Abstract
Background
Contrast-induced acute kidney injury (CI-AKI) is a complication commonly associated with invasive angiographic procedures and is considered the leading cause of hospital-acquired acute kidney injury. CI-AKI can lead to a prolonged hospital stay, with a substantial economic impact, and increased mortality. The DyeVert™ PLUS EZ system (FDA approved and CE marked) is a device that has been developed to divert a portion of the theoretical injected contrast media volume (CMV), reducing the overall volume of contrast media injected and aortic reflux, and potentially improving long-term health outcomes.
Objectives
To assess the long-term costs and health outcomes associated with the introduction of the DyeVert™ PLUS EZ system into the UK health care service for the prevention of CI-AKI in a cohort of patients with chronic kidney disease (CKD) stage 3–4 undergoing diagnostic coronary angiography (DAG) and/or percutaneous coronary intervention (PCI), and to compare these costs and outcomes with those of the current practice.
Methods
A de novo economic model was developed based on the current pathway of managing patients undergoing DAG and/or PCI and on evidence related to the clinical effectiveness of DyeVert™ in terms of its impact on relevant clinical outcomes and health service resource use. Clinical data used to populate the model were derived from the literature or were based on assumptions informed by expert clinical input. Costs included in the model were from the NHS and personal social services perspective and obtained from the literature and UK-based routine sources. Probabilistic distributions were assigned to the majority of model parameters so that a probabilistic analysis could be undertaken, while deterministic sensitivity analyses were also carried out to explore the impact of key parameter variation on the model results.
Results
Base-case results indicate that the intervention leads to cost savings (− £435) and improved effectiveness (+ 0.028 QALYs) over the patient’s lifetime compared with current practice. Output from the probabilistic analysis points to a high likelihood of the intervention being cost-effective across presented willingness-to-pay (WTP) thresholds. The overall long-term cost saving for the NHS associated with the introduction of the DyeVert™ PLUS EZ system is over £19.7 million for each annual cohort of patients. The cost savings are mainly driven by a lower risk of subsequent diseases and their associated costs.
Conclusions
The introduction of the DyeVert™ PLUS EZ system has the potential to reduce costs for the health care service and yield improved clinical outcomes for patients with CKD stage 3–4 undergoing angiographic procedures.
Electronic supplementary material
The online version of this article (10.1007/s41669-020-00195-x) contains supplementary material, which is available to authorized users.
Keywords: Contrast-induced acute kidney injury, Diagnostic coronary angiography, Percutaneous coronary intervention, DyeVert™ PLUS EZ system, Economic model, Cost-effectiveness analysis
Key Point for Decision Makers
| Introduction of the DyeVert™ PLUS EZ system into the NHS could lead to cost savings (− £435) and improved effectiveness (+ 0.028 QALYs) over the patient’s lifetime compared with current practice. |
| Results of the economic analysis indicate that the DyeVert™ PLUS EZ system is highly likely to be cost saving and to result in improved patient outcomes. |
Introduction
One common complication associated with angiographic procedures is contrast-induced acute kidney injury (CI-AKI), which is attributed to radiocontrast media [1–3]. CI-AKI is defined as the impairment of renal function, measured as either a 25% increase in serum creatinine (SCr) from baseline or a 0.5 mg/dL (44 µmol/L) increase in absolute SCr within 48–72 h after intravenous contrast administration.
The development of CI-AKI can lead to a prolonged hospital stay, a greater financial burden, and increased mortality [4, 5]. The economic impact of CI-AKI in the UK is significant. It is estimated that in 2010–2011 there were 977,116 excess bed days attributable to AKI in the UK, with an associated cost of £304 million [4]. CI-AKI is considered the leading cause of hospital-acquired AKI and is responsible for one-third of all AKI cases [6]. This condition is closely linked to angiographic procedures: the incidence of CI-AKI in patients undergoing angiographic procedures ranges between 1 and 2% for patients without prior chronic kidney disease (CKD) but is 30% for patients with a combination of risk factors [7–9].
Since there is no definitive treatment for CI-AKI, the focus has been on preventing the condition. The European Society of Cardiology (ESC) published updated guidelines on the prevention of CI-AKI [10] that provide a framework for the use of evidence-based preventative strategies. Among these strategies, they recommend the identification of patients at risk of CI-AKI, appropriate periprocedural hydration, and minimising contrast volume in at-risk patients. Previous studies have shown that CI-AKI is associated with increased risks of death, myocardial infarction, bleeding and recurrent renal injury after discharge [11, 12].
The DyeVert™ PLUS EZ system (FDA approved and CE marked) is a device that adjusts contrast media (CM) during manual injection. This is achieved by diverting a portion of the theoretical injected contrast media volume (CMV), which reduces the overall volume of contrast media injected and aortic reflux [13]. This reduction in CMV has been shown to be nonlinearly associated with a decreased risk of CI-AKI, which can be linked to a reduction in short- and long-term costs and consequences [14]. The economic analysis reported in the present work was performed to estimate the cumulative difference in costs and effectiveness with and without the use of the DyeVert™ PLUS EZ system in patients undergoing diagnostic coronary angiography (DAG) and/or percutaneous coronary intervention (PCI) from the UK NHS and personal social services (PSS) perspective. As per the protocol for clinical studies [13, 14] of the DyeVert™ PLUS EZ system, other renal protection strategies such as hydration, pre- and postprocedural laboratory studies, and the continuation or discontinuation of specific medications were implemented at the discretion of the study investigators. Therefore, the incremental benefit in terms of absolute reduction in contrast media injection was achieved while DyeVert was used on top of the other strategies, which is reflective of current practice, so we compared the application of the DyeVert™ PLUS EZ system to current practice without the use of the DyeVert™ PLUS EZ system.
Methods
A de novo economic model was developed that reflected the current management pathway of patients undergoing DAG and/or PCI. The model was built upon evidence related to the clinical effectiveness of DyeVert™ PLUS EZ system, measured as the reduction in contrast media (CM) volume and the subsequent incidence of CI-AKI, as well as economic evidence related to associated NHS resource use. For each treatment arm, costs and outcomes were aggregated on the basis of a series of decisions and events. The structure of the model remained unchanged between the two treatment options (i.e. with and without using the DyeVert™ PLUS EZ system).
