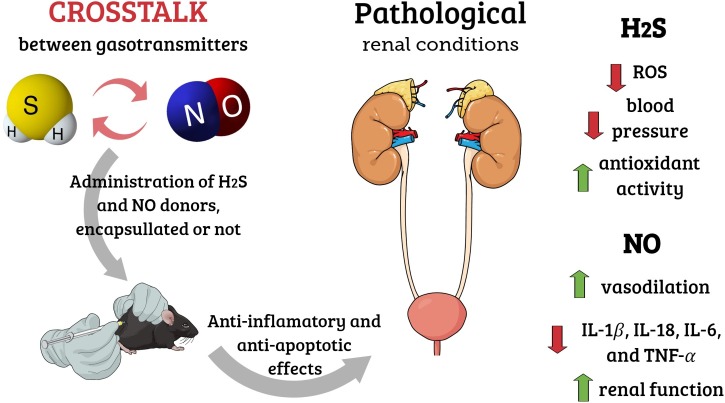
Graphical abstract
Keywords: Acute kidney injury, Hydrogen sulfide, Nitric oxide, Nanomaterials
Abstract
Acute kidney injury (AKI) is a syndrome affecting most patients hospitalized due to kidney disease; it accounts for 15 % of patients hospitalized in intensive care units worldwide. AKI is mainly caused by ischemia and reperfusion (IR) injury, which temporarily obstructs the blood flow, increases inflammation processes and induces oxidative stress. AKI treatments available nowadays present notable disadvantages, mostly for patients with other comorbidities. Thus, it is important to investigate different approaches to help minimizing side effects such as the ones observed in patients subjected to the aforementioned treatments. Therefore, the aim of the current review is to highlight the potential of two endogenous gasotransmitters - hydrogen sulfide (H2S) and nitric oxide (NO) - and their crosstalk in AKI treatment. Both H2S and NO are endogenous signalling molecules involved in several physiological and pathophysiological processes, such as the ones taking place in the renal system. Overall, these molecules act by decreasing inflammation, controlling reactive oxygen species (ROS) concentrations, activating/inactivating pro-inflammatory cytokines, as well as promoting vasodilation and decreasing apoptosis, hypertrophy and autophagy. Since these gasotransmitters are found in gaseous state at environmental conditions, they can be directly applied by inhalation, or in combination with H2S and NO donors, which are compounds capable of releasing these molecules at biological conditions, thus enabling higher stability and slow release of NO and H2S. Moreover, the combination between these donor compounds and nanomaterials has the potential to enable targeted treatments, reduce side effects and increase the potential of H2S and NO. Finally, it is essential highlighting challenges to, and perspectives in, pharmacological applications of H2S and NO to treat AKI, mainly in combination with nanoparticulated delivery platforms.
1. Introduction: pathophysiology of acute renal injury (AKI)
Acute kidney injury (AKI) is defined as a rapid loss of kidney function observed through fast creatinine level increase and urine output decrease, which can last from hours to days [1]. Initially, this syndrome disables electrolyte, acid-base and water balances [2]. Most patients hospitalized with some kidney disease have AKI; these patients account for 10–15 % of all intensive care hospitalizations worldwide [3]. AKI is often silent, since most patients are exposed to other conditions associated with AKI, such as sepsis.
According to the Kidney Disease Improving Global Outcomes (KDIGO), AKI is divided into three main stages associated with stress, damage and dysfunction [4]. At stage 1, creatinine level, an important marker of kidney injury, is ≥ 1.5-fold the baseline or increases to ≥ 0.3 mg/dl within 48 h. At stage 2, creatinine level is ≥ 2-fold the baseline representing damage stage. Finally, at stage 3, creatinine level is ≥ 3-fold the baseline or increases to ≥ 4 mg/dL, or acute dialysis. Urine volume, given as an option to observe the glomerular filtration rate (GFR) and expressed by quantity of urine produced per unit of time (mL/min), can be modulated in all stages, oscillating according to the osmolarity [4].
High incidence and prevalence of advanced AKI stages stood out for over a decade. Advanced and chronic disease stages require dialysis treatment, whereas chronic cases require kidney transplantation [5]. AKI does not present linear progression. AKI stage 1 can lead to chronic kidney disease (CKD) depending on stress severity, whereas CKD can regress the normal stage without going through AKI. It happens when i) kidney dysfunctions or damage remain for a long period-of-time without medical diagnosis [6] and ii) kidney diseases result from different conditions likely simultaneous to different AKI and CKD stages [1]. In other words, AKI is not associated with a single disease, but with a set of them, such as sepsis, urinary tract obstruction, inflammation, among others.
Ischemia and reperfusion (IR) injury is the main cause of AKI [7]; it is a temporary blood flow obstruction condition [8]; many IR models have been described in the literature. It is undeniable that IR causes renal tubular injury, as well as increases inflammatory compound and oxidative stress levels, which leads to systemic injury [7]. IR is followed by a re-oxygenation process named reperfusion. Many cascades of deleterious responses (mainly cellular responses) [9], such as oxidative stress and the formation of reactive oxygen species (ROS), take place during reperfusion. These responses are followed by fibrosis and renal remodeling [10].
Renal fibrosis is strongly associated with AKI and CKD. Studies have investigated myofibroblast populations triggering fibrogenic process after the IR. Moreover, these studies associated them with inflammatory processes in the organ [11]. The tight interaction between kidney and heart, called cardiorenal syndrome (CRS), is well-established. Briefly, CRS is described as a spectrum of clinical changes simultaneously involving the heart and kidneys; which leads to a sequence of interdependent events and mechanisms damaging both organs [[12], [13], [14]]. In other words, CRS is a multifactorial, bidirectional and dynamic process.
Several components are released into the systemic circulation during kidney injuries such as AKI; among them, one finds vasopeptides, catecholamines and cytokines, which can induce a series of changes in cardiac tissue and lead to a wide variety of cardiovascular diseases (CVD) [15,16]. Patients with CKD often show increased prevalence of CVDs such as hypertension, peripheral vascular disease and congestive heart failure. In addition, patients with terminal-stage kidney disease present significantly increased cardiovascular morbidity and mortality rates. Although the cellular process and mechanisms involved in AKI have been the subject of considerable interest, they remain poorly understood [[17], [18], [19]].
AKI has many causes; thus, different treatments have been used against it. The most common pharmacological strategies comprise i) Renin-Angiotensin-Aldosterone System (RAAS) blockers and ii) Sympathetic Nervous System (SNS) antagonists, which are capable of improving AKI by enhancing renal hemodynamics and tubule-glomerular feedback, as well as of improving blood pressure levels [20]. In addition to bringing benefits to renal tissue, angiotensin II blockers also avoid heart and inflammatory impairment in unilateral IR model [21]. Antioxidants also play an important role after the IR since they help preventing the deleterious effects of AKI. Lee et al. have described a powerful treatment based on alpha-lipoic acid (ALA), which was capable of eliminating free radicals [22,23]. More recently, another strategy based on the klotho treatment (antioxidant and anti-inflammatory agent) has shown promising results, since its exogenous administration helped preventing AKI [11].
Although important progress has been achieved in studies involving different AKI treatments, few therapies have led to clinical discoveries. Moreover, pharmacological tools have advantages and disadvantages, since patients may have other comorbidities. Thus, it is necessary investigating new approaches to treat AKI with minimum side effects. The aim of this current work is to highlight the recent progress in investigations about the biological effects of two important gasotransmitters - hydrogen sulfide (H2S) and nitric oxide (NO) – on AKI treatment, as well as their clinical potential to be used in association with nanomaterials.
2. Biological importance of H2S
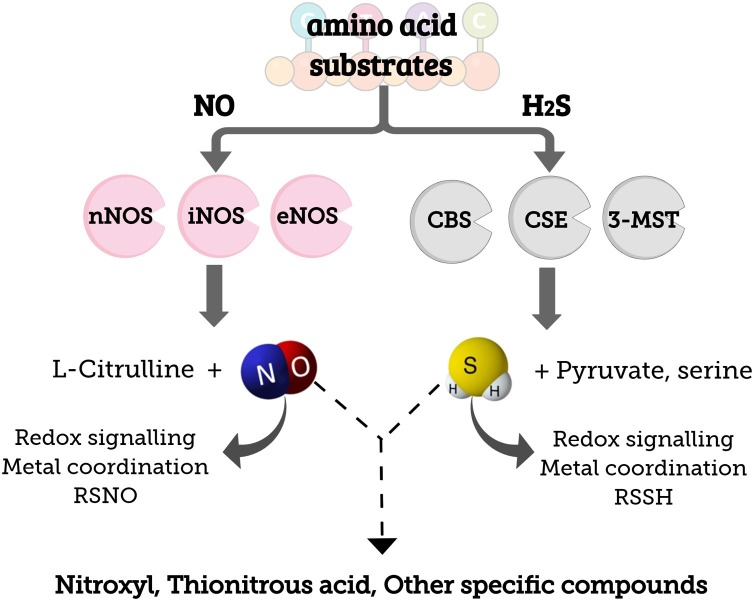
H2S is an endogenous gasotransmitter involved in several physiological and pathophysiological processes taking place in the cardiovascular, neuronal, renal, gastrointestinal and immune systems [24]. Along with NO and carbon monoxide (CO), H2S is part of a family of endogenous gas mediators; it was the last member identified in this family [25]. Although H2S has been known as toxic gas for decades, its biochemistry got better understood in recent years. Since H2S was first described as neuronal modulator in the 1990s [26], there has been intensive research on H2S biochemistry, which is involved in several biological processes. Under physiological conditions, nitrate and nitrite can be recycled in tissues and blood, leading to NO and several enzymes, such as persulfide dioxygenase, quinone oxireductase, sulfite oxidase and rhodanese, catalyze the oxidation of this gasotransmitter into sulfate and thiosulfate [24, [27], [28], [29], [30]]. Nowadays, H2S is acknowledged as an important antioxidant, anti-inflammatory and anti-apoptotic agent [31], although, this molecule has already been shown as a pro-inflammatory agent [32]. NO and H2S have complex and dichotomy effects in biological system, acting as pro- and anti-inflammatory agents, depending on their concentrations, cell redox state, the disease model evaluated, and the rate of H2S generated from a donor (thus, H2S flux is crucial) [32,33]. Generally, at nano to low micromolar concentrations, H2S acts as anti-oxidant agent, whereas at superior concentrations, opposite effects are observed (pro-inflammatory effects). Indeed, at high concentrations, H2S donors have been reported to increase the production of inflammatory mediators (IL-1β, IL-6, TNF-α, prostaglandin E2 and NO), while at low concentrations (up to 100 μM), H2S inhibits their production [33]. For instance, at concentrations between 25–250 μM, H2S protected human neuroblastoma (SH-SY5Y) cells against peroxynitrite (ONOO−) [34]. In contrast, at higher concentrations (200–500 μM), H2S caused apoptosis in human aortic smooth muscle cells [35]. Similar to NO, H2S was first reported to be a pro-inflammatory agent, and during sepsis, patients have been reported to have high levels of H2S in plasma [33]. At low concentrations, H2S acts as cytoprotective agent by reducing the expression of pro-inflammatory cytokines, enzymes, and chemokines, and to significantly suppress the activation of nuclear transcription factor- κB (NF- κB). Thus, H2S has been exploited in the design of effective anti-inflammatory drugs [32]. In this direction, it has been demonstrated that the inhibition of endogenous H2S production activated inflammatory response and promoted apoptosis in renal IR. In this sense, the effect of H2S on toll-like receptors-mediated inflammatory pathways in renal IR was evaluated in rats. Toll-like receptors are involved in innate immunity and inflammatory responses. It has been demonstrated that endogenous H2S has important anti-oxidant effect in rat renal IR by suppressing inflammation and apoptosis by inhibiting the activation of toll-like receptors constitutively expressed in renal tubular epithelial cells [34]. Thus, the majority of recent work have demonstrated H2S as an anti-inflammatory agent, although further research is still required to better comprehend the two faces of H2S [36,37]. Particularly, in renal system and under physiological conditions, H2S regulates the excretory function of the kidney, the release of renin from juxtaglomerular cells, controlling blood pressure. Changes of H2S concentrations have been associated with important pathological conditions, including IR, diabetic nephropathy, obstructive nephropathy, and hypertensive nephropathy [31].
