Abstract
Biologic therapeutics are the medicines of the future and are destined to transform the approaches by which the causes and symptoms of diseases are cured and alleviated. These approaches will be accelerated through the development of novel strategies that target multiple pharmacologically active sites using a combination of different biologics, or mixtures of biologics and small molecule therapeutics. However, for this potential to be realised, advancements in co-formulation strategies for biologic therapeutics must be established. This review describes the current and emerging developments within this field and highlights the challenges and potential solutions, that will pave-the-way towards their clinical translation.
Keywords: Biologics, Co-formulation, Combination therapy, Synergistic benefit, Protein, Nucleic acid, Vaccine
Graphical abstract

1. Current state-of-art
Biologics are macromolecular pharmaceuticals, such as peptides, enzymes, antibodies, antibody fragments, synthetic proteins, antibody drug conjugates, vaccines and nucleic acid-based gene therapies. They are manufactured through biological processes, rather than chemical synthesis, due to their structural complexity [1]. The functional macromolecular chemical architecture of biologics has the potential to afford greater pharmacodynamic specificity and therapeutic efficacy. These advantages far exceed their drawbacks, which include challenges associated with their manufacturing, medium to long-term biologic stability and delivery [2]. As a result, these advantages are accelerating the research and development efforts to produce new and improved biologics to cure disease, or to alleviate the symptoms of disease. In 2016, ~50% of new molecular entities approved by the FDA were biologics, while there were more than 800 macromolecular entities under development. Furthermore, the biologics market is anticipated to grow by 9.5% per annum (doubling approximately every 7 years), having been valued at $254.9Bn in 2017 and is expected to reach $580.5 Bn by 2026 [3].
1.1. Biologic challenges
The structural complexity of biologics provides downstream challenges in the formulation of these therapeutics. The challenges are predominantly associated with stability and delivery, as well as their characterization for changes in structural integrity [4]. Biologics are typically polymers of repeating monomer units e.g. amino-acids, monosaccharides & nucleosides, that are assembled into proteins, carbohydrates & nucleic acids. Their inherent complexity, and their existence as highly heterogeneous forms of closely related forms even upon purification, confirms the challenges associated with their characterization and differentiation between stable and unstable therapeutics.
Biologics are susceptible to various forms of degradation, including aggregation, deamidation, isomerization, hydrolysis, oxidation and denaturation [5]. This is due to their delicate chemistry which can be irreversibly damaged when subjected to subtle changes in temperature, pH, ionic strength, as well as mechanical and chemical stimuli [6]. From a patient perspective, incorrectly manufactured biologics could lead to a loss in therapeutic efficacy and unwanted side effects. From the perspective of medicine development, this also leads to an increased cost per dose. This could be the result of an increase in dosage concentration required, or through additional downstream processing required to maximise product purity and quality. Therefore, the manufacture, storage and administration of biologics are all carefully controlled and regulated, to maximise therapeutic efficacy, safety, and commercial viability.
Conventional control methods for limiting unwanted degradation of biologics during transport and storage include the use of: 1) cold chain storage and transport; 2) formulation with biologically and physiologically compatible buffers and excipients; and 3) freeze- and spray-drying to produce solid formulations of biologics [7]. More recently, sophisticated formulation approaches have been developed that enable biologics to tolerate greater physicochemical extremes, whilst maintaining or improving their therapeutic efficacy. Examples of such technologies include innovative microparticles and [8] nanoparticles [9], hydrogels [10] and 3D printing [11] that permit controlled and targeted delivery.
In general, most biologics are delivered via injection because large molecules are challenging to transport across skin, mucosa & cell membranes. Biologics are also rapidly hydrolysed if delivered into the GI tract [12]. Many vaccines [13] and antibodies [14] are delivered by intravenous infusion, requiring administration and monitoring within a clinic or hospital setting, which increases the associated costs of delivery incurred by healthcare systems. Similarly, intra-muscular injections also require administration by a skilled person. Subcutaneous injections require relatively lower skill for administration and can in many cases even be self-administered by patients. For these reasons, this has become a common and preferred method of delivery [15].
Although delivery by injection is the most convenient method for biologic delivery it is not always ideal. This is primarily because of the pharmacokinetics of biologics, which means they are rapidly cleared from systemic circulation [16]. Regulatory guidelines also limit the volume, and so subcutaneous injections require the biologic to be at a high concentration so that they can be delivered in sufficiently low volume via pre-filled syringe. This introduces new challenges associated with greater susceptibility to aggregation, and increased viscosity of injectables [17]. As a result, multiple doses of biologic injections may often be required, to achieve a concentration within the therapeutic window that produces maximum therapeutic benefit.
To overcome these challenges, enhanced formulations are being prepared that improve the methods by which biologics are delivered whilst augmenting their bioavailability [18]. For example, controlled sub-cutaneous infusion devices have been developed recently to enable larger volume dosage forms, although this may not be amenable to self-administration [19]. Furthermore, 3D printing, a future manufacturing technology that is leading innovations for the pharmaceutical industry, has already been implemented to produce diagnostics [20,21], combination therapeutics for oral delivery [22] as well as implants [23]. These technologies could provide sustained release of therapeutics and be readily translated to co-formulating biologics to provide local target therapy or sustained systemic action [24].
1.2. Landscape of co-formulation
Co-formulation of therapeutics is an emerging strategy that aims to capitalise on advances in therapeutic effects observed through the co-administration of biologics [25]. It is important to first highlight that co-formulation is ambiguous terminology, and is often interchanged with co-administration, such that it can mean many different things from one individual to another. However, we have identified that the term combination therapy can be used to associate the main concepts of both co-formulation and co-administration, where the differences are highlighted by how and when the pharmaceuticals are delivered. Therefore, to clarify the terminology used in the field, a definitions map has for combination therapy has been produced. The landscape of definitions diagram for combination therapy (Fig. 1 ) demonstrates how terminology can be clearly differentiated, to classify the breadth of medicinal products that are administered together. This includes sequential administration, co-administration, combination products, and co-formulation, which are described in detail in this section.
Fig. 1.
The landscape of definitions diagram for combination therapy. The terminology clusters defined here can be used to group a variety of medicinal products that are administered together. This includes sequential administration, co-administration, combination products, co-formulation and medicinal devices.
Co-administered therapeutics are delivered at the same time, whereas sequentially administered therapeutics are dosed at intervals. Sequential administration is generally implemented to enhance therapeutic efficacy, the prevention of pharmacokinetic and pharmacodynamic drug-drug interactions [26] or where a first line therapy is supported by a second line therapy to enhance clinical outcomes [27]. Pharmacokinetic drug-drug interactions can be described when a therapeutic alters the absorption, distribution, metabolism and excretion of another therapeutic. Whereas pharmacodynamic drug interactions can be described as when therapeutics alter the way another therapeutic is able to directly exert its activity at target site or induce biological changes that indirectly modify its activity. Where there are no adverse pharmacokinetic or pharmacodynamic drug-drug interactions, rather a synergistic benefit, co-administration strategies are implemented.
Co-administration envelops several delivery strategies that vary in clinical, technical and regulatory challenge, and range from co-administration alone, to combination products and co-formulation. Co-administration alone can be described as the simultaneous delivery of two or more existing formulations. For example, this could include delivery of two or more of the same dosage forms (e.g. tablet and capsules) or a mixture of multiple formulation types (e.g. solid dosage forms, suspensions and injections). This is the most common form of combination therapy and is implemented widely as polypharmacy for individuals with co-morbidities or when individuals have an advanced stage disease that requires more than one therapeutic for effective disease management. The main challenge here is optimising clinical efficacy, as the technical and regulatory challenges having already been established for the individual drug therapeutic approval processes.
