Abstract
We report a case with extramedullary tumors affecting the supraclavicular region that presented as a relapse of acute myeloid leukemia (AML) with FLT3-ITD mutation after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Treatment with gilteritinib resulted in remarkable response with disappearance of both the medullary and extramedullary tumors. Subsequently, a 2nd allo-HSCT was performed in an attempt to cure his AML and complete molecular response has been sustained with gilteritinib resumption without worsening GVHD. Targeted therapy with gilteritinib for medullary and extramedullary relapse of FLT3-ITD AML could be effective and suitable as a bridging therapy for allo-HSCT.
Keywords: Myeloid sarcoma, Acute myeloid leukemia, FLT3-ITD, Gilteritinib
1. Introduction
Mutations of fms-like tyrosine kinase 3 (FLT3) gene are frequently detectable in acute myeloid leukemia (AML) patients and the most common form of the mutations is an internal tandem duplication (ITD) mutation, being a poor prognostic factor for AML [1]. Currently, several FLT3 inhibitors have been developed in order to improve the dismal prognosis of AML patients harboring FLT3 mutations [2], and gilteritinib, 2nd generation selective FLT3 inhibitor, was recently approved for relapsed/refractory adult AML patients with FLT3 mutations in Japan [3].
Myeloid sarcoma (MS) is a solid collection of leukemic cells occurring at extramedullary sites other than the bone marrow (BM) [4], with the skin and the gums being the most frequently involved areas. MS may occur de novo, may precede or coincide with myeloid neoplasms such as AML, myeloproliferative neoplasm, and myelodysplastic syndromes [5]. MS can be the initial manifestation of AML relapse after allogenic stem cell transplantation (allo-HSCT) [6]. Extramedullary relapse occurs in 5–12% of patients who have undergone allo-HSCT for AML [7,8], however, the efficacy of FLT3 inhibitors for extramedullary tumors bearing FLT3-ITD remains to be determined. Here, we report a case of FLT3-ITD mutated AML with both medullary and extramedullary relapse following allo-HSCT, and gilteritinib showed remarkable clinical and molecular response.
2. Case presentation
A previously healthy 38-year-old male was initially diagnosed as having AML with myelodysplasia-related changes with FLT3-ITD. Induction therapy with idarubicin and cytarabine was immediately started, however, hematological complete remission was not achieved. Because severe pancytopenia persisted, we directly performed allogenic bone marrow transplantation (allo-BMT) from an HLA-DR.. one locus mismatched unrelated donor using a myeloablative conditioning regimen consisting of busulfan and cyclophosphamide three months after the initial diagnosis. A minimal residual disease monitoring was performed using Wilms tumor gene-1 (WT-1) expression in the peripheral blood. WT-1 was negative on day 26 after allo-BMT, however, it increased to 102 copies/μgRNA on day 53, suggesting imminent hematological relapse of AML. Immunosuppressants were stopped and then two courses of DLI were administrated on day 119 and 155. WT-1 became negative on day 173, however, idiopathic pneumonia syndrome, dry eye, and dry mouth caused by chronic GVHD were observed, for which he was initially treated with prednisolone (1 mg/kg/day) and cyclosporine in an attempt to control the GVHD. Within 4 weeks, an improvement in the clinical manifestations was observed, and the immunosuppressants could be tapered to the level of prednisolone (5 mg/day), cyclosporine (off) without worsening the GVHD.
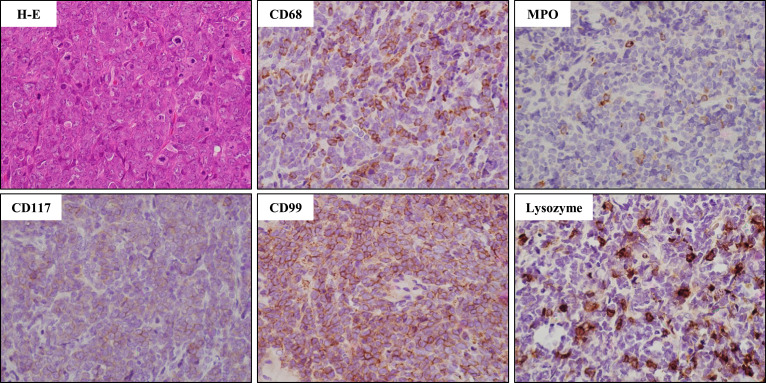
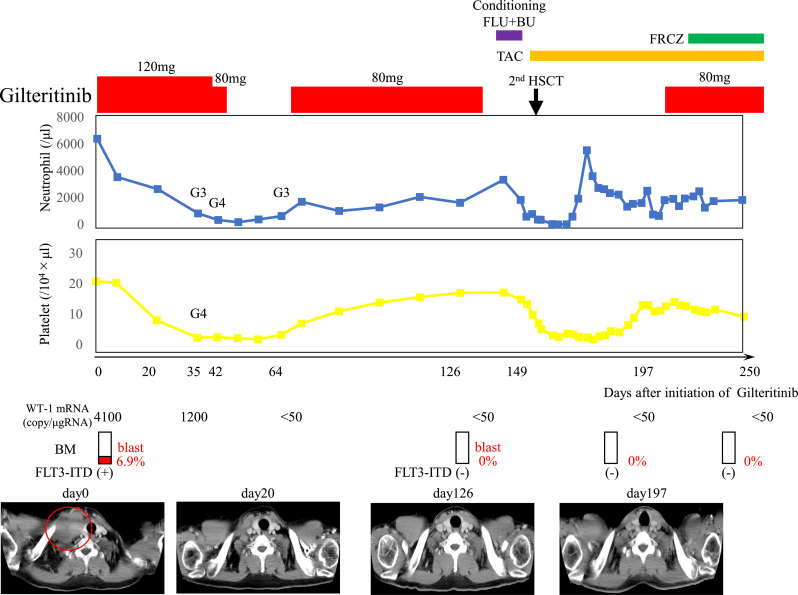