H2S is a weak acid that can dissolve in water. Thus, in biological system, the gasotransmitter is dissolved, actuating as solute [31,38]. By considering the pKa value of H2S under physiological conditions (37 °C, and pH 7.4), H2S is found as ca. 86 % of HS−, 14 % of H2S gas and trace of S2- [31,38]. Similar to NO, the lipophilic nature of H2S allows it to easily diffuse through cell membranes and to act as cell signaling agent [39]. This molecule is significantly synthesized in the kidney, suggesting its importance to the physiological function of this organ [31]. H2S regulates the excretory activity of the kidneys under normal conditions; it prevents the presence of sodium transporters on tubular cells, as well as the modulation of renin release from juxtaglomerular cells, thus controlling blood pressure. As expected, and similar to NO, pathological renal conditions, such as diabetic nephropathy, renal IR injury, obstructive nephropathy and hypertensive nephropathy, can be associated with changes in H2S levels [31]. H2S plays different roles in CKD, whose progression is induced by renal hypoxia; among these roles, one mainly finds the preservation of medullary oxygenation, prevention of inflammatory response, stabilization of the hypoxia inducible factor and suppression of oxidative stress [40].
Given the important role played by H2S in biological system, several H2S donors have been used in biomedical applications to treat different diseases. These donors comprise inorganic sulfide salts such as NaHS (the most used one), nucleoside phosphorothioates, different organic donors, non-steroidal anti-inflammatory drugs, cysteine-derived compounds and even polysulfides deriving from garlic extract [24]. Similar to NO, the use of H2S-donors/generators has been recently evaluated in the treatment of different diseases such as atherosclerosis, renal and heart failure, pulmonary and arterial hypertension, IR injury, inflammatory conditions, diabetic nephro- and retinopathy, Alzheimer’s and Parkinson’s disease, sexual dysfunction, among others [24,41]. Interestingly, the anti-inflammatory properties of H2S donors may be used to mitigate kidney toxicity caused by treatments based on cisplatin, which is an anti-neoplastic drug that causes renal toxicity due to the formation of reactive oxygen species (ROS) [42]. Indeed, H2S acts by disrupting cisplatin bioconversion into nephrotoxic metabolites due to its anti-inflammatory properties.
In addition to the direct use of H2S donors to treat these diseases, many drugs can regulate endogenous H2S levels; these mechanisms have been the object of intensive research. Importantly, recent evidence has suggested crosstalk between NO and H2S under physiological and pathophysiological conditions [43]. The crosstalk between both gasotransmitters may lead to distinct responses: (i) decrease in the NO and NOS expression and bioavailability, and (ii) NOS functions and NO bioavailability restoration [[44], [45], [46], [47]]. However, it is necessary conducting additional studies to help better understanding this topic, which is further discussed in section 7.
3. Pharmacotherapeutic applications of H2S donors in AKI
Several studies have investigated the role played by H2S in AKI. It is well-known that renal cells produce H2S; however, its production decreases under disease condition [48]. Besides, the role played by H2S in the cardiovascular system is also object of studies. H2S is a key player in blood pressure control since it enables vasodilatation and has cardioprotective action [37,49]. Similar to NO, H2S action as vasodilator depends on its concentration, which can have beneficial and harmful effects on cardiac tissue [24]. The role played by H2S donors as therapeutic strategy has already been investigated. Studies have suggested the anti-inflammatory and antioxidant activity of H2S [50]. According to Kubo et al., NaHS (a H2S donor) is capable of inhibiting endothelial nitric oxide synthase (eNOS) activity and may have systolic effect on blood vessels in the absence of NO [51]. Moreover, changes in H2S levels have been linked to the development and progression of kidney diseases, from the acute to the chronic stage [41,42,[52], [53], [54], [55]]. In addition, the administration of H2S donors, in combination with angiotensin, can convert enzyme inhibitors or adrenergic receptor blockers, as well as improve renal function, which turns it into an interesting approach to treat renal fibrosis in CKD [56].
Two strategies can be used to target H2S in biomedical applications: (i) direct administration of H2S donors, and (ii) approaches focused on increasing endogenous H2S production. The aim of this section is to present and discuss the recent progress on the use of H2S donors against AKI. Strategies focused on increasing endogenous H2S production have been investigated in several studies [24,31,57]. H2S donors have been used to treat renal diseases since they help mitigating inflammation, actuate as a ROS scavenger, impair the activation of fibrosis-related cells, decrease cytokine formation, allow vascular remodeling, decrease blood pressure, trigger tubular cell regeneration, as well as decrease apoptosis, hypertrophy and autophagy [58].
Inorganic salt NaHS is the most used H2S donor. Although important results have been achieved with the use of this donor, NaHS has limitations due to its pharmacokinetic profile and short desirable biological effect [24]. Therefore, the design of long-lasting H2S donors/generators is an important topic yet under investigation. Several experimental models of renal IR injury have used NaHS as protective agent [24].
Renal IR injury is the major cause of AKI, which is featured by calcium overload, ATP depletion, ROS generation, apoptosis and inflammation [31]. Accordingly, H2S can mitigate, at least partially, these deleterious effects. Impaired H2S generation is observed during renal IR injury; thus, the administration of H2S donors may have positive effects on it [24,31,59]. Indeed, NaHS administration has renal protective effects since it works as anti-apoptotic, anti-inflammatory and antioxidant agent [31]. Likewise, the water-soluble slow-release H2S donor P-(4-methoxyphenyl)-P-4-morpholinyl-phosphinodithioic acid (GYY4137) can protect tissues from damages by inhibiting the activation of MAPK and NF-κB signaling [60]. Moreover, AP39 (a mitochondria-targeted H2S donor, namely [10-oxo-10-[4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy]decyl]triphenyl-phosphonium) has inhibited ROS formation caused by glucose oxidase and protected kidneys from IR injury in animal models [61].
The impact of H2S donor AP39 was investigated in vitro (kidney epithelial cells of NRK-49 F rats) and in vivo (rat model of renal ischemia reperfusion injury) [61]. Cells pre-treated with H2S donor (30–300 nmol/L) have shown protective effect against glucose oxidase induced by oxidative stress, in a concentration-dependent manner, and mitigated mitochondrial dysfunction. Glucose oxidase has decreased intracellular ATP levels, increased ROS and led to necrosis. These deleterious effects were avoided through cell incubation with AP39. Moreover, rats subjected to renal IR injury have shown high blood creatinine and urea levels, which were seen as damage indicators that have led to increased oxidative stress, enhanced neutrophil infiltration and high plasma IL-12 levels in comparison to the control group. All these pathophysiological changes were mitigated, in a concentration-dependent manner, in rats pre-treated with AP30 (0.1, 0.2 and 0.3 mg/kg); the best results were observed for the highest tested H2S concentration [61]. AP30 renal protection mechanisms may be associated with its antioxidant effect, which, in its turn, mitigates several feed-forward pathways of oxidative and inflammatory processes.
Besides the renal system, the action of H2S donors in the cardiovascular system has been investigated. It is worth highlighting the important role played by crosstalk between heart and kidneys in several pathological conditions. Thus, the cardioprotective role played by different H2S donors (sodium sulfide (Na2S), thiovaline (TV), GYY4137 and AP39) was investigate in animal models with myocardial failure (ischemia-reperfusion injury) [62]. Interestingly, the authors of the aforementioned study have shown that the beneficial effects of Na2S were blocked due to nitric oxide synthase (NOS) inhibition, a fact that suggested crosstalk between H2S and NO. H2S donors presented different actions in the injury, since they enhanced the phosphorylation of endothelial NOS found in Na2S treatment, but not under AP39 addition [62]. This result suggests that H2S donors should be selected for specific applications; however, this issue should be further explored.
H2S donor has shown beneficial effects on rat model of Crush syndrome, which can lead to AKI [27]. Crush injury was induced through hindlimb compression for 6 h; animals were treated with H2S donor NaHS (100 mmol/Kg ip). These animals presented reduced kidney injury, as seen in decreased neutrophil gelatinase-associated lipocalin, transforming growth factor-β, tumor necrotizing factor-α and ROS levels, as well as in increased total antioxidant contents in kidneys. Moreover, these animals showed reduced serum urea, creatine kinase and creatinine levels, decreased renal failure and reduced apoptosis upon NaHS treatment in comparison to untreated animals. Thus, NaHS administration helped preventing AKI in animal models with crush injury by reducing oxidative stress, inflammation and apoptosis [27].
The effects of H2S on contrast-induced AKI were recently evaluated in experiments conducted in vivo focused on investigating endogenous H2S up-regulation [63]. Experiments in vitro were also performed; NRK-52E rat kidney epithelial-like cells were treated with iopromide (to create contrast-induced AKI), which was followed by NaHS administration. Significant improvement in renal dysfunction and morphological changes were observed in animals treated with atorvastatin in comparison to the control group; it also reduced ROS formation, inflammation and apoptosis. Furthermore, increased serum H2S levels and renal expression of cystathionine γ-lyase and cystathionine-β synthase (two H2S synthetases) were reported. Results in vitro have shown that cells incubated with NaHS presented considerably reduced inflammation and cell death levels in comparison to the control group. Thus, the renal protection effects of atorvastatin derive from the H2S pathway [63]. Further studies are required to help better understanding this mechanism.
H2S can have anti- or pro-inflammatory effects, depending on its flow (concentration and action time) and on the redox state of the biological site. For instance, rats were treated with NaHS (2 mg/kg) in a study conducted with animal models presenting endotoxemia. Short course infusion of H2S donor was capable of reducing kidney and lung injury, whereas the opposite effect was observed for systemic NaHS infusion; this case showed increased pro-inflammatory response - elevated TNF-α and IL-10 levels were observed during endotoxemia [64]. Therefore, caution must be taken with H2S doses in biomedical applications.
4. Biological importance of NO in AKI
AKI and CVD share common pathological pathways and factors such as oxidative stress. In mechanistic terms, oxidative stress and NOS system lead to endothelial dysfunction and reduced NO bioavailability, as observed during CRS [65]. NO is produced in the body through the action of three NOS isoforms that are divided into two groups, namely: constitutive (neural- nNOS and endothelial-eNOS) and inducible (iNOS) [66]. NOS produces NO through oxidation of L-arginine to L-citrulline. NO and its oxidized products (NOx) are capable of changing different macromolecules such as proteins, lipids and nucleic acids in order to produce both physiological and pathophysiological effects [67]. Healthy renal function depends on the balance ROS and NO metabolism [68].
