Abstract
Background
Obstructive sleep apnea (OSA) is closely related to the incidence and progression of coronary artery disease (CAD), and the mechanisms linking OSA and CAD are multifactorial. C1q/TNF-related protein-9 (CTRP9) is a novel adipokine that protects the heart against ischemic injury and ameliorates cardiac remodeling. We aimed to ascertain the clinical relevance of CTRP9 with OSA prevalence in patients with CAD.
Methods
From August 2016 to March 2019, consecutive eligible patients with CAD (n = 154; angina pectoris, n = 88; acute myocardial infarction [AMI], n = 66) underwent cardiorespiratory polygraphy. OSA was defined as an apnea-hypopnea index (AHI) ≥15 events·h−1. Plasma CTRP9 concentrations were measured by ELISA method.
Results
Moderate/severe OSA was present in 89 patients (57.8%). CTRP9 levels were significantly decreased in the moderate/severe OSA group than in the no/mild OSA group (4.7 [4.1-5.2] ng/mL vs. 4.9 [4.4-6.0] ng/mL, P = 0.003). The difference between groups was only observed in patients with AMI (3.0 [2.3-4.9] vs. 4.5 [3.2-7.9], P = 0.009). Correlation analysis showed that CTRP9 levels were negatively correlated with AHI (r = −0.238, P = 0.003) and oxygen desaturation index (r = −0.234, P = 0.004) and positively correlated with left ventricular ejection fraction (r = 0.251, P = 0.004) in all subjects. Multivariate analysis showed that male gender (OR 3.099, 95% CI 1.029-9.330, P = 0.044), BMI (OR 1.148, 95% CI 1.040-1.268, P = 0.006), and CTRP9 levels (OR 0.726, 95% CI 0.592-0.890, P = 0.002) were independently associated with the prevalence of moderate/severe OSA.
Conclusions
Plasma CTRP9 levels were independently related to the prevalence of moderate/severe OSA in patients with CAD, suggesting that CTRP9 might play a role in the pathogenesis of CAD exacerbated by OSA.
1. Background
Obstructive sleep apnea (OSA) is an increasingly recognized chronic disorder in adults [1, 2]. Recent evidence indicates OSA is closely related to the incidence and progression of coronary artery disease (CAD), and the prevalence of OSA is high (38% to 65%) in CAD patients [3, 4]. Prior reports and our study have shown that OSA was associated with an increased risk of recurrent cardiovascular events in patients with CAD and/or undergoing PCI [5–8]. However, the molecular mechanisms linking OSA and CAD are multifactorial.
OSA-mediated intermittent hypoxemia and sleep fragmentation triggers metabolic disorder, which is involved in cardiovascular impairment [9, 10]. Adiponectin is a cardioprotective adipokine, which have important role in insulin sensitivity, inflammation, and glucose homeostasis [11]. Previous studies have demonstrated that adiponectin levels were reduced in patients with severe OSA [12, 13]. Recently, a new family of secreted proteins, C1q tumor necrosis factor-related proteins (CTRPs), was found to have the same modular organization with adiponectin [14]. In all CTRPs families, C1q/TNF-related protein 9 (CTRP9) shares the highest degree (54%) of homology with adiponectin. CTRP9 levels are 100 times more than adiponectin in the myocardium [15]. Accumulating studies reveal that CTRP9 can protect the heart by attenuating atherosclerosis, alleviating acute ischemic injury, and attenuating adverse cardiac remodeling [16–18]. However, whether CTRP9 levels were altered by OSA in CAD patients remains undetermined. Therefore, we aimed to investigate the clinical relevance of CTRP9 with parameters of OSA, and whether CTRP9 is significantly associated with OSA prevalence in patients with CAD.
2. Methods
2.1. Study Design and Subjects
From August 2016 to March 2019, we consecutively enrolled patients aged 18 to 85 years with CAD (including angina pectoris (AP) and acute myocardial infarction (AMI)) and receiving overnight sleep study at the Emergency & Critical Care Center of Beijing Anzhen Hospital, Capital Medical University. AP included stable angina pectoris (SAP) and unstable angina pectoris. AMI included ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction. Among them, AMI is defined as the rise and/or fall of cardiac biomarker values such as CK-MB and/or troponin-T with at least one value above the 99th percentile upper reference limit and at least one of the following symptoms: ischemic, electrocardiogram changes indicative of new ischemia, development of pathologic Q waves, and imaging evidence of new loss of viable myocardium or new regional wall motion abnormality [19]. SAP's criteria include symptom of angina being stable for at least 6 months and having at least 50% luminal stenosis in at least one major coronary artery confirmed by coronary angiography. The criteria for unstable angina included symptoms of angina at rest, a new-onset exertional angina, or a recent acceleration of angina.
