Abstract
The global epidemiology of coronavirus disease 2019 (COVID-19) suggests a wide spectrum of clinical severity, ranging from asymptomatic to fatal. Although the clinical and laboratory characteristics of COVID-19 patients have been well characterized, the pathophysiological mechanisms underlying disease severity and progression remain unclear. This review highlights key mechanisms that have been proposed to contribute to COVID-19 progression from viral entry to multisystem organ failure, as well as the central role of the immune response in successful viral clearance or progression to death.
Keywords: coagulation, COVID-19, cytokine storm, multisystem organ failure, pathophysiolog
Introduction
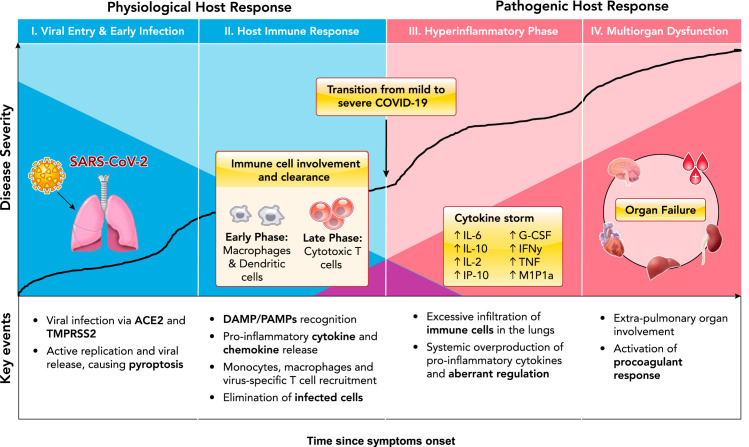
Coronavirus disease 2019 (COVID-19) is caused by a novel beta-coronavirus known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). As of June 15, 2020, the number of global confirmed cases has surpassed 8 million, with over 400,000 reported mortalities. The unparalleled pathogenicity and global impact of this pandemic has rapidly engaged the scientific community in urgently needed research. Preliminary reports from the Chinese Center for Disease Control and Prevention have estimated that the large majority of confirmed SARS-CoV-2 cases are mild (81%), with ~14% progressing to severe pneumonia and 5% developing acute respiratory distress syndrome (ARDS), sepsis, and/or multisystem organ failure (MOF) (144). Although more data is urgently needed to elucidate the global epidemiology of COVID-19 (80), a wide spectrum of clinical severity is evident, with most patients able to mount a sufficient and appropriate immune response, ultimately leading to viral clearance and case resolution. However, a significant subset of patients present with severe clinical manifestations, requiring life-supporting treatment (51). The pathophysiological mechanisms behind key events in the progression from mild to severe disease remain unclear, warranting further investigation to inform therapeutic decisions. Here, we review the current literature and summarize key proposed mechanisms of COVID-19 pathophysiological progression (FIGURE 1).
FIGURE 1.
Characterization of key events in COVID-19 disease pathophysiological progression
The dark blue shading indicates physiological viral host response over time, and the dark red shading indicates pathogenic hyperinflammatory host response over time. Figure adapted from Ref. 124, with permission from the Journal of Heart and Lung Transplantation.
Key Pathophysiological Mechanisms: Our Current Understanding
Viral Invasion
The first step in COVID-19 pathogenesis is viral invasion via its target host cell receptors. SARS-CoV-2 viral entry has been described in detail elsewhere (138). In brief, SARS-CoV-2 consists of four main structural glycoproteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N). The M, E, and N proteins are critical for viral particle assembly and release, whereas the S protein is responsible for viral binding and entry into host cells (33, 76, 89, 143, 148). Similar to SARS-CoV, several researchers have identified human angiotensin converting enzyme 2 (ACE2) as an entry receptor for SARS-CoV-2 (75, 99, 148, 156). SARS-CoV-2 is mostly transmissible through large respiratory droplets, directly infecting cells of the upper and lower respiratory tract, especially nasal ciliated and alveolar epithelial cells (161). In addition to the lungs, ACE2 is also expressed in various other human tissues, such as the small intestine, kidneys, heart, thyroid, testis, and adipose tissue, indicating the virus may directly infect cells of other organ systems when viremia is present (77). Interestingly, although the S proteins of SARS-CoV-2 and SARS-CoV share 72% homology in amino acid sequences, SARS-CoV-2 has been reported to have a higher affinity for the ACE2 receptor (18, 21, 143).
Following host cell binding, viral and cell membranes fuse, enabling the virus to enter into the cell (89). For many coronaviruses, including SARS-CoV, host cell binding alone is insufficient to facilitate membrane fusion, requiring S-protein priming or cleavage by host cell proteases or transmembrane serine proteases (9, 10, 90, 94, 108). Indeed, Hoffman and colleagues demonstrated that S-protein priming by transmembrane serine protease 2 (TMPRSS2), which may be substituted by cathepsin B/L, is required to facilitate SARS-CoV-2 entry into host cells (58). In addition, unlike other coronaviruses, SARS-CoV-2 has been reported to possess a furin-like cleavage site in the S-protein domain, located between the S1 and S2 subunits (31, 138). Furin-like proteases are ubiquitously expressed, albeit at low levels, indicating that S-protein priming at this cleavage site may contribute to the widened cell tropism and enhanced transmissibility of SARS-CoV-2 (123). However, whether furin-like protease-mediated cleavage is required for SARS-CoV-2 host entry has yet to be determined. Blocking or inhibiting these processing enzymes may serve as a potential antiviral target (130). Interestingly, SARS-CoV-2 has developed a unique S1/S2 cleavage site in its S protein, characterized by a four-amino acid insertion, which seems to be absent in all other coronaviruses (4). This molecular mimicry has been identified as an efficient evolutionary adaptation that some viruses have acquired for exploiting the host cellular machinery. Once the nucleocapsid is deposited into the cytoplasm of the host cell, the RNA genome is replicated and translated into structural and accessory proteins. Vesicles containing the newly formed viral particles are then transported to and fuse with the plasma membrane, releasing them to infect other host cells in the same fashion (33, 89, 105). Although much progress has been made in our understanding of the mechanisms underlying SARS-CoV-2 invasion, additional research is needed to delineate exactly how cleavage of the S proteins by TMPRSS2 confers viral particle entry as well as how S-protein cleavage by membrane proteases contributes to viral penetration.