The model was based on a hypothetical cohort of patients with CKD stage 3–4 undergoing DAG and/or PCI. As per NICE requirements, and in order to fully capture the differences in costs and benefits between the two scenarios, a lifetime time horizon was used in the base-case analysis [15]. The recommended discount rate in the UK (3.5% per annum) was applied to both costs and benefits [16]. The model considered all costs from the UK NHS and personal social services perspective, and was developed in Microsoft Excel.
Model Structure
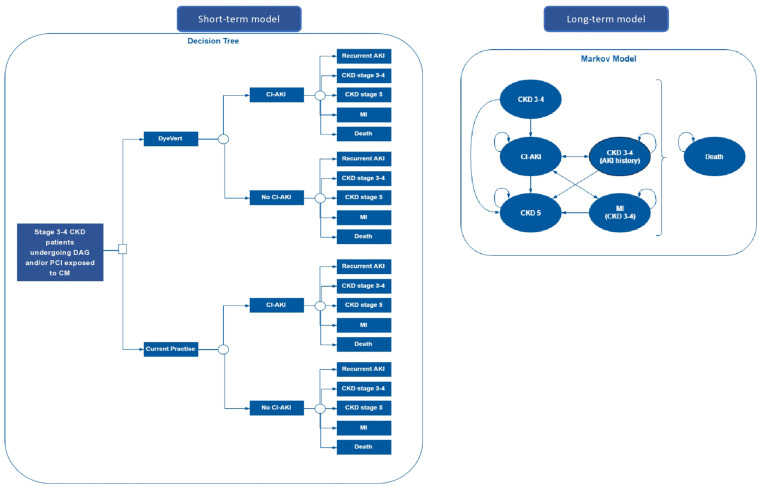
The model is structured as a decision tree followed by a Markov model with six health states. This model has a lifetime time horizon with costs and benefits estimated in the decision tree for the first 3 months and in the Markov model for the remainder of the patient’s lifetime. The Markov model has a cycle length of 3 months. Figure 1 provides a schematic of the model structure. The model was used to simulate the management of patients undergoing DAG and/or PCI with reduced kidney function (i.e. eGFR 15–60 ml/min/1.73 m2). In each strategy, patients may or may not experience a CI-AKI requiring further treatment. Patients may then either remain in the state ‘CKD stage 3–4’ (or the state ‘CKD stage 3–4 (AKI history)’ if they previously had CI-AKI) or progress according to the natural progression of CKD to the state ‘CKD stage 5’. Patients with nonsevere renal insufficiency (i.e. CKD stage 3–4) can experience a recurrent AKI or a myocardial infarction (MI) at any point. Patients who enter CKD stage 5 are assumed to either remain in this state or die, as they will be receiving dialysis and treatments to prevent a MI. Patients in each model state will incur associated costs and quality-adjusted life-years (QALYs).
Fig. 1.
Model structure. AKI acute kidney injury, CI-AKI contrast-induced acute kidney injury, CKD chronic kidney disease, CM contrast media, MI myocardial infarction, PCI percutaneous coronary intervention
Simulated patients are at risk of death from all causes during any given cycle period. Risk of death is conditional on CKD stage, history of AKI and/or MI, and age. The all-cause mortality rates were derived from general population mortality statistics reported in national life tables [17] and were adjusted to reflect the extra mortality associated with CI-AKI and renal insufficiency.
In order to evaluate the face validity of the model, the model structure, input parameters and results were presented to clinical experts in the team. The experts are among the most well-respected clinicians in this field in the UK and have extensive experience. They were asked to evaluate the model structure and assumptions in comparison to real-world circumstances. A wide range of sensitivity analyses were also conducted to assess the internal validity. Null and extreme values were assigned to input parameters and the model was run to test the robustness of the outputs.
Model Inputs
All inputs used to populate the economic model are described in the following section and are presented in Table 2. Patients at model entry were those undergoing DAG and/or PCI with some kidney function impairment (CKD stage 3–4). The base-case population was 72 years old [14]. The number of patients undergoing DAG and/or PCI was derived from the NHS reference costs for percutaneous coronary angioplasty (HRG code EY40–EY41) and cardiac catheterisation (HRG code EY42–EY43) [18]. It was assumed, based on Gurm et al. [14], that 26% of these procedures would be DAG combined with PCI.
Table 2.
