Abstract
Background and aims
Evidence from randomized controlled trials establishes that medication treatment with methadone and buprenorphine reduces opioid use and improves treatment retention. However, little is known about the role of such medications compared with non-medication treatments in mitigating overdose risk among U.S. patient populations receiving treatment in usual care settings This study compared overdose mortality among those in medication versus non-medication treatments in specialty care settings.
Design
Retrospective cohort study using statewide treatment data linked to death records. Survival analysis was used to analyze data in a time-to-event framework.
Setting
Services delivered by 757 providers in publicly-funded outpatient specialty treatment programs in Maryland, USA between January 1st, 2015 and December 31st, 2016.
Participants
A total of 48,274 adults admitted to outpatient specialty treatment programs in 2015–2016 for primary diagnosis of opioid use disorder.
Measurements
Main exposure was time in medication treatment (methadone/buprenorphine), time following medication treatment, time exposed to non-medication treatments and time following non-medication treatment. Main outcome was opioid overdose death during and after treatment. Hazard ratios were calculated using Cox proportional hazard regression. Propensity score weights were adjusted for patient information on sex, age, race, region of residence, marital and veteran status, employment, homelessness, primary opioid, mental health treatment, arrests, and criminal justice referral.
Findings
The study population experienced 371 opioid overdose deaths. Periods in medication treatment were associated with substantially reduced hazard of opioid overdose death compared with periods in non-medication treatment (adjusted hazard ratio [aHR]: 0.18 (0.08–0.40)). Periods after discharge from non-medication treatment (aHR: 5.45 (2.80–9.53)) and medication treatment (aHR: 5.85 (3.10–11.02)) had similar and substantially elevated risk compared with periods in non-medication treatments.
Conclusions
Among Maryland USA patients in specialty opioid treatment, periods in treatment are protective against overdose compared with periods out of care. Methadone and buprenorphine are associated with significantly lower overdose death compared with non-medication treatments during care but not after treatment is discontinued.
Introduction
With 47,600 lives lost to opioid overdose in 2017, the opioid epidemic continues to be a leading cause of morbidity and mortality across the United States (1). A major public health strategy to address opioid harms is to expand access to treatment for opioid use disorders (OUD) (2,3). A large evidence base establishes the effectiveness of long-term use of the opioid agonist medications methadone and buprenorphine in improving outcomes among persons with OUD (4,5). Compared to detoxification alone or behavioral treatments that do not involve medications, treatments with opioid agonists increase retention, reduce illicit opioid use, and prevent infectious disease transmission (6,7). However, in many care settings in the U.S., these medications are underutilized: Approximately 70% of persons entering specialty treatment for OUD across the U.S. do not receive opioid agonist medications (8), and most specialty treatment programs do not offer such medications (9,10). Instead, most treatment programs offer behavioral services such as counseling, substance use screening and assessments, drug testing, outreach and case management, skill development, mentoring/peer support, education, and mental health services (11). Still, there is a paucity of evidence about the impact of medication versus non-medication treatments on overdose risk in usual care, outside of randomized controlled trials. While some cohort studies have identified a protective effect of medications on overdose death compared to not being in treatment at all (12–14), studies comparing overdose risk among those in medication versus non-medication treatments are rare. A cohort study in the UK found lower overdose risk among those on medications compared to those receiving psychological interventions alone (15). A California study found no significant differences in hazard of drug-related mortality but overall lower mortality risk among those in methadone maintenance versus those in three week detoxification programs (16). Moreover, while overdose risk is known to increase immediately following cessation of medication treatments (17,18), it remains unclear how this risk compares to the period following cessation of non-medication treatments, which also holds a high risk of overdose (19,20).
The effectiveness of medication versus non-medication treatment in usual care contexts could differ from clinical trials, where patients are known to be characteristically different than those receiving care in real-world settings (21). Key populations may be excluded from clinical trials (e.g., pregnant and justice-involved persons) and the comprehensiveness and fidelity of treatment is likely to be lower as patients often transition in and out of care (22,23). As a result, it is unclear what length of time in treatment is needed to achieve long-term outcomes (24,25). Previous investigation of medication treatments in usual care settings has involved fairly narrow cohorts in specific settings, or population-level data from countries outside the U.S. that maintain national health and death registries (12,17,19,20,26,27). The current study presents a retrospective analysis of overdose mortality among a cohort of patients in Maryland who are being treated for OUD, using hazard modeling to compare periods in medication treatment, non-medication treatments, and periods following each of these types of care. We hypothesized that medications would be protective against overdose compared to non-medication treatments both during care and in the period following discharge. As the analysis was not pre-registered on a publicly available platform, the results should be considered exploratory.
Methods
Study Design and Setting
We linked Maryland statewide outpatient substance use specialty treatment claims from January 1, 2015 to December 31, 2016 to opioid overdose death records. Data linkage was facilitated by Maryland’s state-designated health information exchange, the Chesapeake Regional Information System for our Patients, which applied a probabilistic algorithm that uses demographic data to link individuals across datasets using a unique, encrypted person-identifier (28). Both the Maryland Department of Health and Johns Hopkins Bloomberg School of Public Health Institutional Review Boards approved this study.
Treatment Record Inclusion
OUD treatment data were obtained from Beacon Health Options, which manages public specialty behavioral health services in Maryland paid for by Medicaid or the State. These services account for the majority of OUD treatment provided across the state, but do not include care provided by private physician offices or clinics. Data were limited to outpatient substance use treatment records in 2015–2016 for patients ≥18 years with a primary diagnosis of OUD, defined using ICD-9 codes 304.00–304.03, 304.70–304.73, and 305.50–305.53 and ICD-10 codes F11*. To limit comparisons of medication and non-medication treatments to those provided in clinical outpatient settings, we excluded claims for recovery supports (e.g. case management and peer services) and inpatient/residential care, which each respectively made up only 2% of claims. We also excluded claims records for drug tests.
