Abstract
In recent months, the presence of an emerging disease of infectious etiology has paralyzed everyone, already being a public health problem due to its high rate of infection, a life-threatening disease. The WHO has named it COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV2). New studies provide information of the role of the environment in COVID-19 transmission process, mortality related to this infectious disease and the impact on human health. The following review aims to analyze information on the implications of COVID-19 infection on human health and the impact of its presence on the environment, from its transmission capacity and the role of air pollutants and climatological factors to reducing the air pollution during confinement. Likewise, it provides a vision of the impact on the environment and human health of exposure to disinfectants and the presence of COVID-19 in wastewater, among other actions.
Keywords: SARS-CoV-2, Transmission, Climate conditions, Air pollution, Aquatic toxicity
1. Implications of COVID-19 on human health
The emergence of a disease of infectious etiology in late 2019 by WHO as “coronavirus disease (COVID-19)" in the city of Wuhan, China (Adhikari et al., 2020; H. Lu et al., 2020a, 2020b; Xu et al., 2020), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as a causative agent of pneumonia (Di Gennaro et al., 2020; C. Huang et al., 2020a, 2020b; Sohrabi et al., 2020; Zhou et al., 2020; N. Zhu et al., 2020a, 2020b). More than 4 million confirmed cases of COVID-19 and more than 285,000 deaths have been reported worldwide as of May 15, 2020 (WHO, 2020b). (Table 1 , Fig. 1, Fig. 2 )
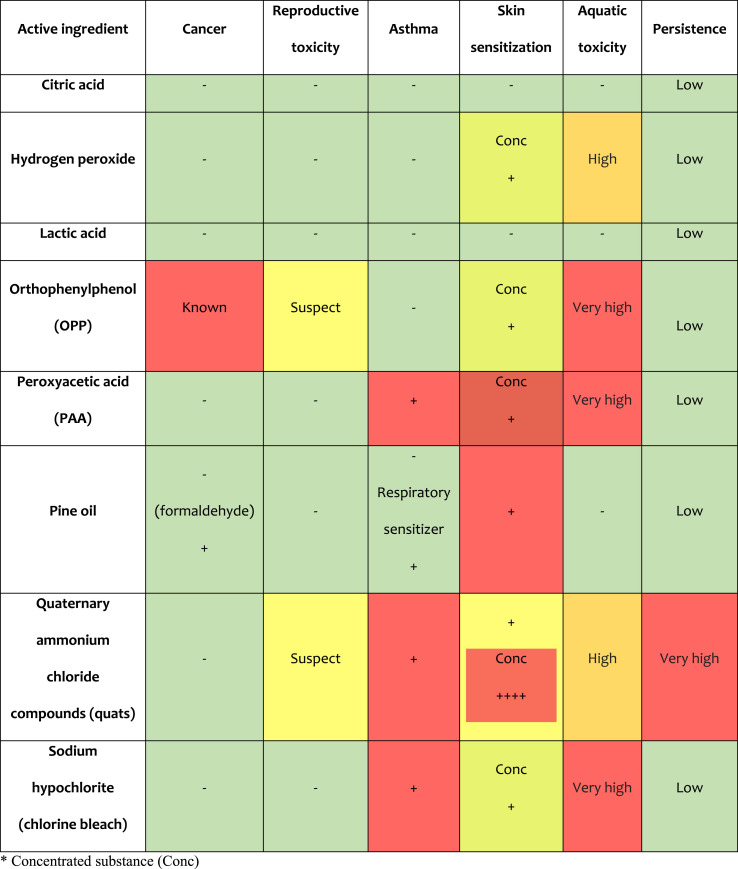
Table 1.
Environmental and health risks of active ingredients for commonly used surface disinfectants. ∗ Concentrated substance (Conc).
Fig. 1.
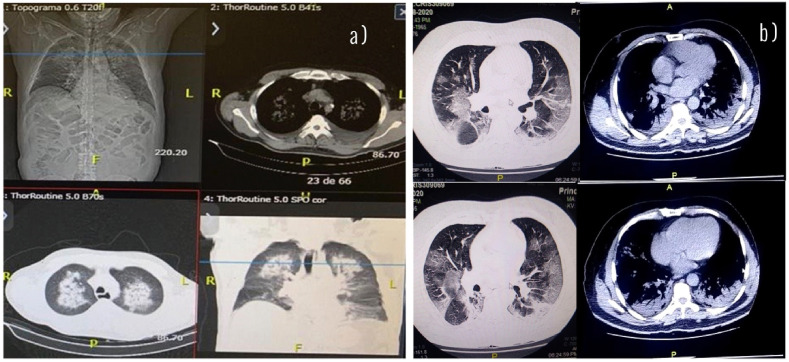
COVID-19 coronavirus disease is a causative agent of pneumonia and symptoms of the upper respiratory tract. The main coronavirus death cases occur mainly in older people, however they are reported as susceptible population: people with underlying noncommunicable diseases, chronic respiratory disease, cardiovascular disease, cancer, diabetes and obesity. a) Computed tomography and chest radiograph of a 70-year-old male high blood pressure and diabetes infected with SARS-CoV-2. Areas of opacity and ground glass and consolidation are observed with prominent involvement of the upper lobes of both lungs. b) Chest CT scan of an obese, 55-year-old man infected with SARS-CoV2. Areas of ground glass opacity, consolidations, and intralobular interstitial thickening are seen, with prominent involvement of the lower lobes of both lungs.
Fig. 2.
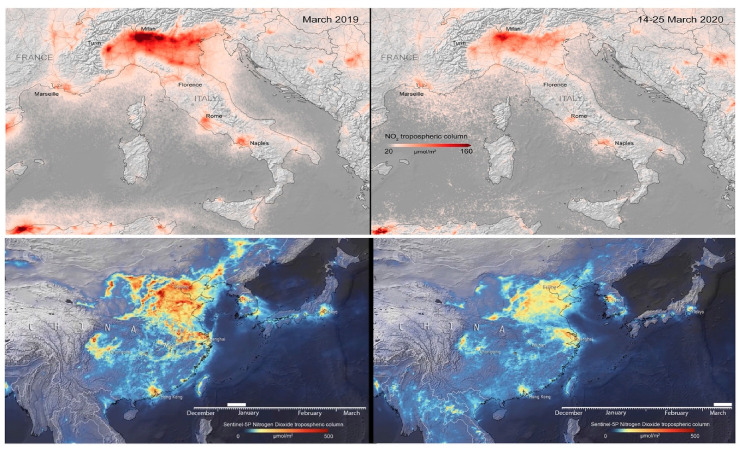
Nitrogen dioxide concentrations over Italy and China (before and during the pandemic). Source (ESA, 2020a, 2020b).