Combination products are technically challenging to produce and are engineered to simultaneously deliver two different therapeutics, that are typically of the same formulation type. Examples of innovations that best describe combination products include multi-barrel syringes [28] or infusion bags that dispense therapeutics via a single IV line. The advantages of this approach are not always clear, as therapeutics in existing formulations could be co-administered, which could overcome the dedication of resources to engineering technologies for a combination product. However, should the technology reduce adverse effects of combination therapy, whilst enhancing patient compliance and concordance and extending commercial rights for a product, there may be an incentive to consider this approach.
Co-formulation can be considered as the purist view of a combination therapy and can be described as consisting of more than one drug substance in a single formulation, with the intention of delivering multiple therapeutic agents at the same time for maximum therapeutic benefit. These products should be subject to the same clinical pharmacology studies for each of the individual new investigational drugs in the formulation as would be performed if the drugs were being developed independently [29]. This includes assessment of safety, bioavailability, characterization of pharmacokinetics, and as a result are the most challenging to produce and face many obstacles to obtain a product license in terms of demonstrable clinical efficacy, technological development and regulatory barriers. This is because a co-formulated product must not significantly impede clinical efficacy, such as pharmacokinetic and pharmacodynamic performance, or adversely affect product shelf life, whilst also demonstrating marked improvement over co-administered therapeutics. It is important to note from our definitions a biologic coformulation must consist of 2 or more drug substances, rather than a drug substance and another macromolecular entity such as an inactive protein, such as albumin. Albumin is classified as a pharmaceutical excipient and been implemented as a useful model to study co-formulation and advanced formulation strategies. [30,31]
1.3. Rationale for co-formulation
Co-formulated therapies are thought to effectively enhance clinical benefit, as well as extend intellectual property rights for pharmaceutical companies, to ensure long-term return on investment (Table 1 ). The drive to co-formulate therapeutics has been predominantly conducted for small molecule drugs (< 500 MW) to control the symptoms of chronic respiratory, cardiovascular, neurological and infectious diseases. A few co-formulated biologic-based products that have been recently approved or are under clinical development suggests an increasing interest in industry to combine biologic therapeutic agents into a single dosage form (Table 2 ). The application of these products include, but are not limited to, diabetes, where the drug substances are short- and long-acting insulin, [32] solid tumours, where 2–6 antibodies targeting same or different antigens were mixed [33,34], and blood diseases, where 25 antibodies were mixed for the treatment of primary immune thrombocytopenia [35]. Therapies have also been developed that use a combination of small molecules and macromolecular biologics [36] for the treatment of complex infectious diseases [36,37]. An example of a new biologic co-formualtion is HyQvia (immune globulin infusion 10% (human) with recombinant human hyaluronidase), a once-monthly treatment for adult patients with primary immunodeficiency. [38]
Table 1.
Benefits, prerequisites and risks in the development of co-formulated pharmaceuticals
| Benefit | Prerequisite | Risk |
|---|---|---|
| Single instead of multiple drug product delivered to the patient, increasing compliance while reducing medical error | Proven synergistic medical benefit and safety from combinational clinical trials | Possible reduction in dosage flexibility Number of patients that can accept combination therapy could show population and disease variance |
| Simplified CMC and logistics | Integration in process development Stability of each DS does not change significantly in the mixture Analytics to characterise the degradation of individual drug component in the mixture |
More complex control strategy Availability of analytics Change in delivery route if co-formulated products have lower concentration tolerance (e.g. from SC to IV) Additional clinical trials are needed to prove the safety of co-formulated products |
| New market/product differential strategy | Potential IP extension of existing drugs | New regulation landscape needs to be introduced (potential delay in BLA approval) |
DS: drug substance, CMC: Chemistry Manufacturing & Control, PK: pharmacokinetics, PD: pharmacodynamics, SC: subcutaneous, IV: intravenous, BLA: biologics license application.
Table 2.
Selection of approved and clinical studies of co-formulated biologics.
| Pharmaceutic | Product and formulation detail | Indication/clinical stage |
|---|---|---|
| Insulin | Humalog® Mix75/25™ Humalog® Mix50/50™ Eli Lilly 1). Pre-mixed form of intermediate-acting insulin (insulin lispro protamine) and rapid-acting (insulin lispro) in 75:25 (Humalog® Mix75/25™) and 50:50 ratio (Humalog® Mix50/50™) 2). Pre-mixed form of intermediate-acting insulin isophane (HumulinN) and rapid-acting regular human insulin (HumulinR) in 70:30 ratio (Humulin® 70/30) Each product contains a total of 100 units of rapid- and intermediate-acting insulin per mL, which is filled in 3 or 10 mL multi-dose vial, or 3 mL single-patient-use prefilled pen (KwikPen®) for subcutaneous injection |
Diabetes, approved |
| Insulin + GLP-1 | SQLIQUA® 100/33 Sanofi Pre-mixed form of long-acting insulin analogue (insulin glargine) with GLP-1 receptor agonist (lixisenatide) The product is formulated with 100 units per mL of insulin glargine and 33 μg per mL lixisenatide in a 3 mL single-patient-use prefilled pen for subcutaneous injection |
Diabetes, approved |
| XULTOPHY® 100/3.6 Novo Nordisk Pre-mixed form of long-acting insulin analog (insulin degludec) with glucagon-like peptide 1 (GLP-1) receptor agonist (liraglutide) The product is formulated with 100 units per mL of insulin degludec and 3.6 mg per mL liraglutide in a 3 mL single-patient-use prefilled pen for subcutaneous injection |
Diabetes, approved | |
| Antibody | Sym004 (Symphogen) 1:1 mixture of antibody candidate 992 and 1024 from mSymplex EGFR-specific antibody repertoire |
Epithelial cancers, such as non-small-cell lung, head-and-neck, and brain cancers (Phase 2b completed) Glioblastoma (Phase 3) |
| Sym013 (Symphogen) Six-antibody mixture: anti-EGFR antibodies 1277 and 1565; anti-HER2 antibodies 4384 and 4517; anti-HER3 antibodies 5038 and 5082 from Symplex antibody repertoires |
A broad range of solid tumour indications with high medical needs globally including breast, lung, colorectal, pancreatic and gastric cancer (Phase 1) | |
| Sym015 (Symphogen) 1:1 mixture of two MET-targeting mAb Hu9006 and Hu 9338 from Symplex antibody repertoires |
Solid tumours showing alterations and/or amplification of the MET proto-oncogene including certain lung cancers and colorectal cancer (Phase 2a) |
|
| Sym029 (Symphogen) Mixture of anti-FLT3, anti-AXL and anti-CD40 antibodies for the mobilization and activation of dendritic cells |
Cancer (Discovery/pre-clinical stage) |
|
| Durvalumab + tremelimumab Astra Zenecca Mixing of durvalumab (anti-PD-L1, Imfinzi®) and tremelimumab (anti-CTLA-4). Durvalumab and tremelimumab were formulated into individually stable formulations and mixed to achieve a design space of new liquid and lyophilized co-formulations at various concentration ratios. [US Patent: 20170306025A1] |
Solid tumours (Phase1) Gastric cancer (Phase 2) Biliary tract, oesophageal (Phase 2) 1st-line bladder cancer (Phase 3) 2nd-line head and neck squamous cell carcinoma (Phase 3) 1st-line hepatocellular carcinoma (Phase 3) |
|
| Nivolumab + ipilimumab Bristol-Myers Squibb 1:1 mMixing of nivolumab or pembrolizumab (anti-PD-1) to a second antibody (anti-CTLA4) in about 50:1, 40:1, 30:1, 20:1 10:1, 5:1, 3:1, 1:1, 1:3, 1:5, 1:10, 1:20, 1:30, 1:40 or 1:50 [US Patent: 20160304607A1] |
Melanoma (approved) | |
| Rituximab (Rituxan®) + Hyaluronidase fixed-dose combination Roche Subcutaneous injection |
Hematologic cancers (approved) | |
| Pertuzumab (Perjeta®) + trastuzumab (Herceptin®) + Hyaluronidase fixed-dose combination Roche Subcutaneous injection |
HER-2 positive breast cancer (approved) |
|
| REGN-COV2 (Regeneron) Dual human IgG1 cocktail formulation |
SARS-CoV-2 virus Phase 3 |
|
| REGN-EB3 (Regeneron) Triple human IgG1 cocktail formulation |
Ebola virus Under Priority Review for BLA |
|
| Cytokines | IRX Therapeutics, pipeline IRX-2 A cell-free mixture comprised of a variety of naturally-derived cytokines, including IL-1, −2, −6, −8, −10, −12, TNF-α, IFN-γ and CSFs) obtained from normal, unrelated donor lymphocytes with potential immune-stimulatory activity |
Squamous cell carcinoma of the head and neck (Phase 2b) Breast cancer (Phase 2b) |
Combination products containing biologics and small molecules, such as antibody + chemotherapy drugs, have not been included.