Similar to H2S, NO plays an important role in kidney function, since this free radical is involved in processes such as the regulation of renal hemodynamics, modulation of medullary blood flow, mediation of pressure-natriuresis, blunting of tubuloglomerular feedback, modulation of renal sympathetic neural activity and inhibition of tubular sodium reabsorption [69]. Human body must function at normal NO level, based on each tissue type. High NO levels (in the micro-milli molar range) can inhibit mitochondrial respiration due to competition for mitochondrial cytochrome oxidase oxygen [68]; in addition, it is associated with endothelial function progression in hypertensive patients [70]. On the other hand, NO deficiency has already been associated with AKI progression to CKD and with hypertension. Reduced plasma NO concentrations have been observed in hypertensive patients [71], whereas transgenic (TG) mice knocked out by eNOS have spontaneously developed hypertension [72]. It becomes evident that interventions in redox imbalance and NO bioactivity can lead to promising therapeutic strategies [65,73]. Thus, recent progress in NO donor administration to treat AKI will be presented in the next section.
5. NO-based pharmacotherapy applications in AKI
This section highlights publications selected based on the recent progress in NO/NO donor application in individuals with acute renal failure. Direct NO gas administration can be used in clinical settings, since it can enhance arterial oxygenation in patients with acute respiratory distress syndrome [74]. NO gas administration in newborns treated for pulmonary hypertension is approved by the US Food and Drug Administration (FDA), and it can be used as recue treatment in patients with hypoxic COVID-19 symptoms [75]. Accordingly, Gozdzik et al. observed the beneficial effects of inhaled NO (initial 80 ppm dose followed by 30 ppm) used in association with intravenous steroid administration (corticosteroids, 25 mg x 3) in IR injury models with aortic clamping. The authors observed that hydrocortisone and inhaled NO administration decreased kidney messenger RNA toll-like receptor 4 expression to pre-ischemic conditions; in addition, it significantly improved systemic hemodynamics and tissue oxygenation, as well as decreased the systemic inflammatory response [75].
An observational study was conducted to investigate the association between inhaled NO therapy and the incidence of AKI in patients subjected to lung transplantation. It was done by taking into consideration that NO gas-based therapy is often applied to lung transplantation recipients in clinical settings and that AKI plays a critical role in the prognosis of lung transplantation recipients [75]. The authors did not find correlation between inhaled NO therapy and incidence of pos-lung transplantation AKI. Inhaled NO plays an important role in lung transplantation cases since it significantly enhances lung oxygenation, reduces pulmonary vascular resistance, prevents reperfusion injury and does not have side effects on kidneys. On the contrary, inhaled NO has positive effects on renal, hepatic and splanchnic perfusion [75].
Besides the direct gaseous NO administration, NO donors/generators have been used in several biomedical applications such as AKI treatment. Thus, nitrate or nitrite intake can increase the NO levels and have cardiorenal-protective effects on animal models with cardiorenal diseases such as IR injury [65,76]. Under physiological conditions, nitrate and nitrite can be recycled in tissues and blood, leading to NO and other nitrogenated species with bioactivity [77,78]. This pathway might lead to the formation of nitrogen dioxide (NO2), which can dimerize forming dinitrogen tetroxide (N2O4) that is hydrolyzed producing equimolar amounts of nitrate and nitrite. Moreover, NO2 can react with NO leading to the formation of dinitrogen trioxide (N2O3), a nitrosating agent, which can be hydrolyzed to nitrite, thus reactive intermediates can also be formed [80]. Nitrite has shown beneficial effects on renal IR injury in mice, whose vasodilation has significantly enhanced during hypoxia at low pH [79]. Interestingly, the acidic environment found in hypoxic tissues enabled nitrite reduction to NO since nitrite protonation produces nitrous acid, which forms N2O3 that undergoes disproportionation, leading to the formation of NO and NO2 [17]. In general, as these mechanisms are enhanced during hypoxia and acidosis, they represent an important pathway, as NOS activity may be compromised in such conditions [80]. In these specific conditions, the nitrite reduction to NO has shown positive effects, such as cellular response to ischemic stress, hypoxic signaling, and vasodilation [81,82]. Based on similar approach applied to experimental rat models with crush syndrome, sodium nitrite (200 mmol/Kg) administration prevented damages associated with IR injury by decreasing systemic inflammation [83].
Red beets and leafy greens are rich in nitrate, which can be reduced to nitrite and NO in the human body due to the action of enzymes such as xanthine oxidoreductase [76,84]. Nitrate-nitrite-NO pathway has important antioxidant and anti-inflammatory effects. Thus, chronic supplementation with inorganic nitrate/nitrate is indicated for several renal and cardiovascular conditions [65]. Indeed, nitrate-rich beetroot intake helped lowering the renal resistive index and blood pressure in patients with kidney disease [85]. Ingested nitrate is concentrated and secreted into the oral cavity by salivary glands, and reduced to nitrite by oral bacteria, followed by its absorption by the upper intestine [86]. Saliva containing nitrite is absorbed in the stomach and enters the blood stream, where it is reduced to NO by different NO reductase pathways [67]. However, caution must be taken with nitrate-based supplementation since certain concentrations of it may lead to increased oxidative stress and endothelial dysfunction, as well as impair kidney function [87].
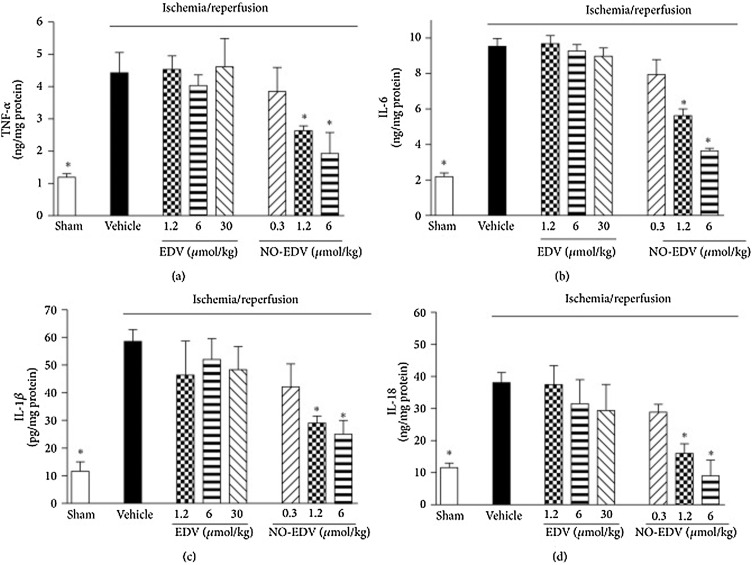
To overcome this issue, in a different approach, the NO donor (5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, EDV), characterized by a furoxan moiety of edaravone, was evaluated in renal IR injury [88]. EDV is a free-radical scavenger known to mitigate organ ischemic injury. NO was coupled to EDV yielding NO-EDV. Wistar rats were subjected to renal IR, and treated with EDV (1.2–6–30 μmol/kg, i.v.) or NO-EDV (0.3–1.2–6 μmol/kg, i.v.). Both treatments were capable of mitigating renal dysfunction, in a concentration-dependent manner, as seen in serum creatinine and urea, urine flow, creatinine clearance, neutrophil gelatinase-associated lipocalin/lipocalin-2 and urinary N-acetyl-β-d-glucosaminidase levels. However, NO-EDV was more effective as protective agent than EDV, since NO-EDV presented renal protection effects at dose-range 1.2–6.0 μmol/kg, whereas higher EDV dose (30 μmol/kg) was necessary to obtain the same protection effect. Moreover, NO-EDV and EDV have modulated oxidative stress and lipid peroxidation in renal tissue. Only NO-EDV has enabled blunted IR upregulation of inducible NOS, activated endothelial NOS, and inhibited the overproduction of proinflammatory cytokines such as IL-1β, IL-18, IL-6, and TNF-α (Fig. 1 ) [88]. Together, these results have suggested that NO-donor EDV codrugs can be used as pharmacological approach to treat AKI.
Fig. 1.
Effects of EDV and NO-EDV on cytokine production in kidney samples. TNF-α (a), IL-6 (b), IL-1β (c), and IL-18 (d) levels were measured in the kidney of sham-operated rats (sham) and rats that underwent 45 min ischemia and 6 h reperfusion in the absence (vehicle) or presence of EDV (1.2–30 μmol/kg, i.v.) or NO-EDV (0.3–6 μmol/kg, i.v.). Data are mean S.E.M. *P < 0.05 versus vehicle. Reproduced from Chiazza et al. 2015 under the Creative Commons Attribution License of open access article [88].
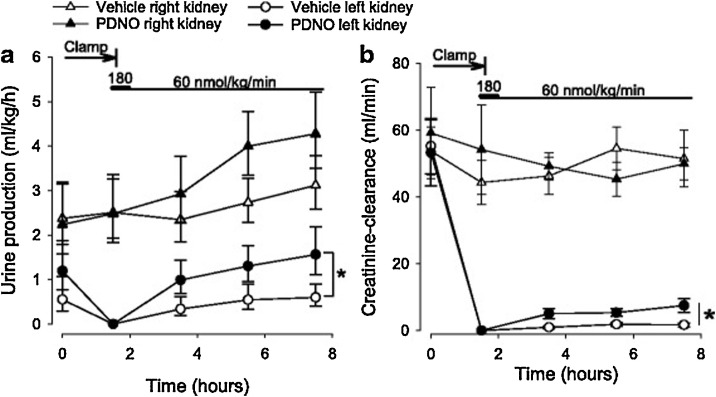
Organic mononitrites of 1,2-propanediol (PDNO), which are capable of generating NO in the blood stream, were synthesized and used to treat AKI in sheep with renal IR injury [89]. Intravenous PDNO infusion has significantly increased creatinine clearance and diuresis in kidneys (Fig. 2 ), as well as enhanced renal oxygen and decreased mean arterial blood pressure, in comparison to the control group. Overall, PDNO was capable of enhancing renal function after ischemia [89].
Fig. 2.
Urine output (a) and creatinine clearance (b) in 16 anesthetized sheep subjected to left renal ischemia and reperfusion. Renal ischemia was caused by clamping of the renal artery for 90 min. Fifteen minutes prior to the release of the clamp, intravenous infusions with either the organic mononitrites of 1,2-propanediol (PDNO, n = 8) or vehicle (1,2-propanediol + inorganic nitrite, n = 8) were commenced. The infusions continued for 6 h. Data are expressed as mean and SEM. Significant (p < 0.05) differences in response to PDNO compared to vehicle is indicated by an asterisk. Reproduced from Nilsson et al. 2017 under the Creative Commons Attribution 4.0 License of open access article [89].
S-nitrosothiols (RSNOs), such as S-nitrosoglutathione (GSNO), act as spontaneous NO donors due to homolytic S—N cleavage with free NO release [90]. In biological medium, RSNOs can be decomposed by different mechanisms, such as: heat, ultraviolet light, nucleophiles, metal ions, and other agents that may lead either to homolytic or to heterolytic cleavages [91]. Despite being able to release NO in biological medium, S-nitrosothiols stand out due to the transnitrosylation reactions rather than the S—N cleavage for NO release [92]. Proteins nearby RSNOs or even NO generated from NOS, may interact with RSNOs leading to protein-SNOs [93]. Thus, small RSNOs such as GSNO may transfer the NO group from one thiol to another (S—NO → protein thiols), leading to S-nitrosated proteins, which is a mechanism dependent on the cell’s surrounding chemical environment [93]. GSNO is an endogenous molecule that increases NO bioavailability and has NO-like activity, such as vasodilation [94], anti-oxidant effects [95], tissue repair [96,97], anti-microbial activity [98,99], among others. GSNO (50 μg/kg body weight) has shown renoprotective effects on the treatment of sepsis-induced AKI applied to rat models with lipopolysaccharide-induced sepsis [100]. GSNO-treatment applied to LPS-challenged animals has significantly increased IL-10, PPAR-γ and GSH levels, as well as reduced caspase-3, iNOS, TNF-α,T lymphocyte infiltration, in comparison to the untreated animals [100]. Similarly, S-nitrosated human serum albumin (50 μM) has shown protective effects in animal models with kidney disease by inhibiting fibrosis factors such as IL-6 and TGF-β, decreasing oxidative stress and increasing erythropoietin (anti-fibrosis factor) and VEGF expression [101].