Construction of Treatment and Episode Types
To study the association between different treatment modalities and overdose mortality, outpatient treatment records were classified as either, “medication treatment” which was any outpatient treatment record whose service codes indicated use of the opioid agonists methadone or buprenorphine; or “non-medication treatment” which was any outpatient treatment record that did not include service codes for methadone or buprenorphine. As we were interested in comparing the effect of opioid agonist medications to all other types of treatment that did not involve medications, this category combined intensive or non-intensive outpatient, ambulatory detoxification, and partial hospitalization programs that did not indicate use of methadone or buprenorphine. Such treatments often involve a combination of assessments, psychotherapies, group and individual counseling and educational programming and are generally categorized based on setting and the American Society for Addiction Medicine “levels of care” criteria (29). Only forty records involved extended-release naltrexone and were also excluded from analyses given naltrexone’s differential clinical course and mechanism of action as an antagonist medication (30).
Claims data were then collapsed to create medication and non-medication episodes of care that constructed the risk sets for time-to-event analyses. Each treatment episode was defined as a consecutive period of time for which a person had a claim for either medication treatment services (“during medication”) or non-medication treatment services (“during non-medication”) with interruptions of no more than 14 days between dates of service (16,31,32). For example, if a patient attended treatment once every two weeks for three months, this was considered one continuous treatment episode. Patients could have more than one episode of treatment, but in cases where medication and non-medication services overlapped, the time involving medication was considered “medication treatment” given pharmacology was involved in the patients’ care. Periods of time during which there were no services (either between treatment episodes or between the end of a treatment episode and the end of follow up at the end of 2016) were categorized as “after-treatment” if they occurred directly after a medication treatment episode or “after non-medication” if they occurred directly after a non-medication treatment episode.
As analyses focused on data from specialty care settings, we excluded episodes during which patients receiving non-medication services filled buprenorphine prescriptions as evident in linked Prescription Drug Monitoring Program (PDMP) data, indicating they were likely receiving medication treatment from an office-based clinician outside the specialty treatment system. The final analytic dataset resulted in a total of 48,274 individual patients with 185,568 treatment episodes (53.45% during treatment and 46.55% after treatment) (Table 1). All analyses were conducted using Stata version 15. (33),(34),(35),
Table 1:
Description of patient episodes, follow up time and overdose deaths during study period 2015–2016
| Number of Patients | 48,274 |
| Patients with medication treatment episodes only | 23,992 (49.70%) |
| Patients with non-medication treatment episodes only | 13,497 (27.96%) |
| Patients with both medication and non-medication treatment episodes | 10,785 (22.34%) |
| Number of Follow-Up Episodes | 185,568 |
| During non-medication treatment | 44,176 (23.81%) |
| During medication treatment* | 55,016 (29.65%) |
| After non-medication treatment | 37,863 (20.40%) |
| After medication treatment | 48,513 (26.14%) |
| Average Length of Follow up Episodes | 123 days |
| During non-medication treatment | 22 days |
| During medication treatment | 248 days |
| After non-medication treatment | 118 days |
| After medication treatment | 79 days |
| Average In-Treatment Episodes per Patient | 2 |
| Substances Involved in Opioid Overdose Deaths During Follow Up | 371 |
| Prescription opioids | 130 (35.04%) |
| Methadone | 114 (30.73%) |
| Heroin | 241 (64.96%) |
| Fentanyl | 213 (57.41%) |
Of the 55,016 medication treatment episodes, 93.31% involved methadone, and 6.69% involved buprenorphine
Linkage to Overdose Death Outcomes and Patient Covariates
Patient records were linked to records from the Office of the Chief Medical Examiner of Maryland. Opioid overdose deaths included all investigated deaths classified as caused by opioids (heroin, fentanyl, methadone, or prescription opioids). All other medical examiner-investigated deaths (including deaths due to injuries, homicide, suicide, or otherwise untimely, suspicious, or not attended by a physician) were included in analyses for censoring purposes so that patients who were known to have died were no longer included in follow up. Covariates derived from treatment records included age and sex, and whether a patient ever had any of the following during the two-year study period: received mental health treatment in the public behavioral health system; reported to be homeless, unemployed, or a veteran; sought treatment primarily for heroin (compared to other opioids); had a past-year arrest; or had been referred to treatment by a criminal justice source. Additional demographic covariates including race, marital status, and county of residence were obtained from linked hospital records from 2013–2016 (available for 93% of sample) provided by the Maryland Health Services Cost Review Commission, an all-payer administrative database of Maryland acute-care non-federal hospitals. Approximately 30% of all patients were missing data on one or more covariates of interest and multiple imputation using chained equations (36) was used to impute missing values.
Identifying Correlates of Medication Treatment
The main exposure was receipt of medications for OUD. We therefore first examined patient factors independently associated with ever using medications as part of treatment during the study period using multivariable logistic regression. To account for possible confounders and emulate a “pseudo-population” of medication vs. non-medication treatment episodes that were similar in terms of observed covariates, Inverse Probability of Treatment Weights (IPTW) for medication treatment receipt were constructed from propensity scores derived with logistic regression. Propensity scores were constructed using covariates available at baseline at the patient-level along with an indicator for missing hospital record, which accounted for 7% of the missing data (37). This allowed us to adjust for the observed differences in baseline characteristics of individuals who received medication vs. non-medication treatment (38,39). Weighting led to a substantial reduction in differences in patient covariates across groups (Appendix Table 1).