The probable origin of COVID-19 has been suggested to be zoonotic (Bassetti et al., 2020; Ji et al., 2020). The genomic sequence of SARS-CoV-2 indicates belongs to the betacoronavirus genus, an identity of 80–96% is reported with SARS-CoV and 50% with MERS-CoV that originate in bats (Cui et al., 2019; Lu et al., 2020a, 2020b; Ren et al., 2020; Rothan and Byrareddy, 2020). Of the seven subtypes of this virus, betacoronaviruses can cause severe illness and death, while alphacoronaviruses cause mild or asymptomatic infection. This virus has the ability, due to its structure, to produce the receptor binding of angio-tensin converting enzyme 2 (ACE2) from host cells (Cao et al., 2020a, 2020b; Jaimes et al., 2020; Kucharski et al., 2020; Wang et al., 2020a, 2020b, 2020c).
The incubation period and the onset of symptoms of SARS-CoV-2 is an average of 5.2 days, it is reported that the death of the infected person occurs between this period of 6 and 41 days with an average of 14 days (Li et al., 2020a, 2020b, 2020c; Wang et al., 2020a, 2020b, 2020c). The main reported symptoms of the upper and lower respiratory tract are: dry cough, runny nose, sore throat and dyspnea, associated with headache and fever; meanwhile, some patients have reported gastrointestinal symptoms such as diarrhea or even have very mild symptoms or be asymptomatic carriers. Bilateral opacities in the form of ground glass are identified on chest radiographs and tomography (Assiri et al., 2013; Rothan and Byrareddy, 2020). Given the above, it is proposed to analyze urine and faecal samples to exclude or identify alternative routes of transmission.
Evidence suggests that person-to-person transmission is the likely route to spread COVID-19 infection (Carlos et al., 2020; Chan et al., 2020; Q. Li et al., 2020a, 2020b, 2020c; D. Wang et al., 2020a, 2020b, 2020c), in addition to the contact of contaminated surfaces through drops that expand when coughing or sneezing (Ghinai et al., 2020; C. Huang et al., 2020a, 2020b; Yu et al., 2020).
The main cases of coronavirus death occur mainly in older people, probably due to a poor immune system. People or children with underlying noncommunicable diseases such as diabetes, chronic cardiovascular and lung diseases or even high blood pressure and cancer are at a higher risk of coronavirus infection (Fang et al., 2020; Giannis et al., 2020; Huang et al., 2020a, 2020b; Qiu et al., 2020; Shekerdemian et al., 2020; Wu et al., 2020a, 2020b; Xiao et al., 2020; Zhou et al., 2020).
A meta-analysis reports that the probability of developing severe Covid-19 disease for hypertension, respiratory disease, and cardiovascular disease is between 2.4 and 3.5 times (Yang et al., 2020). Likewise, it is reported that obesity and smoking were associated with higher risks (Huang et al., 2020a, 2020b; Wang et al., 2020a, 2020b, 2020c). Based on current data, the median case fatality rate for those younger than 60 years is estimated to be <0.2%, compared to 9.3% for adults older than 80 years (Ferguson et al., 2020). Comorbidities increase the risk of mortality up to five times (Jordan et al., 2020). A death rate from COVID-19 is reported in Wuhan of 5.0%, close to that of the world (4.2%), while higher case fatality rates are reported in Italy (9.3%), Iran (7.8%) and Spain (6.0%) (Jin et al., 2020; X. Li et al., 2020a, 2020b, 2020c).
Until now, there are no specific pharmacological treatment or vaccines against COVID-19 infection for potential therapy in humans, so extensive isolation measures and the use of disinfection products have been implemented to reduce their transmission from person to person. person and current outbreak; therefore, it is imperative to continue such measures to avoid the potential effects of COVID-19 infection.
2. Transmission of COVID-19: stability on surfaces
Evidence reports that COVID-19 significantly pollutes the air and the environment from the surfaces of the environment built by aerosol flow (Dietz et al., 2020; Ong et al., 2020; Rothan and Byrareddy, 2020). Current estimates of contagion for each infected person (known as Ro) is 1.5–3.9 people (Du et al., 2020; Q. Li et al., 2020a, 2020b, 2020c; Riou and Althaus, 2020), or even more 6.5 (Guo et al., 2020; Liu et al., 2020). While within the built environment it is between 5 and 14 ((Poon and Peiris, 2020; Zhang et al., 2020a, 2020b). A transmissibility similar to that of other β-coronaviruses, such as SARS-CoV (Ro = 2.2–3.6) and the estimated Ro value of MERS-CoV is 2.0–6.7 (Lipsitch, 2003; Majumder et al., 2014; L. Wang et al., 2020a, 2020b, 2020c). From some published studies, Ro are estimated for two moderately transmissible viruses, the coronaviruses of severe acute respiratory syndrome 2 to 4, Influenza A (H1N1) pdm09 1.4 to 1.6 to 2 and HIV 2 to 5, and for two highly transmissible viruses, smallpox Ro 4–10 and measles 12–18 (Baldo et al., 2016; Fraser et al., 2004). It is concluded then that since the beginning of the epidemic the Ro is 2.38 and according to some current studies up to 5.7 (Li et al., 2020a, 2020b, 2020c; Wu et al., 2020a, 2020b), which indicates that the SARS-CoV-2 has a relatively high sustained transmissibility.
SARS-CoV-2 is reported to be even more contagious (but thankfully less fatal) than SARS-CoV. The virus has intermediate levels of both respiratory and fecal-oral transmission potential according to a model that measures the percentage of intrinsic disorder (PID) of membrane (M) and nucleocapsid (N) proteins in viruses (Goh et al., 2012, 2020a). The main tool uses AI technology to recognize the intrinsic disorder, given the protein sequence. The model is based on the premise that viruses that remain in hostile environments require harder, that is, less disordered, shells to survive (Goh et al., 2019). Furthermore, higher levels of inner layer disorder could be associated with higher infectivity, especially with respect to viruses with high potential for respiratory transmission (Goh et al., 2020b, 2020c, 2013). Evidence of the protective role of outer shells is seen in a wide variety of viruses. Sexually transmitted viruses (eg, HIV, HSV-2, HCV) have PID from the upper outer layer (Goh et al., 2019, 2020b; 2015; Goh and Foster (2019)). Also, viruses that are known to last a long time in the environment, such as smallpox virus, have low outer layer PID. SARS-CoV-2 is very rare with one of the hardest outer protective layers (PID M = 6%) among coronaviruses (Goh et al., 2020a).
It is likely that this peculiarity is responsible for its high level of contagion, since the hardness of its outer layer could provide the virus with greater resistance to conditions outside the body and in body fluid, since the harder layer will better protect the virion from damage. As a result of the hostile environment and the action of digestive enzymes found in body fluids. The ability of SARS-CoV-2 to remain infectious outside the body for a longer period than SARS-CoV could mean that it requires fewer viral particles for greater chances of infection. As a result, the infected body is likely to be able to remove more infectious particles that are more likely to infect a person throughout their life. All of this may explain not only the high spread of COVID-19 but also the reported ability of this virus to spread even before the patient begins to show symptoms. The mechanism by which the virus acquires increased virulence through inner coat disorder arises from the ability of the viral protein to bind promiscuously to the host protein. This ability provides rapid replication of viral proteins and particles (Goh et al., 2019, 2020b; 2020a, 2020c, 2016).