Sodium nitroprusside (SNP) is a well-know NO donor [90]. Renal protective effects of SNP, losartan (angiotensin II type 1 receptor antagonist), captopril, and BQ-123 (endothelin type A receptor antagonist) in rat models with renal IR injury were evaluated [102]. SNP and losartan have shown the highest therapeutic potential to mitigate the deleterious effects of acute renal damage and renal function impairment. Moreover, rats treated with NG-nitro-L-arginine-methyl ester (L-NAME), which is a NOS inhibitor, developed prominent lesions in the kidney tissue, along with significantly reduced Na-K ATPase activity [102].
Glutamyl-protected N‑hydroxyguanidine NO donor drugs were prepared and used in individuals with acute renal failure [103]. Synthesized N-hydroxyguanidine NO donor drugs were effective against spontaneous NO release due to linkage to glutamyl adducts that can be cleaved by γ-glutamyl transpeptidase (γ-GT), which, in its turn, is mainly found in renal tissue. This prodrug acted as an important vasodilator in isolated perfused rat kidneys (EC50 ∼ 50 μM), since it prevented the damaging effects of vasoconstriction on individuals with acute renal failure [103].
Important progress has been achieved due to NO/NO donor administration in several AKI models in vitro and in vivo. Table 1 summarizes the main results referring to H2S and NO use in AKI.
Table 1.
Summary of recent progress of using H2S- and NO-based treatment to AKI.
| Specie | Donor molecule | Concentration | Model | Main biological effects | Ref |
|---|---|---|---|---|---|
| H2S | AP39 | 30 – 300 nM, 0.1−0.3 mg/kg | In vitro kidney epithelial cell (NRK-F), in vivo rat reperfusion model | Inhibition of ROS caused by glucose oxidation and protection from ischemia | [46] |
| GYY4137 | 12.5–50 mg/(kg ∙ day) | In vivo male Sprague-Dawley rats reperfusion model | Protective effects against reperfusion injury through attenuation of oxidative stress and apoptosis | [45] | |
| Na2S, TV, GYY4137, AP39 | 1 μmol/L, 4 μmol/L, 26 μmol/L, and 250 nmol/L, respectively | Ischemia-reperfusion injury animal model | Important results were observed regarding NOS inhibition and phosphorylation | [47] | |
| NaHS | 400−800 μmol/L | In vitro and in vivo contrast-induced acute kidney injury models | Considerable reduction in levels of inflammation and cell death | [48] | |
| NaHS | 100 μmol/kg | Acute kidney injury animal model | Decrease of neutrophil gelatinase-associated lipocalin, tumor necrotizing factor-α, ROS and increase of antioxidative levels | [27] | |
| NaHS | 2 mg/kg | Animal model of endotoxemia | pro-inflammatory was observed by higher levels of TNF-α and IL-10 | [49] | |
| NO | Inhaled NO | During surgery | Patients with acute lung injuries undergoing lung transplantation | Inhaled NO demonstrated positive effects on renal, hepatic and splanchnic perfusion | [80] |
| Nitrite | 10−9-10-4 mol/L | Mice model of ischemia-reperfusion injury | Vasodilation effects due to nitrite was significantly enhanced during hypoxia with low pH | [81] | |
| Nitrite | 200 mmol/kg | Rat experimental model of crush syndrome | Inflammation decrease and prevention of damages associated with ischemic injury | [63] | |
| Beetroot juice | 300 mg | Patients with kidney disease | Treatment led to lower renal resistive index and blood pressure | [65] | |
| NO-EDV | 1.2; 6 and 30 μmol/kg | Rat model of renal ischemia and reperfusion | Mitigation of renal dysfunction, in a concentration dependent manner. Protective effect in higher concentration. | [68] | |
| PDNO | 60−180 nmol/(kg •min) | Sheep mode of kidney ischemia and reperfusion | Increased creatinine clearance, diuresis, renal oxygen and decreased mean arterial blood pressured | [69] | |
| GSNO | 50 μg/kg | Rat mode of lipopolysaccharide-induced sepsis | Renoprotective effects by inhibiting fibrosis factors | [77] | |
| S-nitrosated HSA | 50 μmol/L | Kidney disease rat model | Protective effect through the inhibition of IL-6 and TGF-β, decreasing oxidative stress | [78] | |
| SNP | Pre-treatment (5 mg/kg), post-treatment (10 μg/kg •min) | Pat model of renal ischemia-reperfusion | Alleviation of deleterious effects, NOS inhibition and reduction in the Na-K ATPase activity | [79] | |
| Glutamyl-protected N‑hydroxyguanidin | 50 μmol/L | Rat isolated perfused kidneys | Prevention of the damaging effects of vasoconstriction in acute renal failure | [82] |
6. Challenges and potential uses of H2S/NO-releasing nanomaterials in AKI
The spatiotemporal controlled generation of gasotransmitters such as H2S and/or NO is highly desirable to avoid side effects such as systemic blood pressure decrease, and to enhance the therapeutic effects of H2S or NO on target organs such as kidneys. The development of different classes of H2S and NO donors represented an important pharmacological progress that has enabled the broad use of these gasotransmitters, in comparison to the direct applications in their gas form, which presents clinical limitations. In addition, there are different classes of H2S and NO donors that have short and long-lasting half-life [90,104]. An overview of potential applications of both gasotransmitters (H2S and NO) is schematically represented in Fig. 3 .
Fig. 3.
Schematic representation of potential benefits of exogenous administration of H2S and NO, and their beneficial effects in kidney.
Although important progress has been achieved in H2S or NO administration in a wide range of diseases such as kidney injury, H2S- or NO-based therapies still face limitations in clinical settings due to lack of effective approaches capable of properly delivering these gasotransmitters to a specific organ, such as kidneys. The major issues associated with H2S and NO administration in medical applications can be summarized as: (i) uncontrolled gasotransmitter release, either too fast or too slow, mainly in the case of H2S donors; H2S release mechanism remains poorly known, and (ii) in the case of H2S, some donors (such as thioisocyanates, thioamides, dithioperoxyanhydrides) can deplete biological thiols generating H2S. Low and controlled H2S donor concentrations should be used (nano- low micro molar) due to the biological concentrations of endogenous thiols, such as glutathione at milimolar levels. Caution must be taken in the use of H2S donors in order to avoid excessive consumption of endogenous free thiols and, consequently, significant changes in the thiol redox balance [105]. The combination between H2S/NO donors and nanomaterials is a promising approach that has been subjected to intensive investigation focused on helping to overcome these issues.
Overall, advantages of combining H2S/NO with nanomaterials lie on the likelihood to: (i) promote the sustained release/generation of therapeutic amounts of these gasotransmitters; (ii) target the nanomaterial towards the desired organ, depending on the chemical nature of the nanomaterial; (iii) adapt H2S or NO concentrations to the amounts necessary to enable successful therapy; (iv) co-administrate H2S or NO with other pharmacological agents in a given nanomaterial in order to achieve synergist effect; and (v) conjugate H2S or NO to nanoparticles that intrinsically have therapeutic effects. These progresses may enhance the effectiveness of H2S or NO, with minimum side effects. There has been intensive research focused on designing smart nanovehicles capable of releasing active agents upon external stimuli such as pH, presence of some enzymes, light, oxygen tension, redox state of the environment, temperature, among others, in recent years [106]. It has already been reported that NO donors encapsulated in polymeric and metallic nanoparticles present slow and sustained release. Alginate nanoparticles containing S-nitroso-mercaptosuccinic acid promoted sustained NO release for 12 h, which improved antibacterial applications [107]. Similarly, GSNO encapsulated in chitosan nanoparticles also evidenced sustained release through Fickian-diffusion and reached a plateau after 4 monitoring hours [108]. Moreover, the encapsulation of tert-dodecane S-nitrosothiol donor in co-polymer nanoparticles did not change the NO release profile; it actually enabled photoactivated NO release, which promoted vascular hyperpermeability [109].
Although this topic is poorly explored, few publications have indicated promising nanoparticle applications to enable controlled H2S release. Another interesting approach lies on the use of H2S donors-containing mesoporous silica nanoparticles [110,111]. The incorporation of H2S donors in mesoporous silica nanoparticles enabled a sustained H2S release in cell culture medium and in rat plasma for 24 and 72 h, respectively. The incorporation of H2S donor into the nanomaterial was considered as a superior effective strategy in comparison to the free GYY4137 H2S donor (non-encapsulated), enabling a controlled and slow H2S generation from the nanomaterial [110,111]. Besides, near infrared-controlled H2S release by using upconversion nanoparticles based on LiYF4:Yb/Tm and carried with propane-2,2-diylbis((1-(4,5-dimethoxy-2-nitrophenyl)ethyl)sulfane) as innovative H2S donor was also suggested [112]. Results have evidenced that nanoparticle irradiation with 980-nm laser enabled H2S release in a spatially- and temporally controlled manner, as well as opened room for several biomedical applications such as AKI.
Although several important publications have described the preparation and uses of H2S and NO-releasing nanomaterials in different biomedical applications [113,114], their use in AKI remain poorly explored. Moreover, nanoparticles comprising both H2S and NO donors may have beneficial effects on different pathologies and should be further explored. The crosstalk between H2S and NO appears to have key regulatory effects on some pathophysiological conditions. Both H2S and NO donors can mitigate the deleterious effects of AKI. Several important studies have suggested the likely interaction between these two gases to control important biological processes. Interestingly, H2S pathway appears to be significantly relevant when NO bioactivity is impaired [25]. It is necessary conducting further studies to help better understanding the association between H2S and NO under normal and pathological conditions. We hope the present review opens new room for the progress of smart and versatile nanomaterials capable of generating H2S and/or NO in AKI treatment.
7. Crosstalk between gasotransmitters: possible mechanisms of interaction between H2S and NO and the biological consequences
There are three different gasotransmitters in biological system, namely: CO, NO and H2S; they can interact with each other, affecting their availability and reactivity [115]. Although their interaction mechanism is not fully understood, most studies overall focus on investigating the crosstalk between NO and H2S in cardiovascular system [[116], [117], [118]]. It is well-known that NO and H2S present comparable biological profiles, enable cell protection and act in signaling pathways [44]. The first study about this intercommunication was published in 2006; the authors have shown that H2S was capable of inhibiting the sodium nitroprusside (SNP) mechanism in aorta relaxation [119]. More recently, a study has evidenced that H2S interacts with NO and forms small S-nitrosothiol compounds such as thionitrous acid (HSNO), besides nitroxyl (HNO) and other unknown compounds [116], as shown in Fig. 4 . However, further studies on this topic are necessary.
Fig. 4.
Schematic representation of the biosynthesis of NO, mediated by nitric oxide synthase isoforms: neuronal, inducible and endothelial (nNOS, iNOS, eNOS, respectively) and the biosynthesis of H2S, mediated by cystathionine β-synthase, cystathionine γ-lyase and 3-mercaptopyruvate sulfotransferase (CBS, CSE, 3-MST, respectively). Further, the products resultant from the interaction between the two signaling molecules are shown.