Host Response
Initial Physiological Immune Response
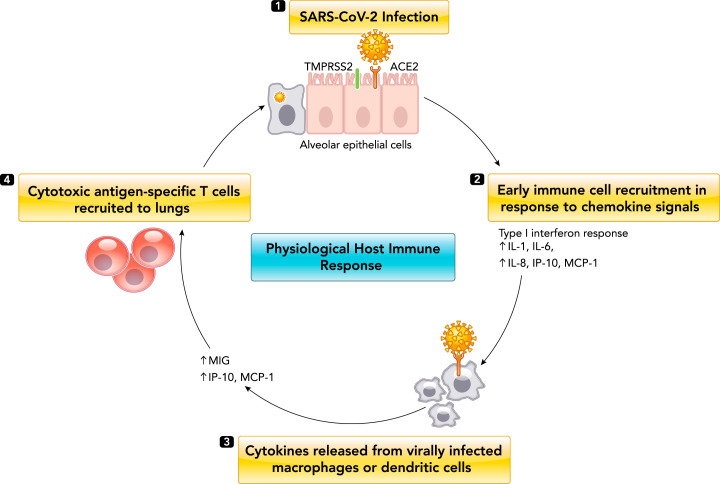
A timely, localized, and well-coordinated immune response presents the first line of physiological defense against SARS-CoV-2 infection (FIGURE 2). Similar to other cytopathic viruses, SARS-CoV-2 infection induces cellular death and injury in airway epithelial cells through diverse processes such as pyroptosis (19, 153). Viral-mediated cell death causes release of various damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), which are believed to be recognized by pattern-recognition receptors on alveolar macrophages and endothelial cells. For example, Toll-like receptors (TLRs) recognize PAMPs in mostly the extracellular space, triggering induction of proinflammatory cytokine transcription factors such as NF-κβ, as well as activating interferon regulatory factors that mediate the type I interferon-dependent antiviral response (122, 125). In contrast, nucleotide-binding domain leucine-rich repeat (NLR) proteins recognize DAMPs expressed intracellularly, thus triggering activation of inflammasomes and conversion of proIL-1β to active IL-1β (122, 125). Circulating levels of IL-1β in COVID-19 patients suggests local inflammasome activation with no systemic manifestations (61). In total, these processes foster an increased secretion of proinflammatory cytokines and chemokines, such as IL-6, type II interferon (IFNγ), monocyte chemoattractant protein 1 (MCP1), and interferon gamma-induced protein 10 (IP-10), as well as subsequent pulmonary recruitment of immune cells, including macrophages and dendritic cells. Direct viral infection of macrophages and/or dendritic cells is estimated to propagate further cytokine and chemokine release, subsequently activating late-phase immune-cell recruitment of antigen-specific T cells to destroy virally infected alveolar cells (61, 130, 132, 149). In addition to cytokine release and immune cell recruitment, another potential mechanism that could contribute to successful viral clearance is antibody neutralization. Current literature suggests seroconversion in COVID-19 patients occurs ~7–14 days post symptom onset (12). However, antibody kinetics of different immunoglobulins have not been well characterized, and reported findings are conflicting (12). Although currently available commercial serological assays do not provide information on whether SARS-CoV-2 antibodies confer immune protection, recent reports using specialized laboratory-based neutralization assays have observed a marked correlation between the levels of SARS-CoV-2 spike/receptor binding domain (RBD) antibodies and the neutralization capacity of patient sera, suggesting its potential beneficial role in clearance (3, 98, 103, 107, 160).
FIGURE 2.
Physiological host immune response to SARS-CoV-2 infection
1: SARS-CoV-2 enters alveolar epithelial cells by binding to angiotensin converting enzyme 2 (ACE2) through surface spike (S) protein mediated by transmembrane serine protease 2 (TMPRSS2). 2: pulmonary recruitment of macrophages and dendritic cells in response to chemokine and cytokine release (early phase). 3: direct viral infection of pulmonary macrophages and dendritic cells causes expression of several proinflammatory cytokines and chemokines. 4: dendritic cells phagocytose virus in the lungs, migrate to secondary lymphoid organs, and activate antigen-specific T cells, which travel to the lungs and destroy virally infected alveolar cells.
In most COVID-19 patients, the combined immune response of initial cytokine release and activation of antiviral interferon response followed by immune-cell recruitment should result in successful SARS-CoV-2 clearance from the lungs (FIGURE 2). However, as has been reported extensively, viral infection can progress to severe disease due to dysregulated immune response.
Induction of Marked and Pathogenic Proinflammatory Cytokine Storm
Several cohort studies have observed markedly elevated levels of circulating proinflammatory cytokines and chemokines, significantly correlating to disease severity and mortality. The main drivers of this response have been postulated and thoroughly reviewed elsewhere (125, 130, 151). Notably, increased levels of IL-6, IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (G-CSF), IP-10, MCP1, IFNγ, macrophage inflammatory protein 1α (MIP1α), and tumor necrosis factor (TNF) have all been implicated in COVID-19 severity, suggesting a combined T-helper type 1 (Th1) and Th2 cell response (61, 130). In particular, IL-6 has emerged as a candidate treatment target due to its robust association with disease progression. A recent meta-analysis suggested serum IL-6 cut-offs of >55 pg/ml and >80 pg/ml to identify patients at high risk for severe COVID-19 and mortality, respectively (5). Prospective validation of these proposed cut-offs across different assay methodologies and patient populations are urgently awaited to establish clinical utility. Notably, the cytokine concentrations observed in hospitalized COVID-19 patients are rarely elevated to the same extent as in secondary hemophagocytic lymphohistiocytosis and cytokine release syndrome following CAR-T cell treatment (64).
In addition to the observed maladaptive cytokine release, elevations in more traditional biochemical markers of acute infection, including C-reactive protein (CRP) and ferritin (both positive acute phase reactants), as well as continual decreases in lymphocytes and significant elevations in neutrophils, are evident (43, 79). As such, the neutrophil-to-lymphocyte ratio appears to be a useful indicator of disease prognostication and management (83). The mechanisms behind progressive lymphopenia in severe COVID-19 remain unclear, although T-cell redistribution via pulmonary recruitment, exhaustion, as well as depletion through TNF-α-mediated apoptosis or even direct cytopathic injury have been suggested (35, 147). It is also important to note that immune-cell infiltration can lead to the excessive secretion of proteases and reactive oxygen species, fostering further damage and hyperinflammation (130). In addition, direct viral infection of immune cells such as monocytes and macrophages have been proposed to contribute to dysregulated immune response, as has been observed in SARS (23, 52, 136). Nevertheless, the exact contribution of direct viral immune cell infection is unknown and highly debated (155). Finally, recent data also suggest SARS-CoV-2-specific antibody titers are elevated in patients with severe disease (98). It is unclear whether increased antibody prevalence in severe COVID-19 patients suggests potential antibody-dependent enhancement (ADE) or is simply a result of higher viral antigen exposure. Further studies are needed to evaluate the contribution of antibodies to both physiological and pathogenic host response (39, 160).
Prominent Changes in Hematology and Coagulation
Since a hyperinflammatory profile consistent with cytokine storm has been robustly associated with COVID-19 severity and suggested as the predominant cause of patient mortality, most initial literature has focused on the dysregulation of immune response in COVID-19 patients and the potential value of immune modulating treatments. However, evidence of alarming coagulation abnormalities and high incidence of thrombotic events in COVID-19 patients is prevalent (70). Early reports from Wuhan, China demonstrated prolonged activated partial thromboplastin time (aPTT) and prothrombin time, and elevated D-dimer as well as thrombocytopenia (20, 139, 155). In a more in-depth study of 183 patients by Tang et al., 71.4% of non-survivors and 0.6% of recovered cases met the criteria for disseminated intravascular coagulation during hospitalization (128). In addition to prolonged prothrombin time, studies in other cohorts have reported high prevalence of lupus anticoagulant in the circulation (13). Recent autopsy data from Italy also observed fibrin thrombi in pulmonary small arterial vessels in 87% of fatal cases examined, suggesting the contribution of coagulation in diffuse alveolar and endothelial damage (15). These data clearly suggest a state of hypercoagulability in severe COVID-19.
