Abstract
Neighborhood perceptions is an important predictor of both allostatic load in the form of biological dysregulation and major depressive disorder. Furthermore, biological dysregulation is predictive of major depressive disorder. Yet to date, the use of biological dysregulation as one potential causal pathway linking neighborhood perceptions to depression has not been explored. This study examined the relationship between neighborhood perception, biological dysregulation in the form of allostatic load, and depression among individuals who participated in the three waves of the Midlife Development in the United States study (1996–2014). Two-to-one propensity score matching was employed prior the use of causal mediation analyses. Lower neighborhood perceptions at wave one were associated with increased allostatic load at wave two. Allostatic load at wave two is associated with depression at wave three. The mediation analysis shows that 6.0% of the relationship between neighborhood perception and depression is mediated by biological dysregulation. These results can inform future prevention and treatment methods by supporting efforts to integrate individuals and community-level interventions to aid in addressing both the environmental conditions and biological factors associated with depression.
Keywords: Stress, Allostasis, Biological dysregulation, Major depressive disorder
Highlights
-
•
Lower neighborhood perceptions are associated with biological dysregulation.
-
•
Biological dysregulation is associated with depression.
-
•
Allostatic load partially mediates neighborhood perception's effect on depression.
Introduction
Neighborhood conditions have important implications for individual health and well-being, with individuals’ perceptions of their neighborhoods being key factors that influence health outcomes. A large body of epidemiological research has demonstrated that more positive neighborhood perceptions are associated with better self-reported well-being (Toma, Hamer, & Shankar, 2015), lower risk of stroke (Kim, Park, & Peterson, 2013), better self-rated health (Weden, Carpiano, & Robert, 2008), and fewer chronic health conditions (Robinette, Charles, & Gruenewald, 2016). Moreover, perceived neighborhood conditions are independent, and sometimes more predictive, of health outcomes, than objective neighborhood measures (Ross & Mirowsky, 2001; Sampson & Raudenbush, 2004; Weden et al., 2008; Wilson-Genderson & Pruchno, 2013). In addition to physical health, neighborhood perceptions are also important factors for mental health. For example, perceived neighborhood disorder (Curry, Latkin, & Davey-Rothwell, 2008), fear of neighborhood violence (Tonorezos et al., 2008), and perceived physical disorder and decay (Mair, Diez Roux, & Morenoff, 2010) are all associated with greater risk of depression among community members.
While the body of evidence linking perceived neighborhood conditions to mental health—specifically depression—is well established, the physiological and biological pathways that connect them have not been well explored (Turner, Shattuck, Hamby, & Finkelhor, 2013). Allostatic load, in the form of cumulative wear and tear due to long-term exposure to stress (McEwen, 1998), is one theoretical framework that can be applied to explicate how perceived neighborhood conditions and negative mental health outcomes are connected. More specifically, biological dysregulation in the form of allostatic load may mediate the relationship between neighborhood perceptions and mental health. Aspects of this relationship have already been established. For example, negative neighborhood perceptions are associated with higher levels of biological dysregulation as measured by a range of biomarkers (Carbone, 2020; van Deurzen et al., 2016). Additionally, increased levels of allostatic load are associated with negative mental health outcomes (e.g., Berger, Juster, et al., 2018; Berger, Lavoie, et al., 2018; Juster et al., 2011; Juster, Sasseville, Giguere, Consortium, & Lupien, 2018). In spite of this evidence, biological dysregulation as the mediating factor that links neighborhood perceptions to mental health outcomes has not been explicitly investigated. This study seeks to explore this link via a cumulative measure of biological dysregulation.
Biological dysregulation and depression
The etiology of major depressive disorder is complex with multiple potential causal pathways and diverse presentations. This diversity of depressive conditions may be the reason that 30–60% of patients do not respond to antidepressant medications (Hodes, Ménard, & Russo, 2016). To date, a range of biological systems—and dysregulation within those systems—have been associated with depression. For example, increased HPA axis activity has been recorded in a majority of patients with depression (Pace & Miller, 2009; Stetler & Miller, 2011). This relationship is likely to be complex and reciprocal, with increased cortisol levels resulting in hippocampal atrophy, which can inhibit cortisol regulation leading to even higher cortisol levels (Bowers & Yehuda, 2017; Pariante & Miller, 2001).
For some individuals with major depressive disorder, immune system dysregulation and metabolic system dysregulation are important aspects of the disease. Biomarkers of inflammation (e.g., IL-6) are often elevated in individuals experiencing depression (Bob et al., 2010; Hodes et al., 2016; Khandaker, Perason, Zammit, Lewis, and Jones, 2014; Sasayama et al., 2013), while comorbidities between depression and metabolic disorders are common (Dunbar et al., 2008; Lamer et al., 2018; McIntyre et al., 2007). In addition, the link between inflammation and depression is bidirectional. While inflammatory markers are elevated among individuals with depression (see Dowlati et al., 2010 and Haapakoski, Mathieu, Ebmeier, Alenius, & Kivimäki, 2015 for reviews), depression can exacerbate inflammation (Chiang, Bower, Irwin, Taylor, & Fuligni, 2017). Multiple mechanisms may account for this relationship, with one potential explanation being that a number of depression risk factors are also pro-inflammatory (e.g., adverse childhood experiences, obesity) (Miller & Raison, 2016).
Parasympathetic dysregulation is another key system for understanding the biological underpinnings of depression. Parasympathetic dysregulation, as operationalized by heart rate variability (HRV) and respiratory sinus arrythmia (i.e., HRV with respiration), is inversely associated with mental health outcomes. A systematic review of 150 studies found that individuals with diagnosed psychiatric disorders presented with lower HRV than controls without psychiatric disorders (Alvares, Quintana, Hickie, & Guastella, 2016) and a meta-analysis found similar results of decreased HRV among individuals with depression (Brown et al., 2018).
