TGA test was performed before its pyrolysis study. The TGA measurements were conducted under both the N2 and CO2 atmospheres between 35 and 900 ˚C. Thermolytic profiles of the face mask were plotted as residual mass and differential thermogram (DTG) curves in Fig. 3 . Because the negligible thermal degradation (mass change) was shown below 200 ˚C and above 600 ˚C, the mass loss and DTG curves were selectively described from 200 to 600 ˚C. Thermolytic patterns of the face mask were compared with those of two major constituents (i.e., PP and PE) of the face mask (Fig. 3(a)). Major thermal degradation of the face mask initiated around 330 ˚C and ended up at 495 ˚C. These start and end temperatures were positioned between those of PP and PE. This is plausible because the major constituents of the face mask are PP and PE as defined in the Fig. 1 and Table 1. The residual mass of 5.28 wt% at 600 ˚C in the N2 condition indicates the existence of inorganic compounds, which are of Fe primarily, and small amounts of Zn, Ti, Ca, and Mn.
Fig. 3.
(a) Mass loss and DTG curves of face mask, PP, and, low density PE between 200 and 600 ˚C under the N2 environment, and (b) mass loss and DTG curves of face mask under the N2 and CO2 environments.
The TGA results of the face mask under the CO2 atmosphere was also compared in reference to N2 environment to determine the impact of CO2 on face mask pyrolysis (Fig. 3(b)). TGA results under the both conditions showed the identical thermolytic patterns, confirming that no additional heterogeneous reactions between CO2 and the solid face mask were occurred. Note that the Boudouard reaction (BR) is a thermodynamically preferred heterogeneous reaction between gas phase CO2 and solid carbon at ≥ 710 ˚C [42]. Since there was negligible amount of solid carbon left at ≥ 500 ˚C, the BR could not occur according to the TGA study.
It should be noted that any gas phase reactions from face mask pyrolysis could not be elucidated due to instrumental limitations of TGA unit. Also, gaseous products evolved from face mask pyrolysis were not able to be clarified with the TGA unit, because TGA study only offers a mass change at the given thermolysis temperature ranges. Thus, further studies to reveal the gaseous and liquid products should be performed to understand the thermolysis mechanism of the face mask and the resulting products from it.
3.2. Non-catalytic pyrolysis of disposable face mask
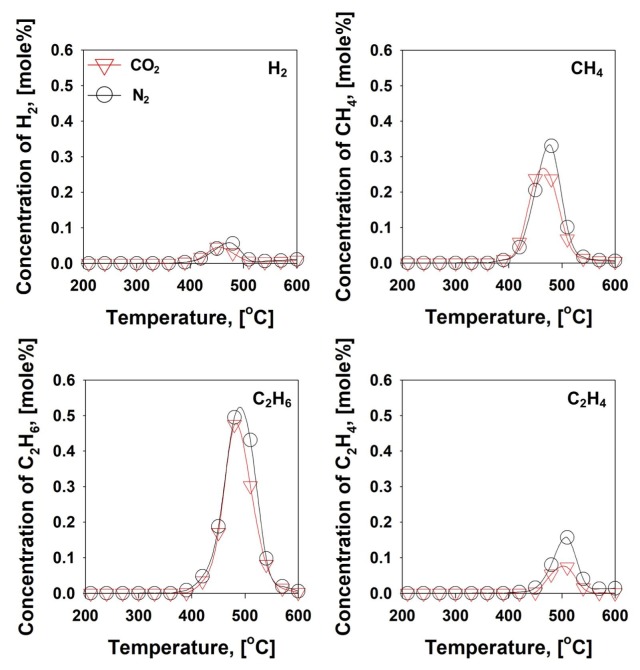
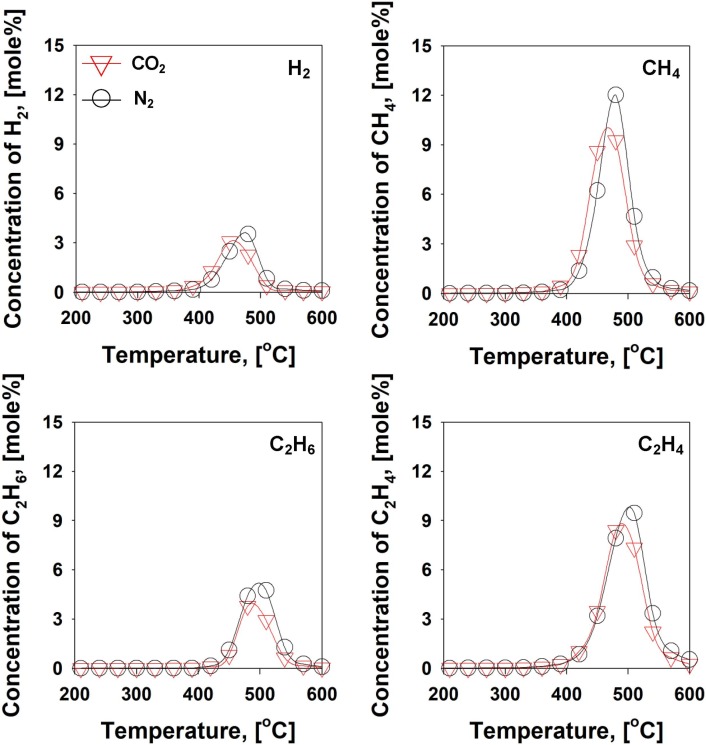