The stability of viruses in the environment is essential in risk analysis. Temperature has been the most studied factor and is recognized as the most influential. The high temperature causes a faster viral inactivation, the opposite happens with the low temperature, viruses can survive for long periods of time (Dublineau et al., 2011; Pinon and Vialette, 2018).
The virus transmitted by blood and body fluids such as the human immunodeficiency virus (HIV) has the potential to be used as a vector, surrounded by a high organic load and its sliding envelope, which protects the internal viral components from the effects of dehydration and that carries a high potential to remain viable for long periods. Persistence of HIV on a glass surface from 30 to 35 h up to broader ranges of 4–8 weeks and survival of HIV for several days in stored refrigerated or non-refrigerated corpses are reported. There is a substantial loss of endogenous infectious virus in plasma samples at room temperature for more than 3 h or a few after venipuncture. The environmental survival of viruses is particularly affected by relative humidity and can vary considerably. Despite its long survival time, there is no known evidence that HIV can be transmitted through contaminated fomites, although the possibility of such risk cannot be excluded (Valtierra, 2008; Van Bueren et al., 1994).
Some other viruses are easily transmitted through the aerosol route such as influence virus and coronavirus, their persistence as infectious potential is stable in fine aerosols for prolonged periods of time. This stability is affected by exposure to environmental stressors, such as relative humidity. Specifically for the Influenza virus, the potential to persist on surfaces for hours in physiological drops depends on relative humidity (RH), low RH, and high RH in cool, dry, or wet and rainy conditions, facilitating virus survival, and the intermediate RH decreases the stability of the virus. Minor fluctuations in temperature, pH and salinity improve or reduce stability and its transmission. Colder temperatures improve virus survival and transmission. There is a significant interaction between particulate matter and mean temperature, while the relationship between ozone level and influenza incidence was independent of temperature (Kormuth et al., 2019; Sooryanarain and Elankumaran, 2015; Xu et al., 2013). Influenza viruses can survive for approximately 24–72 h on hard non-porous surfaces such as stainless steel and plastic, and up to 12 h on porous surfaces such as cloth and paper at 28 °C and humidity levels of 35%–40% As well as banknotes, it has a viability that ranges from 2 h to five days. The influenza virus has been found in more than 50% of fomites and hands in contact on different surfaces in homes and daycare centers (Valtierra, 2008; World Health Organization, 2017).
Transmission of COVID-19 by air occurs in 2 different ways and requires no physical contact: droplet sprays produced by coughing, sneezing, or speaking (vocalization emits an imperceptible aerosol “cloud”) that directly impact a subject susceptible to or lodged on a surface or by inhaling aerosols with viral particles that can last in the air for hours (Asadi et al., 2020; N. Zhu et al., 2020a, 2020b).
A report demonstrated the presence of SARS-CoV-2 virus particles in ventilation systems in a hospital serving patients with COVID-19. Finding virus particles in these systems is more consistent with the hypothesis of the existence of a turbulent gas cloud as a means of transmission of the disease with respect to COVID-19 (Bourouiba, 2020; Ong et al., 2020); therefore, WHO advises healthcare personnel and anyone to keep a distance of 3 feet (1 m) and 6 feet from an infected person (WHO, 2020a). The Center for Disease Control and Prevention recommends a 6 foot (2 m) gap. However, these distances do not estimate the time scale and persistence over which the cloud travels and its pathogenic load, thus generating an underestimated range of potential exposure for a health worker or healthy person. Protective and source control masks, as well as other protective equipment, must have the ability to repeatedly resist the type of turbulent gas cloud that can be expelled during a sneeze or cough and virus exposure (Bourouiba, 2020).
The built environment serves as a contact vector of the surfaces for COVID-19 infection, since the virus can survive for hours on surfaces like fomites, but a simple disinfectant can eliminate it (Lai et al., 2020). The literature indicates that coronaviruses can remain for hours or days according to the physical characteristics of the surfaces: plastic surfaces 6.8 h (half-life = 15.9 h), copper (3.4 h), cardboard (8.45 h) and stainless steel 5.6 h (half-life = 13.1 h) and shorter in aerosol form 1.1–1.2 h (2.7 h); however, aerosol survival was determined at 65% relative humidity (Kampf et al., 2020; van Doremalen et al., 2020). The COVID-19 virus does not resist temperatures above 26 °C, but can survive for approximately 5–10 min on the skin, six to 12 h in plastic materials, 12 h in metal (Nazari Harmooshi et al., 2020). Likewise, the faecal-oral route is reported as a probable route of transmission of the virus, since it is present in the faeces (Xiao et al., 2020).
Precautions to take to slow the spread of COVID-19 infection include: washing hands for at least 20–30 s with soap and water or 60–80% alcohol-based hand sanitizers and implementing cleaning protocols for surfaces by chemical deactivation of viral particles (Kampf et al., 2020; Ong et al., 2020).
Transmission of aerosol SARS-CoV-2 is well documented, while transmission through fomites via abiotic surfaces via the fecal-oral route; however these mechanisms need to be considered. The social distancing and confinement policies currently implemented given the spatial dynamics of the spread of the SARS-CoV-2 virus are of vital importance; however other less well-known routes of transmission will have to be considered and addressed to reduce the spread of this virus, especially the measures that must be taken during the stay within areas of the built environment.
3. Environmental factors and their influence on COVID-19 infection
3.1. Air quality
Beyond the effects of social distancing, the COVID-19 pandemic shows a way to achieve positive environmental change. The reduction of greenhouse gas emissions is identified, due to the decline in industrial activity and refineries; as well as the use of vehicles and transportation systems decreased considerably (He et al., 2020).
In Asia, Europe and America, air pollution levels are reported to be declining in several cities, specifically concentrations of nitrogen dioxide (NO2), particulate matter less than 2.5 μm in diameter (PM), black carbon (CN)·In addition, a reduction in PM10 (−28 to −31.0%) and an increase in ozone (O3) concentrations of around 50% were observed (Tobías et al., 2020).
NASA satellites and of the Copernicus Atmosphere Monitoring Service of the European Space Agency (ESA) have documented significant reduction in air pollution in major cities around the world. It is predicted that during 2 months of improving air quality in China alone, thousands of children and older adults could be saved. A similar 20–30% reduction in pollution in the world’s major cities could generate significant health benefits (Dutheil et al., 2020; Nelson, 2020).
Given the magic and illusion of the positive environmental effects of COVID 19 that could be perceived, there is also the counterpart. As the economy reopens, curbing polluting activities and the emission of greenhouse gases and particles associated with respiratory illness, these will have a long-term negative impact from the Covid-19 pandemic in large cities.
3.2. COVID-19 and atmospheric particles
There is scientific evidence that exposes a high correlation between the presence of ozone (O3), sulfur dioxide (SO2), nitrogen dioxide (NO2) and fine particles in the induction of hyperexpression of proinflammatory interleukins (Kurai et al., 2018; Perret et al., 2017). NO2 is a common marker of air pollution/industrial activity, associated with morbidity and mortality (He et al., 2020).