Analysis
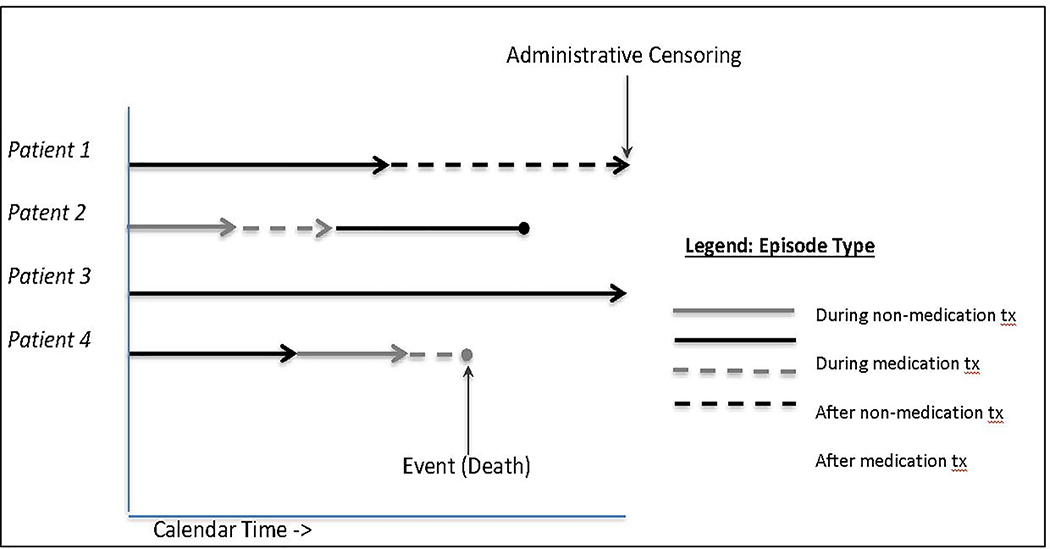
Episode type was constructed as a time-varying variable with four categories: “during non-medication treatment” (reference) “during medication treatment,” “after non-medication treatment,” and “after medication treatment.” For “during treatment” categories, risk set entry was the first date of service and exit was the last date of service, death, or end of follow up (Dec 31, 2016). For “after treatment” categories, risk set entry was the day following last date of service, and exit was the day before entry into a new service, death, or end of follow up. Figure 1 presents four hypothetical patients moving through different episodes. A death was considered to have occurred during treatment if the death was on last date or one day after last date of service; deaths after these dates were considered to have occurred after treatment.
Figure 1:
Examples of four hypothetical patients contributing to risk sets of different episode treatment (tx) types
Crude and propensity-score weighted hazards ratios (40) were calculated using Cox proportional hazards regression to determine the association between episode type and opioid overdose death among groups balanced on observed covariates. Weighted Kaplan-Meier survival curves for opioid overdose death based on medication receipt were plotted for periods during and after treatment. Robust standard errors were applied to account for propensity score weights (41) and clustered to account for multiple episodes of care.
Results
Population Characteristics
Sociodemographic characteristics of persons who ever (72%) and never (28%) received medication treatment, and odds ratios for ever receiving medication are presented in Table 2. Persons who received medications were more likely to be female (46% vs. 38%), older than 35 (62% vs. 45%), married (14% vs. 10%), employed (47% vs. 38%), and not homeless (85% vs. 72%); to primarily use heroin (88% vs. 60%); to live in the Baltimore Metro Area (55% vs. 45%); to not have sought treatment for mental health (52% vs. 38%); to not report an arrest in the past year (90% vs. 81%); and to have been referred to treatment by a non-criminal justice source (89% vs. 61%) (Table 2).
Table 2:
Patient characteristics based on whether received medication treatment with opioid agonists in 2015–2016
| Total N=48,274 | Never Medication Treatment N=13,495 (27.96%) | Ever Medication Treatment N=34,779 (72.04%)* | Adjusted Odds Ratio with 95% Confidence Intervals of Ever Received Medication Treatment | |
|---|---|---|---|---|
| Sex | ||||
| Female | 21265 (44.05%) | 5151 (38.17%) | 16114 (46.33%) | 1 |
| Male | 26960 (55.85%) | 8327 (61.70%) | 18633 (53.58%) | 0.70 [0.66,0.74] |
| - | 49 (0.10%) | 17 (0.13%) | 32 (0.09%) | |
| Age Group | ||||
| 18–25 | 4419 (9.15%) | 2108 (15.62%) | 2311 (6.64%) | 1 |
| 26–35 | 16276 (33.72%) | 5286 (39.17%) | 10990 (31.60%) | 1.84 [1.67,2.03] |
| 36–45 | 10124 (20.97%) | 2467 (18.28%) | 7657 (22.02%) | 2.54 [2.29,2.83] |
| 46–55 | 11570 (23.97%) | 2555 (18.93%) | 9015 (25.92%) | 2.77 [2.48,3.09] |
| 56–65 | 5304 (10.99%) | 1015 (7.52%) | 4289 (12.33%) | 3.00 [2.63,3.42] |
| 66 and over | 581 (1.20%) | 64 (0.47%) | 517 (1.49%) | 5.41 [3.67,7.98] |
| Race | ||||
| White | 26055 (53.97%) | 7382 (54.70%) | 18673 (53.69%) | 1 |
| Black | 15754 (32.63%) | 4488 (33.26%) | 11266 (32.39%) | 0.55 [0.51,0.59] |
| Other | 725 (1.50%) | 275 (2.04%) | 450 (1.29%) | 0.55 [0.45,0.68] |
| - | 5740 (11.89%) | 1350 (10.00%) | 4390 (12.62%) | |
| Region of Residence | ||||
| Baltimore Metro | 25306 (52.42%) | 6069 (44.97%) | 19237 (55.31%) | 1 |
| Eastern Shore | 4366 (9.04%) | 2012 (14.91%) | 2354 (6.77%) | 0.34 [0.32,0.38] |
| Southern | 1036 (2.15%) | 609 (4.51%) | 427 (1.23%) | 0.32 [0.27,0.37] |
| National Capital | 704 (1.46%) | 316 (2.34%) | 388 (1.12%) | 0.33 [0.27,0.40] |
| Northwest | 4650 (9.63%) | 1470 (10.89%) | 3180 (9.14%) | 0.97 [0.89,1.06] |
| - | 12212 (25.30%) | 3019 (22.37%) | 9193 (26.43%) | |
| Marital Status | ||||
| Not married | 38201 (79.13%) | 11092 (82.19%) | 27109 (77.95%) | 1 |
