Abstract
Diabetic retinopathy (DR) is triggered by retinal cell damage stimulated by the diabetic milieu, including increased levels of intraocular free fatty acids. Free fatty acids may serve as an initiator of inflammatory cytokine release from Müller cells, and the resulting cytokines are potent stimulators of retinal endothelial pathology, such as leukostasis, vascular permeability, and basement membrane thickening. Our previous studies have elucidated a role for peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in promoting several steps in the pathologic cascade in DR, including angiogenesis and expression of inflammatory mediators. Furthermore, PPARβ/δ is a known target of lipid signaling, suggesting a potential role for this transcription factor in fatty acid-induced retinal inflammation. Therefore, we hypothesized that PPARβ/δ stimulates both the induction of inflammatory mediators by Müller cells as well the paracrine induction of leukostasis in endothelial cells (EC) by Müller cell inflammatory products. To test this, we used the PPARβ/δ inhibitor, GSK0660, in primary human Müller cells (HMC), human retinal microvascular endothelial cells (HRMEC) and mouse retina. We found that palmitic acid (PA) activation of PPARβ/δ in HMC leads to the production of pro-angiogenic and/or inflammatory cytokines that may constitute DR-relevant upstream paracrine inflammatory signals to EC and other retinal cells. Downstream, EC transduce these signals and increase their synthesis and release of chemokines such as CCL8 and CXCL10 that regulate leukostasis and other cellular events related to vascular inflammation in DR. Our results indicate that PPARβ/δ inhibition mitigates these upstream (MC) as well as downstream (EC) inflammatory signaling events elicited by metabolic stimuli and inflammatory cytokines. Therefore, our data suggest that PPARβ/δ inhibition is a potential therapeutic strategy against early DR pathology.
Keywords: Peroxisome proliferator-activated receptors, Diabetic Retinopathy, Inflammation
1.1. Introduction
Diabetic retinopathy (DR) is a leading cause of blindness in the United States (1). DR is a complication of diabetes consisting of two stages: an early stage called nonproliferative diabetic retinopathy (NPDR) characterized by microvascular changes, such as development of microaneurysms and hemorrhages; and a later stage called proliferative diabetic retinopathy (PDR) characterized by neovascularization. PDR is responsible for the irreversible vision loss observed in diabetics. Current treatments target PDR; however, providing therapies for early disease pathology could prevent the irreversible damage that accompanies progression to this later disease stage.
DR is a neurovascular inflammatory disease associated with metabolic dysfunction and chronic elevation of retinal inflammatory mediators. Systemic metabolic changes are associated with retinal cell activation, leading to increased synthesis and release of inflammatory cytokines. Notably, we have recently identified that metabolic stimuli associated with dyslipidemia specifically activate Müller cells (MC) to increase their production of inflammatory cytokines (2, 3). This supports a conceptual framework in which MC-derived inflammatory cytokines exert their actions through autocrine amplification of these cytokines and paracrine effects on retinal endothelial cells, among others. These amplification steps would then ultimately reach levels commensurate with severe inflammation-mediated tissue damage (4–6). Retinal endothelium respond to these inflammatory cytokines with increased expression of chemokines and leukocyte adhesion molecules that facilitate leukocyte homing, tethering and rolling, all leading to leukostasis (firm adhesion of leukocytes) (7–10). Leukostasis can cause vaso-occlusion, or the arrested leukocytes can transmigrate into perivascular tissues further escalating chronic vascular inflammation. Leukostasis has been linked to EC tight junction dysfunction, blood-retina barrier breakdown, and capillary dropout (11–13). Since leukostasis occurs before any other clinical sign of DR and is linked to DR progression, it constitutes an attractive target for early DR therapy (14).
Mounting evidence supports the investigation of fatty acids as relevant stimuli to model diabetes-linked dyslipidemia in DR (2, 3, 15–17). Serum and tissue profiles from diabetic patients and experimental models of diabetes demonstrate that one saturated fatty acid, palmitic acid (PA), is elevated above others (17–19). Increased retinal levels of PA, as well as the unsaturated fatty acids oleic acid (OA) and linoleic acid (LA), have been observed early in rodent models of diabetes (17, 20). Notably, there is evidence that these fatty acids elicit inflammatory responses in Müller cells and retinal microvascular endothelial cells (RMEC) (3, 15, 16). These data suggest that elevated fatty acids may be causally linked to retinal inflammation occurring early in DR pathogenesis, and provides a novel target for DR therapeutic intervention.
Our data and those from other labs, have shown that MC amplify tumor necrosis factor alpha (TNFα), interleukin-1β (IL-1β), interleukin-6 (IL-6) interleukin-8 (IL-8) and Angptl4 through autocrine and paracrine signaling pathways and each has been linked to angiogenic and/or inflammatory component of DR (4, 21–24). Tumor necrosis factor alpha (TNFα) induces vascular inflammation, and increased serum and vitreous levels of TNFα correlate with the onset and progression of DR (25–29). TNFα stimulates retinal endothelial cells to express inflammatory chemokines and the leukocyte adhesion molecules ICAM-1, VCAM-1, and E-selectin (30, 31). IL-1β, IL-6 and IL-8 are inflammatory cytokines and increased retinal/vitreous levels have been observed in DR animal models and patients (25, 32–36). Thus, these cytokines constitute rational targets for therapeutic intervention in order to limit DR progression. Furthermore, drug targets that manipulate the production of multiple cytokines may be more efficacious than targeting any one cytokine alone.
PPARβ/δ is a transcription factor that is primarily known for its role in lipid metabolism, however our group and others have shown that it can regulate both inflammation and angiogenesis (37–40). Angiopoietin-like 4 (Angptl4) is one of its known target genes (41). Notably, Angptl4 has been firmly established as a potent angiogenic/permeability factor in DR (24, 42–44). GSK0660 is a selective antagonist of PPARβ/δ, which also has inverse agonist activity when used alone (45). Our lab generated evidence that GSK0660 inhibits the TNFα-induced expression of multiple chemokines human retinal microvascular endothelial cells (HRMEC) (40), however the functional significance of these transcriptional changes has not been evaluated. Additionally, using an unbiased approach, we recently observed that several target of PPARβ/δ activity were altered in PA-treated Müller cells (2). As such, we hypothesized that PPARβ/δ constitutes a rational therapeutic target for DR because it may exert its effects at multiple steps in the pathologic cascade. Specifically, we used GSK0660 to interrogate the role of PPARβ/δ in: 1) the induction of pro-inflammatory cytokines in human MC (HMC), 2) the paracrine effect of one of these cytokines, TNFα, on leukocyte adhesion, and 3) TNFα-stimulated retinal leukostasis. These findings represent novel extensions of our previous work (2, 40). Importantly, we identify a role of GSK0660 in the upstream production of inflammatory mediators by Müller cells. Moreover, we demonstrate that transcriptional changes associated with GSK0660 treatment in retinal endothelial cells elicits significant changes in inflammatory behaviors that occur early in DR pathogenesis. These findings further support the clinical potential of PPARβ/δ manipulation in the treatment of DR.
2.1. Materials and Methods
2.2. Reagents
The PPARβ/δ inhibitor GSK0660 and the PPARβ/δ agonist GW0742 were purchased from Tocris (Minneapolis, MN, USA). Palmitic acid and fatty acid-free BSA was purchased from Sigma (St. Louis, MO, USA). Human retinal microvascular endothelial cells (HRMEC) and attachment factor were purchased from Cell Systems (Kirkland, WA, USA). Peripheral blood mononuclear cells were purchased from Sanguine Biosciences (Valencia, CA, USA). TNFα, CCL8, and CXCL10 were purchased from R&D Systems (Minneapolis, MN, USA).