Exclusion criteria included cardiogenic shock, cardiac arrest, history of malignancy, hypertension (≥160/100 mmHg), diabetes, cancer, chronic kidney disease, valvular disease, stroke, thyroid disease, central sleep apnea, and patients without adequate and satisfactory sleep study or known use of continuous positive airway pressure (CPAP) treatment. Finally, one hundred and fifty-four CAD patients (88 AP and 66 AMI) were recruited. This study conformed to the Declaration of Helsinki. The Ethics Committee of Beijing Anzhen Hospital, Capital Medical University approved the study (2013025). All patients provided informed consent.
2.2. Overnight Sleep Study
All patients underwent an overnight sleep study after clinical stabilization during hospitalization (median 2 days (1 to 3 days) after admission) using a portable cardiorespiratory monitoring device (ApneaLink Air, Resmed, Australia). Nasal airflow, arterial oxygen saturation, thoracoabdominal movements, and snoring episodes were recorded. An apnea was defined by an absence of airflow lasting for ≥10 seconds. A hypopnea was defined as a reduction in airflow of >30% for ≥10 seconds and associated with a decrease in arterial oxygen saturation (SaO2) >4%. The apnea-hypopnea index (AHI) was defined as the number of apneas or hypopneas per hour of total recording time. Patients were divided into 2 groups: moderate/severe OSA group (AHI ≥15 events·h−1) and no/mild OSA group (AHI <15 events·h−1). All sleep studies were scored according to the American Academy of Sleep Medicine (AASM) 2007 guidelines. A minimum of 3 h of satisfactory signal recording was considered as a valid test. All studies were scored manually twice by independent sleep technologists (XW and JF) and reviewed by a senior consultant (YW) in cases of discrepancy.
2.3. Laboratory Measurements
All fasting venous blood samples were obtained the morning after the completion of overnight sleep study and overnight fast. We followed the manufacturer's recommendations. Blood samples were drawn into EDTA tubes and immediately centrifuged at 4°C, and plasma was frozen at -80°C for subsequent assays. Plasma glucose, cholesterol, triglycerides, high-sensitivity C-reactive protein, and homocysteine levels were analyzed using standard protocols of biochemistry laboratory. Plasma CTRP9 concentrations were determined with the sandwich method using a commercial ELISA kit (AVISCERA BIOSCIENCE, CA, USA; intra- and interassay CVs: 4-6% and 8-12%, respectively). We did not observe significant cross-reactivity or interference between human CTRP9 and analogs in our previous experiment. Samples were assayed in duplicate, and all results were reported as median.
2.4. Statistical Analysis
Continuous variables were presented as mean ± standard deviation (SD) or median (first and third quartiles) and were compared by Student's t-test or Mann–Whitney U test. Categorical variables were exhibited as the number (percentage) and were compared using chi-square test or Fisher's exact test. The correlations between plasma CTRP9 concentration and baseline and sleep parameters were determined by Spearman's correlation analysis. To identify independent factors of OSA incidence, binary logistic regression analysis was performed. Baseline variables that showed a univariate relationship with outcome were entered into the logistic regression models. All tests were 2-sided, and the value of P < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS (version 25.0 IBM SPSS Inc, Armonk, NY).
3. Results
3.1. Baseline Characteristics of Subjects with Moderate/Severe OSA and No/Mild OSA
In total, 165 consecutive eligible patients with CAD were prospectively enrolled, of whom 157 underwent a successful overnight sleep study. After exclusion of patients according to predefined criteria, 154 patients were included in the final analysis (Figure 1). Baseline characteristics of 154 CAD patients were listed in Table 1. Patients with moderate/severe OSA were more likely to be male and current smokers and had significantly higher body mass index (BMI), waist-to-hip ratio, and neck circumference compared with those with no/mild OSA. Other baseline information was generally well matched between moderate/severe OSA group and no/mild OSA groups.
Figure 1.
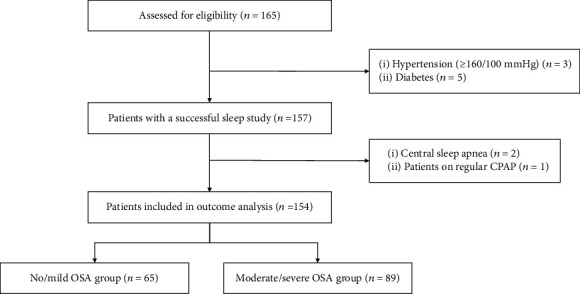
Study flowchart. CPAP: continuous positive airway pressure; OSA: obstructive sleep apnea.
Table 1.