Allostatic load theory posits that biological systems are interrelated and it is important to consider the cumulative effect of biological dysregulation across systems (McEwen & Seeman, 1999; McEwen & Stellar, 1993). Some researchers have begun to apply this theory of multisystem biological dysregulation to their study of depression. To date, cumulative allostatic load has been linked to depression in both cross-sectional (Kobrosly, Seplaki, Cory-Slechta, Moynihan, & van Wijngaarden, 2013 & Kobrosly, van Wijngaarden, Seplaki, Cory-Slechta, & Moynihan, 2014) and prospective studies (Juster et al., 2011). Yet research in this sphere remains limited. This longitudinal study seeks to expand on the current literature by testing if neighborhood perception at wave one is predictive of depression at wave three and testing if this association is mediated—partially or fully—by allostatic load at wave two.
Materials and methods
Data and sample
Data from the existing three waves of the Midlife Development in the United States (MIDUS) study were used in this analysis (Brim et al., 1996; Ryff et al., 2006, 2014). MIDUS is a longitudinal study of non-institutionalized adults in the United States aimed at better understanding the social, physical, and psychological factors that influence health and well-being as individuals age. The study began as a national probability sample of telephone surveys in 1995 and 1996 with wave two telephone surveys occurring between 2004 and 2006, and data collection for wave three telephone surveys in 2013 and 2014. In addition to the initial telephone surveys, MIDUS is comprised of a number of sub-studies that draw from the larger MIDUS sample. One such sub-study is the biomarkers project that collected data from participants at wave two and is currently collecting data from the same participants as part of wave three data collection. The preliminary analytic sample for this study includes individuals who (1) completed the initial survey during wave one, (2) participated in and completed both the survey and the biomarkers sub-study at wave two, and (3) completed the survey at wave three.
Variables
Depression
The dependent variable of depression is a dichotomized variable (yes, no) based on the Composite International Diagnostic Interview Short Form scales (CIDI-SF) (Kessler, Andrews, Mroczek, Ustun, & Wittchen, 1998; Wang, Berglund, & Kessler, 2000; World Health Organization, 1990) and includes a series of questions about experiences with depressed affect and anhedonia. Seven questions about depressed affect and six about experiences of anhedonia, all over at least a two-week period in the previous twelve months, were asked of participants. If an individual said yes to four or more statements about depressed affect or anhedonia and they stated that they felt that they “Everyday” or “Almost every day”“, a new, dichotomous variable (yes, no) was coded as “yes” to show that the individual met the criteria for depression. The variable was dichotomized as this is a validated and accepted tool for clinical diagnostic purposes (e.g., Haro et al., 2006; Kessler et al., 2004) and has even been utilized as the gold standard against which other measures are assessed (Dang, Dong, & Mezuk, 2020).
Neighborhood perception
Neighborhood perception is a dichotomized variable based on seven questions that represent three domains of neighborhood conditions. For each question, respondents were asked to rate their agreement with the statement on a four-point scale (A lot, Some, A little, Not at all). The three domains, and their respective questions, are perceived neighborhood safety (“I feel safe being out alone in my neighborhood during the daytime,” “I feel safe being alone in my neighborhood at night”), perceived trust in neighbors (“I could call on a neighbor for help if I needed it,” “People in my neighborhood trust each other”), and perceived neighborhood conditions (“Buildings and streets in my neighborhood are kept in very good repair,” “I feel very good about my home and neighborhood,” “My neighborhood is kept clean”). While these questions represent three distinct domains, combined they have a Cronbach's alpha of 0.81. Answers to the seven questions were averaged and reverse coded so that a higher score represents more positive neighborhood perceptions (range = 1–4, M = 3.085, SD = 0.45). For the causal analysis described below, a dichotomous variable was created so that respondents in the lowest quartile of the sample distribution for the mean neighborhood perception score (2.86 or less) were coded as having low-rated neighborhood perceptions and the remainder of respondents were coded as having high-rated neighborhood perceptions. Dichotomizing neighborhood perceptions variables is a common approach in the literature (e.g., Hale et al., 2013; Tamayo et al., 2016; Wee et al., 2019) and allows for a comparison, in this instance, between individuals with the most negative perceptions of their neighborhoods and the rest of the sample.
Allostatic load
A cumulative measure of allostatic load was created in a manner consistent with other approaches in the literature (e.g., Friedman, Karlamangla, Gruenewald, Koretz, & Seeman, 2015; Hamdi, South, & Krueger, 2016; Slopen, Chen, Priest, Albert, & Williams, 2016; Vadiveloo & Mattei, 2017; Zilioli, Slatcher, Ong, & Gruenewald, 2015). First, twenty-five biomarkers from seven biological systems were dichotomized into high or low risk (1 = high risk, 0 = low risk) based on high-risk quartiles of the sample distribution as displayed in Table 1. The seven biological systems, and their associated biomarkers, that were included in the measure are as follows: Hypothalamic-Pituitary-Adrenal (HPA) axis (cortisol, D-HEAS), sympathetic nervous system (epinephrine, norepinephrine, dopamine), parasympathetic nervous system (heart rate, low-frequency heart rate variability (LFHRV), high-frequency heart rate variability (HFHRV), root mean squared successive differences of the beat-to-beat interval (RMSSD), standard deviation of heart cycle length variability (SDRR)), inflammatory system (C-reactive protein, interleukin-6 (IL-6), fibrinogen, E-Selectin, intercellular adhesion molecule 1 (ICAM-1)), cardiovascular system (systolic blood pressure, diastolic blood pressure, pulse pressure), glucose metabolism (HbA1c, fasting glucose, HOMA), and lipid metabolism (high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, total cholesterol-to-HDL cholesterol, triglycerides). Next, systems level scores were calculated by averaging biomarker scores across the seven systems so that a value between zero and one was created for each biological system. This approach balances systems and ensures that biological systems with more biomarkers do not have an outsized impact on the overall allostatic load score (Gruenewald et al., 2012). Finally, scores were summed across systems resulting in cumulative, systems level allostatic load scores that ranged from zero to seven.
Table 1.