2.3. Culture and treatment of human Müller cells (HMC)
The vehicle for PA was prepared by dissolving fatty acid free BSA in PBS at 100mg/ml, hereafter referred to as BSA. PA was dissolved in EtOH at 200mM and incubated with BSA for 90 minutes at 37°C to achieve 5mM. BSA-conjugated PA was then diluted in culture media to a final concentration of 250μM, hereafter referred to as PA. GSK0660 and GW0742 were dissolved in DMSO to achieve a 1mM solution. These solutions were added to cell culture media to achieve 1mM concentration and 0.1% DMSO. Preliminary dose response experiments were performed with PA and GSK0660, confirming no significant changes in cell viability (data not shown).
Retinal tissues for Müller cell isolation were harvested from a 42 year old, caucasion male under NDRI protocol #ND06065 and isolated as described previously (46). HMC were cultured in DMEM containing 10% fetal bovine serum (FBS) and 1x antibiotic/antimycotic solution hereafter referred to as HMC growth medium. Passages 4 through 6 were used for all experiments. HMC were cultured in growth medium in 6-well dishes until approximately 70% confluent. Growth medium was aspirated replaced with 2% FBS or 2% + GSK0660 for 12 hours. These media were aspirated and HMC were treated with fresh 2% FBS + BSA (vehicle), PA, PA + GSK0660, or GW0742 for 24 hours.
2.4. Culture and treatment of HRMEC
HRMEC were cultured on attachment factor-coated plates in endothelial basal medium (EBM) supplemented with 10% FBS. Cultures were grown to 70% confluency in a humidified cell culture incubator at 37°C in 5% CO2. For studies using GSK0660, medium was changed to 2% FBS with either vehicle (0.1% DMSO) or GSK0660 for 24hrs. HRMEC were then stimulated with 1ng/ml TNFα in combination with vehicle or GSK0660 for an additional 4hrs. Similarly, in experiments using CCL8 or CXCL10, recombinant proteins (50ng/ml) were added concomitantly with GSK0660 24 hours prior to TNFα treatment. Concentrations of TNFα were determined from previous, published studies (47, 48). For studies using GW0742, medium was changed to 2% FBS for 12hrs, and then treated in 2% FBS plus vehicle (0.1% DMSO) or GW0742. For blocking antibody studies, anti-CCL8 (Fisher Scientific Company LLC; Suwanee GA, USA) was used at 1μg/ml and anti-CXCL10 (Fisher Scientific Company LLC) was used at 4μg/ml. These were added coincident with TNFα, 4 hours before use in the parallel plate flow chamber.
2.5. Quantitative real-time PCR (qRT-PCR)
Cells were lysed and total RNA was isolated from cell lysates using an RNeasy kit (Qiagen; Valencia, CA, USA) according to the manufacturer’s directions. RNA was reverse transcribed to cDNA using the High-Capacity cDNA Archive Kit (Applied Biosystems; Carlsbad, CA, USA). The target genes (TNFA, IL1β, IL6, IL8 (CXCL8), CCL8, CXCL10, CCL17, ANGPTL4) vs ACTB or 18S were amplified by qRT-PCR using gene-specific TaqMan Gene Expression Assays (Applied Biosystems). Primer ID and target exons are included in Table 1. Data were analyzed using the comparative Ct method and Ct values were normalized to ACTB or 18S levels.
Table 1.
Primer IDs, exons boundary, and amplicon length of Taqman primers used in this study.
| Gene | Taqman Primer Assay ID | Exon boundary | Amplicon Length |
|---|---|---|---|
| TNFA | Hs00174128_m1 | 3–4 | 80 |
| IL1B | Hs01555410_m1 | 3–4 | 91 |
| IL6 | Hs00985639_m1 | 2–3 | 66 |
| IL8 (CXCL8) | Hs00174103_m1 | 1–2 | 101 |
| ANGPTL4 | Hs01101127_m1 | 6–7 | 92 |
| CCL8 | Hs04187715_m1 | 1–2 | 67 |
| CXCL10 | Hs01124251_g1 | 2–3 | 135 |
| CCL17 | Hs00171074_m1 | 2–3 | 51 |
| ACTB | Hs99999903_m1 | 1–1 | 171 |
| 18S | Hs99999901_s1 | 1–1 | 187 |
2.6. Parallel plate flow chamber
Peripheral blood mononuclear cells were resuspended in Hank’s Buffered Salt Solution (HBSS) at a concentration of 5×105 cells/ml and loaded into a syringe. HRMEC were grown in monolayers on glass slides coated with attachment factor and treated with drugs as outlined in the above sections. After treatment, slides were mounted in a rectangular parallel plate flow chamber with a silicon rubber gasket (GlycoTech; Gaithersburg, MD, USA). The chamber had a flow width of 1.00cm and a height of 0.0127cm. The chamber was connected to inlet and outlet syringes with Silastic™ tubing (GlycoTech) with an inner diameter of 1/16 inch. A syringe pump (World Precision Instruments; Sarasota, FL, USA) was used to pull PBMC across HRMEC monolayers at a rate of 150μl/min for 7min. Non-adherent cells were washed off the plate with HBSS at a flow rate of 300μl/min for 2min. Eight fields of view were captured using an IMT-2 inverted microscope (Olympus; Tokyo, Japan) and Q-Color3 digital camera (Olympus) at 20x magnification. Adherent cell counts were performed by masked observers using ImageJ (NIH; Bethesda, MD, USA) and averaged for each slide.
2.7. Intravitreal Injections
All experiments using mice were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were performed in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. Eight-week old, male C57BL/6N mice (Charles River Laboratories; Wilmington, MA, USA) were anesthetized by isoflurane (Butler Animal Health Supply; Minneapolis, MN, USA) inhalation. Before intravitreal injection, 0.5% proparacaine (Allergan, Troy Hills, NJ, USA) was topically applied to the cornea. The globe was penetrated approximately 0.5mm posterior to the ora serrata, using a 30-gauge needle with a 19° bevel and a 10μL syringe (Hamilton Co., Reno, NV, USA). The needle was advanced to the posterior vitreous at a steep angle to avoid contact with the lens. The injection bolus was delivered near the trunk of the hyaloid artery proximal to the posterior pole of the retina. After injection, a topical antibiotic suspension (Vigamox; Alcon, Fort Worth, TX, USA) was applied. Mice were given a 1μl injection of vehicle (0.1% DMSO in PBS), GW0742 (1μM), 50ng/ml TNFα plus vehicle, 50ng/ml TNFα plus 1μM GSK0660, or 50ng/ml recombinant CCL8 plus 50ng/ml recombinant CXCL10.
2.8. In vivo leukostasis
Retinal leukostasis was performed as described previously (47). Briefly, 6hrs after injection, mice were anesthetized with ketamine and xylazine and perfused through the left ventricle with 0.9% saline at physiological pressures followed by 2.5ml of 40μg/ml FITC-conjugated concanavalin-A (Vector Laboratories; Burlingame, CA, USA). Non-adherent leukocytes were washed out with 0.9% saline. Eyes were enucleated and retinas dissected in 4% paraformaldehyde. Retinas were flat-mounted and fluorescent images captured with an AX70 upright microscope (Olympus) with a DP71 digital camera (Olympus). Lumenal leukocytes were counted by masked observers using ImageJ and normalized to the retinal vascular area.
2.9. Statistics
The data collected were relative mRNA expression levels, leukocytes adhered per mm2, and leukocytes adhered per retina. For all experiments, statistical significance was determined using either a one-way or two-way ANOVA with a student’s t post hoc analysis using the software JMP (SAS Institute; Cary, NC, USA). Data are considered significant with p < 0.05.