To identify the gaseous effluents from the mask pyrolysis, experimental setup for a single stage (one-stage) pyrolysis was established. The disposable face mask (1 g) was loaded into the tubular reactor within the temperature-programmed tubular furnace (35 to 600 ˚C at 10 ˚C min−1) under both the N2 and CO2 environments. This temperature program was chosen to match with that of TGA study to identify the thermolysis mechanism of the face mask. The resulting gaseous effluents (H2, CH4, C2H6, and C2H4) are displayed as a function of pyrolysis temperature in the Fig. 4 .
Fig. 4.
Gas evolution profiles from mask pyrolysis with one-stage setup under the N2/CO2 atmospheres.
From mask pyrolysis, small quantities (<0.6 mol%) of H2 and C1-2 hydrocarbons (HCs) were detected between 390 and 600 ˚C under both the N2 and CO2 conditions. Also, there was no notable difference shown between them. These results were contrast to the TGA result (Fig. 3), which indicated the substantial thermal degradation of the face mask between 320 and 490 ˚C. The mass loss of the face mask at this temperature range from the TGA study indicates the evolution of gaseous effluents. In fact, the production of H2 and C1-2 HCs from face mask pyrolysis was up to two orders of magnitude lower than that of other lignocellulosic biomass [43], [44], [45]. From the FT-IR analyses, it was confirmed that the main constituents of the face mask are PP, PE, and nylon. The repeating units of PP, PE, and nylon are propylene, ethylene, and long-chain amides, respectively. Therefore, it is estimated that volatile fraction of these plastics is indeed higher than that of lignocellulosic biomass [46], [47] because the plastics did not produce a char at 600 ˚C as demonstrated in the TGA study (Fig. 3). However, the concentration of gaseous effluents from mask pyrolysis was lower. Thus, it can be inferred that the formation of long-chain HCs was occurred after face mask pyrolysis using this single-stage setup.
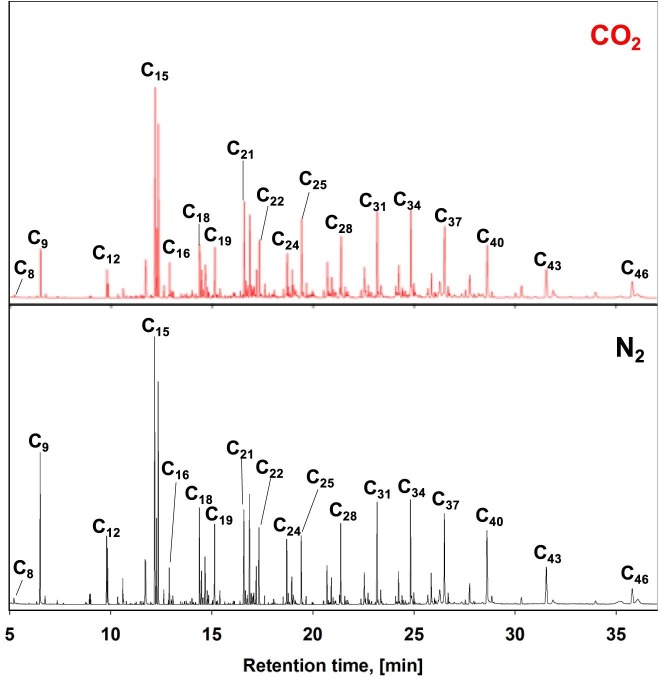
To confirm the presence of the long-chain HCs from one-stage pyrolysis, condensable HCs evolved from mask pyrolysis were collected through a cold solvent trap, filled with a dichloromethane at low temperature (– 1 ˚C). The chemical compositions of the condensed gaseous products from one-stage pyrolysis were analyzed using GC-TOF/MS. Fig. 5 and Table 2 represent the compositional matrixes of condensable pyrolysates. Major chemical constituents obtained from face mask pyrolysis were C9-46 HCs. Both the N2 and CO2 conditions showed similar chromatogram patterns. This confirmed that face mask pyrolysis led to thermal degradation of plastics into long-chain condensable HCs, but it did not fully convert long-chain HCs into H2 and C1-2 HCs. In addition, the effectiveness of CO2 was not observed under the one-stage pyrolysis.
