Abstract
Critical drug shortages have been widely documented during the coronavirus disease 2019 (COVID-19) pandemic, particularly for IV sedatives used to facilitate mechanical ventilation. Surges in volume of patients requiring mechanical ventilation coupled with prolonged ventilator days and the high sedative dosing requirements observed quickly led to the depletion of “just-in-time” inventories typically maintained by institutions. This manuscript describes drug shortages in the context of global, manufacturing, regional and institutional perspectives in times of a worldwide crisis such as a pandemic. We describe etiologic factors that lead to drug shortages including issues related to supply (eg, manufacturing difficulties, supply chain breakdowns) and variables that influence demand (eg, volatile prescribing practices, anecdotal or low-level data, hoarding). In addition, we describe methods to mitigate drug shortages as well as conservation strategies for sedatives, analgesics and neuromuscular blockers that could readily be applied at the bedside. The COVID-19 pandemic has accentuated the need for a coordinated, multi-pronged approach to optimize medication availability as individual or unilateral efforts are unlikely to be successful.
Key Words: critical care, disaster, drugs
Abbreviations: API, active pharmaceutical ingredient; COVID-19, coronavirus disease 2019; FDA, Food and Drug Administration
The World Health Organization designates access to essential drugs as a critical concern due to persistent shortages and escalating costs.1 , 2 Drug shortages are a function of demand and supply mismatches that can be affected by manufacturing and distribution, as well as by regulatory, economic, or political considerations. Prior to the coronavirus disease 2019 (COVID-19) pandemic, numerous countries frequently documented such shortages, mostly with injectable drugs, to include antimicrobials, anesthetics, cardiovascular and neurologic drugs, nutrition, electrolytes, and cancer chemotherapy.2 , 3 The COVID-19 pandemic dramatically shows how a large and sudden surge in demand can lead to shortages when local, national, and international supply chains cannot keep pace, in particular for medications with limited therapeutic alternatives for critical care. The current article highlights the available literature on possible causes and mitigation strategies to manage shortages of critical care drugs from the local, institutional level to the global stage in a worldwide crisis such as a pandemic.
Global Context
The COVID-19 pandemic resulted in international shortages of multiple critical drugs.4, 5, 6, 7, 8 Sedatives, analgesics, and paralytics needed to support critical care were particularly affected by the surge in volumes of patients requiring mechanical ventilation, combined with high-dose requirements often used to manage severe hypoxemia. The result was a quick depletion of limited “just-in-time” inventories at many hospitals. This situation was exacerbated by the inability to access supplies to match these new demands in either local, national, or international networks.4, 5, 6, 7, 8, 9, 10, 11, 12 There are also disparity concerns at the international level, such as preferential access and hoarding by higher income countries, and, conversely, ineffective stock management, notification systems, and demand forecasting in low- and middle-income countries.2 , 10, 11, 12 Further complicating the issue are the absence of international surveillance and notification systems for medication demands and a lack of globally accepted best practices for dealing with shortages.2
Supply chain disruptions leading to medication shortages are more likely to occur when there is limited diversification in the development and distribution of required active pharmaceutical ingredients (APIs). The problem is further exacerbated when the manufacturing process is arduous or lengthy, or the supply of raw materials is disrupted. For example, approximately 80% of active ingredients required for pharmaceutical compounding hail from India and China, one of which (China) was the first country affected by COVID-19.13 In addition, > 80% of new molecular entities are patented with varying degrees of market exclusivity and availability according to country.14 Of 220 new molecular entities launched between 2011 and 2017 in 36 countries, the percent available according to country varied from a high of 87% in the United States to < 10% in other countries.15 There are also additional concerns with the supply of generic medications, which comprise at least 70% of the market in Canada, Germany, the Netherlands, the United States, and the United Kingdom.16 In a study of nonprescription, generic oral forms approved by the US Food and Drug Administration (FDA) since 1939, only 19% had a manufacturer approved by four or more of seven non-US regulators evaluated.17 Of the 26% of medications with no FDA-approved generic, only 48% were available from at least one manufacturer approved by one of the seven non-US regulators.
