Abstract
In light of the numerous US FDA-approved humanized monoclonal antibodies (mAbs) for cancer immunotherapy, it is surprising that the advancement of B-cell epitope vaccines designed to elicit a natural humoral polyclonal antibody response has not gained traction in the immune-oncology landscape. Passive immunotherapy with humanized mAbs (Trastuzumab [Herceptin®]; Pertuzumab [Perjeta®]) has provided clinical benefit to breast cancer patients, albeit with significant shortcomings including toxicity problems and resistance, high costs, sophisticated therapeutic regimen and long half-life. The role of B-cell humoral immunity in cancer is under appreciated and underdeveloped. We have advanced the idea of active immunotherapy with chimeric B-cell epitope peptides incorporating a ‘promiscuous’ T-cell epitope that elicits a polyclonal antibody response, which provides safe, cost–effective therapeutic advantage over mAbs. We have created a portfolio of validated B-cell peptide epitopes against multiple receptor tyrosine kinases (HER-1, HER-3, IGF-1R and VEGF). We have successfully translated two HER-2 combination B-cell peptide vaccines in Phase I and II clinical trials. We have recently developed an effective novel PD-1 vaccine. In this article, I will review our approaches and strategies that focus on B-cell epitope cancer vaccines.
Keywords: : B-cell epitopes, combination immunotherapy, CT-26/HER-2, immuno-oncology, PD-1, peptide cancer vaccines, syngeneic model
Graphical abstract
B-cell epitope peptide cancer immunotherapies. Cancer is a major cause of death in developed countries and world-wide, whereas in the USA cancer death is a close second to cardiovascular disease. The financial burden of this disease, and more importantly, the suffering it causes, is immense. There is an obvious and urgent need to speed the development and application of new, more efficacious anticancer therapies. The field of oncology is considerable and encompasses a number of indications. The current clinical landscape of immune-oncology (IO) since 2006, 3362 trials have been launched to test PD-1/PD-L1 monoclonal antibodies (mAbs) alone or in combination with other agents, and 2975 of them are still active as of September 2019 [1]. Although oncology continues to be one of the most active areas in terms of drug development, there is still a significant unmet need.
B-cell epitope vaccines have lagged behind in immunotherapeutic cancer strategies compared with T-cell vaccine attempts both in preclinical and clinical studies. There are only a few groups that have dedicated their research goals to advance B-cell epitope cancer vaccines. There are few if any reviews concerning B-cell epitope cancer vaccines except for our own reviews on the subject likely due to the paucity of research in this area as a majority of studies are focused on T-cell vaccines. I have summarized in a number of review articles our B-cell epitope cancer vaccine strategies on humoral immunity for the past two decades [2–5]. A more detailed review in Comprehensive Medicinal Chemistry III, Volume 6 (http://dx.doi.org/10.1016/B978-0-12-409547-2.12422-9) summarizes our work entitled ‘Peptide-Based Cancer Vaccines and Therapeutics for Solid Tumors Overexpressing HER-1, HER-2, HER-3, VEGF and IGF-1R.’ The precise role of B cells in humoral immunity and in the tumor microenvironment is beginning to be appreciated as it relates to a better understanding of their functions, and to the design of new immunotherapeutic strategies [6]. An excellent article on B-cell epitope-based vaccination therapy by Kametani et al., reviews the various strategies in this area of research and concludes that peptide vaccines that induce B-cell epitope-specific polyclonal antibodies may be useful against cancers that express high levels of antigens and that respond to passive antibody treatment [7]. A broad review on peptide-based vaccines in various diseases is summarized by Malonis and colleagues [8]. An excellent review on peptide materials for cancer immunotherapy discusses the various strategies being applied to the cancer landscape by Zhang and colleagues [9].
This article is not intended to cover a comprehensive assessment of the field of cancer vaccines or T-cell peptide vaccines in breast cancer and immunotherapy as there are numerous authoritative reviews that cover these topics in depth focusing entirely on T-cell vaccines, and immunotherapies with check point inhibitors. In this article, I hope to focus the readers and researchers in that there is an immediate opportunity to develop B-cell peptide-based epitope cancer vaccines reminiscent of vaccines for infectious diseases establishing a new urgent paradigm change for humoral immunity.
This article will summarize B-cell epitope-based vaccines for cancers that overexpress HER-2 in particular breast, colorectal and gastric cancers focusing exclusively on strategies to develop B-cell epitope vaccines for receptor tyrosine kinases (RTKs). I will discuss our more recent contribution in developing B-cell vaccines in IO with emphasis on checkpoint inhibitors (PD-1) and their combination with HER-2-based B-cell vaccines. In this article, I will briefly overview our HER-2 vaccine approaches from bench to the clinic and strategies to overcome resistance mechanism with development of other B-cell vaccines that could be used in combination. Finally, I will elucidate new IO strategies developing PD-1 B-cell epitope vaccines in combination with other vaccines (e.g., HER-2 vaccine).
Therapeutic cancer vaccines
Cancer vaccines targeting various antigens/oncogenes have been subject to a variety of strategies that include dendritic cell (DC) cell-based vaccines, peptide/protein vaccines, whole-cell, dendritic cell, and adoptive therapy, DNA and RNA vaccines and tumor cell vaccines. An excellent review by Hollingsworth and Jansen [10] summarizes the recent advances in the field of therapeutic vaccines. Therapeutic cancer vaccines have not provided clinical benefit except the ones targeting virus-associated cancers. Thus far, only a tiny handful of trials have shown significant impact for vaccines in patients with advanced cancer. Approval of the first therapeutic vaccine sipuleucel-T (Provenge by the FDA) for the treatment of prostate cancer [11] was obtained in 2010. The past failures of cancer vaccines are largely due, in part, to the fact that these trials have been conducted in severely compromised cancer patients who have received innumerable immune-suppressing chemotherapeutic regimens. Notwithstanding the complexity of the immune system, the intricate interplay within the tumor microenvironment, and an understanding of the depth of immune suppression and evasion which are just beginning to be appreciated may contribute to the successful design of future cancer vaccines. Additionally, cancer vaccines that have been tested in preclinical studies targeting tumor-associated antigens (TAAs), which are unmutated self-proteins overexpressed in tumor cells, thus necessitating breaking immune tolerance to elicit T-cell responses. The main hurdle that had to be overcome in developing peptide vaccines to TAAs is to ‘break tolerance’ through various methods such as strong adjuvants or repeated immunization. These strategies are further detailed in specialized reviews [12,13]. Recently, mutation-derived antigens (neoantigens) have been identified and utilized as targets for cancer vaccines. The realization these neoantigens may be more immunogenic that the previously identified CD8+ cytotoxic T lymphocytes (CTLs) of TAAs has invigorated the T-cell vaccine landscape.
Peptide T-cell-based cancer vaccines have not delivered on the promise
The development of peptide cancer vaccines has focused largely on strategies that induce cellular antitumor immune responses targeting CD8+ CTLs. Peptide-based cancer vaccines (comprehensively reviewed by Hirayama and Nishimura [14]) focused exclusively on CTLs epitopes identified by numerous predictive algorithms. Although these short peptides (9–10 amino acid) were able to elicit antitumor responses in vivo, their clinical benefit was largely ineffective in many Phase I and II clinical trials. More recently, the design of CD4+ T-helper cell vaccines has also been advanced to induce effective CD8+ antitumor responses. Long peptide vaccines (30 multimer) to include both helper and cytotoxic epitopes have also been proposed to be more effective immunogens than the CD8+ T-cell epitopes of 8–10 amino acid in length. The inclusion of CD4+ T-helper cell epitopes in the vaccine with longer peptides can provide some clinical benefit in a small number of cancer patients. There is an ongoing debate whether short peptides representing exact CD4+ or CD8+ T-cell epitopes should be used, or rather long peptides which need to be processed intracellularly before presentation on MHC molecules. Many of these peptide vaccines are undergoing clinical trials. T-cell cancer vaccines have been shown to have limited clinical impact despite being capable of generating human CD8+ T-cell responses to defined cancer antigens. The lack of effective T-cell-based peptide vaccines is likely due to the fact that these tumor-specific T cells were unable to cause regression and rejection of established cancers. A plethora of predictive algorithms to predict CTL epitopes (NetCTL.1.2; www.cbs.dtu.dk/services/NetCTL/) is an online server and a raft of software tools for predicting MHC (class I and II binders) are available to explore binding motifs (http://cancerimmunity.org/resources/webtools/). The recent advancements in the technological and bioinformatics fields enable computer-based approaches for rational design of peptide vaccines [15]. These predictive algorithms provide tools for identifying the best vaccine candidates. However, none of these algorithms has produced an effective T-cell-based peptide vaccine. The likelihood that single epitopes would be effective in a cancer vaccine is unlikely to be realized in the short term. A greater understanding of tumor immune suppression and failure of T-cell vaccination was provided by Allison and colleagues [16], who showed that blocking inhibitory checkpoint receptors on T cells could release limits on the activation and maintenance of T-cell effector function. Thus, the future development of successful cellular T-cell vaccines will likely depend on reversing tumor-induced T-cell dysfunction/exhaustion with agents targeting immune checkpoint blockade [17,18].
Current approved therapies are not ideal & have certain limitations: a case for alternatives
Over the past two decades, there has been a multitude of agents developed targeting oncogenic RTKs [19–21] and many of the FDA-approved therapies have been shown to exhibit significant toxicities [22–25]. There have been major advances in the treatment of HER-2-positive breast cancer since the introduction of FDA-approved anti-HER-2 mAbs: trastuzumab (Herceptin® [25]), followed by pertuzumab (Perjeta® [26,27]). Combined trastuzumab and pertuzumab has been shown to be a more effective therapeutic strategy in preclinical studies [28] as well as in Phase III clinical trials. Trastuzumab-DM1 (T-DM1) is a novel chemistry-driven conjugated HER-2 mAb in which the trastuzumab is conjugated with a fungal toxin DM1 (maytansine) is FDA approved [29]. Fam-trastuzumab deruxtecan-nxki (Enhurtu; DS820100 is a recent antibody-drug conjugate comprising three components: a humanized anti-HER-2 IgG1 mAb with the same amino acid sequence as trastuzumab; a topoisomerase I inhibitor payload, an exatecan derivative; and a tetrapeptide-based cleavable linker has been granted an accelerated approval by the FDA (23 December 2019) for treatment of adult patient with unresectable or metastatic HER-2-positive breast cancer who have received at least two prior lines of anti-HER-2-based regimens in the metastatic setting [30].
Many of the FDA-approved humanized mAbs have improved patient survival significantly. The success of Herceptin has engendered the development of other therapeutic antibodies such as rituximab (humanize anti-CD20); cetuximab (chimeric anti-HER-1 antibody) and bevacizumab (humanized anti-VEGF) antibody. Many of these mAbs exert the effects through several mechanisms such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC). More recently, the approval of several checkpoint mAbs such as nivolumab, pembrolizumab and atelozumab has created excitement in the field of IO.
Despite improvements and advances in current drugs treating metastatic breast cancer (MBC) many patients demonstrate disease progression and show problems of selectivity and efficacy, development of resistance and tolerability issues and unacceptable safety profiles that continue to hamper their clinical acceptance and remain a major therapeutic challenge. Novel combinations with improved clinical activity are therefore needed in patients with metastatic HER-2-positive breast cancer and represent an unmet clinical need. Thus, there has been an increasing demand for safer and newer immunotherapeutic approaches that exploits both the high specificity of vaccines targeting the adaptive immune response and the immunological memory.
Strengths of B-cell epitope peptide-based cancer vaccine
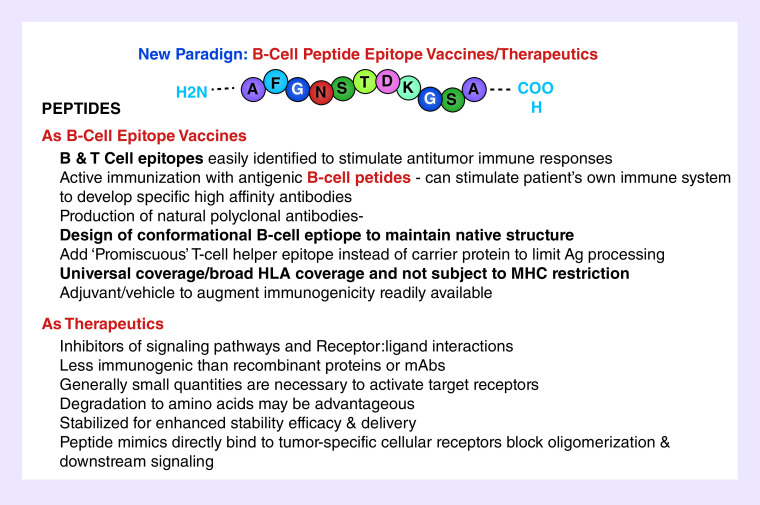
The advantages of peptide approaches are summarized in Table 1. In general, synthetic peptides are safe, easily synthesized and characterized, and are cost-effective and lack of toxicity. There are several advantages of peptide vaccines (B- or T-epitope-based vaccines) and therapeutics over other cancer vaccine approaches. Active immunotherapy offers many advantages, including tumor specificity and the activation of immune responses against antigens that are selectively expressed by tumor cells. Additional advantages of chimeric B- and T-cell vaccines are exquisite specificity and the potential for a durable treatment effect that can be recalled due to immunologic memory [2]. Therapeutic peptides have been advanced as anticancer agents from basic research to clinical studies and eventually to the pharmaceutical market as they exhibit high selectivity and affinity [31,32]. Peptide mimics, however, modulation of the immune system with peptide mimics as inhibitors could offer several advantages that might be complementary and potentially synergistic to mAb. To overcome the stability problem of the L-peptide inhibitors, retro-inverso D-amino acid peptides can be designed successfully to resist enzymatic degradation and represent potential therapeutic agents with long half-lives in vivo and even oral bioavailability [33–38]. The advantages and limitations of T-cell epitope vaccines have been extensive reviewed and addressed [39].