Fig. 5.
GC-TOF/MS results of condensable HCs obtained from mask pyrolysis under the N2/CO2 atmospheres.
Table 2.
Peaks areas of chemical constituents obtained from condensable HCs under N2 and CO2 atmospheres.
| Peak Notation | Component | Retention Time | Molecular Weight | Area (CO2)× 10-6 | Area (N2)× 10-6 |
|---|---|---|---|---|---|
| C8 | 4-methylheptane | 5.222 | 114 | 1.09 | 1.45 |
| C9 | 2,4-Dimethyl-1-heptene | 6.517 | 126 | 18.54 | 38.19 |
| C12 | 2,4,6-trimethyl-1-nonene (meso form) | 9.804 | 168 | 7.29 | 15.04 |
| 2,4,6-trimethyl-1-nonene (racemic form) | 9.853 | 168 | 5.38 | 11.50 | |
| C15 | 2,4,6,8-tetramethyl-1-undecene (isotactic) | 12.180 | 210 | 47.54 | 58.80 |
| 2,4,6,8-tetramethyl-1-undecene (heterotactic) | 12.266 | 210 | 17.81 | 21.28 | |
| 2,4,6,8-tetramethyl-1-undecene (syndiotactic) | 12.353 | 210 | 39.56 | 49.43 | |
| C16 | 2,4,6,8,10-pentamethyl-1,10-undecadiene (isotactic) | 12.909 | 222 | 8.66 | 84.17 |
| C18 | 2,4,6,8,10-pentamethyl-1-tridecene (isotactic) | 14.391 | 252 | 18.19 | 22.73 |
| C19 | 2,4,6,8,10,12-hexamethyl-1,12-tridecadiene (isotactic) | 15.139 | 264 | 14.44 | 20.57 |
| C21 | 2,4,6,8,10,12-hexamethyl-1-pentadecene (isotactic) | 16.588 | 294 | 26.38 | 24.75 |
| C22 | 2,4,6,8,10,12,14-heptamethyl-1,14-pentadecadiene (isotactic) | 17.335 | 306 | 22.86 | 19.36 |
| C24 | 2,4,6,8,10,12,14-heptamethyl-1-heptadecene (isotactic) | 18.713 | 336 | 15.71 | 17.52 |
| C25 | 2,4,6,8,10,12,14,16-octamethyl-1,16-heptadecadiene (isotactic) | 19.429 | 348 | 26.93 | 19.38 |
| C28 | 2,4,6,8,10,12,14,16,18-nonamethyl-1,18-nonadecadiene (isotactic) | 21.378 | 390 | 22.35 | 21.34 |
| C31 | 2,4,6,8,10,12,14,16,18,20-decamethyl-1,20-henicosadiene (isotactic) | 23.174 | 432 | 24.45 | 27.49 |
| C34 | 2,4,6,8,10,12,14,16,18,20,22-undecamethyl-1,22-tricosadiene(isotactic) | 24.831 | 474 | 33.27 | 30.14 |
| C37 | 2,4,6,8,10,12,14,16,18,20,22,24-dodecamethyl-1,24-pentacosadiene (isotactic) | 26.504 | 516 | 32.95 | 34.64 |
| C40 | 2,4,6,8,10,12,14,16,18,20,22,24,26-tridecamethyl-1,26-heptacosadiene (isotactic) | 28.608 | 558 | 26.77 | 33.42 |
| C43 | 2,4,6,8,10,12,14,16,18,20,22,24,26,28-tetradecamethyl-1,28-nonacosadiene (isotactic) | 31.884 | 600 | 21.23 | 25.84 |
| C46 | 2,4,6,8,10,12,14,16,18,20,22,24,26,28,30-pentadecamethyl-1,30-untriacontadiene (isotactic) | 35.802 | 642 | 15.95 | 22.12 |
To enhance the conversion of condensable HCs into more value-added gaseous products (H2 and C1-2 HCs), pyrolysis setup was modified. For more thermal cracking, additional furnace was installed right next to the one-stage pyrolysis setup. The second furnace (second heating zone) operated at 600 ˚C isothermally, while the first heating zone operated from 35 to 600 ˚C with a heating rate of 10 ˚C min−1. The gaseous pyrolysates produced from two-stage pyrolysis are shown in the Fig. 6 . From the two-stage pyrolysis the formations of H2 (3.5 mol%), CH4 (12.0 mol%), C2H6 (4.8 mol%), and C2H4 (9.5 mol%) were substantially enhanced from results of one-stage pyrolysis (<0.6 mol% of H2 and C1-2 HCs) under the N2 condition. This result well agreed with the hypothesis that condensable HCs can be converted into gaseous HCs with additional thermal energy. Considering that chemical structures of PP, PE, and nylon are mostly composed of C–C and C–H bonds [48], the thermal energy through the second heating zone significantly contributed to the improvement of reaction kinetics for C–C and C–H bonds scissions for the production of gaseous effluents (H2 and C1-2 HCs). The production of C1-2 HCs was likely due to the C–C bond scissions [48], while the production of H2 was attributed to C–H bond scissions [49]. The results of pyrolysis studies of the disposable face mask were identical in both the N2 and CO2 environments. This offers that CO2 can be a viable purge gas for pyrolysis of the plastic waste in the replacement of the conventional purge gas, N2.