1.4. Clinical benefit
The clinical benefit of co-formulated biologics is derived from enhancements in therapeutic efficacy, or improved patient compliance and concordance. Therapeutic benefit can be enhanced by targeting multiple pharmacologically active sites synergistically to cure or alleviate the acute and chronic symptoms of disease.
This methodology has been applied to improve disease management for respiratory diseases, such as asthma and chronic obstructive pulmonary diseases (emphysema and chronic bronchitis), using small molecule-based APIs. Here, pharmacological therapy is introduced via inhalers to directly prevent acute symptoms, to improve peak respiratory flow, followed by long-term control, to prevent subsequent exacerbations. Combination therapy achieves this by combining overcoming the short falls of short-acting bronchodilators (e.g. salbutamol, terbutaline) with longer acting bronchodilators and corticosteroids. Long-acting bronchodilators are typically β2-receptor agonists (formoterol and salmeterol), which relax smooth muscle and dilate respiratory passages to improve airflow. Whereas long-term control is achieved through inhalation of corticosteroids (e.g. beclomethasone, budesonide, fluticasone), to improve respiratory airflow by supressing inflammation, oedema and secretion of obstruction mucus. Preparations that combine long-acting bronchodilators and corticosteroids include Salmeterol (salmeterol and fluticasone), Fostair (beclomethasone and formoterol), and Symbicort (formoterol and budesonide).
1.5. Synergistic benefit 6Cs
Synergistic benefit of co-formulation is enhanced through the 6Cs - Combination, Compliance, Concordance, Convenience, Carriage and Cure-all.
Therapeutic efficacy can be maximised using complementary drug substances, where one drug substance improves the performance of another. An excellent example of combination therapy that implements this strategy is the antibiotic co-amoxiclav (Augmentin), which combines two small molecule APIs amoxicillin and clavulanic acid. [39] Amoxicillin is a semi-synthetic derivative of penicillin that exerts its pharmacological effect by preventing peptidoglycans from cross-linking effectively during the latter stages of bacterial cell-wall synthesis. However, bacteria have developed strategies to inactivate penicillin-based antibiotics by enzymatically degrading the β-lactam ring found in its chemical structure, through the secretion of β-lactamase enzymes. Therefore, to enhance therapeutic efficacy of penicillin-based antibiotics, the clavulanic acid in co-amoxiclav inhibits the action of β-lactamases, to ‘augment’ the efficacy of amoxicillin.
In addition to these enhancements in therapeutic benefit and efficacy, patient care is also enriched through improvements in compliance and concordance. Compliance is improved through the elimination of complex therapeutic dosage regimens when more than one therapeutic is taken at the same time or at different times of the day. Furthermore, concordance is improved through effective transfer of information from the health care professional to the patient. For example, the healthcare professional only has to demonstrate the use of one inhaler, rather than two or more. Therefore, co-formulation is convenient for patient and healthcare provider alike. This convenience may also be true for the producer who only has to produce a single therapy, which in the long-term may be more economical in terms of manufacturing packing and carriage. It is important to mention carriage here to demonstrate the changes in global practices that are required to overcome accelerated climate change. Combined international efforts are required to slow and reverse increases in global temperatures, where co-formulated therapies can play a role.
Cure all, or panacea treatments, such as polyvalent vaccines are established examples of biologic co-formulations. Polyvalent vaccines prevent diseases with multiple serotypes, through the mixture of serotype-specific immunogens, or by discovery and use of an immunogen that is conserved among serotypes. For example, the poliovirus vaccine, which contains three poliovirus serotypes, was first used as a polyvalent vaccine for reducing poliomyelitis with a success >99% [40]. However, polyvalent vaccines bring many challenges, [41] which include confirmation of physical, chemical, and immunological responses, as well as stability, for simultaneous administration of multiple antigens, in comparison to the individual antigens separately [42]. In response to the coronavirus pandemic research and development of polyvalent vaccine development has accelerated. This potentially could include co-formulation of spike for proteins for severe acute respiratory syndrome coronavirus 1 & 2 (SARS-CoV, (2003) & SARS-CoV-2, (2019), respectively) [43], as well as other promising platforms which include mRNA, DNA, non-replicating vectors, replicating vector and virus-like particles. [44] The knowledge established through the development of polyvalent vaccines, will form the basis for the future of co-formulated biologics.
1.6. Clinical & regulatory challenges
As co-formulated therapeutics are classified as new pharmaceutical entities, the clinical evaluation of the therapeutic benefit becomes inherently more complex. This is because additional safety concerns could be raised for co-formulating existing pharmaceuticals, even though the efficacy and safety have already been established through independent clinical trials. Therefore, additional clinical trials will be required to provide further safety and efficacy characterization of the co-formulated product. The clinical trial of the combination is thus designed based on the previous safety and efficacy data of the individual component and the dosage of the components tends to be fixed. A Phase III, two-arm, multicentre and randomized study is likely to be conducted to establish the pharmacokinetics, efficacy and safety of the co-formulated product, which was observed in a recently approved co-formulated product [45].
The co-formulation of two new co-developed drugs, however, will be challenging when compared to the mixing of two existing drugs. The Food and Drug Administration (FDA) has published guidance for the co-development of two investigational new drugs, that highlights the difficulty in obtaining clinical safety and efficacy data for the co-developed drugs, in comparison to data that would be obtained if they were developed independently. Therefore, the FDA will request for pre-clinical evidence that demonstrates the superiority of the co-formualtion over the individual components, and the reasons why each component cannot be developed independently [29].
1.7. Technical challenges in therapeutic co-formulation of biologics
The development of co-formulated products has led to innovations in both their formulation and analysis, given the additional complexity, while many challenges remain.
Non-native interactions, and the formation of heterogeneous aggregates, could be major obstacles when attempting to co-formulate two or multiple biologics for long term storage. In the formulation development of single-agent products, proteins are stabilised at the buffer and excipient concentrations that prevent undesired interactions between proteins. However, to mix different proteins in solution, a compromise formulation may be produced, such that each mixing component could be relatively destabilised compared to their independent formulations. This is especially true in formulations where proteins in the mixture have very different preferences for pH, excipient type and ionic strength. Moreover, it is challenging to predict whether a protein in each mixture will demonstrate the same stability and degradation kinetics compared to its monotherapy formulation.
These challenges are further complicated by considering the interaction between the protein and the excipients, as well as the change in viscosity at the higher combined total protein concentration. For example, if an anti-CTLA-4 antibody is formulated at 4.62 mg/mL and mixed with an anti-PD-L1 antibody of 1.54 mg/mL, the total protein concentration in solution for this anti-PD-1 antibody becomes 4.5 times higher [46]. Subcutaneous (SC) injection of biologics uses highly concentrated agents compared to intravenous (IV) infusion for administration. As a result, the concentration of protein agents in SC monotherapy formulation could increase from 10 mg/mL up to 150 mg/mL [47]. This concentration could double in a combination therapy when drug substances are mixed in a 1:1 ratio, such that the total protein concentration would need to be 300 mg/mL in the solution. It is important to note the overall concentration could be further increased if combined in 1:2 and 1:3 ratios.