Descriptive statistics of matched sample (N = 714).
| Variables | High Neighborhood Perception (n = 476) |
Low Neighborhood Perception (n = 238) |
p-valuea,b |
|---|---|---|---|
| n (%) | N (%) | ||
| Depression reported at wave 1 | p > 0.05 | ||
| No | 415 (87.18) | 197 (82.77) | |
| Yes | 61 (12.82) | 41 (17.23) | |
| Depression reported at wave 3 | p < 0.001 | ||
| No | 444 (93.28) | 198 (83.19) | |
| Yes | 32 (6.72) | 40 (16.81) | |
| Age (mean (SD)) | 44.68 (9.92) | 41.66 (10.02) | p < 0.001 |
| Sex | p > 0.05 | ||
| Female | 262 (55.04 | 140 (58.82) | |
| Male | 214 (44.96) | 98 (41.18) | |
| Race | p > 0.05 | ||
| White | 447 (93.91) | 214 (89.92) | |
| Non-White or multiracial | 25 (5.25) | 22 (9.24) | |
| Not reported | 4 (0.84) | 2 (0.84) | |
| Marital Status | p < 0.001 | ||
| Married/living with spouse | 353 (74.16) | 144 (60.50) | |
| Other | 123 (25.84) | 94 (39.50) | |
| Homeownership Status | p < 0.001 | ||
| Own home outright | 78 (16.39) | 38 (15.97) | |
| Own home with a mortgage | 330 (69.33) | 120 (50.42) | |
| Rent | 68 (14.29) | 80 (33.61) | |
| Educational Attainment | p > 0.05 | ||
| High school diploma or less | 123 (25.84) | 74 (31.09) | |
| Some college/associates | 138 (28.99) | 72 (30.25) | |
| At least a bachelor's degree | 215 (45.17) | 92 (38.66) | |
| Years in Neighborhood at wave 1 (mean (SD)) | 10.65 (10.22) | 9.37 (10.09) | p > 0.05 |
| Lived in the same neighborhood for waves 1 & 2 | p < 0.01 | ||
| No | 249 (52.31) | 154 (64.71) | |
| Yes | 227 (47.69) | 84 (35.29) | |
Chi-square tests were employed to if there were statistically significant differences between the high and low neighborhood perceptions groups for eaxh dichotomous or categorical variable. Welch two-sample T-tests were used for continuous variables.
p-values less than 0.05 are bolded.
Covariates and confounders
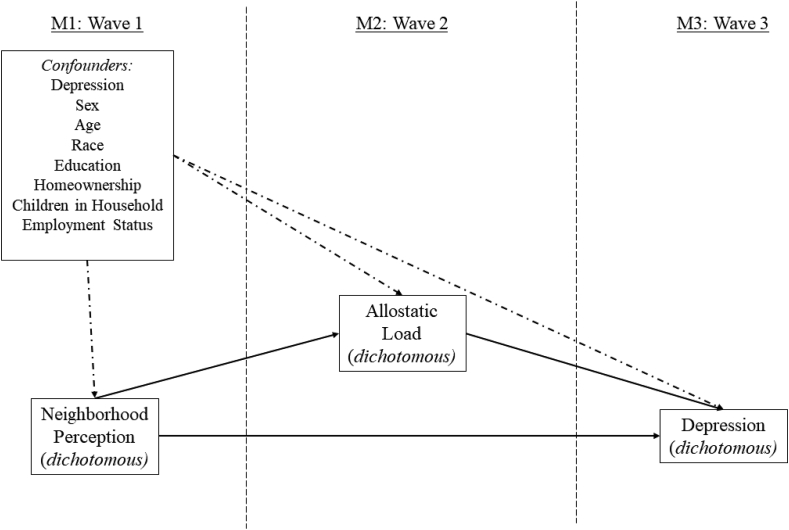
Potential confounders of the hypothesized relationships are shown as part of the conceptual model presented in Fig. 1. These variables, all of which were measured at wave one, include baseline depression (yes, no), age (continuous), sex (male, female), race (white, non-white/multiracial, did not specify), marital status (married and living with spouse, other), homeownership status (own home outright, own home with a mortgage, rent or other), educational attainment (high school diploma or less, some college/associates degree, at least a bachelor's degree), and years living in the neighborhood (continuous). In addition, the final regression model within the mediation analysis that predicts depression included a dichotomous variable (yes, no) for whether or not individuals lived in the same neighborhood at waves one and two.
Fig. 1.
Conceptual mediated model of neighborhood perceptions, allostatic load, and depression.
Analysis
Analysis was completed in multiple steps using the statistical software R (R, version 1.1.383). First, propensity score matching was used to match low-rated neighborhood perception observations to high-rated neighborhood perception observations on the confounding variables (age, sex, race, marital status, educational attainment, homeownership status, years of neighborhood residency, and baseline depression). Next, descriptive statistics for the matched data were completed. Third, a mediation analysis was completed to assess the degree to which allostatic load mediated the relationship between neighborhood perception and depression. Finally, a sensitivity analysis was employed to assess the degree to which unmeasured confounding may have biased the results.
Matching
To strengthen causal inference and reduce the degree of bias introduced by confounders, individuals in the low-rated neighborhood perceptions group were matched to those in the high-rated neighborhood perceptions group on multiple sociodemographic confounders via the MatchIt package (Ho, Imai, King, & Stuart, 2011). Two-to-one optimal matching was selected, as this method provides matches that, on average, have the smallest absolute distance between each pair of low-rated and high-rated neighborhood perception observations (Ho et al., 2011). Matching resulted in 238 low-rated neighborhood perception observations being matched to the 476 high-rated neighborhood perception observations (total N = 714). The remaining unmatched high-perception observations (n = 55) were dropped from the analysis.
Mediation analysis
The R Mediation package (Tingley, Yamamoto, Hirose, Keele, & Imai, 2014) was used to test the hypothesized models. These include both the multivariate logistic regression models as well as the mediation models. Including both the logistic regression results and the mediation results can aid in better understanding the relationships between the variables in the analysis.
