Abstract
Traditional approaches to clinical risk assessment utilize age as a marker of increased vulnerability to stress. Relatively recent advancements in the study of aging have led to the concept of the frailty syndrome, which represents a multidimensional state of depleted physiologic and psychosocial reserve and clinical vulnerability that is related to but variably present with advancing age. The frailty syndrome is now a well-established clinical entity that serves as both a guide for clinical intervention and a predictor of poor outcomes in the primary and acute care settings. The biological aspects of the syndrome broadly represent a network of interrelated perturbations involving the age-related accumulation of molecular, cellular, and tissue damage that leads to multisystem dysregulation, functional decline, and disproportionately poor response to physiologic stress. Given the complexity of the underlying biologic processes, several well-validated approaches to define frailty clinically have been developed, each with distinct and reasonable considerations. Stemming from this background, the past several years have seen a number of observational studies conducted in intensive care units that have established that the determination of frailty is both feasible and prognostically useful in the critical care setting. Specifically, frailty as determined by several different frailty measurement tools appears associated with mortality, increased health care utilization, and disability, and has the potential to improve risk stratification of intensive care patients. While substantial variability in the implementation of frailty measurement likely limits the generalizability of specific findings, the overall prognostic trends may offer some assistance in guiding management decisions with patients and their families. Although no trials have assessed interventions to improve the outcomes of critically ill older people living with frailty, the particular vulnerability of this population offers a promising target for intervention in the future.
Historically, advanced age has been used as an indicator of increased vulnerability to physiologic stress. Indeed, increasing age is associated with the advancement of medical complexity and reduced life expectancy.1 However, chronological age is not a universal predictor of poor clinical outcome. 2 Some older adults retain a substantial ability to recover from stress, while some young individuals lack significant physiologic reserve. The variable ability to recover from physiologic insults, independent of chronologic age, is perhaps best encapsulated within the concept of the “frailty syndrome.” Broadly speaking, the frailty syndrome is common in older adult populations and represents the state of declining health and capacity to respond to stress. In the past 2 decades, significant study has been performed to better understand the biological and nonbiological underpinnings that lead to frailty and the associated declining functional capacity, increased health care utilization, and poorer health outcomes. Recently, investigations of frailty in the intensive care unit (ICU) have demonstrated that people living with frailty are distinctly vulnerable, independent of age. This narrative review discusses the definitions, biologic mechanisms, and the importance of recognizing frailty in the ICU—which may help intensivists optimally strategize care for these high-risk patients.
COMPONENTS OF THE FRAILTY SYNDROME
Underlying the frailty syndrome is a complex, heterogeneous physiologic state which lacks universally recognized symptoms or discrete biomarkers. Nevertheless, it is important to describe the syndrome with reasonable specificity to compare outcomes among individuals with “frailty.” Some authors have generated a set of defining characteristics implicit in the contemporary understanding of frailty across its variety of applications in the published research literature.3 Briefly, 5 components describe frailty as a clinically measurable state of vulnerability, with multifactorial etiology and heterogeneous presentation, predisposing to an increased risk of adverse outcomes (Table 1).3 These components are broadly in line with the views of health care providers of people living with frailty, and these providers also explicitly acknowledge the influence of nonphysical and nonbiological processes on frailty.4
Table 1.
Core Components of Frailty Implicit in the Frailty Literature
| Component | Description |
|---|---|
| State of vulnerability | Acute or chronic stressors elicit a maladaptive response disproportionate to the degree of insult. |
| Multifactorial etiology | Complex biological processes interact through network effects involving multisystem dysregulation and the age-associated accumulation of molecular, cellular, and tissue damage. |
| Heterogeneous presentation | Multiple points of entry and dynamic, nonlinear disease progression produce variability in observed characteristics in those affected. |
| Clinically measurable | Operationalized measurement tools are able to provide a diagnosis of frailty, although a gold standard is notably absent. |
| Increased risk of adverse outcomes | Patients are subject to increased rates of adverse outcomes including functional decline, decreased quality of life, increased health care utilization, and mortality. |
There are 5 general elements central to the definition of frailty that are common throughout the scientific literature examining the condition as a medical syndrome. These elements recognize the presumed underlying biological underpinnings of the development of frailty, along with the clinically relevant implications of the syndrome.
These components reflect the significant interindividual variability in frailty presentation and emphasize its complexity. While these components do not necessarily lead to an operationalizable definition of frailty, they reflect the ambiguity inherent in the clinical description of frailty as a diagnosis. Some of the ambiguity implicit in both the description of and diagnosis of frailty is due to the complex biologic underpinnings of frailty and the variable influence of external factors on its course.
WORKING MODELS OF FRAILTY
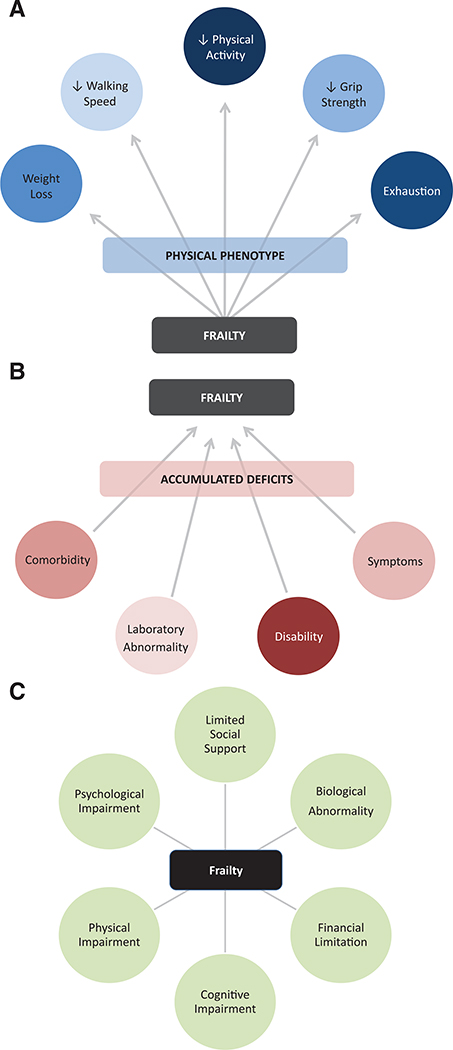
Several clinical models of frailty have been developed to permit recognition of the syndrome. These can be described as the “physical phenotype” model, the “accumulated deficit” model, and the “multidimensional” model (Figure 1).
Figure 1.
Conceptual models of frailty. Working definitions of frailty use 3 broad approaches. Each approach has established merit and validated measurement instruments that can be used for their assessment in clinical practice. A, The frailty physical phenotype model evaluates frailty by a set of 5 clinically measurable phenotypic traits that develop as a result of underlying frailty physiology. B, The accumulated deficits model views frailty as a function of a network of a diverse set of age-associated deficits that interact to produce a state of vulnerability. C, The multidimensional model formalizes frailty as a syndrome of multiple interrelated factors, including both biological and nonbiological impairments.
