Abstract
Artificial intelligence (AI) has penetrated the field of medicine, particularly the field of radiology. Since its emergence, the highly virulent coronavirus disease 2019 (COVID-19) has infected over 10 million people, leading to over 500,000 deaths as of July 1st, 2020. Since the outbreak began, almost 28,000 articles about COVID-19 have been published (https://pubmed.ncbi.nlm.nih.gov); however, few have explored the role of imaging and artificial intelligence in COVID-19 patients—specifically, those with comorbidities.
This paper begins by presenting the four pathways that can lead to heart and brain injuries following a COVID-19 infection. Our survey also offers insights into the role that imaging can play in the treatment of comorbid patients, based on probabilities derived from COVID-19 symptom statistics. Such symptoms include myocardial injury, hypoxia, plaque rupture, arrhythmias, venous thromboembolism, coronary thrombosis, encephalitis, ischemia, inflammation, and lung injury. At its core, this study considers the role of image-based AI, which can be used to characterize the tissues of a COVID-19 patient and classify the severity of their infection. Image-based AI is more important than ever as the pandemic surges and countries worldwide grapple with limited medical resources for detection and diagnosis.
Keywords: COVID-19, Comorbidity, Pathophysiology, Heart, Brain, Lung, Imaging, Artificial intelligence, Risk assessment
We conclude that imaging and AI-based tissue characterization, when considered alongside COVID-19 symptoms and their pre-test probabilities, offer a compelling solution for assessing the risk of comorbid patients. These methods show the potential to become an integral part of tracking and improving the healthcare system, both during the pandemic and beyond.
1. Introduction
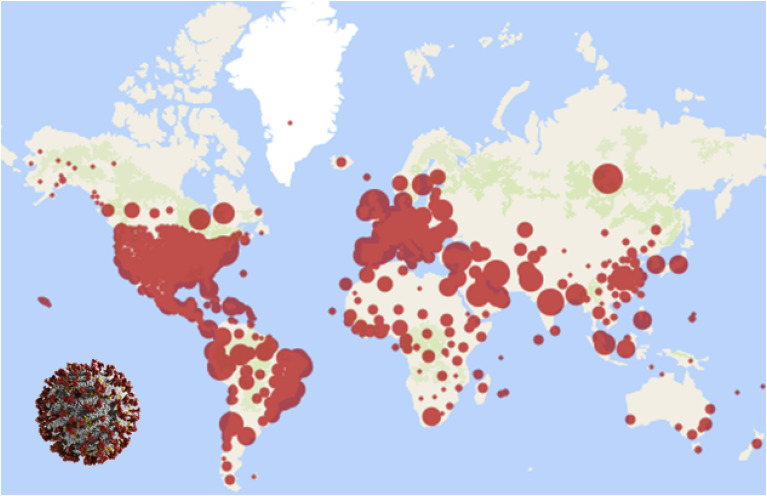
In December 2019, a novel coronavirus referred to as “severe acute respiratory distress syndrome coronavirus 2” (SARS-CoV-2) [1] appeared in Wuhan, the capital of Hubei Province in PR China. The disease caused by the virus was initially named “novel coronavirus pneumonia” (NCP) by the Chinese government but was subsequently renamed “coronavirus disease 2019” (COVID-19) by the World Health Organization (WHO). On January 30th, 2020, it was declared a public health emergency of international concern (PHEIC) [2]. It is believed that SARS-CoV-2 is primarily transmitted through saliva droplets or nasal discharge [3]. The first evidence of human-to-human transmission was found by Jasper Fuk-Woo Chan et al. in their study at The University of Hong Kong-Shenzhen Hospital [4]. Due to its contagiousness (Ro = 2.7), the virus has reached epidemic levels, affecting 213 countries and causing over 10 million infections and more than 500,000 deaths as of July 1st, 2020 [5] (shown in Fig. 1 ).
Fig. 1.
World map showing COVID-19 spread over 213 countries (courtesy: John Hopkins University).
Recent literature suggests that patients with pre-existing diseases are likely to experience severe complications from COVID-19 [[6], [7], [8], [9], [10]]. In one study on admitted diabetic (48, 24.9%) and non-diabetic (145, 75.1%) COVID-19 patients, the mortality rate (81.3% vs. 47.6%) and the rate of admission to the intensive care unit (ICU) (66.7% vs. 41.4%) were significantly higher for diabetic patients. Diabetic patients also experienced severe inflammatory responses and cardiac, hepatic, and renal coagulopathy [11]. The prevalence of heart and brain injuries was also higher in COVID-19 patients with concomitant chronic conditions like diabetes, kidney disease, dyslipidemia, hypertension [[12], [13], [14], [15]], chronic obstructive pulmonary disease (COPD), and cardiovascular diseases [16]. Recent studies have shown that SARS-CoV-2 invades the thin lining of the epithelial cells of the arteries, leading to atherosclerosis [[17], [18], [19]] and arterial inflammatory disease—one of the major causes of cardiovascular diseases (CVDs), which also causes heart and brain injuries [12,20,21]. This could be due to a reduced expression of angiotensin-converting enzyme 2 (ACE2), causing endothelial dysfunction, which, in turn, aggravates existing atherosclerosis [22,23]. It has also been observed that comorbid patients, when subjected to image-screening, show mild to severe pre-test probability (PTP) for COVID-19 [24]. The conventional cardiovascular risk factors (CCVRF) in these comorbid patients appear strongly correlated either to their heart imaging or to surrogate biomarkers of coronary artery disease, such as carotid artery disease. Both imaging and biomarkers could be helpful in severity predictions for COVID-19 [[25], [26], [27], [28], [29], [30]]. Fig. 2 illustrates the associations between SARS-CoV-2 and other comorbidities, such as diabetes, as well as the comparative survival rates for COVID-19 patients with and without diabetes.
Fig. 2.
(a) Association of SARS-CoV-2, with other comorbidities, and (b) comparison of the mortality rate of diabetic and non-diabetic COVID-19 patients (reproduced with permission [11]).
ACE2 expression causes scars in the vessels and can even rupture the walls of the arteries [[31], [32], [33], [34]]. For this reason, CCVRF should be considered alongside imaging in patients who present with COVID-19 and many comorbidities [35]. The second stage is the one at which a patient is most severely affected by COVID-19 and has the highest probability of cardiac injury or release of troponin T (TnT). Imaging has been shown to offer benefits in monitoring the tissue scars caused by COVID-19 [[35], [36], [37], [38], [39]].
Multiple modalities can be utilized to determine whether a patient has the sequelae of COVID-19, including magnetic resonance imaging [40], computed tomography [41], and ultrasound [[41], [42], [43], [44]]. The advantage of these imaging modalities is the visual access they provide to the scar tissue caused by the disease. A disadvantage, however, is their inability to provide a “risk assessment.” The application of artificial intelligence (AI) can enhance the information provided by these imaging modalities, resulting in a more accurate characterization of the tissue and the disease process [[45], [46], [47], [48], [49], [50], [51]]. The combination of AI and medical imaging has been shown to improve diagnosis and risk stratification, speed up patient evaluation, enhance disease monitoring, and accelerate early intervention [40,48,[52], [53], [54], [55], [56], [57]]. Thus, this review will focus on the use of AI-based tissue characterization of images of comorbid patients affected by COVID-19.
The layout of this paper is as follows: Section 2 presents the pathophysiology of the four pathways leading to brain and heart injury. Section 3 summarizes the evidence related to the use of imaging during the COVID-19 pandemic. Section 4 elaborates on the use of AI-based tissue characterization for risk assessment. Finally, the paper concludes with a critical discussion.
2. The pathophysiology of SAR-CoV-2 leading to brain and heart injury
Several studies suggest that SARS-CoV-2 uses the ACE2 receptor to gain access to cells by binding to the SPIKE protein (‘S’ protein) on their surface [[58], [59], [60]] (see Fig. 2). ACE2 and angiotensin-converting enzyme 1 (ACE1) are homolog carboxypeptidase enzymes that have different vital functions in the renin-angiotensin-aldosterone system (RAAS) pathway [61]. ACE2 is widely expressed in myocardial cells [61], type 2 pneumocytes, enterocytes, and astrocytes (in the brain) [15,62,63]. Thus, it is recognized as a cause of extra-pulmonary complications.
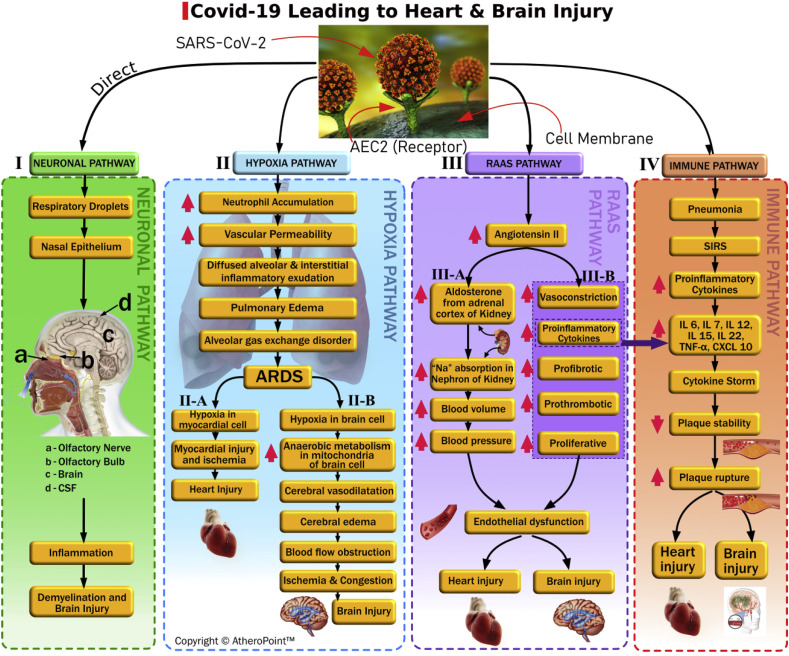
Fig. 3 shows the overall picture of how SARS-CoV-2 causes brain and heart injuries via four different pathways. These include (i) the neuronal pathway, (ii) the hypoxia pathway, (iii) the RAAS pathway, and (iv) the immune pathway. We will discuss these pathways and the injuries they lead to, which may manifest as viral encephalitis, infectious toxic encephalopathy, or acute cerebrovascular disease.