Table 1. . Advantages of B-& T-Cell Epitopes and Peptide Therapeutics.
| As peptide vaccines | As peptide therapeutics/T-cell vaccines |
|---|---|
| Safe, nontoxic, highly stable, cost effective | Safe and viable therapeutic goal |
| Easily manufactured/synthesized/characterized | Effective blocking signaling pathways |
| Epitopes are easily identified and predicted | High affinity, selectivity and potency |
| Break tumor tolerance | Retro-inverso D-amino acid peptides are stable |
| No oncogenic material included and minimal toxicity | Reduces off-target side effects |
| Administered by simple routes s.c, im injections | Large number of peptide-based drugs being marketed |
| Multi-epitope approach leads to broad antigen recognition and universal coverage | No accumulation in specific organs such as kidney and liver minimizing side-effects |
| High affinity, high specificity, strong potency and improved safety profiles | Increased bioavailability in vivo |
| Booster vaccinations | Water soluble, non-immunogenic, low cost production, enhanced shelf life and to easily cross tissue barriers |
| Elicits B and T cell memory responses | MHC class I & II T cell epitopes easily identified |
| Sustainable production of antibodies in vivo | Induction of effective CD8 or CD4 T cell responses in vivo by targeting immune checkpoint blockade |
| Clinical grade peptides easily synthesized for rapid translation into Phase I/II clinical trials | Easy monitoring of T cell responses |
There are currently several mAbs approved for the treatment of cancers that work by targeting different receptors or immune checkpoint. mAbs-targeting immunologic checkpoints and especially the PD-1/PD-L1 axis provided spectacular results in cancer therapy in the recent years [40]. The disadvantages and limitations of present immunotherapies are summarized in Table 2. Highly specific and successful therapeutic mAbs have been developed for many disease indications. There are some disadvantages to antibody drugs, such as production cost, stability and immunogenicity. Humanized mAbs approved for treatment of several cancers are fraught with a number of concerns. Antibody-based immunotherapies have several limitations such as high production cost of the antibodies. Treatment is expensive and has a limited duration of action, necessitating repeated administrations of the mAb. The half-life of IgG administered intravenously can range from 5 to 21 days. Thus, repeated treatments are necessary; patients typically receive the mAb every week to 3 weeks. The repeated treatment with mAb raises the cost of passive immunotherapy with this mAb to US$150,000 a year. Small molecules have begun to grow into another important treatment modality in this field, and have become an active research field in the cancer drug discovery in addition to antibodies, engineered cells and vaccines [41].
Table 2. . Disadvantages and Limitations of Present Immunotherapies.
| Humannized mAbs | Peptide therapeutics/T-cell vaccines | Small molecule RTKs |
|---|---|---|
| Poor penetration across tissues | In vivo instability, short half-life | Highly toxic, non-specific activity |
| Ineffective tumor targeting | Low bioavailability, susceptibility to proteases, formulation and manufacturing challenges | Serious side effects |
| Half life 12 days – requires weekly infusion | Class I MHC restriction limits relevance of individual peptides to certain HLA types | |
| Large quantities of hmAbs resulting intoxicity | Peptides with low affinity for MHC may be poorly immunogenic | |
| Treatment is very expensive | Immune responses transient and/or of low magnitude | |
| Cross-linking leads potential | Large number of peptides required to be useful across a wide range of patients | |
| Immunogenicity | Short peptides may bind directly to MHC which may induce tolerance | |
| Cardiotoxicity, GI perforation | ||
| No immunological memory | ||
| Treatment not a cure | ||
| Resistance to targeted therapies |
GI: Gastrointestinal; mAb: Monoclonal antibodies; RTK: Receptor tyrosine kinase.
Neoantigens: the new kid on the block
Recently, attention has shifted to neoantigens. Targeting an individual’s tumor-specific mutations is attractive because these peptides are new to the immune system and are not found in normal tissues. Compared with tumor-associated self-antigens, neoantigens elicit T-cell responses not subject to host central tolerance in the thymus and also produce fewer toxicities arising from autoimmune reactions to nonmalignant cells. Tumor-specific neoantigens are aggressively being explored as targets for personalized cancer vaccines. It has been reported that high mutational loads are strongly associated with increased tumor antigenicity (or immunogenic neoantigens) as well as high frequency of tumor-infiltrating lymphocytes such as CD8+ T cells. More recently, however, attention has shifted to neoantigens and the identification of neoepitopes to develop cancer vaccines has been suggested to hold great promise, but they have serious misgivings. It is a major challenge to develop cancer vaccines to neoantigens [42]. The promise, progress and challenges for improving neoantigen-targeted T-cell immunotherapies for cancer are discussed by Yamamoto et al. [43].
Immunotherapy makes a comeback: immune checkpoint blockade
Current enthusiasm about cancer immunotherapy stems from the success of some agents targeting immune checkpoint molecules such as PD-1 and CTLA-4 [44,45]. Recent advances in cancer immunology have documented the importance of T-cell-mediated antitumor immunity against human cancers, and inhibitory receptors expressed by T cells have become important targets for cancer immunotherapy. The development of humanized mAbs to checkpoint proteins to inhibit the suppressive effects on T-cell activity has provided the ability to induce prolonged remission in some patients with incurable solid and hematologic malignancies. Checkpoint inhibitor blockade with antibodies specific for CTLA-4 or PD-1 has shown remarkable clinical success in the treatment of cancer and demonstrated impressive activity across a broad set of cancer subtypes, even at advanced and metastatic stages of disease. While mAbs to CTLA-4 and PD-1/PD-L1 have produced remarkable and durable responses in a subset of patients, the majority of patients between 70 and 80% patients receiving anti-PD-1 therapy (nivolumab/pembrolizumab) remain resistant to monotherapy due to the complexity of resistance mechanisms and will not respond or will relapse, leaving a substantial unmet medical need. A plethora of reviews are available that summarizes the impact of checkpoint inhibitors on cancer vaccines which has now commandeered the oncology field [46,47]. With complex mechanisms of resistance limiting the efficacy of checkpoint inhibitor monotherapy, it is critical to develop combination approaches to allow more patients to benefit from immunotherapy.
HER-2 is an attractive target for immunotherapy
HER-2 is a 185-kDa protein and a member of the HER family of RTKs that includes EGFR (EGF receptor, erbB1), HER-3 (erbB3) and HER-4 (erbB4). HER-2 plays a major coordinating role in this network, since each receptor with a specific ligand seems to prefer HER-2 as its heterodimeric partner [48,49]. HER-2 containing heterodimers potently amplify signaling because HER-2 reduces the rate of ligand dissociation, allowing strong and prolonged activation of downstream signaling pathways [50,51], regulating cell growth and differentiation. This receptor plays a central role in the pathogenesis of several human cancers including breast, ovarian, renal, colon and lung carcinomas [52–56]. When overexpressed or mutated HER-2 forms homo- and hetero-dimers with other members of the EGFR family that results in the transduction of positive growth signals in a ligand-independent manner. HER-2 is amplified and overexpressed in about 20–30% of invasive breast cancers and overexpression is associated with aggressive disease and poor prognosis [57]. Thus, HER-2 is an attractive target for receptor-directed antitumor therapy.
Breast cancer is one of the leading causes of death around the world. In 2020, it is estimated by the American Cancer Society that 276,430 women will be diagnosed with breast cancer and 42,170 people will die as the consequence of this disease. The treatment landscape for MBC for HER-2-positive patients have a diverse number of regimens pioneered by use of humanized mAbs, mostly targeting RTKs such as the EGFRs (ErbB) and VEGF receptor (VEGFR) [58–67]. HER-2 overexpressing breast cancer represents about 20–30% of all breast cancer cases and is associated with markedly aggressive forms of cancer with a worse prognosis [57,68–70].
HER-2 B-cell epitope peptide vaccines approaches: preclinical & clinical
Over the past two decades, we have been developing novel combination HER-2 peptide vaccines [71–74] that are safe, tolerable and efficacious. Extensive preclinical data conducted over two decades in our labs have established the strategy of engineering conformational B-cell epitope vaccines [75–78], designed to stimulate a patient’s immune system to produce its own antibodies (natural) to a known and validated target for cancer such as RTK receptors. Our chimeric B-cell vaccines incorporating ‘promiscuous’ T-cell epitopes [79,80], unlike T-cell vaccines can be used for all patient types irrespective of their ‘haplotypes’ an issue that impacts T-cell vaccines. Additionally, B-cell vaccines unlike T-cell vaccines are not dependent on inhibiting checkpoint inhibitors to enhance immune responses. The ensuing polyclonal Abs produce a more powerful antitumor effect that is long lasting and inhibits tumor recurrence.
Prediction of B-cell epitopes & computer-assisted approaches to rational design of peptide vaccines
The selection of candidate B-cell epitopes expressed on the surface of a known protein sequence can be accomplished by an in-house (Peptide Companion™, 5x.com) computer-aided analysis using six correlates of antigenicity reviewed by Kaumaya et al. [81]: The profiles of chain flexibility and mobility (Karplus and Schultz) [82]; hydropathy profiles (Kyte and Doolittle) [83]; hydrophilicity (Hopp and Woods) [84]; analysis of solvent exposure algorithm of Rose et al. [85]; protrusion indices (Thornton et al.) [86]; antigenicity (Welling et al.) [87]. The best scoring epitopes were further ranked by correlation with their secondary structural attributes; for example, an amphiphilic α-helical sequence or a β-turn loop regions are preferred over a random coil fragments. Computer programs by Chou and Fasman [88] and Novotny et al. [89] were used to predict the secondary structure (α-helix, β-strand/sheet, β-turn/loop, random coil) and α-helical amphiphilic moment. Finally, consideration was given to the individual amino acid sequence. Electrostatic ion pairs and helix dipole interaction in helical segment were also considered (e.g., hydrophobic/hydrophilic balance).
The recent advancements in the technological and bioinformatics fields enable computer-based approaches for prediction bioinformatics tools for epitope prediction [90]; A range of computational methods have been developed for predicting which of an antigen’s residues are likely to form part of a B-cell epitope: computational prediction of vaccine potential epitopes and 3D structure [91]; an analysis of B-cell epitope discontinuity [92], B-cell epitope identification and production of neutralizing murine antibodies [93]; structural analysis of B-cell epitopes in antibody:protein complexes [94]. In silico peptide development approaches to predict B-cell epitope online analysis resource at IEDB (www.iedb.org/) and http://tools.immuneepitope.org/toolsElliPro/.
Identification of HER-2 B-cell epitopes as potential vaccine candidates
Eight B-cell epitopes of HER-2 extracellular domain (ECD) were identified by antigenicity predictive algorithms (reviewed by Kaumaya et al. [81] in 2000). These peptide B-cell sequences were engineered with a measles virus fusion protein (MVF, amino acids 288–302) ‘promiscuous’ T-cell epitope as potential chimeric vaccines. A series of in vitro and in vivo studies identified two epitopes HER-2 (628–647) and HER-2 (316–339) [71,72] as viable vaccines. MVF-HER-2 (628–647) elicited exceptionally high antibody titers that bind the native HER-2 protein as assessed by immunoprecipitation, flow cytometry and indirect ELISA and in vivo studies in transgenic mice demonstrated that the 628–647 epitope had the highest activity by eliciting antibodies able to cause ADCC as measured by lysing overexpressing cell lines BT474 and SKBR3. The vaccine was effective in preventing mammary tumors [71] in proto-oncogene (NEU) transgenic mice. Similarly, the MVF-316-339 elicited high-specific antibodies to the native HER-2 that caused inhibition of phosphorylation. The most effective combination vaccine HER-2 sequences 316–339 and 628–647 [72] elicited the highest titers and also caused the highest receptor downmodulation, similar to that produced by the control antibody HER-2 mAb L26, able induce a higher amount of IFN-γ in the presence of effector human peripheral blood mononuclear cell (PBMCs) compared with single epitope vaccines. The efficacy of the combo vaccine was shown by in vivo tumor protection in a BALB/c syngeneic model challenged with human HER-2 (RENCA/lacZ/HER-2). The most effective combination vaccine was found to be identified entirely serendipitously through our peptide strategy. It turns out later that these two sequences overlapped the pertuzumab- and trastuzumab-binding sites of HER-2. We initiated a Phase I clinical trial with a combination of these two peptides in 2002 at the Ohio State University Comprehensive Cancer Center.
Other B-cell epitope peptide HER-2 vaccines
In 2003, the Wiedermann group in Vienna was also developing HER-2 peptide-based vaccines in similar fashion by computer-aided analyses that identified seven putative B-cell epitopes of HER-2 [95]. These peptide epitopes were coupled to tetanus toxoid and used for immunization in BALB/c mice. Among these peptides, immunizations with two single peptides or a combination of two peptides induced antipeptide antibody levels, primarily of the IgG1 isotype. It was confirmed that immunization with HER-2 peptides successfully induced humoral immune response with antitumor activity in an animal model. A Phase I clinical trial with an anti-HER-2 vaccine-construct of immune-potentiating reconstituted influenza virosomes with the three peptides in patients with MBC [96]. The HER-2 multipeptide vaccine was safe, well tolerated and effective in overcoming immunological tolerance to HER-2. More recently, Wiedermann group developed a new vaccine: three short single peptides (P4, P6 and P7) representing different HER-2 ECD B-cell epitopes fused as a single hybrid peptide P467 on either virosomes or to diphtheria toxoid CRM197 (CRM) [97]. The formulation P467-CRM-Montanide induced higher serum IgG antibody titers, compared with P467-CRM-Alum. Fusion of the B-cell peptides has led to additional generation of CD4 T-cell epitopes, and this P467-multiepitope vaccine was found to induce polyclonal antibody responses with antiproliferative capacity against Her-2/neu. The hybrid vaccine together with Montanide induced higher and long-lasting antibody levels, Th1-biased cellular responses being superior to vaccination with the single B-cell peptides. This vaccine formulation is now planned to be evaluated in a Phase Ib/II study in HER-2 overexpressing cancer patients (IMU-131 [HerVaxx]). Another group led by Mahdavi and colleagues [98] has also designed a discontinuous chimeric peptide representing B- and T-cell epitopes from subdomain III of HER-2-ECD following design principles we have advanced. The author’s claim their findings can be applied for mAb production targeting the distinct epitope of HER-2 receptor compared with the two broadly used anti-HER-2 mAbs, Herceptin and Perjeta.
Other B-cell epitope approaches targeting VEGF & EGFR
VEGF B-cell vaccine
Wentink and colleagues [99] designed 3D-structured peptide mimicking the VEGF β5-turn-β6 loop-binding site of bevacizumab-elicited neutralizing antipeptide antibodies. Similar to our work in designing conformational VEGF vaccine, VEGF peptide mimics as well as combination VEGF + HER-2 vaccine by Kaumaya et al. [100–103], they demonstrated that structured B-cell epitope vaccine was superior to the linear unstructured peptides. This work is an independent proof and confirmation that B-cell epitope-based vaccines are often required to elicit high affinity polyclonal antibodies that are efficacious in vivo. This vaccine is currently being investigated in a Phase I clinical trial (NCT02237638) to demonstrate the potential to outperform anti-VEGF treatment strategies.