The physical phenotype model, originally developed by Fried et al5 in the Cardiovascular Health Study, defines frailty by the presence of 3 or more of 5 criteria: unintentional weight loss of 4.5 kg or more in the previous year, weak grip strength, selfreported exhaustion, slow walking speed, and low physical activity. Physical frailty has a strong foundation of evidence to support its biological basis and predictivity for outcomes. Conceptually, physical frailty is rooted in the presumption of an underlying set of physiologic processes that produce a clinically observable phenotype that correlates with vulnerability and adverse outcomes.
The second model of frailty comes from Rockwood and Mitnitski’s Canadian Study of Health and Aging in which the degree of frailty is determined by an index of accumulated deficits across a range of domains including signs, symptoms, functional impairments, and laboratory values.6 This approach is powerful in its capacity to accommodate the complexity of frailty in its use of any number of chosen deficits (optimal utilizing >30 attributes) as long as they increase in prevalence with age and do not saturate at a young age.7 Frailty indexes (FI) have been broadly adapted to different datasets, generally with strong predictive power.8 The accumulated deficits primarily act as a measure of vulnerability as a function of the quantity but not type of age-related deficits.
Third, frailty can be conceptualized by the multidimensional approach.9 While the phenotype model views frailty as primarily physical in nature and the accumulation of deficits model is relatively agnostic to the types of age-related pathology attributable to frailty, the multidimensional model formalizes frailty as a syndrome affecting multiple areas of functioning including physical, cognitive, psychological, and social. This approach stems from consideration of the multisystemic nature of frailty and the impact of external factors on its clinical trajectory.
All 3 approaches possess a strong theoretical framework and supporting evidence for their validity; however, no strong consensus currently exists to justify the use of one approach over another, despite that they likely measure correlated but distinct phenomena. As the 2 original conceptualizations of frailty, some have suggested that, in particular, “physical frailty” and “deficit accumulation frailty” be formally differentiated in ongoing work such that their respective use in context-specific clinical practice might be better elucidated.10
BIOLOGICAL MECHANISMS ASSOCIATED WITH FRAILTY
The unifying biochemical origins of frailty are not entirely known. Rather, the clinical manifestations and consequences of frailty naturally arise from a complex network of physiologic perturbations.11 Generally speaking, patients living with frailty have genetic findings related to dysregulation of inflammation, muscle function, β-adrenergic receptors, and mitochondrial DNA.11 Many biomarkers may be relevant in frailty, although none are explicitly associated with frailty alone or are definitively identifiable in all instances of frailty. Nevertheless, many patients living with frailty possess an imbalance of anabolic and catabolic hormones, a deficiency of vitamin D, a decrease in sirtuins, an increase in some inflammation-related factors and decrease in others, an increase in glycoproteins, renal dysfunction, endothelial dysfunction, and peripheral insulin resistance.11 A variety of potential physiologic maladaptations interact to affect these observable biomarkers in addition to the physical phenotypic findings central to the syndrome such as sarcopenia and functional decline.12 Candidate mechanisms that produce such dysfunction include oxidative stress,13 cellular senescence,14 and inflammation,15 which may in themselves be interconnected. While not definitively causal, environmental exposures including exercise, smoking, and diet along with chronic comorbid disease play a likely role in altering the development and progression of the disease.13–15 Furthermore, diverse socioeconomic factors including race, marital status, education, and income have been linked to the development of frailty.16
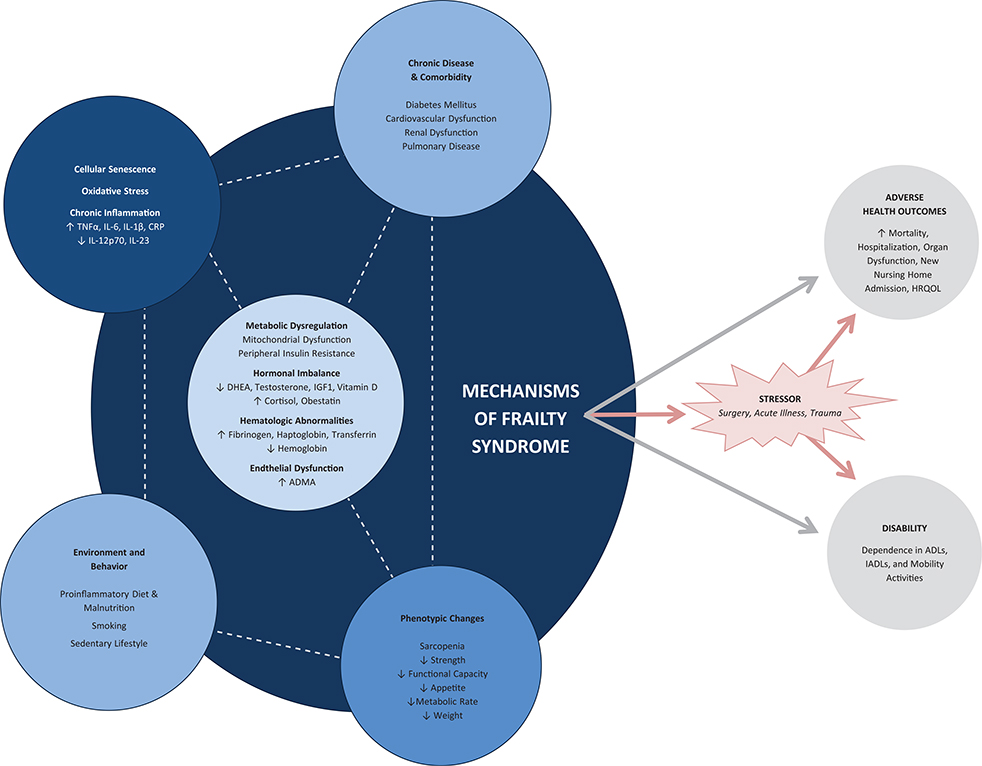
The complexity of relationships inherent to the underlying mechanisms of frailty leads to a number of interconnected feedback loops that ultimately produce the frailty syndrome and subsequent vulnerability to adverse health outcomes (Figure 2). No singular factor within the network of findings associated with frailty has been identified as strictly necessary, except perhaps some physical phenotypic derangement. As such, the defining biological characteristics of the syndrome are debatable as evident in the range of approaches to clinically operationalizing the diagnosis.
Figure 2.
Mechanisms of frailty. The physiologic underpinnings of frailty are complex and involve a range of interacting elements. A number of physiological perturbations have been noted to be common among frail individuals, but none have specifically been shown to be necessary for the development of frailty. Frailty-related changes occur across multiple biological systems as both an innate element of aging and a response to environmental exposures. These systems interact through a network of interconnected feedback loops to produce the vulnerable state of frailty, including disability, dependence, and adverse health outcomes, particularly following stress. ADL indicates activities of daily living; ADMA, asymmetric dimethylarginine; CRP, C-reactive protein; DHEA, dehydroepiandrosterone; HRQOL, health-related quality of life; IADL, instrumental activities of daily living; IGF1, insulin-like growth factor 1; IL, interleukin; TNFα, tumor necrosis factor α.