Fig. 6.
Gas evolution profiles from mask pyrolysis with two-stage setup under the N2/CO2 atmospheres.
3.3. Catalytic pyrolysis of disposable face mask
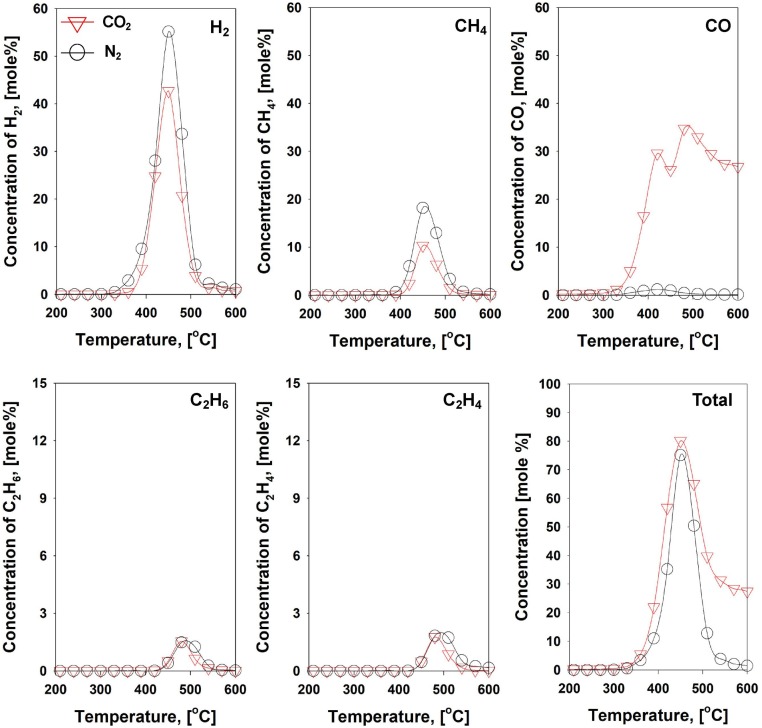
As discussed in the TGA experiments (Fig. 2 and Table 1), the disposable face mask contained higher than 95 wt% of volatile compounds (PP, PE, and nylon), which are mostly composed of C–C and C–H bonds. As a means to enhance the reaction kinetics for C–C and C–H bonds scissions, additional heating zone was introduced in the two-stage pyrolysis. Although the two-stage pyrolysis substantially improved the C–C and C–H bonds scissions with the formations H2 and C1-2 HCs, there was still remained long-chain HCs. Also, much higher concentrations of C1-2 HCs than the H2 were shown. These indicate that the further C–C and C–H bonds scissions of various HCs can facilitate H2 formations. For this reason, Ni-based catalyst (Ni/SiO2) was employed to conduct catalytic pyrolysis of the face mask in the two-stage setup. Ni catalyst was selected because of its known catalytic performance for C–H bond scissions (dehydrogenation for H2 production) [36], [50], [51]. The evolution profiles of gaseous pyrolysates (H2, CH4, CO, C2H6, and C2H4) from catalytic pyrolysis against first heating zone temperature are plotted in Fig. 7 .
Fig. 7.
Gas evolution profiles from mask pyrolysis with 5 wt% Ni/SiO2 catalyst under the N2/CO2 atmospheres.