This approach may significantly change the colloidal stability and protein-protein interactions of antibodies in solution that drives the mixture to heterogeneous aggregation. Moreover, the mixing ratio of the components may need to be varied to fulfil the specific dosage requirements of different patient groups. This further complicates the stability prediction of the product of different mixing profiles. Other factors, such as freeze-dry processing, agitation during transportation and temperature change in storage, will also potentially change the shelf-life of the co-formulated products relative to their respective monotherapies. These challenges also increase the burden on the analytics used to assess product degradation for the quality control of products.
For single-agent biologics, the stability, viscosity, aggregation, and fragmentation can be assessed using an established analytical toolkit. Aggregation, for example, can be analysed using a combination of size-exclusion chromatography (SEC) or ultraperformance size-exclusion chromatography (UPSEC), and light scattering methods such as static and dynamic light scattering (SLS & DLS), to assess the monomer retention and the development of aggregation species in different formulations [[48], [49], [50], [51], [52], [53], [54], [55]]. Similarly, the viscosity is easily assessed using rheometers, or flow rates through syringe needles at constant pressure [56,57]. The thermal stability of the product under different pH can be characterised using differential scanning calorimetry (DSC) or fluorimetry (DSF) [[58], [59], [60], [61]]. Moreover, any structural alteration of the biologics in different formulations and their ligand binding can be quantitatively assessed by circular dichroism (CD) [62,63].
The quality assessment and control for co-formulated biologics raises additional challenges compared to single-agent products, as the analytics must accurately characterise multiple biologics in one solution. Therefore, it would also require additional sensitivity or resolution to deconvolute and distinguish the signal from each component, as well as their respective degradation products.
Current analytical tools demonstrate advantages and limitations when characterising the biological activity, molecular interaction, stability, aggregation and viscosity of proteins in co-formulations (Fig. 2 ). For example, SEC can characterise the respective monomer loss in the solution if the mixed proteins are not co-eluted. SEC with multi-angle light scattering (SEC-MALS) could further characterise the size of the oligomer species in the solution [64,65]. This is valuable information, especially for monitoring the development of early-forming aggregates. However, it is important to note SEC-MALS may not readily provide information on the identity of the aggregates. The idenitity of aggregates is beneficial to understand the formulation development and may provide insight to whether aggregates are are formed selectively from only one of the biologics, or through the co-aggregation of multiple biologic species in the solution. The degradation of antibodies results in the fragmentation of light chain and heavy chain domains, which could co-elute in SEC. Furthermore, if the combined proteins have similar hydrodynamic radii, then the SEC method would be unable to separate them and therefore would only measure the total monomer retention.
Fig. 2.
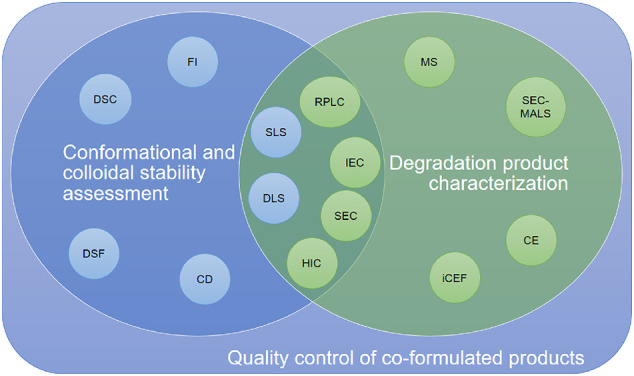
Venn Diagram highlighting the current analytical tools that can be applied to assess the quality control of co-formulated products. The analytics for either stability assessment or degradation product characterization are listed in blue or green circles, respectively. Analytical tools that can be applied to characterise both stability or degradation are presented in the overlapped zone. Abbreviations - DSC: differential scanning calorimetry; DSF: differential scanning fluorimetry; FI: fluorescence intensity; CD: circular dichroism; SLS: static light scattering; DLS: dynamic light scattering; RPLC: reverse-phase liquid chromatography; IEC: ion-exchange chromatography; SEC: size-exclusion chromatography; HIC: hydrophobic interaction chromatography; MS: mass spectrometry; SEC-MALS: size-exclusion chromatography multiple angle light scattering; CE: capillary electrophoresis; iCEF: imaged capillary isoelectric focusing. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Additional chromatographic analytics, such as ion-exchange chromatography (IEC), reverse-phase liquid chromatography (RPLC) and hydrophobic interaction chromatography (HIC), could then be explored in tandem, to separate the species that are co-eluted in SEC. RPLC has been widely used for the separation of antibodies and their fragments. Although RPLC exhibits limited selectivity for closely related proteins when compared to IEC, it has the advantage of producing sharp and highly resolved separation peaks. This can be attributed to the fast-kinetic interactions in the reversed-phase mode. Therefore, RPLC could afford enhanced separations in antibodies mixtures. Similarly, HIC provides comparable separation to RPLC but is performed under non-denaturing conditions. It has been previously applied as a control strategy for bispecific antibodies and antibody-drug conjugates (ADC) [66]. However, as RPLC denatures the protein sample, it can measure the degree of fragmentation whereas HIC will not separate the fragmented heavy or light chains from the whole antibody. Therefore, HIC is less sensitive to map the entire degradation landscape of the proteins in mixture.
Capillary electrophoresis (CE) and imaged capillary isoelectric focusing (iCIEF), separate the mixed biologics based on their charge heterogeneity [67,68]. These methods can be used along with chromatography to map out the degradation species in solution. A recent study of a mixture of two antibodies tested the feasibility and limitations, of combining HPLC and CE in the analysis of co-formulated products [67]. It was previously suggested that mass spectrometry (MS) could be coupled to CE to characterise complex mixtures of antibodies [69]. This is because MS is capable of detecting weakly associated heterogeneous dimers in co-formulations. MS also adds sensitivity to characterise the molecular identity of different degradation products. However, none of these separation methods guarantees a non-overlapping separation of the co-formulated biologics and their degradation products. Whether these analytics fulfil the quality control requirement would be case specific. Therefore, it may be more promising to apply 2D chromatography which performs two separation methods (such as size-based and charge-based) in tandem to characterise the co-formulated system.
Labelling of proteins with fluorescent dyes provides an extremely sensitive insight into the dynamics and inter-molecular interactions of proteins in solution. Proteins are usually labelled on primary amines, disulphide linkages and glycosylation sites. Non-natural amino acid residues can also be introduced into the sequence to provide intrinsic fluorescence at desired sites. However, these labels could potentially change the behaviour of the protein, which is undesired for control strategy. Labelling on primary amines is difficult to control the sites being labelled. It is also postulated that the charge density of labelled proteins can be unfavourably altered. Maleimide chemistry could cross-link the disulphide bond in antibodies to introduce a chemical group into the protein. This was previously applied to introduce a fluorescent dye into a nanobody for imaging, and the development of novel antibody-drug conjugates [70]. However, it is challenging to predict whether the labelling site could report protein-protein interactions in the mixture, as disulphide bonds are usually buried within the protein structure. Nevertheless, it is still a very promising analytical tool to assess the formation of heterogeneous oligomers in the solution.
Labelling of carbohydrate molecules on the glycosylation sites of proteins may have the least influence on the protein structure. However, the distance to the labelled site could also be too far from the protein-protein interaction sites, such that it is not sensitive enough to probe any interaction events. Moreover, it also needs to account for the heterogeneous glycosylation ensembles of the antibody. Finally, the impact of labelling on the stability and degradation of the therapeutic proteins must be verified before this route is taken for analytics.