The diversity of pathophysiology associated with frailty could indicate a relationship between frailty and specific disease states observed frequently in the ICU. Without understanding the precise physiology underlying frailty, definitive statements cannot be made regarding such associations. With that said, substantial overlap between frailty biomarkers and those of illnesses relevant to ICU practice suggests that people with frailty may be predisposed to an increased incidence or severity of certain illnesses while in the ICU. For example, frailty and delirium share an association with similarly altered inflammatory states, atherosclerosis, and malnutrition.17 Patients with the hyperinflammatory subtype of acute respiratory distress syndrome (ARDS) and sepsis may also exhibit inflammatory changes common to those often seen in frail patients.18,19 The mitochondrial dysfunction and oxidative stress associated with frailty have also been linked to the pathophysiology of acute kidney injury, sepsis, and ARDS.20–22 While only speculative in nature, these examples highlight that the biology of frailty syndrome may directly relate to many important diseases affecting critically ill patients.
EVALUATION OF FRAILTY IN THE ICU
While the biological mechanisms of frailty remain somewhat obscure, identification of the general phenotype has been better defined. Recognition of frailty may encourage clinicians to provide careful, considerate care to patients with reduced reserve to recover from stress. This continues to be explored in frail presurgical populations, who might benefit from prehabilitation, alternative operative and anesthetic plans, and discussions of goals of care that better account for patients’ individual risk profiles.23
To some extent, within the ICU, researchers have been successful in utilizing all of the aforementioned approaches to operationalize the definition of frailty, although barriers prevent their more widespread use.24 The physical phenotype definition, despite its potential in ambulatory care settings, is not as easily diagnosed in the ICU given the measurements required. For this reason, a small but growing number of studies have assessed premorbid physical phenotype in intensive care, with 2 based on a single longitudinal outpatient cohort of patients and others using questionnaires to assess preadmission status.25–30 The questionnaire-based approach, while having demonstrated predictivity in the ICU, should be recognized as a derivative of rather than equivalent to the initial conception of Fried et al.5 The accumulated deficits approach is also subject to several challenges within the ICU. A well-constructed FI is typically cumbersome and requires dozens of data points, with many elements difficult to identify in the ICU setting.7 Direct comparisons of FIs should be performed cautiously given variability in evaluated attributes and analytical approaches because these are not necessarily uniform across studies. The relationship between FI scores and outcomes cannot be assumed to be independent of the attributes utilized even though similar mechanics have been observed across similarly constructed FIs. In particular, FIs with a small number of attributes with distinct evaluated domains should not be presumed to be equivalent to other FI scores, but even well-constructed FIs adherent to sound theoretical principles might not behave equally well in all clinical contexts.7,31,32 Furthermore, this approach may suffer from less certain construct validity when multimorbidity is equated with frailty in the frequently used 11-criteria modified FI.33,34 With that said, the accumulated deficits approach has been successfully applied to ICU patients.35–38 The multidimensional approach has been infrequently used in the ICU, relying on questionnaire-based scores to assess mobility, functional status, physical fitness, vision, hearing, nourishment, comorbidity, cognition, and psychosocial factors.39–41 For all 3 of these approaches, much of the challenge in assessment on arrival to the ICU stems from their cumbersome nature along with an inability to assess patients at their true baseline, relying on factors that are potentially altered by critical illness or depending on patient—or more often proxy—report of premorbid status with less reliability.
To address the difficulties of applying complex measurement tools in an ICU setting, other clinical measures have been trialed as well. The simplest clinical approaches use single-item proxies such as an equivalent diagnosis in retrospective review or the ability to leave home independently.42,43 While these techniques can be applied thoughtfully, they should be used with caution in light of the risk of oversimplifying a complex syndrome.
The most widely used frailty measure in the ICU is the Clinical Frailty Scale (CFS), a rating scale designed to holistically assess frailty according to physical activity, functional status, chronic illness burden, and cognition. The scale was originally validated as a measure of mortality risk in a primary care setting and was highly correlated with the accumulated deficit FI (correlation = 0.80) in this outpatient population.44 Given its ease of use, this has been utilized frequently in the ICU.27,29,41,45– 56 The earliest conception of this scale ranged from 1 to 7; however, the revised and more common usage utilizes a scale that ranges from 1 to 9. Generally, scores of 1 to 3 are considered nonfrail; a score of 4 is considered vulnerable or prefrail; scores of 5 to 8 are considered frail; and a score of 9 indicates terminal illness, usually regarded as categorically distinct (Figure 3).44 An important consideration in the intentional design of CFS is that disability (and to a lesser extent comorbidity) is the explicit defining element of frailty according to embedded descriptions of the scale. Such conflation of disability with frailty within the design of the scale may or may not extend to common clinical application, but it does suggest that study design should recognize the predefined relationship between CFS and disability, particularly when intended as primary exposure and outcome. Overall, this scale is applied as a composite assessment of frailty status based on information often readily obtained from medical records along with patient or family interviews.
Figure 3.
The CFS. The 9-point CFS was adapted from the 7-point scale used in the Canadian Study of Health and Aging. CFS is the most frequently used frailty measurement instrument in the ICU. Categorically, the scale distinguishes among fit (1–3), vulnerable (4), frail (5–8), and terminally ill but not otherwise frail (9) patients. The scale is intended to be self-evident in its application, and notably relies on the characterization of disability in IADLs and ADLs to determine the transition from vulnerability to frailty. ADL indicates activities of daily living; CFS, Clinical Frailty Scale; IADL, instrumental activities of daily living; ICU, intensive care unit. Reprinted with permission from Geriatric Medicine Research, Dalhousie University, Halifax, Nova Scotia.
While CFS provides a streamlined approach to assigning frailty scores, there is some ambiguity in its application. Multiple studies have highlighted that the subjectivity of the score predisposes it to lower interrater reliability, particularly across clinical training backgrounds or lack thereof.24,57,58 Although large variation in ICU populations may be possible, Montgomery et al’s52 retrospective study of a large-scale standardized implementation of CFS in all ICUs in Alberta, Canada, produced an unexplained and large range of 9%–43% frailty across the 17 ICUs despite a multifaceted education strategy and a visual analog guide at the time of score assignment. Despite these shortcomings, CFS has repeatedly been shown to be a robust and valid tool in the ICU even in direct comparison with contemporaneously assessed markers of the physical frailty phenotype and multidimensional frailty.26,27,29,41 Moreover, Shears et al59 provide a reliability study of the assignment of CFS scores by chart review, patient interview, and family interview by research coordinators, occupational therapists, and geriatrics residents in the ICU and found no clinically meaningful differences in CFS scores across groups of assessors, demonstrating that well-trained personnel may provide reliable implementation. While a more rigorous and reproducible approach to applying CFS to ICU populations needs to be disseminated, it likely remains a useful frailty assessment tool in the ICU and continues to be the most widely adopted in this setting.