Under the N2 environment, the performance of catalytic pyrolysis over Ni/SiO2 was much greater than non-catalytic pyrolysis. In detail, the maximum concentration of H2 increased from 3.5 to 55.1 mol%, and that of CH4 increased from 12.0 to 18.2 mol%. In contrast, the concentrations of C2 HCs were reduced. Thus, it is inferred that C–H and C–C bond scissions of C2 and longer chain HCs over Ni/SiO2 catalyst contributed to H2 and CH4 formations. The dominant production of H2 over other HCs indicates that Ni catalyst selectively contributed to C–H bond scissions over C–C bond scissions.
In the CO2 environment, the similar evolution trends were shown, which are dominant production of H2 and CH4 with the reduction of C2H6 and C2H4 formations (Fig. 7). Nonetheless, concentrations of H2 and C1-2 HCs were relatively lower, comparing to the results in the N2 condition. The most noticeable difference of catalytic pyrolysis under the CO2 with other pyrolysis setups was CO formation as a major gaseous product. Because of the major constituents of the face mask (i.e., PP, PE, and nylon) have limited quantity of oxygen, the high quantity of CO cannot be expected without the presence of CO2 as an oxygen source. As discussed in the TGA result, the heterogeneous reaction (i.e., BR) between gaseous CO2 and solid carbon could not occur at this temperature region. Therefore, there are three possibilities to produce CO in the presence of CO2 over Ni/SiO2 catalyst: (1) reverse water–gas-shift (RWGS) reaction (, (2) CO2 dry reforming (e.g., ), and (3) gas phase homogeneous reactions (GPRs) between CO2 and gaseous HCs evolved from the face mask pyrolysis through the second heating zone at 600 ˚C. The first suggestion is closely related to the presence of both H2 and CO2. Indeed, the concentration of H2 was reduced at ≥ 330 ˚C in the presence of CO2, comparing to N2 condition. This temperature range was overlapped with the temperature window for CO production. In addition, CO2 dry reforming also could be plausible due to the reduction of CH4 concentration in the presence of CO2, comparing to the pyrolysis result in the N2 condition. Since both the RWGS and CO2 dry reforming reactions produce CO, consuming H2 and CH4, the total molar concentrations of H2, CO, and CH4 should be matched when only these two reactions were occurred for CO formation (Fig. 7). However, higher concentrations of gaseous effluents were observed in the CO2 condition than the N2 condition (Fig. 7). Thus, it can be inferred that CO formation was not entirely ascribed to the RWGS and CO2 dry reforming, thereby suggesting additional GPRs between CO2 and gaseous HCs evolved from face mask pyrolysis. In other words, CO2 acted as a soft oxidant for catalytic pyrolysis of the face mask over Ni/SiO2. Therefore, the second highest peak of CO shown at 480 ˚C was likely ascribed to the GPRs (Fig. 7). Through the catalytic pyrolysis, the cumulative production of gaseous effluents increased 25 times and 165 times higher than two-stage pyrolysis and one-stage pyrolysis, respectively, under the CO2 condition (Fig. 8 ).
Fig. 8.
Comparison of overall gas production from one-stage pyrolysis, two-stage pyrolysis, and catalytic pyrolysis (5 wt% Ni/SiO2) of the disposal face mask under the CO2 environment.
By switching purge gases of the face mask catalytic pyrolysis between N2 and CO, different H2/CO ratio was achieved from the face mask catalytic pyrolysis (Fig. 7). This immediate control of H2/CO ratios can be technically and industrially beneficial. Both H2 and CO are useful industrial chemicals and fuels to be used as direct fuels and intermediates for the production of more value-added chemicals through well-defined chemical processes. In the chemical processes, the control of H2/CO ratios is crucial to selectively produce desired chemicals such as saturated HCs [52], olefins [52], and alcohols [53].