EGFR-based vaccine
Using a mimotope approach, two peptides were identified and epitope-specific immunization has produced an effective anti-EGFR immunotherapy that can elicit the production of ‘cetuximab-like’ antibodies in vivo [104]. The two peptides are capable of mimicking the conformational structure of EGFR-panitumumab-binding sites inducing both humoral and cellular immune responses against EGFR, could serve as candidate vaccines for active immunotherapy against EGFR-positive cancers. Zhu et al. [105] targeting the dimer interface of EGFR in patients synthesized a chimeric peptide, comprising a linear B-cell epitope peptide from the highly conservative β-hairpin loop of dimer interface of human EGFR (EGFR 237–267) and a ‘promiscuous’ Th-cell epitope MVF from the MVF protein. These peptides were highly immunogenic, stimulated high production of antibodies in animal models, and significantly inhibited tumor growth in patients. The chimeric peptide immunization was able to significantly inhibit the growth of subcutaneously transplanted LLC cells in C57BL6 mice. Therefore, the MVF-EGFR 237–267 construct represents a promising candidate for active anti-EGFR immunotherapy and provides a novel targeting strategy for the anti-EGFR therapy.
CIMAvax-EGF vaccine
Another group led by Garcia and colleagues in Cuba has designed EGF-based cancer vaccine (CIMAvax EGF®) conjugate of human recombinant EGF with the P64K protein of Neisseria meningitides (acting as a carrier protein) [106]. The vaccine was designed to induce specific anti-EGF antibodies with the Montanide ISA 51, as adjuvant. The vaccine induced antibodies against EGF that results in EGF withdrawal. CIMAvax-EGF has been demonstrated to be safe and immunogenic in advanced non-small-cell lung cancer (NSCLC) patients (reviewed by Saavedra and colleagues [107]). CIMAvax-EGF vaccine is an innovative immunotherapy that exerts its anticancer activity by inducing a B-cell response. Through an historic partnership with Cuba’s Centro de Inmunología Molecular, or CIM, Roswell Park is helping to develop several innovative and potentially life-saving cancer therapies. The first of these new approaches to be available to US patients is CIMAvax-EGF, an immunotherapy for lung cancer.
Several clinical trial are ongoing to explore the combination of CIMAvax and anti-PD-1 antibodies. Final results from the first US clinical study of a Cuban immunotherapy show that CIMAvax-EGF, a treatment targeting a particular cancer survival protein, EGF, is safe and showed promising efficacy as part of a treatment combination with nivolumab (Opdivo) in patients with advanced NSCLC. The team reports that the combination of these two immunotherapies was safe and well tolerated. They observed promising efficacy in patients whose tumors have low PD-L1 expression and who would not, therefore, be likely to respond well to nivolumab alone. A Phase II study (NCT02955290) is ongoing at Roswell Park, NY, USA, now expanded to include two additional groups of participants as well as those with recurrent NSCLC: Patients with advanced, recurrent squamous-cell head and neck cancer, who will receive the CIMAvax-nivolumab combination. Patients with advanced NSCLC evidencing high PD-L1 levels, who will receive CIMAvax in combination with pembrolizumab (Keytruda®) as first-line or initial treatment for NSCLC. Nearly $4 million in donations is funding Roswell Park’s initial CIMAvax clinical trials. These studies are being conducted in collaboration with scientists from the CIM in Havana, Cuba, and innovative Immunotherapy Alliance, an historic biotech venture formed by Roswell Park and the CIM.
From bench to clinic: first-generation HER-2 B-cell epitope Phase I clinical trial
An NCI-funded (CA84356), OSU cancer IRB approved (2001C0108) and FDA approved (BB-IND-9803) Phase I clinical trial with a combination of two HER-2 Chimeric B-cell MVF 316–339 epitopes and MVF 628–647 (Figure 1) [108–111] emulsified with nor-MDP as adjuvant and ISA 720 vehicle successfully completed at the James Cancer Hospital. The trial evaluated the maximum-tolerated dose, safety profile and immunogenicity. Eligible patients with metastatic and/or recurrent solid tumors received three inoculations on days 1, 22 and 43 at doses of total peptide that ranged from 0.5 to 3.0 mg. 24 patients received three inoculations at the intended dose levels, which elicited antibodies able to recognize native HER-2 receptor and inhibited both the proliferation of HER-2-expressing cell lines and phosphorylation of the HER-2 protein. The maximum-tolerated dose was determined to be the highest dose level of 3.0 mg of the combination vaccine. There was a significant increase from dose level 1 (0.5 mg) to dose level 4 (3.0 mg) in HER-2-specific antibodies. Four patients (one each with adrenal, colon, ovarian and squamous cell carcinoma of unknown primary) were judged to have SD; two patients (one each with endometrial and ovarian cancer) had partial responses; and 11 patients had progressive disease. Patients with SD received 6-month boosts, and one patient received a 20-month boost. The combination vaccines were safe and effective in eliciting antibody responses in a subset of patients (62.5%) and were associated with no serious adverse events, autoimmune disease or cardiotoxicity. There was preliminary evidence of clinical activity in several patients.
Figure 1. . Preclinical second-generation conformational HER-2 peptides mimicking trastuzumab and pertuzumab-binding sites.
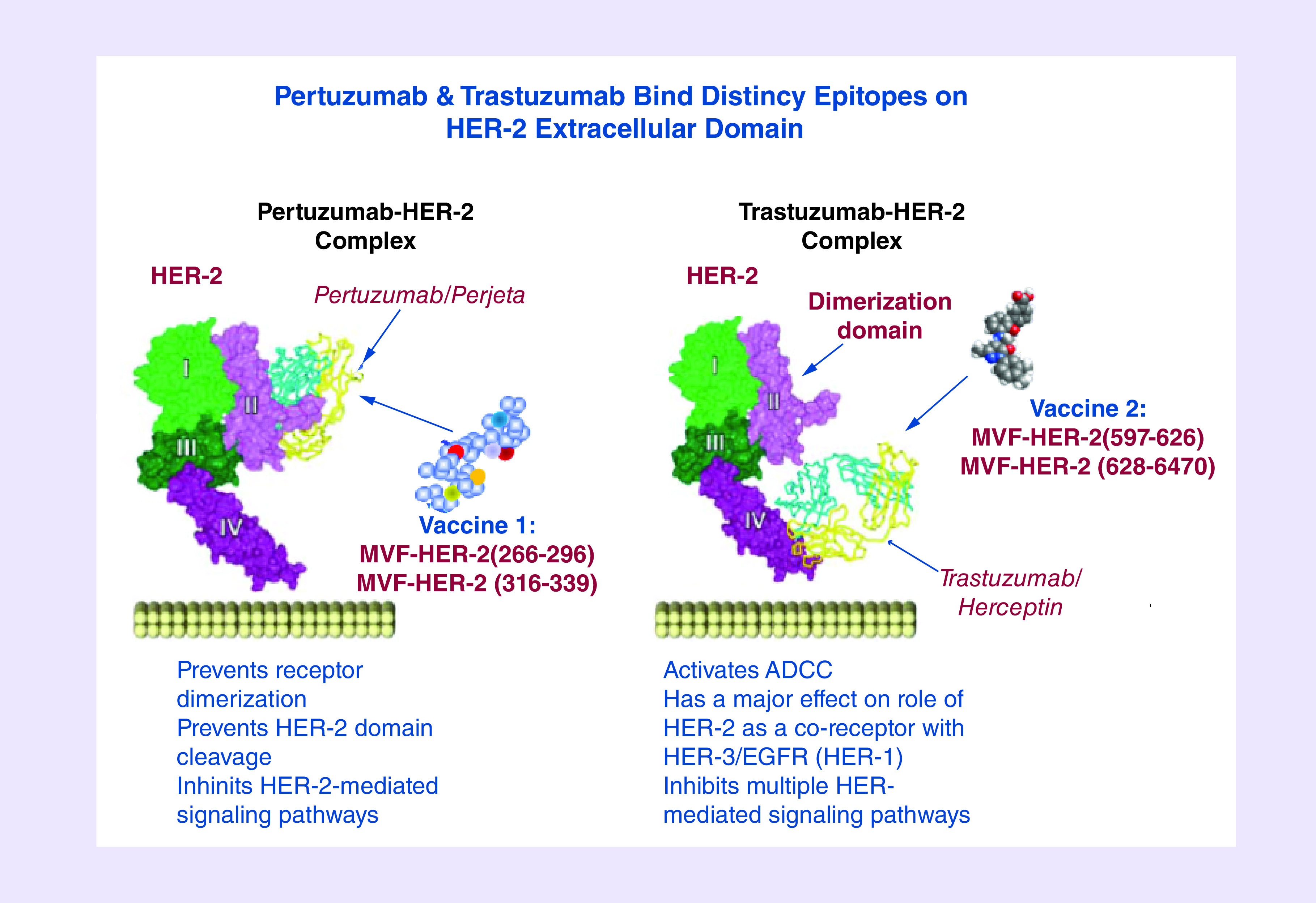
Peptide vaccine HER-2-binding sites
Preclinical second-generation conformational HER-2 peptides mimicking trastuzumab- and pertuzumab-binding sites
The x-ray structures of the HER-2-trastuzumab and -pertuzumab complexes [109–111] have led us to rationally design more effective HER-2 conformational epitope vaccines such as the trastuzumab-binding epitope (597–626) and the pertuzumab-binding epitope (266–296; Figure 1) [112] with potentially increased efficacy for preventing and inhibiting tumor growth.
Trastuzumab-binding conformational B-cell epitope
The 3D structure of the complex between human HER-2 and trastuzumab revealed that the region of HER-2-spaning residues 563–626 of the antigen-binding domain harbors a complex disulfide bonding pattern [109,110]. The structure of soluble HER-2-trastuzumab Fab complex showed that the trastuzumab-binding region is located on the C-terminus of the HER-2 ECD domain IV and this complex buries 1350 Å2 of the HER-2 surface with three loops residues 579–583, 615–625 and 592–595. In order to minimally dissect the interacting region of HER-2 binding domain, four synthetic peptides having with different levels of structural flexibility were designed and synthesized (75). The interacting loops in subdomain IV comprise residues in loop 1: 579–583 (two disulfide pairings between C563–C576, and between C567–C584), loop 2: 592–595 (cysteine disulfide pairing between C587–C596) and loop 3: 615–625 (cysteine disulfide between C600–C623). Chimeric peptides incorporating the MVF ‘promiscuous’ T-cell epitope via a four-residue linker sequence were synthesized, purified and characterized. All conformationally restricted peptides were recognized by trastuzumab and prevented the function of trastuzumab-inhibiting tumor cell proliferation, with 563–598 and 597–626 showing greater reactivity. All epitopes were immunogenic in FVB/n mice with antibodies against 597–626 and 613–626 recognizing HER-2. The 597–626 epitope was immunogenic in outbred rabbits eliciting antibodies which recognized HER-2, competed with trastuzumab for the same epitope, inhibited proliferation of HER-2-expressing breast cancer cells in vitro and caused their ADCC. Moreover, immunization with the 597–626 epitope significantly reduced tumor burden in transgenic BALB-neuT mice. Thus, the trastuzumab-like MVF-597–626 epitope can be included in a combination therapy with the pertuzumab-like MVF266–296 epitope.
Pertuzumab-binding conformational B-cell epitopes
The crystal structure of pertuzumab bound to the ECD of HER-2 elucidated the details of interacting region of residues 266–333 [111]. This structure provides a model in which pertuzumab sterically interferes with HER-2 dimerizing with other members of the HER family. We designed and studied the important binding sequences spanning residues 266–296, 298–333 and 315–333 to define the most biologically relevant conformational epitope that mimic the pertuzumab-binding conformational region for effective vaccination. We designed three conformational peptide constructs to mimic regions of the dimerization loop of the receptor and to characterize the in vitro and in vivo antitumor efficacy. Chimeric peptides incorporating the MVF ‘promiscuous’ T-cell epitope via a four-residue linker sequence were synthesized, purified and characterized [111]. All the constructs elicited high affinity antipeptide antibodies and all the antipeptide antibodies showed ADCC to varying degrees with the 266–296 constructs being equally effective as compared with trastuzumab. The MVF-266–296 elicited high-specific antibodies to the native HER-2 that caused inhibition of phosphorylation. The 266–296 peptide vaccine statistically reduced tumor onset in both transplantable tumor models (FVB/n and BALB/c) and significant reduction in tumor development in a transgenic mouse tumor model (Balb-neuT) confirming its validity to be included in a combination immunotherapy to be tested in a Phase I trial [113].
IND #14633 (Kaumaya); NIH CA18902; Phase I active immunotherapy trial with a combination of two chimeric HER-2 B-cell peptide vaccine MVF-HER-2 (597–626; trastuzumab-like) and MVF-HER-2 (266–296; pertuzumab-like) emulsified in ISA 720 and nor-MDP adjuvant in patients with advanced solid tumors. Patients were immunized with the vaccine constructs emulsified with nor-muramyl-dipeptide adjuvant in a water-in-oil Montanide ISA 720VG vehicle (Figure 2).
Figure 2. . Vaccination and Dose levels.