Individuals of any age group, including healthy people who are in areas with high levels of long-term air pollution, are at increased risk of developing chronic and infectious respiratory diseases. Fine particles 2.5 mm in diameter (PM 2.5) suspended in the air have a greater possibility of entering the lower respiratory tract, leading to the development of a progressive and chronic inflammatory stimulus characterized by excessive mucus production and dysfunction of the ciliary epithelium (first defense mechanism in the respiratory tract) and induce persistent modifications of the immune system, which makes individuals more likely to develop severe respiratory diseases and viral infections (Yu et al., 2020; Conticini et al., 2020; Martelletti and Martelletti, 2020; Tsai et al., 2019).
It has been hypothesized that the SARS-COV-2 virus has the ability to bind to PM and in conditions of atmospheric stability improves its persistence in the atmosphere, due to the presence of RNA from this virus in said particles, promoting its diffusion through the air (McNeill, 2020), since the role of short-term exposure with PM and transmission of COVID-19 has been reported (Y. Zhu et al., 2020a, 2020b); however, a study in Italy reports that the PM concentration and cases of COVID-19 virus infection are not evident, therefore it is not possible to conclude that the diffusion mechanism of COVID-19 also occurs through the air, using PM as a carrier (Bontempi, 2020; Setti et al., 2020). However, the evaluation of PM as a chronic stressor that makes the population more vulnerable to an epidemic has been reinforced by multiple studies. Chronic exposure to air pollutants may represent a risk factor in determining the severity of Covid-19 syndrome and the high incidence of fatal events (N. Chen et al., 2020; Dutheil et al., 2020; D. Wang et al., 2020a, 2020b, 2020c; F. Wu et al., 2020a, 2020b). A correlation is reported between the high level of contamination (particles with an exposure diameter less than 2.5 μm (PM 2.5) and the case fatality rate in northern Italy (Conticini et al., 2020). While the chronicity of Exposure of atmospheric pollutants NO2, O3, PM 2.5 and PM10 were significantly correlated with the spread of cases (mortality) of the Covid-19 virus in provinces in Italy, the mortality rate varied from 18% (Fattorini and Regoli, 2020).
This allows us to conclude that there are still studies to establish the factors that affect the routes of diffusion and transmission of the SARS-COV-2 virus, such as the evaluation of the geophysical and climatic characteristics of the study areas and the relationship between the dynamics of the populations and their relationship with atmospheric pollutants.
Although, the information provided by these studies on the correlation between exposure to particles derived from air pollution, it is a priority to consider the impact that respiratory involvement brings on long-term COVID-19 infection, towards the prevalence of infections and chronic inflammatory processes; these implications need to be determined for the proposal of a future environmental policy. An unexpected advantage may be provided to help understand how environmental health can be altered, through better environmental regulation from technology. A special consideration in the future will be the identification of the effects of the reduction of air pollution from the different emission sources that will be a starting point to evaluate other air quality policies.
3.3. Climate indicators and COVID-19
In particular, in addition to person-to-person transmission, weather parameters (temperature, wind speed and humidity) are classified as the main predictors of infectious respiratory diseases according to the viability, transmission and range of spread of the virus. This reveals a possible association between the accumulation of atmospheric pollutants and the combination of specific weather factors that promote a greater permanence of viral particles in the air and their diffusion, specifically for infection by COVID-19 (Frontera et al., 2020; van Doremalen et al., 2020). Ambient temperature and air quality have been estimated to be correlated with the spread of COVID-19. The mean temperature is related to the high risk of virus transmission (with a threshold of 3 °C, when the temperature is < 3 °C) (Tosepu et al., 2020; Xie and Zhu, 2020). A limited number of studies have shown that humidity and temperature likely affect COVID-19 activity and transmissibility (Nazari Harmooshi et al., 2020). In Hubei province in China, low relative humidity and daytime temperature were found to have a greater impact on COVID-19 infection, while extreme daily temperature negatively influenced this virus (Liu et al., 2020; Pirouz et al., 2020).
When talking about COVID-19 mortality, weather conditions could also contribute to the decline. In Wuhan, China, temperature variation, humidity, and wind speed were reported to influence COVID-19 case fatality and number (Chen et al., 2020; Şahin, 2020). Decreasing one unit in the daytime temperature range increases the risk of COVID-19 cases and deaths by 2.92 times. On the other hand, the increase of 1 unit of temperature as the absolute humidity were related to the decrease in death from this virus (Ma et al., 2020). Therefore, increased mortality from COVID-19 may also be related to lower humidity in winter.
Following the analysis of associations between weather conditions, air pollution and COVID-19 infection, related theories are proposed in the population distribution and the presence of the disease. Some researchers propose that the epidemic could gradually decrease as a result of rising temperatures in the coming months. While others suggest the possibility of this disease becoming seasonal during the autumn-winter months (Hellewell et al., 2020; Maier and Brockmann, 2020). Meanwhile, it is imperative to further strengthen prevention measures against potential transmission; therefore, strict compliance with confinement, hand washing and personal hygiene is necessary to record a lower incidence of COVID-19 and mortality.
3.4. COVID- 19 and wastewater
Transmission of COVID-19 has been established to take place from person to person through direct contact of secretions from an infected individual and contaminated surfaces or objects; however, this virus has been found in faeces and urine (Holshue et al., 2020; Pan et al., 2020; Xiao et al., 2020; Y. Zhang et al., 2020a, 2020b). Some studies report detection of SARS-CoV-2 viral RNA in fecal samples from infected humans in Australia, the Netherlands and Italy in untreated wastewater, which could spread the virus through excreta (Ahmed et al., 2020; La Rosa et al., 2020; Medema et al., 2020). Until now, sewage or drinking water has not been reported as a route of infection for this virus (WHO, 2020a). The proposal that this virus can survive on surfaces for hours or days suggests that it is a potential pathogen capable of being transmissible through untreated wastewater, untreated waste and soil, or its entry into other forms of life allowing its diffusion into the environment and under its influence to change its characteristics.
Recent studies as a result of this short and long-term public health problem propose the evaluation of wastewater as a potential means of transmission of SARS-CoV-2 (C. Daughton, 2020a, 2020b; Sims and Kasprzyk-Hordern, 2020). Wastewater-based epidemiology (WBE) is an efficient approach with great potential for early warning of infectious disease transmission and outbreaks, which aims to trace the source of the virus, identify the locations of potential carriers, and provide early warning effective. Also, if linked to an effective response system, WBE can be useful for epidemic surveillance. Analyzing infectious disease biomarkers in wastewater taken from various collection points reports infectious disease transmission in certain areas that can be comprehensively monitored in near real time (Daughton, 2020a, 2020b; Mao et al., 2020). The WBE measures biochemical signatures in wastewater, such as fragment biomarkers for Coronavirus Severe Acute Respiratory Syndrome 2 (SARS-CoV-2), simply by applying the type of clinical diagnostic test (designed for individuals) to the signature collective of entire communities (Daughton, 2020a, 2020b; Venugopal et al., 2020). However, it is known that you will encounter a number of challenges, many of which are shared by existing WBE methods for other test targets (such as chemical micro-pollutants). Challenges include: statistically representative sampling of wastewater, which is heterogeneous. Accurate estimation of population size to account for temporal fluctuations in population size. In addition, additional revisions to the procedures to be considered are required as there is a pressing need for data on the prevalence and persistence of SARS-CoV-2 in wastewater to better understand related transmission. Given the complexity of the wastewater matrix, the need arises for new biomarker extraction techniques and the development of tools for cost-effective, sensitive, selective and multi-residue analysis of powerful biomarker groups spanning from genes to proteins. Establish efficient methods to evaluate the survival of these viruses under natural conditions (different temperatures and types of water), evaluate the efficiency of water and disinfection treatments to avoid contamination of urban and hospital wastewater; as well as establishing a surveillance system through the monitoring of residual waters of the potential circulation of the virus. This scope requires the cooperation of various related research fields and the generation of public policies that regulate such functions. So much work remains to be done on a larger scale, and rapid progress is needed to address some of the key challenges mentioned above (Carducci et al., 2020; C. Daughton, 2020a, 2020b; Mao et al., 2020; Nghiem et al., 2020; Sims and Kasprzyk-Hordern, 2020).