In sum, this work offered a versatile thermo-chemical process to valorize the disposable COVID-19 face mask. To suggest an environmentally benign process, CO2 was utilized as a reaction medium for pyrolysis of the disposable face mask. Prior to pyrolysis study, chemical constituents of the disposable mask were investigated. Because major compounds of the face mask were plastics (PP, PE, and nylon), its pyrolysis resulted in the production of H2 and various ranges of HCs. To enhance the generations of value-added H2 and C1-2 HCs, multi-stage pyrolysis setup was employed for C–C and C–H bonds scissions of long-chain HCs. Further pyrolysis over Ni/SiO2 catalyst led to substantial conversion of longer chain (≥C2) HCs into H2 and CH4. During catalytic pyrolysis, H2 production was substantially improved under the N2 condition, while significant CO formation was shown under the CO2 environment due to RWGS, CH4 dry reforming, and gas phase reactions of CO2. Thus, H2/CO ratio was controlled in the catalytic pyrolysis when the reaction gas medium was changed. In contrast, CO2 worked as an inert gas for non-catalytic pyrolysis. These findings offer that CO2 can be considered a versatile purge gas and soft oxidant in various thermo-chemical processes, controlling exit gaseous effluents. Formations of syngas and C1-2 HCs from plastic waste (disposable face mask) and greenhouse gas (CO2) can contribute to the reduction of CO2 emission by counterbalancing fossil fuel production. This also could be an environmentally benign and process-efficient way to dispose of COVID-19 plastic waste, simultaneously generating value-added products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was also supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (NRF-2019H1D3A1A01070644 and NRF-2019R1A4A1027795).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cej.2020.126658.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.D.E.L. Promislow, A geroscience perspective on COVID-19 mortality, The journals of gerontology. Series A, Biological sciences and medical sciences (2020). [DOI] [PMC free article] [PubMed]
- 2.WHO, WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020, 2020.
- 3.WHO, Coronavirus disease (COVID-19). World Health Organization Situation Report – 134, 2020.
- 4.CDC, Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings (Centers for Disease Control and Prevention: CDC), 2020.
- 5.PHAC, Coronavirus disease (COVID-19): For health professionals (Public Health Agency of Canada: PHAC), 2020.
- 6.WHO, Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) - World Health Organization (WHO), 2020.
- 7.ECDC, Guidance for wearing and removing personal protective equipment in healthcare settings for the care of patients with suspected or confirmed COVID-19 (European Center for Disease Prevention and Control: ECDC), 2020.
- 8.Klemeš J.J., Fan Y.V., Tan R.R., Jiang P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew. Sustain. Energy Rev. 2020;127 doi: 10.1016/j.rser.2020.109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aalto-Korte K., Ackermann L., Henriks-Eckerman M.L., Välimaa J., Reinikka-Railo H., Leppänen E., Jolanki R. 1,2-Benzisothiazolin-3-one in disposable polyvinyl chloride gloves for medical use. Contact Dermatitis. 2007;57:365–370. doi: 10.1111/j.1600-0536.2007.01278.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee S., Cho A.R., Park D., Kim J.K., Han K.S., Yoon I.J., Lee M.H., Nah J. Reusable Polybenzimidazole Nanofiber Membrane Filter for Highly Breathable PM 2.5 Dust Proof Mask. ACS Appl. Mater. Interfaces. 2019;11:2750–2757. doi: 10.1021/acsami.8b19741. [DOI] [PubMed] [Google Scholar]
- 11.Aslan S., Kaplan S., Çetin C. An investigation about comfort and protection performances of disposable and reusable surgical gowns by objective and subjective measurements. J. Text. Inst. 2013;104:870–882. [Google Scholar]
- 12.Bdour A., Altrabsheh B., Hadadin N., Al-Shareif M. Assessment of medical wastes management practice: A case study of the northern part of Jordan. Waste Manage. 2007;27:746–759. doi: 10.1016/j.wasman.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Wu S.-L., Kuo J.-H., Wey M.-Y. Thermal degradation of waste plastics in a two-stage pyrolysis-catalysis reactor over core-shell type catalyst. J. Anal. Appl. Pyrol. 2019;142 [Google Scholar]