3.1. Results
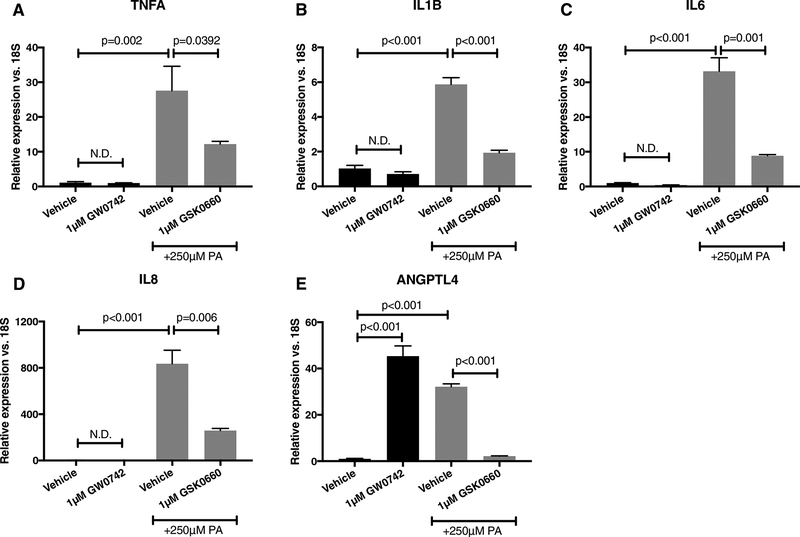
3.2. The PPARβ/δ inverse agonist GSK0660 attenuates palmitic acid-induced cytokine expression in HMC
Based on our previous findings that palmitic acid stimulates transcription of several inflammatory mediators (2), we tested the hypothesis that PA-induced cytokine expression was PPARβ/δ-dependent. Indeed, in HMC, PA increased the following cytokine transcriptional targets relative to vehicle value +/− SD: TNFA, 27.6 ± 12.3; IL1B, 5.9 ± 0.7; IL6, 33.2 ± 6.8; IL8, 835.3 ± 203; and ANGPTL4, 32.2 ± 2.1. Co-treatment of HMC with GSK0660 reduced PA-stimulated expression of TNFA by 55.8% (Fig. 1A); IL1B by 67.2% (Fig. 1B); IL6 by 73.3% (Fig. 1C); IL8 by 69% (Fig. 1D); and ANGPTL4 by 93.1% (Fig 1E). To test PPARβ/δ-dependency of these effects, HMC were treated with PPARβ/δ agonist GW0742. We observed a 45.3-fold increase in ANGPTL4 mRNA (Fig. 1E). However, no increases in TNFA, IL1B, IL6, and IL8 were observed (Fig. 1A–D), suggesting that GSK0660’s effect on inflammatory cytokine transcription were, in part, PPARβ/δ-independent. Our observations reported herein are not likely to be related to any toxicity imposed by these compounds. In a previous study, we tested GSK0660 and GW0742 in HRMEC proliferation and tube formation assays with no evidence of toxicity (39). In the same study, we also tested these drugs on the normal retinal vascular development of rat pups and neither showed any effect.
Figure 1. GSK0660 inhibits PA-stimulated inflammatory cytokines in HMC in a PPARβ/δ-independent manner.
HMC were treated for 24 hours with BSA alone, BSA with 1μM GW0742, 250μM PA, or 250uM with 1μM GSK0660. (A) TNFA, (B) IL1B, (C) IL6, (D) IL8, or (E) ANGPTL4 were assessed by qRT-PCR. All data are relative to BSA alone. Bars represent mean ± SEM (n=3).
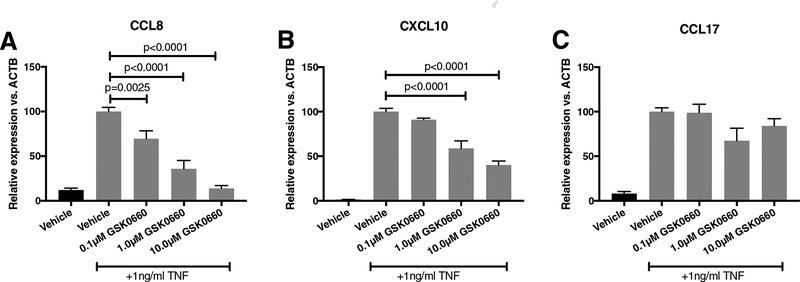
3.3. PPARβ/δ inhibition by GSK0660 attenuates TNFα-induced chemokine expression
We next investigated the effect of one of the PA-stimulated cytokines, TNFα, on HRMEC to query the role of PPARβ/δ on inflammation from the cross-talk of HMC to HRMEC. While our previous studies identified and confirmed changes in CCL8 and CXCL10 with a single GSK0660 treatment by RNA-seq (40), we tested a range of doses in the present study to optimize further treatments of HRMEC. TNFα increased expression of CCL8 and CXCL10. HRMEC pre-treated with GSK0660 attenuated TNFα-induced expression of CCL8 and CXCL10 in a dose-dependent manner (Fig. 2A–B, Supplementary Fig. 1). At the highest concentration of GSK0660 (10μM), CCL8 and CXCL10 were inhibited by 86.1% (p<0.0001) and 59.7% (p<0.0001), respectively. TNFα-induced CCL17 was not significantly decreased with any dose of GSK0660 (Fig. 2C).
Figure 2. GSK0660 inhibits TNFα-stimulated CCL8 and CXCL10 in HRMEC.
HRMEC were pre-treated with increasing concentrations of GSK0660 for 24hrs before stimulation with 1ng/ml TNFα for 4hrs. (A) CCL8, (B) CXCL10, and (C) CCL17 were assessed in treated HRMEC by qRT-PCR. All data are relative to the TNFα plus vehicle-treated samples. Bars represent mean ± SEM (n= 6).
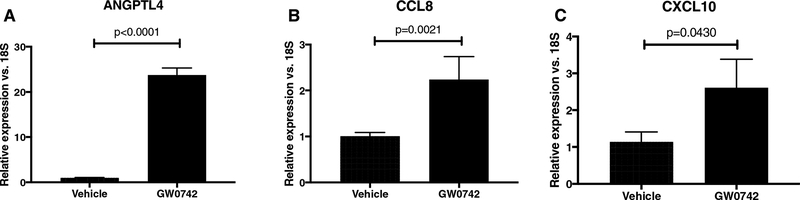
3.4. PPARβ/δ activation by GW0742 increases CCL8 and CXCL10 expression
To further examine the PPARβ/δ-dependency of the effects of GSK0660, we complimented our studies with use of a PPARβ/δ agonist. PPARβ/δ activation in HRMEC was accomplished using the agonist GW0742, and verified by expression of the target gene, ANGPTL4 (23.5-fold; p<0.0001; Fig. 3A) (39, 49, 50). GW0742 induced the expression of CCL8 by 2.2-fold (p=0.0021; Fig. 3B) and CXCL10 by 2.3-fold (p=0.430; Fig. 3C) following 12hrs of treatment. Taken together, these data support the idea that GSK0660 exerts its effect on HRMEC chemokine expression at least in part through a PPARβ/δ-dependent mechanism.
Figure 3. GW0742 stimulates CCL8 and CXCL10 expression in HRMEC.
HRMEC were treated with vehicle or 1μM GW072 for 12hrs. PPARβ/δ activation was confirmed by (A) ANGPTL4 expression. (B) CCL8 and (C) CXCL10 were assessed by qRT-PCR. Bars represent mean ± SEM (n=6).