Molecular detection of the virus has been proposed using paper-based tools in wastewater samples (Ahmed et al., 2020; Lodder and de Roda Husman, 2020; Mao et al., 2020). The use of specific and effective techniques such as bio-sorbents is recommended to inactivate and control its environmental spread (Martínez-Puchol et al., 2020; Nag et al., 2020; Núñez-Delgado, 2020).
Although there are not yet conclusive studies that demonstrate the role of wastewater as a transmission medium for COVID-19, however it has been established that it can potentially occur through the fecal-oral route. It would be useful to assess the characteristics of wastewater, its impact on the environment, and future implications for human and environmental health.
3.5. Impact of Covid-19 on soil and water
Among the changes in the environment due to the presence of the SARS-CoV-2 virus in the world, it is observed that the water of rivers, coasts and seas is clearer and cleaner given the reduction in the number of tourists, of the use of motorboats and decreased sediment agitation, while the presence of water pollutants have also been reduced accordingly. Derived from the policies of social confinement around the world for the mitigation of Covid-19, a cleaning of the recreation spaces (beaches, parks, gardens, etc.) is observed given the null or little human influence in these spaces. At the beginning of the pandemic, a decrease in the production of solid waste of up to 30% was reported in China, in contrast to what was observed in the generation of medical waste (infectious and non-infectious), which increased considerably (+370.00%) in Hubei province (Klemeš et al., 2020). Safe waste management has been critical during the COVID-19 emergency. Some countries have abandoned their recycling and waste management programs due to the risk of spreading the virus, this being a factor of possible secondary effects on health and the environment (Zambrano-Monserrate et al., 2020). The increase in the volume of medical care waste and the delay in municipal waste recycling activities can negatively affect the environment (Kulkarni and Anantharama, 2020).
The increase in the volume of plastic waste, particularly for products used for personal protection for health care purposes and for the general public such as face masks, that are being used to take precautionary measures in the face of this recent outbreak of COVID-19, made of durable materials, are being disposed of in large quantities, empty bottles of hand sanitizer together, solid waste, paper and cardboard, are becoming an environmental problem important due to growing concerns about pollution in natural terrestrial and marine habitats.
Among the measures proposed are the modification of urban waste management, in the handling, separation and storage in homes and hospitals, appropriate transportation, treatment and disposal, up to safety protocols and training for waste collection teams. during the pandemic (Zand and Heir, 2020). The crucial priority at this time is the destruction of residual pathogens for the safe disposal of waste (Klemeš et al., 2020).
4. Covid-19: exposure to disinfectants
Common coronavirus viruses spread through the respiratory and gastrointestinal tracts; meanwhile, the nose and mouth are its two main routes of entry. The outflow and spread of the virus from the body occurs within about six days after infection and peaks four days later (Nishiura et al., 2020). Actions that can help prevent the risk of infection, such as frequent hand washing with soap and water or an alcohol-based sanitizing gel for 20–30 s are of utmost importance. The WHO also recommends a 70% concentration of ethanol for disinfecting small surfaces and for proper cleaning (WHO, 2020a).
Currently, for most surface disinfectant products they are classified as biocides, available on the European Union market in accordance with Biocide Regulation No. 528/2012 (European Parliament and of the Council, 2012). Biocides that have virucidal activity and are licensed are effective against the SARS-CoV-2 coronavirus. For more information and to obtain the list of authorized disinfectant products, visit the European Chemicals Agency (ECHA) at https://echa.europa.eu/covid-19 and the Environmental Protection Agency (US EPA) https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2.
The selection of disinfectants must comply with the requirements of local authorities, including regulations (WHO, 2020b). The following disinfectants and defined concentrations can be used on surfaces to achieve> 3 log10 reduction of human coronavirus: 70–90% ethanol, 0.1% (1000 ppm) chlorine-based products (eg hypochlorite) for surface disinfection in general, or 0.5% (5000 ppm) for body fluid spills (such as blood) and hydrogen peroxide> 0.5% (Kampf et al., 2020).
Most of the products used to disinfect that meet the criteria of the US Environmental Protection Agency. USA EPA for use against SARS-CoV-2, contain the active ingredient quaternary ammonium (benzalkonium chloride). Others contain mixtures of hydrogen peroxide; peroxyacetic acid, lactic acid, isopropanol, ethanol, sodium hypochlorite, aldehyde (formaldehyde), phenols and their derivatives. These compounds are characterized by having negative health effects when used regularly, especially children and people who are particularly sensitive to toxic effects (acute poisoning, irritation of the skin or mucous membranes, asthma, carcinogenic effect, etc.) (Yari et al., 2020). EPA registers all disinfectants and surface disinfectants without food contact as pesticides. Commonly used disinfectants are described below taking into account the health and environmental effects (Culver et al., 2014):
Taken and modified from (Culver et al., 2014).
Exposure to biocidal agents used for chemical disinfection (cleaners and disinfectants) and the direct attribution of these exposures to efforts to prevent or treat COVID-19 are not yet available. The possibility of improper use of cleaners and disinfectants is a waste, such as using more than indicated on the label, mixing various chemicals and storing them within reach of children. Misuse of disinfecting substances leads to many incidents of acute toxicity in the home. However, knowledge of the use and preparation of disinfecting solutions in accordance with the manufacturer’s recommendations for volume, compatibility of chemical disinfectants with mixing, and product stability is imperative. The impact or efficacy for health, as well as the risk of toxicity due to acute or chronic chemical exposure of disinfecting substances (carcinogenic effect, respiratory sensitizer or corrosive damage to the eyes or skin) is of importance for public health. It should be noted that the continuous use of these substances leads to their presence in the environment and cause serious biological damage, such as its potential to persist in the environment (through its ability to bioaccumulate and biomagnify), irreversible damage to fish or other aquatic organisms.