Baseline characteristics of subjects with moderate/severe OSA and no/mild OSA.
| Variables | All (n = 154) | Moderate/severe OSA (n = 89) | No/mild OSA (n = 65) | P |
|---|---|---|---|---|
| Age (years) | 54.9 ± 9.4 | 54.4 ± 8.8 | 55.1 ± 10.3 | 0.619 |
| Male (%) | 136 (88.3) | 83 (93.3) | 53 (81.5) | 0.025 |
| BMI (kg/m2) | 27.2 ± 3.7 | 27.9 ± 3.7 | 26.4 ± 3.9 | 0.012 |
| Waist-to-hip ratio | 0.97 (0.94-1.01) | 0.98 (0.95-1.02) | 0.97 (0.93-1.00) | 0.032 |
| Neck circumference (cm) | 40.0 (38-42) | 40.5 (38-42) | 40.0 (37-44) | <0.001 |
| Systolic BP (mm/Hg) | 126 (116-139) | 129 (115-140) | 125 (115-136) | 0.389 |
| Diastolic BP (mm/Hg) | 76 (70-86) | 79 (70-87) | 74 (70-85) | 0.176 |
| Hypertension (%) | 89 (57.8) | 54 (60.7) | 35 (53.8) | 0.397 |
| Hyperlipidemia (%) | 38 (24.7) | 23 (25.8) | 15 (23.1) | 0.694 |
| Current smoking (%) | 76 (49.4) | 53 (59.6) | 23 (35.4) | 0.015 |
| Previous CAD (%) | 49 (31.8) | 29 (32.6) | 20 (30.8) | 0.811 |
| Previous myocardial infarction (%) | 19 (12.3) | 12 (13.5) | 7 (10.8) | 0.613 |
| Previous PCI (%) | 24 (15.6) | 18 (20.2) | 6 (9.2) | 0.063 |
| LDL-cholesterol (mmol/L) | 2.3 (1.8-3.1) | 2.4 (1.9-3.0) | 2.1 (1.7-3.0) | 0.635 |
| HDL-cholesterol (mmol/L) | 1.03 (0.89-1.18) | 1.03 (0.91-1.20) | 1.01 (0.89-1.13) | 0.010 |
| Total cholesterol (mmol/L) | 3.9 (3.3-5.0) | 4.2 (3.45-4.9) | 3.8 (3.2-4.8) | 0.948 |
| Triglyceride (mmol/L) | 1.4 (1.0-2.3) | 1.5 (1.0-2.2) | 1.3 (0.9-2.1) | 0.448 |
| LVEF (%) | 60 (55-65) | 62 (60-65) | 55 (48-60) | 0.582 |
| CTRP9 (ng/mL) | 4.9 (4.3-5.5) | 4.7 (4.1-5.2) | 4.9 (4.4-6.0) | 0.003 |
| hsCRP (mg/L) | 1.5 (0.6-5.1) | 1.2 (0.5-3.3) | 2.0 (0.6-5.9) | 0.103 |
| HCY (μmol/L) | 12.7 (9.4-19.1) | 12.7 (9.3-16.0) | 11.9 (9.5-19.2) | 0.618 |
| HbA1c (%) | 5.8 (5.4-6.0) | 5.8 (5.5-6.2) | 5.6 (5.4-6.0) | 0.293 |
| Fasting glucose (mmol/L) | 5.48 (5.07-5.95) | 5.54 (5.07-6.00) | 5.48 (5.12-5.86) | 0.220 |
| AHI (events/h) | 19.1 (9.9-33.5) | 29.0 (21.9-38.8) | 8.5 (4.3-10.9) | <0.001 |
| ODI (events/h) | 20.7 (11.7-30.7) | 27.2 (21.2-36.2) | 10.5 (4.6-13.9) | <0.001 |
| Minimum SaO2 (%) | 85 (81-89) | 83 (79-87) | 88 (83-90) | <0.001 |
| Mean SaO2 (%) | 94 (93-95) | 93 (93-95) | 94 (94-95) | 0.001 |
| Time with SaO2 <90% (%) | 3.0 (0.2-8.0) | 5.0 (2.0-14.0) | 0.8 (0-3.3) | <0.001 |
Data are presented as mean ± SD: median (first quartile: third quartile): or n (%). AHI: apnea-hypopnea index; BMI: body mass index; BP: blood pressure; CAD: coronary artery disease; CTRP9: C1q/TNF-related protein 9; HbA1c: glycated hemoglobin; HCY: homocysteine; HDL: high-density lipoprotein; hsCRP: high-sensitivity C-reactive protein; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; ODI: oxygen desaturation index; OSA obstructive sleep apnea; PCI: percutaneous coronary intervention; SaO2: arterial oxygen saturation.