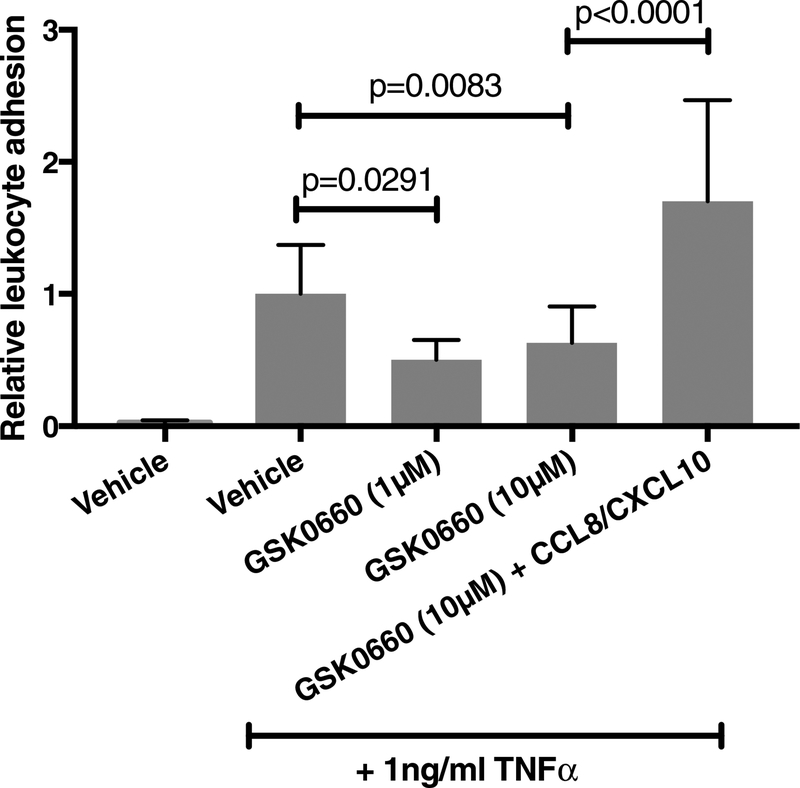
3.5. Inhibition of PPARβ/δ reduces TNFα-induced cell adhesion
To determine the behavioral effects of PPARβ/δ manipulation, we assessed PPARβ/δ inhibition in leukocyte behavior. As shown in Fig. 4A, treatment of HRMEC with both 1μM and 10μM GSK0660 significantly inhibited TNFα-induced cell adhesion by 49.7% (p=0.0291) and 36.8% (p=0.0083), respectively. The addition of recombinant CCL8 and CXCL10 with GSK0660 to HRMEC monolayers prevented the effect of GSK0660 on TNFα-induced cell adhesion.
Figure 4. GSK0660 inhibits TNFα-induced PBMC adhesion to HRMEC via its effect on CCL8 and CXCL10.
HRMEC monolayers were treated with vehicle, 1μM, or 10μM GSK0660 for 24hrs before stimulation with 1ng/ml TNFα. An additional group was treated with 50ng/ml of both CCL8 and CXCL10 in addition to 10μM GSK0660. PBMC were flowed over treated monolayers in a parallel plate flow chamber and adherent leukocytes were quantified. Data are shown as relative to vehicle control. Bars represent mean ± SEM (n=6).
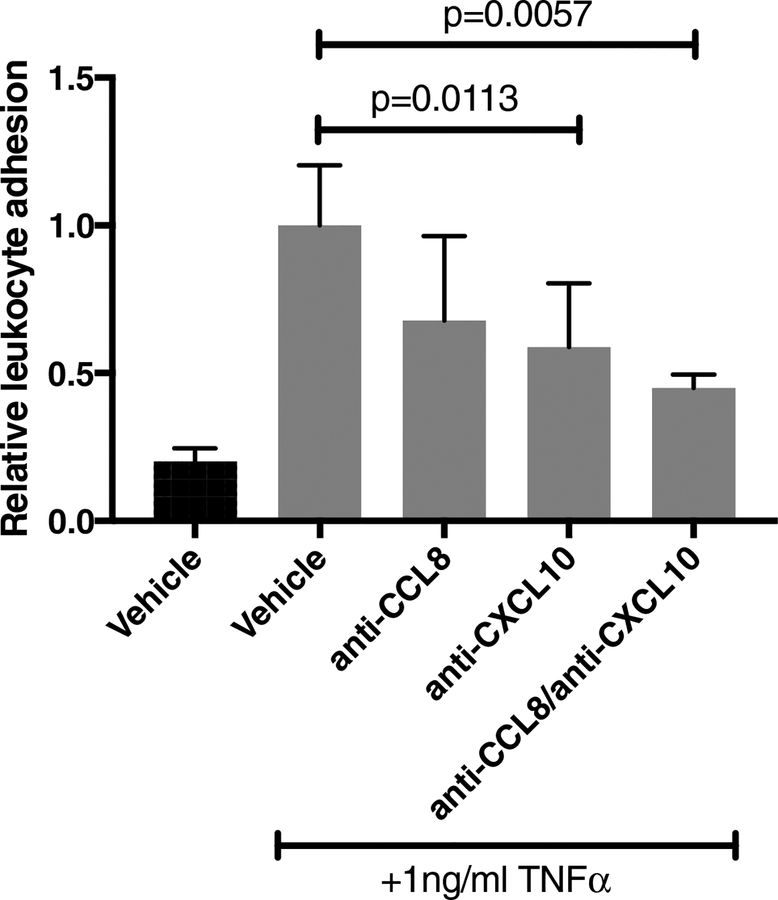
3.6. CCL8 and CXCL10 are involved in TNFα-induced leukocyte adhesion
HRMEC monolayers were treated with maximal doses of antibodies against CCL8 and CXCL10 (as determined from preliminary experiments), alone or in combination. Addition of anti-CCL8 did not significantly affect TNFα-induced PBMC adhesion to HRMEC monolayers (Fig. 5). However, addition of anti-CXCL10 significantly reduced TNFα-induced cell adhesion by 41.2% (p=0.0113). Combination treatment with anti-CCL8 and anti-CXCL10 further reduced TNFα-induced PBMC adhesion, inhibiting induction by 55.1% (p=0.0057).
Figure 5. CCL8 and CXCL10 mediate TNFα-induced PBMC adhesion to HRMEC.
HRMEC monolayers were treated with 1μg/ml anti-CCL8, 4μg/ml anti-CXCL10, or a combination of both CCL8 and CXCL10, concomitant with 1ng/ml TNFα stimulation. PBMC were flowed over treated monolayers in a parallel plate flow chamber and adherent leukocytes were quantified. Data are shown as relative to vehicle control. Bars represent mean ± SEM (n=6).
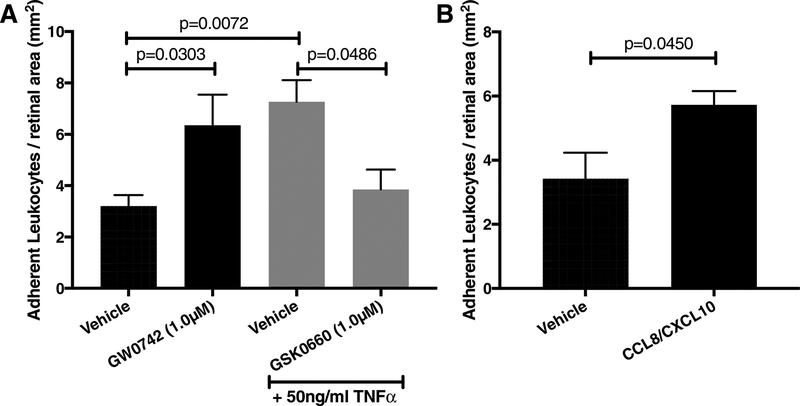
3.7. PPARβ/δ is involved in TNFα-induced retinal leukostasis in vivo
Intravitreal injections of TNFα increased the number of adherent leukocytes in retinal vessels by 2.3-fold (p=0.0072; Fig. 6A). PPARβ/δ agonism by GW0742 induced leukostasis by 2-fold (p=0.0303) while co-treatment of TNFα and GSK0660 reduced TNFα-induced leukostasis by 47.1% (p=0.0486). Similar to TNFα injection, recombinant CCL8 (50ng/ml) and CXCL10 (50ng/ml) significantly increased adherent leukocytes by 1.7-fold (p=0.045; Fig. 6B).