The International Pharmaceutical Federation held a summit involving multiple stakeholders in 2013 to address the persistent issue of drug shortages.18 The federation recommended the compilation of publicly available lists of known national drug shortages and the creation of national surveillance bodies to gather and share information about demand. The goal was to develop mitigation strategies for times of shortage, remove unnecessary variability in regulatory practices, and develop a global process for determining vulnerable products. A scoping review evaluated publicly available listings of drugs at risk of shortage on an international level, providing a listing according to country of publicly available databases, along with frequency of updating and whether reporting was mandatory or voluntary.3 Although many countries were identified in the scoping review to have mandatory reporting, only eight countries required daily updating: Australia, Belgium, Canada, Czechia, Latvia, Portugal, Sweden, and the United States. Two multicountry collaborations were identified that analyzed the causes of shortages and provided frameworks to ensure patient safety: the South American Institute of Government in Health and the European Cooperation in Science and Technology (COST) research collaboration in Europe.3 , 19 , 20 Based on examples from these networks, a larger international task force could reasonably facilitate preparation, mitigation, and coordination and minimize the impact of shortages at the front lines of care.2
National or Regional Considerations
The root causes of drug shortages involve every aspect of the supply chain, from manufacturing to delivery to an individual institution’s case mix and preferences.1 , 2 In the United States, several measures to improve reporting of production interruptions have been implemented, but regulatory bodies cannot mandate a pharmaceutical company into drug production. The FDA Drug Shortages Task Force report described three major root causes.21 The first was the lack of incentive for manufacturers to produce less-profitable drugs. Many of these shortages involve older or generic drugs, whose manufacturers contend with intense price competition and uncertain revenues. In an analysis of data from 2013 to 2017, injectables identified in shortage were in the 33rd percentile by cost of all marketed drugs. The decision to increase manufacturing of a less-profitable but needed drug could force production tradeoffs with more-profitable drugs.
The second factor described was the expense of maintaining mature quality management systems. Many drugs in shortage have been available for > 35 years, and quality maintenance requires continual technical improvement, involving investments that markets may not reward in the short term. A failure to comply with required production standards or Good Manufacturing Practice regulations, however, can result in supply interruption and consequently drug shortages. Finally, logistical and regulatory challenges may make it difficult for markets to recover after supply disruptions. As drug supply chains become longer and more fragmented (with increased overseas production), the ability to rapidly increase production during a shortage is diminished.
Additional causes of drug shortages also require consideration. Health systems typically obtain their drug products through wholesale distributors. Restrictive distribution methods may limit product availability to suppliers or systems that comply with manufacturer agreements based on market approval requirements.22 Shortages can also occur when a disproportionate number of hospitals in a region use the same wholesale distributor. Most institutions use “just-in-time” inventory practices for budgetary reasons; this inventory strategy is highly reliant on a functional supply chain, making them vulnerable to unexpected shortages during a natural disaster or pandemic. Furthermore, rumors of a disruption in the supply chain can lead to hoarding, potentially preventing drugs from reaching regions where affected patients are concentrated. The impact of rumors and fears on supply chains was dramatically illustrated to the world with the “Great Toilet Paper Panic of 2020,” in which sales ballooned to > 700% nearly overnight.23 Although individual institutions may have good intentions when ordering increased stock in anticipation of future events, the cumulative impact of such practices across an entire region or country can be enormous.
Next, allocations for some drugs are based on historical usage, meaning institutions are restricted to ordering only their usual allotment even if patient volumes escalate. Local shortages can therefore occur with drastic increases in hospital census (eg, a large number of patients who are ventilated requiring deep sedation) or when use of a medication increases for a new indication (eg, azithromycin, hydroxychloroquine, tocilizumab). Last, in the United States, distribution of remdesivir, a novel antiviral agent with activity against COVID-19, fell under federal control for use consistent with an emergency use authorization. Uncertainty about access has led to calls for transparency from multiple organizations.24
Careful assessment and forecasting of essential drugs are vital components of an ICU surge plan.25 , 26 The American College of Chest Physicians’ Task Force for Mass Critical Care recommends that institutions should stock necessary drugs to support ICU care and be prepared to provide emergency mass critical care for up to 10 days.27 However, the stock quantities suggested by the task force now require reexamination, given the greater-than-expected demand to manage patients with COVID-19 with mechanical ventilation, as described in Italy, the United Kingdom, and the United States, where institutional supplies were quickly “burned”. National stockpiles are available in some countries, but drugs are typically not available for immediate use and represent only a fraction of the essential drugs required to provide critical care (eg, common antimicrobials). There are prediction models available to quantify the amount of drug required to support an ICU surge, but these are largely based on assumptions pertaining to expected census (ie, size of the surge), estimated use (ie, percentage of patients receiving the drug), and estimates of daily dose and duration of therapy.25 , 26
Several factors specific to the COVID-19 pandemic illustrate the challenges with estimating drug needs and the need for real-time data for predicting drug supplies. First is the variance reported in key outcomes that dictate drug usage. For example, one large case series from New York City noted a median (interquartile range) duration of mechanical ventilation (for survivors) of 27 (15-32) days, whereas data from the Seattle region reported a median of 10 (7-12) days.28 , 29 These differences would greatly influence the amount of sedatives, analgesics, and paralytics required to facilitate mechanical ventilation, especially given a very high reported daily drug consumption per patient that far exceeds the average needs for ventilated patients, leading to rapid supply depletion.