2. Future perspectives for co-formulation
Co-formulation provides a powerful methodology to combine multiple therapeutics for maximum synergistic benefit. However, this approach, and subsequent methodological innovation, will ultimately be driven by clinical need. This is because preparation of more than one therapeutic entity is a complex process and is associated with technical, analytical, and regulatory challenges, which may contribute to unsurmountable economical obstacles. Therefore, if the clinical benefit of the biologic co-formulation can be justified by the additional barriers, then co-formulation should ensue.
Strategies are emerging, alongside small molecule therapeutics co-formulation, that could reduce the size of the obstacle through demonstration of promising proof of concept results. This can be achieved through the development of formulation technology, such as micro/nano encapsulation and 3D printing, and of medicinal devices that take advantage of a variety of routes of administration, e.g. pulmonary, transdermal and oral delivery pathways.
Micro and nanoparticle-based formulations possess enhanced biophysical properties (large surface to volume ratio), are readily manipulated through chemical modification for targeted delivery [71], and are able to encapsulate a variety of different small and macromolecular structures. As a result, they could be excellent candidates to co-formulate biological medicines. Continuously manufactured poly(lactic-co-glycolic acid) (PLGA) nanoparticles have been shown to encapsulate two model proteins in the form of green and red fluorescently labelled bovine serum albumins (BSAs) [72]. Nanoparticles demonstrated high protein association and enhanced co-delivery of green and red model proteins to sub-cellular spaces, when compared non-formulated proteins. Disruptive technology, such as 3D printing, has demonstrated strong potential in the pharmaceutical industry, to produce personalised and co-formulated therapeutics [22]. Poly-pills have been produced for small molecule APIs, each for different co-morbidities and tailored to a bespoke dose to maximise patient compliance concordance. This formulation strategy could in-time be translated for biologics should they demonstrate appropriate pharmacokinetic and pharmacodynamic profiles.
Typically, biologics have been administered by intravenous injection, however formulation pathways that take advantage of alternate non-invasive delivery pathways, such as subcutaneous, transdermal, implants, inhalation, oral nasal and buccal routes delivery, are being developed and explored [12]. Intravenous administration affords immediate short-term solutions and advantages for the evaluation of therapeutic efficacy for co-formulated medicinal products to achieve clinical and regulatory approval. Although, non-invasive delivery methods possess research and development barriers, they present clear advantages in the long-term over intravenous therapy for patients, which include of ease administration, patient compliance and concordance [73]. This facilitates translation of biologic medicine away from clinics and hospitals, where specialist care is required, and into local healthcare practices, pharmacies, and homes.
This important transition is heavily highlighted in the literature methods to improve care for diabetic patients [74]. Co-formulated insulin-based therapies have been developed for administration via subcutaneous, transdermal [75] buccal [76] and inhalation pathways [77]. Non-invasive biologic therapies have seen the greatest success as co-formulated subcutaneous insulin products, containing long and short-acting analogues [78] [79], that provide a wide treatment window to prevent repeated administration to the patient, and suggests reasons why non-invasive delivery pathways are extremely attractive to develop co-formulated medicines [80]. The latest developments in diabetes care include MultipepT1De microneedles. These microneedles use a mixture of co-formulated peptides from islet autoantigens to attenuate the autoimmune attack that is associated with the development of Type-1 diabetes [81].
Innovations in formulation and platform technologies that accelerate translation of co-formulated therapies from invasive to non-invasive modalities, especially for those therapies which have short circulation half-lives will be essential for long-term self-managed patient care outside of clinics. Examples of existing formulation innovations and platform technologies include preparations that include hyaluronidase and pharmaceutical implants. Hyaluronidase, like clavulanic acid, assists the drug substance to exert its therapeutic benefit effectively. Hyaluronidase achieves this by catalysing the degradation of hyaluronic acid in extracellular matrices to enhance the efficacy of subcutaneous administration. This formulation pathway has been used to co-formulate subcutaneous administration of the fixed-dose combination of two monoclonal antibodies, trastuzumab and pertuzumab, in combination with chemotherapy in HER2-positive early breast cancer [45]. Whereas implants, that are also positioned sub-cutaneously, consisting of biologics have begun to demonstrate their strong potential for their ease of application, owing to their sustained release properties, but also their ability to protect macromolecules against degradation [82].
The fast development of co-formulated antibody products, termed “antibody cocktail therapy”, also demonstrates their potential in the treatment of viral infection. The rationale behind this is that multi-targeting drug substances are applied to neutralize the virus by binding to non-overlapping epitopes to reduce the chances of viral resistance, which was successfully tested in the treatment of Ebola virus infection [83]. With respect to the COVID-19 global public health crisis, another co-formulated antibody cocktail was developed with potent binding to the SARS-COV-2 spike protein [84]. Antibody co-formulation could become a powerful strategy to control future virus pandemics.
Co-formulation is an attractive therapeutic option to address multiple disease targets using two or more co-administrated pharmaceuticals. From the perspective of the manufacturer, this process also can have the benefit of extending intellectual property protection. However, it is important to note, there are competing technological solutions, other than co-formulation, that are being developed to effectively address multiple disease targets. Although beyond the scope of this review, bispecific antibodies [85], which can be classified as new therapeutic entities and require their own research and development programmes and regulatory approval, are an excellent example of another technology with the potential to bind and neutralize multiple targets in one or more diseases [86].
In summary, although there are many advantages proposed for co-formulation therapies, their development and clinical uptake is a case-by-case debate, whereby the patient benefits are balanced against the technical and regulatory limits imposed by co-formulation.
Declaration of Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council [grant numbers EP/R025282/1]. Financial and in-kind support from a consortium of industrial users is also acknowledged. This work was funded by a Nottingham Research Fellowship from the University of Nottingham (VMC).
References
- 1.Rathore A.S., Shareef F. The influence of domestic manufacturing capabilities on biologic pricing in emerging economies. Nat. Biotechnol. 2019;37:498–501. doi: 10.1038/s41587-019-0116-0. [DOI] [PubMed] [Google Scholar]
- 2.Mitragotri S., Burke P.A., Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.researchandmarkets, Global Biologics Market Size, Market Share, Application Analysis, Regional Outlook, Growth Trends, Key Players, Competitive Strategies and Forecasts, 2018 To 2026, in: researchandmarkets (Ed.) Acute Market Reports, researchandmarkets, https://www.researchandmarkets.com/reports/4564281/global-biologics-market-size-market-share, 2018, pp. Biologics market 2018–2026.