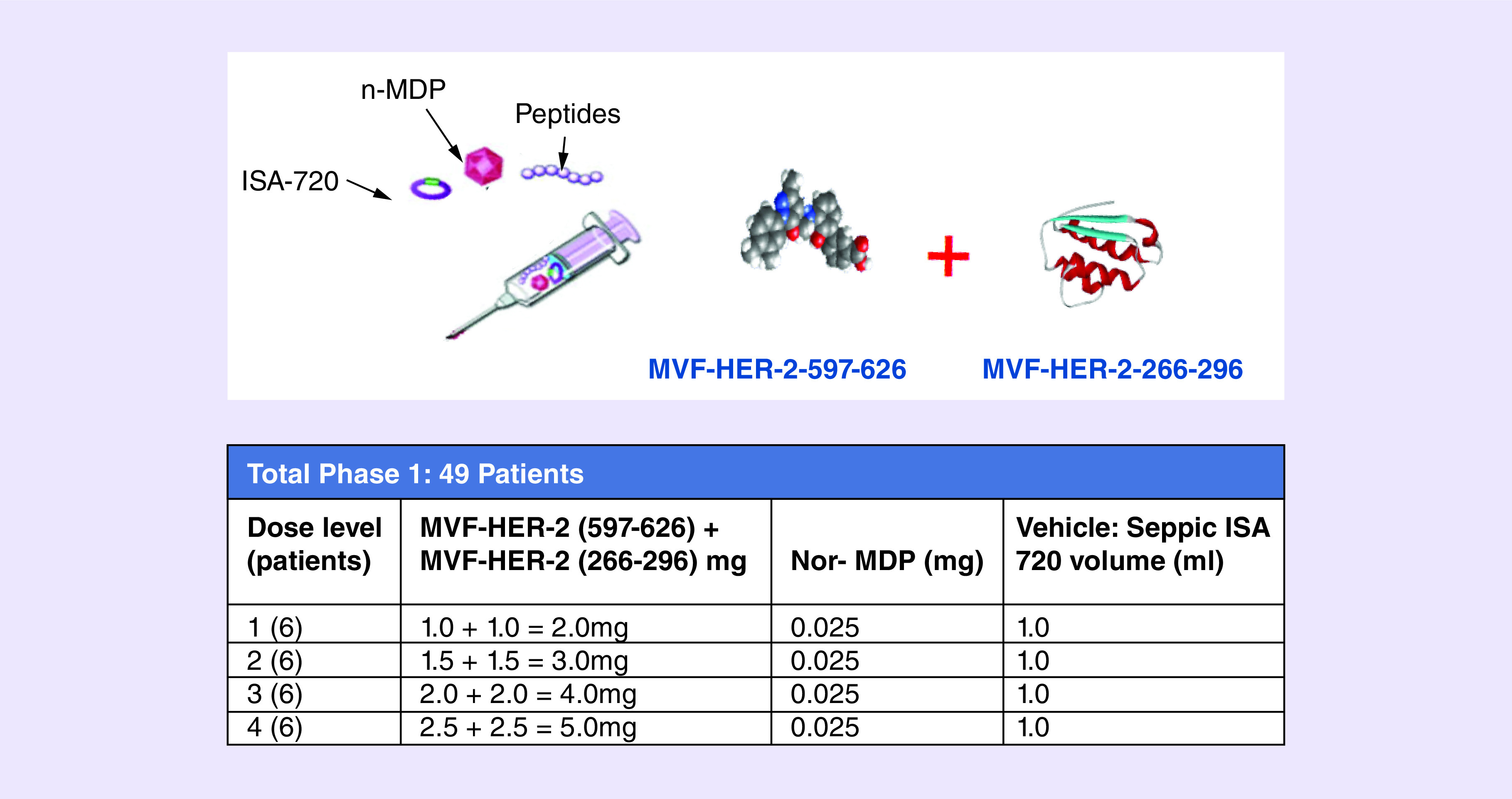
Eligible patients with metastatic and/or recurrent solid tumors received three inoculations every 3 weeks. The trial is a dose-escalating study consisting of four cohorts of six patients. Of the 49 patients with metastatic and/or recurrent solid tumors with a median of four prior lines of chemotherapy, only 28 patients completed the three vaccination regimens. No serious adverse reactions or dose-limiting toxicities were observed. The vaccine was well tolerated with dose level 2 as the recommended Phase II dose. The most common related toxicities in all patients were injection site reactions (24%). Two patients had a partial response, 14 had stable disease (SD) and 19 had progressive disease. Six patients received one 6-month boost, with one patient receiving as much as seven 6-month boosts. The study vaccine was safe, exhibited antitumor activity and showed preliminary indication that peptide vaccination may avoid therapeutic resistance and offer a promising alternative to mAb therapies. Given the initial promise, continuous development of the vaccine is ongoing in a Phase II trial at the suggested optimal biological dose (OBD) in a less heavily pretreated patient population in breast and/or gastrointestinal malignancies with HER-2/EGFR overexpression. The clinical data are summarized in the waterfall (Figure 3). One HER-2-positive patient received seven 6-monthly booster vaccinations suggesting that B-cell vaccination does not result in resistance to therapy as is well documented for other HER-2 therapies. A majority of the patient antibodies showed potent antitumor activity (induction of ADCC and apoptosis, inhibition of proliferation and phosphorylation).
Figure 3. . Phase I clinical trial HER-2 combination vaccine (B-Vaxx).
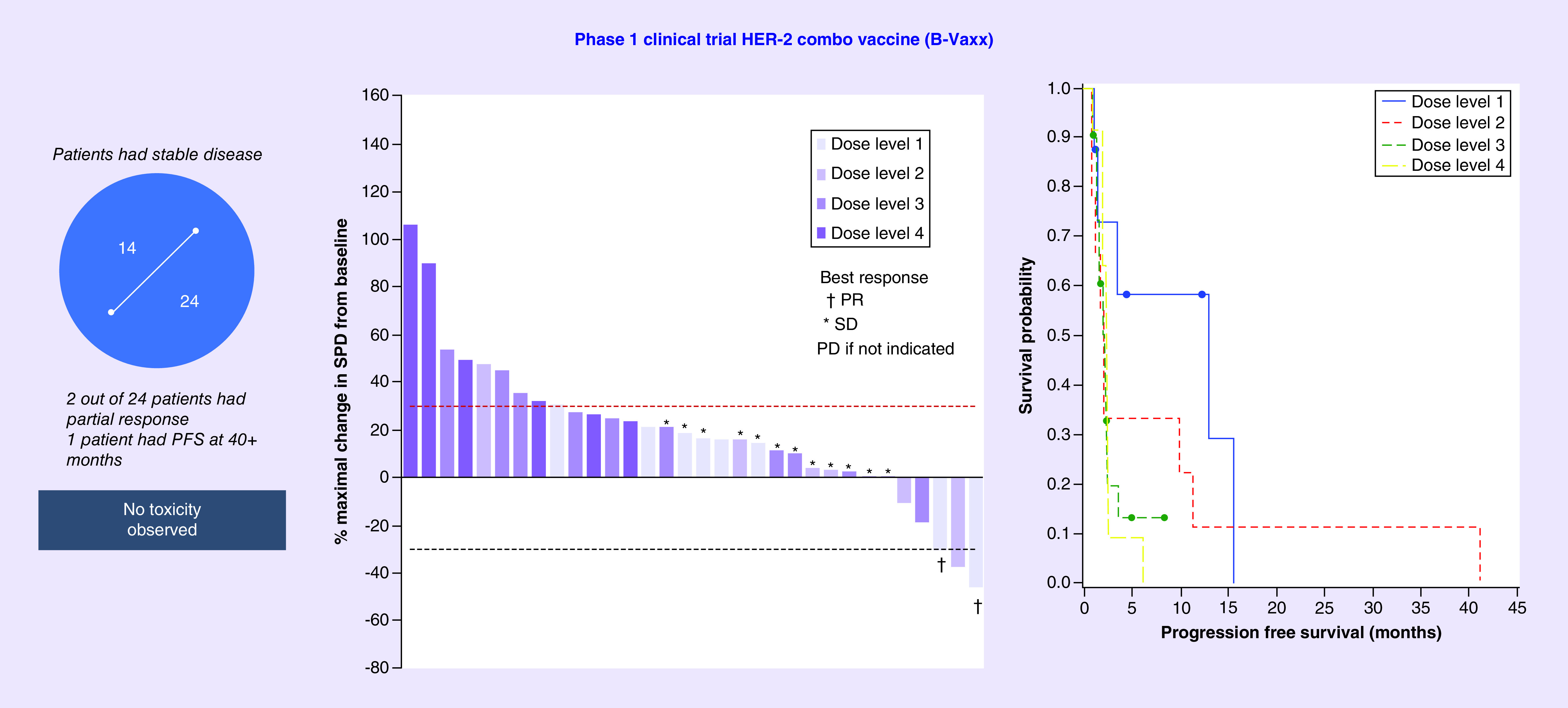
While Herceptin and Perjeta have been approved for clinical use, patients often develop resistance to these therapies. This study of the combination of the two HER-2 B-cell peptide vaccines to safely deliver curative and transformative cancer immunotherapies to advanced cancer patients is a validation of the effectiveness of B-cell immunotherapy strategies. This is a new paradigm in immunotherapy that focuses on humoral responses based on vaccination with conformational B-cell epitope vaccines comprising two chimeric HER-2 B-cell peptide vaccines incorporating a ‘promiscuous T-cell epitope.’
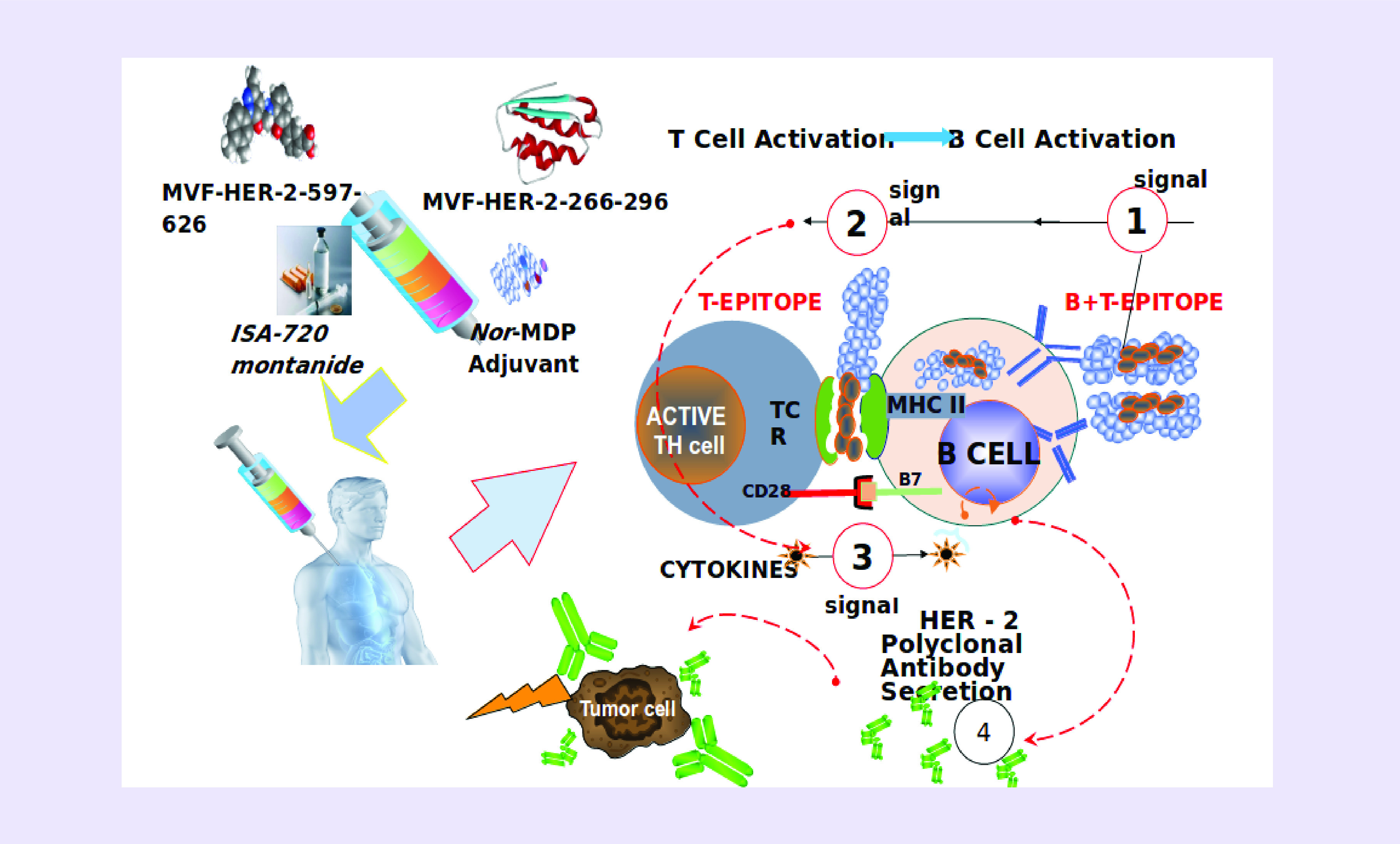
The vaccine works in innovative ways (Figure 4) in which the peptide vaccine is given intramuscularly at 3 weeks interval. The T-cell epitope of the chimeric construct binds MHC class II directly without processing activating the T cell with cytokine release that helps the B cell to produce natural polyclonal antibodies that can bind the tumor cell.
Figure 4. . The B-cell epitope peptide vaccine works in innovative ways.
Other HER-2 clinical trials
Clinical trial NCT02795988: A study of IMU-131 (HER-Vaxx) and chemotherapy compared with chemotherapy only in patients with HER-2-positive advanced gastric cancer. Imugene Ltd, an Australian immuno-oncology company announced comprehensive clinical data results from the Phase Ib clinical study of its HER-Vaxx anticancer vaccine in gastric cancer patients overexpressing HER-2 target protein. Imugene Ltd’s HER-Vaxx is a B-cell peptide cancer vaccine designed to treat tumors that overexpress the HER-2/neu receptor, such as gastric, breast, ovarian, lung and pancreatic cancers. This small study’s data in 68 patients showed a 100% objective response rate in three patients who received the optimal dose of 50 μg.
Clinical trial NCT02795988: A study of IMU-131 (HER-Vaxx) and chemotherapy compared with chemotherapy only in patients with HER-2-positive advanced gastric cancer. The Phase Ib study aims to determine the safety and tolerability of IMU-131 and identify the Recommended Phase II Dose of IMU-131 in combination with chemotherapy in HER-2/neu overexpressing ACS to carry into the Phase II dose-expansion study. The Phase II component is presently ongoing. Phase II will be designed to further characterize the safety and to explore clinical activity of IMU-131 in combination with chemotherapy in HER-2/neu overexpressing ACS. The Phase II study is a randomized, open-label comparison of IMU-131 plus standard of care chemotherapy versus standard of care chemotherapy alone.
Mechanisms of resistance to HER-2-targeted therapy
Cross-talk and compensatory signaling networks limit the activity of most targeted therapies, including HER-2-targeted agents. Multiple mechanisms of trastuzumab resistance have been proposed by Peake and Nahta [114]. The mechanisms through which trastuzumab blocks tumor growth include reduced downstream signaling, inhibition of angiogenesis and increased immune activity, primarily ADCC [115]. Other proposed mechanisms include Inhibition of homo- or hetero-dimerization, receptor downregulation through endocytosis, upregulation of HER-2 downstream signaling pathway. Signaling through an alternate receptor pathway, and failure to trigger immune-mediated mechanisms to destroy tumor cells.