Main inputs used in the model
| Parameters | Mean | Distribution | Lower limit | Upper limit | Sources |
|---|---|---|---|---|---|
| Population size | |||||
| % of patients undergoing DAG and/or PCI with CKD stage 3–4 | 27% | Fixed | NA | NA | Dangas et al. [3] |
| Number of patients undergoing DAG only | 145,046 | Fixed | NA | NA | NHS reference cost [22] |
| Number of patients undergoing PCI only | 20,843 | Fixed | NA | NA | |
| Number of patients undergoing DAG and PCI | 58,286 | Fixed | NA | NA | |
| % of extended hospital admissions compared to new admissions | 50% | Fixed | NA | NA | Assumption |
| Transition probabilities | |||||
| Decision tree (3 month) probabilities | |||||
| CKD 3–4 to CI-AKI | 0.30 | Beta | 0.26 | 0.352 | Dangas et al. [3] |
| RR reduction of CI-AKI due to DyeVert™ | 0.21 | Log normal | 0.16 | 0.268 | Gurm et al. [23] |
| HR of CI-AKI to death | 2.13 | Log normal | 2.01 | 2.260 | Valle et al. [12] |
| Markov model (long term) | |||||
| CI-AKI to CKD 5 | 3.28% | Beta | 3.10% | 3.46% | James et al. [21] |
| CKD 3–4 to CKD 5 | |||||
| < 69 years | 0.02% | Beta | Alpha 5.50 | Beta 3043.00 | Eriksen et al. [20] and CG169 [24] |
| 70–79 years | 0.10% | Beta |
Alpha 3.1 |
Beta 3045.0 | |
| > 79 years | 0.08% | Beta |
Alpha 2.3 |
Beta 3046.0 | |
| Risk of recurrent AKI, first 3 months (without previous CI-AKI) | 1.78% | Beta | 1.74% | 1.82% | Valle et al. [12] |
| Risk of recurrent AKI, subsequent (without previous CI-AKI) | 0.91% | Beta | 0.49% | 0.50% | |
| Risk of recurrent AKI, first 3 months (with previous CI-AKI) | 6.61% | Beta | 6.24% | 6.96% | |
| Risk of recurrent AKI, subsequent (with previous CI-AKI) | 2.26% | Beta | 2.20% | 2.32% | |
| Risk of MI, acute phase (with previous CI-AKI) | 2.58% | Beta | 2.35% | 2.82% | |
| Risk of MI, subsequent (with previous CI-AKI) | 1.23% | Beta | 1.18% | 1.28% | |
| Risk of MI, acute phase (without previous CI-AKI) | 1.42% | Beta | 1.36% | 2.35% | |
| Risk of MI, subsequent (without previous CI-AKI) | 0.67% | Beta | 0.64% | 1.11% | |
| Risk of AKI requiring dialysis, acute phase (with previous CI-AKI) | 0.79% | Beta | 0.65% | 0.93% | |
| Risk of AKI requiring dialysis, subsequent (with previous CI-AKI) | 0.16% | Beta | 0.15% | 0.18% | |
| Risk of AKI requiring dialysis, acute phase (without previous CI-AKI) | 0.11% | Beta | 0.11% | 0.12% | |
| Risk of AKI requiring dialysis, subsequent (without previous CI-AKI) | 0.04% | Beta | 0.04% | 0.04% | |
| Mortality | |||||
| CKD 3–4 to death RR (conditional on age and gender) | |||||
| Male < 69 years | 3.60 | Log normal | 2.60 | 5.000 | Eriksen et al. [20] |
| Female < 69 years | 2.70 | Log normal | 2.00 | 3.700 | |
| Male 70–79 years | 2.40 | Log normal | 2.00 | 2.900 | |
| Female 70–79 years | 1.80 | Log normal | 1.50 | 2.100 | |
| Male > 79 | 2.30 | Log normal | 2.00 | 2.600 | |
| Female > 79 | 2.10 | Log normal | 1.90 | 2.300 | |
| CKD 5 to death RR (conditional on age and gender) | |||||
| Male 18–64 years | 10.00 | Log normal | 7.10 | 13.700 | Villar et al. [25] |
| Female 18–64 years | 16.40 | Log normal | 9.60 | 26.300 | |
| Male > 64 years | 4.80 | Log normal | 3.90 | 5.800 | |
| Female > 64 years | 7.10 | Log normal | 5.40 | 9.200 | |
| Relative risk of stage 5 CKD after CI-AKI | 4.81 | Log normal | 3.04 | 7.62 | See et al. [5] |
| MI (acute) to death SMR | 5.84 | Log normal | 4.38 | 7.300 | TA236 [26] |
| MI (subsequent) to death SMR | 2.21 | Log normal | 1.66 | 2.763 | |
| Costs | |||||
| Health-state costs/cycle | |||||
| CKD stage 3–4/cycle | £279.78 | Gamma | £209.83 | £349.72 | NHS reference cost [22] |
| CKD stage 5 in first cycle/cycle | £7006.45 | Gamma | £5254.84 | £8758.06 | |
| CKD stage 5 in subsequent cycles/cycle | £6048.95 | Gamma | £4536.71 | £7561.19 | |
| Cost of MI (initial)/cycle | £6249.79 | Gamma | £6109.65 | £6389.92 | Walker et al. [27] |
| Cost of MI (subsequent)/cycle | £502.56 | Gamma | £447.99 | £557.12 | |
| Event costs and other | |||||
| CI-AKI cost of index admission | £2673.79 | Gamma | £2005.35 | £3342.24 | NHS digital 2017–2018 NHS reference cost [22] |
| CI-AKI cost of extended hospital admission | £1726.34 | Gamma | £1294.75 | £2157.92 | NHS digital 2017–2018 NHS reference cost [22] |
| DyeVert™ cost | £350.00 | Gamma | Osprey Medical Corporation [28] | ||
| Cost of DAG | £1766.21 | Gamma | £1324.66 | £2207.77 | NHS digital 2017–2018 NHS reference cost [22] |
| Cost of PCI | £2937.22 | Gamma | £2202.91 | £3671.52 | NHS digital 2017–2018 NHS reference cost [22] |
| Health utilities | |||||
| CKD stage 3–4/cycle | 0.17 | Beta | 0.12 | 0.22 | Tajima et al. [29] and Kind et al. [30] |
| CKD stage 5/cycle | 0.16 | Beta | 0.11 | 0.20 | |
| CI-AKI/cycle | 0.13 | Beta | 0.07 | 0.20 | Sullivan et al. [31] |
| Post myocardial infarction/cycle | 0.17 | Beta | 0.12 | 0.22 | Assumption |
AKI acute kidney injury, DAG diagnostic coronary angiography, CI-AKI contrast-induced acute kidney injury, CKD chronic kidney disease, MI myocardial infarction, PCI percutaneous coronary intervention, RR relative risk
Clinical Effectiveness
Evidence has shown that when DyeVert™ PLUS EZ system is used in patients undergoing DAG and/or PCI, the contrast media volume is significantly reduced [14]. To estimate the reduction in risk of CI-AKI after using DyeVert™, we analysed the CI-AKI rate based on the definition used in the Mehran risk score (i.e. an increase of 0.5 mg/dL in serum creatinine) [9], and found the Mehran risk scores for the population, ultimately arriving at a projected risk rate for the population. The projected absolute rate was 14% and the actual rate was 11%. Therefore, the estimated risk reduction was estimated to be 21.4% (the corresponding relative risk was 78.6%). The estimated absolute risk reduction was used to adjust the risk of CI-AKI in the intervention arm for the base-case analysis. Alternatively, the absolute risk reduction was estimated using data from Gurm et al. [14, 19] (Table 1).
Table 1.