Next is the highly volatile state of prescribing based on less-rigorous sources of data and noncomparative trials. The explosion of information, ranging from traditional journalism to social media and hearsay, has led to drastic shifts in drug demand. For example, lopinavir-ritonavir, an older antiretroviral agent, was described as a potential therapy given its in vitro activity against severe acute respiratory syndrome coronavirus 2, and it was used widely early in the pandemic. Demand for lopinavir-ritonavir promptly decreased following the publication of a single randomized trial that showed no benefit to its use.30 Similarly, the perceived benefit of hydroxychloroquine has changed throughout the pandemic, with initial use largely influenced by a single small, nonrandomized study.31 As subsequent studies have failed to show benefit, the demand for hydroxychloroquine decreased.32 Another challenge with estimating drug usage during the pandemic is that doses and treatment durations for newer or off-label medications are not readily available, making it challenging to estimate needed quantities. Dosing strategies for tocilizumab, an IL-6 receptor antagonist, have been described, including a weight-based dose of 4 to 8 mg/kg or a fixed nonweight-based dose of 400 mg. No guidance is provided regarding the most appropriate weight metric to use (total, ideal, or adjusted body weight) or if there is benefit with larger doses. As such, real-time data are needed to optimize the process of estimating supply requirements. This type of drug supply planning must occur not just at the institutional level but at regional and national levels, permitting coordination of the critical care response with input from scientific advisory committees and experts. Similarly, incident command systems must include pharmacy experts in operations, logistics, and planning.
Regional and national multidisciplinary teams that proactively plan ahead for alternatives and prepare recommendations for substitution or conservation strategies prior to supplies being exhausted are more robust than any single-center effort.3 , 19 , 20 , 33 , 34 Regional and national dashboards that include drug quantities available can provide valuable information when drug shortages do arise and can allow drugs to be moved to where the patients are concentrated. When supplies within an institution are exhausted, borrowing is common between hospitals within the same health system, but very few countries or regions have organized methods for borrowing (personal communication). This process becomes more complex when hospitals are from different systems, especially during a pandemic when hospitals are unlikely to “return” that medication (ie, a vial for a vial) and have to compensate monetarily. Additional concerns arise when new products are obtained (eg, the acquisition of 2% propofol in a country where 1% propofol is routinely used); there are significant patient safety efforts required to ensure compatibility with technology such as bar coding, pump programming, and electronic documentation. Vendor support is needed to ease the burden of making safe product changes within their systems.
Pharmaceutical Manufacturing
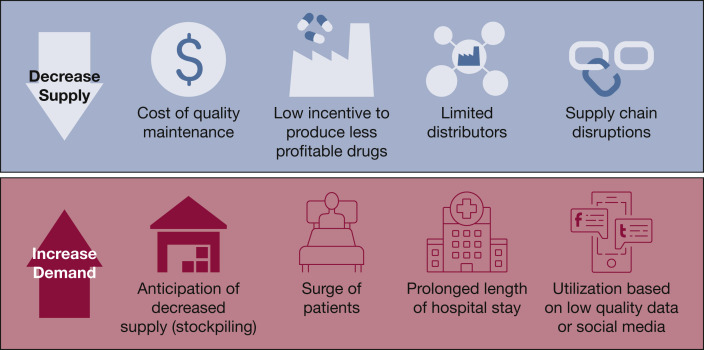
Pharmaceutical manufacturing involves multiple contributors involved in processes that include the synthesis of APIs and inactive excipients, the production of an administrable form, packaging, and labeling. The manufacturing of biologic drugs involves the use of microorganisms, plants, or animals, resulting in a longer and more complicated process.1 As noted, pharmaceutical and API manufacturers are at the heart of the supply chain, and they play a major role both in the creation and the mitigation of drug shortages. Manufacturing difficulties related to regulatory or quality issues, including product sterility, impurities, or degradation, are among the most common causes of drug shortages (Fig 1 ).3 , 22 , 35, 36, 37 In addition, pharmaceutical manufacturers often depend on a limited number of API suppliers; any disruption in production of ingredients, such as due to a regulatory concern or a natural disaster, will consequently affect manufacturing.35 , 38 Production interruptions and capacity concerns, such as line breakdowns, are also potential causes of shortages, especially for sterile parenteral drugs.3 , 22 , 37, 38, 39, 40 These drugs demand highly specialized production lines, are expensive to produce, and are typically among the drugs needed to manage mass critical care. This process results in low profit margins, and very few manufacturers are consequently interested in producing them.41 , 42
Figure 1.