5. Conclusions, future trends and perspectives
The impact of COVID-19 infection on human health represents at this time a negative effect due to the scarce information on behavior under natural conditions according to its stability in the environment of this new virus and is reflected in the lack of health systems worldwide; meanwhile, the impact on the environment in the short term brings with it temporary positive effects on air quality that is manifested in health, by reducing the transmissibility and infection of other viral pathogens according to their seasonality. This study is exploratory in nature and aims to carry out a thorough investigation of the influence that the environment generates on the survival and behavior of the COVID-19 virus and how it affects different environmental environments. In the long term, the evaluation of the impact that the SARS-CoV-2 virus infection brings is uncertain at the environmental level and on human health, so it is imperative that epidemiological actions should focus on possible transmission routes, influence of the environment on the spread of the virus between people and possible reservoirs. There are still some outstanding questions to consider. However, today the use of technology will allow better regulation of an environmental policy to be implemented by promoting preventive measures of the highest priority. The direction of future research can provide a better insight into the fight against the SARS-CoV-2 virus, by generating more flexible policies that allow for accurate and comprehensive education, by implementing adequate prevention and control in an uncertain to medium future. and long-term to decrease the risk of Covid-19 infection.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Jian-Ying Hu
References
- Adhikari S.P., Meng S., Wu Y.-J., Mao Y.-P., Ye R.-X., Wang Q.-Z., Sun C., Sylvia S., Rozelle S., Raat H., Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infectious Diseases of Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol. Sci. Technol. 2020;54:635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F., Makhdoom H.Q., Zumla A.I., Memish Z.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo V., Bertoncello C., Cocchio S., Fonzo M., Pillon P., Buja A., Baldovin T. The new pandemic influenza A/(H1N1)PDM09 virus: is it really “new”? Journal of Preventive Medicine and Hygiene. 2016;57:E19–E22. doi: 10.15167/2421-4248/jpmh2016.57.1.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M., Vena A., Giacobbe D.R. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur. J. Clin. Invest. 2020;50 doi: 10.1111/eci.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020;186:109639. doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Cao Yu, Chen M., Dong D., Xie S., Liu M. Environmental pollutants damage airway epithelial cell cilia: implications for the prevention of obstructive lung diseases. Thoracic Cancer. 2020;11:505–510. doi: 10.1111/1759-7714.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Yu-chen, Deng Q., Dai S. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Trav. Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making Waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179:115907. doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel wuhan (2019-nCoV) coronavirus. Am. J. Respir. Crit. Care Med. 2020;201:P7–P8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., Tsoi H.-W., Lo S.K.-F., Chan K.-H., Poon V.K.-M., Chan W.-M., Ip J.D., Cai J.-P., Cheng V.C.-C., Chen H., Hui C.K.-M., Yuen K.-Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Liang H., Yuan X., Hu Y., Xu M., Zhao Y., Zhang B., Tian F., Zhu X. Roles of meteorological conditions in COVID-19 transmission on a worldwide scale. medRxiv. 2020;11 doi: 10.1101/2020.03.16.20037168. 2020.03.16.20037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020;261:114465. doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver A., Geiger C., Simon D. 2014. Comprehensive Report on Safer Disinfectant Products. (San Francisco) [Google Scholar]
- Daughton C. The international imperative to rapidly and inexpensively monitor community-wide Covid-19 infection status and trends. Sci. Total Environ. 2020;726:138149. doi: 10.1016/j.scitotenv.2020.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736:139631. doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gennaro F., Pizzol D., Marotta C., Antunes M., Racalbuto V., Veronese N., Smith L. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int. J. Environ. Res. Publ. Health. 2020;17:2690. doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz L., Horve P.F., Coil D.A., Fretz M., Eisen J.A., Van Den Wymelenberg K. 2019 novel coronavirus (COVID-19) pandemic: built environment considerations to reduce transmission. mSystems. 2020;5 doi: 10.1128/mSystems.00245-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Wang L., Cauchemez S., Xu X., Wang X., Cowling B.J., Meyers L.A. Risk for transportation of coronavirus disease from wuhan to other cities in China. Emerg. Infect. Dis. 2020;26:1049–1052. doi: 10.3201/eid2605.200146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dublineau A., Batéjat C., Pinon A., Burguière A.M., Leclercq I., Manuguerra J.-C. Persistence of the 2009 pandemic influenza A (H1N1) virus in water and on non-porous surface. PloS One. 2011;6 doi: 10.1371/journal.pone.0028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutheil F., Baker J.S., Navel V. COVID-19 as a factor influencing air pollution? Environ. Pollut. 2020;263:114466. doi: 10.1016/j.envpol.2020.114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESA No title. 2020. https://www.esa.int/Applications/Observing_the_Earth/Copernicus/Sentinel-5P/COVID-19_nitrogen_dioxide_over_China accessed 7.24.20.
- ESA No title. 2020. https://www.esa.int/Applications/Observing_the_Earth/Copernicus/Sentinel-5P/Coronavirus_lockdown_leading_to_drop_in_pollution_across_Europe accessed 7.24.20.
- European Parliament and of the Council Regulation (EU) No 528/2012. Official Journal of the European Union. 2012 [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet Respiratory Medicine. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.M., Laydon D., Nedjati-Gilani G., Imai N., Ainslie K., Baguelin M., Bhatia S., Boonyasiri A., Cucunubá Z., Cuomo-Dannenburg G., Dighe A., Dorigatti I., Fu H., Gaythorpe K., Green W., Hamlet A., Hinsley W., Okell L.C., Van Elsland S., Thompson H., Verity R., Volz E., Wang H., Wang Y., Gt Walker P., Walters C., Winskill P., Whittaker C., Donnelly C.A., Riley S., Ghani A.C. Imperial College; 2020. Report 13: Estimating the Number of Infections and the Impact of Non-pharmaceutical Interventions on COVID-19 in 11 European Countries. [DOI] [Google Scholar]