Figure 6. GSK0660 inhibits TNFα-induced retinal leukostasis in a PPARβ/δ-dependent manner.
(A) Mice were injected with vehicle, 1μM GW0742, 50ng/ml TNFα vehicle, or 50ng/ml TNFα with 1μM GSK0660. (B) Mice were injected with vehicle or 50ng/ml recombinant CCL8 with 50ng/ml recombinant CXCL10. Six hrs after intravitreal injection, mice were perfused with FITC-conjugated concanavalin-A to label adherent leukocytes. Bars represent mean ± SEM (A: vehicle n=13; GW0742 n=5; TNFα vehicle n=4; TNFα + GSK0660 n=8; B: vehicle n=5; CCL8/CXCL10 n=5).
Discussion
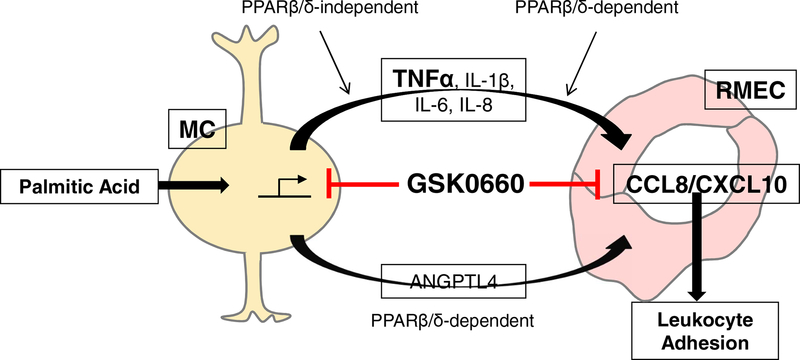
The homeostatic functions of Müller cells decline in animal models of DR, perhaps leading to their activation (gliosis) and release of angiogenic growth factors and/or inflammatory cytokines. Gliosis, as defined by elevated glial fibrillary acidic protein (GFAP) occurs early in disease progression, preceding the vascular changes that characterize DR (51). In vitro studies support the theory that diabetes-relevant stimuli elicit these events. For example, Müller cells cultured in high glucose media increase expression of the activation marker glial fibrillary acidic protein (GFAP) and increase their release of inflammatory cytokines (52). However, the interpretation of these and related studies is sometimes obscured depending on the species, the use of normal or transformed Müller cells, and/or the incorporation of an osmotic control into the experimental design (2). Informed by studies suggesting a role for dyslipidemia in DR pathogenesis (2, 3), we used saturated fatty acids as a way to model an initiating step in the DR pathogenic cascade (Fig. 7).
Figure 7. Model of GSK0660 on upstream (Müller cell) and downstream (endothelial cell) inflammation.
Palmitic acid stimulates the release of inflammatory cytokines (TNFα, IL-1β, IL-6, IL-8) and a pro-angiogenic factor (Angptl4). GSK0660 reduces the stimulation of these factors in PPARβ/δ-independent and -dependent manners, respectively. TNFα can then act in a paracrine manner to stimulate CCL8 and CXCL10 production by endothelial cells. GSK0660 blocks the production of these chemokines in a PPARβ/δ-dependent manner, thereby limiting leukostasis.
PA is consistently elevated in the sera and tissues of diabetics, and we found it to be a potent inducer of TNFA, IL1B, IL6, IL8, and ANGPTL4 in non-diabetic donor HMC. Each plays a significant role in DR pathogenesis. Increased concentrations of Angptl4 are observed in the aqueous of PDR patients and correlates with neovascular disease and retinal edema, independent of VEGF (42, 44). Experiments in hypoxic Müller cells and in a mouse model of oxygen-induced retinopathy revealed that increased Angptl4 expression was hypoxia–inducible factor (HIF)-dependent suggesting that hypoxia is the primary inducer of Angptl4 in PDR (44). However, free-fatty acids (FFAs) bind and activate PPARβ/δ, and Angptl4 is one of its target genes. Therefore, our findings suggest that PA induces a PPARβ/δ-dependent increase in Angptl4 that may elicit pathologic changes in response to dyslipidemia, independent of ischemia-induced hypoxia. Notably, we observed that GSK0660 mitigated ANGPTL4 expression, suggesting a potential therapeutic strategy to intervene at the earliest stages of disease progression. As further support of PPARβ/δ as a drug target, ANGPTL4 expression was increased in HMC by GW0742, suggesting that transcriptional regulation of this particular target is PPARβ/δ-dependent.
We also observed that GSK0660 decreased PA-induced TNFA, IL1B, IL6 and IL8 expression in HMC. However, unlike ANGPTL4, GW0742 did not increase expression of these inflammatory cytokines, suggesting that GSK0660 has off-target, but nonetheless beneficial effects. Further studies are warranted to elucidate these GSK0660 targets to facilitate the design, synthesis and implementation of novel inhibitors.
Since inflammatory cytokines released from Müller cells may activate inflammation pathways through paracrine signaling to other retinal cells, we investigated whether GSK0660 reduces TNFα-mediated inflammatory changes HRMEC. In previous experiments, we found that GSK0660 mitigates TNFα-induced CCL8 and CXCL10 gene expression in HRMEC. As part of this study, we confirmed these expression changes by qRT-PCR, and also determined similar effects at the protein level (Supplementary Fig. 1). There are data suggesting roles for CCL8 and CXCL10, also called MCP-2 and IP-10, respectively in DR. For example, in experimental DR, VEGFR1 blockade reduced retinal leukostasis and permeability in part through down-regulation of ccl8 and cxcl10 (53). Interestingly, CCL8 activates CCR5, and leukocytes expressing high levels of CCR5 were found to be the primary leukocyte subset involved in retinal capillary leukostasis (54, 55). CXCL10 may also contribute to DR pathogenesis, because its levels are significantly elevated in the vitreous of DR patients (56, 57). Guided by these studies, we performed additional experiments to investigate the functional significance of these transcriptional changes. We observed that GSK0660 reduces CCL8- and/or CXCL10-dependent leukocyte adhesion in in vitro assays (Fig. 5) and leukostasis in mice (Fig. 6B). Since CCL8 and CXCL10 have not been previously characterized as direct targets of PPARβ/δ, we performed additional tests to determine if the observed effects of GSK0660 were PPARβ/δ-dependent. Pharmacologic agonism with the well-characterized PPARβ/δ agonist, GW0742 increased CCL8 and CXCL10 expression (Fig. 3), suggesting PPARβ/δ-dependency. We also identified peroxisome proliferator response element (PPRE) motifs in the upstream 5’ promoter regions of the human CCL8 and CXCL10 genes by screening against a collection of 235 PPRE motifs (58). Six PPREs were found in CXCL10 and 3 were found in CCL8. This further suggests that reduced CCL8 and CXCL10 expression by GSK0660 is PPARβ/δ-dependent.
PPARβ/δ has been studied in the context of vascular inflammation, with a majority of work suggesting that PPARβ/δ agonism is anti-inflammatory. In human umbilical vein endothelial cells (HUVECs), PPARβ/δ agonists inhibited TNFα-induced expression of VCAM-1 and ICAM-1 (37, 59). Furthermore, PPARβ/δ agonists have been shown to inhibit the expression of some chemokines. In HUVECs, the PPARβ/δ agonist GW501516 reduced TNFα-induced release of CXCL10, and in cultured human monocytes it prevented VLDL-induced expression of MIP-1α (CCL3) (37). Activation of PPARβ/δ prevented TNFα-induced inflammation in adipocytes, HUVECs, and proximal tubular cells (37, 59–61). Additionally, PPARβ/δ agonists have been shown to be protective against inflammation associated with hyperoxia-induced lung injury and spinal cord injury in rodents (62, 63). Interestingly, in the present study we observed that PPARβ/δ agonism with GW0742 stimulates both chemokine expression (Fig. 3) and retinal leukostasis (Fig. 6A).