Sensitivity analysis
To assess the potentialfor unmeasured confounding, a leave-one-out sensitivity analysis was completed (Chen et al., 2015; Hernán & Robins, 2020; Noyce et al., 2017; Zeng, Yu, & Xu, 2019). This analysis was employed to determine the degree of potential confounding added to the analysis by removing—and no longer controlling for—each of the covariates. This allows for a ballpark estimate of the degree to which potential unmeasured confounding could be biasing the results. If it is assumed that any unmeasured confounding had an effect on the results that did not exceed the degree of confounding introduced by any individual covariate included in the analysis, then a reasonable assumption could be made that the magnitude of potential unmeasured confound would not drastically change the results of the analysis (Hernán & Robins, 2020). Given that the analysis includes baseline depression, which is likely to be the largest potential confounder, it is highly probable that this analysis measures the true relationships between the variables of interest.
Results
Descriptive statistics
Complete descriptive statistics for the matched sample are presented in Table 2. This includes the results of either a chi square test or Welch two-sample T-test to determine of there was a statistically significant difference between the high and low neighborhood perception groups on each of the covariates. 17.23% of individuals in the low neighborhood perceptions group reported depression at wave one as compared to 12.82% in the high neighborhood perception group (p > 0.05). At wave three, there was a statistically significant difference between the low and high neighborhood perception group on depression (6.72% and 16.81%, respectively; p < 0.001). Individuals in the low neighborhood perception group were, on average, younger (M = 41.66 years) than those in the high neighborhood perception group (M = 44.68 years) (p < 0.001). A higher proportion on individuals in the high neighborhood perception group (74.16%) than the low neighborhood perception group (60.50%) reported being married and living with a spouse (p < 0.001). A statistically significant difference (p < 0.001) was also present for housing tenure, as the high neighborhood perception group had higher rates of home ownership with (69.33%) and without (16.39%) a mortgage as compared to the low neighborhood perception group (50.42% and 15.97%, respectively). There was not a statistically significant difference between the two groups in terms of number of years individuals lived in their current neighborhoods (p > 0.05), yet those in the high neighborhood perception group were more likely to live in the same neighborhood at wave one and wave two (47.69%) than those in the low neighborhood perception group (35.29%) (p < 0.01).
Table 2.
High risk quartile biomarker cutoff values (N = 762).
| System | Biomarker | Cutoff value |
|---|---|---|
| HPA Axis | ||
| Urine cortisol adjusted for creatine (ug/g) | ≤4.40 or≥28.00 | |
| Blood DHEA-S (ug/dL) |
≤30.00 or≥187.00 |
|
| Sympathetic Nervous System | ||
| Urine Epinephrine adjusted for creatine (ug/g) | ≥2.464 | |
| Urine Norepinephrine adjusted for creatine (ug/g) | ≥2.60 | |
| Urine Dopamine adjusted for creatine (ug/g) |
≥182.979 |
|
| Parasympathetic Nervous System | ||
| Heart rate (beats per minute)1 | ≥79.80 | |
| RMSSD | ≤2.49 | |
| SDRR (milliseconds) | ≤3.15 | |
| Low frequency heart rate variability (0.04–0.15 Hz) | ≤4.64 | |
| High frequency heart rate variability (0.15–0.50 Hz) |
≤4.02 |
|
| Inflammatory System | ||
| Serum interleukin-6 (IL6) (pg/mL) | ≥3.47 | |
| Blood C-Reactive protein (ug/mL) | ≥3.66 | |
| Blood fibrinogen (ug/dL) | ≥399.00 | |
| Serum soluble E-Selectin (ng/mL) | ≥51.89 | |
| Serum soluble ICAM-1 (ng/mL) |
≥335.34 |
|
| Cardiovascular System | ||
| Systolic blood pressure2 | ≥143.00 | |
| Diastolic blood pressure2 | ≥82.00 | |
| Pulse pressure3 |
≥64.00 |
|
| Lipid Metabolism | ||
| HDL cholesterol4 | ≤43.00 | |
| LDL cholesterol4 | ≥127.00 | |
| Total to HDL cholesterol4 | ≥4.43 | |
| Triglycerides4 |
≥156.00 |
|
| Glucose Metabolism | ||
| Blood fasting glucose levels mg/dL | ≥105.00 | |
| Blood hemoglobin (HbA1c) percentage | ≥6.242 | |
| Insulin resistance (HOMA-IR) | ≥4.36 | |
Causal mediation analysis
The causal mediation analysis tests the direct and indirect effects of low-rated neighborhood perceptions at wave one on depression at wave three. The indirect effect is the proportion of the relationship mediated by allostatic load at wave two. The results of the mediation model are shown in Table 3. The total effect of neighborhood perception at wave one on depression at wave three is 0.083 (95% CI: 0.034, 0.129). Of that, 0.078 (95% CI: 0.030, 0.125) is the average direct effect and 0.005 (95% CI: 0.001, 0.013) is the indirect effect. That is, 6.0% of the effect of neighborhood perception at wave one on depression at wave three is mediated by allostatic load at wave two.
Table 3.
Causal mediation analysis results for the mediating effect of allostatic load on the relationship between neighborhood perception and depression (N = 771).
| Estimate | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| Average indirect effect | 0.005* | 0.001 | 0.013 |
| Average direct effect | 0.078*** | 0.030 | 0.125 |
| Total effect |
0.083*** |
0.034 |
0.129 |
| Prop. Mediated (average) | 6.0%* | 0.7% | 20.4% |
*p < 0.05, ***p < 0.001.
Multivariate regression models
Two multivariate regression models were estimated as part of the initial mediation analysis. The results of these models are reported to further disentangle and better explicate the relationship between the key variables in the analysis. The first is a multivariate, linear model that assessed the association between neighborhood perception at wave one and allostatic load at wave two while controlling for all the covariates previously listed. Individuals in the low neighborhood perception group had higher allostatic load (B = 0.15, p < 0.05). Overall, the model accounted for 7.5% of the variance in allostatic load (adjusted R2 = 0.075). The second model was a multivariate logistic regression and results are reported as adjusted odds ratios (aORs). These results show that individuals who reported depression at wave three had more than twice the odds of being in the low neighborhood perception group at wave one (aOR = 2.19, 95% CI: 1.31, 3.69). Additionally, depression at wave three was associated with 53% greater odds of higher allostatic load at wave two (aOR = 1.53, 95% CI: 1.18, 1.97).