NO has shown pro- and anti-inflammatory properties intrinsically associated with its concentration and application site [116]. With respect to H2S, it was firstly hypothesized that this gasotransmitter plays pro-inflammatory role; although it is well-known that in order to have functions similar to those of NO, it also acts as anti-inflammatory agent [32]. Two different articles have shown that the administration of the H2S donor inhibits NO overproduction and, simultaneously, enables the inhibition of pro-inflammatory mediators such as IL-1β, IL-6, TNF-α. This outcome indicates the possible positive effect on inflammation resulting from the crosstalk between H2S and NO [117,118]. Interestingly, opposite effects were also observed in different models. Investigations conducted with endothelial cells have evidenced that the exogenous administration of the H2S donor (NaHS) has directly increased NO levels due to eNOS stimulation; this process has potentiated proliferative effects on endothelial cells [120]. Still, acute H2S therapy has successfully restored NO concentration and eNOS functions in individuals with IR injury, which suggested cytoprotective signaling-dependent pathway in NO production stimulation [44].
Data about the interaction between gasotransmitters in renal injuries remain scarce in the literature. A decade ago, two different research groups have evidenced that the crosstalk between NO and H2S had the potential to mitigate renal damages induced by IR, mostly through iNOS activation by exogenous H2S administration [121,122]. These results have been supported by recent studies about exogenous H2S administration, whose promising effects on chronic kidney injury in animal models were evaluated, by taking into consideration the presence and absence of NOS inhibitor [123]. Overall, this treatment protected the animal models from kidney injuries, mostly due to improved antioxidant/oxidant balance, reduced apoptosis and autophagy and decreased number of genes associated with inflammation. Moreover, the authors of the aforementioned study suggested that NO plays an intrinsic role in H2S-induced renoprotection via the previously described patterns; they concluded that NOS isoforms can reduce kidney damage [123]. Although this pathway is not fully understood, these authors have also suggested that NOS isoforms can accelerate chronic kidney damage depending on their concentration and site; however, further studies should be conducted to help better understanding this mechanism. Similar pattern was also observed for renal injury model induced by gentamicin and treated with exogenous H2S donor, in the presence and absence of CO synthesis inhibitor [124]. The treatment was capable of reducing renal NO levels and iNOS isoform overexpression, as well as of preventing eNOS degradation and inducing its phosphorylation. Interestingly, this crosstalk was capable of minimizing toxic peroxynitrite formation and, at the same time, it promoted ideal NO release enough to enable blood vessel dilation [124]. Moreover, the authors have shown that effects of H2S were minimized in the presence of CO inhibitor, suggesting that all three gasotransmitters presented important and linked pathways in the biological system [124].
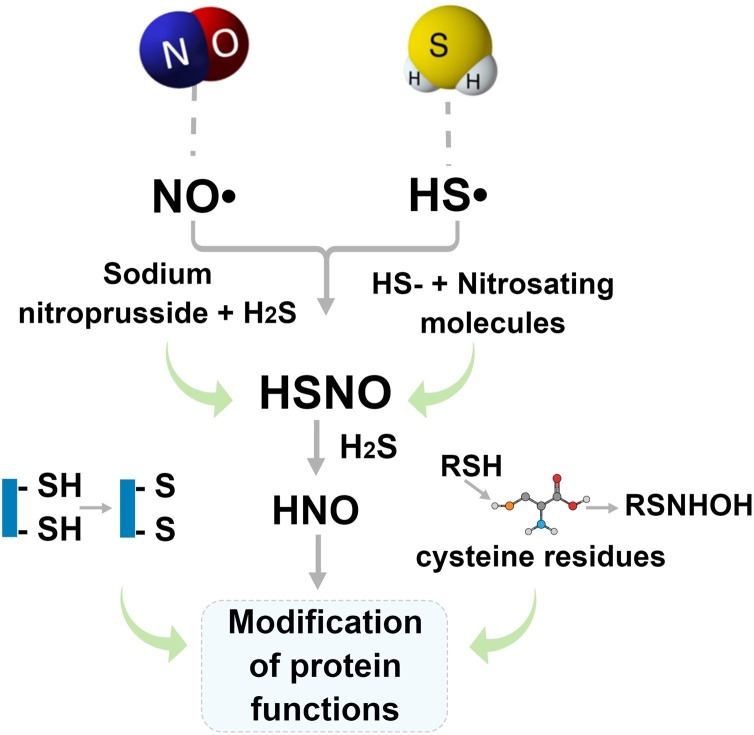
Little is known about the interaction mechanism among H2S, NO and CO in biological systems, under different conditions, sites and concentrations. H2S acts as reducing and nucleophilic agent under physiological conditions, which might react with several NO-derived species [122]. H2S can lead to chemical complexes with S-nitrosothiols, nitrite, nitrate and peroxinitrates [122]. In fact, some studies have proposed a chemical interaction between NO and H2S in biological system [[125], [126], [127]]. The interaction between H2S and nitrogenated species produces two different intermediates: thionitrous acid (HSNO) and nitroxyl (HNO), as demonstrated in Fig. 5 [125]. HNO is known to induces vasodilation, via multiple mechanisms, including via activation of cyclic guanilyl monophosphate (cGMP)-dependent pathway [35]. Moreover, HNO intermediate generated is responsible for modifying the functions of proteins, mostly by two different routes: (i) by forming disulfide bonds between thiol groups, or (ii) converting thiolated groups (RSH) in cysteine residues into N-hydroxysulfenamide (RSNHOH) [[127], [128], [129]]. This pathway has been mostly studied due to the interesting mechanisms regarding the intermediates HSNO and HNO in the cardiovascular system [127]. HNO is very reactive with thiol-containing biomolecules, forming sulfinamides or disulfides [126].
Fig. 5.
Schematic representation of the chemical crosstalk between NO and H2S. From the NO• and HS• the intermediates HSNO and HNO (nitroxyl) are formed, directly influencing in the protein functions by two distinct pathways: (i) induction of disulfide bonds and (ii) conversion of thiolated groups in cysteine residues to N-hydroxysulfenamide (RSNHOH).
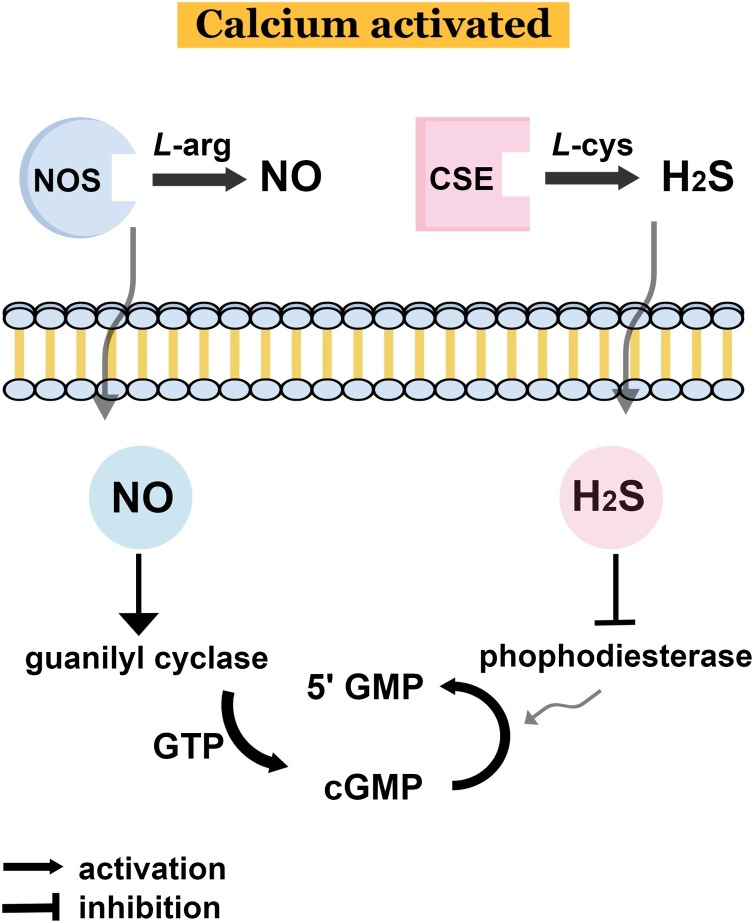
Considering the interaction previously described in Fig. 4, the crosstalk between L-arginine/NO and L-cysteine/H2S was overviewed in a schematic representation (Fig. 6 ). Through different possible mechanisms, such as H2S binding to Zn-containing enzymes and/or S-sulfhydration reactions, the phosphodiesterase (PDE) activity is inhibited by H2S [34]. This inhibition directly enhances the expression of cGMP and 5′GMP (5′ guanilyl monophosphate) [34]. Thus, H2S can modify some cysteine residues impairing PDE activity and enhancing cGMP/protein kinase G signaling [123]. Regarding NO, the activation of guanilyl cyclase increases the guanilyl triphosphate increasing the expression of cGMP and 5′GMP, evidencing the crosstalk between both species with direct consequences in hyperpolarization and relaxation [126]. Administration of exogenous H2S increased cGMP levels in time/concentration dependent manner. In the endothelium, H2S inhibits PDE enhancing cGMP accumulation [123]. Indeed, NO and H2S are both necessary to elicit vasodilation and angiogenesis by converging at cGMP. Increase in intracellular levels of cGMP, in a NO-dependent manner, was observed upon exposure endothelial cells to H2S, in addition to the activation of protein kinase G (PKG). In other words, both H2S and NO mutually control vascular function by enhancing intracellular levels of cGMP, which is essential for PKG, angiogenesis and dilation of blood vessels. Moreover, in vivo studies confirmed that NO- and H2S-induced vasorelaxation is cooperative on the cGMP pathway, and both gasotransmitters are required for angiogenesis [130].
Fig. 6.
Schematic representation of the endogenous crosstalk between L-arginine/NO and L-cysteine/H2S leading to an increased expression of cGMP and 5′GMP, with direct consequence in hyperpolarization and relaxation. 5′GMP: (5′ guanilyl monophosphate); cGMP: (cyclic guanilyl monophosphate); GTP: guanilyl triphosphate; NOS: nitric oxide synthase; CSE: cystathionine gamma lyase.
In a further study, rabbits subjected to IR and treated with NaHS presented increased levels of cardiac cGMP and reduction of infarct size [131]. These results reinforced that H2S donors have cardioprotective effects via cGMP/PKG pathway by activating NOS, which in turn generates NO. In fact, H2S facilitates NO-mediated cellular signaling events [132,133]. It is assumed that H2S stabilizes soluble guanylate cyclase (sGC) in its NO-responsive form. By inhibiting vascular cGMP phosphodiesterase, H2S can prolong the bioavailability of cGMP. Moreover, polysulfides (derived from H2S) active cGMP-dependent PKG [133]. Finally, NO and H2S interaction might also affect each other synthesizing enzymes affecting their generation [134]. For more detailed discussion and mechanisms regarding the convergence of NO and H2S in biological system, we recommend important review articles in this topic [25,95,133,134].
Although several progresses have been achieved pointing the crosstalk between H2S and NO, much work still need to be done.