-
(i)
The Neuronal Pathway (Fig. 3, the pathway I): Recent epidemiological studies have demonstrated similarities at the genomic level between SARS-CoV-1, MERS, and SARS-CoV-2 [6,64,65]. Meanwhile, previous experimental studies have shown that beta coronaviruses in general—such as SARS-CoV-1 and MERS—can spread into and directly infect the brain when inhaled as droplets via the nasal epithelium [66,67]. Fig. 3 depicts the olfactory nerve and the olfactory bulb [[68], [69], [70]]—labeled as “a” and “b,” respectively—on the image of the sagittal brain in the neuronal pathway. Based on recent reports, we are aware that patients infected by SARS-CoV-2 show symptoms of dysgeusia (loss of taste) and anosmia (loss of smell) [64,[71], [72], [73]]. Bohmwald et al. further validate that coronaviruses that infect through the olfactory nerve and bulb can reach the brain and cerebrovascular fluid (CSF) within seven days. Additionally, these viruses have been observed to cause inflammation and demyelination [74]. The authors demonstrated in an experimental study of mice that removing the olfactory bulb from the pathway can lead to the restriction of CoV in the central nervous system (CNS) [74]. Based on this evidence, we believe that the neuronal pathway is one possible track for SARS-CoV-2.
-
(ii)
The Hypoxia Pathway (Fig. 3, pathway II): In this pathway, decreased levels of ACE2 proliferate in the lung parenchyma cells after the coronavirus has passed through, causing exaggerated neutrophils accumulation, enhanced vascular permeability, and the formation of diffuse alveolar and interstitial exudates. This ultimately leads to pulmonary edema and acute respiratory distress syndrome (ARDS) [75]. ARDS is characterized by severe abnormalities in blood gas composition resulting from an oxygen and carbon dioxide mismatch, which leads to low blood oxygen levels [76,77]. This ongoing hypoxia can lead to myocardial ischemia and heart injury [78,79] (see Fig. 3, pathway II-A). Hypoxia in the brain increases anaerobic metabolism in the mitochondria of the brain cells [80], leading to cerebral vasodilatation, edema, and impaired flow. This can result in cerebral ischemia and acute cerebrovascular diseases such as acute ischemic stroke [71,80] (see Fig. 3, pathway II-B).
-
(iii)
The RAAS Pathway after SARS-CoV-2 (Fig. 3, pathway III): The RAAS pathway is crucial in regulating blood pressure, as well as the balance of fluid and electrolytes. Any disturbance in this pathway can trigger the pathogenesis of cardiovascular diseases [15]. Before a SARS-CoV-2 infection triggers the RAAS, Angiotensin I (Ang I) cleaves to Angiotensin II (Ang II) via ACE1. Ang II causes vasospasm. It is also a pro-inflammatory agent with prothrombotic and proliferative effects that are detrimental to vascular tone and hemostasis [77,80]. Thus, as a counter-regulatory mechanism, ACE2 degrades Ang II and generates Ang (1–7), which counteracts the negative impacts of Ang II [75,78]. Both ACE2 and Ang (1–7) have cardio-cerebral vascular protective effects [61]. After the triggering of SARS-CoV-2 infection, its results are in the deregulation of RAAS causing heart and brain injury in two different pathways. The main culprit is an increase in Ang II, which is caused by the decrease in ACE2 levels (Fig. 3, pathway III-A). First, an increase in Ang II levels stimulates the adrenal cortex of the kidney, resulting in an increased production of aldosterone. Aldosterone is a steroid hormone that causes sodium and water reabsorption to increase at the distal tubule and collecting duct of the nephron [81]. This reabsorption increases blood volume and causes an elevation in blood pressure, which results in the endothelial dysfunction that causes brain and heart injury [82]. The second effect of an increase in Ang II levels (i.e., as a consequence of decreased ACE2 levels) is endothelial dysfunction leading to intimal damage in the arterial walls [21], which can be seen during the imaging of the arterial wall (see Fig. 3, pathway III-B). This pathway can also trigger a cytokine storm, as high levels of Ang II can cause an increase in pro-inflammatory cytokines (see the bridge line between the RAAS and immune pathways).
-
(iv)
The Immune Pathway (Fig. 3, pathway IV): Several recent studies have reported SARS-Cov-2 viral pneumonia [7,77,83,84] having an exaggerated inflammatory response known as a “cytokine storm.” This response appears to present at advanced stages of severe COVID-19, with increased levels of inflammatory cytokines leading to multiple-organ failure [[85], [86], [87]]. The rise in inflammatory markers—including IL-6, IL-7, IL-12, IL-15, IL-22, TNF-α, and CXCL-10—results in the destabilization of plaque. This, in turn, can cause plaque rupture, resulting in heart and brain injury [74,78,88].
Fig. 3.
We have shown in four pathways how COVID-19 can cause Brain and heart injury. Brain image in pathway I: http://debuglies.com/2020/01/23/olfactory-disturbances-have-implications-in-mental-and-emotional-well-being-health/(Courtesy of Debug Lies).
3. The role of imaging in comorbid patients with COVID-19
As the previous section discussed, COVID-19 uses four pathways (i.e., neuronal, hypoxia, RAAS, and immune) to cause critical heart and brain injuries in patients with comorbidities. The prevalence of myocardial injury and brain injury caused by COVID-19 [37,[68], [69], [70],80,[85], [86], [87],[89], [90], [91]] points to a need for increased use of medical imaging to expedite assessments, differential diagnoses, and patient management [35,36,92] with proper safety measures [[93], [94], [95], [96], [97]]. The seriousness of a patient's COVID-19 symptoms helps to determine which imaging modality is appropriate: portable or non-portable, and invasive or non-invasive. B-Mode ultrasound imaging is portable and can be used for low-risk patients. Meanwhile, X-ray, magnetic resonance imaging [40] and computed tomography [41] are non-portable and can be used for medium-risk patients. Intravascular ultrasound (IVUS) [98] and ventriculography are invasive imaging modalities used in highly critical cases [42,43,99,100]. Amongst all the imaging modalities, ultrasound is noteworthy because it is radiation-free, portable, quick, repeatable, inexpensive, and can be performed in isolation, thus lowering the chance of spreading the COVID-19 infection [101,102].
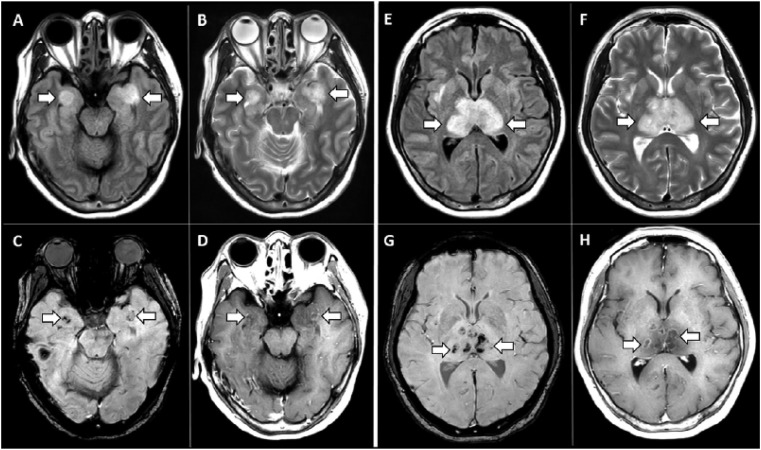
There are several examples of medical imaging that have led to proper treatment and healthcare management during the pandemic, ultimately reducing the mortality rate. X-ray imaging of the chest has demonstrated irregular, patchy, hazy, reticular, and widespread ground-glass opacities, indicating the progression of COVID-19 at various stages; this information can support the healthcare team in developing the most appropriate treatment plan [103]. Chest CT scans of 21 COVID-19 patients revealed in almost 18 (86%) of the patients that the disease was affecting at least one of the five lobes of their lungs [104]. Chest MRI scans of 11 COVID-19 patients showed pulmonary tissue consolidation in six (50%), diffusion-restricted regions in six (50%), and lung damage in seven (58%) [105]. Meanwhile, heart MRI studies of 26 recovered patients showed that 14 (54%) of the patients had myocardial edema. At the same time, late gadolinium enhancement was found in 8 (31%), implying that COVID-19–related cardiac injury is longstanding and requires frequent monitoring even after recovery [106]. In a different study, MR scans of a COVID-19 patient revealed myocardial inflammation, signifying myocardial injury due to a cytokine storm related to the SARS-CoV2 infection (as discussed in Section 2, Pathway IV) [107]. Several studies have also evaluated the effects of COVID-19 on the brain. In one, MRI scans revealed hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions [108] (shown in Fig. 4 ). In another, brain MRI scans were completed for 27 patients, 12 (44%) of which produced abnormal findings [109]. Additionally, evidence of liver injury (27%) and gall bladder abnormality (83%) was found in a joint CT and ultrasound study of the abdomen [110]. Recent MRI scans of COVID-19 patients’ olfactory bulbs have revealed the cause of olfactory function loss to be the interaction between SARS-CoV2 and the ACE2 protein expressed by the olfactory epithelium, which leads to inflammatory obstruction [111].
Fig. 4.
MRI scan of COVID-19 patient showing hemorrhage. MRI images demonstrate T2 FLAIR hyperintensity within the bilateral medial temporal lobes and thalami (A, B, E, F) with evidence of hemorrhage indicated by hypointense signal intensity on susceptibility-weighted images (C, G) and rim enhancement on postcontrast images (D, H) (reproduced with permission [108]).