Beyond clinical assessment tools that possess a significant degree of subjectivity, the measurement of more objective proxies of clinical frailty has been sssssexplored. To date, this has primarily focused on assessments of sarcopenia and body composition using radiographic or ultrasonographic assessment of muscle mass, adiposity, and osteopenia.35,39,60,61 An additional derivative assessment of malnutrition and muscle mass has been trialed using serum creatinine to cystatin C ratio.62,63 While certainly notable for potential prognostic (or interventional) utility, these alternatives do not purport to directly measure frailty but rather sarcopenia, a distinct though demonstrably correlated clinical concept that has not been as thoroughly explored in the ICU to date.
CHARACTERISTICS OF PATIENTS LIVING WITH FRAILTY ON ARRIVAL TO THE ICU
As to be expected, patients living with frailty often present to the ICU with several differences compared to nonfrail patients (Table 2). In support of the validity of clinical frailty measurement instruments, patients living with frailty admitted to the ICU have a greater number of comorbidities, are more likely to be disabled at baseline, are less likely to be admitted from home, and are generally older.25,52 Each of these factors individually likely contributes to poorer outcomes in the ICU.
Table 2.
Key Findings of Studies of Frailty in the ICU
| Clinical Finding | Association With Frailty in the ICU |
|---|---|
| Admission | |
| Indication | On a proportional basis, frailty is associated with more frequent admission to the ICU for acute indications such as acute medical illness or emergency surgery compared to admission following elective surgery. |
| Severity of illness | Inconsistent results across studies. Patients living with frailty are generally more likely admitted to the ICU, with higher illness severity scores such as APACHE and SOFA. |
| Advanced directives | Patients living with frailty who are admitted to the ICU are more likely to have orders for limitation of care, such as “do not resuscitate” and “DNI.” |
| ICU course | |
| Mortality | Frailty is associated with increased mortality in the ICU, particularly in the subset of ICU patients with lower illness severity scores. Multivariable analyses do not consistently demonstrate frailty as an independent predictor of ICU mortality. |
| Organ supports and complications | Inconsistent results across studies with potential for significant confounding potentially due to orders for limitation of care. General tendency toward increased use of organ supports and increased incidence of ICU complications. Possible less frequent use of invasive mechanical ventilation and more frequent use of noninvasive ventilation, likely attributable to DNI orders. |
| Length of stay | Inconsistent but larger studies suggest increased LOS in the ICU for patients living with frailty. |
| Post-ICU course | |
| Mortality | Frailty is associated with increased in-hospital mortality and long-term mortality, performing well as an independent predictor of mortality in multivariable analyses. |
| Disability | Among survivors of critical illness, frailty is associated with an increased total burden of disability through at least 6 mo. |
| Nursing home admission | Among those survivors of critical illness who are community dwelling at baseline, frailty is associated with an increased risk of admission to a nursing home following ICU discharge. |
A number of relatively consistent differences have been observed between patients living with and without frailty in the ICU. The diversity and inconsistency of implementation of frailty measurement tools suggest that aggregate results may be hard to interpret with regards to specific values and likelihoods, but confirmation across an adequate number of observational trials suggest that frailty confers a number of disadvantages with regards to the clinical course of critical illness.
Abbreviations: APACHE, Acute Physiologic and Chronic Health Evaluation; DNI, do not intubate; ICU, intensive care unit; LOS, length of stay; SOFA, Sequential Organ Failure Assessment.
Several studies have observed that patients living with frailty are proportionally more often admitted to an ICU for acute indications rather than elective surgery compared to nonfrail patie nts.47,49,51,54,64 Furthermore, patients living with frailty are more likely than robust patients to require ICU admission following emergency general surgery.65 In addition, patients deemed frail by CFS, physical frailty, and FI tend to have significant increases in illness severity as measured by several different acute illness severity scores. While smaller studies have not consistently achieved statistical significance on this point, Montgomery et al’s52 retrospective study of CFS applied to more than 15,000 ICU patients found a substantial difference in Acute Physiologic and Chronic Health Evaluation II (APACHE II; frail 22 versus nonfrail 17). Although this could partially reflect a degree of CFS score inflation in sicker patients, Zampieri’s retrospective study of the less subjective modified FI further affirms the relationship between admission illness severity and frailty.33 The differences in acute indications for admission and illness severity predispose to differences in clinical outcomes to a significant extent.
Also pertinent to the interpretation of study results, at least one-third of patients living with frailty have orders for limitations of care.26,47,49,51,66 Patients determined to be frail by CFS are roughly twice as likely to have medical care withheld or withdrawn in the ICU.67 Furthermore, in one study using a simplified marker of the physical frailty phenotype, orders for limitation of care interact with the association of frailty with mortality.28 While frailty in the subgroup of patients with treatment limitations was not associated with a greater risk of mortality, the subgroup without treatment limitations displayed a greater incidence of mortality for patients living with frailty compared to those without.28 Notably, this factor is generally unaccounted for in the frailty ICU literature.
As a whole, critically ill patients with frailty often possess several distinctions on arrival to the ICU compared to their robust counterparts, and these distinctions are likely enough to drive meaningful differences in their course of critical illness.
CLINICAL COURSE OF CRITICALLY ILL PATIENTS LIVING WITH FRAILTY
Beyond admission characteristics, there have been a number of both prospective and retrospective studies assessing the association of frailty with a variety of outcomes. Unfortunately, the limitations of the measurement of frailty demand caution in the generalization of specific results: there remains a lack of a gold standard instrument for diagnosing frailty, and contemporary frailty measurement tools are undoubtedly applied in nonuniform and imperfectly reliable ways. With that said, consistent patterns have emerged that suggest that frailty is associated with adverse outcomes among ICU patients in several predictable ways that adhere to current models of frailty (Table 2; Supplemental Digital Content, Table 1, http://links.lww.com/AA/D10).
In an assessment of the earliest studies of frailty in the ICU, Muscedere et al68 have previously provided a systematic review of outcomes differences in frail compared to robust patients. Their meta-analysis unadjusted for covariates of 3030 ICU patients from 10 studies found increased risk of hospital and long-term mortality (relative risk [RR], 1.71 and 1.53, respectively) but not ICU mortality, no statistically significant difference in ICU or hospital length of stay (LOS), no difference in the use of mechanical ventilation or vasopressor therapy, decreased likelihood of discharge to home (RR 0.59), and relatively reduced health-related quality of life (HRQOL) at 1 year. Montgomery et al’s52 more recent retrospective cohort of 15,238 patients along with other recent study has extended these results and found statistical significance not found in this smaller sample of patients, including differences in the use of organ supports and LOS. The rapidly growing literature on frailty in the ICU frequently finds that frailty may help prognosticate many of the aforementioned outcomes.