The signaling networks consisting of IGF-1R, EGFR/HER-1, HER-2 and HER-3 play major roles in trastuzumab resistance [116–121]. Recent evidence also suggests that HER-3 also plays a central role and contributes to escape from therapeutic suppression by several tyrosine kinase inhibitors in breast cancer [2,122,123]. HER-3 has also been shown to be involved in acquired resistance to HER-2-targeted therapies and other inhibitors that directly or indirectly antagonize PI3K signaling [124,125]. Trastuzumab has been shown to be mediated by increased HER-3 signaling in part due to heterodimerization of HER-2 and HER-3 that is critical for the growth and progression of HER-2-positive breast cancers [2,122,123,126,127], or HER-2 and IG-1R and in part through the formation of heterotrimers involving HER-2, HER-3 and IGF-1R [117]. Increased expression of IGF-1R also reduces the growth inhibitory activity of trastuzumab [119]. The formation of a unique receptor complex containing IGF-1R and HER-2 in resistant cells facilitates cross-talk from IGF-1R to HER-2, resulting in sustained HER-2 phosphorylation in resistant cells [117,121,128].
Therapeutic strategies that target single molecular pathways eventually succumb to problems of intrinsic or acquired resistance due to extensive signaling ‘cross-talk.’ Targeting two or more receptors have the potentials to inhibit cross-talk and development of resistance that occurs in single treatment. For these reasons, we have targeted multiple signaling pathways that can offer hope, circumvent resistance mechanisms, provide synergy and enhance tumor effects. The combination of different vaccine and therapeutic strategies to target specific molecular pathways that are dysregulated in tumors may create clinical breakthroughs for safe and efficacious cancer cures.
The EGF receptors (HER-1, HER-2, HER-3 and HER-4), VEGF receptor (VEGFR) [58–67,129,130] and IGF receptor-1 (IGF-1R) [131,132] are members among RTKs. A plethora of FDA-approved agents targeted against RTK signaling pathways [19–21] are directed against HER-2 (trastuzumab, pertuzumab, lapatinib, Kadcyla [T-DM1] and Fam-trastuzumab deruxtecan-nxki), EGFR (cetuximab, gefitinib, erlotonib) or VEGF (bevacizumab, sunitinib). These agents have markedly improved survival but demonstrate significant toxicities [22–24].
We have developed novel approaches such as active immunization against RTKs that offer an alternative and effective treatment options (reviewed by Kaumaya [2]). The strategies focus on the design of chimeric B- & T-cell novel vaccines to HER-2 [71–74], VEGF [100], EGFR/HER-1 [133], HER-3 [134] and IGF-1R [123,134] specifically aimed at eliciting specific high affinity antibodies (B cell). Recently, we have also developed novel VEGF strategies and combination HER-2/VEGF [135,136]. We have identified crucial peptides that target HER-1, HER-2, HER-3 and IGF-1R; these peptides effectively reduce tumor growth in xenograft models of cancer. We have identified and validated the most effective combinations of EGFR (HER-1), HER-2, HER-3 and IGF-1R peptide vaccines/mimics to selectively inhibit multiple signaling pathways in rigorous in vitro studies.
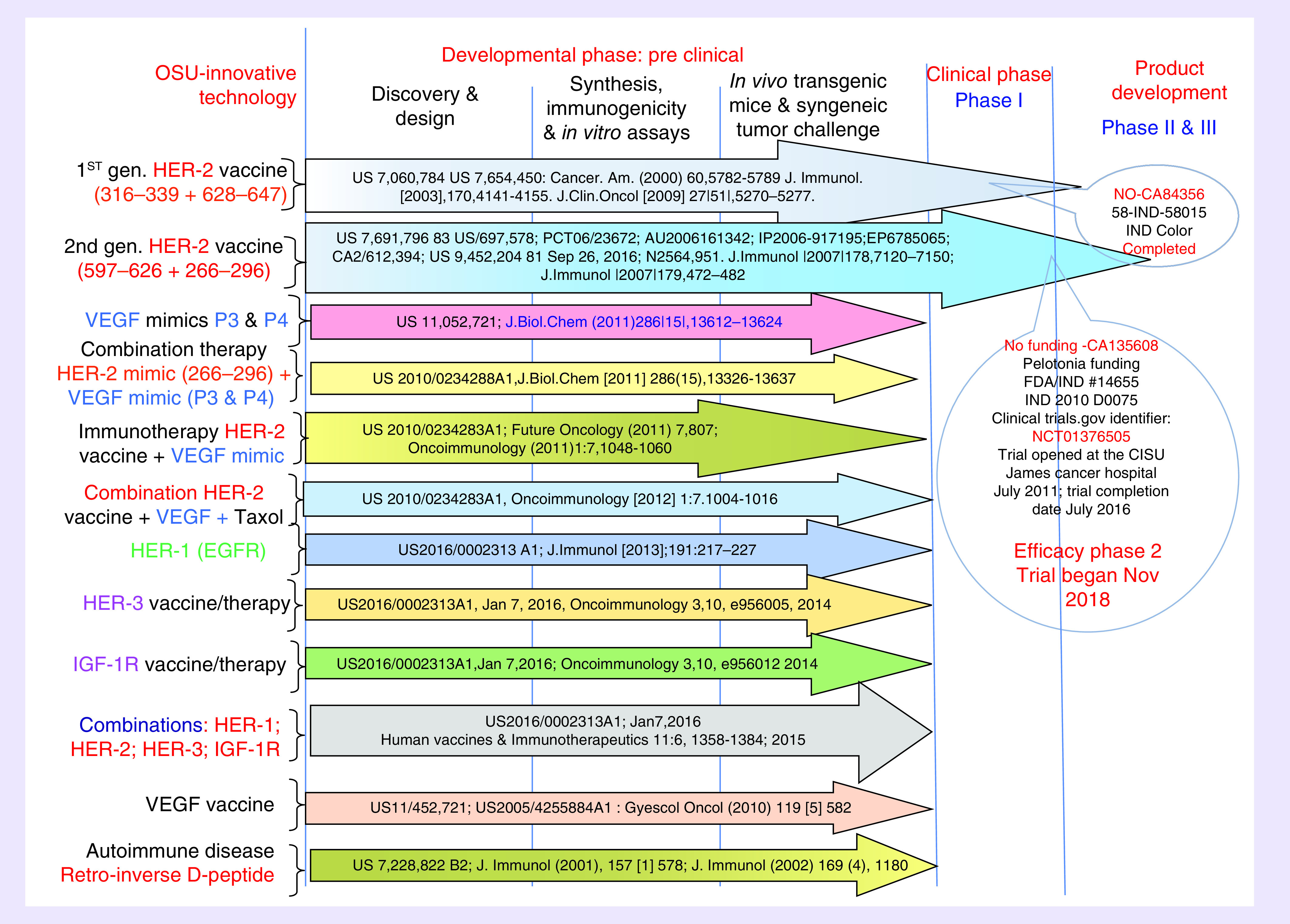
Having established a portfolio (Figure 5) of validated peptide epitopes as either vaccine candidates or peptide mimics, we have initiated a series of combination therapies to determine in vitro antitumor effects prior to verifying their efficacy in vivo in animal models. Ultimately, our goal was to identify the most biologically effective combinations of EGFR (HER-1), HER-2, HER-3 and/or IGF-1R peptide vaccines to selectively inhibit multiple receptors and signaling pathways to overcome the extensive receptor cross-talk that drives the biology and resistance of HER-overexpressing cancers. Optimal combinations of anti-HER-2 agents delivered with anyone of the following other growth factors such as HER-1, HER-3, VEGF and IGF-1R may provide the best therapy for breast cancers and other solid tumors including pancreatic, colon, gastrointestinal stromal tumor (GIST) and lung. This strategy holds the promise of achieving durable cures for multiple types of cancers that can be translated to human clinical trials.
Figure 5. . Peptide B-cell vaccine portfolio.
Combination immunotherapies to overcome resistance to targeted therapies
We have demonstrated that combination therapies with HER-2 and IGF-1R or HER-2 and HER-3 (BT-474 and JIMT-1) and HER-1 and IGF-1R (TNBC, MDA-MB-231) exhibit enhanced antitumor responses in breast cancer cell lines [134,137]:
HER-2 & IGF-1R in breast cancer
Resistance toward anti-HER-2 antibodies trastuzumab has been shown to be mediated by increased IGF-1R signaling [138,139]. Combination treatment with α-HER-2-(597–626) and α-IGF-1R-(56–81) peptide antibodies in trastuzumab-resistant (JIMT-1) and trastuzumab-sensitive (BT-474) human breast cancer cells inhibits proliferation, receptor phosphorylation and significantly induces apoptosis, ADCC and cellular invasion. These results indicate that cotargeting HER-2 and IGF-1R produce significant antitumor effects, synergistically blocks tumor growth of breast cancers and can be used to overcome trastuzumab resistance in breast cancer and points to the potential benefits of dual targeting. Cotargeting HER-2 and IGF-1R produces significant antitumor effects, and potentially overcomes trastuzumab resistance in breast cancer supporting the concept of dual targeting IGF-1R and HER-2 in this setting. Additionally, we also recently showed that combining the IGF-1R-56-81 peptide antibody with HER-2 mAb trastuzumab suppresses invasion and induces ADCC in JIMT-1 trastuzumab-resistant breast cancer cells [123], further supporting our dual strategy.
HER-2 & HER-3 in breast cancer
Dual-specific antibodies against HER-2:HER-3 or EGFR:HER-3 heterodimers are being evaluated [140,141], but none have yet been found to be useful. Our results show that combination treatment with HER-2 and HER-3 peptide vaccine antibodies in two different cell lines BT-474 breast cancer cell line and JIMT-1 a trastuzumab-resistant cell line caused an increased rate of inhibition of proliferation versus single treatments. Phosphorylated levels of HER-2 and HER-3 following combined treatment with both HER-2 and HER-3 peptide antibodies caused enhanced inhibition of phosphorylation as compared with individual treatment. Significant inhibition was achieved in the BT-474 breast cancer cell that has high HER-2 and HER-3 overexpression. Overall, the results point to the potential benefits of a combination approach targeting HER-3 and HER-2 in breast, pancreatic and colon cancers. Combination treatment with α-HER-2 and α-HER-3 peptide vaccine antibodies on cancer cell induced apoptosis and caused ADCC.
HER-1 & HER-2 in colon cancer
Human colorectal cancer (CRC) is one of the most common malignancies and remains largely incurable. HER-1 and HER-2 are highly implicated in CRCs [142,143], and cetuximab, a blocking anti-EGFR mAb, is effective in combination with chemotherapy or as single agent for the treatment of patients with KRAS wild-type metastatic CRC [144,145]. After an initial response, secondary resistance invariably ensues, thereby limiting the clinical benefit of this drug [146], leading to treatment failure. Combination treatment with anti-HER-1-418 and anti-HER-2-266 peptide vaccine antibodies in Caco-2 cells inhibited cell proliferation, inhibited receptor phosphorylation more than treatment with single-peptide antibody alone, and mediated ADCC using HT-29 colon cancer. Finally, we showed that HER-1 and HER-2 peptide antibodies were capable of inducing apoptosis in Caco-2 cells via a caspase activation assay, inhibited cell proliferation more than single treatment, corroborating our results obtained with the peptide antibodies
HER-1 in combination with HER-3 or IGF-1R human pancreatic cancer
An estimated 57,600 new cases of pancreatic cancer (PC) are expected to occur in the USA during 2020 with an estimated 47,050 deaths (American Cancer Society) [147]. Several studies demonstrate that HER-1 overexpression correlates with poor prognosis and increased metastasis of PCs [148]. Several recent studies also show that HER-3 is frequently upregulated in cancers with HER-1 (EGFR) overexpression. It is clear that innovative approaches are needed for the prevention and treatment of PC [149]. IGF-1R has also been implicated in the growth and development of multiple tumor types [150] and is expressed in 50–60% of PCs [151,152]. The IGF-1R:IGF-1 pathway is implicated in the development of resistance to anticancer drugs [131,153] as well as in PC [154]. HER-1 is implicated in aggressive PCs with poor patient outcome [155,156] and decrease overall survival [157–159]. Increased expression of IGF-1R increases expression of HER-1 and results in the formation of IGF-1R/HER-1 dimers [160,161]. There is considerable evidence of cross-talk between HER-1 and IGF-1R in PC cells [162]. In this aim, we will test the hypothesis that either combined inhibition of HER-1 and HER-3 or HER-1 and IGF-1R will enhance the inhibition of tumor growth in xenograft models of PCs.
HER-1 & IGF-1R
Combination treatment with HER-1-418 and IGF-1R-56 peptide antibodies in BxPC-3 pancreatic cells inhibited proliferation, receptor phosphorylation and significantly induced apoptosis, ADCC and cellular invasion. Previous studies in our laboratory established the HER-1-418 epitope as a novel inhibitor of HER-1-dependent signaling in vitro and in vivo [163]. These results indicate that cotargeting HER-1 and IGF-1R produces significant antitumor effects and is a promising approach for inhibiting PCs.
HER-1-418 & HER-3-461
Combination HER-3-461 + HER-1-418 peptide antibodies caused significant inhibition of proliferation in BxPC3 PC cells and decreased receptor phosphorylation, significantly delayed tumor growth in mice challenged with BxPC3 cells demonstrated synergistic antitumor effects and significantly induced apoptosis and ADCC. Significant apoptosis was observed with HER-3 peptide mimics in BxPC-3 cells; the peptide constructs HER-3 (461–479). These data provide strong rationale for cotargeting HER-1 and HER-3 in PCs.
The potential of B-cell peptide cancer vaccines
Taken together, the work summarized here strongly highlights the potential of B cells for vaccine immunotherapy and their applicability in a clinical setting. In a recent article, Wennhold et al. [164] surmised that it can be expected that the near future will see the first clinical trials of B-cell-based cancer vaccines. Wennhold et al. concluded that these trials will show if B cells deserve a place in the oncologist’s toolbox. We can argue that our work over the past three decades and translation of two HER-2 B-cell vaccine in a Phase I clinical trial in 2009 [108] and 2019 [165] is testament to the viability of B-cell cancer vaccines in our armamentarium of IO landscape.