Overall, the reports selected for the current review highlight the potential of these gasotransmitters and the importance of better understanding the roles played by them under different physiological and pathological conditions. It is clear that treatments based on the use of exogenous H2S or NO donors can have beneficial effects on individuals with renal injury; however, it is necessary conducting further studies to help better understanding this mechanism and enabling advances in this field. It should be noted that as several important works have demonstrated the convergence of the NO and H2S signaling pathways in cardio/renal diseases, administration of either NO or H2S donors, individually, might not be sufficient to restore vascular homeostasis. In this sense, the supplementation of both gasotransmitters might have a superior therapeutic effect in the treatment of several vascular dysfunctions. Moreover, the articles analyzed in this review did not evaluate the likelihood of a controlled and targeted release promoted by the association of H2S and NO with nanoparticles. Since this mechanism depends on the concentration of each gasotransmitter, it is expected that different release approaches can lead to different roles of the gasotransmitters, which may help improving their applications in renal injuries and other pathologies.
8. Conclusion and perspectives
Either H2S or NO plays a key role in AKI. Although relevant progress has been achieved in understanding the role played by H2S/NO in pathological conditions, as the ones presented in the current study, it is necessary conducting further investigations to help better understand their action mechanisms, as well as to clarify the association between these gasotransmitters under physiological and pathological conditions. The combination of H2S- and/or NO- donors with nanomaterials may enable designing new and versatile materials that can be translated into therapeutically useful approaches to treat several diseases such as renal failure. Finally, both H2S and NO can have positive effects on AKI, although further studies focused on underlying the molecular interaction mechanism of these gasotransmitters in renal failure should be conducted to enable developing new therapeutic tools to be associated with nanotechnology with significant benefits.
CRediT authorship contribution statement
Joana Claudio Pieretti: Conceptualization, Writing - review & editing. Carolina Victoria Cruz Junho: Conceptualization, Writing - review & editing. Marcela Sorelli Carneiro-Ramos: Supervision, Conceptualization, Writing - review & editing. Amedea Barozzi Seabra: Supervision, Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We have appreciated the support from FAPESP (2018/08194-2, 2018/02832-7, 2018/03089-6, 2018/03089-6, 2015/19107-5), CNPq (404815/2018-9, 313117/2019-5). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoalde Nível Superior - Brasil (CAPES) - Finance Code 001. We would like to thank GoodDeal for revising our manuscript text.
References
- 1.Ronco C., Bellomo R., Kellum J.A. Acute kidney injury. Lancet. 2019;394:1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 2.Mercado M.G., Smith D.K., Guard E.L. Acute kidney injury: diagnosis and management. Am. Fam. Physician. 2019;100:687–694. [PubMed] [Google Scholar]
- 3.Al-Jaghbeer M., Dealmeida D., Bilderback A., Ambrosino R., Kellum J.A. Clinical decision support for in-hospital AKI. J. Am. Soc. Nephrol. 2018;29:654–660. doi: 10.1681/ASN.2017070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckardt K.U., Kasiske B.L. Improving global outcomes. Nat. Rev. Nephrol. 2009;5:650–657. doi: 10.1038/nrneph.2009.153. [DOI] [PubMed] [Google Scholar]
- 5.Kelly K.J., Williams W.W., Colvin R.B., Meehan S.M., Springer T.A., Gutiérrez-Ramos J.C., Bonventre J.V. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siew E.D., Peterson J.F., Eden S.K., Hung A.M., Speroff T., Ikizler T.A., Matheny M.E. Outpatient nephrology referral rates after acute kidney injury. J. Am. Soc. Nephrol. 2012;23:305–312. doi: 10.1681/ASN.2011030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malek M., Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015;4:20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharfuddin A.A., Molitoris B.A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre J.V. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–1178. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 10.Yang L., Besschetnova T.Y., Brooks C.R., Shah J.V., Bonventre J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphreys B.D. Mechanisms of renal fibrosis. Annu. Rev. Physiol. 2018;80:309–326. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 12.Ronco C., Di Lullo L. Cardiorenal syndrome. Heart Fail. Clin. 2014;10:251–280. doi: 10.1016/j.hfc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Kumar U., Wettersten N., Garimella P.S. Cardiorenal syndrome: pathophysiology. Cardiol. Clin. 2019;37:251–265. doi: 10.1016/j.ccl.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Lullo L., Bellasi A., Barbera V., Russo D., Russo L., Di Iorio B., Cozzolino M., Ronco C. Pathophysiology of the cardio-renal syndromes types 1–5: an uptodate. Indian Heart J. 2017;69:255–265. doi: 10.1016/j.ihj.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yogasundaram H., Chappell M.C., Braam B., Oudit G.Y. Cardiorenal syndrome and heart failure—challenges and opportunities. Can. J. Cardiol. 2019;35:1208–1219. doi: 10.1016/j.cjca.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basile D.P., Anderson M.D., Sutton T.A. Compr. Physiol. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2012. Pathophysiology of acute kidney injury; pp. 1303–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Xu X., Shang R., Chen Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide - Biol. Chem. 2018;78:113–120. doi: 10.1016/j.niox.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie J., Rainone F., Green D., Alderson H., Chiu D., Middleton R., O’Donoghue D., Kalra P.A. Extreme elevations in blood pressure and all-cause mortality in a referred CKD population: results from the CRISIS study. Int. J. Hypertens. 2013;2013 doi: 10.1155/2013/597906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iimori S., Naito S., Noda Y., Nishida H., Kihira H., Yui N., Okado T., Sasaki S., Uchida S., Rai T. Anaemia management and mortality risk in newly visiting patients with chronic kidney disease in Japan: the CKD-ROUTE study. Nephrology. 2015;20:601–608. doi: 10.1111/nep.12493. [DOI] [PubMed] [Google Scholar]
- 20.Chawla L.S., Busse L., Brasha-Mitchell E., Davison D., Honiq J., Alotaibi Z., Seneff M.G. Intravenous angiotensin II for the treatment of high-output shock (ATHOS trial): a pilot study. Crit. Care. 2014;18:534. doi: 10.1186/s13054-014-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panico K., Abrahão M.V., Trentin-Sonoda M., Muzi-Filho H., Vieyra A., Carneiro-Ramos M.S. Cardiac inflammation after ischemia-reperfusion of the kidney: role of the sympathetic nervous system and the renin-angiotensin system. Cell. Physiol. Biochem. 2019;53:587–605. doi: 10.33594/000000159. [DOI] [PubMed] [Google Scholar]
- 22.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J. Am. Soc. Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.R., Jeong M.H., Lim S.Y., Hong S.N., Kim K.H., Sohn I.S., Hong Y.J., Park H.W., Kim J.H., Kim W., Ahn Y.K., Cho J.G., Park J.C., Kang J.C. The effect of alpha lipoic acid (Thioctacid HR®) on endothelial function in diabetic and hypertensive patients. Korean Circ. J. 2006;36:559–564. doi: 10.4070/kcj.2006.36.8.559. [DOI] [Google Scholar]
- 24.Bełtowski J. Hydrogen sulfide in pharmacology and medicine - an update. Pharmacol. Rep. 2015;67:647–658. doi: 10.1016/j.pharep.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Cirino G., Vellecco V., Bucci M. Nitric oxide and hydrogen sulfide: the gasotransmitter paradigm of the vascular system. Br. J. Pharmacol. 2017;174:4021–4031. doi: 10.1111/bph.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/jneurosci.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tekşen Y., Kadıoğlu E., Koçak C., Koçak H. Effect of hydrogen sulfide on kidney injury in rat model of crush syndrome. J. Surg. Res. 2019;235:470–478. doi: 10.1016/j.jss.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 28.Yu M., Sturgill-Short G., Ganapathy P., Tawfik A., Peachey N.S., Smith S.B. Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp. Eye Res. 2012;96:124–131. doi: 10.1016/j.exer.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruger W.D., Cox D.R. 1994. A Yeast System for Expression of Human Cystathionine f3-synthase: Structural and Functional Conservation of the Human and Yeast Genes (homcystlnuria/inherlted Metabolic dlsorder/Sacchromyces cerevisiae/CYS4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diwakar L., Ravindranath V. Inhibition of cystathionine-γ-lyase leads to loss of glutathione and aggravation of mitochondrial dysfunction mediated by excitatory amino acid in the CNS. Neurochem. Int. 2007;50:418–426. doi: 10.1016/j.neuint.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Cao X., Bian J.S. The role of hydrogen sulfide in renal system. Front. Pharmacol. 2016;7:385. doi: 10.3389/fphar.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallace J.L., Ferraz J.G.P., Muscara M.N. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxidants Redox Signal. 2012;17:58–67. doi: 10.1089/ars.2011.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteman M., Li L., Rose P., Tan C.H., Parkinson D.B., Moore P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxidants Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabõ C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 35.Yang G., Wu L., Wang R. Pro‐apoptotic effect of endogenous H 2 S on human aorta smooth muscle cells. FASEB J. 2006;20:553–555. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- 36.Tan Z., Shi Y., Yan Y., Liu W., Li G., Li R. Renal Failure Impact of endogenous hydrogen sulfide on toll-like receptor pathway in renal ischemia/reperfusion injury in rats Impact of endogenous hydrogen sulfide on toll-like receptor pathway in renal ischemia/reperfusion injury in rats. Ren. Fail. 2015;37:727–733. doi: 10.3109/0886022X.2015.1012983. [DOI] [PubMed] [Google Scholar]
- 37.Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life. 2005;57:603–606. doi: 10.1080/15216540500217875. [DOI] [PubMed] [Google Scholar]
- 38.Li L., Hsu A., Moore P.K. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation - a tale of three gases! Pharmacol. Ther. 2009;123:386–400. doi: 10.1016/j.pharmthera.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Mathai J.C., Missner A., Kügler P., Saparov S.M., Zeidel M.L., Lee J.K., Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16633–16638. doi: 10.1073/pnas.0902952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dugbartey G.J. The smell of renal protection against chronic kidney disease: hydrogen sulfide offers a potential stinky remedy. Pharmacol. Rep. 2018;70:196–205. doi: 10.1016/j.pharep.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Dugbartey G.J. H2S as a possible therapeutic alternative for the treatment of hypertensive kidney injury. Nitric Oxide - Biol. Chem. 2017;64:52–60. doi: 10.1016/j.niox.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Dugbartey G.J., Bouma H.R., Lobb I., Sener A. Hydrogen sulfide: a novel nephroprotectant against cisplatin-induced renal toxicity. Nitric Oxide - Biol. Chem. 2016;57:15–20. doi: 10.1016/j.niox.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Kolluru G.K., Yuan S., Shen X., Kevil C.G. Methods Enzymol. Academic Press Inc.; 2015. H2S regulation of nitric oxide metabolism; pp. 271–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King A.L., Polhemus D.J., Bhushan S., Otsuka H., Kondo K., Nicholson C.K., Bradley J.M., Islam K.N., Calvert J.W., Tao Y.X., Dugas T.R., Kelley E.E., Elrod J.W., Huang P.L., Wang R., Lefer D.J. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai W.K.C., Kan M.Y. Homocysteine-induced endothelial dysfunction. Ann. Nutr. Metab. 2015;67:1–12. doi: 10.1159/000437098. [DOI] [PubMed] [Google Scholar]