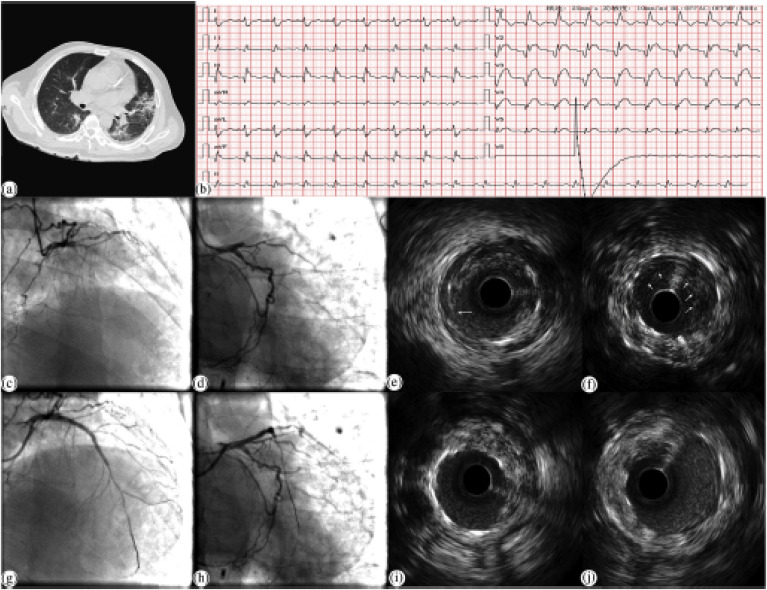
Invasive imaging is another option for diagnosing COVID-19 patients who have critical comorbidities. In one such study, IVUS, along with stenting, was performed with precautions on a COVID-19 patient with myocardial infarction [112] (shown in Fig. 5 ). A detailed discussion of precautions is included in section 5. In another study, takotsubo syndrome, a form of myocardial injury triggered by COVID-19, was detected using ventriculography [113]. In various studies, the medical imaging of COVID-19 patients had been crucial to ascertaining the extent of tissue damage and critical infection, although there were no visible symptoms [39,114]. Therefore, medical imaging is the preferred way to ascertain the extent of cardiac and brain tissue damage throughout the lifetime of COVID-19 patients. COVID-19 patients with comorbidities are especially vulnerable, and so they need to be screened through medical imaging from the first day of their diagnosis. Medical imaging can help patients with deep vein thrombosis (DVT), as they are highly susceptible to severe tissue damage from COVID-19. A study showed that COVID-19 patients suffering from DVT had a worse prognosis than patients without DVT. Patients with DVT were admitted to the ICU more frequently (18.2%), discharged less frequently (48.5%), and suffered more deaths (38.5%) than those without DVT [115].
Fig. 5.
Application of chest CT and IVUS for a COVID-19 patient suffering from myocardial infarction (a) Chest CT scan with viral pneumonia showing fibrinous, focal exudative changes. (b) When the patient complained of chest pain, the ECG report showed the ST-segments elevations in V1–V5 lead. (c, d) CAG radiology that the proximal segment of LAD was occluded. (e, f) The blood flow of LAD restoration after 2 DESs was implanted. (g) The dissection distal shown by IVUS to the stent in LAD from 7 to 12 o'clock. (h) The low echogenic shadow with scattered higher echogenic flicker, indicating a thrombus. (i) After a DES was implanted, and the stent was well expanded, the dissection could not be seen (j) The thrombus disappearance after the intervention (reproduced with permission [112]).
Although medical imaging can be very useful to patients and doctors alike, the exponential pandemic curve, inadequate medical facilities [[116], [117], [118]], and a limited number of radiologists make the assessment, diagnosis, and management processes challenging, tedious, and error-prone. Therefore, although medical imaging can make diagnoses faster, as stated above, it will be of limited use. Given this fact, new age techniques such as artificial intelligence (AI) [[119], [120], [121]] applications in medical imaging for tissue characterization can make computer-aided assessments and diagnoses faster. The main reason for this is that the AI can be scaled up to match the pandemic curve, thereby meeting the immediate demands of medical image diagnoses during the COVID-19 pandemic.
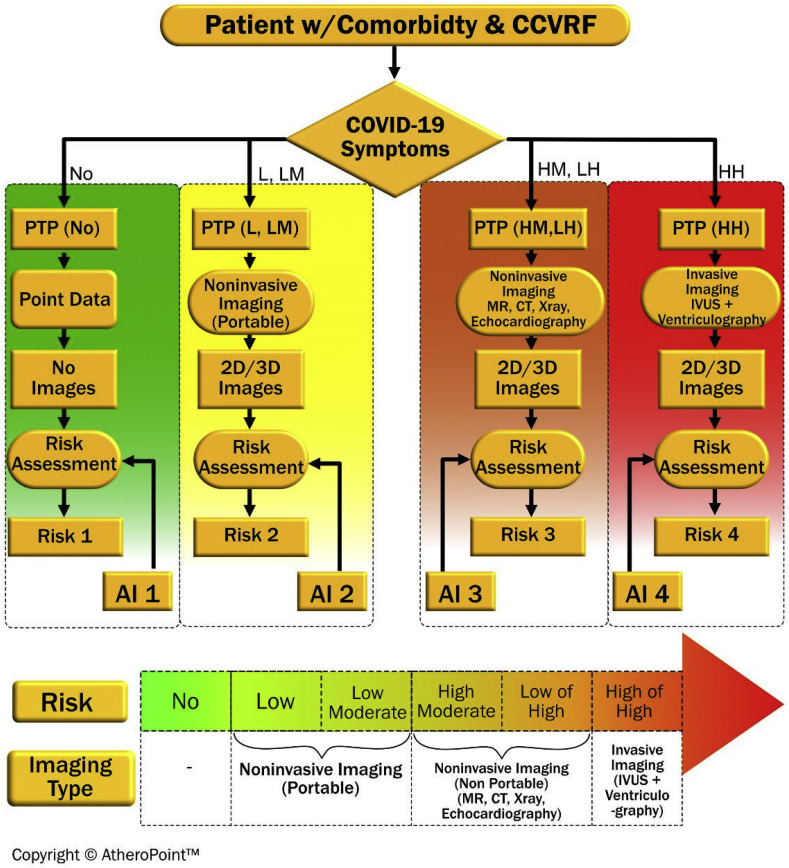
AI-based tests can categorize the nature of a patient's risk in one of the categories namely no-risk, low, low-medium (LM), high-medium (HM), low-high (LH), or high-high (HH) risk depending on the patient's symptoms and their severity [120,122] as shown in Fig. 6 . The imaging modality also varies with the degree of risk as follows: no imaging for no-risk, portable imaging for low and LM risk, non-portable imaging for HM and LH, and invasive imaging for HH. A probability (PTP) is performed to accurately interpret diagnostic results to categorize the patient into one of the four groups [[123], [124], [125], [126]]. After that, for no-risk patients, non-imaging biomarkers can be collected for risk assessment using AI-based data protocols. For low-risk patients, portable 2D/3D imaging, such as ultrasound, is used, whereas non-portable and invasive 2D/3D imaging such as MRI/CT/X-Ray/echocardiography can be used for LM patients. For HH patients, invasive imaging techniques such as IVUS and ventriculography can be used. Based on the data provided by various 2D/3D scans, AI-based medical imaging is applied for risk assessment. Further treatment is then planned based on this imaging process. In the next section, deep learning (DL)-based medical imaging is proposed for medical imaging scans, particularly ultrasounds for COVID-19 patients.
Fig. 6.
Role of AI-based risk assessment on COVID-19 patients having comorbidity.
4. Machine learning and Deep Learning for tissue characterization
Using AI and associated technologies in healthcare can significantly slow down diagnosis times, especially during the COVID-19 pandemic, as patient numbers are continually growing and there are few specialists available [119].
Although some caution must be exercised regarding its full-scale deployment [127], its overall usefulness in healthcare management during times of crisis cannot be ignored [54,55,128,129]. In general, AI in healthcare refers to all artificial intelligence-based technologies that make educated decisions regarding a patient's diagnosis, monitoring, treatment, and management. The importance of AI has specifically increased many folds when imaging comes into play, mainly because of large volumetric data sizes and the extensive need to characterize and quantify the disease via lesion images [[130], [131], [132]]. Tissue imaging and its characterization is of prime importance since it has a direct influence on decisions related to COVID-19 severity for a patient [[133], [134], [135]]. The main benefit of AI methods is that the machines can be used to train (by mimicking the physician's cognitive actions), and such trained models can be used to predict the disease's severity in asymptomatic patients. Within a short period, several machine learning (ML)-based techniques used the power of AI to manage COVID-19 [136,137].
4.1. ML and DL architectures
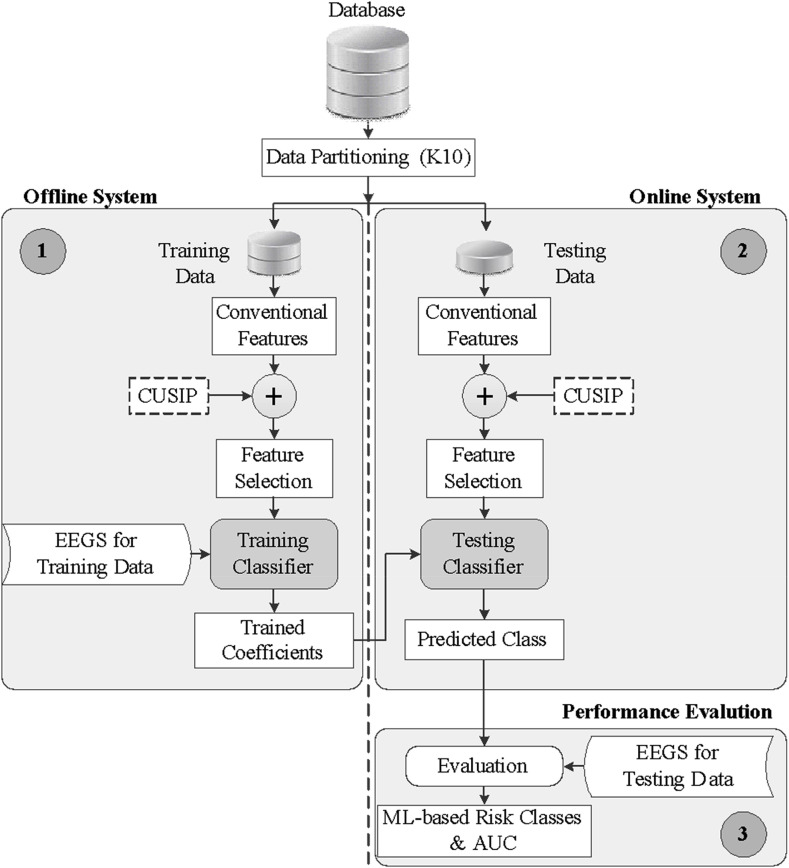
ML Architecture: ML is a two-stage process. In stage I, different features are extracted from the lesion COVID images; the extractions are then operated on by an ML statistical model (called a training system), to generate offline coefficients. These coefficients are then transformed by the test lesion images, which yield an intelligent classification or inference. A typical ML system for predicting risk class is shown in Fig. 7. CUSIP is an image-based phenotype that uses the event equivalent gold standard (EEGS) [57,138,139] model.
Fig. 7.
Typical low-cost machine learning architecture utilizing the EEGS model.