Effectiveness of DyeVert™ PLUS EZ system
| Effectiveness | Value (SD) | Source |
|---|---|---|
| Base-case approach | ||
| % Reduction in risk of CI-AKI due to the use of the DyeVert™ PLUS EZ system | 21.4% | Calculated |
| Alternative approach | ||
| % Contrast volume reduction per procedure (DyeVert™) | 40.1% (8.8) | [14] |
| % Reduction in risk of CI-AKI associated with 40.1% reduction in contrast media for patients with eGFR ≤ 60 ml/min/1.73 m2 | 15.1% | [19] |
Transition Probabilities
The incidence of CI-AKI in patients with CKD undergoing PCI was based on a cohort study of 1473 patients and was estimated to be 30% [3, 9]. Due to the limited evidence, it was assumed that the risk for patients receiving PCI and DAG at the same time or DAG alone were the same and equal to 30% in the base-case analysis. Different levels of risk of CI-AKI (± 25%, ± 50% and ± 75%) were explored in the sensitivity analyses. The baseline transition probability associated with the progression of patients from CKD stage 3–4 to CKD stage 5 for different age groups was based on the 10-year cumulative incidence rate in a cohort study of 3047 patients [20]. The probability of transitioning to stage 5 CKD following a CI-AKI after the first cycle (first 3 months) was obtained from clinical guidelines from CG169 and James et al. [21] and from a study by Valle et al. [12]. The probability of MI for patients with a history of CI-AKI was taken from Valle et al. [12]. For patients who had not experienced an AKI throughout the model, the probability of MI was 1.42% and 0.67% in the first 3 months and the subsequent cycles, respectively [12]. For patients who had experienced CI-AKI in the previous cycles, the equivalent probabilities were 2.58% and 1.23%, respectively [12].
The probability of recurrent AKI was taken from the same study as the probability of MI [12]. Based on the cumulative incidence in the third month and the first year of the study follow-up, we estimated the probability of recurrent AKI in the first 3 months after the DAG and/or PCI, and for subsequent 3-month cycles. For patients who had not experienced a CI-AKI after the procedure, the probability of recurrent AKI was 1.78% and 0.91% in the first 3 months and the subsequent cycles, respectively. For patients who had experienced CI-AKI during the procedure, the equivalent probabilities were 6.83% and 2.43%, respectively.
Probability of Death
Standardised mortality ratios for each health state included in the model were applied to the relevant age-dependent mortality rates and are shown in Table 2. These mortality ratios were derived from the literature.
Costs
Resource Consequences
Unit costs for all resource use estimates were extracted from the literature or obtained through other relevant sources such as NHS reference costs [22], the Personal Social Services Research Unit [32], and the British National Formulary and manufacturer price lists [33]. Costs were obtained in sterling (£) for the year 2018 and were discounted at 3.5% per annum, where appropriate. All costs included in the model are shown in Table 2 and the Electronic supplementary material (ESM).
The choice of cost items was mainly informed by a NICE clinical guidelines model developed to evaluate prevention strategies for CI-AKI using different hydration methods [34]. However, updated unit costs and dosages of drugs were extracted from the literature. In the instances where unit costs of relevant outcomes were not available, the costs of items used in the NICE guidelines model (CG169 [24]) were inflated to reflect current prices. This was done by applying an inflation index provided by the Bank of England [35].
Intervention Cost
The cost of the DyeVert™ PLUS EZ technology was estimated to be £350, including the cost of the smart syringe and module [28]. Osprey Medical Corporation has indicated that free training in the use of the technology will be provided to clinical staff, and that a smart monitor will be provided free of charge. Therefore, these costs were not included.
CI-AKI Costs
Two different methods were used in the model to estimate the CI-AKI event costs: (1) for patients who have to be readmitted to the hospital due to CI-AKI after a DAG and/or PCI procedure, the cost of an index admission due to AKI was used; (2) for patients admitted for DAG and/or PCI who have a prolonged length of stay due to CI-AKI, the cost of these additional bed days was considered. Given that the analysis was conducted from the NHS and PSS perspective and the fact that both of these situations are possible in the real world, an average cost of these two situations were used in the base-case analysis.
Health State Costs
Patients with CKD stage 3–4 are expected to incur costs associated with consultations with the nephrologist [34] along with lab resource costs and an assumed 5-min period with a phlebotomist to measure the patient’s eGFR. Additional costs would include 9% of patients requiring epoetin alfa to treat anaemia, as recommended by the clinical guidelines for anaemia treatment in patients with CKD [34]. Epoetin alfa dosage was estimated for a 77 kg individual on average, according to ONS. To account for patients who require diuretics, an assumption was made based on the guidelines model [34] that about a quarter of the patients (26%) with CKD stage 3–4 had CKD stage 4, 60% of whom were on a 40 mg daily dose of furosemide [24]. The total cost of CKD stage 3–4 per cycle was estimated to be £279.80.
Patients with CKD stage 5 will incur drug costs. However, patients at this stage will also incur costs associated with renal replacement therapy (RRT) or conservative management. At this stage, patients are expected to incur costs such as RRT procedures, anaemia management, specialist appointments, EGFR measurements and diuretics. During the cycle that a patient progresses to CKD stage 5, it is assumed that the intensity of treatment will be greater than in later stages, as the costs of initiating treatment are included at this stage. It was assumed, based on the NICE guidelines model [24], that 90% of patients in this model state would receive RRT. To estimate RRT costs, a pooled average of NHS reference costs for 2017–2018 was taken, accounting for the national usage of different treatment modalities such as haemodialysis and filtration [22]. Patients entering CKD stage 5 will receive an access procedure that allows permanent access for RRT. It was assumed that there would be no further access procedure-related costs in subsequent cycles. Drugs and check-ups are also required and are more frequent in CKD stage 5. It was assumed that all patients at this stage would have an eGFR more frequently (on a weekly basis) and two nephrologist appointments per 3 months. In this state, epoetin was assumed to be administered to 33% of the patients at the same dosage as for patients with CKD stage 3–4 [34]. Patients on conservative management (10%) will receive monthly home visits by a specialist nurse as well as telephone calls on a weekly basis. It was assumed [34] that diuretics would be used by 90% of the patients with CKD stage 5 at double the dosage compared to patients with CKD stage 3–4 (80 mg). The cost of AMI was taken from [27]. In this study, the cost of AMI was estimated to be £6869 for the first year and £1780 for subsequent years. These costs were inflated to represent prices in 2018 and then adjusted to estimate the cost in the acute phase/first 3 months (first cycle). It was assumed that the cost of subsequent cycles from cycle 2 onward is constant and equal to one-quarter of the cost in subsequent years. The inflated costs in the first and subsequent years were £7757 and £2010, respectively. The cost for the first cycle was estimated as follows: £7757 − £2010 × (3/4) = £6249.79.