3.2. Sleep Study Results
Based on the criteria of AHI ≥15, the prevalence of moderate/severe OSA was 57.8% in this CAD cohort. Patients with moderate/severe OSA exhibited higher AHI and oxygen desaturation index (ODI), lower minimum and average oxygen saturation, and more time of SaO2 <90% compared with those with no/mild OSA (Table 1).
3.3. Plasma CTRP9 Concentrations in Subjects with Moderate/Severe OSA and No/Mild OSA
Plasma CTRP9 concentrations were significantly decreased in the moderate/severe OSA group than in the no/mild OSA group (4.7 [4.1-5.2] ng/mL vs. 4.9 [4.4-6.0] ng/mL, P = 0.003) (Table 1 and Figure 2(a)). When we stratified the CAD patients into AP and AMI subgroup, the plasma CTRP9 levels were significantly lower in the moderate/severe OSA group only in patients with AMI (3.0 [2.3-4.9] ng/mL vs. 4.5 [3.2-7.9] ng/mL, P = 0.009), but not in patients with AP (5.0 [4.7-5.3] ng/mL vs. 5.1 [4.7-5.9] ng/mL, P = 0.571) (Figure 2(b)).
Figure 2.
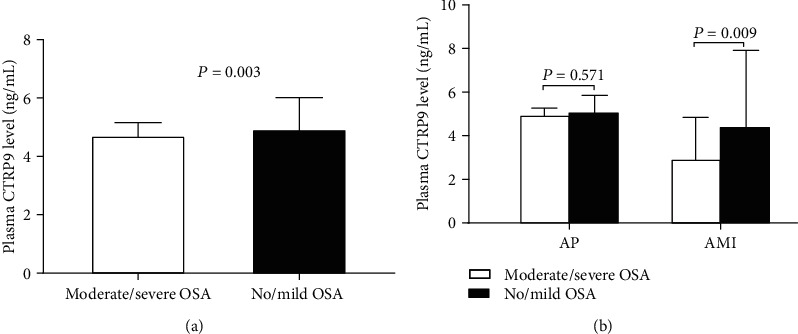
Plasma CTRP9 levels between moderate/severe OSA and no/mild OSA groups in patients with CAD (a). Plasma CTRP9 levels between moderate/severe OSA and no/mild OSA groups in AP and AMI subgroups (b). AMI: acute myocardial infarction; AP: angina pectoris; CTRP9: C1q/TNF-related protein 9; OSA: obstructive sleep apnea.
3.4. Correlation of CTRP9 Levels with Sleep Parameters and LVEF
Scatter plot showed the correlation of CTRP9 levels with sleep parameters and LVEF (Figure 3). CTRP9 levels were negatively correlated with AHI (r = −0.238, P = 0.003) and oxygen desaturation index (r = −0.234, P = 0.004) and positively correlated with left ventricular ejection fraction (LVEF) (r = 0.251, P = 0.004) in all subjects. On the other hand, CTRP9 concentrations had no significant correlation with minimum SaO2, mean SaO2, and the time of SaO2 <90%. In subgroup patients with AP and AMI, there was also a significant correlation between CTRP9 and AHI (Figure 4).
Figure 3.
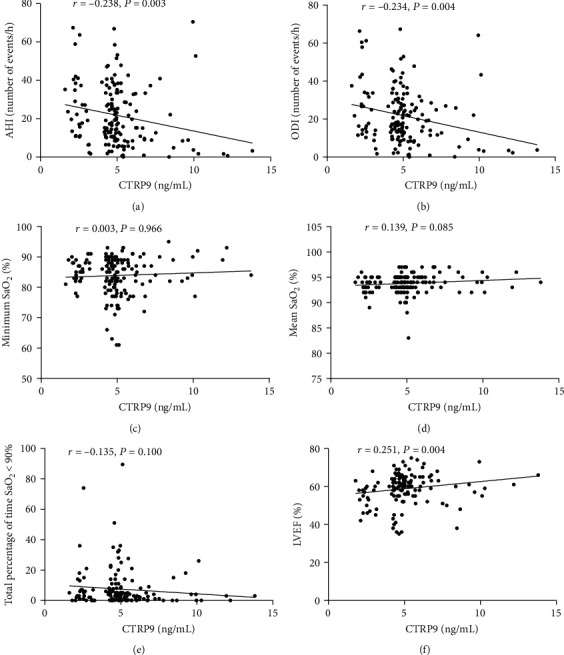
Correlation between CTRP9 and parameters of sleep study (a–e) and LVEF (%) (f). AHI: apnea-hypopnea index; LVEF: left ventricular ejection fraction; ODI: oxygen desaturation index; SaO2: arterial oxygen saturation.
Figure 4.