Although prominent changes in blood coagulation may be a contributing mechanism to COVID-19 mortality, its pathogenesis is estimated to be tightly linked to inflammation and cytokine release. Specifically, immunothrombosis is a phenomenon known to occur as a result of host defense against various pathogens, including viral infection (30). For example, the activation of complement pathways can lead to initiation of the coagulation cascade (30, 127). Complement-mediated pulmonary tissue damage and microvascular injury have been observed in small cohorts with severe COVID-19 (85). Procoagulant response is also associated with the inflammatory effects of cytokines in the vascular endothelium, including increased vascular permeability and damage as a result of immune-cell infiltration (62). Given the correlation of IL-6 levels with increased fibrinogen and D-dimer in severe COVID-19 patients, it is likely that cytokine-mediated procoagulant changes are partially responsible for the specific thrombosis profile observed in critically ill patients (41, 110). Presence of neutrophil extracellular traps (NETs) are also possibly linked to COVID-19 thrombosis via activation of intrinsic coagulation (8, 50, 162). Overall, the predominant mechanism seems that encompassing SARS-CoV-2-induced endothelial damage fosters monocyte recruitment and activation, along with tissue factor exposure, which then activates blood coagulation. Recruitment of neutrophils by activated endothelial cells can also synthesize and release multiple cytokines into the circulation, further accelerating this process (93). In addition to the coagulopathy observed in COVID-19, severe bleeding in patients is rare in comparison to other RNA-type viruses with hemorrhagic manifestations (30). However, a recent report in Blood characterized bleeding as a significant cause of morbidity in COVID-19 patients, emphasizing the need for randomized trials on the benefit of escalated prophylaxis (1). By taking these data into consideration, a close connection between the inflammatory and coagulation response of COVID-19 patients appears to exist, wherein treatment options for both contributing factors should be explored.
Extrapulmonary Involvement and Progression to Multisystem Organ Failure
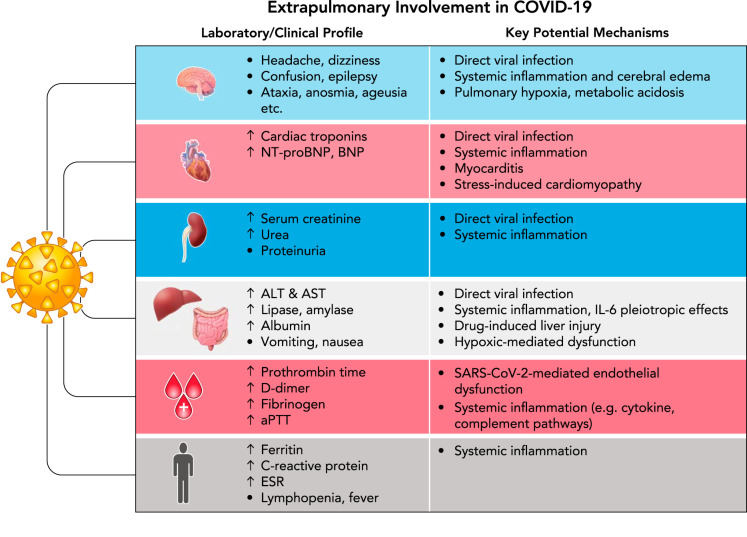
One of the key hallmarks of COVID-19 severity is the progression to systemic disease characterized by multisystem organ damage or failure. Many groups have suggested extrapulmonary involvement in COVID-19 is a direct result of unrestrained inflammation. However, other contributing mechanisms have been proposed and are explored below (FIGURE 3).
FIGURE 3.
Laboratory/clinical profile and key potential mechanisms underlying extrapulmonary manifestations observed in severe COVID-19 patients
NT-proBNP, NH2-terminal-proB-type natriuretic peptide; ALT, alanine aminotransferase; AST, aspartate aminotransferase; aPTT, activated partial thromboplastin time; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; ESR, erythrocyte sedimentation rate.
Cardiovascular Complications
Significant cardiovascular damage has been observed in severe COVID-19 patients. Several studies have demonstrated significantly elevated levels of classical markers of cardiac injury and failure [i.e., cardiac troponin and brain natriuretic peptides (BNP)] in patients with greater disease severity (53a, 78). Notably, increasing cardiac troponin levels have been correlated to other inflammatory markers, such as CRP, ferritin, and IL-6, suggesting inflammatory damage as opposed to primary myocardial injury (28). Maladaptive cytokine release is known to directly affect cardiomyocytes as well as to lead to endothelial cell reprogramming and dysfunction, supporting their causative role in COVID-19 cardiovascular manifestations (71, 131). However, it is important to note that a handful of studies have described patients presenting with primary cardiac symptoms, suggesting myocarditis and stress-related cardiomyopathy due to respiratory failure and hypoxemia (60, 63, 152). Currently, there is insufficient evidence to support direct viral infection of cardiomyocytes, although SARS-CoV-2 genomes have been effectively detected in endomyocardial biopsies, mostly involving immune cell infiltrates (40, 149). Previous data from the SARS epidemic suggests 35% of heart specimens showed presence of viral RNA in the myocardium. Given the homology between these viruses, such direct viral invasion should not be discounted (100, 106).
Renal Injury and Failure
In addition to cardiovascular damage, renal involvement is frequently observed in COVID-19, varying from mild proteinuria and minor serum creatinine elevations to acute kidney injury (AKI) and renal failure. Initial studies have reported varying incidences (3–15%) of AKI during illness (20, 22, 155). Now considered a valuable prognostic indicator for COVID-19 survival, AKI is estimated to affect 20–40% of critically ill patients in intensive care, necessitating renal replacement therapy and extracorporeal support therapies such as blood purification (112, 155). An understanding of the complex and likely multifactorial pathophysiological mechanisms behind kidney failure in COVID-19 is thus needed for early recognition and appropriate treatment selection. Direct renal infection and damage presents one potential contributing mechanism. ACE2 is expressed in the kidney, and although previous studies suggested absence of viral particles in postmortem renal specimens from SARS patients (27), electron microscopic examination of 26 postmortem COVID-19 patients demonstrated direct virulence in tubular epithelium and podocytes (126). Direct SARS-CoV-2 infection of the renal epithelium is estimated to result in mitochondrial dysfunction, acute tubular necrosis, and protein leakage (72, 118). In addition to direct infection, uncontrolled cytokine release, thrombosis, and ischemia can also result in further kidney dysfunction, characterized by intrarenal inflammation, increased vascular permeability, and volume depletion (88). Cytokine-mediated inflammatory AKI has been described previously in the literature in other clinical contexts such as CAR-T-cell treatment in cancer patients (102, 104, 117).
Gastrointestinal, Hepatic, and Pancreatic Manifestations
The involvement of the gastrointestinal (GI) tract and hepatic system in COVID-19 disease progression is being increasingly reported. The most common GI manifestations reported in both adult and especially pediatric COVID-19 patients include diarrhea, nausea, vomiting, and abdominal pain (16, 133, 157). Similar to renal COVID-19 involvement, there is evidence of direct SARS-CoV-2 GI infection through isolation of viral RNA from GI epithelial cells (146). It is thus hypothesized that the GI manifestations observed in COVID-19 are a result of SARS-CoV-2 infection of intestinal enterocytes and subsequent dysfunction in the ileum and colon (16). The association of GI manifestations with disease severity is not well described, with many conflicting results reported (25, 139, 154).