Utilities
Utilities for CKD stages were obtained from a Japanese study [29] used in both the NICE guidelines (CG169) [24, 34] and a NHS report on kidney care [36]. An adjustment for the UK population was made by multiplying the values by the general UK population average utility values for people aged 65–75. Utility values for the stages of CI-AKI were taken from Sullivan et al. [31], who reported in a catalogue of UK EQ-5D-derived utilities that a utility value of 0.525 (n = 194) was associated with kidney injury (‘renal failure’). The same utility as for CKD stage 3–4 was assumed for the MI state.
Analysis
A Monte Carlo simulation was conducted (10,000 iterations) to derive cumulative estimates of the costs and effects of each strategy in the model. Probabilistic distributions were assigned to the majority of model parameters (Table 2) so that a probabilistic analysis could be undertaken, while deterministic sensitivity analyses were also carried out to explore the impact of key parameter variation on the model results. Total cost savings for the entire cohort of patients receiving the intervention in the UK over a lifetime time horizon were also calculated.
Results
This section presents the results of the economic analysis; base-case results are presented first, followed by the results of the sensitivity analyses.
Base-Case Analysis
The results of the base-case analysis presented in Table 3 indicate that the introduction of the DyeVert™ PLUS EZ system leads to cost savings of £448 per patient over a lifetime time horizon. Additionally, the intervention leads to improved effectiveness over the patient’s lifetime (+ 0.028 QALYs). Therefore, DyeVert™ PLUS EZ system is considered a dominant strategy (less costly and more effective) compared to current practice.
Table 3.
Base-case probabilistic results
| Base-case probabilistic results (lifetime time horizon) | Current practice | DyeVert™ PLUS EZ system |
|---|---|---|
| Cost (£) | £23,932 | £23,484 |
| Incremental cost (£) | − £448 | |
| QALYs | 4.633 | 4.661 |
| Incremental QALYs | + 0.028 | |
| ICER (£) (∆cost/∆QALYs) | DyeVert™ PLUS EZ system is dominant | |
| Probability of being cost-effective at £20,000 WTP threshold | 100% | |
| Probability of being cost saving | 99.8% | |
ICER incremental cost-effectiveness ratio, QALYs quality-adjusted life-years, WTP willingness to pay
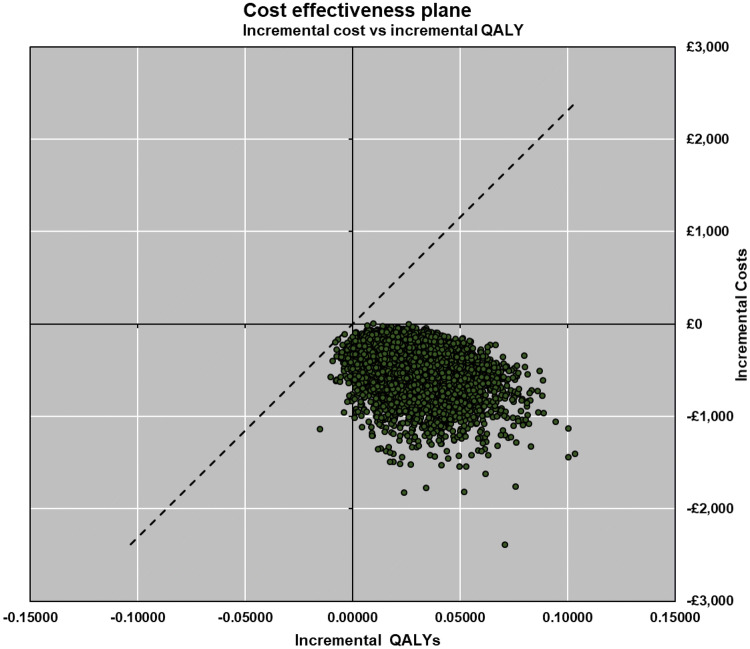
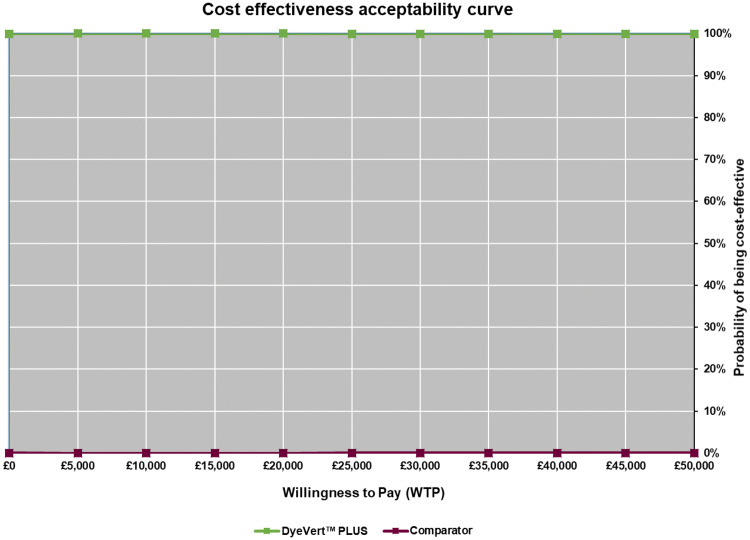
The scatter plot produced from the probabilistic analysis (Fig. 2) shows that the vast majority of points from the 10,000 iterations of the model are in the southeast quadrant of the cost-effectiveness plane (less costly and more effective), while all simulations indicate that the intervention is less costly than the comparator. Additionally, the cost-effectiveness acceptability curve (CEAC) shown in Fig. 3 (which displays the probability that the intervention is cost-effective across a range of willingness-to-pay (WTP) thresholds) indicates that the DyeVert™ PLUS EZ system has a 100% probability of being cost-effective across all WTP thresholds presented. The overall long-term cost savings for the NHS for the cohort of patients is over £19.7 million (Table 4). Total cost savings are calculated based on the total number of patients receiving the intervention in the UK over a lifetime time horizon. The cost savings are mainly driven by a lower risk of subsequent diseases and their associated costs.