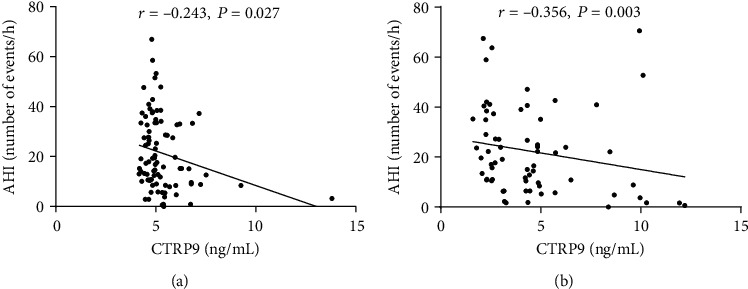
Correlation between CTRP9 and AHI in patients with AP (a) and AMI (b). AHI: apnea-hypopnea index; AMI: acute myocardial infarction; AP: angina pectoris.
3.5. Baseline Characteristics according to Tertiles of CTRP9 Levels
All subjects were categorized into trisection according to CTRP9 tertiles (T1: <4.47 ng/mL; T2: 4.47-5.07 ng/mL; T3: >5.07 ng/mL). The baseline characteristics of each category are shown in Table S1. There were significant differences among the tertiles in terms of systolic and diastolic blood pressure, LVEF, AHI, and ODI.
3.6. Plasma CTRP9 and the Prevalence of OSA
To evaluate the association between CTRP9 and OSA, univariate and multivariate logistic regression analyses were performed. In univariate logistic regression, we found that gender, BMI, neck circumference, HDL-cholesterol, and CTRP9 levels were significantly associated with the prevalence of OSA. In the multivariate model, only male gender (OR 3.099, 95% CI 1.029-9.330, P = 0.044), BMI (OR 1.148, 95% CI 1.040-1.268, P = 0.006), and CTRP9 levels (OR 0.726, 95% CI 0.592-0.890, P = 0.002) were independently associated with the prevalence of OSA (Table 2). Neck circumference did not enter into the multivariate regression due to its multicollinearity.
Table 2.
Independent factors associated with OSA in binary logistic regression models.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| β ± SE | OR (95% CI) | P | β ± SE | OR (95% CI) | P | |
| Age | −0.008 ± 0.017 | 0.992 (0.959-1.026) | 0.630 | |||
| Male | 1.142 ± 0.530 | 3.132 (1.108-8.851) | 0.031 | 1.131 ± 0.562 | 3.099 (1.029-9.330) | 0.044 |
| BMI | 0.113 ± 0.046 | 1.119 (1.023-1.225) | 0.015 | 0.138 ± 0.051 | 1.148 (1.040-1.268) | 0.006 |
| Waist-to-hip ratio | 0.979 ± 1.908 | 2.663 (0.063-112.055) | 0.608 | |||
| Neck circumference | 0.186 ± 0.055 | 1.205 (1.082-1.341) | 0.001 | |||
| Systolic BP | 0.004 ± 0.010 | 1.004 (0.985-1.023) | 0.693 | |||
| Diastolic BP | 0.018 ± 0.014 | 1.018 (0.991-1.047) | 0.197 | |||
| Hypertension | 0.279 ± 0.330 | 1.322 (0.692-2.526) | 0.414 | |||
| Hyperlipidemia | 0.150 ± 0.381 | 1.162 (0.550-2.452) | 0.694 | |||
| Current smoking | 0.835 ± 0.465 | 2.304 (0.926-5.732) | 0.073 | |||
| Previous CAD | 0.084 ± 0.351 | 1.087 (0.546-2.165) | 0.811 | |||
| Previous myocardial infarction | 0.256 ± 0.506 | 1.291 (0.479-3.484) | 0.614 | |||
| Previous PCI | 0.913 ± 0.503 | 2.493 (0.930-6.685) | 0.069 | |||
| LDL-cholesterol | −0.060 ± 0.162 | 0.942 (0.686-1.294) | 0.713 | |||
| HDL-cholesterol | −1.602 ± 0.792 | 0.202 (0.043-0.951) | 0.043 | −1.220 ± 0.851 | 0.295 (0.056-1.565) | 0.152 |
| Total cholesterol | −0.064 ± 0.140 | 0.938 (0.712-1.235) | 0.647 | |||
| Triglyceride | 0.088 ± 0.169 | 1.092 (0.784-1.520) | 0.603 | |||
| LVEF | 0.003 ± 0.021 | 1.003 (0.962-1.046) | 0.871 | |||
| CTRP9 | −0.256 ± 0.093 | 0.774 (0.645-0.929) | 0.006 | −0.320 ± 0.104 | 0.726 (0.592-0.890) | 0.002 |
| hsCRP | 0.024 ± 0.026 | 1.024 (0.973-1.077) | 0.357 | |||
| HCY | 0.010 ± 0.016 | 1.010 (0.979-1.041) | 0.532 | |||
| HbA1C | 0.525 ± 0.344 | 1.690 (0.861-3.318) | 0.127 | |||
| Fasting glucose | 0.406 ± 0.208 | 1.501 (0.999-2.254) | 0.051 | |||
BMI: body mass index; BP: blood pressure; CAD: coronary artery disease; CTRP9: C1q/TNF-related protein 9; HbA1c: glycated hemoglobin; HCY: homocysteine; HDL: high-density lipoprotein; hsCRP: high-sensitivity C-reactive protein; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention.