Supply and demand impact on manufacturing.
Given the lack of incentives to produce these drugs and limited competition among generic drug manufacturers, the number of producers is generally low.42 Countries such as Canada and the United States often depend on only one or two suppliers of essential drugs, including many critical care drugs, and production interruptions rapidly cause shortages.38 This was illustrated in 2010 with propofol, when two of three manufacturers in the United States simultaneously shut down production for regulatory reasons.40 , 43 In the context of shortages, pharmaceutical manufacturers may also have to deal with internal competition, as many essential products will generally share the same production lines.37 In the case of biological drugs such as vaccines, limited manufacturing options due to the complexity of production may also escalate shortages.
Because pharmaceutical and especially API production is highly concentrated in a limited number of countries, pandemics, unplanned large-scale political events, and natural disasters have the potential to cause global shortages. Capacity reduction and lockdown of pharmaceutical or API production sites because of government-imposed restrictions or staffing shortages during a quarantine may halt production, or natural disasters may disrupt the manufacturing plant itself.44 Fear of local shortages or a surge in consumption, as seen with COVID-19, can push governments to impose restrictions on exports, further preventing drugs from reaching patients.10 , 45 Unforeseen increases in demand, such as for the off-label use of hydroxychloroquine, may also inflict shortages and affect the management of patients previously stabilized on indicated therapy.38
Pharmaceutical manufacturers, regulatory bodies, and professional organizations need to collaborate to assess acute and long-term needs and determine products to be prioritized in production.34 , 46 Because regulatory and quality problems are often at the heart of drug shortages, continuous improvement of pharmaceutical processes is fundamental to avoid these difficulties and ensure the continuous supply of products.24 Manufacturers have shortage prevention and management plans in place, as well as internal procedures and forecasting to track and avoid shortages in normal circumstances. In the case of unforeseen shortages, pharmaceutical manufacturers should plan to increase manufacturing capacity to favor those agents needed to manage critical patient populations.38 Manufacturers can further decrease the risk of shortages using methods to increase system resiliency, such as decentralizing production to multiple sites rather than one single site, investing in redundancy of critical production steps, and ensuring availability of active and inactive pharmaceutical ingredients through optimal inventory management and the development of relationships with alternative producers. Regulatory agencies in North America and Europe have legislation in place to ensure that drug shortages are reported in a timely manner, but manufacturers must also maintain transparent real-time inventories and actively participate in the rapid notification of shortages on national registries.
Institutional and Patient Levels
At the hospital pharmacy level, strategies for mitigating drug shortages involve identifying drugs at risk and anticipating their demand and supply, balancing inventory with allocations from distributors, while implementing conservation and therapeutic alternative strategies.34 Shortages are sometimes predictable and anticipated (eg, preplanned production gaps for maintenance), allowing for increased stockpiling of medication and planning for larger allocations of alternative options. For example, the manufacturing of premixed unfractionated heparin infusion bags was deemed by the FDA in 2018 to be noncompliant with Good Manufacturing Practice regulations, resulting in a drug shortage in multiple countries.47 Hospitals had time to consider other suppliers, alternative products, and the possibility of local preparation. Unfortunately, most drug shortages usually have little advanced warning, as seen during the COVID-19 pandemic. Sedatives, opioid analgesics, and paralytics have been among the most vulnerable classes of drugs in short supply in this pandemic.48 , 49 Professional societies must be engaged in the process of developing and disseminating guidelines to list class alternatives and aid broad distribution, rather than individual institutions working in isolation.