In addition to GI manifestations, several studies have reported elevated liver enzymes and higher rates of liver injury in patients with severe COVID-19. Specifically, in a study of 417 COVID-19 patients, 76.3% had abnormal liver tests, and 21.5% had liver injury during hospitalization (14). Patients with abnormal liver function tests, particularly elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST), also had significantly higher risk of developing severe pneumonia (14). These findings have been observed in numerous studies, and several potential pathophysiological mechanisms have been proposed (11, 20, 42, 61, 74, 139, 141). First, there is potential for ACE2-mediated liver dysfunction. Although hepatocytes have not been shown to exhibit high ACE2 expression, previous studies have demonstrated a high level of ACE2 expression in cholangiocytes, suggesting direct bile duct infection/damage as a potential cause of abnormal liver enzymes (17). This, however, is unlikely since significant increases in circulating levels of common bile duct injury markers (e.g., serum bilirubin, gamma glutamyltransferase, and alkaline phosphatase) have not been extensively reported (7). Contrary to earlier studies, a recent study by Wang et al. observed abundant SARS-CoV-2 viral particles in hepatocytes of postmortem specimens, prompting further research on hepatic viral infection/clearance (141). Additional pathophysiological mechanisms underlying liver injury include drug-induced liver injury as well as hypoxic hepatitis. However, the validity of these mechanisms have been debated, since abnormal liver enzymes have been reported at hospital admission before any drug treatment as well as in patients without the need for mechanical ventilation (7). A more plausible mechanism behind liver dysfunction in COVID-19 is the observed systemic inflammatory response, as described previously, leading to cytotoxic T-cell-mediated necrosis and MOF. The pleiotropic hepatic effects of IL-6 could play a particularly important role, inducing expression of serum amyloid A, fibrinogen, and CRP (121).
Pancreatic injury has also been reported in patients with COVID-19. Single-cell RNA sequencing suggests that ACE2 is expressed in both the exocrine and islet cells of the pancreas (81). In terms of exocrine-related damage, a study by Wang et al. reported that 17% of COVID-19 patients in their cohort (n = 52) had serologic evidence of exocrine pancreatic injury, defined as elevated amylase or lipase (140). In a more recent study, hyperlipasemia was reported in 12.1% of COVID-19 patients (n = 71) but was not associated with worse outcome (91). Due to the low specificity of lipase elevations, exocrine pancreatic injury and inflammation is challenging to confirm without abdominal imaging (32). Few case reports have observed acute pancreatitis in COVID-19 patients (2, 45, 54), although it is expected to be quite uncommon. Some authors have proposed this is due to direct exocrine damage, whereas others suggest it is likely resultant from the gastrointestinal symptoms observed in many COVID-19 patients (32). In addition to exocrine damage, there is much debate regarding the impact of COVID-19 on the endocrine pancreas and its subsequent effect on glucose regulation. Acute diabetes has been previously observed in SARS-CoV patients (150). Although direct damage of pancreatic β-cells has been proposed as a plausible mechanism behind this phenotype, immune destruction of β-cells has also been suggested in addition to bystander death due to exocrine infection (101). Importantly, COVID-19 appears to enhance complications in patients with diabetes, likely due to viral-induced pancreatic dysfunction as well as associated immune dysregulation, vasculopathy, and coagulopathy (29, 37).
Central Nervous System Involvement
The neurological manifestations of COVID-19 have not been of much focus in the literature, but a few published reports are concerning. In a case study series of 214 patients diagnosed with COVID-19, neurological symptoms were observed in 36.4% of patients, and this percentage increased to 45.5% when examining patients with severe infection (86). The reported neurological manifestations of COVID-19 include headache, dizziness, confusion, epilepsy, ataxia (lack of voluntary muscle movement), altered sense of smell (hyposmia/anosmia), loss of taste (ageusia), and Guillain-Barré syndrome, among others (97, 115, 134). Altered sense of taste or smell can be present in up to 80% of patients with mild to moderate COVID-19 (73). The underlying pathophysiology of the loss of these olfactory and gustatory perceptions have been postulated to be related to direct damage of the supporting cells of the olfactory epithelium, olfactory bulb and altered function of the olfactory neurons, altered ACE2 signal transmission, and accelerated gustatory particle degradation by sialic acid (87, 137). Autopsy findings in SARS-CoV infections have shown strong evidence of neuro-invasion, with demonstrated viral presence in the cerebrospinal fluid (6, 95). The pathophysiological mechanisms behind the neurological manifestations of COVID-19 have not been well elucidated. Potential mechanisms include 1) viral entry via ACE2 receptors into the endothelia that line the blood capillaries and subsequent neuro-invasion, 2) neurological edema and brain stem compression as a result of breached blood-brain barrier, 3) neurological edema and hypercoagulability as a result of cytokine storm syndrome, and 4) propagation via mechanoreceptors and chemoreceptors in the lung and lower respiratory airways (65). Importantly, it is possible that the neurological manifestations of COVID-19 could be a result of hypoxia, respiratory, and/or metabolic acidosis at end-stage disease (6).
Pediatrics and Pregnancy: Special Considerations
The pathophysiological mechanisms proposed above primarily relate to observations in nonpregnant adult patients. Although the clinical picture of COVID-19 in pediatrics and pregnancy is less understood, their respective characteristics appear different when compared with nonpregnant adults.
Pediatrics
In a case study series of >2,000 children with suspected or confirmed COVID-19 in China, 5% of symptomatic children had dyspnea or hypoxemia, and only 0.6% progressed to ARDS or MOF (36). A multicenter European study of children with PCR-confirmed SARS-CoV-2 infection also reported that 8% of pediatric patients required ICU admission, 4% required mechanical ventilation, 3% required inotropic support, and <1% required extracorporeal membrane oxygenation (49). Although these reports indicate a milder COVID-19 profile in pediatric patients compared with adults (159), reports from China and the CDC indicate that the documented hospitalization and mortality rates in pediatric cases are concerning and emphasize the importance of comprehensive studies to examine the clinical picture of pediatric disease (15a, 36). Interestingly, current evidence suggests that the laboratory profile observed in pediatric COVID-19 patients is different from that of adults. A recent meta-analysis identified 24 studies, including a total of 624 pediatric cases with PCR-confirmed COVID-19, and reported common laboratory abnormalities in mild and severe disease. Their study demonstrated frequent elevations in CRP, procalcitonin, and LDH in severe pediatric COVID-19, similar to adult findings (56). However, no consistent trend in lymphocyte count was reported (56). This is surprising since lymphopenia has been estimated to be one of the most consistent laboratory abnormalities in adult patients with severe COVID-19 illness (57). It is important to note that the heterogeneous standards used to interpret laboratory tests in pediatrics could contribute to the variation observed in study findings. More comprehensive studies based on larger sample sizes are needed to better characterize the laboratory and clinical profile of mild versus severe pediatric COVID-19 and to help develop our understanding of immune pathogenesis.