- 46.Upchurch G.R., Welche G.N., Fabian A.J., Freedman J.E., Johnson J.L., Keaney J.F., Loscalzo J. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J. Biol. Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 47.Eberhardt M., Dux M., Namer B., Miljkovic J., Cordasic N., Will C., Kichko T.I., De La Roche J., Fischer M., Suárez S.A., Bikiel D., Dorsch K., Leffler A., Babes A., Lampert A., Lennerz J.K., Jacobi J., Martí M.A., Doctorovich F., Högestätt E.D., Zygmunt P.M., Ivanovic-Burmazovic I., Messlinger K., Reeh P., Filipovic M.R. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014;5:1–17. doi: 10.1038/ncomms5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun H.J., Wu Z.Y., Cao L., Zhu M.Y., Liu T.T., Guo L., Lin Y., Nie X.W., Bian J.S. Hydrogen sulfide: recent progression and perspectives for the treatment of diabetic nephropathy. Molecules. 2019;24 doi: 10.3390/molecules24152857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szabó G., Veres G., Radovits T., Ger D., Módis K., Miesel-Gröschel C., Horkay F., Karck M., Szabó C. Nitric Oxide - Biol. Chem., Nitric Oxide. 2011. Cardioprotective effects of hydrogen sulfide; pp. 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozatik F.Y., Teksen Y., Kadioglu E., Ozatik O., Bayat Z. Effects of hydrogen sulfide on acetaminophen-induced acute renal toxicity in rats. Int. Urol. Nephrol. 2019;51:745–754. doi: 10.1007/s11255-018-2053-0. [DOI] [PubMed] [Google Scholar]
- 51.Kubo S., Doe I., Kurokawa Y., Nishikawa H., Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology. 2007;232:138–146. doi: 10.1016/j.tox.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 52.Lobb I., Davison M., Carter D., Liu W., Haig A., Gunaratnam L., Sener A. Hydrogen sulfide treatment mitigates renal allograft ischemia-reperfusion injury during cold storage and improves early transplant kidney function and survival following allogeneic renal transplantation. J. Urol. 2015;194:1806–1815. doi: 10.1016/j.juro.2015.07.096. [DOI] [PubMed] [Google Scholar]
- 53.Aminzadeh M.A., Vaziri N.D. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol. Dial. Transplant. 2012;27:498–504. doi: 10.1093/ndt/gfr560. [DOI] [PubMed] [Google Scholar]
- 54.Dugbartey G.J. Diabetic nephropathy: a potential savior with ‘rotten-egg’ smell. Pharmacol. Rep. 2017;69:331–339. doi: 10.1016/j.pharep.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Dugbartey G.J., Peppone L.J., de Graaf I.A.M. An integrative view of cisplatin-induced renal and cardiac toxicities: molecular mechanisms, current treatment challenges and potential protective measures. Toxicology. 2016;371:58–66. doi: 10.1016/j.tox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dugbartey G.J. The smell of renal protection against chronic kidney disease: hydrogen sulfide offers a potential stinky remedy. Pharmacol. Rep. 2018;70:196–205. doi: 10.1016/j.pharep.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y., Xing Q.Q., Tu J.K., Bin Tang W., Yuan X.N., Xie Y.Y., Wang W., Peng Z.Z., Huang L., Xu H., Qin J., Xiao X.C., Tao L.J., Yuan Q.J. Involvement of hydrogen sulfide in the progression of renal fibrosis. Chin. Med. J. (Engl). 2019;132:2872–2880. doi: 10.1097/CM9.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu D., Wang J., Li H., Xue M., Ji A., Li Y. 2015. Role of Hydrogen Sulfide in Ischemia-Reperfusion Injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng G., Wang J., Xiao Y., Bai W., Xie L., Shan L., Moore P.K., Ji Y. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J. Biomed. Res. 2015;29:203–213. doi: 10.7555/JBR.28.20140037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmad A., Olah G., Szczesny B., Wood M.E., Whiteman M., Szabo C. AP39, A mitochondrially targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury in vivo. Shock. 2016;45:88–97. doi: 10.1097/SHK.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatzianastasiou A., Bibli S.I., Andreadou I., Efentakis P., Kaludercic N., Wood M.E., Whiteman M., Di Lisa F., Daiber A., Manolopoulos V.G., Szabó C., Papapetropoulos D.A. Cardioprotection by H2S donors: nitric oxide-dependent and -Independent mechanisms. J. Pharmacol. Exp. Ther. 2016;358:431–440. doi: 10.1124/jpet.116.235119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan L., Jiaqiong L., Yue G., Xiaoyong L., Xuexian T., Ming L., Yinglan L., Xinxue L., Zena H. Atorvastatin protects against contrast-induced acute kidney injury via upregulation of endogenous hydrogen sulfide. Ren. Fail. 2020;42:270–281. doi: 10.1080/0886022X.2020.1740098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aslami H., Beurskens C.J.P., Beer F.Md., Kuipers M.T., Roelofs J.J.T.H., Hegeman M.A., Van der Sluijs K.F., Schultz M.J., Juffermans N.P. A short course of infusion of a hydrogen sulfide-donor attenuates endotoxemia induced organ injury via stimulation of anti-inflammatory pathways, with no additional protection from prolonged infusion. Cytokine. 2013;61:614–621. doi: 10.1016/j.cyto.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Carlstrom M., Montenegro M.F. Therapeutic value of stimulating the nitrate-nitrite-nitric oxide pathway to attenuate oxidative stress and restore nitric oxide bioavailability in cardiorenal disease. J. Intern. Med. 2019;285:2–18. doi: 10.1111/joim.12818. [DOI] [PubMed] [Google Scholar]
- 66.Zago A.S., Zanesco A. Nitric oxide, cardiovascular disease and physical exercise. Arq. Bras. Cardiol. 2006;87:e264–e270. doi: 10.1590/S0066-782X2006001900029. [DOI] [PubMed] [Google Scholar]
- 67.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 68.Aksu U., Demirci C., Ince C. Contrib. Nephrol. Karger Publishers; 2011. The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide; pp. 119–128. [DOI] [PubMed] [Google Scholar]
- 69.Lee J.U. Nitric oxide in the kidney: its physiological role and pathophysiological implications. Electrolyte Blood Press. 2008;6:27–34. doi: 10.5049/EBP.2008.6.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Higashi Y., Sasaki S., Kurisu S., Yoshimizu A., Sasaki N., Matsuura H., Kajiyama G., Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in Normotensive As well As hypertensive subjects. Circulation. 1999;100:1194–1202. doi: 10.1161/01.CIR.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 71.Node K., Kitakaze M., Yoshikawa H., Kosaka H., Hori M. Reduced plasma concentrations of nitrogen oxide in individuals with essential hypertension. Hypertension. 1997;30:405–408. doi: 10.1161/01.HYP.30.3.405. [DOI] [PubMed] [Google Scholar]
- 72.Huang P.L., Huang Z., Mashimo H., Bloch K.D., Moskowitz M.A., Bevan J.A., Fishman M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 73.Hsu C.-N., Tain Y.-L. Regulation of nitric oxide production in the developmental programming of hypertension and kidney disease. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20030681. 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen L., Liu P., Gao H., Sun B., Chao D., Wang F., Zhu Y., Hedenstierna G., Wang C.G. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin. Infect. Dis. 2004;39:1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gebistorf F., Karam O., Wetterslev J., Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst. Rev. 2016;6:1465–1858. doi: 10.1002/14651858.CD002787.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi E.K., Jung H., Kim K.J., Kang S.J., Kim H.J., Lim J.A., Kwak K.H., Lim D.G. Sodium nitrite attenuates hepatic Ischemia-Reperfusion injury in rats. Exp. Clin. Transplant. 2019;17:348–354. doi: 10.6002/ect.2018.0169. [DOI] [PubMed] [Google Scholar]
- 77.Zweier J.L., Wang P., Samouilov A., Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 78.Benjamin N., O’Driscoll F., Dougall H., Duncan C., Smith L., Golden M., McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 79.Liu M., Zollbrecht C., Peleli M., Lundberg J.O., Weitzberg E., Carlström M. Nitrite-mediated renal vasodilatation is increased during ischemic conditions via cGMP-independent signaling. Free Radic. Biol. Med. 2015;84:154–160. doi: 10.1016/j.freeradbiomed.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 80.Gladwin M.T., Raat N.J.H., Shiva S., Dezfulian C., Hogg N., Kim-Shapiro D.B., Patel R.P. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Hear. Circ Physiol. 2006;291:2026–2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 81.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X., Huang K.T., Shields H., Kim-Shapiro D.B., Schechter A.N., Cannon R.O., Gladwin M.T. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 82.Bryan N.S., Rassaf T., Maloney R.E., Rodriguez C.M., Saijo F., Rodriguez J.R., Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murata I., Miyake Y., Takahashi N., Suzuki R., Fujiwara T., Sato Y., Inoue Y., Kobayashi J., Kanamoto I. Low-dose sodium nitrite fluid resuscitation prevents lethality from crush syndrome by improving nitric oxide consumption and preventing myoglobin cytotoxicity in kidney in a rat model. SHOCK. 2017;48:112–118. doi: 10.1097/SHK.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 84.Zweier J.L., Wang P., Samouilov A., Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 85.Kemmner S., Lorenz G., Wobst J., Kessler T., Wen M., Günthner R., Stock K., Heemann U., Burkhardt K., Baumann M., Schmaderer C. Dietary nitrate load lowers blood pressure and renal resistive index in patients with chronic kidney disease: a pilot study. Nitric Oxide - Biol. Chem. 2017;64:7–15. doi: 10.1016/j.niox.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 86.Lundberg J.O., Gladwin M.T., Ahluwalia A., Benjamin N., Bryan N.S., Butler A., Cabrales P., Fago A., Feelisch M., Ford P.C., Freeman B.A., Frenneaux M., Friedman J., Kelm M., Kevil C.G., Kim-Shapiro D.B., Kozlov A.V., Lancaster J.R., Lefer D.J., McColl K., McCurry K., Patel R.P., Petersson J., Rassaf T., Reutov V.P., Richter-Addo G.B., Schechter A., Shiva S., Tsuchiya K., Van Faassen E.E., Webb A.J., Zuckerbraun B.S., Zweier J.L., Weitzberg E. Nat. Chem. Biol. Nature Publishing Group; 2009. Nitrate and nitrite in biology, nutrition and therapeutics; pp. 865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krishnan S.M., Kraehling J.R., Eitner F., Bénardeau A., Sandner P. The impact of the nitric oxide (no)/soluble guanylyl cyclase (sGC) signaling cascade on kidney health and disease: a preclinical perspective. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061712. 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chiazza F., Chegaev K., Rogazzo M., Cutrin J.C., Benetti E., Lazzarato L., Fruttero R., Collino M. A nitric oxide-donor furoxan moiety improves the efficacy of edaravone against early renal dysfunction and injury evoked by Ischemia/Reperfusion. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nilsson K.F., Sandin J., Gustafsson L.E., Frithiof R. The novel nitric oxide donor PDNO attenuates ovine ischemia-reperfusion induced renal failure. Intensive Care Med. Exp. 2017;5:1–13. doi: 10.1186/s40635-017-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seabra A.B. 1st edition. Academic Press; 2017. Nitric Oxide Donors : Novel Biomedical Applications and Perspectives; p. 364. [Google Scholar]