Sensitivity analysis
Table 4 shows the results of the leave-one-out sensitivity analysis. This analysis included a series of multivariate logistic regression models whereby wave one neighborhood perceptions predicted depression at wave three while systematically removing potential confounding variables from the analysis one at a time to assess the impact on the coefficient for neighborhood perception. The results show that age, followed by wave one depression, had the largest impact on the effect of wave one neighborhood perception on wave three depression. When age is removed from the analysis, the bias introduced to the model shows an increase in the effect of neighborhood perceptions on depression by roughly 14% (i.e., the difference between not removing any confounders, B = 0.080, and age, B= 0.091). It should also be noted that the point estimates for removing each confounder fall within the 95% confidence interval for not removing any confounders (95% CI: 0.023, 0.138).
Table 4.
Leave-one-out sensitivity analysis.
| Coefficient for Neighborhood Perception | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| None | 0.080 | 0.023 | 0.138 |
| Age | 0.091 | 0.036 | 0.145 |
| Sex | 0.085 | 0.029 | 0.140 |
| Years in neighborhood | 0.085 | 0.030 | 0.141 |
| Marital status | 0.084 | 0.029 | 0.140 |
| Homeownership status | 0.084 | 0.030 | 0.139 |
| Race | 0.082 | 0.027 | 0.137 |
| Educational attainment | 0.080 | 0.023 | 0.138 |
| Baseline depression | 0.089 | 0.033 | 0.145 |
Note: The table shows change in the effect of neighborhood perception on depression when each covariate is individually dropped from the multivariate logistic regression analysis.
Discussion
This study sought to disaggregate the role of allostatic load in the relationship between neighborhood perceptions and depression. The findings show that a portion of the relationship between neighborhood perception and depression is mediated by allostatic load. Previous studies found that connections between negative perceptions of one's community can result in biological dysregulation that is associated with negative physical health outcomes such as cardiovascular disease and type 2 diabetes mellitus (Donath & Shoelson, 2011; Font-Burgada, Sun, & Karin, 2016; Juster & Lupien, 2012; Mattei, Demissie, Falcon, Ordovas, & Tucker, 2010; Rosmond & Bjorntorp, 2000). This study expands that work to the arena of mental health. The findings are consistent with a growing body of literature that is seeking to better understand the linkages between biological dysregulation and depression (e.g., Geisler, Fuchs, Sperner-Unterweger, & Gostner, 2018; Milaneschi et al., 2017). Considering the new and developing nature of this area of research, these findings add to the understanding of the effect that biological dysregulation in the form of allostatic load can have on depression as well as the role of environmental factors on these forms of biological dysregulation.
Practice implications
Understanding how individuals view their communities can have important implications for clinical practice. For example, exercise is considered to be beneficial for individuals experiencing depression (Schuch et al., 2016). Yet negative perceptions of neighborhood safety or physical conditions influence one's decision to engage in active, outdoor activities (Galaviz, Zytnick, Kegler, & Cunningham, 2016; Maisel, 2016). While clinicians cannot fix these community-level issues directly, they should be aware of how they influence individual behaviors as well as the underlying biological linkages between community factors—specifically perceptions—and health outcomes.
In addition, understanding how individuals perceive their communities can have important implications for the development and implementation of interventions at multiple levels. Community interventions, which are comprised of efforts by individuals across multiple sectors—including community members—and involved community-based service delivery (e.g., community centers, schools) (Castillo et al., 2019), is one category of intervention that can draw on these findings. While not a new approach, the use of community interventions to address a wide range of health problems has grown internationally in recent years. Examples of the diversity of community interventions include efforts to strengthen social networks among older adults in Japan (Harada et al., 2017), address mental health in low- and middle-income countries (Kohrt et al., 2018), and support caregivers of individuals living with dementia (Paúl, Teixeira, Duarte, Pires, & Ribeiro, 2019). Expanding community intervention work to integrate how individuals experience their communities, such as through neighborhood and community perceptions, as well as the mediating effects of biological dysregulation may lead to more effective interventions.
Limitations and opportunities for future research
There are limitations to this study. Attrition from wave to wave of the study may lead to self-selection bias within the study sample. Additionally, study attrition reduces the overall sample size so that the analysis cannot be subset to only individuals who lived in the same neighborhood for the duration of the study and instead resulted in controlling for this in the analysis. The fact that only one wave of biomarker data is currently available prevents the ability to consider or control for changes in biological dysregulation over time. This limits the ability to make broader causal statements and any statement about the allostatic load variable must be clear to note that it is based on biological dysregulation as measured at a given point in time without a baseline measure. An additional wave of biomarker data is set to be released as part of the MIDUS wave three. These data will enable future analyses to address this issue. Future research projects should focus efforts on longitudinal biomarker data collection. Although some researchers have begun to structure research projects in this manner (e.g., Chyu & Upchurch, 2018; Tampubolon & Maharani, 2018), it is still a new approach, most likely due to the expense of conducting such studies. Longitudinal studies will also allow for a better understanding of the complex and bidirectional relationship between allostatic load and depression in addition to identifying the specific biological pathways that link neighborhood perceptions to mental health.
While the causal model allows for the controlling of confounders and leads to more accurate estimates that are less model dependent, the need to dichotomize or establish specific cut offs for key variables of interest means that some nuance in the data may be lost. This is especially true for the neighborhood perception variable. Future studies should also seek to integrate more complex ways to control for changes in neighborhood perceptions and conditions over time. In addition, the data used in the analysis is observational and while statistical methods can strengthen the ability to make causal inferences, such conclusions are still limited by the fact that this is not experimental data.