All potential supply chain options should be explored, including different manufacturers and distributors, requiring the formation of partnerships with regulatory bodies.35 Interchangeable products, such as therapeutic alternatives within the same class (eg, morphine instead of fentanyl), may be readily available and can be considered at the bedside. Conservation strategies may involve different routes of administration (eg, enteral opioids instead of IV options) or alternative drugs from other classes (eg, midazolam instead of propofol). We illustrate an example of applying potential alternatives and conservation options for the use of analgesics, sedatives, and paralytics in the setting of drug shortages in Table 1 . Specific recommendations for therapeutic alternatives and conservation strategies for many essential drug classes are detailed in other publications.34 , 50
Table 1.
Examples of Mitigation Strategies and Assessing Alternatives in the Setting of Drug Shortages Supporting Mechanical Ventilation
| Preferred Agents (Clinical Pearls) | Potential Conservation Strategies | Potential Alternatives (Depending on Market Availability) |
|---|---|---|
| Analgesia | ||
Fentanyl:
|
|
Morphine:
|
| Sedation | ||
Propofol:
|
|
Midazolam:
|
| Neuromuscular Blocking Agents | ||
Cisatracurium
|
|
Vecuronium:
|
Opportunities to minimize drug wastage must also be considered (eg, dose rounding, central batch preparation by pharmacy, selection of appropriate vial sizes and concentrations). In the ICU, therapeutic escalation strategies can be used to conserve sedatives and opioid analgesics, using intermittent enteral dosing as the preferred option, followed by intermittent IV dosing, and finally continuous infusions only when required.34 , 50 Additional strategies include ensuring that the lowest effective dose is being used (eg, by using sedation and analgesic targets), critically evaluating how essential a medication is for each patient (eg, agents for stress ulcer prophylaxis), drug rotation (ie, not depleting the supply of one drug but instead rotating between drug alternatives while awaiting new shipments), and prioritization (eg, reserving selected drugs for specific situations). Although the impact of drug shortages for COVID-19 may focus on critical care areas, conservation strategies may apply to other areas that require similar drugs (eg, operating rooms, palliative care). For example, smaller vial sizes of propofol may be less vulnerable to shortages and could be reserved for procedural sedation or within the operating room, while larger vial sizes are conserved for ICU patients requiring prolonged sedation.
We must recognize that conservation strategies and therapeutic alternatives may not always be possible, may not represent best practices, or could involve the use of locally unfamiliar medications that could compromise patient safety. Each institution should engage administrators, educators, and bedside clinicians to evaluate the risks and benefits of proposed strategies in their institution. In addition to efficacy and safety, the feasibility, suitability, and logistics must be considered within the institution. Any change in clinical practice will require time and resources to disseminate information and educate staff. Hospitals using electronic health records with prescriber order entry may need to develop new ordering pathways and program IV pumps for new drugs, concentrations, or bag sizes (eg, changing from 1% to 2% propofol).
During a global health crisis such as the COVID-19 pandemic, there are always competing interests that must be considered in addition to drug shortages. In these times, it can be expected that drug shortages will occur at the same time as disruption of other essential resources, such as staff and space, as well as the availability of personal protective equipment, IV pumps, and supplies for medication preparation and administration. Some strategies that mitigate drug shortages may negatively affect other essential resources. For example, bid or tid intermittent dosing of subcutaneous unfractionated heparin for thromboembolic prophylaxis requires more frequent personal protective equipment usage to support the room entries for nurses, compared with a single daily dose of enoxaparin. Consolidating medication administration times to coincide with other activities (eg, routine assessments, obtaining laboratory samples) can further reduce room entries. When considering conservation strategies for drugs or other resources, consultation with end-users is essential.