Mortality appears to be significantly influenced by the presence of frailty even when controlling for covariates. Notably, there is some heterogeneity at different time points. When adjusting for illness severity, mortality while in the ICU has been shown to be increased with greater FI and with incremental increases in the number of markers of the physical frailty phenotype.26,40 This is contrary to the more numerous studies utilizing CFS, in which frailty is a predictor of ICU mortality but does not perform well as an element of multivariable analysis, including in Montgomery’s large study.26,45,47,50–52 More broadly, in-hospital mortality for patients living with frailty who have been admitted to the ICU is more consistently increased across several different patient populations and measures of frailty, and the effect is increased with higher frailty scores.33–35,42,51,52 While the modified FI has been reasonably critiqued as a measure of multimorbidity rather than a direct measure of frailty, Zampieri’s retrospective study of nearly 130,000 ICU patients across Brazil adds an important nuance likely translatable to other measures: the effect size of frailty on mortality is substantially greater for patients with decreased illness severity as measured by Sequential Organ Failure Assessment.33 Assessments of longerterm outcomes indicate an independent association of frailty with mortality over 30 days, 6 months, and up to 1 year, with CFS, physical frailty, and FI measures reliably demonstrating increased risk with incremental frailty severity across multiple studies.25,36,37,43,47–49,51,56 Taken together, these findings suggest that the capacity of frailty for predicting mortality is most useful as an indicator of persistent vulnerability following recovery from the initial critical insult. While patients living with frailty are at increased risk of death in the ICU in univariate analyses, this may be driven primarily by other correlates of frailty including increased severity of illness on presentation to the ICU and confounded by possible discharge from the ICU for comfort care before anticipated death.
Beyond mortality, the overall clinical trajectory of patients living with frailty differs significantly compared to nonfrail patients in the ICU. While Muscedere et al’s68 analysis did not find a difference in LOS or organ support, Zampieri et al’s33 and Hamidi et al’s34 large retrospective studies using modified FI identified an independent association of MFI with an increased use of organ supports, increased complications, and decreased likelihood of discharge from the ICU (adjusted hazards ratio [HR], 0.942) and from the hospital (adjusted HR, 0.792). In contrast, though Montgomery et al52 found patients living with frailty to have longer ICU and hospital LOS, they also observed that these patients were less likely to be treated with invasive mechanical ventilation and vasopressors and more likely to be treated with noninvasive ventilation. Once started, however, these interventions were used for longer durations. In Fernando et al’s53 prospective study of 1510 septic patients in the ICU, CFS predicted longer ICU and hospital LOS along with a greater number of interventions and risk of adverse events, including hospital-acquired infection, delirium, and cardiac complications.52 The particular association of the development of delirium with presurgical frailty as measured by adaptations of both physical frailty and accumulated deficits has also been observed in patients recovering from cardiac surgery.69–71 In particular, weight loss and grip strength, 2 sarcopeniarelated frailty indicators, were most strongly linked to delirium as opposed to pre-existing cognitive impairment.69 As a net effect of these findings, Fernando et al53 identified increased ICU and total health care utilization along with costs in their study. These observations taken together suggest that critically ill patients living with frailty most likely suffer from greater rates of organ dysfunction requiring intervention, and the significantly higher rate of withholding and withdrawal of treatment among frail patients may ultimately blunt the observed effect on rates of interventions utilized in this population.
To further assist in prognostication, several studies have assessed factors related to the anticipated course of recovery of frail survivors of critical illness. This has likely best been assessed in Ferrante et al’s25,30 longitudinal cohort of 754 patients ≥70 years of age who underwent serial evaluations of Fried et al’s5 physical frailty, cognitive status, and disability every 18 months from 1998 to 2014. Community-dwelling patients with frailty had a substantially increased rate of incident nursing home admission following ICU discharge compared to robust patients: 58.8% of frail, 37.7% of prefrail, and 23.5% of robust patients were newly admitted to nursing homes (adjusted odds ratio [OR], 3.52 for frail versus robust). All groups of patients were likely to acquire new disability in the immediate period following critical illness, but patients living with frailty had a significantly increased total burden of disability during the 6 months after critical illness (adjusted RR 1.41 for frail versus robust, 1.28 for prefrail versus robust). Significantly increased disability persisted through 6 months for frail and prefrail patients compared to robust patients who generally returned to their baseline. In addition, preexisting frailty interacted with pre-existing cognitive impairment: among ICU survivors with any cognitive impairment (even mild), each additional frailty criterion (from 0 to 5) was associated with a 54% higher burden of disability during the 6 months following a critical illness (RR, 1.54; 95% confidence interval [CI], 1.37–1.74), whereas the increase among cognitively intact patients was only 18%. These findings are consistent across other studies of frailty in the ICU that have assessed both younger and older patient populations across different measures of frailty.27,43,48,51,57 As an extension of disability, measures of HRQOL including a 36-Item Short Form Survey (SF)-36, SF-12, and EuroQol- 5 Dimension (EQ-5D) in patients living with frailty demonstrate often several-fold increased risk of such problems through 1 year following ICU admission.37,48,51,56,64 In general, all patients leaving the ICU should anticipate challenges in their recovery, but patients living with frailty are particularly susceptible to a more severe and sustained decline from an already reduced baseline level of function and wellbeing.
FRAILTY INTERVENTIONS
The ultimate diagnostic utility of the frailty syndrome may eventually be the generation of effective interventions to improve the clinical course of this vulnerable patient population. Unfortunately, to date, there have been no specific interventional trials aimed at patients with frailty in the ICU, but various related studies offer some promise toward the improved management of these patients.
On its own, identification of frailty may passively encourage more proactive efforts to involve additional team members for care planning at an earlier stage and may alter clinical decision-making in an as yet undetermined way such that morbidity and mortality are reduced; this effect has previously been demonstrated as part of a frailty screening initiative in preoperative elective surgical patients.72 More specific interventions in frail populations may also be helpful, including physical exercise and nutrition strategies as has been repeatedly shown to be effective in primary and secondary care settings.73 This is more likely to manifest as early mobilization in the ICU, which has broadly supported benefit in all ICU patients (although not specifically studied in frail patients), and postillness rehabilitation.74 Various pharmacologic agents have some support as means of halting or even reversing the course of frailty as a chronic condition, including metformin, angiotensinconverting enzyme inhibitors, and to a lesser extent anabolic steroids; some study has also begun investigating the possibility of cell-based therapy with mesenchymal stem cells.75 Unfortunately, while these potential therapeutic mechanisms have gained some traction as preventative care strategies, none have yet been investigated in the critical care setting, and more study is needed to assess their potential benefit in ICU survivors.