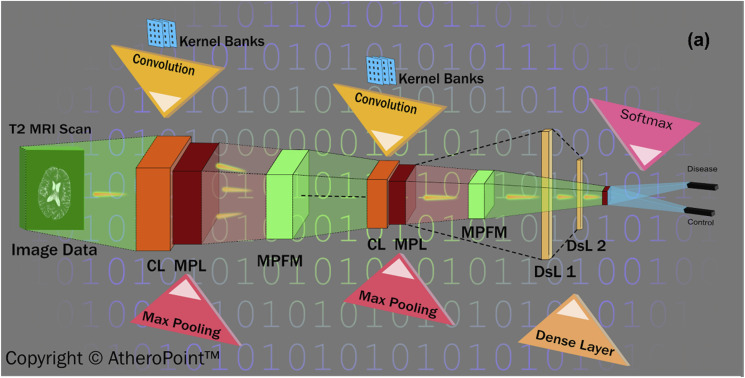
DL Architecture: DL refers to a visual cortex that imitates multiple layers of a neural network applied directly to tissue images to extract features and for characterization purposes [54]. The convolution neural network (CNN) [140] (shown in Fig. 8) is one such DL network architecture that is widely used to characterize medical images. It performs a series of convolution max-pooling operations to extract features and perform characterizations. ML and DL both follow the supervised learning approach by which models are trained using offline data.
Fig. 8.
A convolution neural network (courtesy of AtheroPoint™, CA, USA).
As discussed in previous sections, there are four pathways through which a COVID-19 infection leads to heart and brain injuries. AI can be used via medical imaging to detect the extent of tissue damage in these pathways and help medical professionals to develop an effective treatment plan for patients. There are several instances in which the AI paradigm has been used for tissue characterization based on medical images during the pandemic as well as during standard times. Some uses of AI are described organ-wise following a proposed model for characterizing DL-based tissues.
4.2. ML Architecture used for tissue characterization for stroke risk stratification
Two types of tissue characterization that can be carried out using AI: (i) ML-based [63,141], and (ii) DL-based [142]. Various ML-based technologies have been developed to classify symptomatic and asymptomatic plaque from ultrasound images. For example, an ML-based technique based on support vector machines (SVM) was developed to characterize the symptomatic and asymptomatic plaques of 346 carotid scans that indicated the presence of plaque [143,144]. SVM classifiers work by determining the maximum margin between two data clusters. First, a texture analysis [145] is used to extract the features (i.e., standard deviation, entropy, symmetry, and run percentage) in the feature extraction phase [146]. SVM with a radial basis function (RBF) kernel was then applied to the features to characterize the plaque tissue lesions. The performance accuracy was 82.4%. The higher-order spectra [130] domain provides evidence of compelling tissue characterization features related.
In other work, an SVM-based (RBF kernel) classifier [46] was developed using a combination of HOS [130,147], discrete wavelet transforms (DWTs) [148], and texture features [146] taken from 146 patient scans. This classifier's accuracy was 91.7%. Polynomial kernels of order two were used to characterize tissues when adapting DWT-based features, yielding an accuracy of 83.7%. Several combinations of classifiers were applied to two different carotid plaque cohorts (Portugal and the UK) comprising 346 scans. The focus was to compare and contrast different classifiers, such as SVM [45], the Gaussian mixture model (GMM) [149], radial basis probabilistic neural network (RBPNN) [150], decision tree (DT) [151], k-nearest neighbor (KNN) [152], naive Bayes classifier (NBC) [153], and fuzzy classifier [154]. The main features used were trace transform [155], fuzzy gray level co-occurrence matrix (FGLCM) [156], and fuzzy run-length matrix (FRLM) [157]. The highest fuzzy classifier accuracy achieved for the Portugal cohort was 93.1%, while the NBC and SVM-RBF kernel performed equally (85.3%). Different methods for CVD risk stratification using the AI paradigm for plaque characterization have been published [27,28,158].
4.3. ML/DL used for vessel characterization, measurement and risk stratification
Recently, Jamthikar et al. [159,160] explained the role of ML for CVD/stroke risk assessment within a big data framework by fusing image-based phenotypes and conventional risk factors for CCA and bulb segments [161]. In another study, the authors discussed the preventive cardiovascular framework for coronary artery disease management in ML [63] and the big data framework. Similarly, ML and DL algorithms have been applied to CVD risk assessments in several other areas [158,162]. Highly accurate techniques for lumen characterization [164], stenosis estimation [165], and cIMT measurement [56] have been developed using the deep fully convolutional network (FCN) [163] in segmentation models.
4.4. AI used for chest CT and liver disease classification
ML and DL technologies are being applied during the COVID-19 pandemic to characterize lung CT images [[166], [167], [168]] with varying degrees of success. Kang et al. [169] applied representation learning to characterize non-infected chest CT scans from COVID-19 patients with an accuracy of 95.5%. Wang et al. [170] used a DL-based network to differentiate COVID-19 patients’ CT scans from scans of non-infected people, yielding a ROC having an AUC of 0.959.
Different DL-based methodologies for tissue characterization and segmentation have been implemented to differentiate diseased (fatty liver) ultrasound images (with 100% accuracy) [171,172] and assess liver fibrosis stages using DL radionics of shear wave elastography [173]. This method is ideal for characterizing and classifying COVID-19 patients.
4.5. AI-based tissue characterization and risk stratification in lung CT
Several studies have appeared recently in the area of lung CT classification using AI methods. They are divided into two kinds, depending on the number of classes used for risk stratification. The first set of studies compared COVID-19 pneumonia patients against non-COVID-19 pneumonia, i.e., two-class scenarios. The second set consisted of multiclass paradigms. Wang et al. [174] used DenseNet 121 for lung mask creation and segmentation and used DenseNet to classify COVID-19 and control patients, yielding an AUC of 0.9, a sensitivity of 78.93% and a specificity of 89.93%. Zhang et al. [175] used a combination of lung segmentation combined with a classification for three classes (i.e., COVID-19, community pneumonia, and normal). The authors used the DeepLabv3 model for lung segmentation and 3D ResNet-18 for classification, yielding an accuracy of 92.49% and an AUC of 0.98.
Other authors have applied AI in their research protocol for CT lung scans. Li et al. [176] recently developed a CT lung DL system to predict the severity and progression of COVID-19. Recently, Chen et al. [177] developed a UNet++ architecture for segmenting COVID-19-infected lung regions in CT scans. This system is accessible worldwide [178]. Yang et al. [179] illustrated the lung segmentation in CT images by identifying pulmonary parenchyma, followed by DenseNet-based classification. This method yielded an accuracy of 92% and an AUC of 0.98. In other work, Oh et al. [180] used X-ray CHEST images as inputs for segmentation and classification. The authors used DenseNet for segmentation. The authors then used the same network for classification, adapting patch-based strategy, and demonstrating an accuracy of 88.9%.
4.6. AI-based plaque tissue characterization and risk stratification for cardiac health
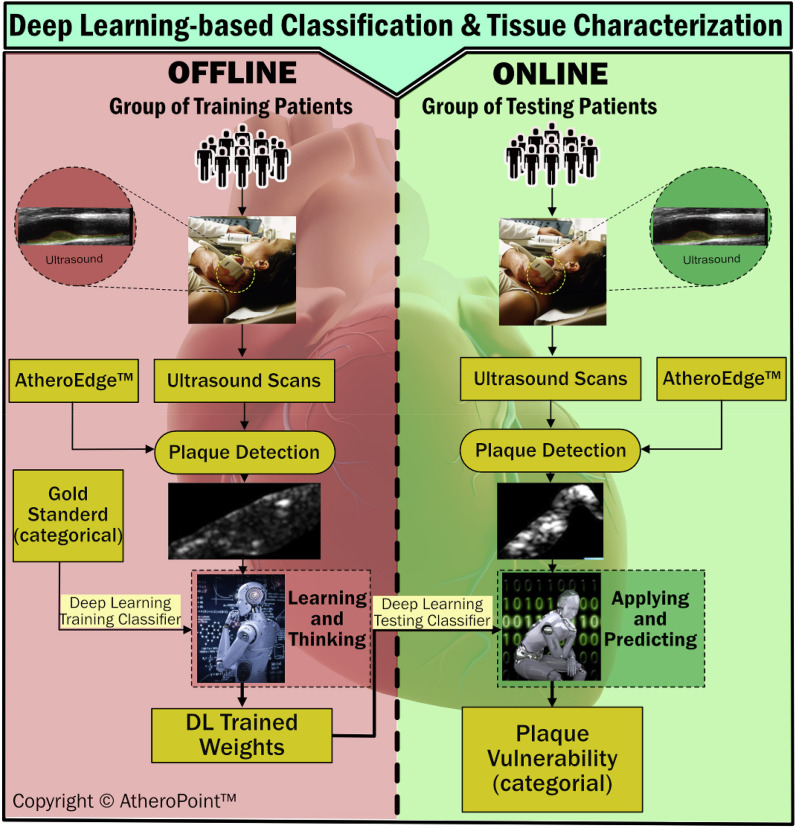
A well-implemented DL-based platform is proposed to treat COVID-19 patients with comorbidities (see Fig. 9 ). The proposed DL system is trained using offline data collected from COVID-19 patients across the globe. The data is given in the form of several ultrasound scans taken from COVID-19 patients with comorbidities following specific guidelines [[93], [94], [95], [96], [97]]. The ROI of the tissue is obtained from the scan using the AtheroEdge™ system, which can detect plaque automatically and segment the plaque region. Similarly, ultrasound scans are taken from online patients, and ROI is extracted using the same AtheroEdge™ system. Once the DL parameters are trained using the offline data, they are used to predict the plaque vulnerability of the online data obtained from the testing patients. The predictions are used to further assess and support the clinical viability of the DL system.
Fig. 9.
Proposed DL-based system for tissue characterization and classification of COVID-19 severity with patients with comorbidities (courtesy of AtheroPoint™, CA, USA).
5. Summary
In this review, we summarized several imaging studies on COVID-19 patients to explore the extent to which major organs, such as the heart [106,107], brain [108,109], lungs [104,105], and liver, undergo due to COVID-19 infection. All the imaging studies were done on COVID-19 patients with mild to severe symptoms who were referred to the medical team for appropriate patient management. Although there are multiple imaging modalities, ultrasound is preferred over others because of its portability, which allows patients to stay in their isolation zones. Similar portability is advised for MRI [181,182] and CT [183] scanning to decrease the spread of infection among different isolation wards. A mass portable imaging test for admitting COVID-19 patients would allow medical practitioners to devise treatment plans quickly and help save lives. For patients with severe cases of COVID-19, IVUS [98] and, ventriculography (with proper precautions) are proposed [42,43,99,100].
The exponential pandemic curve makes it impossible to examine and diagnose all medical images due to the limited number of radiologists and scarce medical resources. In this respect, AI-based medical imaging can be used to examine and diagnose COVID-19 patients and help with risk stratification. Because AI systems can handle millions of images at a time, they can be scaled to make mass diagnoses to match the pandemic curve.