- 4.Frokjaer S., Otzen D.E. Protein drug stability: a formulation challenge. Nat. Rev. Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty A.L., Mrsny R.J. Formulation and delivery issues for monoclonal antibody therapeutics. Adv. Drug Deliv. Rev. 2006;58:686–706. doi: 10.1016/j.addr.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Yu B.B., Zeng L.M., Yang H. Determination of acceptance criteria and sample sizes for accelerated stability comparability studies for biologics. Biologicals. 2017;49:46–50. doi: 10.1016/j.biologicals.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Weiss W.F., Young T.M., Roberts C.J. Principles, approaches, and challenges for predicting protein aggregation rates and shelf life. J. Pharm. Sci. 2009;98:1246–1277. doi: 10.1002/jps.21521. [DOI] [PubMed] [Google Scholar]
- 8.Al-Qadi S., Grenha A., Carrion-Recio D., Seijo B., Remunan-Lopez C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: in vivo evaluation of insulin-loaded formulations. J. Control. Release. 2012;157:383–390. doi: 10.1016/j.jconrel.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Fuller J.E., Zugates G.T., Ferreira L.S., Ow H.S., Nguyen N.N., Wiesner U.B., Langer R.S. Intracellular delivery of core-shell fluorescent silica nanoparticles. Biomaterials. 2008;29:1526–1532. doi: 10.1016/j.biomaterials.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Pechenov S., Shenoy B., Yang M.X., Basu S.K., Margolin A.L. Injectable controlled release formulations incorporating protein crystals. J. Control. Release. 2004;96:149–158. doi: 10.1016/j.jconrel.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Mathew E., Pitzanti G., Larraneta E., Lamprou D.A. 3D printing of pharmaceuticals and drug delivery devices. Pharmaceutics. 2020;12:9. doi: 10.3390/pharmaceutics12030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anselmo A.C., Gokarn Y., Mitragotri S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019;18:19–40. doi: 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- 13.Sahni N., Cheng Y., Middaugh C.R., Volkin D.B. In: Drug Delivery: Principles and Applications. 2nd edition. Wang B., Hu L., Siahaan T.J., editors. John Wiley & Sons Inc; Hoboken: 2016. Vaccine delivery: Current routes of administration and novel approaches; pp. 623–654. [Google Scholar]
- 14.Schweizer D., Serno T., Goepferich A. Controlled release of therapeutic antibody formats. Eur. J. Pharm. Biopharm. 2014;88:291–309. doi: 10.1016/j.ejpb.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Mathaes R., Koulov A., Joerg S., Mahler H.C. Subcutaneous injection volume of biopharmaceuticals-pushing the boundaries. J. Pharm. Sci. 2016;105:2255–2259. doi: 10.1016/j.xphs.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Di L. Strategic approaches to optimizing peptide ADME properties. Aaps J. 2015;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav S., Shire S.J., Kalonia D.S. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J. Pharm. Sci. 2010;99:4812–4829. doi: 10.1002/jps.22190. [DOI] [PubMed] [Google Scholar]
- 18.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bittner B., Richter W., Schmidt J. Subcutaneous Administration of Biotherapeutics: an overview of current challenges and opportunities. Biodrugs. 2018;32:425–440. doi: 10.1007/s40259-018-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orsi G., De Maria C., Montemurro F., Chauhan V.M., Aylott J.W., Vozzi G. Combining inkjet printing and sol-gel chemistry for making pH-sensitive surfaces. Curr. Top. Med. Chem. 2015;15:271–278. doi: 10.2174/1568026614666141229114738. [DOI] [PubMed] [Google Scholar]
- 21.Orsi G., De Maria C., Montemurro F., Chauhan V., Aylott J., Vozzi G. Nanoparticles doped sol-gel ink for inkjet printers. J. Tissue Eng. Regen. Med. 2014;8:197–198. [Google Scholar]
- 22.Khaled S.A., Burley J.C., Alexander M.R., Yang J., Roberts C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015;494:643–650. doi: 10.1016/j.ijpharm.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 23.Stewart S.A., Dominguez-Robles J., McIlorum V.J., Mancuso E., Lamprou D.A., Donnelly R.F., Larraneta E. Development of a biodegradable subcutaneous implant for prolonged drug delivery using 3D printing. Pharmaceutics. 2020;12:16. doi: 10.3390/pharmaceutics12020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capel A.J., Rimington R.P., Lewis M.P., Christie S.D.R. 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2018;2:422–436. [Google Scholar]
- 25.Wu L., Leng D.L., Cun D.M., Foged C., Yang M.S. Advances in combination therapy of lung cancer: rationales, delivery technologies and dosage regimens. J. Control. Release. 2017;260:78–91. doi: 10.1016/j.jconrel.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Aronson J.K. Classifying drug interactions. Br. J. Clin. Pharmacol. 2004;58:343–344. doi: 10.1111/j.1365-2125.2004.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cramer P., von Tresckow J., Bahlo J., Robrecht S., Langerbeins P., Al-Sawaf O., Engelke A., Fink A.M., Fischer K., Tausch E., Seiler T., von Weikersthal L.F., Hebart H., Kreuzer K.A., Bottcher S., Ritgen M., Kneba M., Wendtner C.M., Stilgenbauer S., Eichhorst B., Hallek M. Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19:1215–1228. doi: 10.1016/S1470-2045(18)30414-5. [DOI] [PubMed] [Google Scholar]
- 28.Moore B.D., New R.R.C., Butcher W., Mahood R., Steward J., Bayliss M., MacLeod C., Bogus M., Williamson E.D. Dual route vaccination for plague with emergency use applications. Vaccine. 2018;36:5210–5217. doi: 10.1016/j.vaccine.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 29.USFDA . In: Codevelopment of Two or More New Investigational Drugs for Use in Combination. USFDA, editor. USFDA; 2013. Codevelopment of two or more new investigational drugs for use in combination. , https://www.fda.gov/regulatory-information/search-fda-guidance-documents/codevelopment-two-or-more-new-investigational-drugs-use-combination. Guidance. [Google Scholar]
- 30.Quinlan G.J., Martin G.S., Evans T.W. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–1219. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 31.Farrugia A. Albumin usage in clinical medicine: tradition or therapeutic? Transfus. Med. Rev. 2010;24:53–63. doi: 10.1016/j.tmrv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Minze M.G., Chastain L.M. Combination therapies in the management of type 2 diabetes: the use of insulin degludec/liraglutide. Ther. Clin. Risk Manag. 2016;12:8. doi: 10.2147/TCRM.S73579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poulsen T.T., Grandal M.M., Skartved N.J.O., Hald R., Alifrangis L., Koefoed K., Lindsted T., Frohlich C., Pollmann S.E., Eriksen K.W., Dahlman A., Jacobsen H.J., Bouquin T., Pedersen M.W., Horak I.D., Lantto J., Kragh M. Sym015: a highly efficacious antibody mixture against MET-amplified Tumors. Clin. Cancer Res. 2017;23:5923–5935. doi: 10.1158/1078-0432.CCR-17-0782. [DOI] [PubMed] [Google Scholar]
- 34.Cruz E., Kayser V. Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy. Biologics Targets Therapy. 2019;13:33–51. doi: 10.2147/BTT.S166310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robak T., Windyga J., Trelinski J., Prondzinski M.V., Giagounidis A., Doyen C., Janssens A., Alvarez-Roman M.T., Jarque I., Loscertales J., Rus G.P., Hellmann A., Jedrzejczak W.W., Kuliczkowski K., Golubovic L.M., Celeketic D., Cucuianu A., Gheorghita E., Lazaroiu M., Shpilberg O., Attias D., Karyagina E., Svetlana K., Vilchevska K., Cooper N., Talks K., Prabhu M., Sripada P., Bharadwaj T.P.R., Naested H., Skartved N.J.O., Frandsen T.P., Flensburg M.F., Andersen P.S., Petersen J. Rozrolimupab, a mixture of 25 recombinant human monoclonal RhD antibodies, in the treatment of primary immune thrombocytopenia. Blood. 2012;120:3670–3676. doi: 10.1182/blood-2012-06-438804. [DOI] [PubMed] [Google Scholar]
- 36.Patel A., Gupta V., Hickey J., Nightlinger N.S., Rogers R.S., Siska C., Joshi S.B., Seaman M.S., Volkin D.B., Kerwin B.A. Coformulation of broadly neutralizing antibodies 3BNC117 and PGT121: analytical challenges during Preformulation characterization and storage stability studies. J. Pharm. Sci. 2018;107:3032–3046. doi: 10.1016/j.xphs.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M.X., Lee D., Obi C.R., Freeberg J.K., Farr-Jones S., Tomic M.T. An ambient temperature-stable antitoxin of nine co-formulated antibodies for botulism caused by serotypes A, B and E. PLoS One. 2018;13:16. doi: 10.1371/journal.pone.0197011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda, Takeda, editors. HyQvia. Takeda; 2020. HyQvia. https://www.hyqvia.com/. pp. HyQvia - once-monthly treatment for adult patients with primary immunodeficiency. [Google Scholar]
- 39.Todd P.A., Benfield P. Amoxicillin CLAVULANIC acid - an update of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1990;39:264–307. doi: 10.2165/00003495-199039020-00008. [DOI] [PubMed] [Google Scholar]
- 40.Hinman A.R., Orenstein W.A., Schuchat A. Vaccine-preventable diseases, immunizations, and the epidemic intelligence service. Am. J. Epidemiol. 2011;174:S16–S22. doi: 10.1093/aje/kwr306. [DOI] [PubMed] [Google Scholar]
- 41.Schlingmann B., Castiglia K.R., Stobart C.C., Moore M.L. Polyvalent vaccines: high-maintenance heroes. PLoS Pathog. 2018;14:7. doi: 10.1371/journal.ppat.1006904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.USFDA, Guidance for Industry for the Evaluation of Combination Vaccines for Preventable Diseases: Production, Testing and Clinical Studies, in: USFDA (Ed.) Guidance Document, USFDA, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-evaluation-combination-vaccines-preventable-diseases-production-testing-and, 1997, Pp. the Center for Biologics Evaluation and Research (CBER) is providing this document to provide further guidance and advice on the production, testing and clinical study of combination vaccines.