- Fraser C., Riley S., Anderson R.M., Ferguson N.M. Factors that make an infectious disease outbreak controllable. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6146–6151. doi: 10.1073/pnas.0307506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera A., Martin C., Vlachos K., Sgubin G. Regional air pollution persistence links to COVID-19 infection zoning. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K., Rubin R., Morales-Estrada S., Black S.R., Pacilli M., Fricchione M.J., Chugh R.K., Walblay K.A., Ahmed N.S., Stoecker W.C., Hasan N.F., Burdsall D.P., Reese H.E., Wallace M., Wang C., Moeller D., Korpics J., Novosad S.A., Benowitz I., Jacobs M.W., Dasari V.S., Patel M.T., Kauerauf J., Charles E.M., Ezike N.O., Chu V., Midgley C.M., Rolfes M.A., Gerber S.I., Lu X., Lindstrom S., Verani J.R., Layden J.E., Brister S., Goldesberry K., Hoferka S., Jovanov D., Nims D., Saathoff-Huber L., Hoskin Snelling C., Adil H., Ali R., Andreychak E., Bemis K., Frias M., Quartey-Kumapley P., Baskerville K., Murphy E., Murskyj E., Noffsinger Z., Vercillo J., Elliott A., Onwuta U.S., Burck D., Abedi G., Burke R.M., Fagan R., Farrar J., Fry A.M., Hall A.J., Haynes A., Hoff C., Kamili S., Killerby M.E., Kim L., Kujawski S.A., Kuhar D.T., Lynch B., Malapati L., Marlow M., Murray J.R., Rha B., Sakthivel S.K.K., Smith-Jeffcoat S.E., Soda E., Wang L., Whitaker B.L., Uyeki T.M. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh Dunker, Foster Uversky. Zika and flavivirus shell disorder: virulence and fetal morbidity. Biomolecules. 2019;9:710. doi: 10.3390/biom9110710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K.-M., Dunker A.K., Foster J.A., Uversky V.N. Shell disorder analysis predicts greater resilience of the SARS-CoV-2 (COVID-19) outside the body and in body fluids. Microb. Pathog. 2020;144:104177. doi: 10.1016/j.micpath.2020.104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K.-M., Dunker A.K., Foster J.A., Uversky V.N. Nipah shell disorder, modes of infection, and virulence. Microb. Pathog. 2020;141:103976. doi: 10.1016/j.micpath.2020.103976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K.-M., Dunker A.K., Foster J.A., Uversky V.N. Rigidity of the outer shell predicted by a protein intrinsic disorder model sheds light on the COVID-19 (Wuhan-2019-nCoV) infectivity. Biomolecules. 2020;10:331. doi: 10.3390/biom10020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K.-M., Dunker A.K., Foster J.A., Uversky V.N. HIV vaccine mystery and viral shell disorder. Biomolecules. 2019;9:178. doi: 10.3390/biom9050178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K.-M., Dunker A.K., Uversky V. Prediction of intrinsic disorder in MERS-CoV/HCoV-emc supports a high oral-fecal transmission. PLoS Currents. 2013 doi: 10.1371/currents.outbreaks.22254b58675cdebc256dbe3c5aa6498b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K.-M., Dunker A.K., Uversky V.N. Correlating Flavivirus virulence and levels of intrinsic disorder in shell proteins: protective roles vs. immune evasion. Mol. Biosyst. 2016;12:1881–1891. doi: 10.1039/C6MB00228E. [DOI] [PubMed] [Google Scholar]
- Goh G.K.-M., Dunker A.K., Uversky V.N. Shell disorder, immune evasion and transmission behaviors among human and animal retroviruses. Mol. Biosyst. 2015;11:2312–2323. doi: 10.1039/C5MB00277J. [DOI] [PubMed] [Google Scholar]
- Goh G.K.-M., Dunker A.K., Uversky V.N. Understanding viral transmission behavior via protein intrinsic disorder prediction: coronaviruses. Journal of Pathogens. 2012;2012:1–13. doi: 10.1155/2012/738590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Medical Research. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M.Z., Kinney P.L., Li T., Chen C., Sun Q., Ban J., Wang J., Liu S., Goldsmith J., Kioumourtzoglou M.-A. Short- and intermediate-term exposure to NO2 and mortality: a multi-county analysis in China. Environ. Pollut. 2020;261:114165. doi: 10.1016/j.envpol.2020.114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W., Munday J.D., Kucharski A.J., Edmunds W.J., Funk S., Eggo R.M., Sun F., Flasche S., Quilty B.J., Davies N., Liu Y., Clifford S., Klepac P., Jit M., Diamond C., Gibbs H., van Zandvoort K. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. The Lancet Global Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Zhu L., Xue L., Liu L., Yan Xuebing, Wang J., Zhang B., Xu T., Ji F., Zhao Y., Cheng J., Wang Y., Shao H., Hong S., Cao Q., Li C., Zhao X., Zou L., Sang D., Zhao H., Guan X., Chen X., Shan C., Xia J., Chen Y., Yan Xiaomin, Wei J., Zhu C., Wu C. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ m1198. 2020 doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemeš J.J., Fan Y. Van, Tan R.R., Jiang P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew. Sustain. Energy Rev. 2020;127:109883. doi: 10.1016/j.rser.2020.109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormuth K.A., Lin K., Qian Z., Myerburg M.M., Marr L.C., Lakdawala S.S. mSphere 4; 2019. Environmental Persistence of Influenza Viruses Is Dependent upon Virus Type and Host Origin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski A.J., Russell T.W., Diamond C., Liu Y., Edmunds J., Funk S., Eggo R.M., Sun F., Jit M., Munday J.D., Davies N., Gimma A., van Zandvoort K., Gibbs H., Hellewell J., Jarvis C.I., Clifford S., Quilty B.J., Bosse N.I., Abbott S., Klepac P., Flasche S. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect. Dis. 2020;20:553–558. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni B.N., Anantharama V. Repercussions of COVID-19 pandemic on municipal solid waste management: challenges and opportunities. Sci. Total Environ. 2020;743:140693. doi: 10.1016/j.scitotenv.2020.140693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurai J., Onuma K., Sano H., Okada F., Watanabe M. Ozone augments interleukin-8 production induced by ambient particulate matter. Gene Environ. 2018;40:14. doi: 10.1186/s41021-018-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Liu Y.H., Wang C.-Y., Wang Y.-H., Hsueh S.-C., Yen M.-Y., Ko W.-C., Hsueh P.-R. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J. Microbiol. Immunol. Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., Zhang C., Yue J., Zhang Z., Renz H., Liu X., Xie J., Xie M., Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Trav. Med. 2020;27 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. The Lancet Gastroenterology & Hepatology. 2020;5:533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Stratton C.W., Tang Y. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Yan J., Niu J., Zhou J., Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;724:138226. doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B.F., Brockmann D. Effective containment explains subexponential growth in recent confirmed COVID-19 cases in China. Science. 2020;368:742–746. doi: 10.1126/science.abb4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder M.S., Rivers C., Lofgren E., Fisman D. Estimation of MERS-coronavirus reproductive number and case fatality rate for the spring 2014 Saudi arabia outbreak: insights from publicly available data. PLoS Currents. 2014 doi: 10.1371/currents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K., Zhang K., Du W., Ali W., Feng X., Zhang H. The potential of wastewater-based epidemiology as surveillance and early warning of infectious disease outbreaks. Current Opinion in Environmental Science & Health. 2020;17:1–7. doi: 10.1016/j.coesh.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelletti L., Martelletti P. Air pollution and the novel covid-19 disease: a putative disease risk factor. SN Comprehensive Clinical Medicine. 2020;2:383–387. doi: 10.1007/s42399-020-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Puchol S., Rusiñol M., Fernández-Cassi X., Timoneda N., Itarte M., Andrés C., Antón A., Abril J.F., Girones R., Bofill-Mas S. Characterisation of the sewage virome: comparison of NGS tools and occurrence of significant pathogens. Sci. Total Environ. 2020;713:136604. doi: 10.1016/j.scitotenv.2020.136604. [DOI] [PubMed] [Google Scholar]