Fig. 2.
Scatter plot at £20,000 WTP threshold. QALYs quality-adjusted life-years, WTP willingness to pay
Fig. 3.
Cost-effectiveness acceptability curve at various WTP thresholds (£0–50,000). WTP willingness to pay
Table 4.
Total long-term cost results (per cohort of patients (n = 45,421))
| Costs | Current practice | DyeVert™ PLUS EZ system | Difference |
|---|---|---|---|
| Cost of procedure (DAG and/or PCI) | 161,965,325 | 161,965,325 | £0 |
| Cost of DyeVert™ PLUS EZ system | £0 | 15,897,192 | 15,897,192 |
| Cost of CI-AKI and related complications (first 3 months) | 43,589,729 | 36,781,340 | − 6,808,389 |
| Cost of subsequent disease management | 880,159,158 | 851,308,760 | − 28,850,398 |
| Total costs | 1,085,714,211 | 1,065,952,617 | − 19,761,595 |
DAG coronary angiography, CI-AKI contrast-induced acute kidney injury, PCI percutaneous coronary intervention
Sensitivity Analyses
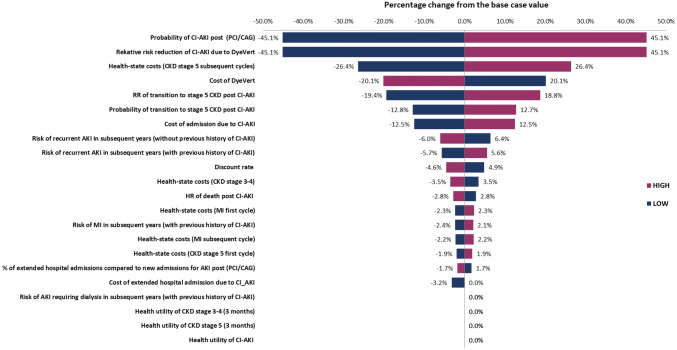
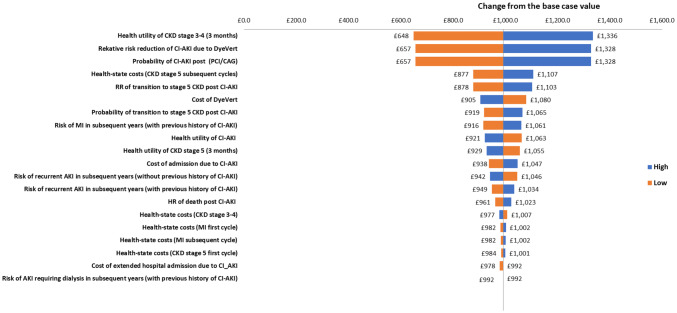
A number of model parameters were also varied in sensitivity analyses. Multiple one-way sensitivity analyses were carried out to explore the impact of increasing/decreasing relevant parameter values by 25%. Figure 4 presents the impacts of varying each input parameter value by 25% on the incremental cost of the intervention as compared to the comparator. Parameters are displayed in order, with those that have the greatest impact on incremental cost at the top and those which have the least impact at the bottom. In the base-case analysis, the incremental cost of the DyeVert™ PLUS EZ system was £448. As seen in Fig. 4 below, the probability of CI-AKI post PCI/DAG and the absolute risk reduction for CI-AKI due to the use of the DyeVert™ PLUS EZ system have the greatest impact on the incremental cost of the intervention (± 45.1%). All other parameters have moderate or minimal impacts on the incremental costs. The intervention was still cost saving (− £203) when an alternative approach (15.1%) was used to estimate the reduction in risk of CI-AKI associated with 40.1% reduction in contrast media.
Fig. 4.
Tornado diagram showing the impacts of changing the input parameters by ± 25% on the estimated incremental cost. AKI acute kidney injury, DAG diagnostic coronary angiography, CI-AKI contrast-induced acute kidney injury, CKD chronic kidney disease, MI myocardial infarction, PCI percutaneous coronary intervention, RR relative risk
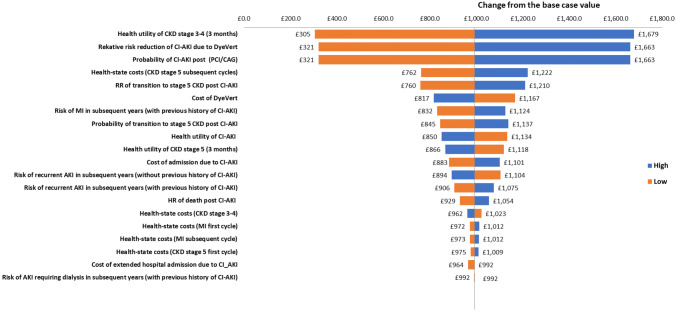
Figures 5 and 6 show the change in net monetary benefit (NMB) upon varying each parameter by 25% and 50% in either direction. The NMB was calculated as
As in the previous analysis, varying the probability of CI-AKI post-PCI/DAG, the absolute risk reduction for CI-AKI due to the use of the DyeVert™ PLUS EZ system, and the health utility of CKD (stage 3–4) have the greatest impacts on the incremental cost of the intervention. However, regardless of the direction in which these input parameters are varied (± 25% and ± 50%), the NMB of the intervention is still positive. Indeed, for all of the parameters included in this analysis, the NMB of the DyeVert™ PLUS EZ system remains positive, meaning that it is preferable to current practice from a health economic perspective.
Fig. 5.
Impact of changing the input parameters by ± 25% on the estimated NMB. AKI acute kidney injury, DAG diagnostic coronary angiography, CI-AKI contrast-induced acute kidney injury, CKD chronic kidney disease, MI myocardial infarction, PCI percutaneous coronary intervention, RR relative risk
Fig. 6.