Summary and Future Direction
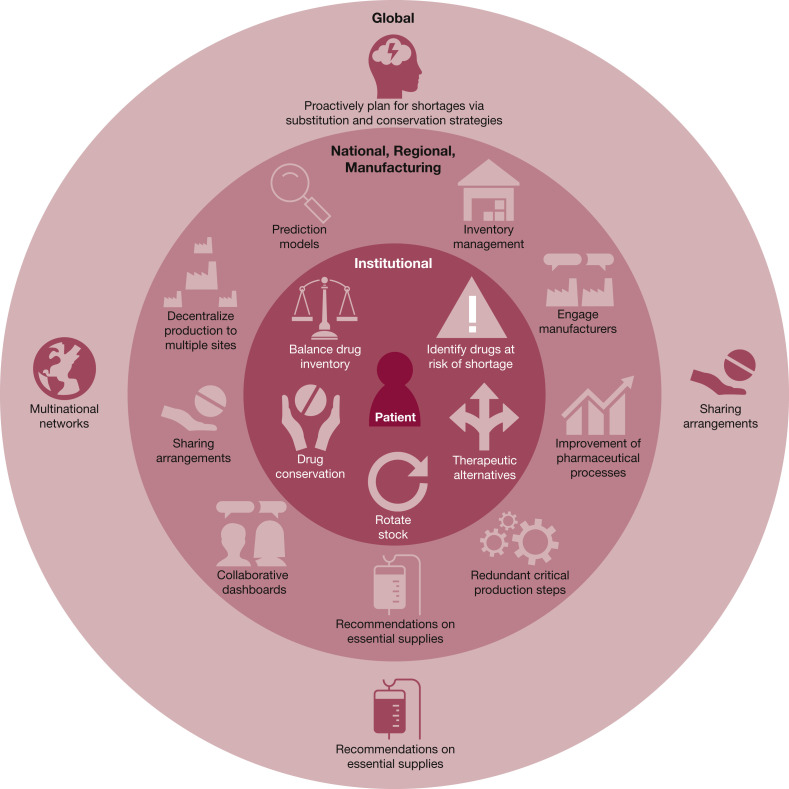
The goal of this article was to highlight the issues associated with anticipating and managing shortages of essential critical care drugs from global, national, regional, and institutional perspectives during a disaster. Important issues associated with drug shortages have been reported in the literature in the last 5 years, providing us with an understanding of the root causes of these shortages and potential mitigation strategies. Figure 2 summarizes take-home messages that stakeholders must consider for future steps during a global disaster. Dealing with shortages of essential drugs during the pandemic has taught us “that coordination, communication and transparency should be fundamental principles in all actions between stakeholders at the regional, national and global level”2 is needed to mobilize essential therapies to the front lines of patient care.
Figure 2.
Approach to drug shortages.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. F. B. serves as a consultant for Wolters Kluwer. One of the authors (R. C. M.) is a US military member, and this work was prepared as part of his official duties. Title 17 U.S.C. §105 provides that ‘‘Copyright protection under this title is not available for any work of the US Government.’’ Title 17 U.S.C. §101 defines a US Government work as a work prepared by a military service member or official employee of the US Government as part of that person’s official duties. None declared (L. B. D., D. W., S. K., J. D., M. D. C., J. G., B. L. E.).
Other contributions: The authors thank Christopher Tse, PharmD, for creating the figures in the document and the international pharmacy community who answered their many questions and helped them broaden their perspective. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government.
References
- 1.Executive Board, 142. (2018). Addressing the global shortage of, and access to, medicines and vaccines. World Health Organization. https://apps.who.int/iris/handle/10665/273811. Accessed September 9, 2020.
- 2.World Health Organization Medicines shortages: global approaches to addressing shortages of essential medicines in health systems. WHO Drug Information. 2016;30:2. [Google Scholar]
- 3.Acosta A., Vanegas E.P., Rovira J., Godman B., Bochenek T. Medicine shortages: gaps between countries and global perspectives. Front Pharmacol. 2019;10:763. doi: 10.3389/fphar.2019.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan Z. FDA reports shortage of sedation drug used for putting COVID-19 patients on ventilators. https://endpts.com/fda-reports-shortage-of-sedation-drug-used-for-putting-covid-19-patients-on-ventilators/
- 5.ASHP COVID-19 Resource Center. COVID-19 periodic pharmacy resources survey results. https://www.ashp.org/COVID-19/Bi-weekly-PPE-Survey-Results-Covid-19?loginreturnUrl=SSOCheckOnly. Accessed September 9, 2020.
- 6.The Star. Ottawa seeks new drug providers as COVID-19 fights sends demand soaring and triggers fears of a shortage. https://www.thestar.com/politics/federal/2020/04/20/ottawa-seeks-new-drug-providers-as-covid-19-fights-sends-demand-soaring-and-triggers-fears-of-a-shortage.html. Accessed September 9, 2020.
- 7.Siow W.T., Tang S.H., Agrawal R.V. Essential ICU drug shortages for COVID-19: what can frontline clinicians do? Crit Care. 2020;24:260. doi: 10.1186/s13054-020-02971-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Independent. Revealed: Hospitals fear shortage of essential pain relief and sedation drugs will make ventilators useless for worst-hit coronavirus patients. https://www.independent.co.uk/news/world/americas/ventilators-coronavirus-drugs-painkillers-medicine-covid-19-a9458511.html. Accessed September 9, 2020.