| Married | 6276 (13.00%) | 1364 (10.11%) | 4912 (14.12%) | 1.33 [1.22,1.45] |
| - | 3797 (7.87%) | 1039 (7.70%) | 2758 (7.93%) | |
| Employment | ||||
| Not Employed | 26838 (55.60%) | 8398 (62.23%) | 18440 (53.02%) | 1 |
| Employed | 21436 (44.40%) | 5097 (37.77%) | 16339 (46.98%) | 1.68 [1.58,1.79] |
| Veteran | ||||
| Not Veteran | 46913 (97.18%) | 13092 (97.01%) | 33821 (97.25%) | 1 |
| Veteran | 1361 (2.82%) | 403 (2.99%) | 958 (2.75%) | 0.87 [0.74,1.03] |
| Homelessness | ||||
| Not Homeless | 39133 (81.06%) | 9656 (71.55%) | 29477 (84.76%) | 1 |
| Homeless | 9141 (18.94%) | 3839 (28.45%) | 5302 (15.24%) | 0.46 [0.43,0.50] |
| Primary Opioid Use | ||||
| Primary Prescription Opioids | 9398 (19.47%) | 5368 (39.78%) | 4030 (11.59%) | 1 |
| Primary Heroin | 38876 (80.53%) | 8127 (60.22%) | 30749 (88.41%) | 6.89 [6.43,7.38] |
| Mental Health Treatment | ||||
| No Mental Health Treatment | 23324 (48.32%) | 5116 (37.91%) | 18208 (52.35%) | 1 |
| Mental Health Treatment | 24950 (51.68%) | 8379 (62.09%) | 16571 (47.65%) | 0.61 [0.57,0.65] |
| Past Year Arrest | ||||
| No Arrest | 42063 (87.13%) | 10931 (81.00%) | 31132 (89.51%) | 1 |
| Arrest | 6211 (12.87%) | 2564 (19.00%) | 3647 (10.49%) | 0.92 [0.85,1.00] |
| Referral Source | ||||
| Non-Criminal Justice Source | 39164 (81.13%) | 8238 (61.04%) | 30926 (88.92%) | 1 |
| Criminal Justice Source | 9110 (18.87%) | 5257 (38.96%) | 3853 (11.08%) | 0.23 [0.21,0.24] |
Notes:
• 33.01% of persons who ever had medication treatment also had at least 1 episode of care that did not involve medications
• Employment, veteran status, homelessness, heroin as primary substance, past year arrest and criminal justice referral all derived from treatment claims authorization records. Value reflects ever having a positive value on an authorization record during 2015–2016
• Mental health treatment indicates ever having any claim for a psychiatric treatment episode in the specialty treatment system during 2015–2016
• Marital status, region of residence, and race group derived from the modal value found in linked hospital records during 2013–2016
Risk of Overdose Death by Episode Type
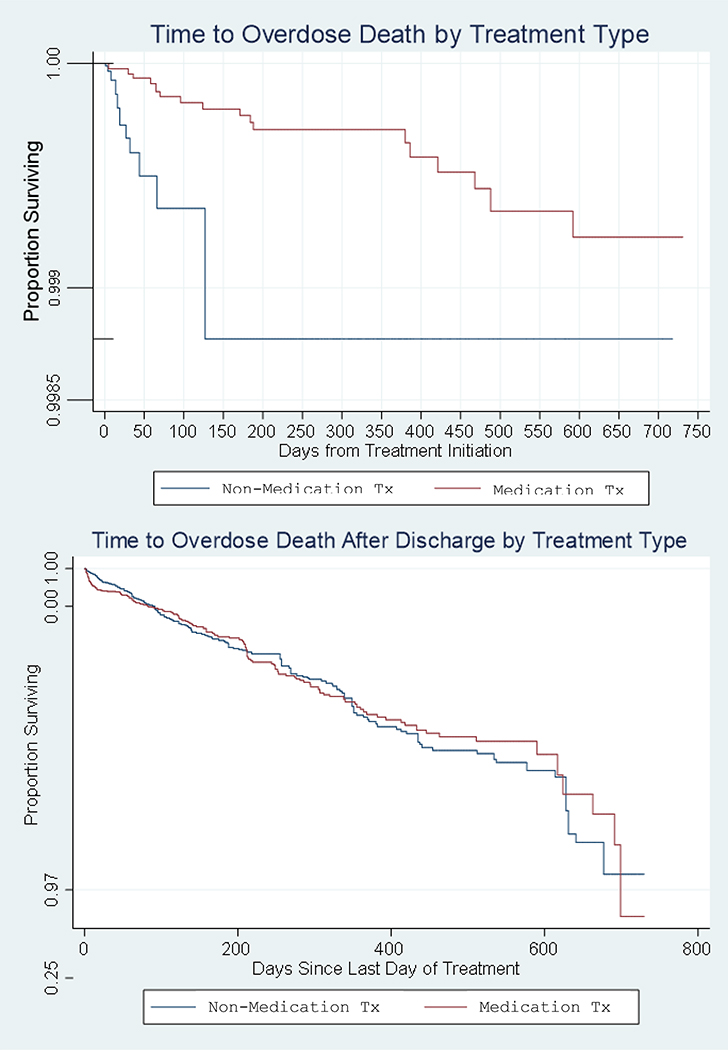
The number and types of opioids involved in overdose deaths are presented in Table 1. Most of the 371 overdose deaths during follow up occurred in the first month of each follow up period, with the exception of time during medication treatment (Appendix Figure 1). An additional 182 non-overdose deaths were investigated by the medical examiner and were used for censoring purposes and in sensitivity analyses. Table 3 presents opioid overdose death rates and crude and weighted hazard ratios for overdose death based on episode type. Overdose death rates were lowest during medication treatment (0.48/1000 person-years), followed by during non-medication treatment (4.13/1000 person-years), after non-medication treatment (13.22/1000 person-years), and after medication treatment (17.21/1000 person-years). Weighed hazard of opioid overdose death were significantly lower during medication compared to during non-medication treatment periods (Hazard Ratio (HR): 0.18 (0.08–0.40)). Periods after non-medication (HR: 5.43 (2.88–10.23)) and after medication treatment (HR: 5.84 (3.10–10.99)) both had substantially elevated risk compared to non-medication treatment periods, indicating an overall protective effect of being in treatment, regardless of type. We did not detect a statistically significant difference in risk between periods after medication vs. after non-medication treatment (p= 0.55). Figure 2 displays weighted survival curves illustrating differential survival from overdose death between people in medication and non-medication treatment during (3A) and after care (3B). Persons receiving medications had a greater survival rate while in treatment, but had similar rates to persons receiving non-medication treatments once no longer actively enrolled in care. There were no indications of departures from the proportional hazards assumption on the basis of Schoenfeld residuals.