Given the absence of evidence to support frailty detection and intervention in ICU settings, frailty is currently more likely to play a role in facilitating conversations about goals of care and advanced care planning. On its own, a diagnosis of frailty likely increases the likelihood that a goal of care discussion occurs at all,76 and these discussions could reasonably be informed by the prognostic information connected to that diagnosis. While the goals of survivorship specific to critically ill patients with frailty have not been reported, many elderly patients with frailty express general concerns associated with their increasing dependence and functional disability, and they are likely to feel increasingly isolated, particularly when moved into a care facility.77 As such, the shared decision-making process for the determination of patient disposition and treatment intensity (including withholding or withdrawal of care) could be aided by an understanding of conferred risk of frailty of an increased burden of disability, new admission to a skilled nursing facility, and the persistently greater risk of mortality despite recovery from the acute critical insult. Further along these lines, frailty could theoretically contribute to the determination of treatment futility in certain settings, although no research has yet been conducted to provide frailty-driven guidelines for ICU triage. While specific recommendations cannot be made in this regard based on the currently available literature, frailty could reasonably be incorporated into the decision-making process for patient disposition.
FUTURE DIRECTIONS
Frailty in the ICU has been thoroughly examined in both retrospective and prospective observational studies, and the evidence supports that frailty is strongly associated with critical care outcomes, including mortality, health care utilization, and disability. Unfortunately, these findings have come from the use of a large number of different frailty measurement instruments. While a true gold standard tool for frailty assessment has yet to be established in any context, the CFS continues to be the most frequently utilized instrument in the ICU despite some degree of uncertainty in its reliability. This suggests a more rigorous implementation protocol in the ICU should be introduced along with its validation against a well-established instrument of frailty measured in the premorbid setting. In addition, the increased adoption of an adaptation of the physical frailty phenotype may avoid some of the shortcomings of CFS and better connect with other domains of frailty research in the United States that more widely use Fried et al’s5 model. Alternatively, the powerful accumulated deficits approach may find a streamlined path to more widespread implementation in the era of electronic health records. An FI comprised of an adequate number of diverse attributes that can be easily assessed in the ICU setting and are unaffected by acute illness may offer an effective means of evaluating frailty among critically ill patients in the future. Finally, the use of measures of frailty aimed explicitly at assessing the multidimensional nature of the syndrome may also provide a better assessment of the impact of the psychosocial and economic factors of frailty on the course of critical illness.
In addition, although frailty may eventually help target treatment strategies for critically ill patients, there are currently no studies that have evaluated any specific therapy or intervention to improve the outcomes of critically ill patients living with frailty. In particular, research assessing physical rehabilitation and nutrition tailored to patients with frailty in the ICU may offer possible targeted therapies to achieve improved outcomes in this population. Furthermore, the formal incorporation of frailty into risk stratification models may help improve shared medical decision-making in the ICU to reduce the potential burden of overmedicalization with the benefit of improved morbidity and mortality for this vulnerable population.
CONCLUSIONS
Frailty is a complex multisystemic syndrome that profoundly alters a patient’s capacity to recover from an acute insult. The frailty syndrome has been measured by several approaches in the ICU including the adaptation of the Fried et al5 physical frailty phenotype, FI, and CFS, and these tools have been validated for their predictive capacities. Presently, the generalizability of specific results is limited because of uncertainty in the reliability and varied implementation of many of the current frailty measurement tools used in the ICU. Despite such shortcomings, the current evidence suggests that the vulnerability of frailty extends to the ICU in the form of increased mortality, health care utilization, and disability in addition to increased severity of illness at presentation. The additional prognostic information carried with a frailty diagnosis may assist patients, their families, and their health care team in the determination of goals of care in the setting of critical illness, but other specific interventions to improve outcomes in this population have not yet been identified.
Supplementary Material
Funding:
L.E.F. is supported by a Paul B. Beeson Emerging Leaders in Aging Research Career Development Award from the National Institute on Aging (K76AG057023). D.H.K. is supported by R01AG062713 from the National Institute on Aging. S.S. is supported by a grant for Early Medical/ Surgical Specialists’ Transition to Aging Research (GEMSSTAR) Award (R03AG060179) from the National Institute on Aging and a Mentored Clinical Scientist Research Career Development Award from the National Institute of General Medical Sciences (K08GM134220).
GLOSSARY
- ADL
activities of daily living
- ADMA
asymmetric dimethylarginine
- APACHE
Acute Physiology And Chronic Health Evaluation
- ARDS
acute respiratory distress syndrome
- CFS
Clinical Frailty Scale
- CI
confidence interval
- CRP
C-reactive protein
- DHEA
dehydroepiandrosterone
- DNI
do not intubate
- EQ-5D
EuroQol- 5 Dimension
- FI
frailty indexes
- HR
hazards ratio
- HRQOL
health-related quality of life
- IADL
instrumental activities of daily living
- ICU
intensive care unit
- IGF1
insulin-like growth factor 1
- IL
interleukin
- LOS
length of stay
- OR
odds ratio
- RR
relative risk
- SF
short form
- SOFA
Sequential Organ Failure Assessment
- TNFα
tumor necrosis factor α
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
Listen to this Article of the Month podcast and more from OpenAnesthesia.org® by visiting http://journals.lww.com/anesthesia-analgesia/pages/default.aspx.
Reprints will not be available from the authors.
DISCLOSURES
Name: Justin C. De Biasio, MD.
Contribution: This author helped in the conception and design, acquisition, analysis or interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the submitted manuscript.
Name: Aaron M. Mittel, MD.
Contribution: This author helped in the conception and design, acquisition, analysis or interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the submitted manuscript.
Name: Ariel L. Mueller, MA.
Contribution: This author helped in the acquisition, analysis, or interpretation of the data, critical revision of the manuscript for important intellectual content, and final approval of the submitted manuscript.
Name: Lauren E. Ferrante, MD.
Contribution: This author helped in the conception and design, acquisition, analysis, or interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the submitted manuscript.
Name: Dae H. Kim, MD.
Contribution: This author helped in the conception and design, acquisition, analysis, or interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the submitted manuscript.
Name: Shahzad Shaefi, MD, MPH.
Contribution: This author helped in the conception and design, acquisition, analysis, or interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the submitted manuscript.
This manuscript was handled by: Robert Whittington, MD.