- 9.Jakhar D., Kaur I. Potential of chloroquine and hydroxychloroquine to treat COVID-19 causes fears of shortages among people with systemic lupus erythematosus. Nat Med. 2020;26(5):632. doi: 10.1038/s41591-020-0853-0. [DOI] [PubMed] [Google Scholar]
- 10.ASH Clinical News. U.K. bans exports on drugs in short supply. https://www.ashclinicalnews.org/online-exclusives/u-k-bans-exports-drugs-short-supply/. Accessed September 9, 2020.
- 11.Global News. Canada. Coronavirus: Canadian drug wholesaler takes steps to prevent hoarding during COVID-19 pandemic. https://globalnews.ca/news/6692752/drug-wholesaler-restricting-shipments-covid-19/. Accessed September 9, 2020.
- 12.The Pharmaceutical Journal. Ways to safeguard UK drug supplies during COVID-19 and beyond. https://www.pharmaceutical-journal.com/news-and-analysis/opinion/comment/ways-to-safeguard-uk-drug-supplies-during-covid-19-and-beyond/20207897.article?firstPass=false. Accessed September 9, 2020.
- 13.Huang Y. The coronavirus outbreak could disrupt the U.S. drug supply. Council on Foreign Relations. https://www.cfr.org/in-brief/coronavirus-disrupt-us-drug-supply-shortages-fda
- 14.Keyhani S., Wang S., Hebert P., Carpenter D., Anderson G. US pharmaceutical innovation in an international context. Am J Public Health. 2010;100:1075–1080. doi: 10.2105/AJPH.2009.178491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haninger K. New analysis shows that more medicines worldwide are available to U.S. patients. The Catalyst. https://catalyst.phrma.org/new-analysis-shows-that-more-medicines-worldwide-are-available-to-u.s.-patients
- 16.Sarnak D.O., Squires D., Bishop S. Paying for prescription drugs around the world: why is the U.S. an outlier? The Commonwealth Fund. https://www.commonwealthfund.org/publications/issue-briefs/2017/oct/paying-prescription-drugs-around-world-why-us-outlier [PubMed]
- 17.Gupta R., Bollyky T.J., Cohen M., Ross J.S., Kesselheim A.S. Affordability and availability of off-patent drugs in the United States—the case for importing from abroad: observational study. BMJ. 2018;60:k831. doi: 10.1136/bmj.k831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FIP FIP addressing global medicines shortages. https://www.fip.org/medicines-shortages
- 19.ISAGS 2017 Situation of essential medicines at risk of supply shortage with emphasis on South American countries. http://isags-unasur.org/en/publicacao/situation-of-essential-medicines-at-risk-of-supply-shortage-with-emphasis-on-south-american-countries-2/
- 20.COST 2018 European Union European Medicines Shortages Research Network—addressing supply problems to patients (Medicines Shortages). Medicines Shortages in Europe. eCOST Action CA15105. http://www.medicinesshortages.eu/
- 21.US Food and Drug Administration Drug shortages: root causes and potential solutions. https://www.fda.gov/media/131130/download
- 22.Fox E.R., McLaughlin M.M. ASHP guidelines on managing drug product shortages. Am J Health-Syst Pharm. 2018;75:1742. doi: 10.2146/ajhp180441. [DOI] [PubMed] [Google Scholar]
- 23.Fortune. The case of the missing toilet paper: How the coronavirus exposed U.S. supply chain flaws. https://fortune.com/2020/05/18/toilet-paper-sales-surge-shortage-coronavirus-pandemic-supply-chain-cpg-panic-buying/. Accessed September 9, 2020.