Table 3:
Opioid overdose death rates and hazard ratios (HR) with 95% confidence intervals (CI) during 2015–2016 by treatment (tx) episode type
| Person-years | Number of opioid overdose deaths | Opioid overdose death rate per 1000 person-years | Crude HR for opioid overdose death (95% CI) | HR for opioid overdose death adjusted with IPTW (95% CI) | |
|---|---|---|---|---|---|
| During non-medication tx | 2664 | 11 | 4.13 | 1 | 1 |
| During medication tx | 37371 | 18 | 0.48 | 0.16 (0.07–0.35) | 0.18 (0.08–0.40) |
| After non-medication tx | 12251 | 162 | 13.22 | 4.14 (2.24–7.65) | 5.45 (2.89–10.27) |
| After medication tx | 10458 | 180 | 17.21 | 5.17 (2.80–9.53) | 5.85 (3.10–11.02) |
Notes:
• There was no evidence of non-proportional hazards (weighted analysis, p = 0.68)
• Post-estimation test found no statistical difference between HR after non-medication and medication treatment compared to during non-medication treatment (weighted analysis, p = 0.55)
Figure 2:
Kaplan-Meier Survival Curves for opioid overdose death by medication treatment status during treatment (A) and after discharge (B), adjusted for inverse probability of treatment-weights
We then specifically calculated differences in risk of death during and after the initial four weeks since treatment discharge in patients leaving medication and non-medication treatments by calculating death rates and rate ratios for these time frames. Risk was significantly higher in the first four weeks since treatment discharge compared to the remainder of time out of treatment for both in patients who received medication (RR:2.91 (2.14–3.95) and non-medication treatment (RR: 1.59 (1.04–2.27)). We also graphically explored whether length of time in medication treatment, specifically, differentially impacted risk of opioid overdose in the period following discharge by plotting cumulative incidence of overdose deaths in the 30, 90 and 180-days after medication treatment discharge by number of months in care. The sample was limited to new patients (excluding treatment episodes from first 14 days) with up to 18 months of medication treatment to allow for a period of observation of at least 180 days post-discharge. While there was no detectable linear trend, locally weighted scatterplot smoothing (LOWESS) indicated a general reduction in cumulative overdose death incidence post-discharge with longer months in treatment, especially following 12+ months (Appendix Figure 2).
Supplementary Analyses
Multiple supplementary analyses supported consistency of results under different conditions (Appendix Tables 2–9). First, we excluded episodes from the first 14 days of the study to control for possible bias due to prevalent users who may have been in treatment for long periods before follow up began(42), which did not change results. Second, we extended treatment discharge to 14 days after the last date of record due to potential error stemming from claims records. The relationships between episode types and overdose remained qualitatively consistent but relative hazards of overdose death between periods in- and out of-treatment were attenuated. Third, we limited our definition of medication treatment to methadone only (not buprenorphine), which resulted in qualitatively and quantitatively similar findings. Fourth, we tested three alternative methods of handling episodes with overlapping office-based buprenorphine prescriptions: a) analyzing all episodes regardless of a simultaneous buprenorphine prescription; b) excluding all records for patients who received any buprenorphine prescription at any point during the study period; and c) reclassifying episodes as medication treatment if a patient had any buprenorphine prescription during the period of care. Only the third method resulted in a differential finding with a greater hazard in the period after non-medication than after medication treatment. Fifth, we repeated analyses with the outcome of all investigated deaths (n=553) to see whether medication and non-medication treatments had a differential impact on other causes of death investigated by the Medical Examiner. Results were qualitatively similar, with the difference between in-treatment medication vs. non-medication slightly attenuated and periods out of treatment exhibiting an even larger risk compared to being in non-medication treatment, especially after treatment with medication. Finally, we conducted the primary analysis using covariate adjusted- instead of propensity-score weighted- Cox regression as an alternate strategy to account for confounding, which also resulted in qualitatively similar findings.
Discussion
The present study examined the association between receipt of medication treatment versus treatment that does not involve medications in a U.S. statewide sample of individuals receiving specialty care for OUD. This is one of the first studies in the U.S. to longitudinally assess overdose risk in persons seeking outpatient specialty substance use treatment across an entire state, and to compare outcomes of medication and non-medication treatment seekers during periods in and out of care. Consistent with previous studies, periods out of treatment had the highest relative risk of fatal overdose compared to periods during care, especially in the first few weeks following discharge, regardless of treatment modality (15,20). Hazard of overdose death after both medication and non-medication treatments was over five-fold that of the period during non-medication treatment. Opioid overdose mortality rates in out of treatment periods were higher than those reported in the international literature (12), likely reflecting the much higher potency of opioid drug supply in the U.S. and in Maryland, specifically, in recent years (43).