AI is categorized into two types: ML and DL [54]. Predictions made by ML models depend on feature extraction algorithms. Meanwhile, DL models can extract features directly from medical imaging, thus making them clearer. We propose that a risk assessment model using AI-based imaging should be used. Initially, based on PTP tests [[123], [124], [125], [126]], patients are categorized as wither no-risk, low-risk, LM, MH, LH, or HH [120,122]. Medical imaging is then performed according to the patient's risk level. Finally, AI is applied to medical images to assess risk. DL-based tissue characterization is also proposed for ultrasound scans and extended to other imaging modalities. The DL-based system is trained using offline data and tested using online data. This system is capable of ascertaining the degree of tissue damage caused by a COVID-19 infection.
In addition to AI, telemedicine and social media can be useful for monitoring patients' health. Telemedicine can be used to track patients' health using Internet of Things devices, thereby augmenting infection containment efforts [184]. Also, through the use of big data analytics, social media can be used to track patients’ health and share essential research findings [[185], [186], [187]].
A short note on precautions that should be taken during the medical imaging of COVID-19 patients.
Guidelines must be followed by medical staff to prevent infection [[188], [189], [190]]. Specifically, medical staff members are required to wear eye protection, a disposable water-resistant gowns, and disposable gloves. Portable imaging equipment must be used to avoid moving patients. All medical equipment that needs to be touched should be disinfected after each use (as shown in Fig. 10 (a)). To prevent contact, imaging equipment can be used outside the isolation room, and images can be taken through the isolation room glass (as shown in Fig. 10 (b)). A disposable sterile protection cover (as shown in Fig. 10 (c)) can be used to contact devices (e.g., ultrasound probes).
Fig. 10.
a) Safety guidelines to be followed by medical staff before performing imaging (reproduced with permission [188]); (b) Images being taken through glass (reproduced with permission [189]); (c) disposable sterile sheath for covering probe. (reproduced with permission [190]).
6. Conclusion
COVID-19 leads to brain and heart injury via four pathways (i.e., neuronal, hypoxia, RAAS, and immune). Portable/non-portable invasive imaging techniques must be carried out following proper precautions depending upon the level of risk associated with a patient's symptoms. Although medical imaging could significantly enhance a patient's chances of survival, the scarcity of trained radiologists limits its usage. Thus, AI techniques, such as ML- and DL-based methods, are proposed to speed up assessments and diagnoses based on medical imaging. COVID-19 causes severe health hazards in patients with comorbidities, and so a DL-system is proposed for COVID-19 diagnosis and risk stratification.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Yuen K.-S., Ye Z.-W., Fung S.-Y., Chan C.-P., Jin D.-Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10(1):1–5. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus (COVID-19) outbreak [https://www.who.int/westernpacific/emergencies/covid-19].
- 3.Coronavirus [https://www.who.int/health-topics/coronavirus#tab=tab_1].
- 4.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronavirus [https://www.worldometers.info/coronavirus/].
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao F., Zheng K.I., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., Wang T.Y., Chen Y.P., George J., Zheng M.H. Metabolic associated fatty liver disease increases COVID‐19 disease severity in non‐diabetic patients. J. Gastroenterol. Hepatol. 2020 doi: 10.1111/jgh.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., Yu X., Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Research and Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001343. e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020:E139–E596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams V.R., Scholey J.W. Angiotensin-converting enzyme 2 and renal disease. Curr. Opin. Nephrol. Hypertens. 2018;27(1):35–41. doi: 10.1097/MNH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 16.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12(7):6049. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng H., Wang Y., Wang G.Q. Organ‐protective effect of angiotensin‐converting enzyme 2 and its effect on the prognosis of COVID‐19. J. Med. Virol. 2020 doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby P. The heart in COVID19: primary target or secondary bystander? JACC (J. Am. Coll. Cardiol.): Basic to Translational Science. 2020 doi: 10.1016/j.jacbts.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., Jain S.S., Burkhoff D., Kumaraiah D., Rabbani L. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 20.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 21.Suri J.S., Kathuria C., Molinari F. Springer Science & Business Media; 2010. Atherosclerosis Disease Management. [Google Scholar]
- 22.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong B., Zhang C., Feng J.B., Zhao Y.X., Li S.Y., Yang Y.P., Dong Q.L., Deng B.P., Zhu L., Yu Q.T. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008;28(7):1270–1276. doi: 10.1161/ATVBAHA.108.164715. [DOI] [PubMed] [Google Scholar]
- 24.Mossa-Basha M., Meltzer C.C., Kim D.C., Tuite M.J., Kolli K.P., Tan B.S. Radiology department preparedness for COVID-19: radiology scientific expert panel. Radiology. 2020 doi: 10.1148/radiol.2020200988. 200988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotsis V., Jamthikar A.D., Araki T., Gupta D., Laird J.R., Giannopoulos A.A., Saba L., Suri H.S., Mavrogeni S., Kitas G.D. Echolucency-based phenotype in carotid atherosclerosis disease for risk stratification of diabetes patients. Diabetes Res. Clin. Pract. 2018;143:322–331. doi: 10.1016/j.diabres.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Khanna N.N., Jamthikar A.D., Gupta D., Araki T., Piga M., Saba L., Carcassi C., Nicolaides A., Laird J.R., Suri H.S. Effect of carotid image-based phenotypes on cardiovascular risk calculator: AECRS1. 0. Med. Biol. Eng. Comput. 2019;57(7):1553–1566. doi: 10.1007/s11517-019-01975-2. [DOI] [PubMed] [Google Scholar]
- 27.Khanna N.N., Jamthikar A.D., Araki T., Gupta D., Piga M., Saba L., Carcassi C., Nicolaides A., Laird J.R., Suri H.S. Nonlinear model for the carotid artery disease 10‐year risk prediction by fusing conventional cardiovascular factors to carotid ultrasound image phenotypes: a Japanese diabetes cohort study. Echocardiography. 2019;36(2):345–361. doi: 10.1111/echo.14242. [DOI] [PubMed] [Google Scholar]
- 28.Cuadrado-Godia E., Jamthikar A.D., Gupta D., Khanna N.N., Araki T., Maniruzzaman M., Saba L., Nicolaides A., Sharma A., Omerzu T. Ranking of stroke and cardiovascular risk factors for an optimal risk calculator design: logistic regression approach. Comput. Biol. Med. 2019;108:182–195. doi: 10.1016/j.compbiomed.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Khanna N.N., Jamthikar A.D., Gupta D., Piga M., Saba L., Carcassi C., Giannopoulos A.A., Nicolaides A., Laird J.R., Suri H.S. Rheumatoid arthritis: atherosclerosis imaging and cardiovascular risk assessment using machine and deep learning–based tissue characterization. Curr. Atherosclerosis Rep. 2019;21(2):7. doi: 10.1007/s11883-019-0766-x. [DOI] [PubMed] [Google Scholar]
- 30.Jamthikar A., Gupta D., Khanna N.N., Araki T., Saba L., Nicolaides A., Sharma A., Omerzu T., Suri H.S., Gupta A. A special report on changing trends in preventive stroke/cardiovascular risk assessment via B-mode ultrasonography. Curr. Atherosclerosis Rep. 2019;21(7):25. doi: 10.1007/s11883-019-0788-4. [DOI] [PubMed] [Google Scholar]
- 31.Schnee J.M., Hsueh W.A. Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc. Res. 2000;46(2):264–268. doi: 10.1016/s0008-6363(00)00044-4. [DOI] [PubMed] [Google Scholar]
- 32.Wu L.L., Yang N., Roe C.J., Cooper M.E., Gilbert R.E., Atkins R.C., Lan H.Y. Macrophage and myofibroblast proliferation in remnant kidney: role of angiotensin II. Kidney Int. 1997;(63) Supplement. [PubMed] [Google Scholar]
- 33.Sun Y., Ramires F.J., Weber K.T. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc. Res. 1997;35(1):138–147. doi: 10.1016/s0008-6363(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 34.Morihara K., Takai S., Takenaka H., Sakaguchi M., Okamoto Y., Morihara T., Miyazaki M., Kishimoto S. Cutaneous tissue angiotensin–converting enzyme may participate in pathologic scar formation in human skin. J. Am. Acad. Dermatol. 2006;54(2):251–257. doi: 10.1016/j.jaad.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 35.Cosyns B., Lochy S., Luchian M.L., Gimelli A., Pontone G., Allard S.D., de Mey J., Rosseel P., Dweck M., Petersen S.E. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. European Heart Journal-Cardiovascular Imaging. 2020 doi: 10.1093/ehjci/jeaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., Cani D.S., Cerini M., Farina D., Gavazzi E. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim I.-C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020;41(19):1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiamanesh O., Harper L., Wiskar K., Luksun W., McDonald M., Ross H., Woo A., Granton J. Lung ultrasound for cardiologists in the time of COVID-19. Can. J. Cardiol. 2020 doi: 10.1016/j.cjca.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zieleskiewicz L., Duclos G., Dransart-Rayé O., Nowobilski N., Bouhemad B. Ultrasound findings in patients with COVID-19 pneumonia in early and late stages: two case-reports. Anaesthesia, Critical Care & Pain Medicine. 2020 doi: 10.1016/j.accpm.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saba L., Tiwari A., Biswas M., Gupta S.K., Godia-Cuadrado E., Chaturvedi A., Turk M., Suri H.S., Orru S., Sanches J.M. Wilson's disease: a new perspective review on its genetics, diagnosis and treatment. Frontiers in bioscience (Elite edition) 2019;11:166–185. doi: 10.2741/E854. [DOI] [PubMed] [Google Scholar]
- 41.Collaborators* NASCET Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 42.Sanches J.M., Laine A.F., Suri J.S. Springer; 2012. Ultrasound Imaging. [Google Scholar]
- 43.Suri J.S., Wilson D., Laxminarayan S. vol. 2. Springer Science & Business Media; 2005. (Handbook of Biomedical Image Analysis). [Google Scholar]
- 44.Suri J.S., Laxminarayan S. CRC press; 2003. Angiography and Plaque Imaging: Advanced Segmentation Techniques. [Google Scholar]