Increasing evidence also suggests the emergence of an associated multisystem inflammatory condition with similar features to Kawasaki disease and toxic shock syndrome in a small subset of pediatric patients (24, 26, 34, 44, 67, 113). This condition appears to be associated with prevalent cutaneous manifestations as well as significant GI symptoms. Some cases of cutaneous manifestations in adult COVID-19 patients have been reported, although varying incidence among patients has been noted (68, 111, 120). The urgent need to appropriately identify these patients has led the World Health Organization (WHO) and other regulatory bodies to develop a preliminary case definition known as Multisystem Inflammatory Disorder in Children and adolescents (MIS-C) (142a). Interestingly, a recent study characterizing a small cohort of previously healthy children and adolescents who developed an inflammatory profile related to COVID-19 in New York City described a unique cytokine pattern characterized by elevated IL-6 and IL-10 production, as well as increased interferon signaling components. Increases in TNF-α were not observed in contrast to adult patients (24). A recent, large, multi-center U.S. study of 186 patients who met the broad CDC criteria for MIS-C reported 92% of patients had at least four laboratory results indicating inflammation, including but not limited to elevated CRP and ferritin, lymphocytopenia, neutrophilia, hypoalbuminemia, thrombocytopenia, anemia, as well as elevated D-dimer and fibrinogen (44). Elevations in troponin and brain natriuretic peptide were also observed in the majority of patients (44). The pathophysiological mechanisms behind this novel disease are unknown. Some have suggested MIS-C is mainly resultant from post-infectious IgG-mediated enhancement, whereas others have proposed it is due to blockage of type I and III interferon responses, leading to uncontrolled viral replication and high viral load (119). Due to the paucity of data in this area, further research is required to elucidate what mechanisms confer protection from COVID-19 in most pediatric patients as well as what factors predispose children to progress to MIS-C.
Pregnancy
Evaluating the risk of severe outcomes of SARS-CoV-2 infection in pregnant women is imperative for both mother and child. Several original studies and systematic reviews have been completed, assessing clinical characteristics of pregnant women with COVID-19 (46, 69, 135). Interestingly, most studies report similar clinical characteristics and mortality rates in pregnant women with COVID-19 compared with nonpregnant women of reproductive age (48). This is in contrast to what has been observed in other respiratory viruses, including SARS-CoV (142). Some have suggested this is likely a result of the physiological immune adaptions that occur during pregnancy, preventing escalation to the hyperinflammatory phase of COVID-19 (48). However, despite evidence of mild COVID-19 in pregnant patients, a recent report by the CDC suggests pregnant women may be at higher risk for more severe outcomes, estimating a higher proportion of pregnant women with COVID-19 undergo hospitalization compared with nonpregnant women (38). This study, however, is limited by the lack of information regarding whether hospital admission was due to COVID-19 illness or pregnancy-related conditions, complicating interpretation (38). In addition to these reports, there is increasing evidence of higher rates of miscarriage and preeclampsia in pregnant women with SARS-CoV-2 infection, suggesting placental involvement (5a). Most studies have reported no evidence of detectable SARS-CoV-2 RNA in the placenta. However, a recent case report showed evidence of SARS-CoV-2 in the syncytiotrophoblast cells of a pregnant COVID-19 patient in the second trimester of gestation with preeclampsia (59). A unique correlation between the laboratory profile observed in pregnant patients with preeclampsia and COVID-19 also appears to exist, prompting questions of shared disease pathways (116). Finally, it is important to note that current evidence suggests vertical transmission of SARS-CoV-2 is unlikely (55). However, as described above, there is potential for SARS-CoV-2 to significantly affect the placenta and thus negatively impact fetal development. Further research is urgently needed to better characterize the clinical picture of COVID-19 at each trimester of pregnancy.
Future Directions and Unanswered Questions
Most of our knowledge on COVID-19 pathophysiological progression has been observed through a laboratory lens, inferring potential causative mechanisms from observed biomarker trends across patients. Based on the current evidence, it is clear that, although direct SARS-CoV-2 infection of multiple organs as well as hypoxia and stress-related injury may contribute to COVID-19 pathophysiological progression, systemic inflammation and aberrant cytokine regulation is a hallmark of disease severity. Considering this, it is still unclear what factors influence the transition from normal physiological to pathogenic hyperinflammatory response. The nuances of age-related immune response appear to play a role, with increasing disease severity observed in older populations (82). Furthermore, limited available data in the pediatric population suggests a distinct and diverse spectrum of disease completely different from adults, further reinforcing the importance of age-related immune responses (84, 145). In addition to age, emerging clinical and epidemiological data suggest sex-specific differences in the clinical characteristics and case-to-fatality ratio of COVID-19, with worse prognosis observed in males (66, 92). This disproportionate clinical epidemiology may be explained by sex-specific regulation of ACE2, increased incidence of preexisting comorbidities in males (i.e., hypertension, diabetes, cardiovascular disease), as well as sex-specific differences in viral immune response, as described elsewhere (47, 109). Genetic predispositions have also been proposed, including polymorphisms in ACE2 and genetic variability in histocompatibility complex (MHC) class I genes (96). Finally, several comorbidities have been associated with poor outcomes, likely due to the fact that organ and immune function may already be compromised and in a state of subclinical inflammation (53, 158). Notably, in a case study series of 5,700 patients from New York City, the most commonly observed comorbidities were hypertension, obesity, and diabetes (112). These factors need to be observed more thoroughly to complete our clinical understanding of COVID-19. In addition to understanding relevant risk factors, there is increasing suspicion of delayed but severe COVID-19 presentation, particularly in children, even after viral clearance (113). This not only suggests the importance of defining the timing of antibody response through serological testing in multiple age groups but also points toward the increasing complexity of COVID-19.
This review presents various potential pathophysiological mechanisms behind SARS-CoV-2 infection. The evidence behind these proposals are based on previous experience with similar coronaviruses, as well as clinical characteristics, laboratory testing, and postmortem pathological analysis of COVID-19 patients around the world. The exact contribution of risk factors to disease progression is still partially undefined. Additionally, further research is needed to examine the main drivers of COVID-19 and their molecular mechanisms of action in both pediatric and adult populations, since this should inform appropriate risk stratification and therapeutic strategies. From our preliminary understanding, immunomodulatory therapies are likely to be equally or more effective than solely targeting viral host cell entry. Furthermore, treatment approaches may be further tailored to the disease course of the patient by bolstering immune response earlier during disease progression to enhance an efficient antiviral response and blocking inflammation once severe disease develops.
To conclude, current evidence highlights that appropriate immune response is fundamental to COVID-19 pathogenesis, but much remains unknown regarding the key drivers of progression. As new therapeutic paradigms emerge, our understanding of disease pathophysiology will undoubtedly advance and not only inform current clinical practice for COVID-19 but fundamentally shape our understanding of immune involvement in systemic disease.
Acknowledgments
This work was supported by a Foundation Grant from the Canadian Institutes of Health Research (CIHR) (grant no. 353989). M.K.B. was supported by a Restracomp Scholarship (Hospital for Sick Children) and an Ontario Graduate Scholarship (OGS).
No conflicts of interest, financial or otherwise, are declared by the author(s).