There has been little work testing the PPARβ/δ antagonist GSK0660 on molecular and cellular events that promote inflammation. Notwithstanding the clear effects of agonism on reducing inflammation, there also exist reports suggesting that GSK0660 might be anti-inflammatory. In monocytes, GSK0660 reverses the effect of carbaprostacyclin (a PPARβ/δ agonist) and TNFα on CXCL8 expression (64). GSK0660 also reduces an inflammatory psoriasis-like skin condition in mice, perhaps by reversing the upregulation of IL-1β (65). In our study in HRMEC, GSK0660 had no effect on TNFα-induced ICAM-1 or VCAM-1 expression (data not shown), but it reduced chemokine expression (Fig. 1), leukocyte adhesion to endothelial monolayers (Fig. 4), and leukostasis. Opposite effects were observed when using PPARβ/δ agonist GW0742, suggesting they were indeed PPARβ/δ-dependent. It is therefore possible that PPARβ/δ is differentially regulated depending on the cell type and tissue queried.
The exact mechanism by which PPARβ/δ agonists and GSK0660 affect inflammation is still unknown. However, there are many reports suggesting these small molecules activate different pathways depending on concentration, duration, cell type, and context. For example, the PPARβ/δ agonist GW0742 reduced TNFα-induced VCAM-1, ICAM-1, and E-selectin expression in a BCL6-dependent manner in umbilical vein cells (59). In the same experiment, GW0742 had no effect on NF-κB translocation, nor did it affect phosphorylation of the three different MAPK pathways JNK, ERK, or p38 MAPK. However, in another endothelial cell line, a PPARβ/δ agonist inhibited TNFα-induced VCAM-1 expression by inhibiting NF-κB translocation (66). Lastly, GSK0660 and PPARβ/δ might act through a non-genomic mechanism to inhibit ERK signaling. PPARβ/δ has been shown to directly activate the Akt signaling pathway by binding to the regulatory subunit of the kinase PI3K (67). Further work should be done to determine the differential activation of signaling pathways by agonists and antagonists of PPARβ/δ in retinal endothelial cells.
In this study we used the C57BL/6 mice that are known to carry the rd8 mutation in Crb1. This gene causes retinal degeneration that could affect ocular inflammatory processes. Schnabolk et al. 2014, investigated potential differences between rd8 positive and negative substrains to susceptibility to laser-induced choroidal neovascularization (LCNV) and inflammatory gene expression(68). They found that LCNV was not affected by the rd8 mutation and that the inflammatory gene expression was blunted. Therefore, it is reasonable to assume that this mutation did not skew our endpoints and if there were any minor effects, they were properly controlled in our experimental design.
In the present study, we demonstrated that GSK0660 is anti-inflammatory in both PPARβ/δ-dependent and -independent manners. Notably, one limitation to the present study is that Müller cells from a single donor were used. Despite this, these findings are an important extension of our previous work, because we confirm that the transcriptional pathways modulated by GSK0660 lead to significant attenuation of DR-related inflammatory processes. We propose a model in which GSK0660 intervenes at multiple steps in DR progression (Fig. 7). The notion of using GSK0660 as an intervention for DR is further bolstered by our previous work in which we demonstrated that PPARβ/δ inhibition with GSK0660 was angiostatic (39), which would be beneficial against the late, proliferative stage of DR as well. Taken together, our findings demonstrate that PPARβ/δ inhibition may be an ideal therapeutic strategy for DR because it is efficacious in multiple pathogenic steps of the disease.
Supplementary Material
Highlights.
The PPARβ/δ inhibitor GSK0660 inhibits palmitic acid-stimulated inflammatory mediator production by Müller cells.
GSK0660 inhibits TNFα-induced leukocyte adhesion in both HRMEC and mouse retina.
GSK0660 blocks TNFα-induced leukostasis by modulating the levels of CCL8 and CXCL10.
GSK0660’s effects on retinal cells are mediated by both PPARβ/δ-dependent and PPARβ/δ-independent pathways.
Funding:
This work was supported by the National Institutes of Health (R01-EY07533; R01-EY023639; P30-EY008126; P30-DK020593; T32-EY07135; 2UL1-TR000445); an unrestricted grant from Research to Prevent Blindness, Inc.; and the Carl Marshall Reeves & Mildred Almen Reeves Foundation, Inc.
Abbreviations
- Angptl4
Angiopoietin-like protein 4
- CCL8
C-C motif chemokine ligand 8
- CXCL10
C-X-C motif chemokine ligand 10
- DR
Diabetic retinopathy
- EC
Endothelial cell
- HMC
Human Müller cell
- HRMEC
Human retinal microvascular endothelial cell
- IL-1β
Interleukin 1β
- IL6
Interleukin 6
- IL8
Interleukin 8
- NPDR
Non-proliferative diabetic retinopathy
- MC
Müller cell
- PA
Palmitic acid
- PBMC
Peripheral blood mononuclear cells
- PDR
Proliferative diabetic retinopathy
- PPARβ/δ
Peroxisome proliferator-activated receptor β/δ
- TNFα
Tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fong DS, Aiello LP, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27(10):2540–53. [DOI] [PubMed] [Google Scholar]
- 2.Capozzi ME, Giblin MJ, Penn JS. Palmitic Acid Induces Muller Cell Inflammation that is Potentiated by Co-treatment with Glucose. Sci Rep. 2018;8(1):5459. doi: 10.1038/s41598-018-23601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capozzi ME, McCollum GW, Cousins DB, Penn JS. Linoleic Acid is a Diabetes-relevant Stimulator of Retinal Inflammation in Human Retinal Muller Cells and Microvascular Endothelial Cells. J Diabetes Metab. 2016;7(12). doi: 10.4172/2155-6156.1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Biarnes Costa M, Gerhardinger C. IL-1beta is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1beta autostimulation. PLoS One. 2012;7(5):e36949. doi: 10.1371/journal.pone.0036949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klen J, Goricar K, Janez A, Dolzan V. NLRP3 Inflammasome Polymorphism and Macrovascular Complications in Type 2 Diabetes Patients. J Diabetes Res. 2015;2015:616747. doi: 10.1155/2015/616747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Y, Pagadala J, Miller D, Steinle JJ. Reduced insulin receptor signaling in retinal Muller cells cultured in high glucose. Mol Vis. 2013;19:804–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SY, Johnson MA, McLeod DS, Alexander T, Hansen BC, Lutty GA. Neutrophils are associated with capillary closure in spontaneously diabetic monkey retinas. Diabetes. 2005;54(5):1534–42. [DOI] [PubMed] [Google Scholar]
- 8.Schroder S, Palinski W, Schmid-Schonbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139(1):81–100. [PMC free article] [PubMed] [Google Scholar]
- 9.Gustavsson C, Agardh CD, Zetterqvist AV, Nilsson J, Agardh E, Gomez MF. Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia. PLoS One. 2010;5(9):e12699. doi: 10.1371/journal.pone.0012699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalfaoui T, Lizard G, Ouertani-Meddeb A. Adhesion molecules (ICAM-1 and VCAM-1) and diabetic retinopathy in type 2 diabetes. J Mol Histol. 2008;39(2):243–9. doi: 10.1007/s10735-007-9159-5. [DOI] [PubMed] [Google Scholar]
- 11.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 12.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86(4):1245–57. [DOI] [PubMed] [Google Scholar]