- 45.Acharya U.R., Mookiah M.R.K., Sree S.V., Afonso D., Sanches J., Shafique S., Nicolaides A., Pedro L.M., e Fernandes J.F., Suri J.S. Atherosclerotic plaque tissue characterization in 2D ultrasound longitudinal carotid scans for automated classification: a paradigm for stroke risk assessment. Med. Biol. Eng. Comput. 2013;51(5):513–523. doi: 10.1007/s11517-012-1019-0. [DOI] [PubMed] [Google Scholar]
- 46.Acharya U.R., Faust O., Sree S.V., Alvin A.P.C., Krishnamurthi G., Sanches J., Suri J.S. Annual International Conference of the IEEE Engineering in Medicine and Biology Society: 2011. IEEE; 2011. Atheromatic™: symptomatic vs. asymptomatic classification of carotid ultrasound plaque using a combination of HOS, DWT & texture; pp. 4489–4492. [DOI] [PubMed] [Google Scholar]
- 47.Acharya U.R., Sree S.V., Kulshreshtha S., Molinari F., Koh J.E.W., Saba L., Suri J.S. GyneScan: an improved online paradigm for screening of ovarian cancer via tissue characterization. Technol. Canc. Res. Treat. 2014;13(6):529–539. doi: 10.7785/tcrtexpress.2013.600273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biswas M., Kuppili V., Edla D.R., Suri H.S., Saba L., Marinhoe R.T., Sanches J.M., Suri J.S. Symtosis: a liver ultrasound tissue characterization and risk stratification in optimized deep learning paradigm. Comput. Methods Progr. Biomed. 2018;155:165–177. doi: 10.1016/j.cmpb.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Acharya U.R., Krishnan M.M.R., Sree S.V., Sanches J., Shafique S., Nicolaides A., Pedro L.M., Suri J.S. Plaque tissue characterization and classification in ultrasound carotid scans: a paradigm for vascular feature amalgamation. IEEE Transactions on Instrumentation and Measurement. 2012;62(2):392–400. [Google Scholar]
- 50.Molinari F., Liboni W., Pavanelli E., Giustetto P., Badalamenti S., Suri J.S. 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society: 2007. IEEE; 2007. Accurate and automatic carotid plaque characterization in contrast enhanced 2-D ultrasound images; pp. 335–338. [DOI] [PubMed] [Google Scholar]
- 51.Acharya U., Vinitha Sree S., Mookiah M., Yantri R., Molinari F., Zieleźnik W., Małyszek-Tumidajewicz J., Stępień B., Bardales R., Witkowska A. Diagnosis of Hashimoto's thyroiditis in ultrasound using tissue characterization and pixel classification. Proc. IME H J. Eng. Med. 2013;vol. 227(7):788–798. doi: 10.1177/0954411913483637. [DOI] [PubMed] [Google Scholar]
- 52.Sharma A.M., Gupta A., Kumar P.K., Rajan J., Saba L., Nobutaka I., Laird J.R., Nicolades A., Suri J.S. A review on carotid ultrasound atherosclerotic tissue characterization and stroke risk stratification in machine learning framework. Curr. Atherosclerosis Rep. 2015;17(9):55. doi: 10.1007/s11883-015-0529-2. [DOI] [PubMed] [Google Scholar]
- 53.Ravì D., Wong C., Deligianni F., Berthelot M., Andreu-Perez J., Lo B., Yang G.-Z. Deep learning for health informatics. IEEE journal of biomedical and health informatics. 2016;21(1):4–21. doi: 10.1109/JBHI.2016.2636665. [DOI] [PubMed] [Google Scholar]
- 54.Saba L., Biswas M., Kuppili V., Godia E.C., Suri H.S., Edla D.R., Omerzu T., Laird J.R., Khanna N.N., Mavrogeni S. The present and future of deep learning in radiology. European journal of radiology. 2019 doi: 10.1016/j.ejrad.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 55.Biswas M., Kuppili V., Saba L., Edla D.R., Suri H.S., Cuadrado-Godia E., Laird J., Marinhoe R., Sanches J., Nicolaides A. State-of-the-art review on deep learning in medical imaging. Front Biosci (Landmark Ed) 2019;24:392–426. doi: 10.2741/4725. [DOI] [PubMed] [Google Scholar]
- 56.Biswas M., Kuppili V., Araki T., Edla D.R., Godia E.C., Saba L., Suri H.S., Omerzu T., Laird J.R., Khanna N.N. Deep learning strategy for accurate carotid intima-media thickness measurement: an ultrasound study on Japanese diabetic cohort. Comput. Biol. Med. 2018;98:100–117. doi: 10.1016/j.compbiomed.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Jamthikar A., Gupta D., Khanna N.N., Saba L., Araki T., Viskovic K., Suri H.S., Gupta A., Mavrogeni S., Turk M. A low-cost machine learning-based cardiovascular/stroke risk assessment system: integration of conventional factors with image phenotypes. Cardiovasc. Diagn. Ther. 2019;9(5):420. doi: 10.21037/cdt.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu K., Peng G., Wilken M., Geraghty R.J., Li F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J. Biol. Chem. 2012;287(12):8904–8911. doi: 10.1074/jbc.M111.325803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel V.B., Zhong J.-C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ. Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020:1–8. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. A Journal of the Pathological Society of Great Britain and Ireland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koyuncu O.O., Hogue I.B., Enquist L.W. Virus infections in the nervous system. Cell Host Microbe. 2013;13(4):379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCray P.B., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., Netland J., Jia H.P., Halabi C., Sigmund C.D. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81(2):813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A.K., Reznikov L.R., Gibson-Corley K.N., Meyerholz D.K., McCray P.B., Jr. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. The Journal of infectious diseases. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baig A.M. Neurological manifestations in COVID‐19 caused by SARS‐CoV‐2. CNS Neurosci. Ther. 2020;26(5):499. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryan W. 2020. There's a New Symptom of Coronavirus, Doctors Say: Sudden Loss of Smell or Taste. Retrieved from. [Google Scholar]
- 73.Hopkins C., Kumar N. Loss of sense of smell as marker of COVID-19 infection. 2020;26(3) doi: 10.1111/coa.13620. https://www entuk org/sites/default/files/files/Loss of sense of smell as marker of COVID pdf 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bohmwald K., Galvez N., Ríos M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H., Baker A. Springer; 2017. Recombinant Human ACE2: Acing Out Angiotensin II in ARDS Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radermacher P., Maggiore S.M., Mercat A. Fifty years of research in ARDS. Gas exchange in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017;196(8):964–984. doi: 10.1164/rccm.201610-2156SO. [DOI] [PubMed] [Google Scholar]
- 77.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiong T.-Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oudit G., Kassiri Z., Jiang C., Liu P., Poutanen S., Penninger J., Butany J. SARS‐coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdennour L., Zeghal C., Deme M., Puybasset L. Annales francaises d'anesthesie et de reanimation. 2012. Interaction brain-lungs; pp. e101–107. [DOI] [PubMed] [Google Scholar]
- 81.Fountain J.H., Lappin S.L. StatPearls [Internet] StatPearls Publishing; 2019. Physiology, renin angiotensin system. [PubMed] [Google Scholar]
- 82.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., Nishigaki I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9(10):1057. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lillie P.J., Samson A., Li A., Adams K., Capstick R., Barlow G.D., Easom N., Hamilton E., Moss P.J., Evans A. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J. Infect. 2020;80(5):578–606. doi: 10.1016/j.jinf.2020.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. Jama. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin C., Wang C., Tang Z., Wen Y., Zhang S., Wang B. Clinical analysis of multiple organ dysfunction syndrome in patients suffering from SARS. Zhongguo wei zhong bing ji jiu yi xue= Chinese critical care medicine= Zhongguo weizhongbing jijiuyixue. 2004;16(11):646–650. [PubMed] [Google Scholar]
- 88.Schoenhagen P., Tuzcu E.M., Ellis S.G. Am Heart Assoc; 2002. Plaque vulnerability, plaque rupture, and acute coronary syndromes: (Multi)-Focal manifestation of a systemic disease process. [DOI] [PubMed] [Google Scholar]
- 89.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tersalvi G., Vicenzi M., Calabretta D., Biasco L., Pedrazzini G., Winterton D. Elevated troponin in patients with Coronavirus Disease 2019 (COVID-19): possible mechanisms. J. Card. Fail. 2020 doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gomes V.A. COVID-19 Cardiac repercussions. Revista Brasileira de Fisiologia do Exercício. 2020;19(2) [Google Scholar]
- 92.Zeng J.H., Liu Y.-X., Yuan J., Wang F.-X., Wu W.-B., Li J.-X., Wang L.-F., Gao H., Wang Y., Dong C.-F. 2020. First Case of COVID-19 Infection with Fulminant Myocarditis Complication: Case Report and Insights. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cieszanowski A., Czekajska E., Giżycka B., Gruszczyńska K., Podgórska J., Oronowicz-Jaśkowiak A., Serafin Z., Szurowska E., Walecki J.M. Management of patients with COVID-19 in radiology departments, and indications regarding imaging studies–recommendations of the Polish Medical Society of Radiology. Pol. J. Radiol. 2020;85:e209. doi: 10.5114/pjr.2020.95022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim D.J., Jelic T., Woo M.Y., Heslop C., Olszynski P. Just the facts: recommendations on point of care ultrasound use and machine infection control during the COVID-19 pandemic. Can. J. Emerg. Med. 2020:1–7. doi: 10.1017/cem.2020.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.An X., Song Z., Gao Y., Tao J., Yang J. To resume noninvasive imaging detection safely after peak period of COVID‐19: experiences from Wuhan China. Dermatol. Ther. 2020 doi: 10.1111/dth.13590. e13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jakhar D., Kaur I., Kaul S. Art of performing dermoscopy during the times of coronavirus disease (COVID‐19): simple change in approach can save the day! J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16412. [DOI] [PubMed] [Google Scholar]
- 97.Skulstad H., Cosyns B., Popescu B.A., Galderisi M., Salvo G.D., Donal E., Petersen S., Gimelli A., Haugaa K.H., Muraru D. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. European Heart Journal-Cardiovascular Imaging. 2020 doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lo S., Yong A., Sinhal A., Shetty S., McCann A., Clark D., Galligan L., El-Jack S., Sader M., Tan R. Consensus guidelines for Interventional Cardiology services delivery during COVID-19 pandemic in Australia and New Zealand. Heart Lung Circ. 2020 doi: 10.1016/j.hlc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Baz A., Jiang X., Suri J.S. CRC Press; 2016. Biomedical Image Segmentation: Advances and Trends. [Google Scholar]
- 100.El-Baz A.S., Acharya R., Mirmehdi M., Suri J.S. vol. 1. Springer Science & Business Media; 2011. (Multi Modality State-Of-The-Art Medical Image Segmentation and Registration Methodologies). [Google Scholar]
- 101.Olusanya O: Ultrasound in Times of COVID-19.