- McNeill V.F. COVID-19 and the air we breathe. ACS Earth and Space Chemistry. 2020;4:674–675. doi: 10.1021/acsearthspacechem.0c00093. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Nag R., Whyte P., Markey B.K., O’Flaherty V., Bolton D., Fenton O., Richards K.G., Cummins E. Ranking hazards pertaining to human health concerns from land application of anaerobic digestate. Sci. Total Environ. 2020;710:136297. doi: 10.1016/j.scitotenv.2019.136297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari Harmooshi N., Shirbandi K., Rahim F. Environmental concern regarding the effect of humidity and temperature on SARS-COV-2 (COVID-19) survival: fact or fiction. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3563403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B. The positive effects of covid-19. BMJ m1785. 2020 doi: 10.1136/bmj.m1785. [DOI] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies in Chemical and Environmental Engineering. 2020;1:100006. doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int. J. Infect. Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Delgado A. What do we know about the SARS-CoV-2 coronavirus in the environment? Sci. Total Environ. 2020;727:138647. doi: 10.1016/j.scitotenv.2020.138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. J. Am. Med. Assoc. 2020;323:1610. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret J., Bowatte G., Lodge C., Knibbs L., Gurrin L., Kandane-Rathnayake R., Johns D., Lowe A., Burgess J., Thompson B., Thomas P., Wood-Baker R., Morrison S., Giles G., Marks G., Markos J., Tang M., Abramson M., Walters E., Matheson M., Dharmage S. The dose–response association between nitrogen dioxide exposure and serum interleukin-6 concentrations. Int. J. Mol. Sci. 2017;18:1015. doi: 10.3390/ijms18051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon A., Vialette M. Survival of viruses in water. Intervirology. 2018;61:214–222. doi: 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- Pirouz B., Shaffiee Haghshenas Sina, Haghshenas Shaffiee, Sami, Piro P. Investigating a serious challenge in the sustainable development process: analysis of confirmed cases of COVID-19 (new type of coronavirus) through a binary classification using artificial intelligence and regression analysis. Sustainability. 2020;12:2427. doi: 10.3390/su12062427. [DOI] [Google Scholar]
- Poon L.L.M., Peiris M. Emergence of a novel human coronavirus threatening human health. Nat. Med. 2020;26:317–319. doi: 10.1038/s41591-020-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L.-L., Wang Y.-M., Wu Z.-Q., Xiang Z.-C., Guo L., Xu T., Jiang Y.-Z., Xiong Y., Li Y.-J., Li X.-W., Li H., Fan G.-H., Gu X.-Y., Xiao Y., Gao H., Xu J.-Y., Yang F., Wang X.-M., Wu C., Chen L., Liu Y.-W., Liu B., Yang J., Wang X.-R., Dong J., Li L., Huang C.-L., Zhao J.-P., Hu Y., Cheng Z.-S., Liu L.-L., Qian Z.-H., Qin C., Jin Q., Cao B., Wang J.-W. Identification of a novel coronavirus causing severe pneumonia in human. Chinese Med J. 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou J., Althaus C.L. Eurosurveillance 25; 2020. Pattern of Early Human-To-Human Transmission of Wuhan 2019 Novel Coronavirus (2019-nCoV) December 2019 to January 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Torboli V., Fontana F., Clemente L., Pallavicini A., Ruscio M., Piscitelli P., Miani A. SARS-Cov-2RNA found on particulate matter of bergamo in northern Italy: first evidence. Environ. Res. 2020 doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N., Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020;139:105689. doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooryanarain H., Elankumaran S. Environmental role in influenza virus outbreaks. Annual Review of Animal Biosciences. 2015;3:347–373. doi: 10.1146/annurev-animal-022114-111017. [DOI] [PubMed] [Google Scholar]
- Tobías A., Carnerero C., Reche C., Massagué J., Via M., Minguillón M.C., Alastuey A., Querol X. Changes in air quality during the lockdown in Barcelona (Spain) one month into the SARS-CoV-2 epidemic. Sci. Total Environ. 2020;726:138540. doi: 10.1016/j.scitotenv.2020.138540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai D.-H., Riediker M., Berchet A., Paccaud F., Waeber G., Vollenweider P., Bochud M. Effects of short- and long-term exposures to particulate matter on inflammatory marker levels in the general population. Environ. Sci. Pollut. Control Ser. 2019;26:19697–19704. doi: 10.1007/s11356-019-05194-y. [DOI] [PubMed] [Google Scholar]
- Valtierra H.N. Stability of viral pathogens in the laboratory environment. Applied Biosafety. 2008;13:21–26. doi: 10.1177/153567600801300104. [DOI] [Google Scholar]
- Van Bueren J., Simpson R.A., Jacobs P., Cookson B.D. Survival of human immunodeficiency virus in suspension and dried onto surfaces. J. Clin. Microbiol. 1994;32:571–574. doi: 10.1128/jcm.32.2.571-574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal A., Ganesan H., Sudalaimuthu Raja S.S., Govindasamy V., Arunachalam M., Narayanasamy A., Sivaprakash P., Rahman P.K.S.M., Gopalakrishnan A.V., Siama Z., Vellingiri B. Novel wastewater surveillance strategy for early detection of coronavirus disease 2019 hotspots. Current Opinion in Environmental Science & Health. 2020;17:8–13. doi: 10.1016/j.coesh.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents. 2020;55:105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organisation; 2020. Interim Guidance April 2020: Water, Sanitation, Hygiene and Waste Management for the COVID-19 virusInterim Guidance April 2020; pp. 1–9. [Google Scholar]
- WHO . 2020. Cleaning and Disinfection of Environmental Surfaces in the Context of COVID-19: Interim Guidance. [Google Scholar]
- World Health Organization . WHO public health research agenda for influenza; 2017. WHO Public Health Research Agenda for Influenza: Limiting the Spread of Pandemic, Zoonotic and Seasonal Epidemic Influenza. [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E.C., Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Hu W., Williams G., Clements A.C.A., Kan H., Tong S. Air pollution, temperature and pediatric influenza in Brisbane, Australia. Environ. Int. 2013;59:384–388. doi: 10.1016/j.envint.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yari S., Moshammer H., Fallah Asadi A., Mosavi jarrahi A. Side effects of using disinfectants to fight COVID-19. Asian Pacific Journal of Environment and Cancer. 2020;3:9–13. doi: 10.31557/apjec.2020.3.1.9-13. [DOI] [Google Scholar]
- Yu Y.X., Sun L., Yao K., Lou X.T., Liang X., Zhao B.W., Mu Q.X., Du H., Zhao Y., Zhang H. [Consideration and prevention for the aerosol transmission of 2019 novel coronavirus] [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2020;56:E008. doi: 10.3760/cma.j.cn112142-20200313-00181. [DOI] [PubMed] [Google Scholar]
- Zambrano-Monserrate M.A., Ruano M.A., Sanchez-Alcalde L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020;728:138813. doi: 10.1016/j.scitotenv.2020.138813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand A.D., Heir A.V. Emerging challenges in urban waste management in Tehran, Iran during the COVID-19 pandemic. Resour. Conserv. Recycl. 2020;162:105051. doi: 10.1016/j.resconrec.2020.105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Diao M., Yu W., Pei L., Lin Z., Chen D. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: a data-driven analysis. Int. J. Infect. Dis. 2020;93:201–204. doi: 10.1016/j.ijid.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J., Song Y., Zhen W., Feng Z., Wu G., Xu J., Xu W. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2:123–124. doi: 10.46234/ccdcw2020.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]