To further explore the risk factors for OSA comorbidity in patients with CAD, we established multiple regression models based on the CTRP9 tertile. In model 1 without adjustment, subjects with low and moderate CTRP9 levels had significantly higher risk of OSA compared with those with high levels. This trend was further intensified after adjustment for age, gender, and BMI (Table S2).
3.7. Correlation between AHI and Other Variables
To evaluate the association between CTRP9 and AHI, correlation analysis and multivariate linear regression analysis were performed (Table S3). Spearman's correlation analysis illustrated that AHI was positively correlated with BMI (r = 0.256, P = 0.001), waist-to-hip ratio (r = 0.184, P = 0.024), and neck circumference (r = 0.347, P = 0.000) and negatively correlated with CTRP9 levels (r = −0.238, P = 0.003) in all subjects. In the multivariate linear regression model, CTRP9 levels, sex, and BMI were independent factors associated with AHI (P < 0.05, respectively). Waist-to-hip ratio and neck circumference were excluded from the model due to their high multicollinearity with BMI.
4. Discussion
In the present study, we first demonstrated the clinical relevance of CTRP9 with OSA in patients with CAD. The plasma CTRP9 levels were significantly decreased in moderate/severe OSA versus no/mild OSA groups, which was driven by the difference in patients with AMI. Lower CTRP9 level was an independent factor related to OSA prevalence even after adjusting for other confounding factors.
Emerging evidence has demonstrated a close relationship between OSA and CAD [4, 5, 20]. Also, prior reports have shown that OSA-mediated chronic intermittent hypoxia (CIH), triggered by repetitive episodes of apneas and hypopneas, exacerbates metabolic dysfunction including insulin resistance and nonalcoholic fatty liver disease [10, 21]. Mechanistically, recurrent cycles of hypoxemia with reoxygenation promote oxidative stress, systemic inflammation, and endothelial dysfunction, all contributing to the pathogenesis of diabetes [22]. Our recent study indicated that OSA was associated with increased risk of 1-year cardiovascular events following acute coronary syndrome only in patients with diabetes or poor glucose control, but not in patients without diabetes [23]. Therefore, metabolic disorders may be involved in OSA-induced incidence and aggravation of CAD.
Findings from animal models and patients have demonstrated that the levels of adiponectin, a classical cardioprotective adipokine, were decreased under CIH/OSA [12, 13, 24]. However, the results were distinct and CPAP intervention did not increase adiponectin levels [25]. C1q tumor necrosis factor-related proteins (CTRPs) is a highly conserved family of adiponectin paralogs, which includes fifteen family members [14]. Among them, CTRP9 shares the greatest homology with adiponectin and is highly expressed in the adult heart. Besides the metabolic regulatory properties, CTRP9 had important local cardiac biological function [15]. CTRP9 supplementation attenuated cardiac remodeling and improved contractile function postmyocardial infarction (MI) [17]. Moreover, clinical studies have shown decreased CTRP9 levels in patients with MI as well as heart failure with reduced ejection fraction [26, 27]. In the present study, we found that in patients with CAD, the CTRP9 levels were further reduced in moderate/severe OSA group compared with no/mild OSA group, and the CTRP9 concentrations were inversely correlated to the AHI levels. The difference between groups was only observed in patients with AMI, because in such circumstances, the heart may be in a more vulnerable state sensitive to the negative consequences of OSA [4]. Also, our recent study found that cardiac CTRP9 gene and protein levels were significantly reduced in CIH+MI animals [28]. These findings indicate that CTRP9 may be involved in the pathogenesis of CAD and related complications exacerbated by OSA.
Obesity is a major risk factor for OSA, because it contributes significantly to pharyngeal airway narrowing [10]. Our study also showed that BMI level significantly predicted higher risk of OSA. Noteworthy, multivariate regression analysis showed that CTRP9 levels were independently associated with the prevalence of OSA. Furthermore, the results from basic study demonstrated that CTRP9 supplementation significantly attenuated CIH-exacerbated post-MI remodeling and improved cardiac function [28]. These findings support CTRP9 as a potential therapeutic target by restoring cardiac function in CAD patients with OSA.