- 102.Smith M., Hayward S., Innes S., Miller A. Point‐of‐care lung ultrasound in patients with COVID‐19–a narrative review. Anaesthesia. 2020 doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jacobi A., Chung M., Bernheim A., Eber C. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin. Imag. 2020 doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., Cui J., Xu W., Yang Y., Fayad Z.A. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vasilev Y., Sergunova K., Bazhin A., Masri A., Vasileva Y., Suleumanov E., Semenov D., Kudryavtsev N., Panina O., Khoruzhaya A. MRI of the lungs in patients with COVID-19: clinical case. medRxiv. 2020 [Google Scholar]
- 106.Huang L., Zhao P., Tang D., Zhu T., Han R., Zhan C., Liu W., Zeng H., Tao Q., Xia L. Cardiac involvement in recovered COVID-19 patients identified by magnetic resonance imaging. JACC (J. Am. Coll. Cardiol.): Cardiovascular Imaging. 2020 doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luetkens J.A., Isaak A., Zimmer S., Nattermann J., Sprinkart A.M., Boesecke C., Rieke G.J., Zachoval C., Heine A., Velten M. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circulation: Cardiovascular Imaging. 2020;13(5) doi: 10.1161/CIRCIMAGING.120.010897. e010897. [DOI] [PubMed] [Google Scholar]
- 108.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. 201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D., Kaya Y., Yildirim D., Tuzuner F., Yildirim M.S. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020 doi: 10.1148/radiol.2020201697. 201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhayana R., Som A., Li M.D., Carey D.E., Anderson M.A., Blake M.A., Catalano O., Gee M.S., Hahn P.F., Harisinghani M. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020 doi: 10.1148/radiol.2020201908. 201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eliezer M., Hautefort C. Academic Radiology; 2020. MRI Evaluation of the Olfactory Clefts in Patients with SARS-CoV-2 Infection Revealed an Unexpected Mechanism for Olfactory Function Loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiao Z., Xu C., Wang D., Zeng H. The experience of treating patients with acute myocardial infarction under the COVID‐19 epidemic. Cathet. Cardiovasc. Interv. 2020 doi: 10.1002/ccd.28951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meyer P., Degrauwe S., Van Delden C., Ghadri J.-R., Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur. Heart J. 2020;41(19):1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J., Zhang C., Li H., Xia X., Kong S. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 116.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., Zhang C., Boyle C., Smith M., Phillips J.P. Mass Medical Soc; 2020. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. [DOI] [PubMed] [Google Scholar]
- 117.Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic's front line. N. Engl. J. Med. 2020;382(20):1873–1875. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 118.ASBe al. Declines in hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic: a multicenter tertiary care experience. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vaishya R., Haleem A., Vaish A., Javaid M. Emerging technologies to combat COVID-19 pandemic. Journal of Clinical and Experimental Hepatology. 2020 doi: 10.1016/j.jceh.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Murphy K., Smits H., Knoops A.J., Korst M.B., Samson T., Scholten E.T., Schalekamp S., Schaefer-Prokop C.M., Philipsen R.H., Meijers A. COVID-19 on the chest radiograph: a multi-reader evaluation of an AI system. Radiology. 2020 doi: 10.1148/radiol.2020201874. 201874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng N., Du S., Wang J., Zhang H., Cui W., Kang Z., Yang T., Lou B., Chi Y., Long H. Predicting COVID-19 in China using hybrid AI model. IEEE transactions on cybernetics. 2020 doi: 10.1109/TCYB.2020.2990162. [DOI] [PubMed] [Google Scholar]
- 122.Chieffo A., Stefanini G.G., Price S., Barbato E., Tarantini G., Karam N., Moreno R., Buchanan G.L., Gilard M., Halvorsen S. EAPCI position statement on invasive management of acute coronary syndromes during the COVID-19 pandemic. Eur. Heart J. 2020;41(19):1839–1851. doi: 10.1093/eurheartj/ehaa381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am. J. Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 124.Dangis A., Gieraerts C., Bruecker Y.D., Janssen L., Valgaeren H., Obbels D., Gillis M., Ranst M.V., Frans J., Demeyere A. Accuracy and reproducibility of low-dose submillisievert chest CT for the diagnosis of COVID-19. Radiology: Cardiothoracic Imaging. 2020;2(2) doi: 10.1148/ryct.2020200196. e200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rubin G.D., Ryerson C.J., Haramati L.B., Sverzellati N., Kanne J.P., Raoof S., Schluger N.W., Volpi A., Yim J.-J., Martin I.B. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Chest. 2020 doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nair A., Rodrigues J., Hare S., Edey A., Devaraj A., Jacob J., Johnstone A., McStay R., Denton E., Robinson G. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin. Radiol. 2020;75(5):329–334. doi: 10.1016/j.crad.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laghi A. Cautions about radiologic diagnosis of COVID-19 infection driven by artificial intelligence. The Lancet Digital Health. 2020;2(5):e225. doi: 10.1016/S2589-7500(20)30079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miotto R., Wang F., Wang S., Jiang X., Dudley J.T. Deep learning for healthcare: review, opportunities and challenges. Briefings Bioinf. 2018;19(6):1236–1246. doi: 10.1093/bib/bbx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Esteva A., Robicquet A., Ramsundar B., Kuleshov V., DePristo M., Chou K., Cui C., Corrado G., Thrun S., Dean J. A guide to deep learning in healthcare. Nat. Med. 2019;25(1):24–29. doi: 10.1038/s41591-018-0316-z. [DOI] [PubMed] [Google Scholar]
- 130.Hosny A., Parmar C., Quackenbush J., Schwartz L.H., Aerts H.J. Artificial intelligence in radiology. Nat. Rev. Canc. 2018;18(8):500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sinha JSS G.R. Cognitive informatics, computer modelling, and cognitive science. Theory, Case Studies, and Applications: Elsevier. 2019;1 [Google Scholar]
- 132.Tang X. The role of artificial intelligence in medical imaging research. BJR| Open. 2019;2(1):20190031. doi: 10.1259/bjro.20190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saeian K., Rhyne T.L., Sagar K.B. Ultrasonic tissue characterization for diagnosis of acute myocardial infarction in the coronary care unit. Am. J. Cardiol. 1994;74(12):1211–1215. doi: 10.1016/0002-9149(94)90550-9. [DOI] [PubMed] [Google Scholar]
- 134.Mavrogeni S., Sfikakis P.P., Gialafos E., Bratis K., Karabela G., Stavropoulos E., Spiliotis G., Sfendouraki E., Panopoulos S., Bournia V. Cardiac tissue characterization and the diagnostic value of cardiovascular magnetic resonance in systemic connective tissue diseases. Arthritis Care Res. 2014;66(1):104–112. doi: 10.1002/acr.22181. [DOI] [PubMed] [Google Scholar]
- 135.Wu J., Pan J., Teng D., Xu X., Feng J., Chen Y.-C. Interpretation of CT signs of 2019 novel coronavirus (COVID-19) pneumonia. Eur. Radiol. 2020:1–8. doi: 10.1007/s00330-020-06915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alimadadi A., Aryal S., Manandhar I., Munroe P.B., Joe B., Cheng X. American Physiological Society. 2020. Artificial intelligence and machine learning to fight COVID-19. Bethesda, MD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vaishya R., Javaid M., Khan I.H., Haleem A. Clinical Research & Reviews; 2020. Artificial Intelligence (AI) Applications for COVID-19 Pandemic. Diabetes & Metabolic Syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jamthikar A., Gupta D., Saba L., Khanna N.N., Araki T., Viskovic K., Mavrogeni S., Laird J.R., Pareek G., Miner M., et al. Cardiovascular/stroke risk predictive calculators: a comparison between statistical and machine learning models. Cardiovasc. Diagn. Ther. 2020 doi: 10.21037/cdt.2020.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jamthikar A., Gupta D., Khanna N.N., Saba L., Laird J.R., Suri J.S. Cardiovascular/stroke risk prevention: a new machine learning framework integrating carotid ultrasound image-based phenotypes and its harmonics with conventional risk factors. Indian Heart J. 2020 Jun 18 doi: 10.1016/j.ihj.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Biswas M., Kuppili V., Edla D.R., Suri H.S., Saba L., Marinhoe R.T., Sanches J.M., Suri J.S. Symtosis: a liver ultrasound tissue characterization and risk stratification in optimized deep learning paradigm. Comput. Methods Progr. Biomed. 2017 doi: 10.1016/j.cmpb.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 141.Bishop C.M. springer; 2006. Pattern Recognition and Machine Learning. [Google Scholar]
- 142.LeCun Y., Bengio Y., Hinton G. Deep learning. nature. 2015;521(7553):436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 143.Suri J.S., Acharya U.R., Faust O., Alvin A.P.C., Sree S.V., Molinari F., Saba L., Nicolaides A. 2011. Symptomatic vs. Asymptomatic Plaque Classification in Carotid Ultrasound. [DOI] [PubMed] [Google Scholar]
- 144.Cortes C., Vapnik V. Support-vector networks. Mach. Learn. 1995;20(3):273–297. [Google Scholar]
- 145.Mirmehdi M. Imperial College Press; 2008. Handbook of Texture Analysis. [Google Scholar]
- 146.Bharati M.H., Liu J.J., MacGregor J.F. Image texture analysis: methods and comparisons. Chemometr. Intell. Lab. Syst. 2004;72(1):57–71. [Google Scholar]
- 147.Acharya U.R., Chua C.K., Lim T.-C., Dorithy, Suri J.S. Automatic identification of epileptic EEG signals using nonlinear parameters. J. Mech. Med. Biol. 2009;9(4):539–553. [Google Scholar]