REFERENCES
- 1.Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. 2018;15:e1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Rooij SE, Govers A, Korevaar JC, Abu-Hanna A, Levi M, de Jonge E. Short-term and long-term mortality in very elderly patients admitted to an intensive care unit. Intensive Care Med. 2006;32:1039–1044. [DOI] [PubMed] [Google Scholar]
- 3.Junius-Walker U, Onder G, Soleymani D, et al. ; ADVANTAGE JA WP4 group. The essence of frailty: a systematic review and qualitative synthesis on frailty concepts and definitions. Eur J Intern Med. 2018;56:3–10. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a delphi method based consensus statement. The frailty operative definition-consensus conference project. J Gerontol. 2013;68:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 6.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. [DOI] [PubMed] [Google Scholar]
- 9.Aguayo GA, Donneau AF, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186:420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walston J, Bandeen-Roche K, Buta B, et al. Moving frailty toward clinical practice: NIA intramural frailty science symposium summary. J Am Geriatr Soc. 2019;67:1559–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viña J, Tarazona-Santabalbina FJ, Pérez-Ros P, et al. Biology of frailty: modulation of ageing genes and its importance to prevent age-associated loss of function. Mol Aspects Med. 2016;50:88–108. [DOI] [PubMed] [Google Scholar]
- 12.Bernabei R, Martone AM, Vetrano DL, Calvani R, Landi F, Marzetti E. Frailty, physical frailty, sarcopenia: a new conceptual model. Stud Health Technol Inform. 2014;203:78–84. [PubMed] [Google Scholar]
- 13.Viña J, Borras C, Gomez-Cabrera MC. A free radical theory of frailty. Free Radic Biol Med. 2018;124:358–363. [DOI] [PubMed] [Google Scholar]
- 14.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulop T, Witkowski JM, Olivieri F, Larbi A. The integration of inflammaging in age-related diseases. Semin Immunol. 2018;40:17–35. [DOI] [PubMed] [Google Scholar]
- 16.Ocampo-Chaparro JM, Reyes-Ortiz CA, Castro-Flórez X, Gómez F. Frailty in older adults and their association with social determinants of Health. The SABE Colombia Study. Colomb Med (Cali). 2019;50:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc. 2011;59 Suppl 2:S262–S268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spadaro S, Park M, Turrini C, et al. Biomarkers for acute respiratory distress syndrome and prospects for personalised medicine. J Inflamm (Lond). 2019;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leger T, Charrier A, Moreau C, et al. Early sepsis does not stimulate reactive oxygen species production and does not reduce cardiac function despite an increased inflammation status. Physiol Rep. 2017;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan JS, Sheikh-Hamad D. Mitochondrial dysfunction in acute kidney injury and sex-specific implications. Med Res Arch. 2019;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montini L, DE Sole P, Pennisi MA, et al. Prognostic value of the reactive oxygen species in severe sepsis and septic shock patients: a pilot study. Minerva Anestesiol. 2016;82:1306–1313. [PubMed] [Google Scholar]
- 22.Kellner M, Noonepalle S, Lu Q, Srivastava A, Zemskov E, Black SM. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv Exp Med Biol. 2017;967:105–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wozniak SE, Coleman J, Katlic MR. The utility of preoperative frailty assessment. Curr Surg Rep. 2016;4:36. [Google Scholar]
- 24.Falvey JR, Ferrante LE. Frailty assessment in the ICU: translation to ‘real-world’ clinical practice. Anaesthesia. 2019;11:14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, LeoSummers LS, Gill TM. The association of frailty with postICU disability, nursing home admission, and mortality: a longitudinal study. Chest. 2018;153:1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Maguet P, Roquilly A, Lasocki S, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. [DOI] [PubMed] [Google Scholar]
- 27.Hope AA, Hsieh SJ, Petti A, Hurtado-Sbordoni M, Verghese J, Gong MN. Assessing the usefulness and validity of frailty markers in critically ill adults. Ann Am Thorac Soc. 2017;14:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueno R, Shiraishi A, Yamamoto R, Kobara S, Hayashi Y. Relationship between community walking ability and in-hospital mortality in elderly patients with sepsis: a single-center retrospective cohort study. J Intensive Care. 2019;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tipping CJ, Hodgson CL, Harrold M, Chan T, Holland AE. Frailty in patients with trauma who are critically ill: a prospective observational study to determine feasibility, concordance, and construct and predictive validity of 2 frailty measures. Phys Ther. 2019;99:1089–1097. [DOI] [PubMed] [Google Scholar]
- 30.Ferrante LE, Murphy TE, Leo-Summers LS, Gahbauer EA, Pisani MA, Gill TM. The combined effects of frailty and cognitive impairment on post-ICU disability among older ICU survivors. Am J Respir Crit Care Med. 2019;200:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. Geroscience. 2017;39:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. [DOI] [PubMed] [Google Scholar]
- 33.Zampieri FG, Iwashyna TJ, Viglianti EM et al. ; ORCHESTRA Study Investigators. Association of frailty with short-term outcomes, organ support and resource use in critically ill patients. Intensive Care Med. 2018;44:1512–1520. [DOI] [PubMed] [Google Scholar]
- 34.Hamidi M, Zeeshan M, Leon-Risemberg V, et al. Frailty as a prognostic factor for the critically ill older adult trauma patients. Am J Surg. 2019;218:484–489. [DOI] [PubMed] [Google Scholar]
- 35.Mueller N, Murthy S, Tainter CR, et al. Can sarcopenia quantified by ultrasound of the rectus femoris muscle predict adverse outcome of surgical intensive care unit patients as well as frailty? A prospective, observational cohort study. Ann Surg. 2016;264:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng A, Song X, Dong J, et al. Mortality in relation to frailty in patients admitted to a specialized geriatric intensive care unit. J Gerontol A Biol Sci Med Sci. 2015;70:1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heyland DK, Garland A, Bagshaw SM, et al. Recovery after critical illness in patients aged 80 years or older: a multicenter prospective observational cohort study. Intensive Care Med. 2015;41:1911–1920. [DOI] [PubMed] [Google Scholar]
- 38.Kara I, Yildirim F, Zerman A, et al. The impact of frailty on noninvasive mechanical ventilation in elderly medical intensive care unit patients. Aging Clin Exp Res. 2018;30:359–366. [DOI] [PubMed] [Google Scholar]
- 39.de Hoogt PA, Reisinger KW, Tegels JJW, Bosmans JWAM, Tijssen F, Stoot JHMB. Functional Compromise Cohort Study (FCCS): sarcopenia is a strong predictor of mortality in the intensive care unit. World J Surg. 2018;42:1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kizilarslanoglu MC, Civelek R, Kilic MK, et al. Is frailty a prognostic factor for critically ill elderly patients? Aging Clin Exp Res. 2017;29:247–255. [DOI] [PubMed] [Google Scholar]
- 41.Darvall JN, Greentree K, Braat MS, Story DA, Lim WK. Contributors to frailty in critical illness: multi-dimensional analysis of the Clinical Frailty Scale. J Crit Care. 2019;52:193–199. [DOI] [PubMed] [Google Scholar]