Conclusion
Environmental factors, such as perceived neighborhood conditions, are important contributors to mental health outcomes such as depression. By better understanding how these factors are linked, specifically by identifying biological mediators, interventions can be developed and implemented at multiple levels. Such a multilevel approach is an innovation that may prove valuable, especially for individuals who experience treatment resistant depression.
Ethics approval
This study utilized publicly available, deidentified data and therefore does not fall under the purview of institutional review board oversight as it does not meet the definition of research with human subjects.
Author Statement
As a sole author manuscript, Jason T. Carbone is responsible for all aspects of the manuscript entitled, “The Mediating Effect of Allostatic Load on the Relationship Between Neighborhood Perceptions and Depression”
Financial disclosures
None.
Declaration of competing interest
None.
Acknowledgements
The author would like th acknowledge Stephen Edward McMillin, Julie Birkenmaier, Jin Huang, Travis Loux, and Michael G. Vaughn for the feedback and comments on this paper.
References
- Alvares G.A., Quintana D.S., Hickie I.B., Guastella A.J. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. Journal of Psychiatry & Neuroscience. 2016;41:89–104. doi: 10.1503/jpn.140217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Juster R.-P., Westphal S., Amminger G.P., Bogerts B., Schiltz K. Allostatic load is associated with psychotic symptoms and decreases with antipsychotic treatment in patients with schizophrenia and first-episode psychosis. Psychoneuroendocrinology. 2018;90:35–42. doi: 10.1016/j.psyneuen.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Berger M., Lavoie S., McGorry P.D., Nelson B., Markulev C., Yuen H.-P. Relationship between allostatic load and clinical outcomes in youth at ultra-high risk for psychosis in the NEURAPRO study. Schizophrenia Research. 2018 doi: 10.1016/j.schres.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Bob P., Raboch J., Maes M., Susta M., Pavlat J., Jasova D. Depression, traumatic stress and interleukin-6. Journal of Affective Disorders. 2010;120:231–234. doi: 10.1016/j.jad.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Bowers M.E., Yehuda R. Chapter 16 - Neuroendocrinology of posttraumatic stress disorder: Focus on the HPA Axis. In: Fink G., editor. ume 2. Academic Press; San Diego: 2017. pp. 165–172. (Stress: Neuroendocrinology and Neurobiology, Handbook of stress). [Google Scholar]
- Brim O.G., Baltes P.B., Bumpass L.L., Cleary P.D., Featherman D.L., Hazzard W.R. Inter-university Consortium for Political and Social Research; Ann Arbor, MI: 1996. National survey of Midlife development in the United States (MIDUS), 1995–1996. [Google Scholar]
- Brown L., Karmakar C., Gray R., Jindal R., Lim T., Bryant C. Heart rate variability alterations in late life depression: A meta-analysis. Journal of Affective Disorders. 2018;235:456–466. doi: 10.1016/j.jad.2018.04.071. [DOI] [PubMed] [Google Scholar]
- Carbone J.T. Neighborhood perceptions and allostatic load: Evidence from Midlife in the United States study. Health & Place. 2020;61:102263. doi: 10.1016/j.healthplace.2019.102263. [DOI] [PubMed] [Google Scholar]
- Castillo E.G., Ijadi-Maghsoodi R., Shadravan S., Moore E., Mensah M.O., Docherty M. Community interventions to promote mental health and social equity. Current Psychiatry Reports. 2019;21(5):35. doi: 10.1007/s11920-019-1017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Yang L., Pu F., Lin H., Wang B., Liu J. High birth weight increases the risk for bone tumor: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health. 2015;12:11178–11195. doi: 10.3390/ijerph120911178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J.J., Bower J.E., Irwin M.R., Taylor S.E., Fuligni A.J. Adiposity moderates links from early adversity and depressive symptoms to inflammatory reactivity to acute stress during late adolescence. Brain, Behavior, and Immunity. 2017;66:146–155. doi: 10.1016/j.bbi.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyu L., Upchurch D.M. A longitudinal analysis of allostatic load among a multi- ethnic sample of midlife women: Findings from the Study of Women's Health across the Nation. Women's Health Issues: Official Publication of the Jacobs Institute of Women’s Health. 2018;28:258–266. doi: 10.1016/j.whi.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry A., Latkin C., Davey-Rothwell M. Pathways to depression: The impact of neighborhood violent crime on inner-city residents in Baltimore, Maryland, USA. Social Science & Medicine. 2008;67:23–30. doi: 10.1016/j.socscimed.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L., Dong L., Mezuk B. Shades of blue and gray: A comparison of the center for epidemiologic studies depression scale and the composite international diagnostic Interview for assessment of depression syndrome in later life. The Gerontologist. 2020;60(4):e242–e253. doi: 10.1093/geront/gnz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurzen I., Rod N.H., Christensen U., Hansen A.M., Lund R., Dich N. Neighborhood perceptions and allostatic load: Evidence from Denmark. Health & Place. 2016;40:1–8. doi: 10.1016/j.healthplace.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nature Reviews Immunology. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Dunbar J.A., Reddy P., Davis-Lameloise N., Philpot B., Laatikainen T., Kilkkinen A. Depression: An important comorbidity with metabolic syndrome in a general population. Diabetes Care. 2008;31(12):2368–2373. doi: 10.2337/dc08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font-Burgada J., Sun B., Karin M. Obesity and cancer: The oil that feeds the flame. Cell Metabolism. 2016;23:48–62. doi: 10.1016/j.cmet.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Friedman E.M., Karlamangla A.S., Gruenewald T.L., Koretz B., Seeman T.E. Early life adversity and adult biological risk profiles. Psychosomatic Medicine. 2015;77:176–185. doi: 10.1097/psy.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaviz K.I., Zytnick D., Kegler M.C., Cunningham S.A. Parental perception of neighborhood safety and children's physical activity. Journal of Physical Activity and Health. 2016;13:1110–1116. doi: 10.1123/jpah.2015-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S., Fuchs D., Sperner-Unterweger B., Gostner J.M. Immunometabolism in the pathogenesis of depressive disorders - therapeutic considerations. Current Topics in Medicinal Chemistry. 2018;18:1408–1415. doi: 10.2174/1568026618666180410141042. [DOI] [PubMed] [Google Scholar]