- 148.Acharya U.R., Faust O., Sree S.V., Molinari F., Suri J.S. ThyroScreen system: high resolution ultrasound thyroid image characterization into benign and malignant classes using novel combination of texture and discrete wavelet transform. Comput. Methods Progr. Biomed. 2012;107(2):233–241. doi: 10.1016/j.cmpb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 149.Reynolds D.A. Gaussian mixture models. Encyclopedia of biometrics. 2009:741. [Google Scholar]
- 150.Huang D-s. Radial basis probabilistic neural networks: model and application. Int. J. Pattern Recogn. Artif. Intell. 1999;13(7):1083–1101. [Google Scholar]
- 151.Quinlan J.R. Ijcai. Citeseer; 1987. Generating production rules from decision trees; pp. 304–307. [Google Scholar]
- 152.Clark P.J., Evans F.C. Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology. 1954;35(4):445–453. [Google Scholar]
- 153.Rish I. IJCAI 2001 Workshop on Empirical Methods in Artificial Intelligence. 2001. An empirical study of the naive Bayes classifier; pp. 41–46. [Google Scholar]
- 154.Ross T.J. University of New Mexico; 2009. „‟ Fuzzy Logic with Engineering Applications‟‟ Wiley. [Google Scholar]
- 155.Kadyrov A., Petrou M. The trace transform and its applications. IEEE Trans. Pattern Anal. Mach. Intell. 2001;23(8):811–828. [Google Scholar]
- 156.Jawahar C., Ray A. Incorporation of gray-level imprecision in representation and processing of digital images. Pattern Recogn. Lett. 1996;17(5):541–546. [Google Scholar]
- 157.Galloway M.M. Texture analysis using grey level run lengths. STIN. 1974;75:18555. [Google Scholar]
- 158.Boi A., Jamthikar A.D., Saba L., Gupta D., Sharma A., Loi B., Laird J.R., Khanna N.N., Suri J.S. A survey on coronary atherosclerotic plaque tissue characterization in intravascular optical coherence tomography. Curr. Atherosclerosis Rep. 2018;20(7):33. doi: 10.1007/s11883-018-0736-8. [DOI] [PubMed] [Google Scholar]
- 159.Jamthikar A., Gupta D., Khanna N.N., Saba L., Araki T., Viskovic K., Suri H.S., Gupta A., Mavrogeni S., Turk M., et al. A low-cost machine learning-based cardiovascular/stroke risk assessment system: integration of conventional factors with image phenotypes. Cardiovasc. Diagn. Ther. 2019;9(5):420. doi: 10.21037/cdt.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jamthikar A., Gupta D., Khanna N.N., Araki T., Saba L., Nicolaides A., Sharma A., Omerzu T., Suri H.S., Gupta A., et al. A special report on changing trends in preventive stroke/cardiovascular risk assessment via B-mode ultrasonography. Curr. Atherosclerosis Rep. 2019;21(7):25. doi: 10.1007/s11883-019-0788-4. [DOI] [PubMed] [Google Scholar]
- 161.Viswanathan V., Jamthikar A.D., Gupta D., Puvvula A., Khanna N.N., Saba L., Viskovic K., Mavrogeni S., Laird J.R., Pareek G., Miner M. Angiology; 2020. Does the carotid bulb offer a better 10-year CVD/stroke risk assessment compared to the common carotid artery? A 1516 ultrasound scan study. 3319720941730. [DOI] [PubMed] [Google Scholar]
- 162.Khanna N.N., Jamthikar A.D., Gupta D., Piga M., Saba L., Carcassi C., Giannopoulos A.A., Nicolaides A., Laird J.R., Suri H.S., et al. Rheumatoid arthritis: atherosclerosis imaging and cardiovascular risk assessment using machine and deep learning–based tissue characterization. Curr. Atherosclerosis Rep. 2019;21(2):7. doi: 10.1007/s11883-019-0766-x. [DOI] [PubMed] [Google Scholar]
- 163.Long J., Shelhamer E., Darrell T. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2015. Fully convolutional networks for semantic segmentation; pp. 3431–3440. [DOI] [PubMed] [Google Scholar]
- 164.Biswas M., Kuppili V., Saba L., Edla D.R., Suri H.S., Sharma A., Cuadrado-Godia E., Laird J.R., Nicolaides A., Suri J.S. Deep learning fully convolution network for lumen characterization in diabetic patients using carotid ultrasound: a tool for stroke risk. Med. Biol. Eng. Comput. 2019;57(2):543–564. doi: 10.1007/s11517-018-1897-x. [DOI] [PubMed] [Google Scholar]
- 165.Saba L., Biswas M., Suri H.S., Viskovic K., Laird J.R., Cuadrado-Godia E., Nicolaides A., Khanna N., Viswanathan V., Suri J.S. Ultrasound-based carotid stenosis measurement and risk stratification in diabetic cohort: a deep learning paradigm. Cardiovasc. Diagn. Ther. 2019;9(5):439. doi: 10.21037/cdt.2019.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dong D., Tang Z., Wang S., Hui H., Gong L., Lu Y., Xue Z., Liao H., Chen F., Yang F. The role of imaging in the detection and management of COVID-19: a review. IEEE Reviews in Biomedical Engineering. 2020 doi: 10.1109/RBME.2020.2990959. [DOI] [PubMed] [Google Scholar]
- 167.Ito R.I.S., Naganawa S. A review on the use of artificial intelligence for medical imaging of the lungs of patients with coronavirus disease 2019. Diagn. Interventional Radiol. 2020 doi: 10.5152/dir.2019.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lu W., Zhang S., Chen B., Chen J., Xian J., Lin Y., Shan H., Su Z.Z. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus disease-19 (COVID-19) by bedside ultrasound. Ultraschall in der Medizin-European Journal of Ultrasound. 2020 doi: 10.1055/a-1154-8795. [DOI] [PubMed] [Google Scholar]
- 169.Kang H., Xia L., Yan F., Wan Z., Shi F., Yuan H., Jiang H., Wu D., Sui H., Zhang C. Diagnosis of coronavirus disease 2019 (covid-19) with structured latent multi-view representation learning. IEEE Trans. Med. Imag. 2020 doi: 10.1109/TMI.2020.2992546. [DOI] [PubMed] [Google Scholar]
- 170.Xinggang Wang X.D., Fu Qing, Zhou Qiang, Zhou Qiang, Feng Jiapei, Ma Hui, Liu Wenyu, Zheng Chuansheng. A weakly-supervised framework for COVID-19 classification and lesion localization from chest CT. IEEE Trans. Med. Imag. 2020 doi: 10.1109/TMI.2020.2995965. [DOI] [PubMed] [Google Scholar]
- 171.Krizhevsky A., Sutskever I., Hinton G.E. Advances in Neural Information Processing Systems. 2012. Imagenet classification with deep convolutional neural networks; pp. 1097–1105. [Google Scholar]
- 172.Szegedy C., Vanhoucke V., Ioffe S., Shlens J., Wojna Z. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2016. Rethinking the inception architecture for computer vision; pp. 2818–2826. [Google Scholar]
- 173.Wang K., Lu X., Zhou H., Gao Y., Zheng J., Tong M., Wu C., Liu C., Huang L., Jiang Ta. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2019;68(4):729–741. doi: 10.1136/gutjnl-2018-316204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang S., Zha Y., Li W., Wu Q., Li X., Niu M., Wang M., Qiu X., Li H., Yu H., Gong W. A fully automatic deep learning system for COVID-19 diagnostic and prognostic analysis. Eur. Respir. J. 2020 doi: 10.1183/13993003.00775-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang K., Liu X., Shen J., Li Z., Sang Y., Wu X., Zha Y., Liang W., Wang C., Wang K., Ye L. Clinically applicable AI system for accurate diagnosis, quantitative measurements, and prognosis. Cell. 2020 doi: 10.1016/j.cell.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li Z., Zhong Z., Li Y., Zhang T., Gao L., Jin D., Sun Y., Ye X., Yu L., Hu Z., Xiao J. medRxiv; 2020. From Community Acquired Pneumonia to COVID-19: A Deep Learning Based Method for Quantitative Analysis of COVID-19 on Thick-Section CT Scans. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen J., Wu L., Zhang J., Zhang L., Gong D., Zhao Y., Hu S., Wang Y., Hu X., Zheng B., Zhang K. Deep learning-based model for detecting 2019 novel coronavirus pneumonia on high-resolution computed tomography: a prospective study. MedRxiv. 2020 doi: 10.1038/s41598-020-76282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.CT Angel. Accessed July 24, 2020. http://121.40.75.149/znyx-ncov/index#/app/index.
- 179.Yang, S., Jiang, L., Cao, Z., Wang, L., Cao, J., Feng, R., Zhang, Z., Xue, X., Shi, Y. and Shan, F.,. Deep learning for detecting corona virus disease 2019 (COVID-19) on high-resolution computed tomography: a pilot study. Ann. Transl. Med., 8(7). 2020. [DOI] [PMC free article] [PubMed]
- 180.Oh Y., Park S., Ye J.C. Deep learning covid-19 features on cxr using limited training data sets. IEEE Trans. Med. Imag. 2020 doi: 10.1109/TMI.2020.2993291. [DOI] [PubMed] [Google Scholar]
- 181.Ren Z.H., Mu W.C., Huang S.Y. Design and optimization of a ring-pair permanent magnet array for head imaging in a low-field portable MRI system. IEEE Trans. Magn. 2018;55(1):1–8. [Google Scholar]
- 182.Cooley C.Z., Stockmann J.P., Armstrong B.D., Sarracanie M., Lev M.H., Rosen M.S., Wald L.L. Two‐dimensional imaging in a lightweight portable MRI scanner without gradient coils. Magn. Reson. Med. 2015;73(2):872–883. doi: 10.1002/mrm.25147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mirvis S.E. Use of portable CT in the R Adams Cowley Shock Trauma Center: experiences in the admitting area, ICU, and operating room. Surg. Clin. 1999;79(6):1317–1330. doi: 10.1016/s0039-6109(05)70080-3. [DOI] [PubMed] [Google Scholar]
- 184.Wang X., Bhatt D.L. COVID-19: an unintended force for medical revolution. J. Invasive Cardiol. 2020;32:E81–E82. [PubMed] [Google Scholar]
- 185.Thamman R., Gulati M., Narang A., Utengen A., Mamas M.A., Bhatt D.L. Twitter-based learning for continuing medical education? Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li L., Zhang Q., Wang X., Zhang J., Wang T., Gao T.-L., Duan W., Tsoi KK-f, Wang F.-Y. Characterizing the propagation of situational information in social media during COVID-19 epidemic: a case study on weibo. IEEE Transactions on Computational Social Systems. 2020;7(2):556–562. [Google Scholar]
- 187.El-Baz A., Suri J.S., editors. Big Data in Multimodal Medical Imaging. CRC Press; 2019. 2019. [Google Scholar]
- 188.Kooraki S., Hosseiny M., Myers L., Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the department of radiology should know. J. Am. Coll. Radiol. 2020 doi: 10.1016/j.jacr.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mossa-Basha M., Medverd J., Linnau K., Lynch J.B., Wener M.H., Kicska G., Staiger T., Sahani D. University of Washington. Radiology; 2020. Policies and Guidelines for COVID-19 Preparedness: Experiences from the. 201326. [DOI] [PubMed] [Google Scholar]
- 190.Buonsenso D., Piano A., Raffaelli F., Bonadia N., Donati K.D.G., Franceschi F. Novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]