- 42.Hope AA, Gong MN, Guerra C, Wunsch H. Frailty before critical illness and mortality for elderly medicare beneficiaries. J Am Geriatr Soc. 2015;63:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López Cuenca S, Oteiza López L, Lázaro Martín N, et al. Frailty in patients over 65 years of age admitted to Intensive Care Units (FRAIL-ICU). Med Intensiva. 2019;43:395–401. [DOI] [PubMed] [Google Scholar]
- 44.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fronczek J, Polok KJ, Nowak-Kózka I, et al. Frailty is associated with an increased mortality among patients ≥ 80 years old treated in Polish ICUs. Anaesthesiol Intensive Ther. 2018;50:245–251. [DOI] [PubMed] [Google Scholar]
- 46.Langlais E, Nesseler N, Le Pabic E, Frasca D, Launey Y, Seguin P. Does the clinical frailty score improve the accuracy of the SOFA score in predicting hospital mortality in elderly critically ill patients? A prospective observational study. J Crit Care. 2018;46:67–72. [DOI] [PubMed] [Google Scholar]
- 47.Muessig JM, Nia AM, Masyuk M, et al. Clinical Frailty Scale (CFS) reliably stratifies octogenarians in German ICUs: a multicentre prospective cohort study. BMC Geriatr. 2018;18:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brummel NE, Bell SP, Girard TD, et al. Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. 2017;196:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flaatten H, De Lange DW, Morandi A, et al. ; VIP1 study group. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years). Intensive Care Med. 2017;43:1820–1828. [DOI] [PubMed] [Google Scholar]
- 50.Fisher C, Karalapillai DK, Bailey M, Glassford NG, Bellomo R, Jones D. Predicting intensive care and hospital outcome with the Dalhousie Clinical Frailty Scale: a pilot assessment. Anaesth Intensive Care. 2015;43:361–368. [DOI] [PubMed] [Google Scholar]
- 51.Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montgomery CL, Zuege DJ, Rolfson DB, et al. Implementation of population-level screening for frailty among patients admitted to adult intensive care in Alberta, Canada. Can J Anaesth. 2019;66:1310–1319. [DOI] [PubMed] [Google Scholar]
- 53.Fernando SM, McIsaac DI, Perry JJ, et al. Frailty and associated outcomes and resource utilization among older ICU patients with suspected infection. Crit Care Med. 2019;47:e669–e676. [DOI] [PubMed] [Google Scholar]
- 54.Jung C, Wernly B, Muessig JM, et al. ; VIP1 study group. Electronic address: hans.flaatten@uib.no. A comparison of very old patients admitted to intensive care unit after acute versus elective surgery or intervention. J Crit Care. 2019;52:141–148. [DOI] [PubMed] [Google Scholar]
- 55.de Lange DW, Brinkman S, Flaatten H, et al. ; VIP1 Study Group. Cumulative prognostic score predicting mortality in patients older than 80 years admitted to the ICU. J Am Geriatr Soc. 2019;67:1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bagshaw M, Majumdar SR, Rolfson DB, Ibrahim Q, McDermid RC, Stelfox HT. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care. 2016;20:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hope AA, Munoz M, Hsieh SJ, Gong MN. Surrogates’ and researchers’ assessments of prehospital frailty in critically ill older adults. Am J Crit Care. 2019;28:117–123. [DOI] [PubMed] [Google Scholar]
- 58.Pugh RJ, Battle CE, Thorpe C, et al. Reliability of frailty assessment in the critically ill: a multicentre prospective observational study. Anaesthesia. 2019;74:758–764. [DOI] [PubMed] [Google Scholar]
- 59.Shears M, Takaoka A, Rochwerg B, et al. ; Canadian Critical Care Trials Group. Assessing frailty in the intensive care unit: a reliability and validity study. J Crit Care. 2018;45:197–203. [DOI] [PubMed] [Google Scholar]
- 60.Jaitovich A, Khan M, Itty R, et al. ICU admission muscle and fat mass, survival, and disability at discharge: a Prospective Cohort Study. Chest. 2018;28:023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaplan SJ, Pham TN, Arbabi S, et al. Association of radiologic indicators of frailty with 1-year mortality in older trauma patients: opportunistic screening for sarcopenia and osteopenia. JAMA Surg. 2017;152:e164604. [DOI] [PubMed] [Google Scholar]
- 62.Barreto EF, Kanderi T, DiCecco SR, et al. Sarcopenia index is a simple objective screening tool for malnutrition in the critically ill. JPEN J Parenter Enteral Nutr. 2019;43:780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barreto EF, Poyant JO, Coville HH, et al. Validation of the sarcopenia index to assess muscle mass in the critically ill: a novel application of kidney function markers. Clin Nutr. 2019;3:1362–1367. [DOI] [PubMed] [Google Scholar]
- 64.Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med. 2015;43:973–982. [DOI] [PubMed] [Google Scholar]
- 65.McIsaac DI, Moloo H, Bryson GL, van Walraven C. The association of frailty with outcomes and resource use after emergency general surgery: a population-based cohort study. Anesth Analg. 2017;124:1653–1661. [DOI] [PubMed] [Google Scholar]
- 66.Heyland D, Cook D, Bagshaw SM et al. ; Canadian Critical Care Trials Group; Canadian Researchers at the End of Life Network. The very elderly admitted to ICU: a quality finish? Crit Care Med. 2015;43:1352–1360. [DOI] [PubMed] [Google Scholar]
- 67.Guidet B, Flaatten H, Boumendil A, et al. ; VIP1 study group. Withholding or withdrawing of life-sustaining therapy in older adults (≥80 years) admitted to the intensive care unit. Intensive Care Med. 2018;44:1027–1038. [DOI] [PubMed] [Google Scholar]
- 68.Muscedere J, Waters B, Varambally A, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43:1105–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogawa M, Izawa KP, Satomi-Kobayashi S, et al. Impact of delirium on postoperative frailty and long term cardiovascular events after cardiac surgery. PLoS One. 2017;12:e0190359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nomura Y, Nakano M, Bush B, et al. Observational study examining the association of baseline frailty and postcardiac surgery delirium and cognitive change. Anesth Analg. 2019;129:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watt J, Tricco AC, Talbot-Hamon C, et al. Identifying older adults at risk of delirium following elective surgery: a systematic review and meta-analysis. J Gen Intern Med. 2018;33:500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg. 2017;152:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kidd T, Mold F, Jones C, et al. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 2019;19:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joseph B, Jehan FS. The mobility and impact of frailty in the intensive care unit. Surg Clin North Am. 2017;97:1199–1213. [DOI] [PubMed] [Google Scholar]
- 75.Florea V, Bagno L, Rieger AC, Hare JM. Attenuation of frailty in older adults with mesenchymal stem cells. Mech Ageing Dev. 2019;181:47–58. [DOI] [PubMed] [Google Scholar]
- 76.Madni TD, Nakonezny PA, Wolf SE, et al. The relationship between frailty and the subjective decision to conduct a goals of care discussion with burned elders. J Burn Care Res. 2018;39:82–88. [DOI] [PubMed] [Google Scholar]
- 77.Ohnsorge K, Rehmann-Sutter C, Streeck N, Gudat H. Wishes to die at the end of life and subjective experience of four different typical dying trajectories. A qualitative interview study. PLoS One. 2019;14:e0210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.