- 13.Lutty GA, Cao J, McLeod DS. Relationship of polymorphonuclear leukocytes to capillary dropout in the human diabetic choroid. Am J Pathol. 1997;151(3):707–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96(19):10836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed IN, Hafez SS, Fairaq A, Ergul A, Imig JD, El-Remessy AB. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia. 2014;57(2):413–23. doi: 10.1007/s00125-013-3101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Jump DB, Grant MB, Esselman WJ, Busik JV. Dyslipidemia, but not hyperglycemia, induces inflammatory adhesion molecules in human retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 2003;44(11):5016–22. [DOI] [PubMed] [Google Scholar]
- 17.Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A, McSorley KM, Renis RL, Kern T, Jump DB, Reid GE, Busik JV. Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2010;59(1):219–27. doi: 10.2337/db09-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chorvathova V, Ondreicka R. The fatty acid composition of the tissues of streptozotocin-diabetic rats. Physiol Bohemoslov. 1983;32(5):466–75. [PubMed] [Google Scholar]
- 19.Korani M, Firoozrai M, Maleki J, Ghahramanpour F, Heidari I, Fallah S, Seifi M. Distribution of fatty acids in adipose tissue of patients with type 2 diabetes. Clin Lab. 2012;58(5–6):457–64. [PubMed] [Google Scholar]
- 20.Hegde KR, Varma SD. Electron impact mass spectroscopic studies on mouse retinal fatty acids: effect of diabetes. Ophthalmic Res. 2009;42(1):9–14. doi: 10.1159/000219679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Ye F, Xiong H, Hu D, Limb GA, Xie T, Peng L, Yang W, Sun Y, Zhou M, Song E, Zhang DY. IL-1beta Upregulates IL-8 Production in Human Muller Cells Through Activation of the p38 MAPK and ERK1/2 Signaling Pathways. Inflammation. 2014;37(5):1486–95. doi: 10.1007/s10753-014-9874-5. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Ye F, Xiong H, Hu DN, Limb GA, Xie T, Peng L, Zhang P, Wei Y, Zhang W, Wang J, Wu H, Lee P, Song E, Zhang DY. IL-1beta induces IL-6 production in retinal Muller cells predominantly through the activation of p38 MAPK/NF-kappaB signaling pathway. Exp Cell Res. 2015;331(1):223–31. doi: 10.1016/j.yexcr.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 23.Liu ST, Zhong SM, Li XY, Gao F, Li F, Zhang ML, Zhu K, Sun XH, Wang X, Miao Y, Yang XL, Wang Z. EphrinB/EphB forward signaling in Muller cells causes apoptosis of retinal ganglion cells by increasing tumor necrosis factor alpha production in rat experimental glaucomatous model. Acta Neuropathol Commun. 2018;6(1):111. doi: 10.1186/s40478-018-0618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sodhi A, Montaner S. Angiopoietin-like 4 as an Emerging Therapeutic Target for Diabetic Eye Disease. JAMA Ophthalmol. 2015;133(12):1375–6. doi: 10.1001/jamaophthalmol.2015.3723. [DOI] [PubMed] [Google Scholar]
- 25.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond). 2006;20(12):1366–9. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- 26.Doganay S, Evereklioglu C, Er H, Turkoz Y, Sevinc A, Mehmet N, Savli H. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond). 2002;16(2):163–70. doi: 10.1038/sj/EYE/6700095. [DOI] [PubMed] [Google Scholar]
- 27.Zorena K, Mysliwska J, Mysliwiec M, Balcerska A, Hak L, Lipowski P, Raczynska K. Serum TNF-alpha level predicts nonproliferative diabetic retinopathy in children. Mediators Inflamm. 2007;2007:92196. doi: 10.1155/2007/92196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koleva-Georgieva DN, Sivkova NP, Terzieva D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (Plovdiv). 2011;53(2):44–50. [DOI] [PubMed] [Google Scholar]
- 29.Ju HB, Zhang FX, Wang S, Song J, Cui T, Li LF, Zhang HY. Effects of fenofibrate on inflammatory cytokines in diabetic retinopathy patients. Medicine (Baltimore). 2017;96(31):e7671. doi: 10.1097/MD.0000000000007671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crane IJ, Wallace CA, McKillop-Smith S, Forrester JV. Control of chemokine production at the blood-retina barrier. Immunology. 2000;101(3):426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest Ophthalmol Vis Sci. 2012;53(9):5906–11. doi: 10.1167/iovs.12-10410. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2(2):96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovic MG, Korosec P, Kosnik M, Hawlina M. Vitreous levels of interleukin-8 in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2007;143(1):175–6. doi: 10.1016/j.ajo.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, Zhang J, Zhu R, Zhao R, Chen J, Jin J, Tian Y, Su SB. The Profile of Angiogenic Factors in Vitreous Humor of the Patients with Proliferative Diabetic Retinopathy. Curr Mol Med. 2017;17(4):280–6. doi: 10.2174/1566524017666171106111440. [DOI] [PubMed] [Google Scholar]
- 35.Tang J, Kern TS. Inflammation in diabetic retinopathy. Progress in retinal and eye research. 2011;30(5):343–58. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammad G, Mairaj Siddiquei M, Imtiaz Nawaz M, Abu El-Asrar AM. The ERK1/2 Inhibitor U0126 Attenuates Diabetes-Induced Upregulation of MMP-9 and Biomarkers of Inflammation in the Retina. J Diabetes Res. 2013;2013:658548. doi: 10.1155/2013/658548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piqueras L, Sanz MJ, Perretti M, Morcillo E, Norling L, Mitchell JA, Li Y, Bishop-Bailey D. Activation of PPARbeta/delta inhibits leukocyte recruitment, cell adhesion molecule expression, and chemokine release. J Leukoc Biol. 2009;86(1):115–22. doi: 10.1189/jlb.0508284. [DOI] [PubMed] [Google Scholar]
- 38.Piqueras L, Reynolds AR, Hodivala-Dilke KM, Alfranca A, Redondo JM, Hatae T, Tanabe T, Warner TD, Bishop-Bailey D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27(1):63–9. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 39.Capozzi ME, McCollum GW, Savage SR, Penn JS. Peroxisome proliferator-activated receptor-beta/delta regulates angiogenic cell behaviors and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2013;54(6):4197–207. doi: 10.1167/iovs.13-11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savage SR, McCollum GW, Yang R, Penn JS. RNA-seq identifies a role for the PPARbeta/delta inverse agonist GSK0660 in the regulation of TNFalpha-induced cytokine signaling in retinal endothelial cells. Mol Vis. 2015;21:568–76. [PMC free article] [PubMed] [Google Scholar]