M.K.B. and K.A. conceived and designed research; M.K.B., A.H., L.S., and K.A. prepared figures; M.K.B., A.H., L.S., B.J., and K.A. drafted manuscript; M.K.B., A.H., L.S., B.J., S.S., and K.A. edited and revised manuscript; M.K.B., A.H., L.S., B.J., S.S., and K.A. approved final version of manuscript.
References
- 1.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JC, Fogerty AE, Waheed A, Goodarzi K, Bendapudi P, Bornikova L, Gupta S, Leaf D, Kuter DJ, Rosovsky RP. COVID and Coagulation: Bleeding and Thrombotic Manifestations of SARS-CoV2 Infection. Blood. In press. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H. COVID-19 presenting as acute pancreatitis. Pancreatology. In press. doi: 10.1016/j.pan.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, Bermudez-Gonzalez M, Kleiner G, Aydillo T, Miorin L, Fierer DS, Lugo LA, Kojic EM, Stoever J, Liu STH, Cunningham-Rundles C, Felgner PL, Moran T, García-Sastre A, Caplivski D, Cheng AC, Kedzierska K, Vapalahti O, Hepojoki JM, Simon V, Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 26: 1033–1036, 2020. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand P, Puranik A, Aravamudan M, Venkatakrishnan AJ, Soundararajan V. SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. eLife 9: e58603, 2020. doi: 10.7554/eLife.58603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J Med Virol. In press. doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Baergen RN, Heller DS, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol 154: 279. 2020. doi: 10.1093/AJCP/AQAA101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11: 995–998, 2020. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 7.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol 5: 529–530, 2020. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Daßler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M, Looney MR, McAllister F, Rayes R, Renaud S, Rousseau S, Salvatore S, Schwartz RE, Spicer JD, Yost CC, Weber A, Zuo Y, Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med 217: e20200652, 2020. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci USA 106: 5871–5876, 2009. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertram S, Glowacka I, Müller MA, Lavender H, Gnirss K, Nehlmeier I, Niemeyer D, He Y, Simmons G, Drosten C, Soilleux EJ, Jahn O, Steffen I, Pöhlmann S. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J Virol 85: 13363–13372, 2011. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver biochemistries in hospitalized patients with COVID-19. Hepatology. In press. doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 12.Bohn MK, Lippi G, Horvath A, Sethi S, Koch D, Ferrari M, Wang C-B, Mancini N, Steele S, Adeli K. Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation of the latest evidence. Clin Chem Lab Med 58: 1037–1052, 2020. doi: 10.1515/cclm-2020-0722. [DOI] [PubMed] [Google Scholar]
- 13.Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, MacDonald V, Green L, Sivapalaratnam S, Pasi KJ, MacCallum P. Lupus anticoagulant and abnormal coagulation tests in patients with COVID-19. N Engl J Med. In press. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: abnormal liver function tests. J Hepatol. In press. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. In press. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Centers for Disease Control and Prevention Coronavirus disease 2019 in children—United States, February 12–April 2, 2020 [Online]. Atlanta, GA: Center for Disease Control and Prevention, 2020. https://www.cdc.gov/coronavirus/2019-ncov/downloads/pui-form.pdf. [Google Scholar]
- 16.Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J Gastroenterol 26: 2323–2332, 2020. doi: 10.3748/wjg.v26.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection (Preprint). bioRxiv 2020.02.03.931766, 2020. doi: 10.1101/2020.02.03.931766. [DOI]
- 18.Chan JFW, Kok KH, Zhu Z, Chu H, To KKW, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9: 221–236, 2020. doi: 10.1080/22221751.2020.1719902. A correction for this article is available at https://doi.org/10.1080/22221751.2020.1737364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen IY, Moriyama M, Chang MF, Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol 10: 50, 2019. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513, 2020. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun 525: 135–140, 2020. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung CY, Poon LLM, Ng IHY, Luk W, Sia S-F, Wu MHS, Chan K-H, Yuen K-Y, Gordon S, Guan Y, Peiris JSM. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol 79: 7819–7826, 2005. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, Milner JD. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. In press. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung KS, Hung IF, Chan PP, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TW, Tam AR, Yip CC, Leung K-H, Yim-Fong Fung A, Zhang RR, Lin Y, Cheng HM, Zhang AJ, To KK, Chan K-H, Yuen K-Y, Leung WK. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. In press. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, Fitzgerald JC, Topjian A, John ARO. Multisystem inflammatory syndrome in children during the Coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc 9: 393–398, 2020. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, Fung KS, Tang HL, Yan WW, Chan HWH, Lai TST, Tong KL, Lai KN. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 67: 698–705, 2005. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and cardiovascular disease. Circulation 141: 1648–1655, 2020. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 29.Cole SA, Laviada-Molina HA, Serres-Perales JM, Rodriguez-Ayala E, Bastarrachea RA. The covid-19 pandemic during the time of the diabetes pandemic: Likely fraternal twins? Pathogens 9: 389, 2020. doi: 10.3390/pathogens9050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 135: 2033–2040, 2020. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 176: 104742, 2020. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de-Madaria E, Siau K, Cárdenas-Jaén K. Increased amylase and lipase in patients with COVID-19 pneumonia: don´t blame the pancreas just yet! Gastroenterology. In press. doi: 10.1053/j.gastro.2020.04.044. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 14: 523–534, 2016. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeBiasi RL, Song X, Delaney M, Bell M, Smith K, Pershad J, Ansusinha E, Hahn A, Hamdy R, Harik N, Hanisch B, Jantausch B, Koay A, Steinhorn R, Newman K, Wessel D. Severe COVID-19 in children and young adults in the Washington, DC metropolitan region. J Pediatr. In press. doi: 10.1016/j.jpeds.2020.05.007. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y. Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19). Front Immunol 11: 827, 2020. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 among children in China. Pediatrics 145: e20200702, 2020. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 37.Drucker DJ. Coronavirus infections and Type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev 41: 457–470, 2020. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, Nahabedian J, Anderson K, Gilboa SM. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep 69: 769–775, 2020. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eroshenko N, Gill T, Keaveney MK, Church GM, Trevejo JM, Rajaniemi H. Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat Biotechnol 38: 789–791, 2020. doi: 10.1038/s41587-020-0577-1. [DOI] [PubMed] [Google Scholar]
- 40.Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, Wenzel P, Hamm C, Westenfeld R, Schultheiss M, Gross U, Morawietz L, Schultheiss H. Detection of viral SARS‐CoV‐2 genomes and histopathological changes in endomyocardial biopsies. ESC Hear Fail. In press. doi: 10.1002/ehf2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res 190: 62, 2020. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol 18: 1561–1566, 2020. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang B, Meng QH. The laboratory’s role in combating COVID-19. Crit Rev Clin Lab Sci 17: 1–15, 2020. doi: 10.1080/10408363.2020.1776675. [DOI] [PubMed] [Google Scholar]