The promise of IO: development of a novel PD-1 vaccine
Therapeutic blockade of the signaling axis between PD-1 and its ligand PD-L1 with mAbs such as pembrolizumab (Keytruda) and nivolumab (Optivo®) has shown remarkable clinical success in some cancer patients [166,167]. Such monotherapies have demonstrated impressive activity across a broad set of cancer subtypes, even at advanced and metastatic stages of disease [168–173]. However, 70–80% of patients receiving anti-PD-1 therapy, such as Keytruda and Optivo, remain resistant to this therapy and will not respond or will relapse, leaving a substantial unmet medical need [174]. PD-1/PD-L1-targeted mAbs are now the standard of care for 16 different types of cancer and tissue-agnostic indication. Since the first PD-1/PD-L1 trial landscape survey conducted in September 2017, 23 additional approvals have been granted to PD-1/PD-L1 mAbs by the FDA, and four new PD-1 mAbs have reached the market, bringing the total on the global market to nine [1].
Thus, it is clear that there is significant need to develop novel immune-based therapies that have the potential to circumvent mechanisms of resistance to achieve long-term control without causing toxicities associated with combination regimens. We have shown that vaccines to HER-2 can overcome many of the obstacles associated with mAb therapies. We therefore set out to develop a vaccine for PD-1 and combine it with our HER-2 vaccine. We have created and established the development of a novel B-cell peptide vaccine (PD1-Vaxx) with high immunogenicity that binds to human PD-1 and produces tumor inhibition in vivo in two animal models of colon cancer. The antitumor activity and toxicity profile was investigated in mice and beagles.
We designed several PD-1 vaccine B-cell epitopes using peptide mapping, predictive antigenicity algorithms and rational design based on 3D structure of PD-1/PD-L1 and PD-1/nivolumab/pembrolizumab. The vaccine was engineered into a B-cell chimeric vaccine based on the ectodomain of PD-1 linked to a ‘promiscuous’ T-helper cell MVF. The specificity of the selected PD-1 peptides to PD-L1 and nivolumab was demonstrated by surface plasmon resonance spectroscopy. The immunogenicity of the various individual peptide epitopes was then established in rabbits and Balb/c mice-eliciting antibodies that recognized the immunogenic sequences and the recombinant human PD-1. The transferable human colorectal CT-26 tumor model in syngeneic Balb/c mice was used to evaluate the effects of vaccination treatments with the four PD-1 MVF-peptide chimeras as inhibitors on the growth of CT-26 tumor cells. Only the 92–110 epitope (PD1-Vaxx) significantly reduced tumor growth compared with treatment with a mouse surrogate antagonist antibody anti-PD-1 mAb (29F.1A12).
Imugene presented the PD1-Vaxx Clinical Plan at the American Association for Cancer Research 2020 Annual Meeting scheduled for 27th–28th April 2020: VPO.CT01 – Phase I clinical trials from 9.00 am Eastern Daylight Time, USA, on Monday, 27th April. The abstract presentation is entitled ‘IMU-201-101 an open-label, multicenter, dose escalation/expansion, Phase I study of IMU-201 (PD1-Vaxx), a B-cell immunotherapy, in adults with NSCLC,’ and was authored by Professor P Kaumaya at the Ohio State University, Ohio, USA; Professor T Bekaii-Saab at the Mayo Clinic, Arizona, USA; Dr T Phan, at St Vincent’s Clinical School, UNSW, AUS, Dr M Marino, Dr N Ede & Dr A Good from Imugene Ltd.
The first-in-human, Phase I, multicenter, dose escalation study of PD1-Vaxx, is targeting patients with NSCLC and will be testing different doses of PD1-Vaxx as monotherapy and in combination with immune checkpoint inhibitors. The primary objective of the Phase I trial is to determine safety and an optimal biological dose as monotherapy and in combination with immune checkpoint inhibitors. Efficacy, tolerability and immune response will also be measured.
Combination IO vaccine strategies
Combinations of checkpoint-blocking antibodies are more efficacious than single inhibitors, but also cause greater immune-related toxicities [174]. A large body of data are evolving on the combinations of immune checkpoint inhibitors [175] with chemotherapy, radiotherapy or targeted therapies [176]. The emerging hypothesis is to move from pragmatic therapeutic combinations to one of rational design, based on the compatibility of mechanisms that can act synergistically. We then set out to combine the PD1-Vaxx with our B-Vaxx (combo HER-2) to examine whether we can obtain higher efficacy in syngeneic Balb/c model. In another colon carcinoma Balb/c model challenged with CT26/HER-2 cell line, PD1-Vaxx outperformed the industry-standard mouse anti-PD-1 antibody in a mouse model of HER-2-positive CRC. Combined triple vaccination (PD1-Vaxx and B-Vaxx) was more effective in the CT-26/HER-2 carcinoma cell line in syngeneic Balb/c that exhibited superior activity compared with the positive gold control antimouse PD-1 (CD279) mAb.
The preliminary results of the development of the PD-1 vaccine was presented at the 2019 AACR meeting (Atlanta) in Proceedings of the American Association for Cancer Research Annual Meeting 2019, March 29–April 3, Atlanta GA. Philadelphia (PA): AACR: Cancer Res 2019: 79 (13 Suppl): Abstract#1453; and also at the 2019 ESMO meeting (Barcelona) Annals Of Oncology (2019) 30 (Suppl_5): V475–V532. 10.1093/Annonc/Mdz253. As far as I know these results are the first combination of B-cell epitope peptide vaccine (HER-2) therapy with a vaccine developed for immune checkpoint inhibitor (PD-1) that acted synergistically to induce antitumor immune responses.
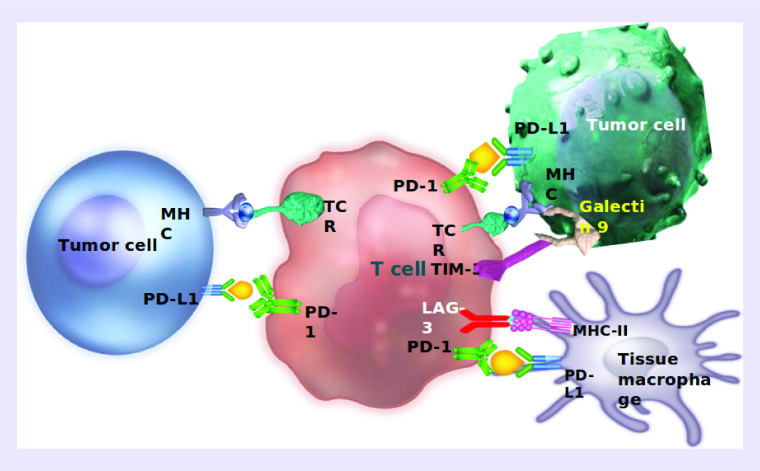
Future IO landscape
There is a need to develop novel vaccination strategies, using them in combination with targeted therapies or other immunotherapeutic agents, such as checkpoint inhibitors, in order to address tumor-induced immunosuppression [177]. The recent developments in IO have opened an unprecedented avenue for the emergence of vaccine strategies. Several other checkpoint molecules are under investigation, such as TIM-3 and LAG-3. TIM-3, as a checkpoint inhibitor, suppresses effector T-cell activation, whereas LAG-3 acts by binding to MHC molecules and also inhibits T-cell activation and proliferation [178,179]. LAG-3 is coexpressed with PD-1 on T cells, making it a suitable candidate for a combinatorial approach with anti-PD-1 agents. Antibodies against TIM-3 and LAG-3 are under clinical investigation showing encouraging efficacy. We are presently pursuing developing vaccines for TIM3 and LAG 3 that is being used in combination with PD1-Vaxx (Figure 6). In conclusion, the IO landscape is rapidly evolving and current enthusiasm will only surge in coming years with emerging novel approaches (Figure 7) that exhibit increased clinical efficacy, unique survival benefits and safety profiles with potential synergistic effects.
Figure 6. . Immuno-oncology and B-cell vaccine landscape.
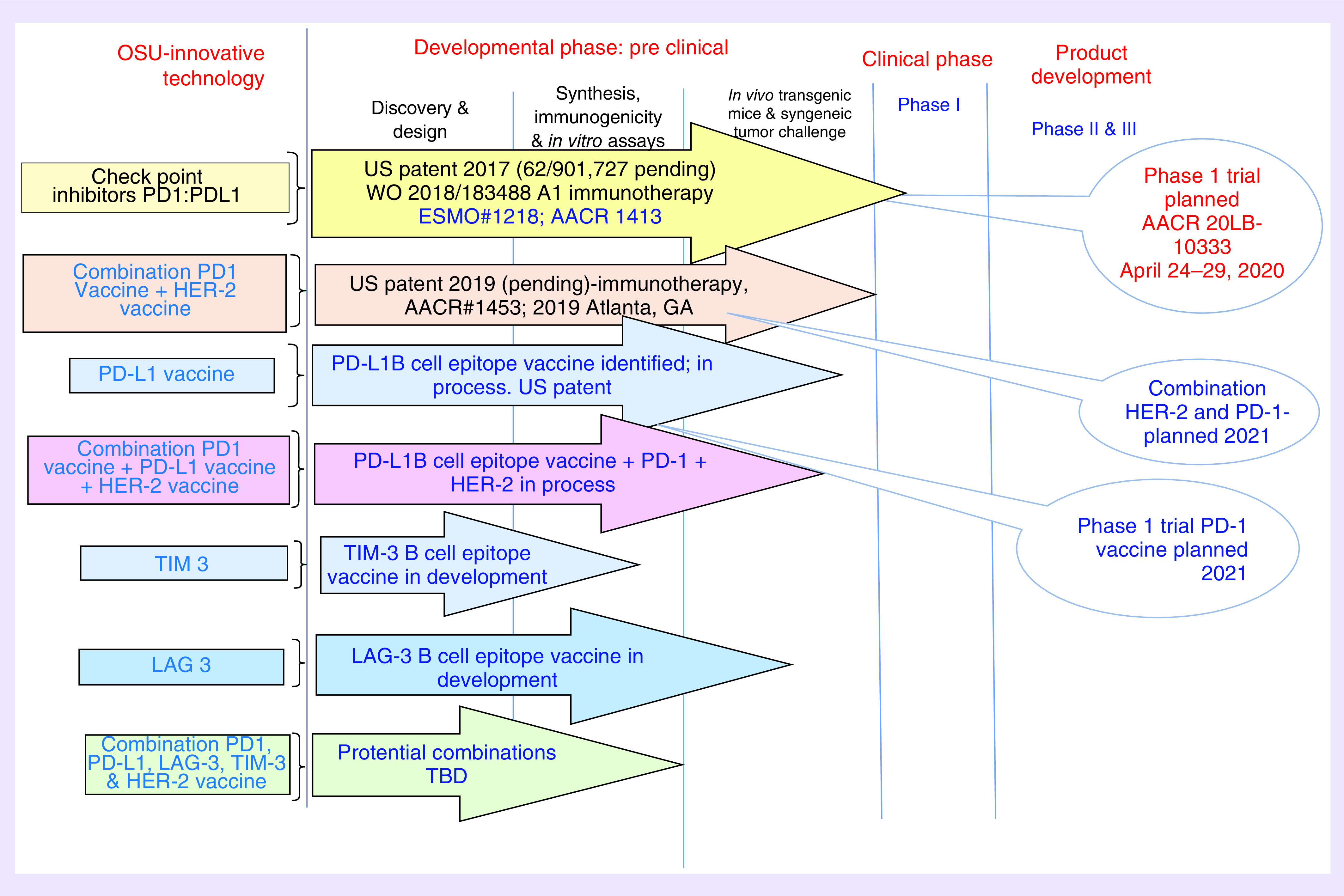
Figure 7. . Future immuno-oncology landscape: evolving approach to combination cancer therapy.
Executive summary.
Peptide-based vaccines have been used in the past with a limited clinical success. However, during the last few years, new knowledge has been provided on the biological characteristics of the peptides and their interaction with the immune system to be used in the clinic.
The role of B-cell humoral immunity in cancer is under appreciated and underdeveloped.
Cancer vaccines based on B-cell peptides are generally composed of an adjuvant and an immunogenic protein containing a B-cell epitope peptide that can induce B cells to create polyclonal antibodies.
Developed novel approaches such as active immunization against receptor tyrosine kinases that offer an alternative and effective treatment options.
The idea of active immunotherapy with chimeric B-cell epitope peptides incorporating a ‘promiscuous’ T-cell epitope that elicits a polyclonal antibody response provides safe, cost-effective therapeutic advantage over monoclonal antibodies.
B-Vaxx peptides were engineered to mimic conformational epitopes on the basis of those defined by the 3D structures of HER-2/pertuzumab and HER-2/trastuzumab complexes.
It is anticipated that combination therapy strategies will be the way forward for immunotherapy in breast cancer, with an improved understanding of tumor, microenvironment and host factors informing treatment combination decisions.
Current enthusiasm about cancer immunotherapy stems from the success of checkpoint inhibitor blockade with antibodies specific for CTL-4 or PD-1. However, 70–80% patients receiving anti-PD-1 therapy remain resistant to this therapy and will not respond or will relapse, leaving a substantial unmet medical need.
More than 3000 clinical trials are evaluating the clinical activity of the PD-1 checkpoint inhibitors as monotherapies and in combinations with other cancer therapies developed and implemented an effective novel PD-1 vaccine that when combined with our HER-2 vaccine acts synergistically to enhance immune mediated tumor killing in a syngeneic model.
Thus, it is clear that there is significant need to develop novel immune-based therapies that have the potential to circumvent mechanisms of resistance to achieve long-term control without causing toxicities associated with combination regimens.
There is a need to develop novel vaccination strategies, using them in combination with targeted therapies or other immunotherapeutic agents, such as checkpoint inhibitors, in order to address tumor-induced immunosuppression.
Combinations of checkpoint-blocking antibodies are more efficacious than single inhibitors, but also cause greater immune-related toxicities.
The emerging hypothesis is to move from pragmatic therapeutic combinations to one of rational design, based on the compatibility of mechanisms that can act synergistically.
Combine the PD1-Vaxx with our B-Vaxx (combo HER-2) to examine whether we can obtain higher efficacy in syngeneic Balb/c model. In another colon carcinoma Balb/c model challenged with CT-26/HER-2 cell line.
PD1-Vaxx outperformed the industry-standard mouse anti-PD-1 antibody in a mouse model of HER-2-positive colorectal cancer. Combined triple vaccination (PD1-Vaxx and B-Vaxx) was more effective in the CT-26/HER-2 carcinoma cell line in syngeneic Balb/c, which exhibited superior activity compared with the positive gold control antimouse PD-1 (CD279) monoclonal antibody.