Contrary to trends seen in U.S. national samples (8), the majority of persons seeking specialty outpatient treatment for OUD in Maryland did receive methadone or buprenorphine as part of their care. However, many individual factors (such as age) and structural factors (such as criminal justice referral), were still highly related to whether a person received medications as part of care, indicating ongoing systematic gaps in access to medication treatments. Compared to patients in non-medication treatment, those in medication treatment had an 80% lower hazard of overdose death during care even after accounting for several clinical and demographic confounders. This is consistent with our clinical understanding of the effectiveness of opioid agonist medications compared to non-medication treatments that are often limited to psychosocial interventions (7,44), and demonstrates the potential magnitude of this effect on overdose reduction across an entire U.S. statewide population. Another observed benefit was that individuals in medication treatment had longer average duration of care than those attending non-medication treatments (248 days versus 22 days). It was not possible to detect a persistent protective effect of medication versus non-medication treatment on overdose in periods after treatment, however.
These findings have important clinical and policy implications. First, they support efforts to increase expansion of and access to medications in community settings as a means of reducing overdose risk, especially among groups who underutilize them. Beyond increasing participation, improving retention in medication remains one of the greatest challenges of the field (3). While treatment discontinuation is partly driven by the relapsing nature of OUD (45), retention could be improved through stronger promotion of long-term maintenance as the standard of care and by eliminating programmatic, logistical and financial barriers. For example, addressing medication stigma (46), and removing burdensome treatment requirements such as daily attendance or zero tolerance (47–49) may encourage better and longer engagement (50,51). The risk that may incur immediately after treatment discontinuation also highlights the critical need to couple care with overdose education and harm-reduction modalities, such as naloxone training and distribution ((52). Lastly, this study shows how integration of data across distinct service systems may serve as a tool for care coordination and research efforts to inform evidence-based practices.
Limitations
This study is subject to several limitations. First, analyses relied on administrative records that did not contain detailed information about patients or nature of treatment episodes. Thus, we could not cluster by service setting, distinguish between persons who had an overdose death after a planned termination of treatment and those who never returned to treatment due to death, or account for important potential confounders such as past treatment history, history of substance use, and previous overdoses. Second, we grouped treatment type as either medication or non-medication treatment and did not assess how different types of services or factors such as switching providers played a role in overdose risk. Future population-based research should further investigate risks and benefits associated with distinct types of medication treatments (e.g. buprenorphine, methadone) and non-medication treatments (e.g. counseling, detox), and whether non-medication adjunct services mitigate overdose risk among those receiving medications. Third, medical examiner data only contained information on investigated deaths in Maryland. Therefore, we were not able to examine the impact of treatment on all-cause mortality nor censor deaths not investigated. Fourth, treatment data only contained information about outpatient care in specialty settings funded by public dollars and excluded private programs paid for by other means. Lastly, while we were able to use prescription drug data to control for misclassification among those receiving buprenorphine from office-based settings, we were not able to investigate overdose risk factors among persons receiving care outside the specialty treatment system. Future studies should incorporate and compare risk among persons seeking buprenorphine treatment in private physician offices and clinics.
Conclusions
Using population-based patient data from Maryland, this study found that engaging in opioid agonist medication treatments for OUD is associated with significant retention in treatment and reduced overdose risk. And yet, many patients are still receiving non-medication and short-term treatments that are less protective against overdose. Policy makers should ensure substance use treatment systems make opioid agonist medications highly accessible to all patients who present with OUD and focus efforts on promoting engagement and retention in these programs.
Supplementary Material
Table 4:
Opioid overdose death rates and rate ratios with 95% confidence intervals (CI) in period following treatment (tx) discharge, comparing the period during and after the first 4 weeks since treatment discharge in patients leaving medication and non-medication treatment
| Person -Years | Number of opioid overdose deaths | Opioid overdose death rate per 1000 person-years | Opioid overdose death rate ratio (95% CI) | |
|---|---|---|---|---|
| After Medication Treatment | ||||
| Up to 4 weeks after tx | 78 | 2176 | 35.85 | 2.91 (2.14–3.95) |
| Post-4 weeks after tx | 102 | 8282 | 12.32 | 1 |
| After Non-Medication Treatment | ||||
| Up to 4 weeks after tx | 43 | 2270 | 18.94 | 1.59 (1.04–2.27) |
| Post-4 weeks after tx | 119 | 9981 | 11.92 | 1 |
Acknowledgements
This project was supported by Grant No. 2015-PM-BX-K002 awarded to the Maryland Department of Health by the Bureau of Justice Assistance of the US Department of Justice. This research was also supported by the National Institute on Drug Abuse (F31DA047021, NK supported). The points of view or opinions in this article are those of the authors and do not necessarily represent the official position or policies of the U.S. Department of Justice, the Maryland Department of Health or any of the State of Maryland agencies who made their data available for this study. Authors would like to thank members of the entire PRECOG team for their intellectual contributions and review of this work, including Kristin E. Schneider, Matthew D. Eisenberg, Tom M. Richards, Lindsey Ferris, and Klaus W. Lemke. We also thank the Maryland Department of Health and Office of the Chief Medical Examiner for providing data for this study.