4.1. Limitations
First, due to the cross-sectional design, the causal relationship could not be confirmed. Second, this is a single-center study that recruited only East-Asian patients. Studies pertaining to other ethnicities are needed. Third, the plasma levels of other members of CTRP family need to be examined in future studies.
5. Conclusions
In conclusion, the present study demonstrated that CTRP9 levels were significantly reduced in moderate/severe OSA versus no/mild OSA groups in patients with CAD. Lower CTRP9 levels were independently associated with OSA prevalence after adjusting for traditional contributing factors. These results support the role of CTRP9 in linking OSA and pathogenesis of CAD.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81870322, 81600209), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201710), Beijing Municipal Administration of Hospitals' Ascent Plan (DFL20180601), Beijing Lab for Cardiovascular Precision Medicine, Beijing, China (PXM2018_014226_000013), the Capital Health Research and Development of Special Fund (2018-1-2061), and Beijing Municipal Science & Technology Commission (Z181100001718060).
Abbreviations
- AASM:
American Academy of Sleep Medicine
- AHI:
Apnea-hypopnea index
- AMI:
Acute myocardial infarction
- AP:
Angina pectoris
- BMI:
Body mass index
- BP:
Blood pressure
- CAD:
Coronary artery disease
- CI:
Confidence interval
- CPAP:
Continuous positive airway pressure
- CTRP9:
C1q/TNF-related protein 9
- ELISA:
Enzyme-linked immunosorbent assay
- HbA1c:
Glycated hemoglobin
- HCY:
Homocysteine
- HDL:
High-density lipoprotein
- hsCRP:
High-sensitivity C-reactive protein
- LDL:
Low-density lipoprotein
- LVEF:
Left ventricular ejection fraction
- ODI:
Oxygen desaturation index
- OR:
Odds ratio
- OSA:
Obstructive sleep apnea
- PCI:
Percutaneous coronary intervention
- SaO2:
Arterial oxygen saturation
- SD:
Standard deviation.
Contributor Information
Xiao Wang, Email: spaceeye123@126.com.
Shaoping Nie, Email: spnie@ccmu.edu.cn.
Data Availability
Data generated or analyzed during this study are included in this published article.
Ethical Approval
This study was conducted in accordance with the amended Declaration of Helsinki. The Ethics Committee of Beijing Anzhen Hospital, Capital Medical University approved this study (2013025). All patients provided written informed consent.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
ZL, XW, and SN conceived the study concept and design. ZL, YD, LJ, JF, RG, and XW performed the acquisition, analysis, or interpretation of data. ZL and XW drafted the manuscript. All authors critically revised the manuscript for important intellectual content. XW and SN obtained funding. XM gathered the administrative, technical, or material support. ZL, XW, and SN had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Supplementary Materials
Table S1: baseline characteristics according to tertiles of CTRP9 levels. Table S2: OSA risk according to CTRP9 tertiles in multivariable logistic regression. Table S3: correlation between AHI and other variables.
References
- 1.Benjafield A. V., Ayas N. T., Eastwood P. R., et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. The Lancet Respiratory Medicine. 2019;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenstone M., Hack M. Obstructive sleep apnoea. BMJ. 2014;348(11):p. g3745. doi: 10.1136/bmj.g3745. [DOI] [PubMed] [Google Scholar]
- 3.Javaheri S., Barbe F., Campos-Rodriguez F., et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. Journal of the American College of Cardiology. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arzt M., Hetzenecker A., Steiner S., Buchner S. Sleep-disordered breathing and coronary artery disease. The Canadian Journal of Cardiology. 2015;31(7):909–917. doi: 10.1016/j.cjca.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Fan J., Wang X., Ma X., Somers V. K., Nie S., Wei Y. Association of obstructive sleep apnea with cardiovascular outcomes in patients with acute coronary syndrome. Journal of the American Heart Association. 2019;8(2, article e010826) doi: 10.1161/JAHA.118.010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., Fan J. Y., Zhang Y., Nie S. P., Wei Y. X. Association of obstructive sleep apnea with cardiovascular outcomes after percutaneous coronary intervention: a systematic review and meta-analysis. Medicine. 2018;97(17, article e0621) doi: 10.1097/MD.0000000000010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C. H., Sethi R., Li R., et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133(21):2008–2017. doi: 10.1161/CIRCULATIONAHA.115.019392. [DOI] [PubMed] [Google Scholar]
- 8.Yumino D., Tsurumi Y., Takagi A., Suzuki K., Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. The American Journal of Cardiology. 2007;99(1):26–30. doi: 10.1016/j.amjcard.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Bauters F., Rietzschel E. R., Hertegonne K. B. C., Chirinos J. A. The link between obstructive sleep apnea and cardiovascular disease. Current Atherosclerosis Reports. 2016;18(1):p. 1. doi: 10.1007/s11883-015-0556-z. [DOI] [PubMed] [Google Scholar]