- 14.Anuar Sharuddin S.D., Abnisa F., Wan Daud W.M.A., Aroua M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manage. 2016;115:308–326. [Google Scholar]
- 15.Ma J., Wang J., Tian X., Zhao H. In-situ gasification chemical looping combustion of plastic waste in a semi-continuously operated fluidized bed reactor. Proc. Combust. Inst. 2019;37:4389–4397. [Google Scholar]
- 16.Al-Salem S.M., Antelava A., Constantinou A., Manos G., Dutta A. A review on thermal and catalytic pyrolysis of plastic solid waste. PSWJ. Environ. Manage. 2017;197:177–198. doi: 10.1016/j.jenvman.2017.03.084. [DOI] [PubMed] [Google Scholar]
- 17.Qin L., Han J., Zhao B., Wang Y., Chen W., Xing F. Thermal degradation of medical plastic waste by in-situ FTIR, TG-MS and TG-GC/MS coupled analyses. J. Anal. Appl. Pyrol. 2018;136:132–145. [Google Scholar]
- 18.Geyer R., Jambeck J.R., Law K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makarichi L., Jutidamrongphan W., Techato K.-A. The evolution of waste-to-energy incineration: A review. Renew. Sustain. Energy Rev. 2018;91:812–821. [Google Scholar]
- 20.Wang X., Ma D., Jin Q., Deng S., Stančin H., Tan H., Mikulčić H. Synergistic effects of biomass and polyurethane co-pyrolysis on the yield, reactivity, and heating value of biochar at high temperatures. Fuel Process. Technol. 2019;194 [Google Scholar]
- 21.Zhao H., Wang J. Chemical-looping combustion of plastic wastes for in situ inhibition of dioxins. Combust. Flame. 2018;191:9–18. [Google Scholar]
- 22.Wey M.-Y., Yu L.-J., Jou S.-I. The influence of heavy metals on the formation of organics and HCl during incinerating of PVC-containing waste. J. Hazard. Mater. 1998;60:259–270. [Google Scholar]
- 23.Buekens A., Cen K. Waste incineration, PVC, and dioxins. J. Mater. Cycles Waste Manage. 2011;13:190–197. [Google Scholar]
- 24.Lu J.-W., Zhang S., Hai J., Lei M. Status and perspectives of municipal solid waste incineration in China: A comparison with developed regions. Waste Manage. 2017;69:170–186. doi: 10.1016/j.wasman.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Quina M.J., Bontempi E., Bogush A., Schlumberger S., Weibel G., Braga R., Funari V., Hyks J., Rasmussen E., Lederer J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018;635:526–542. doi: 10.1016/j.scitotenv.2018.04.150. [DOI] [PubMed] [Google Scholar]
- 26.Burra K.G., Gupta A.K. Kinetics of synergistic effects in co-pyrolysis of biomass with plastic wastes. Appl. Energy. 2018;220:408–418. [Google Scholar]
- 27.Miandad R., Barakat M.A., Aburiazaiza A.S., Rehan M., Nizami A.S. Catalytic pyrolysis of plastic waste: a review. Process Saf. Environ. Prot. 2016;102:822–838. [Google Scholar]
- 28.Singh R.K., Ruj B. Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel. 2016;174:164–171. [Google Scholar]
- 29.Jung S., Park Y.-K., Kwon E.E. Strategic use of biochar for CO2 capture and sequestration. Journal of CO2 Utilization. 2019;32:128–139. [Google Scholar]
- 30.Yao D., Wu C., Yang H., Zhang Y., Nahil M.A., Chen Y., Williams P.T., Chen H. Co-production of hydrogen and carbon nanotubes from catalytic pyrolysis of waste plastics on Ni-Fe bimetallic catalyst. Energy Convers. Manage. 2017;148:692–700. [Google Scholar]
- 31.Navarro M.V., López J.M., Veses A., Callén M.S., García T. Kinetic study for the co-pyrolysis of lignocellulosic biomass and plastics using the distributed activation energy model. Energy. 2018;165:731–742. [Google Scholar]
- 32.Kim S., Park C., Lee J. Reduction of polycyclic compounds and biphenyls generated by pyrolysis of industrial plastic waste by using supported metal catalysts: A case study of polyethylene terephthalate treatment. J. Hazard. Mater. 2020;392 doi: 10.1016/j.jhazmat.2020.122464. [DOI] [PubMed] [Google Scholar]
- 33.Lee T., Jung S., Hong J., Wang C.H., Alessi D.S., Lee S.S., Park Y.K., Kwon E.E. Using CO2 as an Oxidant in the Catalytic Pyrolysis of Peat Moss from the North Polar Region. Environ. Sci. Technol. 2020;54:6329–6343. doi: 10.1021/acs.est.0c01862. [DOI] [PubMed] [Google Scholar]