- 44.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. In press. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gadiparthi C, Bassi M, Yegneswaran B, Ho S, Pitchumoni CS. Hyperglycemia, hypertriglyceridemia, and acute pancreatitis in COVID-19 infection: clinical implications. Pancreas 49: e62–e63, 2020. doi: 10.1097/MPA.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galang RR, Chang K, Strid P, Snead MC, Woodworth KR, House LD, Perez M, Barfield WD, Meaney-Delman D, Jamieson DJ, Shapiro-Mendoza CK, Ellington SR. Severe Coronavirus infections in pregnancy: a systematic review. Obstet Gynecol. In press. doi: 10.1097/AOG.0000000000004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ 11: 29, 2020. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golden TN, Simmons RA. Maternal and neonatal response to COVID-19. Am J Physiol Endocrinol Metab. In press. doi: 10.1152/ajpendo.00287.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U, Lo Vecchio A, Shingadia D, Soriano-Arandes A, Melendo S, Lanari M, Pierantoni L, Wagner N, L’Huillier AG, Heininger U, Ritz N, Bandi S, Krajcar N, Roglić S, Santos M, Christiaens C, Creuven M, Buonsenso D, Welch SB, Bogyi M, Brinkmann F, Tebruegge M, Pfefferle J, Zacharasiewicz A, Berger A, Berger R, Strenger V, Kohlfürst DS, Zschocke A, Bernar B, Simma B, Haberlandt E, Thir C, Biebl A, Vanden Driessche K, Boiy T, Van Brusselen D, Bael A, Debulpaep S, Schelstraete P, Pavic I, Nygaard U, Glenthoej JP, Heilmann Jensen L, Lind I, Tistsenko M, Uustalu Ü, Buchtala L, Thee S, Kobbe R, Rau C, Schwerk N, Barker M, Tsolia M, Eleftheriou I, Gavin P, Kozdoba O, Zsigmond B, Valentini P, Ivaškeviciene I, Ivaškevicius R, Vilc V, Schölvinck E, Rojahn A, Smyrnaios A, Klingenberg C, Carvalho I, Ribeiro A, Starshinova A, Solovic I, Falcón L, Neth O, Minguell L, Bustillo M, Gutiérrez-Sánchez AM, Guarch Ibáñez B, Ripoll F, Soto B, Kötz K, Zimmermann P, Schmid H, Zucol F, Niederer A, Buettcher M, Cetin BS, Bilogortseva O, Chechenyeva V, Demirjian A, Shackley F, McFetridge L, Speirs L, Doherty C, Jones L, McMaster P, Murray C, Child F, Beuvink Y, Makwana N, Whittaker E, Williams A, Fidler K, Bernatoniene J, Song R, Oliver Z, Riordan A; ptbnet COVID-19 Study Group . COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 0: S2352-4642(20)30177-2, 2020. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SHC, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol 34: 1977–1984, 2014. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 51.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of Covid-19 in New York City. N Engl J Med 382: 2372–2374, 2020. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong ASY. Multiple organ infection and the pathogenesis of SARS. J Exp Med 202: 415–424, 2005. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX; China Medical Treatment Expert Group for COVID-19 . Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55: 2000547, 2020. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. In press. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, Gluud LL. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology 20: 665–667, 2020. doi: 10.1016/j.pan.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasnain M, Pasha MF, Ghani I, Budiarto R. Protection challenges of pregnant women against vertical transmission during COVID-19 epidemic: a narrative review. Am J Infect Control. In press. doi: 10.1016/j.ajic.2020.06.206. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henry BM, Benoit. SW, de Oliveira MHS,, Hsieh WC,, Benoit J,, Ballout RA,, Plebani M,, Lippi G,. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): A pooled analysis and review. Clin Biochem 81: 1–8, 2020. doi: 10.1016/j.clinbiochem.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chem Lab Med 58: 1021–1028, 2020. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hosier H, Farhadian SF, Morotti RA, Deshmukh U, Lu-Culligan A, Campbell KH, Yasumoto Y, Vogels CBF, Casanovas-Massana A, Vijayakumar P, Geng B, Odio CD, Fournier J, Brito AF, Fauver JR, Liu F, Alpert T, Tal R, Szigeti-Buck K, Perincheri S, Larsen CP, Gariepy AM, Aguilar G, Fardelmann KL, Harigopal M, Taylor HS, Pettker CM, Wyllie AL, Dela Cruz CS, Ring AM, Grubaugh ND, Ko AI, Horvath TL, Iwasaki A, Reddy UM, Lipkind HS. SARS-CoV-2 infection of the placenta. J Clin Invest. In press. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. In press. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost 16: 231–241, 2018. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 63.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with Coronavirus disease 2019 (COVID-19). JAMA Cardiol 5: 819, 2020. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, Walzer T, François B, Sève P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev 19: 102567, 2020. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jasti M, Nalleballe K, Dandu V, Onteddu S. A review of pathophysiology and neuropsychiatric manifestations of COVID-19. J Neurol. In press. doi: 10.1007/s00415-020-09950-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, Liu S, Yang JK. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 8: 152, 2020. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, Nguyen EL, Barsh GR, Maskatia S, Mathew R. COVID-19 and Kawasaki Disease: novel virus and novel case. Hosp Pediatr 10: 537–540, 2020. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 68.Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol 82: e177, 2020. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khalil A, Kalafat E, Benlioglu C, O’Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Heath P, Ladhani S, von Dadelszen P, Magee LA. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. In press. doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191: 145–147, 2020. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond) 108: 205–213, 2005. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 72.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing glomerulopathy in a patient with Coronavirus Disease 2019 (COVID-19). Kidney Int Rep 5: 935–939, 2020. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277: 2251–2261, 2020, doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Zhou J, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu L, Chen G, Li H, Huang X, Zhang BH, Yuan Y. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. In press. doi: 10.1002/hep.31301. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 5: 562–569, 2020. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li F. Evidence for a common evolutionary origin of coronavirus spike protein receptor-binding subunits. J Virol 86: 2856–2858, 2012. doi: 10.1128/JVI.06882-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 9: 45, 2020. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. In press. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 58: 1131–1134, 2020. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 80.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 - Studies needed. N Engl J Med 382: 1194–1196, 2020. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 81.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. In press. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect 80: e14–e18, 2020. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, Chen L, Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 81: e6–e12, 2020. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109: 1088–1095, 2020. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res 220: 1–13, 2020. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 77: 683–690, 2020. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marinosci A, Landis BN, Calmy A. Possible link between anosmia and COVID-19: sniffing out the truth. Eur Arch Otorhinolaryngol 277: 2149–2150, 2020. doi: 10.1007/s00405-020-05966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martínez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol 318: F1454–F1462, 2020. doi: 10.1152/ajprenal.00160.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Masters PS. The molecular biology of coronaviruses. Adv Virus Res 66: 193–292, 2006. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsuyama S, Ujike M, Morikawa S, Tashiro M, Taguchi F. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc Natl Acad Sci USA 102: 12543–12547, 2005. doi: 10.1073/pnas.0503203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McNabb-Baltar J, Jin DX, Grover AS, Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Shen L, Chan WW. Lipase elevation in patients with COVID-19. Am J Gastroenterol. In press. doi: 10.14309/ajg.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meng Y, Wu P, Lu W, Liu K, Ma K, Huang L, Cai J, Zhang H, Qin Y, Sun H, Ding W, Gui L, Wu P. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog 16: e1008520, 2020. doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20: 355–362, 2020. doi: 10.1038/s41577-020-0331-4. . A correction for this article is available at https://doi.org/10.1038/s41577-020-0353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Millet JK, Whittaker GR. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci USA 111: 15214–15219, 2014. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82: 7264–7275, 2008. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nguyen A, David JK, Maden SK, Wood MA, Weeder BR, Nellore A, Thompson RF. Human leukocyte antigen susceptibility map for severe acute respiratory syndrome Coronavirus 2. J Virol 94: e00510–e00520, 2020. doi: 10.1128/JVI.00510-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci 41: 1667–1671, 2020. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, Lamers MM, Sikkema RS, de Bruin E, Chandler FD, Yazdanpanah Y, Le Hingrat Q, Descamps D, Houhou-Fidouh N, Reusken CBEM, Bosch BJ, Drosten C, Koopmans MPG, Haagmans BL. Severe acute respiratory syndrome Coronavirus 2-specific antibody responses in Coronavirus Disease 2019 patients. Emerg Infect Dis 26: 2020. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11: 1620, 2020. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 39: 618–625, 2009. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pal R, Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest 43: 1027–1031, 2020. doi: 10.1007/s40618-020-01276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol 29: 2039–2052, 2018. doi: 10.1681/ASN.2018050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perera RAPM, Mok CKP, Tsang OTY, Lv H, Ko RLW, Wu NC, Yuan M, Leung WS, Chan JMC, Chik TSH, Choi CYC, Leung K, Chan KH, Chan KCK, Li KC, Wu JT, Wilson IA, Monto AS, Poon LLM, Peiris M. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill 25: 2000421, 2020. doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perico L, Benigni A, Remuzzi G. Should covid-19 concern nephrologists? why and to what extent? The emerging impasse of angiotensin blockade. Nephron 144: 213–221, 2020. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol 7: 439–450, 2009. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pirzada A, Mokhtar AT, Moeller AD. COVID-19 and myocarditis: What do we know so far? CJC Open 0: 278–285, 2020. doi: 10.1016/j.cjco.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Premkumar L, Segovia-Chumbez B, Jadi R, Martinez DR, Raut R, Markmann A, Cornaby C, Bartelt L, Weiss S, Park Y, Edwards CE, Weimer E, Scherer EM, Rouphael N, Edupuganti S, Weiskopf D, Tse LV, Hou YJ, Margolis D, Sette A, Collins MH, Schmitz J, Baric RS, de Silva AM. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 5: eabc8413, 2020. doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qian Z, Dominguez SR, Holmes KV. Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation. PLoS One 8: e76469, 2013. doi: 10.1371/journal.pone.0076469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qin L, Li X, Shi J, Yu M, Wang K, Tao Y, Zhou Y, Zhou M, Xu S, Wu B, Yang Z, Zhang C, Yue J, Cheng C, Liu X, Xie M. Gendered effects on inflammation reaction and outcome of COVID‐19 patients in Wuhan. J Med Virol. In press. doi: 10.1002/jmv.26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 18: 1747–1751, 2020. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 34: e212–e213, 2020. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 112.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP; the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323: 2052–2059, 2020. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395: 1607–1608, 2020. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, Alvarado-Arnez LE, Bonilla-Aldana DK, Franco-Paredes C, Henao-Martinez AF, Paniz-Mondolfi A, Lagos-Grisales GJ, Ramírez-Vallejo E, Suárez JA, Zambrano LI, Villamil-Gómez WE, Balbin-Ramon GJ, Rabaan AA, Harapan H, Dhama K, Nishiura H, Kataoka H, Ahmad T, Sah R; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19). Electronic address: https://www.lancovid.org . Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 34: 101623, 2020. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rolnik DL. Can COVID-19 in pregnancy cause preeclampsia? BJOG. In press. doi: 10.1111/1471-0528.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol 16: 308–310, 2020. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med 8: 738–742, 2020. doi: 10.1016/s2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 1–2: 2020. doi: 10.1038/s41577-020-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, Ziv M, Leshem E, Dodiuk-Gad RP. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci 98: 75–81, 2020. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol 64: 1403–1415, 2016. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 122.Schnappauf O, Chae JJ, Kastner DL, Aksentijevich I. The pyrin inflammasome in health and disease. Front Immunol 10: 1745, 2019. doi: 10.3389/fimmu.2019.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shapiro J, Sciaky N, Lee J, Bosshart H, Angeletti RH, Bonifacino JS. Localization of endogenous furin in cultured cell lines. J Histochem Cytochem 45: 3–12, 1997. doi: 10.1177/002215549704500102. [DOI] [PubMed] [Google Scholar]
- 124.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant 39: 405–407, 2020. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol 39: 2085–2094, 2020. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Subramaniam S, Jurk K, Hobohm L, Jäckel S, Saffarzadeh M, Schwierczek K, Wenzel P, Langer F, Reinhardt C, Ruf W. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood 129: 2291–2302, 2017. doi: 10.1182/blood-2016-11-749879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18: 844–847, 2020. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20: 363–374, 2020. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated troponin in patients with Coronavirus Disease 2019: possible mechanisms. J Card Fail 26: 470–475, 2020. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel Coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 15: 700–704, 2020. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther 51: 843–851, 2020. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, Baldanti F, Daturi R, Postorino P, Cavallini A, Micieli G. Guillain-Barré Syndrome associated with SARS-CoV-2. N Engl J Med 382: 2574–2576, 2020. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Trippella G, Ciarcià M, Ferrari M, Buzzatti C, Maccora I, Azzari C, Dani C, Galli L, Chiappini E. COVID-19 in pregnant women and neonates: a systematic review of the literature with quality assessment of the studies. Pathogens 9: 485, 2020. doi: 10.3390/pathogens9060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tseng C-TK, Perrone LA, Zhu H, Makino S, Peters CJ. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J Immunol 174: 7977–7985, 2005. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- 137.Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol. In press. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181: 281–292.e6, 2020. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with Coronavirus Disease 19 pneumonia. Gastroenterology. In press. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. In press. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, Ng PC, Lam PWY, Ho LC, To WWK, Lai ST, Yan WW, Tan PYH. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 191: 292–297, 2004. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142a.World Health Organization Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19 [Online]. Geneva, Switzerland: WHO, 2020. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. [Google Scholar]
- 143.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases From the Chinese Center for Disease Control and Prevention. JAMA 323: 1239, 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 145.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol 55: 1169–1174, 2020. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158: 1831–1833.e3, 2020. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 12: 8, 2020. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 63: 457–460, 2020. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420–422, 2020. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 47: 193–199, 2010. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect 80: 607–613, 2020. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, Gao H, Wang Y, Dong CF, Li YJ, Xie XJ, Feng C, Liu L. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. In press. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, Zhang S, Cao T, Yang C, Li M, Guo G, Chen X, Chen Y, Lei M, Liu H, Zhao J, Peng P, Wang CY, Du R. Histopathologic changes and SARS-COV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med 172: 629–632, 2020. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75: 1730–1741, 2020. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 155.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of gastrointestinal symptoms on patients infected with COVID-19. Gastroenterology 158: 2294–2297, 2020. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang C, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH, Li H. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing Type 2 diabetes. Cell Metab 31: 1068–1077.e3, 2020. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J 39: 355–368, 2020. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zohar T, Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat Rev Immunol 20: 392–394, 2020. doi: 10.1038/s41577-020-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14: 185–192, 2020. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. Neutrophil extracellular traps in COVID-19. JCI Insight 5: 138999, 2020. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]