- 10.Drager L. F., Togeiro S. M., Polotsky V. Y., Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. Journal of the American College of Cardiology. 2013;62(7):569–576. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrade-Oliveira V., Câmara N. O. S., Moraes-Vieira P. M. Adipokines as drug targets in diabetes and underlying disturbances. Journal Diabetes Research. 2015;2015, article 681612:1–11. doi: 10.1155/2015/681612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bingol Z., Karaayvaz E. B., Telci A., Bilge A. K., Okumus G., Kiyan E. Leptin and adiponectin levels in obstructive sleep apnea phenotypes. Biomarkers in Medicine. 2019;13(10):865–874. doi: 10.2217/bmm-2018-0293. [DOI] [PubMed] [Google Scholar]
- 13.Al Mutairi S., Mojiminiyi O. A., Al Alawi A., Al Rammah T., Abdella N. Study of leptin and adiponectin as disease markers in subjects with obstructive sleep apnea. Disease Markers. 2014;2014:8. doi: 10.1155/2014/706314.706314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seldin M. M., Tan S. Y., Wong G. W. Metabolic function of the CTRP family of hormones. Reviews in Endocrine & Metabolic Disorders. 2014;15(2):111–123. doi: 10.1007/s11154-013-9255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan Y., Lau W. B., Su H., et al. C1q-TNF-related protein-9, a novel cardioprotetcive cardiokine, requires proteolytic cleavage to generate a biologically active globular domain isoform. American Journal of Physiology. Endocrinology and Metabolism. 2015;308(10):E891–E898. doi: 10.1152/ajpendo.00450.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu X. H., Zhang D. W., Zheng X. L., Tang C. K. C1q tumor necrosis factor-related protein 9 in atherosclerosis: mechanistic insights and therapeutic potential. Atherosclerosis. 2018;276:109–116. doi: 10.1016/j.atherosclerosis.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y., Yi W., Yuan Y., et al. C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase a activation. Circulation. 2013;128(11):S113–S120. doi: 10.1161/CIRCULATIONAHA.112.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kambara T., Shibata R., Ohashi K., et al. C1q/tumor necrosis factor-related protein 9 protects against acute myocardial injury through an adiponectin receptor I-AMPK-dependent mechanism. Molecular and Cellular Biology. 2015;35(12):2173–2185. doi: 10.1128/MCB.01518-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thygesen K., Alpert J. S., Jaffe A. S., et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 20.Pafili K., Steiropoulos P., Papanas N. The relationship between obstructive sleep apnoea and coronary heart disease. Current Opinion in Cardiology. 2015;30(4):439–446. doi: 10.1097/HCO.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 21.Aron-Wisnewsky J., Minville C., Tordjman J., et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. Journal of Hepatology. 2012;56(1):225–233. doi: 10.1016/j.jhep.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Reutrakul S., Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Fan J., Du Y., et al. Clinical significance of obstructive sleep apnea in patients with acute coronary syndrome in relation to diabetes status. BMJ Open Diabetes Research & Care. 2019;7(1, article e000737) doi: 10.1136/bmjdrc-2019-000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye J., Gao Z., Yin J., He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. American Journal of Physiology. Endocrinology and Metabolism. 2007;293(4):E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 25.Arnardottir E. S., Mackiewicz M., Gislason T., Teff K. L., Pack A. I. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32(4):447–470. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Hang T., Cheng X.-M., et al. Associations of C1q/TNF-related protein-9 levels in serum and epicardial adipose tissue with coronary atherosclerosis in humans. BioMed Research International. 2015;2015:6. doi: 10.1155/2015/971683.971683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao C., Zhao S., Lian K., et al. C1q/TNF-related protein 3 (CTRP3) and 9 (CTRP9) concentrations are decreased in patients with heart failure and are associated with increased morbidity and mortality. BMC Cardiovascular Disorders. 2019;19(1):p. 139. doi: 10.1186/s12872-019-1117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Y., Wang X., Li L., et al. miRNA-mediated suppression of a cardioprotective cardiokine as a novel mechanism exacerbating post-MI remodeling by sleep breathing disorders. Circulation Research. 2020;126(2):212–228. doi: 10.1161/CIRCRESAHA.119.315067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: baseline characteristics according to tertiles of CTRP9 levels. Table S2: OSA risk according to CTRP9 tertiles in multivariable logistic regression. Table S3: correlation between AHI and other variables.
Data Availability Statement
Data generated or analyzed during this study are included in this published article.