- 91.Stomberski C.T., Hess D.T., Stamler J.S., S-Nitrosylation Protein. Determinants of specificity and enzymatic regulation of S-Nitrosothiol-Based signaling. Antioxidants Redox Signal. 2019;30:1331–1351. doi: 10.1089/ars.2017.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carver J., Doctor A., Zaman K., Gaston B. S-nitrosothiol formation. Methods Enzymol. 2005;396:95–105. doi: 10.1016/S0076-6879(05)96010-2. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura T., Lipton S.A. Emerging role of protein-Protein transnitrosylation in cell signaling pathways. Antioxidants Redox Signal. 2013;18:239–249. doi: 10.1089/ars.2012.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perrin-Sarrado C., Zhou Y., Salgues V., Parent M., Giummelly P., Lartaud I., Gaucher C. S-Nitrosothiols as potential therapeutics to induce a mobilizable vascular store of nitric oxide to counteract endothelial dysfunction. Biochem. Pharmacol. 2020;173:113686. doi: 10.1016/j.bcp.2019.113686. [DOI] [PubMed] [Google Scholar]
- 95.Marozkina N., Gaston B. An update on thiol signaling: S-Nitrosothiols, hydrogen sulfide and a putative role for thionitrous acid. Antioxidants. 2020;9 doi: 10.3390/antiox9030225. 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nascimento M.H.M., Pelegrino M.T., Pieretti J.C., Seabra A.B. How can nitric oxide help osteogenesis? AIMS Mol. Sci. 2020;7:29–48. doi: 10.3934/molsci.2020003. [DOI] [Google Scholar]
- 97.Georgii J.L., Amadeu T.P., Seabra A.B., de Oliveira M.G., Monte-Alto-Costa A. Topical S-nitrosoglutathione-releasing hydrogel improves healing of rat ischaemic wounds. J. Tissue Eng. Regen. Med. 2011;5:612–619. doi: 10.1002/term.353. [DOI] [PubMed] [Google Scholar]
- 98.Cabral F.V., Pelegrino M.T., Sauter I.P., Seabra A.B., Cortez M., Ribeiro M.S. Nitric oxide-loaded chitosan nanoparticles as an innovative antileishmanial platform. Nitric Oxide - Biol. Chem. 2019;93:25–33. doi: 10.1016/j.niox.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 99.Urzedo A.L., Gonçalves M.C., Nascimento M.H.M., Lombello C.B., Nakazato G., Seabra A.B. Cytotoxicity and antibacterial activity of alginate hydrogel containing nitric oxide donor and silver nanoparticles for topical applications. ACS Biomater. Sci. Eng. 2020;6:2117–2134. doi: 10.1021/acsbiomaterials.9b01685. [DOI] [PubMed] [Google Scholar]
- 100.Samuvel D.J., Shunmugavel A., Singh A.K., Singh I., Khan M. S-Nitrosoglutathione ameliorates acute renal dysfunction in a rat model of lipopolysaccharide-induced sepsis. J. Pharm. Pharmacol. 2016;68:1310–1319. doi: 10.1111/jphp.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oshiro S., Ishima Y., Maeda H., Honda N., Bi J., Kinoshita R., Ikeda M., Iwao Y., Imafuku T., Nishida K., Miyamura S., Watanabe H., Otagiri M., Maruyama T. Dual therapeutic effects of an albumin-based nitric oxide donor on 2 experimental models of chronic kidney disease. J. Pharm. Sci. 2018;107:848–855. doi: 10.1016/j.xphs.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 102.Srisawat U., Kongrat S., Muanprasat C., Chatsudthipong V. Losartan and sodium nitroprusside effectively protect against renal impairments after ischemia and reperfusion in rats. Biol. Pharm. Bull. 2015;38:753–762. doi: 10.1248/bpb.b14-00860. [DOI] [PubMed] [Google Scholar]
- 103.Ri H.-S., Son H.J., Oh H.B., Kim S.-Y., Park J.Y., Kim J.Y., Choi Y.J. Inhaled nitric oxide therapy was not associated with postoperative acute kidney injury in patients undergoing lung transplantation. Bull. Sch. Med. Md. 2018;97:e10915. doi: 10.1097/MD.0000000000010915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zaorska E., Tomasova L., Koszelewski D., Ostaszewski R., Ufnal M. Hydrogen sulfide in pharmacotherapy, beyond the hydrogen sulfide-donors. Biomolecules. 2020;10:323. doi: 10.3390/biom10020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y., Pacheco A., Xian M. Handb. Exp. Pharmacol. Springer; New York LLC: 2015. Medicinal chemistry: insights into the development of novel H2S donors; pp. 365–388. [DOI] [PubMed] [Google Scholar]
- 106.Wang W., Jin Y., Xu Z., Liu X., Bajwa S.Z., Khan W.S., Yu H. Stimuli‐activatable nanomedicines for chemodynamic therapy of cancer. WIREs Nanomedicine and Nanobiotechnology. 2020;12 doi: 10.1002/wnan.1614. [DOI] [PubMed] [Google Scholar]
- 107.Urzedo A.L., Gonçalves M.C., Nascimento M.H.M., Lombello C.B., Nakazato G., Seabra A.B. Multifunctional alginate nanoparticles containing nitric oxide donor and silver nanoparticles for biomedical applications. Mater. Sci. Eng. C. 2020;112:110933. doi: 10.1016/j.msec.2020.110933. [DOI] [PubMed] [Google Scholar]
- 108.Pelegrino M.T., Weller R.B., Chen X., Bernardes J.S., Seabra A.B. Chitosan nanoparticles for nitric oxide delivery in human skin. Medchemcomm. 2017;8:713–719. doi: 10.1039/c6md00502k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alimoradi H., Barzegar-Fallah A., Sammut I.A., Greish K., Giles G.I. Encapsulation of tDodSNO generates a photoactivated nitric oxide releasing nanoparticle for localized control of vasodilation and vascular hyperpermeability. Free Radic. Biol. Med. 2019;130:297–305. doi: 10.1016/j.freeradbiomed.2018.10.433. [DOI] [PubMed] [Google Scholar]
- 110.Wang W., Liu H., Lu Y., Wang X., Zhang B., Cong S., Zhao Y., Ji M., Tao H., Wei L. Controlled-releasing hydrogen sulfide donor based on dual-modal iron oxide nanoparticles protects myocardial tissue from ischemia–reperfusion injury. Int. J. Nanomedicine. Volume. 2019;14:875–888. doi: 10.2147/IJN.S186225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun X., Wang W., Dai J., Jin S., Huang J., Guo C., Wang C., Pang L., Wang Y. A long-term and slow-releasing hydrogen sulfide donor protects against myocardial ischemia/reperfusion injury. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-03941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen W., Chen M., Zang Q., Wang L., Tang F., Han Y., Yang C., Deng L., Liu Y.N. NIR light controlled release of caged hydrogen sulfide based on upconversion nanoparticles. Chem. Commun. (Camb.) 2015;51:9193–9196. doi: 10.1039/c5cc02508g. [DOI] [PubMed] [Google Scholar]
- 113.Wu W., Chen M., Luo T., Fan Y., Zhang J., Zhang Y., Zhang Q., Sapin-Minet A., Gaucher C., Xia X. ROS and GSH-responsive S-nitrosoglutathione functionalized polymeric nanoparticles to overcome multidrug resistance in cancer. Acta Biomater. 2020;103:259–271. doi: 10.1016/j.actbio.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 114.Pieretti J.C., Pelegrino M.T., Nascimento M.H.M., Tortella G.R., Rubilar O., Seabra A.B. Small molecules for great solutions: Can nitric oxide-releasing nanomaterials overcome drug resistance in chemotherapy? Biochem. Pharmacol. 2019 doi: 10.1016/j.bcp.2019.113740. [DOI] [PubMed] [Google Scholar]
- 115.Pae H.O., Lee Y.C., Jo E.K., Chung H.T. Subtle interplay of endogenous bioactive gases (NO, CO and H2S) in inflammation. Arch. Pharm. Res. 2009;32:1155–1162. doi: 10.1007/s12272-009-1806-9. [DOI] [PubMed] [Google Scholar]
- 116.Lo Faro M.L., Fox B., Whatmore J.L., Winyard P.G., Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide - Biol. Chem. 2014;41:38–47. doi: 10.1016/j.niox.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 117.Whiteman M., Moore P.K. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J. Cell. Mol. Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hua W., Chen Q., Gong F., Xie C., Zhou S., Gao L. Cardioprotection of H2S by downregulating iNOS and upregulating HO-1 expression in mice with CVB3-induced myocarditis. Life Sci. 2013;93:949–954. doi: 10.1016/j.lfs.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 119.Ali M.Y., Ping C.Y., Mok Y.Y.P., Ling L., Whiteman M., Bhatia M., Moore P.K. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Altaany Z., Yang G., Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J. Cell. Mol. Med. 2013;17:879–888. doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu Z., Prathapasinghe G., Wu N., Hwang S.Y., Siow Y.L. Ischemia-reperfusion reduces cystathionine-β-synthase-mediated hydrogen sulfide generation in the kidney. Am. J. Physiol. - Ren. Physiol. 2009;297:27–35. doi: 10.1152/ajprenal.00096.2009. [DOI] [PubMed] [Google Scholar]
- 122.Tripatara P., Patel N.S.A., Collino M., Gallicchio M., Kieswich J., Castiglia S., Benetti E., Stewart K.N., Brown P.A., Yaqoob M.M., Fantozzi R., Thiemermann C. Generation of endogenous hydrogen sulfide by cystathionine γ-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab. Investig. 2008;88:1038–1048. doi: 10.1038/labinvest.2008.73. [DOI] [PubMed] [Google Scholar]
- 123.Shirazi M.K., Azarnezhad A., Abazari M.F., Poorebrahim M., Ghoraeian P., Sanadgol N., Bokharaie H., Heydari S., Abbasi A., Kabiri S., Aleagha M.N., Enderami S.E., Dashtaki A.S., Askari H. The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: involvement of oxidative stress, autophagy and apoptosis. J. Cell. Physiol. 2019;234:11411–11423. doi: 10.1002/jcp.27797. [DOI] [PubMed] [Google Scholar]
- 124.Aziz N.M., Elbassuoni E.A., Kamel M.Y., Ahmed S.M. Hydrogen sulfide renal protective effects: possible link between hydrogen sulfide and endogenous carbon monoxide in a rat model of renal injury. Cell Stress Chaperones. 2020;25:211–221. doi: 10.1007/s12192-019-01055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bruce King S. Potential biological chemistry of hydrogen sulfide (H2S) with the nitrogen oxides. Free Radic. Biol. Med. 2013;55:1–7. doi: 10.1016/j.freeradbiomed.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bucci M., Cirino G. Hydrogen sulphide in heart and systemic circulation. Inflamm. Allergy - Drug Targets. 2011;10:103–108. doi: 10.2174/187152811794776204. [DOI] [PubMed] [Google Scholar]
- 127.Nagpure B.V., Bian J.-S. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/6904327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kumars M.R., Fukuto J.M., Miranda K.M., Farmer P.J. Reactions of HNO with heme proteins: new routes to HNO-heme complexes and insight into physiological effects. Inorg. Chem. 2010;49:6283–6292. doi: 10.1021/ic902319d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Keceli G., Moore C.D., Labonte J.W., Toscano J.P. NMR detection and study of hydrolysis of HNO-derived sulfinamides. Biochemistry. 2013;52:7387–7396. doi: 10.1021/bi401110f. [DOI] [PubMed] [Google Scholar]
- 130.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Módis K., Panopoulos P., Asimakopoulou A., Gerö D., Sharina I., Martin E., Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bibli S.-I., Andreadou I., Chatzianastasiou A., Tzimas C., Sanoudou D., Kranias E., Brouckaert P., Coletta C., Szabo C., Th Kremastinos D., Iliodromitis E.K., Papapetropoulos A. Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc. Res. 2015;106:432–442. doi: 10.1093/cvr/cvv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Z., Martin E., Sharina I., Esposito I., Szabo C., Bucci M., Cirino G., Papapetropoulos A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol. Res. 2016;111:556–562. doi: 10.1016/j.phrs.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Szabo C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: mechanisms and implications. Am. J. Physiol. Physiol. 2017;312:C3–C15. doi: 10.1152/ajpcell.00282.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu D., Hu Q., Zhu D. An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/4579140. 4579140. [DOI] [PMC free article] [PubMed] [Google Scholar]