Footnotes
Financial & competing interests disclosure
This study was partly funded by NIH R01CA84356, NIH R21CA13508 to PTP Kaumaya. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Xin Yu J, Hodge JP, Oliva C, Neftelinov ST, Hubbard-Lucey VM, Tang J. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat. Rev. Drug Discov. 19(3), 163–164 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Kaumaya PT. A paradigm shift: cancer therapy with peptide-based B-cell epitopes and peptide immunotherapeutics targeting multiple solid tumor types: emerging concepts and validation of combination immunotherapy. Hum. Vaccin. Immunother. 11(6), 1368–1386 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaumaya PT, Foy KC. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol. 8(8), 961–987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaumaya PT. Bridging oncology and immunology: expanding horizons with innovative peptide vaccines and peptidomimetics. Immunotherapy 5(11), 1159–1163 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Miller MJ, Foy KC, Kaumaya PT. Cancer immunotherapy: present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov. Med. 15(82), 166–176 (2013). [PubMed] [Google Scholar]
- 6.Largeot A, Pagano G, Gonder S, Moussay E, Paggetti J. The B-side of cancer immunity: the underrated tune. Cells 8(5), 449 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kametani Y, Miyamoto A, Tsuda B, Tokuda Y. B cell epitope-based vaccination therapy. Antibodies. 4, 225– 239 (2015). [Google Scholar]
- 8.Malonis RJ, Lai JR, Vergnolle O. Peptide-based vaccines: current progress and future challenges. Chem. Rev. 120(6), 3210–3229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Huang Y, Lindstrom AR, Lin TY, Lam KS, Li Y. Peptide-based materials for cancer immunotherapy. Theranostics 9(25), 7807–7825 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 4, 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantoff PW, Higano CS, Shore ND. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363(5), 411–422 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Wong KK, Li WA, Mooney DJ, Dranoff G. Advances in therapeutic cancer vaccines. Adv. Immunol. 130, 191–249 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 18(3), 168–182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirayama M, Nishimura Y. The present status and future prospects of peptide-based cancer vaccines. Int. Immunol. 28(7), 319–328 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Nandy A, Basak SC. A brief review of computer-assisted approaches to rational design of peptide vaccines. Int. J. Mol. Sci. 17(5), pii: E666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271(5256), 1734–1736 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell 33(4), 547–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxena M, Bhardwaj N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer 4(2), 119–137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol. 9(3), 1165–1172 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepard HM, Lewis GD, Sarup JC. et al. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J. Clin. Immunol. 11(3), 117–127 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Ryan AJ, Wedge SR. ZD6474–a novel inhibitor of VEGFR and EGFR tyrosine kinase activity. Br. J. Cancer 92(Suppl. 1), S6–S13 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B, Ogasawara AK, Yang R. et al. KDR (VEGF receptor 2) is the major mediator for the hypotensive effect of VEGF. Hypertension 39(6), 1095–1100 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur. J. Cancer 42(18), 3127–3139 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Grothey A. Recognizing and managing toxicities of molecular targeted therapies for colorectal cancer. Oncology 20(10 Suppl. 14), 21–28 (2006). [PubMed] [Google Scholar]
- 25.Carter P, Presta L, Gorman CM. et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl Acad. Sci. USA 89(10), 4285–4289 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agus DB, Akita RW, Fox WD. et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2(2), 127–137 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Agus DB, Gordon MS, Taylor C. et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J. Clin. Oncol. 23(11), 2534–43 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 64(7), 2343–2346 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Lewis Phillips GD, Li G, Dugger DL. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68(22), 9280–9290 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Modi S, Saura C, Yamashita T. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382(7), 610–621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boohaker RJ, Lee MW, Vishnubhotla P, Perez JM, Khaled AR. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 19(22), 3794–3804 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahrens VM, Bellmann-Sickert K, Beck-Sickinger AG. Peptides and peptide conjugates: therapeutics on the upward path. Future Med. Chem. 4(12), 1567–1586 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Taylor EM, Otero DA, Banks WA, O’Brien JS. Retro-inverso prosaptide peptides retain bioactivity, are stable in vivo, and are blood–brain barrier permeable. J. Pharmacol. Exp. Ther. 295(1), 190–194 (2000). [PubMed] [Google Scholar]
- 34.Srinivasan M, Wardrop RM, Gienapp IE, Stuckman SS, Whitacre CC, Kaumaya PT. A retro-inverso peptide mimic of CD28 encompassing the MYPPPY motif adopts a polyproline type II helix and inhibits encephalitogenic T cells in vitro. J. Immunol. 167(1), 578–585 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan M, Gienapp IE, Stuckman SS. et al. Suppression of experimental autoimmune encephalomyelitis using peptide mimics of CD28. J. Immunol. 169(4), 2180–2188 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Allen SD, Rawale SV, Whitacre CC, Kaumaya PT. Therapeutic peptidomimetic strategies for autoimmune diseases: costimulation blockade. J. Pept. Res. 65(6), 591–604 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Fischer PM. The design, synthesis and application of stereochemical and directional peptide isomers: a critical review. Curr. Protein Pept. Sci. 4(5), 339–356 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Fletcher MD, Campbell MM. Partially modified retro-inverso peptides: development, synthesis, and conformational behavior. Chem. Rev. 98(2), 763–796 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Slingluff CL., Jr The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 17(5), 343–350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 348(6230), 56–61 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Weinmann H. Cancer immunotherapy: selected targets and small-molecule modulators. ChemMedChem 11(5), 450–466 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Finn OJ, Rammensee HG. Is it possible to develop cancer vaccines to neoantigens, what are the major challenges, and how can these be overcome? Neoantigens: nothing new in spite of the name. Cold Spring Harb. Perspect. Biol. 10(11), a028829 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto TN, Kishton RJ, Restifo NP. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat. Med. 25(10), 1488–1499 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J. Clin. Invest. 125(9), 3377–3383 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27(4), 450–461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye Z, Qian Q, Jin H, Qian Q. Cancer vaccine: learning lessons from immune checkpoint inhibitors. J. Cancer 9(2), 263–268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cebon J. Perspective: cancer vaccines in the era of immune checkpoint blockade. Mamm. Genome 29(11–12), 703–713 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tzahar E, Waterman H, Chen X. et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 16(10), 5276–5287 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 16(7), 1647–1655 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beerli RR, Graus-Porta D, Woods-Cook K, Chen X, Yarden Y, Hynes NE. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol. Cell. Biol. 15(12), 6496–6505 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graus-Porta D, Beerli RR, Hynes NE. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol. Cell. Biol. 15(3), 1182–1191 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slamon DJ. Studies of the HER-2/neu proto-oncogene in human breast cancer. Cancer Invest. 8(2), 253 (1990). [DOI] [PubMed] [Google Scholar]
- 53.Cirisano FD, Karlan BY. The role of the HER-2/neu oncogene in gynecologic cancers. J. Soc. Gynecol. Investig. 3(3), 99–105 (1996). [PubMed] [Google Scholar]
- 54.Berchuck A, Rodriguez G, Kinney RB. et al. Overexpression of HER-2/neu in endometrial cancer is associated with advanced stage disease. Am. J. Obstet. Gynecol. 164(1 Pt 1), 15–21 (1991). [DOI] [PubMed] [Google Scholar]
- 55.Kern JA, Schwartz DA, Nordberg JE. et al. p185neu expression in human lung adenocarcinomas predicts shortened survival. Cancer Res. 50(16), 5184–5187 (1990). [PubMed] [Google Scholar]
- 56.Ward RL, Hawkins NJ, Coomber D, Disis ML. Antibody immunity to the HER-2/neu oncogenic protein in patients with colorectal cancer. Hum. Immunol. 60(6), 510–515 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785), 177–182 (1987). [DOI] [PubMed] [Google Scholar]
- 58.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J. Clin. Oncol. 23(11), 2445–2459 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science 312(5777), 1175–1178 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from Phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Pract. Oncol. 3(1), 24–40 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 5(12), 1806–1814 (1991). [DOI] [PubMed] [Google Scholar]
- 62.Cobleigh MA, Langmuir VK, Sledge GW. et al. A Phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin. Oncol. 30(16 Suppl. 5), 117–124 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5(5), 341–354 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 56(4), 549–580 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Zhu Z, Witte L. Inhibition of tumor growth and metastasis by targeting tumor-associated angiogenesis with antagonists to the receptors of vascular endothelial growth factor. Invest. New Drugs 17(3), 195–212 (1999). [DOI] [PubMed] [Google Scholar]
- 66.Oshima RG, Lesperance J, Munoz V. et al. Angiogenic acceleration of Neu induced mammary tumor progression and metastasis. Cancer Res. 64(1), 169–179 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285(21), 1182–1186 (1971). [DOI] [PubMed] [Google Scholar]
- 68.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim. Biophys. Acta 1198(2–3), 165–184 (1994). [DOI] [PubMed] [Google Scholar]
- 69.Scholl S, Beuzeboc P, Pouillart P. Targeting HER2 in other tumor types. Ann. Oncol. 12(Suppl. 1), S81–S87 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Slamon DJ, Godolphin W, Jones LA. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905), 707–712 (1989). [DOI] [PubMed] [Google Scholar]
- 71.Dakappagari NK, Douglas DB, Triozzi PL, Stevens VC, Kaumaya PT. Prevention of mammary tumors with a chimeric HER-2 B-cell epitope peptide vaccine. Cancer Res. 60(14), 3782–3789 (2000). [PubMed] [Google Scholar]
- 72.Dakappagari NK, Pyles J, Parihar R, Carson WE, Young DC, Kaumaya PT. A chimeric multi-human epidermal growth factor receptor-2 B cell epitope peptide vaccine mediates superior antitumor responses. J. Immunol. 170(8), 4242–4253 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Dakappagari NK, Sundaram R, Rawale S, Liner A, Galloway DR, Kaumaya PT. Intracellular delivery of a novel multiepitope peptide vaccine by an amphipathic peptide carrier enhances cytotoxic T-cell responses in HLA-A*201 mice. J. Pept. Res. 65(2), 189–199 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Dakappagari NK, Lute KD, Rawale S. et al. Conformational HER-2/neu B-cell epitope peptide vaccine designed to incorporate two native disulfide bonds enhances tumor cell binding and antitumor activities. J. Biol. Chem. 280(1), 54–63 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Garrett J, Rawale S, Allen S, Philips G,Forni G, Morris J. Novel engineered ttrastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER-2neu. J. Immunol. 178(11), 7120–7130 (1992). [DOI] [PubMed] [Google Scholar]
- 76.Kaumaya PT, Berndt KD, Heidorn DB, Trewhella J, Kezdy FJ, Goldberg E. Synthesis and biophysical characterization of engineered topographic immunogenic determinants with alpha alpha topology. Biochemistry 29(1), 13–23 (1990). [DOI] [PubMed] [Google Scholar]
- 77.Kobs-Conrad S, Lee H, DiGeorge AM, Kaumaya PTP. Engineered topographic determinants with αβ, βαβ, and βαβα topologies show high affinity binding to native protein antigen (lactate dehydrogenase-C4). J. Biol. Chem. 268(34), 25285–25295 (1993). [PubMed] [Google Scholar]
- 78.Lairmore MD, DiGeorge AM, Conrad SF, Trevino AV, Lal RB, Kaumaya PT. Human T-lymphotropic virus type 1 peptides in chimeric and multivalent constructs with promiscuous T-cell epitopes enhance immunogenicity and overcome genetic restriction. J. Virol. 69(10), 6077–6089 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaumaya PTP, Kobs-Conrad S, Seo Young H. et al. Peptide vaccines incorporating a ‘promiscuous’ T-cell epitope bypass certain haplotype restricted immune responses and provide broad spectrum immunogenicity. J. Mol. Recognit. 6(2), 81–94 (1993). [DOI] [PubMed] [Google Scholar]
- 80.Frangione-Beebe M, Albrecht B, Dakappagari N. et al. Enhanced immunogenicity of a conformational epitope of human T-lymphotropic virus type 1 using a novel chimeric peptide. Vaccine 19(9–10), 1068–1081 (2000). [DOI] [PubMed] [Google Scholar]
- 81.Kaumaya PTP, Kobs-Conrad S, DiGeorge AM, Stevens V. Denovo engineering of protein immunogenic & antigenic determinants. : Peptides. Anantharamaiah GMB C. (). Springer-Verlag, Heidelberg, Germany, 133–164 (1994). [Google Scholar]
- 82.Karplus PA, Schulz GE. Refined structure of glutathione reductase at 1.54 A resolution. J. Mol. Biol. 195(3), 701–729 (1987). [DOI] [PubMed] [Google Scholar]
- 83.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157(1), 105–132 (1982). [DOI] [PubMed] [Google Scholar]
- 84.Hopp TP, Woods KR. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl Acad. Sci. USA 78(6), 3824–3828 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rose GD, Geselowitz AR, Lesser GJ, Lee RH, Zehfus MH. Hydrophobicity of amino acid residues in globular proteins. Science 229(4716), 834–838 (1985). [DOI] [PubMed] [Google Scholar]
- 86.Thornton JM, Edwards MS, Taylor WR, Barlow DJ. Location of ‘continuous’ antigenic determinants in the protruding regions of proteins. EMBO J. 5(2), 409–413 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Welling GW, Weijer WJ, van der Zee R, Welling-Wester S. Prediction of sequential antigenic regions in proteins. FEBS Lett. 188(2), 215–218 (1985). [DOI] [PubMed] [Google Scholar]
- 88.Chou PY, Fasman GD. Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzymol. Relat. Areas Mol. Biol. 47, 45–148 (1978). [DOI] [PubMed] [Google Scholar]