- 34.Cho S.-H., Lee S.S., Jung S., Park Y.-K., Lin K.-Y.-A., Lee J., Kwon E.E. Carbon dioxide-cofeeding pyrolysis of pine sawdust over nickle-based catalyst for hydrogen production. Energy Convers. Manage. 2019;201 [Google Scholar]
- 35.Jung S., Choi D., Park Y.-K., Fai Tsang Y., Klinghoffer N.B., Kim K.-H., Kwon E.E. Functional use of CO2 for environmentally benign production of hydrogen through catalytic pyrolysis of polymeric waste. Chem. Eng. J. 2020 [Google Scholar]
- 36.Xu J., Froment G.F. Methane steam reforming, methanation and water-gas shift: I. intrinsic kinetics. AIChE J. 1989;35:88–96. [Google Scholar]
- 37.Kim J.-H., Jung S., Park Y.-K., Kwon E.E. CO2-cofed catalytic pyrolysis of tea waste over Ni/SiO2 for the enhanced formation of syngas. J. Hazard. Mater. 2020;396 doi: 10.1016/j.jhazmat.2020.122637. [DOI] [PubMed] [Google Scholar]
- 38.Siddique R., Khatib J., Kaur I. Use of recycled plastic in concrete: A review. Waste Manage. 2008;28:1835–1852. doi: 10.1016/j.wasman.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 39.M.R. Jung, F.D. Horgen, S.V. Orski, V. Rodriguez C, K.L. Beers, G.H. Balazs, T.T. Jones, T.M. Work, K.C. Brignac, S.-J. Royer, K.D. Hyrenbach, B.A. Jensen, J.M. Lynch, Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms, Marine Pollution Bulletin 127 (2018) 704-716. [DOI] [PubMed]
- 40.Rajandas H., Parimannan S., Sathasivam K., Ravichandran M., Su Yin L. A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polym. Test. 2012;31:1094–1099. [Google Scholar]
- 41.Lee K.H., Kim K.W., Pesapane A., Kim H.Y., Rabolt J.F. Polarized FT-IR study of macroscopically oriented electrospun nylon-6 nanofibers. Macromolecules. 2008;41:1494–1498. [Google Scholar]
- 42.Kwon E.E., Jeon E.-C., Castaldi M.J., Jeon Y.J. Effect of carbon dioxide on the thermal degradation of lignocellulosic biomass. Environ. Sci. Technol. 2013;47:10541–10547. doi: 10.1021/es402250g. [DOI] [PubMed] [Google Scholar]
- 43.Jung S., Kwon D., Tsang Y.F., Park Y.-K., Kwon E.E. CO2-cofeeding catalytic pyrolysis of macadamia nutshell. Journal of CO2 Utilization. 2020;37:97–105. [Google Scholar]
- 44.Lee T., Nam I.-H., Jung S., Park Y.-K., Kwon E.E. Synthesis of nickel/biochar composite from pyrolysis of Microcystis Aeruginosa and its practical use for syngas production. Bioresour. Technol. 2020;300 doi: 10.1016/j.biortech.2019.122712. [DOI] [PubMed] [Google Scholar]
- 45.Kwon E.E., Lee T., Ok Y.S., Tsang D.C.W., Park C., Lee J. Effects of calcium carbonate on pyrolysis of sewage sludge. Energy. 2018;153:726–731. [Google Scholar]
- 46.Park J.H., Park H.-W., Choi S., Park D.-W. Effects of blend ratio between high density polyethylene and biomass on co-gasification behavior in a two-stage gasification system. Int. J. Hydrogen Energy. 2016;41:16813–16822. [Google Scholar]
- 47.Jung J.-M., Oh J.-I., Baek K., Lee J., Kwon E.E. Biodiesel production from waste cooking oil using biochar derived from chicken manure as a porous media and catalyst. Energy Convers. Manage. 2018;165:628–633. [Google Scholar]
- 48.Liu X., Li X., Liu J., Wang Z., Kong B., Gong X., Yang X., Lin W., Guo L. Study of high density polyethylene (HDPE) pyrolysis with reactive molecular dynamics. Polym. Degrad. Stab. 2014;104:62–70. [Google Scholar]
- 49.Gao Z., Amasaki I., Kaneko T., Nakada M. Calculation of activation energy from fraction of bonds broken for thermal degradation of polyethylene. Polym. Degrad. Stab. 2003;81:125–130. [Google Scholar]
- 50.Rodemerck U., Schneider M., Linke D. Improved stability of Ni/SiO2 catalysts in CO2 and steam reforming of methane by preparation via a polymer-assisted route. Catal. Commun. 2017;102:98–102. [Google Scholar]
- 51.Kim S., Kwon E.E., Kim Y.T., Jung S., Kim H.J., Huber G.W., Lee J. Recent advances in hydrodeoxygenation of biomass-derived oxygenates over heterogeneous catalysts. Green Chem. 2019;21:3715–3743. [Google Scholar]
- 52.van Santen R.A., Markvoort A.J., Filot I.A.W., Ghouri M.M., Hensen E.J.M. Mechanism and microkinetics of the Fischer-Tropsch reaction. PCCP. 2013;15:17038–17063. doi: 10.1039/c3cp52506f. [DOI] [PubMed] [Google Scholar]
- 53.Fang K., Li D., Lin M., Xiang M., Wei W., Sun Y. A short review of heterogeneous catalytic process for mixed alcohols synthesis via syngas. Catal. Today. 2009;147:133–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.