Impact of changing the input parameters by ± 50% on the estimated NMB. AKI acute kidney injury, DAG diagnostic coronary angiography, CI-AKI contrast-induced acute kidney injury, CKD chronic kidney disease, MI myocardial infarction, PCI percutaneous coronary intervention, RR relative risk
Discussion
This study provides insight into the potential cost savings that could be made by introducing the DyeVert™ PLUS EZ system into the UK health care system for use amongst patients with CKD stage 3–4 undergoing DAG and/or PCI. CI-AKI is one of the most common forms of AKI. The technology being evaluated is a device that acts by reducing the CMV, which has been shown to be linked to CI-AKI. The clinical efficacy of the technology has been demonstrated in previous clinical studies [13, 23, 37]. Therefore, this innovative device has the potential to improve short-term health outcomes and to achieve cost savings and improved clinical outcomes in the longer term. In order to assess costs and outcomes over the patient’s lifetime, a decision-analytic model was developed.
Results of the analysis presented here point to a high likelihood that the DyeVert™ PLUS EZ system will be cost-effective over the lifetime of the patient. The probabilistic results were conclusive in that the device had a 100% probability of being cost-effective following 10,000 iterations of the model in a Monte Carlo simulation. Similarly, results of the deterministic sensitivity analyses indicated that the intervention would still be cost saving and result in a positive net monetary benefit in all scenarios assessed. Cost savings associated with the introduction of the intervention are primarily related to the impact it has on the occurrence of AKI. Not only are there immediate cost savings associated with reducing the likelihood of experiencing an AKI, but there are also longer-term savings due to the relationship between AKI and the development of CKD. See et al. [5] found that individuals who experienced AKI were at a heightened risk of experiencing new or progressive CKD. These long-term cost savings are captured in the model.
The number of studies that have been conducted to assess the relative cost-effectiveness of interventions to prevent the onset of CI-AKI are limited. As part of their guidelines on AKI [24], the National Clinical Guideline Centre (UK) developed a Markov model in 2013 to assess the cost-effectiveness of various different intravenous fluids for the prevention of this condition. The results of that study indicated that the most cost-effective strategy involved infusion with sodium chloride 0.9% and treatment with N-acetylcysteine. Additionally, a study by Hiremath et al. [38] was identified that focussed on assessing the cost-effectiveness of utilising an iso-osmolar agent, iodixanol, compared to a low-osmolar contrast agent. The results of that study indicated that the iso-osmolar agent was less costly and more effective over the patient’s lifetime than the low-osmolar agent. However, no economic evaluations focussing on the cost-effectiveness of interventions designed to divert an amount of the injected CMV were identified, which makes the study presented here unique.
There are limitations to the analysis presented here. A number of assumptions were made when populating the model due to a lack of appropriate data. Firstly, it was unknown how the risk of developing CI-AKI changes depending on whether the patient receives DAG, PCI or a combination of both. Therefore, in the model it was assumed that this risk was the same regardless of the intervention initially received. This may have the effect of over- or underestimating the risk of developing this complication, depending on the initial intervention received, and the direction of this effect is unknown. Secondly, there were no data to inform the utility value of patients with CKD stage 3–4 who have experienced a MI. Therefore, for the purpose of this analysis, it was assumed that the utility value of those patients would be same as the utility value of patients with CKD stage 3–4 who have not experienced this adverse event. Although the data used to inform the effectiveness of the DyeVert™ PLUS EZ system were derived from robust published clinical evidence, the number of studies available to inform the clinical effectiveness of the device were limited. Ideally, when modelling the impact of a healthcare technology on clinical outcomes, multiple data sources would be available to verify the clinical efficacy data being used, but that was not the case for this analysis. Finally, although evidence exists on the relationship between AKI and long-term clinical outcomes [5, 12], information is limited, which means that there is also a degree of uncertainty around the relevant data included in the model. However, to address this limitation, an extreme sensitivity analysis was performed in which the main input parameters were changed by ± 50%, but the conclusion remained stable. Additional minor assumptions were made when populating the model, but none of those were likely to have a major impact on the final model results.
Despite the limitations highlighted above, a robust decision-analytic model was developed. The model was informed by clinical guidelines, published literature and expert clinical input, and any assumptions that were made in the analysis can be rectified by using more robust data in later studies, as a model now exists for re-analysis once additional information becomes available.
Conclusion
The economic analysis presented in this study has shown that the introduction of the DyeVert™ PLUS EZ system has the potential to reduce costs for the UK health care service and to improve quality of life and clinical outcomes for patients with CKD stage 3–4 undergoing angiographic procedures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to show our gratitude to all team members who provided insight and expertise that greatly assisted the research.
Author Contributions
MJ and AM were responsible for developing and populating the economic model and drafting the first version of the manuscript. All authors provided inputs for the model and read and approved the final draft of the manuscript.
Data Availability Statement
The authors declare that all of the data supporting the findings of this study are available within the article (or the Electronic supplementary material for the article).
Compliance with Ethical Standards
Funding
This report is independent research funded by Osprey Medical Corporation (Grant no. 2019-1).
Conflict of interest
MRH, AZ, YN, DOD, FFO and SW have no conflicts of interest that are directly relevant to the content of this article. Device Access (MJ, AM and MBH) received funds from Osprey Medical Corporation when the study was being conducted.