- Gruenewald T.L., Karlamangla A.S., Hu P., Stein-Merkin S., Crandall C., Koretz B. History of socioeconomic disadvantage and allostatic load in later life. Social Science & Medicine. 2012;74:75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, Behavior, and Immunity. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L., Hill T.D., Friedman E., Nieto F.J., Galvao L.W., Engelman C.D. Perceived neighborhood quality, sleep quality, and health status: Evidence from the survey of the health of Wisconsin. Social Science & Medicine. 2013;79:16–22. doi: 10.1016/j.socscimed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi N.R., South S.C., Krueger R.F. Does education lower allostatic load? A co- twin control study. Brain, Behavior, and Immunity. 2016;56:221–229. doi: 10.1016/j.bbi.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Masumoto K., Katagiri K., Fukuzawa A., Chogahara M., Kondo N. Community intervention to increase neighborhood social network among Japanese older adults. Geriatrics and Gerontology International. 2018;18(3):462–469. doi: 10.1111/ggi.13208. [DOI] [PubMed] [Google Scholar]
- Haro J.M., Arbabzadeh‐Bouchez S., Brugha T.S., De Girolamo G., Guyer M.E., Jin R. Concordance of the composite international diagnostic Interview version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World mental health surveys. International Journal of Methods in Psychiatric Research. 2006;15:167–180. doi: 10.1002/mpr.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán M.A., Robins J.M. Chapman & Hall/CRC; Boca Raton: 2020. Causal inference: What if. [Google Scholar]
- Hodes G.E., Ménard C., Russo S.J. Integrating interleukin-6 into depression diagnosis and treatment. Neurobiology of Stress. 2016;4:15–22. doi: 10.1016/j.ynstr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D.E., Imai K., King G., Stuart E.A. MatchIt: Nonparametric preprocessing for parametric causal inference. Journal of Statistical Software. 2011;42(8):1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- Juster R.P., Lupien S. A sex- and gender-based analysis of allostatic load and physical complaints. Gender Medicine. 2012;9:511–523. doi: 10.1016/j.genm.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Juster R.P., Marin M.F., Sindi S., Nair N.P., Ng Y.K., Pruessner J.C. Allostatic load associations to acute, 3-year and 6-year prospective depressive symptoms in healthy older adults. Physiology & Behavior. 2011;104:360–364. doi: 10.1016/j.physbeh.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Abelson J., Demler O., Escobar J.I., Gibbon M., Guyer M.E. Clinical calibration of DSM‐IV diagnoses in the World mental health (WMH) version of the World health organization (WHO) composite international diagnostic Interview (WMH‐CIDI) International Journal of Methods in Psychiatric Research. 2004;13:122–139. doi: 10.1002/mpr.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Andrews A., Mroczek D., Ustun B., Wittchen H.U. The World health organization composite international diagnostic Interview short-form (CIDI-SF) International Journal of Methods in Psychiatric Research. 1998;7:171–185. [Google Scholar]
- Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.S., Park N., Peterson C. Perceived neighborhood social cohesion and stroke. Social Science & Medicine. 2013;97(Supplement C):49–55. doi: 10.1016/j.socscimed.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Kobrosly R.W., Seplaki C.L., Cory-Slechta D.A., Moynihan J., van Wijngaarden E. Multisystem physiological dysfunction is associated with depressive symptoms in a population-based sample of older adults. International Journal of Geriatric Psychiatry. 2013;28(7):718–727. doi: 10.1002/gps.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly R.W., van Wijngaarden E., Seplaki C.L., Cory-Slechta D.A., Moynihan J. Depressive symptoms are associated with allostatic load among community-dwelling older adults. Physiology & Behavior. 2014;123:223–230. doi: 10.1016/j.physbeh.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt B.A., Asher L., Bhardwaj A. The role of communities in mental health care in low- and middle-income countries: A meta-review of components and competencies. International Journal of Environmental Research and Public Health. 2018;15(6):1279. doi: 10.3390/ijerph15061279. Published 2018 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F., Milaneschi Y., De Jonge P., Giltay E.J., Penninx B.W.J.H. Metabolic and inflammatory markers: Associations with individual depressive symptoms. Psychological Medicine. 2018;48(7):1102–1110. doi: 10.1017/S0033291717002483. [DOI] [PubMed] [Google Scholar]
- Mair C., Diez Roux A.V., Morenoff J.D. Neighborhood stressors and social support as predictors of depressive symptoms in the Chicago Community Adult Health Study. Health & Place. 2010;16:811–819. doi: 10.1016/j.healthplace.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J.L. Impact of older adults' neighborhood perceptions on walking behavior. Journal of Aging and Physical Activity. 2016;24:247–255. doi: 10.1123/japa.2014-0278. [DOI] [PubMed] [Google Scholar]
- Mattei J., Demissie S., Falcon L.M., Ordovas J.M., Tucker K. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Social Science & Medicine. 2010;70:1988–1996. doi: 10.1016/j.socscimed.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Annals Of the New York Academy Of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy Of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. doi: 10.1001/archinte.1993.00410180039004. [DOI] [PubMed] [Google Scholar]
- McIntyre R.S., Soczynska J.K., Konarski J.Z., Woldeyohannes H.O., Law C.W., Miranda A. Should depressive syndromes be reclassified as “metabolic syndrome type II”? Annals of Clinical Psychiatry. 2007;19(4):257–264. doi: 10.3109/10401230701653377. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y., Lamers F., Peyrot W.J., Baune B.T., Breen G., Dehghan A. Genetic association of major depression with atypical features and obesity-related immunometabolic dysregulations. JAMA Psychiatry. 2017;74:1214–1225. doi: 10.1001/jamapsychiatry.2017.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Reviews Immunology. 2016;16:22–34. doi: 10.1038/mp.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce A.J., Kia D.A., Hemani G., Nicolas A., Price T.R., De Pablo-Fernandez E., International Parkinson Disease Genomics Consortium Estimating the causal influence of body mass index on risk of Parkinson disease: A mendelian randomisation study. PLoS Medicine. 2017;14(6) doi: 10.1016/j.trb.2017.01.017. e1002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T.W., Miller A.H. Cytokines and glucocorticoid receptor signaling: Relevance to major depression. Annals of the New York Academy of Sciences. 2009;1179:86. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante C.M., Miller A.H. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biological Psychiatry. 2001;49:391–404. doi: 10.1016/S0006-3223(00)01088-X. [DOI] [PubMed] [Google Scholar]