- 43.Hotez P.J., Corry D.B., Bottazzi M.E. COVID-19 vaccine design: the Janus face of immune enhancement. Nat. Rev. Immunol. 2020;20:347–348. doi: 10.1038/s41577-020-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen J. Vaccine designers take first shots at COVID-19. Science. 2020;368:14. doi: 10.1126/science.368.6486.14. [DOI] [PubMed] [Google Scholar]
- 45.Tan A.R., Im S.-A., Mattar A., Colomer R., Stroyakovskii D., Nowecki Z., De Laurentiis M., Pierga J.-Y., Jung K.H., Schem C., Heeson S., Shivhare M., Kirschbrown W.P., Restuccia E., Crnjevic T.B., Jackisch C. Abstract PD4-07: subcutaneous administration of the fixed-dose combination of trastuzumab and pertuzumab in combination with chemotherapy in HER2-positive early breast cancer: primary analysis of the phase III, multicenter, randomized, open-label, two-arm FeDeriCa study. Cancer Res. 2020;80:PD4–07. [Google Scholar]
- 46.Sadineni V., Quan Y., Kaserer W. In: Compositions comprising a combination of nivolumab and ipilimumab. U.S.P.a.T. Office, editor. 2019. pp. 1–74. https://patents.google.com/patent/US10512689B2/en, Bristol Myers Squibb, United States. [Google Scholar]
- 47.Garidel P., Kuhn A.B., Schafer L.V., Karow-Zwick A.R., Blech M. High-concentration protein formulations: how high is high? Eur. J. Pharm. Biopharm. 2017;119:353–360. doi: 10.1016/j.ejpb.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 48.Chi E.Y., Krishnan S., Kendrick B.S., Chang B.S., Carpenter J.F., Randolph T.W. Roles of conformational stability and colloidal stability in the aggregation of recombinant human granulocyte colony-stimulating factor. Protein Sci. 2003;12:903–913. doi: 10.1110/ps.0235703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Respaud R., Marchand D., Parent C., Pelat T., Thullier P., Tournamille J.F., Viaud-Massuard M.C., Diot P., Si-Tahar M., Vecellio L., Heuze-Vourc'h N. Effect of formulation on the stability and aerosol performance of a nebulized antibody. Mabs. 2014;6:1347–1355. doi: 10.4161/mabs.29938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neergaard M.S., Nielsen A.D., Parshad H., Van de Weert M. Stability of monoclonal antibodies at high-concentration: head-to-head comparison of the IgG(1) and IgG(4) subclass. J. Pharm. Sci. 2014;103:115–127. doi: 10.1002/jps.23788. [DOI] [PubMed] [Google Scholar]
- 51.Schermeyer M.T., Woll A.K., Kokke B., Eppink M., Hubbuch J. Characterization of highly concentrated antibody solution - a toolbox for the description of protein long-term solution stability. Mabs. 2017;9:1169–1185. doi: 10.1080/19420862.2017.1338222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim N., Remmele R.L., Liu D.J., Razinkov V.I., Fernandez E.J., Roberts C.J. Aggregation of anti-streptavidin immunoglobulin gamma-1 involves fab unfolding and competing growth pathways mediated by pH and salt concentration. Biophys. Chem. 2013;172:26–36. doi: 10.1016/j.bpc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Zhao H., Graf O., Milovic N., Luan X.S., Bluemel M., Smolny M., Forrer K. Formulation development of antibodies using robotic system and high-throughput laboratory (HTL) J. Pharm. Sci. 2010;99:2279–2294. doi: 10.1002/jps.22008. [DOI] [PubMed] [Google Scholar]
- 54.Ahrer K., Buchacher A., Iberer G., Josic D., Jungbauer A. Analysis of aggregates of human immunoglobulin G using size-exclusion chromatography, static and dynamic light scattering. J. Chromatogr. A. 2003;1009:89–96. doi: 10.1016/s0021-9673(03)00433-3. [DOI] [PubMed] [Google Scholar]
- 55.Kunitani M., Wolfe S., Rana S., Apicella C., Levi V., Dollinger G. Classical light scattering quantitation of protein aggregates: off-line spectroscopy versus HPLC detection. J. Pharm. Biomed. Anal. 1997;16:573–586. doi: 10.1016/s0731-7085(97)00191-x. [DOI] [PubMed] [Google Scholar]
- 56.Hudson S.D., Sarangapani P., Pathak J.A., Migler K.B. A microliter capillary Rheometer for characterization of protein solutions. J. Pharm. Sci. 2015;104:678–685. doi: 10.1002/jps.24201. [DOI] [PubMed] [Google Scholar]
- 57.Zarraga I.E., Taing R., Zarzar J., Luoma J., Hsiung J., Patel A., Lim F. High shear rheology and anisotropy in concentrated solutions of monoclonal antibodies. J. Pharm. Sci. 2013;102:2538–2549. doi: 10.1002/jps.23647. [DOI] [PubMed] [Google Scholar]
- 58.Barnett G.V., Razinkov V.I., Kerwin B.A., Hillsley A., Roberts C.J. Acetate- and citrate-specific ion effects on unfolding and temperature-dependent aggregation rates of anti-streptavidin IgG1. J. Pharm. Sci. 2016;105:1066–1073. doi: 10.1016/j.xphs.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 59.Brader M.L., Estey T., Bai S.J., Alston R.W., Lucas K.K., Lantz S., Landsman P., Maloney K.M. Examination of thermal unfolding and aggregation profiles of a series of developable therapeutic monoclonal antibodies. Mol. Pharm. 2015;12:1005–1017. doi: 10.1021/mp400666b. [DOI] [PubMed] [Google Scholar]
- 60.Cheng W.Q., Joshi S.B., He F., Brems D.N., He B., Kerwin B.A., Volkin D.B., Middaugh C.R. Comparison of high-throughput biophysical methods to identify stabilizing excipients for a model IgG2 monoclonal antibody: conformational stability and kinetic aggregation measurements. J. Pharm. Sci. 2012;101:1701–1720. doi: 10.1002/jps.23076. [DOI] [PubMed] [Google Scholar]
- 61.Goldberg D.S., Bishop S.M., Shah A.U., Sathish H.A. Formulation development of therapeutic monoclonal antibodies using high-throughput fluorescence and static light scattering techniques: role of conformational and colloidal stability. J. Pharm. Sci. 2011;100:1306–1315. doi: 10.1002/jps.22371. [DOI] [PubMed] [Google Scholar]
- 62.Weichel M., Bassarab S., Garidel P. Probing thermal stability of MAbs by intrinsic tryptophan fluorescence. BioProcess Int. 2008:42–52. doi: 10.1002/biot.200800091. [DOI] [PubMed] [Google Scholar]
- 63.J. Domingues, Stability Assessment of Biopharmaceutical Formulations, in, Allen Institute for AI, https://www.semanticscholar.org/paper/Stability-Assessment-of-Biopharmaceutical-Domingues/87e3d466b5ff37dbab9ffd696ca9b1692204b16a, 2011, pp. Stability Assessment of Biopharmaceutical Formulations.