Approximately 400 days later, a right supraclavicular mass was observed, and computed tomography (CT) scan showed the presence of a right supraclavicular tumor and subcutaneous mass on the right chest. A biopsy of the supraclavicular tumor suggested a hematolymphoid neoplasm with a large number of cells having atypical nuclei and scant cytoplasm, which were positive for CD68, myeloperoxidase, lysozyme, CD99, slightly positive for CD117, and negative for CD34, and terminal deoxynucleotidyl transferase (Fig. 1). BM examination revealed increased AML blasts (6.9%), and these cells both in the extramedullary and medullary harbored FLT3-ITD mutation. He was administered gilteritinib at 120 mg/day from day 446, and a remarkable tumor regression on CT scan was observed 20 days after gilteritinib administration (on day 466) (Fig. 2). Furthermore, both complete molecular response in BM and disappearance of tumors on CT scan were confirmed 126 days after the commencement of gilteritinib (on day 572) (Fig. 2). However, grade 4 adverse events (AEs) with severe neutropenia and thrombocytopenia induced by gilteritinib occurred, requiring several rounds of platelet transfusions. These AEs were attenuated after reducing the dosage or temporally interrupting gilteritinib, and eventually the dose of gilteritinib was fixed on 80 mg/day (Fig. 2).
Fig. 1.
Histological examination of myeloid sarcoma. Hematoxylin-eosin (H-E) and immunostaining, objective magnification, × 40.
Fig. 2.
Clinical course after the administration of gilteritinib. Computed tomography (CT) scan showed the presence of a right supraclavicular tumor (101 mm × 55 mm in size) which involved the right internal jugular vein (in red circle). CT scan also showed the findings after the administration of gilteritinib.
Abbreviation: BM, bone marrow; MS, myeloid sarcoma; G3, grade3; G4, grade4; FLU, fludarabine; BU, busulfan; HSCT, hematopoietic stem cell transplantation; TAC, Tacrolimus; FRCZ, fluconazole.
Consequently, he underwent 2nd allo-BMT from an HLA-DR.. one locus mismatched unrelated donor using a myeloablative conditioning regimen consisting of busulfan and fludarabine in the state of complete molecular remission 149 days after the gilteritinib administration (on day 595). Gilteritinib (80 mg/day) was resumed 48 days after 2nd allo-BMT (on day 643). A complete molecular response (CMR) has been maintained for 150 after 2nd allo-BMT without AEs or worsening of GVHD.
3. Discussion
We presented here a case of medullary and extramedullary relapse of FLT3-ITD AML following allo-BMT. Although DLI induced transient remission for molecular relapse of AML, the leukemic blasts perhaps escaped from the graft versus leukemia (GVL) effect mediated by DLI and manifested as a relapse with extramedullary involvement. The diagnosis was based on a biopsy of the right supraclavicular tumor, and the leukemic blasts both in the extramedullary and medullary sites harbored FLT3-ITD mutation. Eventually, gilteritinib showed a remarkable response, and complete molecular response has been sustained with gilteritinib resumption without worsening of GVHD.
In general, the immune response to AML mediated by the GVL effect after allo-HSCT preferentially maintains BM remission, which is a major mechanism for the curative effect of allo-HSCT. However, it has been reported that extramedullary relapse of AML in the form of MS occurs in 5–12% of patients who have undergone allo-HSCT for AML, and the incidence of relapse after DLI or a 2nd allo-HSCT is even higher [8]. Furthermore, in comparison with patients with BM relapse, those with extramedullary relapse are more likely to have had preceding GVHD [8].
In the present case, the GVL effect for molecular BM relapse and chronic GVHD co-occurred after the DLI, and immunosuppression required to treat the GVHD until when the extramedullary tumors eventually relapsed. Collectively, these observations suggest that the GVL effect associated with an occurrence of GVHD is less effective in preventing the relapse of the extramedullary tumors, however, the precise mechanism remains to be clarified.