References
- 1.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. MMWR Morb Mortal Wkly Rep [Internet]. 2019. December 21 [cited 2019 Jan 6];67(5152). Available from: http://www.cdc.gov/mmwr/volumes/67/wr/mm6751521e1.htm?s_cid=mm6751521e1_w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saloner B, McGinty EE, Beletsky L, Bluthenthal R, Beyrer C, Botticelli M, et al. A Public Health Strategy for the Opioid Crisis. Public Health Rep [Internet]. 2018. November 14 [cited 2019 Apr 17];133(1_suppl):24S–34S. Available from: http://journals.sagepub.com/doi/10.1177/0033354918793627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AR, Nunes EV., Bisaga A, Levin FR, Olfson M. Development of a Cascade of Care for responding to the opioid epidemic. Am J Drug Alcohol Abuse [Internet]. 2019. January 2 [cited 2019 Apr 17];45(1):1–10. Available from: https://www.tandfonline.com/doi/full/10.1080/00952990.2018.1546862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakeman SE. Using science to battle stigma in addressing the opioid epidemic: opioid agonist therapy saves lives. Am J Med. 2016;129(5):455–6. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–6. [DOI] [PubMed] [Google Scholar]
- 6.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63–75. [DOI] [PubMed] [Google Scholar]
- 7.Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2010;30(2):155–66. [DOI] [PubMed] [Google Scholar]
- 8.Krawczyk N, Feder KA, Fingerhood MI, Saloner B. Racial and ethnic differences in opioid agonist treatment for opioid use disorder in a U.S. national sample. Drug Alcohol Depend. 2017. September 1;178:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mojtabai R, Mauro C, Wall MM, Barry CL, Olfson M. Medication Treatment For Opioid Use Disorders In Substance Use Treatment Facilities. Health Aff [Internet]. 2019. January 7 [cited 2019 Apr 18];38(1):14–23. Available from: http://www.healthaffairs.org/doi/10.1377/hlthaff.2018.05162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011. March;5(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Substance Abuse and Mental Health Services Adminisration. 2018. N-SSATS State Profiles [Internet]. Rockville, MD; 2018 [cited 2019 Dec 20]. Available from: https://www.samhsa.gov/data/report/2018-n-ssats-state-profiles [Google Scholar]
- 12.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017. April 26;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality. Ann Intern Med [Internet]. 2018. June 19 [cited 2018 Jun 18]; Available from: http://annals.org/article.aspx?doi=10.7326/M17-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend [Internet]. 2019. May 3 [cited 2019 May 7]; Available from: https://www.sciencedirect.com/science/article/pii/S0376871619301310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce M, Bird SM, Hickman M, Marsden J, Dunn G, Jones A, et al. Impact of treatment for opioid dependence on fatal drug-related poisoning: A national cohort study in England. Addiction. 2016;111(2):298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans E, Li L, Min J, Huang D, Urada D, Liu L, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006–10. Addiction. 2015;110(6):996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornish R, Macleod J, Strang J, Vickerman P, Hickman M. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ. 2010. October 26;341:c5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94(1):151–7. [DOI] [PubMed] [Google Scholar]
- 19.E Ravndal EA. Mortality among drug users after discharge from inpatient treatment: An 8-year prospective study. Drug Alcohol Depend [Internet]. 2010. April 1 [cited 2017 Nov 19];108(1–2):65–9. Available from: http://www.sciencedirect.com/science/article/pii/S0376871609004153#! [DOI] [PubMed] [Google Scholar]
- 20.Davoli M, Bargagli AM, Perucci CA, Schifano P, Belleudi V, Hickman M, et al. Risk of fatal overdose during and after specialist drug treatment: the VEdeTTE study, a national multi-site prospective cohort study. Addiction [Internet]. 2007. December [cited 2019 Apr 18];102(12):1954–9. Available from: http://doi.wiley.com/10.1111/j.1360-0443.2007.02025.x [DOI] [PubMed] [Google Scholar]
- 21.Susukida R, Crum RM, Ebnesajjad C, Stuart EA, Mojtabai R. Generalizability of findings from randomized controlled trials: application to the National Institute of Drug Abuse Clinical Trials Network. Addiction [Internet]. 2017. July 1 [cited 2017 Nov 12];112(7):1210–9. Available from: http://doi.wiley.com/10.1111/add.13789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krebs E, Min JE, Evans E, Li L, Liu L, Huang D, et al. Estimating State Transitions for Opioid Use Disorders. Med Decis Mak [Internet]. 2017. July 27 [cited 2017 Nov 19];37(5):483–97. Available from: http://journals.sagepub.com/doi/10.1177/0272989X16683928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat [Internet]. 2018. February 1 [cited 2019 May 7];85:90–6. Available from: https://www.sciencedirect.com/science/article/pii/S0740547216304135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glanz JM, Binswanger IA, Shetterly SM, Narwaney KJ, Xu S. Association Between Opioid Dose Variability and Opioid Overdose Among Adults Prescribed Long-term Opioid Therapy. JAMA Netw Open [Internet]. 2019. April 19 [cited 2019 Apr 19];2(4):e192613 Available from: http://jamanetworkopen.jamanetwork.com/article.aspx?doi=10.1001/jamanetworkopen.2019.2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis [Internet]. 2016. January 2 [cited 2018 Mar 3];35(1):22–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26467975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cousins G, Teljeur C, Motterlini N, McCowan C, Dimitrov BD, Fahey T. Risk of drug-related mortality during periods of transition in methadone maintenance treatment: a cohort study. J Subst Abuse Treat [Internet]. 2011. October 1 [cited 2018 Feb 1];41(3):252–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21696913 [DOI] [PubMed] [Google Scholar]
- 27.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend [Internet]. 2009. November 1 [cited 2018 Feb 1];105(1–2):9–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19608355 [DOI] [PubMed] [Google Scholar]