- Paúl C., Teixeira L., Duarte N., Pires C.L., Ribeiro O. Effects of a community intervention program for dementia on mental health: The importance of secondary caregivers in promoting positive aspects and reducing strain. Community Mental Health Journal. 2019;55(2):296–303. doi: 10.1007/s10597-018-0345-6. [DOI] [PubMed] [Google Scholar]
- Robinette J.W., Charles S.T., Gruenewald T.L. Vigilance at home: Longitudinal analyses of neighborhood safety perceptions and health. SSM - Population Health. 2016;2:525–530. doi: 10.1016/j.ssmph.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R., Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. Journal of Internal Medicine. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Ross C.E., Mirowsky J. Neighborhood disadvantage, disorder, and health. Journal Of Health And Social Behavior. 2001;42:258–276. doi: 10.2307/3090214. [DOI] [PubMed] [Google Scholar]
- Ryff C., Almeida D.M., Ayanian J., Binkley N., Carr D.S., Coe C. 2014. Midlife in the United States (MIDUS 3) [DOI] [Google Scholar]
- Ryff C., Almeida D.M., Ayanian J., Carr D.S., Cleary P.D., Coe C. 2006. Midlife in the United States (MIDUS 2) [DOI] [Google Scholar]
- Sampson R.J., Raudenbush S.W. Seeing disorder: Neighborhood stigma and the social construction of "broken windows". Social Psychology Quarterly. 2004;4:319–342. doi: 10.1177/019027250406700401. [DOI] [Google Scholar]
- Sasayama D., Hattori K., Wakabayashi C., Teraishi T., Hori H., Ota M. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. Journal of Psychiatric Research. 2013;47:401–406. doi: 10.1016/j.jpsychires.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Schuch F.B., Vancampfort D., Richards J., Rosenbaum S., Ward P.B., Stubbs B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. Journal of Psychiatric Research. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Slopen N., Chen Y., Priest N., Albert M.A., Williams D.R. Emotional and instrumental support during childhood and biological dysregulation in midlife. Preventive Medicine. 2016;84:90–96. doi: 10.1016/j.ypmed.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C., Miller G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosomatic Medicine. 2011;73(2):114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Tamayo A., Karter A.J., Mujahid M.S., Warton E.M., Moffet H.H., Adler N. Associations of perceived neighborhood safety and crime with cardiometabolic risk factors among a population with type 2 diabetes. Health & Place. 2016;39:116–121. doi: 10.1016/j.healthplace.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampubolon G., Maharani A. Trajectories of allostatic load among older Americans and Britons: Longitudinal cohort studies. BMC Geriatrics. 2018;18:1–10. doi: 10.1186/s12877-018-0947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. Mediation: R package for causal mediation analysis. Journal of Statistical Software. 2014;59(5):1–38. doi: 10.18637/jss.v059.i05. [DOI] [Google Scholar]
- Toma A., Hamer M., Shankar A. Associations between neighborhood perceptions and mental well-being among older adults. Health & Place. 2015;34:46–53. doi: 10.1016/j.healthplace.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Tonorezos E.S., Breysse P.N., Matsui E.C., McCormack M.C., Curtin-Brosnan J., Williams D. Does neighborhood violence lead to depression among caregivers of children with asthma? Social Science & Medicine. 2008;67:31–37. doi: 10.1016/j.socscimed.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner H.A., Shattuck A., Hamby S., Finkelhor D. Community disorder, victimization exposure, and mental health in a national sample of youth. Journal of Health and Social Behavior. 2013;54:258–275. doi: 10.1177/0022146513479384. [DOI] [PubMed] [Google Scholar]
- Vadiveloo M., Mattei J. Perceived weight discrimination and 10-year risk of allostatic load among US adults. Annals of Behavioral Medicine. 2017;51(1):94–104. doi: 10.1007/s12160-016-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.S., Berglund P., Kessler R.C. Recent care of common mental disorder in the United States: Prevalence and conformance with evidence-based recommendations. Journal of General Internal Medicine. 2000;15:284–292. doi: 10.1046/j.1525-1497.2000.9908044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weden M.M., Carpiano R.M., Robert S.A. Subjective and objective neighborhood characteristics and adult health. Social Science & Medicine. 2008;66:1256–1270. doi: 10.1016/j.socscimed.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Wee L.E., Tsang Y., Tay S.M., Cheah A., Puhaindran M., Yee J. Perceived neighborhood environment and its association with health screening and exercise participation amongst low-income public rental flat residents in Singapore. International Journal of Environmental Research and Public Health. 2019;16(8):1384. doi: 10.3390/ijerph16081384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Genderson M., Pruchno R. Effects of neighborhood violence and perceptions of neighborhood safety on depressive symptoms of older adults. Social Science & Medicine. 2013;85:43–49. doi: 10.1016/j.socscimed.2013.02.028. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 1990. Composite international diagnostic Interview, CIDI, version 10. [Google Scholar]
- Zeng P., Yu X., Xu H. Association between premorbid body mass index and amyotrophic lateral sclerosis: Causal inference through genetic approaches. Frontiers in Neurology. 2019;10:543. doi: 10.3389/fneur.2019.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilioli S., Slatcher R.B., Ong A.D., Gruenewald T.L. Purpose in life predicts allostatic load ten years later. Journal of Psychosomatic Research. 2015;79(5):451–457. doi: 10.1016/j.jpsychores.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]