References
- 1.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368–375. doi: 10.1016/S0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 2.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–2264. doi: 10.1161/01.CIR.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 3.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95(1):13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 4.Kerr M, Bedford M, Matthews B, O’Donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014;29(7):1362–1368. doi: 10.1093/ndt/gfu016. [DOI] [PubMed] [Google Scholar]
- 5.See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160–72. [DOI] [PubMed]
- 6.Mohammed NMA, Mahfouz A, Achkar K, Rafie IM, Hajar R. Contrast-induced nephropathy. Heart Views. 2013;14(3):106–116. doi: 10.4103/1995-705X.125926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nusca A, Miglionico M, Proscia C, Ragni L, Carassiti M, Lassandro Pepe F, et al. Early prediction of contrast-induced acute kidney injury by a “bedside” assessment of Neutrophil Gelatinase-Associated Lipocalin during elective percutaneous coronary interventions. PLoS One. 2018;13(5):e0197833. doi: 10.1371/journal.pone.0197833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pattharanitima P, Tasanarong A. Pharmacological strategies to prevent contrast-induced acute kidney injury. Biomed Res Int. 2014;2014:236930. doi: 10.1155/2014/236930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 10.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–619. [DOI] [PubMed]
- 11.James MT, Samuel SM, Manning MA, Tonelli M, Ghali WA, Faris P, et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6(1):37–43. doi: 10.1161/CIRCINTERVENTIONS.112.974493. [DOI] [PubMed] [Google Scholar]
- 12.Valle JA, McCoy LA, Maddox TM, Rumsfeld JS, Ho PM, Casserly IP, et al. Longitudinal risk of adverse events in patients with acute kidney injury after percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2017;10:4. [DOI] [PubMed]
- 13.Desch S, Fuernau G, Poss J, Meyer-Saraei R, Saad M, Eitel I, et al. Impact of a novel contrast reduction system on contrast savings in coronary angiography—the DyeVert randomised controlled trial. Int J Cardiol. 2018;257:50–53. doi: 10.1016/j.ijcard.2017.12.107. [DOI] [PubMed] [Google Scholar]
- 14.Gurm HS, Mavromatis K, Bertolet B, Kereiakes DJ, Amin AP, Shah AP, et al. Minimizing radiographic contrast administration during coronary angiography using a novel contrast reduction system: a multicenter observational study of the DyeVert plus contrast reduction system. Catheter Cardiovasc Interv. 2019;93(7):1228–1235. doi: 10.1002/ccd.27935. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence. The guidelines manual. London: National Institute for Health and Care Excellence; 2012.
- 16.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. In: Sculpher MJ, Claxton K, Stoddart GL, Torrance GW, editors. Methods for the economic evaluation of health care programmes. New York: Oxford University Press; 2015. [Google Scholar]
- 17.Office of National Statistics. National life tables, United Kingdom, 1980–1882 to 2015–2017. London: Office of National Statistics; 2018.
- 18.NHS Improvement. NHS reference costs. London: NHS Improvement; 2018. https://improvement.nhs.uk/resources/reference-costs/. Accessed Aug 2019.
- 19.Gurm HS, Seth M, Mehran R. Impact of contrast dose reduction on incidence of acute kidney injury (AKI) among patients undergoing PCI: a modeling study. J Invas Cardiol. 2016;28(4):142–146. [PubMed] [Google Scholar]
- 20.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69(2):375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 21.James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet (London, England). 2010;376(9758):2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 22.Department of Health. Reference costs 2017–2018. London: Department of Health; 2018.
- 23.Gurm HS, Mavromatis K, Bertolet B, Kereiakes DJ, Amin AP, Shah AP, et al. Minimizing radiographic contrast administration during coronary angiography using a novel contrast reduction system: a multicenter observational study of the DyeVert PLUS contrast reduction system. Cathet Cardiovasc Interv; 2019:93(7):1228–35. [DOI] [PubMed]
- 24.National Institute for Health and Care Excellence. CG169. Acute kidney injury: prevention, detection and management. London: National Institute for Health and Care Excellence; 2013. https://www.nice.org.uk/guidance/cg169. Accessed Aug 2019.
- 25.Villar E, Remontet L, Labeeuw M, Ecochard R. Effect of age, gender, and diabetes on excess death in end-stage renal failure. J Am Soc Nephrol. 2007;18(7):2125. doi: 10.1681/ASN.2006091048. [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Care Excellence. Ticagrelor for the treatment of acute coronary syndromes: Technology appraisal guidance (TA236). London: National Institute for Health and Care Excellence; 2011. https://www.nice.org.uk/guidance/ta236.
- 27.Walker S, Asaria M, Manca A, Palmer S, Gale CP, Shah AD, et al. Long-term healthcare use and costs in patients with stable coronary artery disease: a population-based cohort using linked health records (CALIBER) Eur Heart J Qual Care Clin Outcomes. 2016;2(2):125–140. doi: 10.1093/ehjqcco/qcw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osprey Medical Corporation. Corporate website. Minnetonka: Osprey Medical Corporation; 2019. https://ospreymed.com/. Accessed Aug 2019.
- 29.Tajima R, Kondo M, Kai H, Saito C, Okada M, Takahashi H, et al. Measurement of health-related quality of life in patients with chronic kidney disease in Japan with EuroQol (EQ-5D) Clin Exp Nephrol. 2010;14(4):340–348. doi: 10.1007/s10157-010-0304-1. [DOI] [PubMed] [Google Scholar]
- 30.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316(7133):736–41. [DOI] [PMC free article] [PubMed]
- 31.Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Mak. 2011;31(6):800–804. doi: 10.1177/0272989X11401031. [DOI] [PubMed] [Google Scholar]
- 32.Curtis L, Burns A. Unit costs of health and social care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- 33.Joint Formulary Committee. British national formulary online. 2017. https://www.bnf.org/products/bnf-online/ [DOI] [PMC free article] [PubMed]
- 34.NICE. Acute kidney injury: Prevention, detection and management up to the point of renal replacement therapy (guidance). London: National Institute for Health and Clinical Excellence; 2013.
- 35.Bank of England. Inflation calculator. 2018. https://www.bankofengland.co.uk/monetary-policy/inflation/inflation-calculator. Accessed 12 Nov 2018.
- 36.Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27 Suppl 3:iii73–III80. doi: 10.1093/ndt/gfs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corcione N, Biondi-Zoccai G, Ferraro P, Messina S, Maresca G, Avellino R, et al. Contrast minimization with the new-generation DyeVert plus system for contrast reduction and real-time monitoring during coronary and peripheral procedures: first experience. J Invas Cardiol. 2017;29(8):259–262. [PubMed] [Google Scholar]
- 38.Hiremath S, Akbari A, Wells GA, Chow BJW. Are iso-osmolar, as compared to low-osmolar, contrast media cost-effective in patients undergoing cardiac catheterization? An economic analysis. Int Urol Nephrol. 2018;50(8):1477–1482. doi: 10.1007/s11255-018-1874-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all of the data supporting the findings of this study are available within the article (or the Electronic supplementary material for the article).