- 28.Ferris L, Saloner B, Krawczyk N, Schneider K, Jarman M, Jackson K, et al. Predicting Opioid Overdose Deaths Using Statewide Prescription Drug Monitoring Program Data. J Prev Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medicaid Innovation Accelerator Program. Overview of Substance Use Disorder (SUD) Care Clinical Guidelines: A Resource for States Developing SUD Delivery System Reforms [Internet]. 2017. [cited 2019 May 14]. Available from: https://www.medicaid.gov/state-resource-center/innovation-accelerator-program/iap-downloads/learn-hilc- [Google Scholar]
- 30.Krupitsky E, Nunes E V, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet [Internet]. 2011. April 30 [cited 2019 May 7];377(9776):1506–13. Available from: https://www.sciencedirect.com/science/article/pii/S0140673611603589 [DOI] [PubMed] [Google Scholar]
- 31.Saloner B, Daubresse M, Caleb AG. Patterns of Buprenorphine-Naloxone Treatment for Opioid Use Disorder in a Multistate Population. Med Care [Internet]. 2017. July [cited 2018 Nov 2];55(7):669–76. Available from: http://insights.ovid.com/crossref?an=00005650-201707000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce M, Bird SM, Hickman M, Millar T. National record linkage study of mortality for a large cohort of opioid users ascertained by drug treatment or criminal justice sources in England, 2005–2009. Drug Alcohol Depend. 2015;146(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP; 2017. [Google Scholar]
- 34.Leuven E SPELLUTIL: Stata module of utilities for the manipulation of timespan data. 2003. April 3 [cited 2019 Apr 22]; Available from: https://econpapers.repec.org/software/bocbocode/s431701.htm [Google Scholar]
- 35.Kröger H Newspell: Easy Management of Complex Spell Data. Stata J Promot Commun Stat Stata [Internet]. 2015. April 19 [cited 2019 Apr 22];15(1):155–72. Available from: http://journals.sagepub.com/doi/10.1177/1536867X1501500110 [Google Scholar]
- 36.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 37.Haviland A, Nagin D, Rosenbaum P, Tremblay R. Combining group-based trajectory modeling and propensity score matching for causal inferences in nonexperimental longitudinal data. Dev Psychol. 2008;44(2):422–36. [DOI] [PubMed] [Google Scholar]
- 38.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology [Internet]. 2000. September [cited 2017 Nov 16];11(5):550–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10955408 [DOI] [PubMed] [Google Scholar]
- 39.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed [Internet]. 2004. July [cited 2017 Nov 16];75(1):45–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15158046 [DOI] [PubMed] [Google Scholar]
- 40.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med [Internet]. 2014. March 30 [cited 2017 Nov 16];33(7):1242–58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24122911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. J Am Stat Assoc [Internet]. 1989. December [cited 2017 Nov 16];84(408):1074–8. Available from: http://www.tandfonline.com/doi/abs/10.1080/01621459.1989.10478874 [Google Scholar]
- 42.Ray WA. Evaluating Medication Effects Outside of Clinical Trials: New-User Designs. Am J Epidemiol [Internet]. 2003. November 1 [cited 2019 Apr 16];158(9):915–20. Available from: https://academic.oup.com/aje/article-lookup/doi/10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 43.Ciccarone D Fentanyl in the US heroin supply: A rapidly changing risk environment. Int J Drug Policy [Internet]. 2017. August 1 [cited 2019 May 1];46:107–11. Available from: https://www.sciencedirect.com/science/article/pii/S0955395917301810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connery HS. Medication-Assisted Treatment of Opioid Use Disorder. Harv Rev Psychiatry [Internet]. 2015;23(2):63–75. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00023727-201503000-00002 [DOI] [PubMed] [Google Scholar]
- 45.Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect [Internet]. 2002. July [cited 2018 Jan 30];1(1):13–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18567959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krawczyk N, Negron T, Nieto M, Agus D, Fingerhood MI. Overcoming medication stigma in peer recovery: a new paradigm. Subst Abus [Internet]. 2018. February 12 [cited 2018 Feb 17];00–00. Available from: https://www.tandfonline.com/doi/full/10.1080/08897077.2018.1439798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reisinger HS, Schwartz RP, Mitchell SG, Peterson JA, Kelly SM, O’Grady KE, et al. Premature Discharge from Methadone Treatment: Patient Perspectives. J Psychoactive Drugs [Internet]. 2009. September [cited 2019 Mar 18];41(3):285–96. Available from: http://www.tandfonline.com/doi/abs/10.1080/02791072.2009.10400539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truong C, Krawczyk N, Dejman M, Marshall-Shah S, Tormohlen K, Agus D, et al. Challenges on the road to recovery: Exploring attitudes and experiences of clients in a community-based buprenorphine program in Baltimore City. Addict Behav [Internet]. 2019. June 1 [cited 2019 Apr 20];93:14–9. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0306460318308360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kourounis G, Richards BDW, Kyprianou E, Symeonidou E, Malliori M-M, Samartzis L. Opioid substitution therapy: Lowering the treatment thresholds. Drug Alcohol Depend [Internet]. 2016. April 1 [cited 2018 Mar 3];161:1–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26832931 [DOI] [PubMed] [Google Scholar]
- 50.Doernberg M, Krawczyk N, Agus D, Fingerhood MI. Demystifying buprenorphine misuse: Has fear of diversion gotten in the way of addressing the opioid crisis? Subst Abus. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krawczyk N, Buresh M, Gordon MS, Blue T, Fingerhood MI, Agus D. Expanding Low-Threshold Buprenorphine to Justice-Involved Individuals through Mobile Treatment: Addressing a Critical Care Gap. J Subst Abuse Treat. 2019;103:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katzman JG, Takeda MY, Bhatt SR, Moya Balasch M, Greenberg N, Yonas H. An Innovative Model for Naloxone Use Within an OTP Setting: A Prospective Cohort Study. J Addict Med [Internet]. 2018. [cited 2019 Apr 21];12(2):113–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29227321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.