- 64.Sahin E., Roberts C.J. In: Therapeutic Proteins. Voynov V., Caravella J.A., editors. Humana Press; Totwa: 2012. Size-exclusion chromatography with multi-angle light scattering for elucidating protein aggregation mechanisms; pp. 403–423. [DOI] [PubMed] [Google Scholar]
- 65.Patel B.A., Gospodarek A., Larkin M., Kenrick S.A., Haverick M.A., Tugcu N., Brower M.A., Richardson D.D. Multi-angle light scattering as a process analytical technology measuring real-time molecular weight for downstream process control. Mabs. 2018;10:945–950. doi: 10.1080/19420862.2018.1505178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fekete S., Veuthey J.L., Beck A., Guillarme D. Hydrophobic interaction chromatography for the characterization of monoclonal antibodies and related products. J. Pharm. Biomed. Anal. 2016;130:3–18. doi: 10.1016/j.jpba.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Kim J., Kim Y.J., Cao M., De Mel N., Albarghouthi M., Miller K., Bee J.S., Wang J., Wang X. Analytical characterization of coformulated antibodies as combination therapy. mAbs. 2020;12 doi: 10.1080/19420862.2020.1738691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W.-H., Cheung-Lau J., Chen Y., Lewis M., Tang Q.M. Specific and high-resolution identification of monoclonal antibody fragments detected by capillary electrophoresis–sodium dodecyl sulfate using reversed-phase HPLC with top-down mass spectrometry analysis. mAbs. 2019;11:1233–1244. doi: 10.1080/19420862.2019.1646554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L., Wang L., Shion H., Yu C., Yu Y.Q., Zhu L., Li M., Chen W., Gao K. In-depth structural characterization of Kadcyla® (ado-trastuzumab emtansine) and its biosimilar candidate. mAbs. 2016;8:1210–1223. doi: 10.1080/19420862.2016.1204502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Massa S., Xavier C., De Vos J., Caveliers V., Lahoutte T., Muyldermans S., Devoogdt N. Site-specific Labeling of cysteine-tagged Camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug. Chem. 2014;25:979–988. doi: 10.1021/bc500111t. [DOI] [PubMed] [Google Scholar]
- 71.Al-Natour M.A., Yousif M.D., Cavanagh R., Abouselo A., Apebende E.A., Ghaemmaghami A., Kim D.-H., Aylott J.W., Taresco V., Chauhan V.M., Alexander C. Facile dye-initiated polymerization of Lactide–Glycolide generates highly fluorescent poly(lactic-co-glycolic acid) for enhanced characterization of cellular delivery. ACS Macro Lett. 2020;9:431–437. doi: 10.1021/acsmacrolett.9b01014. [DOI] [PubMed] [Google Scholar]
- 72.Martins C., Chauhan V.M., Selo A.A., Al-Natour M., Aylott J.W., Sarmento B. Modelling protein therapeutic co-formulation and co-delivery with PLGA nanoparticles continuously manufactured by microfluidics. React. Chem. Eng. 2020;5:308–319. doi: 10.1039/C9RE00395A. [DOI] [Google Scholar]
- 73.Jin J.F., Zhu L.L., Chen M., Xu H.M., Wang H.F., Feng X.Q., Zhu X.P., Zhou Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adher. 2015;9:923–942. doi: 10.2147/PPA.S87271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Tabakha M.M., Arida A.I. Recent challenges in insulin delivery systems: a review. Indian J. Pharm. Sci. 2008;70:278–286. doi: 10.4103/0250-474X.42968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prausnitz M.R., Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palermo A., Napoli N., Manfrini S., Lauria A., Strollo R., Pozzilli P. Buccal spray insulin in subjects with impaired glucose tolerance: the prevoral study. Diabetes Obes. Metab. 2011;13:42–46. doi: 10.1111/j.1463-1326.2010.01312.x. [DOI] [PubMed] [Google Scholar]
- 77.Rosenstock J., Cefalu W.T., Hollander P.A., Klioze S.S., Reis J., Duggan W.T. Safety and efficacy of inhaled human insulin (Exubera) during discontinuation and Readministration of therapy in adults with type 2 diabetes: a 3-year randomized controlled trial. Diabetes Technol. Ther. 2009;11:697–705. doi: 10.1089/dia.2009.0062. [DOI] [PubMed] [Google Scholar]
- 78.Rosak C. Insulin analogues: structure, properties and therapeutic indications. Part one: short-action insulin analogues. Internist. 2001;42:1523. doi: 10.1007/s001080170043. [DOI] [PubMed] [Google Scholar]
- 79.Rosak C. Insulin analogs: structure, properties and therapeutic indications - part two: long-acting insulin analogs. Internist. 2001;42:1692–1699. doi: 10.1007/s001080170022. [DOI] [PubMed] [Google Scholar]
- 80.Vazquez-Carrera M., Silvestre J.S. Insulin analogues in the management of diabetes. Methods Find. Exp. Clin. Pharmacol. 2004;26:445–461. [PubMed] [Google Scholar]
- 81.Liu Y.F., Powrie J.K., Arif S., Fountoulakis N., Joshi M., Smith E.L., Strimenopoulou F., Thomson M., Peakman M. The MultiPepT1De study-examining the safety of peptide immunotherapy using multiple islet antigens in recent-onset type 1 diabetes. Diabetes. 2018;67:2. [Google Scholar]
- 82.Agarwal P., Rupenthal I.D. Injectable implants for the sustained release of protein and peptide drugs. Drug Discov. Today. 2013;18:337–349. doi: 10.1016/j.drudis.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 83.Pascal K.E., Dudgeon D., Trefry J.C., Anantpadma M., Sakurai Y., Murin C.D., Turner H.L., Fairhurst J., Torres M., Rafique A., Yan Y., Badithe A., Yu K., Potocky T., Bixler S.L., Chance T.B., Pratt W.D., Rossi F.D., Shamblin J.D., Wollen S.E., Zelko J.M., Carrion R., Worwa G., Staples H.M., Burakov D., Babb R., Chen G., Martin J., Huang T.T., Erlandson K., Willis M.S., Armstrong K., Dreier T.M., Ward A.B., Davey R.A., Pitt M.L.M., Lipsich L., Mason P., Olson W., Stahl N., Kyratsous C.A. Development of clinical-stage human monoclonal antibodies that treat advanced Ebola virus disease in nonhuman Primates. J. Infect. Dis. 2018;218:S612–S626. doi: 10.1093/infdis/jiy285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., Fulton B.O., Yan Y., Koon K., Patel K., Chung K.M., Hermann A., Ullman E., Cruz J., Rafique A., Huang T., Fairhurst J., Libertiny C., Malbec M., Lee W.-y., Welsh R., Farr G., Pennington S., Deshpande D., Cheng J., Watty A., Bouffard P., Babb R., Levenkova N., Chen C., Zhang B., Romero Hernandez A., Saotome K., Zhou Y., Franklin M., Sivapalasingam S., Lye D.C., Weston S., Logue J., Haupt R., Frieman M., Chen G., Olson W., Murphy A.J., Stahl N., Yancopoulos G.D., Kyratsous C.A. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;218(suppl_5):S612–S626. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kontermann R.E., Brinkmann U. Bispecific antibodies. Drug Discov. Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 86.Kontermann R. Dual targeting strategies with bispecific antibodies. mAbs. 2012;4:182–197. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]