- 89.Novotny J, Handschumacher M, Haber E. et al. Antigenic determinants in proteins coincide with surface regions accessible to large probes (antibody domains). Proc. Natl Acad. Sci. USA 83(2), 226–230 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soria-Guerra RE, Nieto-Gomez R, Govea-Alonso DO, Rosales-Mendoza S. An overview of bioinformatics tools for epitope prediction: implications on vaccine development. J. Biomed. Inform. 53, 405–414 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Tarek MM, Shafei AE, Ali MA, Mansour MM. Computational prediction of vaccine potential epitopes and 3-dimensional structure of XAGE-1b for non-small cell lung cancer immunotherapy. Biomed. J. 41(2), 118–128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sivalingam GN, Shepherd AJ. An analysis of B-cell epitope discontinuity. Mol. Immunol. 51(3–4), 304–309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kozlova EEG, Cerf L, Schneider FS. et al. Computational B-cell epitope identification and production of neutralizing murine antibodies against Atroxlysin-I. Sci. Rep. 8(1), 14904 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kringelum JV, Nielsen M, Padkjaer SB, Lund O. Structural analysis of B-cell epitopes in antibody:protein complexes. Mol. Immunol. 53(1–2), 24–34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jasinska J, Wagner S, Radauer C. et al. Inhibition of tumor cell growth by antibodies induced after vaccination with peptides derived from the extracellular domain of Her-2/neu. Int. J. Cancer 107(6), 976–983 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Wiedermann U, Wiltschke C, Jasinska J. et al. A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: a Phase I study. Breast Cancer Res. Treat. 119(3), 673–683 (2010). [DOI] [PubMed] [Google Scholar]
- 97.Tobias J, Jasinska J, Baier K. et al. Enhanced and long term immunogenicity of a Her-2/neu multi-epitope vaccine conjugated to the carrier CRM197 in conjunction with the adjuvant Montanide. BMC Cancer 17(1), 118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mahdavi M, Keyhanfar M, Jafarian A, Mohabatkar H, Rabbani M. Immunization with a novel chimeric peptide representing B and T cell epitopes from HER2 extracellular domain (HER2 ECD) for breast cancer. Tumour Biol. 35(12), 12049–12057 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Wentink MQ, Hackeng TM, Tabruyn SP. et al. Targeted vaccination against the bevacizumab binding site on VEGF using 3D-structured peptides elicits efficient antitumor activity. Proc. Natl Acad. Sci. USA 113(44), 12532–12537 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vicari D, Foy KC, Liotta EM, Kaumaya PTP. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J. Biol. Chem. 286(15), 13612–13625 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Foy KC, Liu Z, Phillips G, Miller M, Kaumaya PT. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J. Biol. Chem. 286(15), 13626–13637 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang B, Kaumaya PT, Cohn DE. Immunization with synthetic VEGF peptides in ovarian cancer. Gynecol. Oncol. 119(3), 564–570 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Foy KC, Miller MJ, Moldovan N, Carson WE 3rd>, Kaumaya PT. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. Oncoimmunology 1(7), 1048–1060 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang A, Cui M, Qu H. et al. Induction of anti-EGFR immune response with mimotopes identified from a phage display peptide library by panitumumab. Oncotarget 7(46), 75293–75306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu L, Zhao L, Wu M, Chen Z, Li H. B-cell epitope peptide vaccination targeting dimer interface of epidermal growth factor receptor (EGFR). Immunol. Lett. 153(1–2), 33–40 (2013). [DOI] [PubMed] [Google Scholar]
- 106.Garcia B, Neninger E, de la Torre A. et al. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin. Cancer Res. 14(3), 840–846 (2008). [DOI] [PubMed] [Google Scholar]
- 107.Saavedra D, Neninger E, Rodriguez C. et al. CIMAvax-EGF: toward long-term survival of advanced NSCLC. Semin. Oncol. 45(1–2), 34–40 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Kaumaya PT, Foy KC, Garrett J. et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J. Clin. Oncol. 27(31), 5270–5277 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cho HS, Mason K, Ramyar KX. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421(6924), 756–760 (2003). [DOI] [PubMed] [Google Scholar]
- 110.Garrett TP, McKern NM, Lou M. et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 11(2), 495–505 (2003). [DOI] [PubMed] [Google Scholar]
- 111.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5(4), 317–328 (2004). [DOI] [PubMed] [Google Scholar]
- 112.Allen SD, Garrett JT, Rawale SV. et al. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J. Immunol. 179(1), 472–482 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Kaumaya P. T. P, Wesolowski R, Ramaswamy B. et al. Phase I immunotherapy trial with two chimeric HER-2 B-cell peptide vaccines emulsified in montanide ISA 720VG and Nor-MDP adjuvant in patients with advanced solid tumors. Clin. Cancer Res. 25(12), 3495–3507 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peake BF, Nahta R. Resistance to HER2-targeted therapies: a potential role for FOXM1. Breast Cancer Manag. 3(5), 423–431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat. Clin. Pract. Oncol. 3(5), 269–280 (2006). [DOI] [PubMed] [Google Scholar]
- 116.Dua R, Zhang J, Nhonthachit P, Penuel E, Petropoulos C, Parry G. EGFR over-expression and activation in high HER2, ER negative breast cancer cell line induces trastuzumab resistance. Breast Cancer Res. Treat. 122(3), 685–697 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Huang X, Gao L, Wang S. et al. Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-i receptor in breast cancer cells resistant to herceptin. Cancer Res. 70(3), 1204–1214 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Junttila TT, Akita RW, Parsons K. et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 15(5), 429–440 (2009). [DOI] [PubMed] [Google Scholar]
- 119.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J. Natl Cancer Inst. 93(24), 1852–1857 (2001). [DOI] [PubMed] [Google Scholar]
- 120.Ritter CA, Perez-Torres M, Rinehart C. et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin. Cancer Res. 13(16), 4909–4919 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 65(23), 11118–11128 (2005). [DOI] [PubMed] [Google Scholar]
- 122.Liu B, Ordonez-Ercan D, Fan Z, Edgerton SM, Yang X, Thor AD. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int. J. Cancer 120(9), 1874–1882 (2007). [DOI] [PubMed] [Google Scholar]
- 123.Sanabria-Figueroa E, Donnelly SM, Foy KC. et al. Insulin-like growth factor-1 receptor signaling increases the invasive potential of human epidermal growth factor receptor 2-overexpressing breast cancer cells via Src-focal adhesion kinase and forkhead box protein M1. Mol. Pharmacol. 87(2), 150–161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Amin DN, Sergina N, Ahuja D. et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci. Transl. Med. 2(16), 16ra17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Serra V, Scaltriti M, Prudkin L. et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 30(22), 2547–2557 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yen L, Benlimame N, Nie ZR. et al. Differential regulation of tumor angiogenesis by distinct ErbB homo- and heterodimers. Mol. Biol. Cell 13(11), 4029–4044 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rajkumar T, Stamp GW, Hughes CM, Gullick WJ. c-erbB3 protein expression in ovarian cancer. Clin. Mol. Pathol. 49(4), M199–202 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chakraborty AK, Liang K, DiGiovanna MP. Co-targeting insulin-like growth factor I receptor and HER2: dramatic effects of HER2 inhibitors on nonoverexpressing breast cancer. Cancer Res. 68(5), 1538–1545 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2(2), 127–137 (2001). [DOI] [PubMed] [Google Scholar]
- 130.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 9(7), 463–475 (2009). [DOI] [PubMed] [Google Scholar]
- 131.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer 4(7), 505–518 (2004). [DOI] [PubMed] [Google Scholar]
- 132.Pollak MN. Insulin-like growth factors and neoplasia. Novartis Foundation Symposium 262, 84–98 (2004). [PubMed] [Google Scholar]
- 133.Foy KC, Wygle RM, Miller MJ, Overholser JP, Bekaii-Saab T, Kaumaya PTP. Peptide vaccines and peptidomimetics of EGFR (HER-1) ligand binding domain inhibit cancer cell growth in vitro and in vivo. J. Immunol. 191(1), 217–227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller MJ, Foy KC, Overholser JP, Nahta R, Kaumaya PTP. HER-3 peptide vaccines/mimics: combined therapy with IGF-1R, HER-2, and HER-1 peptides induces synergistic antitumor effects against breast and pancreatic cancer cells. Oncoimmunology 3(11), e956012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vicari D, Foy KC, Liotta EM, Kaumaya PT. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J. Biol. Chem. 286(15), 13612–13625 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Foy KC, Miller MJ, Moldovan N, Carson WE, Kaumaya PTP. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. OncoImmunology 1(7), 0–1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Foy KC, Miller MJ, Overholser J, Donnelly SM, Nahta R, Kaumaya PTP. IGF-1R peptide vaccines/mimics inhibit the growth of BxPC3 and JIMT-1 cancer cells and exhibit synergistic antitumor effects with HER-1 and HER-2 peptides. Oncoimmunology 3(11), e956005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu Y, Zi X, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int. J. Cancer 108(3), 334–341 (2004). [DOI] [PubMed] [Google Scholar]
- 139.Haluska P, Carboni JM, TenEyck C. et al. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol. Cancer Ther. 7(9), 2589–2598 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kamath AV, Lu D, Gupta P. et al. Preclinical pharmacokinetics of MEHD7945A, a novel EGFR/HER3 dual-action antibody, and prediction of its human pharmacokinetics and efficacious clinical dose. Cancer Chemother. Pharmacol. (2011). [DOI] [PubMed] [Google Scholar]
- 141.Schaefer G, Haber L, Crocker LM. et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 20(4), 472–486 (2011). [DOI] [PubMed] [Google Scholar]
- 142.Radinsky R, Risin S, Fan D. et al. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin. Cancer Res. 1(1), 19–31 (1995). [PubMed] [Google Scholar]
- 143.Kluftinger AM, Robinson BW, Quenville NF, Finley RJ, Davis NL. Correlation of epidermal growth factor receptor and c-erbB2 oncogene product to known prognostic indicators of colorectal cancer. Surg. Oncol. 1(1), 97–105 (1992). [DOI] [PubMed] [Google Scholar]
- 144.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 358(11), 1160–1174 (2008). [DOI] [PubMed] [Google Scholar]
- 145.Ciardiello F, De Vita F, Orditura M, Comunale D, Galizia G. Cetuximab in the treatment of colorectal cancer. Future Oncol. 1(2), 173–181 (2005). [DOI] [PubMed] [Google Scholar]
- 146.Karapetis CS, Khambata-Ford S, Jonker DJ. et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359(17), 1757–1765 (2008). [DOI] [PubMed] [Google Scholar]
- 147.Cancer Facts & Figures 2020. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- 148.Friess H, Kleeff J, Korc M, Buchler MW. Molecular aspects of pancreatic cancer and future perspectives. Dig. Surg. 16(4), 281–290 (1999). [DOI] [PubMed] [Google Scholar]
- 149.Lowery MA, O’Reilly EM. New approaches to the treatment of pancreatic cancer: from tumor-directed therapy to immunotherapy. BioDrugs 25(4), 207–216 (2011). [DOI] [PubMed] [Google Scholar]
- 150.Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: lessons from animal models. Cytokine Growth Factor Rev. 16(4–5), 407–420 (2005). [DOI] [PubMed] [Google Scholar]
- 151.Stoeltzing O, Liu W, Reinmuth N. et al. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am. J. Pathol. 163(3), 1001–1011 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ishiwata T, Bergmann U, Kornmann M, Lopez M, Beger HG, Korc M. Altered expression of insulin-like growth factor II receptor in human pancreatic cancer. Pancreas 15(4), 367–373 (1997). [DOI] [PubMed] [Google Scholar]
- 153.Adams TE, Epa VC, Garrett TP, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cell. Mol. Life Sci. 57(7), 1050–1093 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 55(10), 2007–2011 (1995). [PubMed] [Google Scholar]
- 155.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J. Clin. 60(5), 277–300 (2010). [DOI] [PubMed] [Google Scholar]
- 156.Cartwright T, Richards DA, Boehm KA. Cancer of the pancreas: are we making progress? A review of studies in the US Oncology Research Network. Cancer Control. 15(4), 308–313 (2008). [DOI] [PubMed] [Google Scholar]
- 157.Valsecchi ME, McDonald M, Brody JR. et al. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer 118(14), 3484–3493 (2012). [DOI] [PubMed] [Google Scholar]
- 158.Dong M, Nio Y, Guo KJ, Tamura K, Tian YL, Dong YT. Epidermal growth factor and its receptor as prognostic indicators in Chinese patients with pancreatic cancer. Anticancer Res. 18(6B), 4613–4619 (1998). [PubMed] [Google Scholar]
- 159.Sherwood ER, Van Dongen JL, Wood CG, Liao S, Kozlowski JM, Lee C. Epidermal growth factor receptor activation in androgen-independent but not androgen-stimulated growth of human prostatic carcinoma cells. Br. J. Cancer 77(6), 855–861 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 66(20), 10100–10111 (2006). [DOI] [PubMed] [Google Scholar]
- 161.Kuribayashi A, Kataoka K, Kurabayashi T, Miura M. Evidence that basal activity, but not transactivation, of the epidermal growth factor receptor tyrosine kinase is required for insulin-like growth factor I-induced activation of extracellular signal-regulated kinase in oral carcinoma cells. Endocrinology 145(11), 4976–4984 (2004). [DOI] [PubMed] [Google Scholar]
- 162.Guix M, Faber AC, Wang SE. et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J. Clin. Invest. 118(7), 2609–2619 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Foy KC, Wygle RM, Miller MJ, Overholser JP, Bekaii-Saab T, Kaumaya PT. Peptide vaccines and peptidomimetics of EGFR (HER-1) ligand binding domain inhibit cancer cell growth in vitro and in vivo. J. Immunol. 191(1), 217–227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wennhold K, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. B cell-based cancer immunotherapy. Transfus Med. Hemother. 46(1), 36–46 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bekaii-Saab T, Wesolowski R, Ahn DH. et al. Phase I immunotherapy trial with two chimeric HER-2 B-cell peptide vaccines emulsified in montanide ISA 720VG and nor-MDP adjuvant in patients with advanced solid tumors. Clin. Cancer Res. 25(12), 3495–3507 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Emens LA, Ascierto PA, Darcy PK. et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur. J. Cancer 81, 116–129 (2017). [DOI] [PubMed] [Google Scholar]
- 167.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 14, 73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weber JS, D’Angelo SP, Minor D. et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, Phase III trial. Lancet Oncol. 16(4), 375–384 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373(1), 23–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 373(2), 123–135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Motzer RJ, Escudier B, McDermott DF. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373(19), 1803–1813 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Robert C, Ribas A, Wolchok JD. et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a Phase I trial. Lancet 384(9948), 1109–1117 (2014). [DOI] [PubMed] [Google Scholar]
- 173.Borghaei H, Paz-Ares L, Horn L. et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 373(17), 1627–1639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yan Y, Kumar AB, Finnes H. et al. Combining immune checkpoint inhibitors with conventional cancer therapy. Front. Immunol. 9, 1739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161(2), 205–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12(4), 237–251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 16(4), 219–233 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207(10), 2187–2194 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Woo SR, Turnis ME, Goldberg MV. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72(4), 917–927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]