Treatment guidelines for MS have not been established because of the rarity of the disease, especially in the relapse setting after allo-HSCT. Although systemic chemotherapy or a 2nd allo-HSCT should be considered, the prognosis with these treatment strategies remains extremely poor [8]. In recent years, the efficacy of targeted therapies for MS, such as a humanized anti-CD33 monoclonal antibody, tyrosine kinase inhibitors (for FIP1L1-PDGFR, and FLT3-ITD), and DNA methyltransferase inhibitors, have been reported and these agents may change the disease outcome [5,[9], [10], [11]].
Although the molecular abnormalities in MS have not been well investigated, there have been several reports analyzing gene mutations, which have shown the frequent occurrence of FLT3 mutations in MS [7]. Interestingly, a discordance of FLT3-ITD mutational status in leukemic blasts between medullary and extramedullary sites has been demonstrated in some cases. In our case, however, we confirmed FLT3-ITD mutation in the leukemic blasts at both medullary and extramedullary sites.
As for FLT3 inhibitors, 1st generation multikinase inhibitors, such as lestaurtinib, sorafenib, and midostaurin, have been investigated, however, clinical efficacies for AML with FLT3 mutations are still limited, and may contribute to adverse effects due to the inhibition of multiple other kinases [2]. Therefore, next-generation FLT3 inhibitors have been developed to overcome these hurdles. Gilteritinib, which is a 2nd generation selective FLT3 inhibitor, was shown to improve the overall survival for relapsed or refractory AML with FLT3 mutations compared to conventional chemotherapy [3], however, the efficacy for relapsed MS after allo-HSCT was not mentioned in the trial. In our patient, a remarkable response for the extramedullary tumors and a complete molecular response in BM were seen. Although grade 4 hematological AEs were observed, these AEs were manageable and gilteritinib could be administered in the outpatient setting. Because our patient was young with good performance status and his AML relapse co-occurred not only at extramedullary but also at medullary sites, we performed 2nd allo-BMT in an attempt to cure his AML. Furthermore, gilteritinib could be resumed after 2nd allo-BMT without AEs nor worsening of GVHD. Because the efficacy of gilteritinib as maintenance therapy after allo-HSCT remains unknown and the impact on GVHD of FLT3 inhibitors is controversial, future studies are necessary to address these issues.
To our knowledge, this is the first report of medullary and extramedullary relapse of FLT3-ITD AML after allo-BMT successfully treated with gilteritinib followed by 2nd allo-BMT. In the future, large prospective studies for MS are needed to establish the best strategy for using targeted therapies.
Declaration of Competing Interests
Itaru Matsumura
Honoraria: Astellas, Otsuka Pharmaceuticals, Novartis, Sanofi, Shionogi Pharmaceuticals,
No other potential conflicts of interest were reported.
References
- 1.Stirewalt D.L., Radich J.P. The role of FLT3 in haematopoietic malignancies. Nat. Rev. Cancer. 2003;3(9):650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 2.Daver N., Schlenk R.F., Russell N.H., Levis M.J. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312. doi: 10.1038/s41375-018-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perl A.E., Martinelli G., Cortes J.E. Gilteritinib or chemotherapy for relapsed or refractory. N. Engl. J. Med. 2019;381(18):1728–1740. doi: 10.1056/NEJMoa1902688. [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow S.H., Campo E., Pileri S.A. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magdy M., Abdel Karim N., Eldessouki I., Gaber O., Rahouma M., Ghareeb M. Myeloid Sarcoma. Oncol. Res. Treat. 2019;42(4):224–229. doi: 10.1159/000497210. [DOI] [PubMed] [Google Scholar]
- 6.Pileri S.A., Ascani S., Cox M.C. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21(2):340–350. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 7.Ansari-Lari M.A., Yang C.F., Tinawi-Aljundi R. FLT3 mutations in myeloid sarcoma. Br. J. Haematol. 2004;126(6):785–791. doi: 10.1111/j.1365-2141.2004.05124.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara S., Ando T., Ogawa H. Extramedullary relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: an easily overlooked but significant pattern of relapse. Biol. Blood Marrow Transplant. 2012;18(12):1800–1807. doi: 10.1016/j.bbmt.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Védy D., Muehlematter D., Rausch T., Stalder M., Jotterand M., Spertini O. Acute myeloid leukemia with myeloid sarcoma and eosinophilia: prolonged remission and molecular response to imatinib. J. Clin. Oncol. 2010;28(3):e33–e35. doi: 10.1200/JCO.2009.23.6976. [DOI] [PubMed] [Google Scholar]
- 10.Minoia C., de Fazio V., Scognamillo G. Long-Lasting remission in De Novo Breast myeloid sarcoma treated with decitabine and radiotherapy. Diagnostics (Basel) 2019;9(3) doi: 10.3390/diagnostics9030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kida M., Kuroda Y., Kido M., Chishaki R., Kuraoka K., Ito T. Successful treatment with gilteritinib for isolated extramedullary relapse of acute myeloid leukemia with FLT3-ITD mutation after allogeneic stem cell transplantation. Int. J. Hematol. 2020 doi: 10.1007/s12185-020-02855-4. [DOI] [PubMed] [Google Scholar]