- 41.La Paglia L, Listi A, Caruso S, Amodeo V, Passiglia F, Bazan V, Fanale D. Potential Role of ANGPTL4 in the Cross Talk between Metabolism and Cancer through PPAR Signaling Pathway. PPAR Res. 2017;2017:8187235. doi: 10.1155/2017/8187235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babapoor-Farrokhran S, Jee K, Puchner B, Hassan SJ, Xin X, Rodrigues M, Kashiwabuchi F, Ma T, Hu K, Deshpande M, Daoud Y, Solomon S, Wenick A, Lutty GA, Semenza GL, Montaner S, Sodhi A. Angiopoietin-like 4 is a potent angiogenic factor and a novel therapeutic target for patients with proliferative diabetic retinopathy. Proc Natl Acad Sci U S A. 2015;112(23):E3030–9. doi: 10.1073/pnas.1423765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sodhi A, Montaner S. Re: Kwon et al. : Aqueous levels of angiopoietin-like 4 and semaphorin 3E correlate with nonperfusion area and macular volume in diabetic retinopathy (Ophthalmology 2015;122:968–75). Ophthalmology. 2016;123(1):e7–8. doi: 10.1016/j.ophtha.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Xin X, Rodrigues M, Umapathi M, Kashiwabuchi F, Ma T, Babapoor-Farrokhran S, Wang S, Hu J, Bhutto I, Welsbie DS, Duh EJ, Handa JT, Eberhart CG, Lutty G, Semenza GL, Montaner S, Sodhi A. Hypoxic retinal Muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proc Natl Acad Sci U S A. 2013;110(36):E3425–34. doi: 10.1073/pnas.1217091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shearer BG, Steger DJ, Way JM, Stanley TB, Lobe DC, Grillot DA, Iannone MA, Lazar MA, Willson TM, Billin AN. Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist. Mol Endocrinol. 2008;22(2):523–9. doi: 10.1210/me.2007-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capozzi ME, McCollum GW, Penn JS. The role of cytochrome P450 epoxygenases in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2014;55(7):4253–60. doi: 10.1167/iovs.14-14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bretz CA, Savage SR, Capozzi ME, Suarez S, Penn JS. NFAT isoforms play distinct roles in TNFalpha-induced retinal leukostasis. Sci Rep. 2015;5:14963. doi: 10.1038/srep14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capozzi ME, Hammer SS, McCollum GW, Penn JS. Epoxygenated Fatty Acids Inhibit Retinal Vascular Inflammation. Sci Rep. 2016;6:39211. doi: 10.1038/srep39211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adhikary T, Brandt DT, Kaddatz K, Stockert J, Naruhn S, Meissner W, Finkernagel F, Obert J, Lieber S, Scharfe M, Jarek M, Toth PM, Scheer F, Diederich WE, Reinartz S, Grosse R, Muller-Brusselbach S, Muller R. Inverse PPARbeta/delta agonists suppress oncogenic signaling to the ANGPTL4 gene and inhibit cancer cell invasion. Oncogene. 2013;32(44):5241–52. doi: 10.1038/onc.2012.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staiger H, Haas C, Machann J, Werner R, Weisser M, Schick F, Machicao F, Stefan N, Fritsche A, Haring HU. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 2009;58(3):579–89. doi: 10.2337/db07-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Coughlin BA, Feenstra DJ, Mohr S. Muller cells and diabetic retinopathy. Vision Res. 2017;139:93–100. doi: 10.1016/j.visres.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He J, Wang H, Liu Y, Li W, Kim D, Huang H. Blockade of vascular endothelial growth factor receptor 1 prevents inflammation and vascular leakage in diabetic retinopathy. J Ophthalmol 2015;2015:605946. doi: 10.1155/2015/605946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gong W, Howard OM, Turpin JA, Grimm MC, Ueda H, Gray PW, Raport CJ, Oppenheim JJ, Wang JM. Monocyte chemotactic protein-2 activates CCR5 and blocks CD4/CCR5-mediated HIV-1 entry/replication. J Biol Chem. 1998;273(8):4289–92. [DOI] [PubMed] [Google Scholar]
- 55.Serra AM, Waddell J, Manivannan A, Xu H, Cotter M, Forrester JV. CD11b+ bone marrow-derived monocytes are the major leukocyte subset responsible for retinal capillary leukostasis in experimental diabetes in mouse and express high levels of CCR5 in the circulation. Am J Pathol. 2012;181(2):719–27. doi: 10.1016/j.ajpath.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006;17(3):155–65. [PubMed] [Google Scholar]
- 57.Xu Y, Cheng Q, Yang B, Yu S, Xu F, Lu L, Liang X. Increased sCD200 Levels in Vitreous of Patients With Proliferative Diabetic Retinopathy and Its Correlation With VEGF and Proinflammatory Cytokines. Invest Ophthalmol Vis Sci. 2015;56(11):6565–72. doi: 10.1167/iovs.15-16854. [DOI] [PubMed] [Google Scholar]
- 58.Venkatachalam G, Kumar AP, Yue LS, Pervaiz S, Clement MV, Sakharkar MK. Computational identification and experimental validation of PPRE motifs in NHE1 and MnSOD genes of human. BMC Genomics. 2009;10 Suppl 3:S5. doi: 10.1186/1471-2164-10-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan Y, Wang Y, Tang Z, Zhang H, Qin X, Zhu Y, Guan Y, Wang X, Staels B, Chien S, Wang N. Suppression of pro-inflammatory adhesion molecules by PPAR-delta in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(2):315–21. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- 60.Serrano-Marco L, Chacon MR, Maymo-Masip E, Barroso E, Salvado L, Wabitsch M, Garrido-Sanchez L, Tinahones FJ, Palomer X, Vendrell J, Vazquez-Carrera M. TNF-alpha inhibits PPARbeta/delta activity and SIRT1 expression through NF-kappaB in human adipocytes. Biochim Biophys Acta. 2012;1821(9):1177–85. doi: 10.1016/j.bbalip.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Yang X, Kume S, Tanaka Y, Isshiki K, Araki S, Chin-Kanasaki M, Sugimoto T, Koya D, Haneda M, Sugaya T, Li D, Han P, Nishio Y, Kashiwagi A, Maegawa H, Uzu T. GW501516, a PPARdelta agonist, ameliorates tubulointerstitial inflammation in proteinuric kidney disease via inhibition of TAK1-NFkappaB pathway in mice. PLoS One 2011;6(9):e25271. doi: 10.1371/journal.pone.0025271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bao XC, Fang YQ, You P, Zhang S, Ma J. Protective role of peroxisome proliferator-activated receptor beta/delta in acute lung injury induced by prolonged hyperbaric hyperoxia in rats. Respir Physiol Neurobiol. 2014;199:9–18. doi: 10.1016/j.resp.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Paterniti I, Esposito E, Mazzon E, Galuppo M, Di Paola R, Bramanti P, Kapoor A, Thiemermann C, Cuzzocrea S. Evidence for the role of peroxisome proliferator-activated receptor-beta/delta in the development of spinal cord injury. J Pharmacol Exp Ther. 2010;333(2):465–77. doi: 10.1124/jpet.110.165605. [DOI] [PubMed] [Google Scholar]
- 64.Hall JM, McDonnell DP. The molecular mechanisms underlying the proinflammatory actions of thiazolidinediones in human macrophages. Mol Endocrinol. 2007;21(8):1756–68. doi: 10.1210/me.2007-0060. [DOI] [PubMed] [Google Scholar]
- 65.Hack K, Reilly L, Palmer C, Read KD, Norval S, Kime R, Booth K, Foerster J. Skin-targeted inhibition of PPAR beta/delta by selective antagonists to treat PPAR beta/delta-mediated psoriasis-like skin disease in vivo. PLoS One. 2012;7(5):e37097. doi: 10.1371/journal.pone.0037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rival Y, Beneteau N, Taillandier T, Pezet M, Dupont-Passelaigue E, Patoiseau JF, Junquero D, Colpaert FC, Delhon A. PPARalpha and PPARdelta activators inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur J Pharmacol. 2002;435(2–3):143–51. [DOI] [PubMed] [Google Scholar]
- 67.Han JK, Lee HS, Yang HM, Hur J, Jun SI, Kim JY, Cho CH, Koh GY, Peters JM, Park KW, Cho HJ, Lee HY, Kang HJ, Oh BH, Park YB, Kim HS. Peroxisome proliferator-activated receptor-delta agonist enhances vasculogenesis by regulating endothelial progenitor cells through genomic and nongenomic activations of the phosphatidylinositol 3-kinase/Akt pathway. Circulation. 2008;118(10):1021–33. doi: 10.1161/CIRCULATIONAHA.108.777169. [DOI] [PubMed] [Google Scholar]
- 68.Schnabolk G, Stauffer K, O’Quinn E, Coughlin B, Kunchithapautham K, Rohrer B. A comparative analysis of C57BL/6J and 6N substrains; chemokine/cytokine expression and susceptibility to laser-induced choroidal neovascularization. Exp Eye Res. 2014;129:18–23. doi: 10.1016/j.exer.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.