- 24.Ison M.G., Wolfe C., Boucher H.W. Emergency use authorization of remdesivir: the need for a transparent distribution process. JAMA. 2020;323(23):2365–2366. doi: 10.1001/jama.2020.8863. [DOI] [PubMed] [Google Scholar]
- 25.Burry L., Shaffer D.L. How to build ICU surge capacity. In: Farmer J.C., Wax R.S., Baldisseri M.R., editors. Preparing Your ICU for Disaster Response. Society of Critical Care Medicine; Mt Prospect, IL: 2012. [Google Scholar]
- 26.Aziz S., Arabi Y.M., Alhazzani W. Managing ICU surge during the COVID-19 crisis: rapid practice guidelines. Intensive Care Med. 2020;46(7):1303–1325. doi: 10.1007/s00134-020-06092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubinson L., Hick J.L., Hanfling D.G. Definitive care for the critically ill during a disaster: a framework for optimizing critical care surge capacity. From a Task Force for Mass Critical Care Summit Meeting, January 26-27, 2007, Chicago, IL. Chest. 2008;133(suppl 5):18S–31S. doi: 10.1378/chest.07-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatraju P.K., Ghassemieh B.J., Nichols M. COVID-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magagnoli J., Narendran S., Pereira F. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. https://www.medrxiv.org/content/10.1101/2020.04.16.20065920v2 [DOI] [PMC free article] [PubMed]
- 33.Einav S., Hick J.L., Hanfling D. Surge capacity logistics. Care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e17S–e43S. doi: 10.1378/chest.14-0734. [DOI] [PubMed] [Google Scholar]
- 34.Kanji S., Burry L., Williamson D. Therapeutic alternatives and strategies for drug conservation in the intensive care unit during times of drug shortages: a report of the Ontario COVID-19 ICU Drug Task Force. Can J Anaesth. 2020;67(10):1405–1416. doi: 10.1007/s12630-020-01713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A Toolkit for Improved Understanding and Transparency of Drug Shortage Response in Canada. 2017 (Revised) https://www.drugshortagescanada.ca/files/MSSC_Toolkit_2017.pdf
- 36.Ventola C.L. The drug shortage crisis in the United States: causes, impact, and management strategies. P T. 2011;36(11):740–757. [PMC free article] [PubMed] [Google Scholar]
- 37.Dill S., Ahn J. Drug shortages in developed countries—reasons, therapeutic consequences, and handling. Eur J Clin Pharmacol. 2014;70(12):1405–1412. doi: 10.1007/s00228-014-1747-1. [DOI] [PubMed] [Google Scholar]
- 38.Choo E.K., Rajkumar S.V. Medication shortages during the COVID-19 crisis: what we must do. Mayo Clin Proc. 2020;95(6):1112–1115. doi: 10.1016/j.mayocp.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodcock J., Wosinska M. Economic and technological drivers of generic sterile injectable drug shortages. Clin Pharmacol Ther. 2013;93(2):170–176. doi: 10.1038/clpt.2012.220. [DOI] [PubMed] [Google Scholar]
- 40.Hvisdas C., Lordan A., Pizzi L.T., Thoma B.N. US propofol drug shortages: a review of the problem and stakeholder analysis. Am Health Drug Benefits. 2013;6(4):171–175. [PMC free article] [PubMed] [Google Scholar]
- 41.Weaver J.M. Why are there so many drug shortages? Anesth Prog. 2010;57(3):89–90. doi: 10.2344/0003-3006-57.3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez I., Hershey T.B., Donohue J.M. Drug shortages in the United States: are some prices too low? JAMA. 2020;323(9):819–820. doi: 10.1001/jama.2019.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen V., Rappaport B.A. The reality of drug shortages—the case of the injectable agent propofol. N Engl J Med. 2010;363(9):806–807. doi: 10.1056/NEJMp1005849. [DOI] [PubMed] [Google Scholar]
- 44.Chatterjee P. Indian pharma threatened by COVID-19 shutdowns in China. Lancet. 2020;395(10225):675. doi: 10.1016/S0140-6736(20)30459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Post. 3M says Trump officials have told it to stop sending face masks to Canada. Trudeau responds. https://nationalpost.com/news/world/3m-says-trump-officials-have-told-it-to-stop-sending-face-masks-to-canada. Accessed September 9, 2020.
- 46.Musazzi U.M., Di Giorgio D., Minghetti P. New regulatory strategies to manage medicines shortages in Europe. Int J Pharm. 2020;579:119171. doi: 10.1016/j.ijpharm.2020.119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canadian Society of Hospital Pharmacists. CSHP advises health Canada on heparin shortage. https://cshp.ca/cshp-advises-health-canada-heparin-shortage. Accessed September 9, 2020.
- 48.Hanidziar D., Bittner E. Sedation of mechanically ventilated COVID-19 patients: challenges and special considerations. Anesth Analg. 2020;131(1):e40–e41. doi: 10.1213/ANE.0000000000004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gesin G., Barletta J.F., Brown D.R., Shander A. Recommendations for alternative analgesic and sedative agents in the setting of drug shortages. Critical Connections. https://sccm.org/getattachment/36f06b68-e097-4cbe-9a0e-f97066725f91/Recommendations-for-Alternative-Analgesic-and-Seda