Abstract
This cohort study uses National Cancer Database data from 2004 to 2014 to examine the association between overall survival and timing of radiotherapy relative to androgen deprivation therapy in patients with prostate cancer.
The coronavirus disease 2019 (COVID-19) pandemic poses challenges for patients with localized prostate cancer (PC) who require radiotherapy (RT), often administered with 6 to 36 months of androgen deprivation therapy (ADT). Daily hospital trips for RT create many possible points of COVID-19 transmission, and patients with cancer are at high risk of COVID-19 mortality.1 Therefore, an important option for patients being treated with RT and ADT is delayed RT initiation. Two prior randomized trials examined the relative timing of ADT and RT.2,3 Malone et al2 and the NRG Oncology/Radiation Therapy Oncology Group 9413 trial (NRG/RTOG 9413)3 showed no difference in overall survival (OS) when RT was started after ADT initiation vs when RT was started before or concurrently with ADT. However, neither trial had a noninferiority design, they were not powered for OS, and the enrolled patients may have been younger and healthier than the average real-world patient, limiting generalizability. Therefore, we used data from a large, contemporary database of patients with clinically significant localized PC to examine the association between OS and RT timing in patients receiving RT and ADT.
Methods
Using the patient-deidentified National Cancer Database,4 we identified men diagnosed from 2004 to 2014 with PC that met National Comprehensive Cancer Network criteria for unfavorable intermediate-risk PC (Gleason score 4 + 3 = 7 [primary Gleason pattern 4 with secondary pattern 3], or >1 of the following: Gleason score of 7, prostate-specific antigen [PSA] level of 10-20 ng/mL, clinical stage T2b-T2c) (to convert PSA concentration to micrograms per liter, multiply by 1.0) or high-risk or very high-risk PC (Gleason score of 8-10, PSA level >20 ng/mL, clinical stage T3-T4)5 and whose cancer was managed with external beam RT and ADT. The Dana-Farber/Harvard Cancer Center institutional review board deemed this study exempt from the need for review board approval and patient informed consent because deidentified data were used.
To approximate groups from prior trials,2,3 start times were categorized as follows: RT 0 to 60 days before ADT initiation (reference group) (n = 3572), RT 1 to 60 days after ADT initiation (n = 23 207), RT 61 to 120 days after ADT initiation (n = 30 285), and RT 121 to 180 days after ADT initiation (n = 6794). Multivariable Cox regression compared 10-year OS, hazard ratios (HRs), and 95% CIs across the groups. The multivariable Cox survival analysis was adjusted for the following clinical and sociodemographic characteristics: Gleason score, PSA, T stage, race, age, year of diagnosis, facility type, region, county type (rural or urban), distance traveled to the treatment facility, Charlson-Deyo comorbidity score, insurance status, and zip code–wide median household income. A continuous, nonlinear relationship between RT start time and OS was assessed using a restricted cubic spline transformation with 4 knots followed by plotting of the relative HR and 95% confidence bands (reference category: RT started 60 days before ADT).6
Results
Of the 63 858 men included in this study, 79.2% were White individuals, 16.8% were Black individuals, 2.2% were Asian American individuals, and 0.2% were Native American individuals. An additional 1.6% of individuals did not identify as any of the above race/ethnicity categories and were classified as other. The mean (SD) age was 69.9 (7.9) years. The Table shows baseline characteristics. Among men with unfavorable intermediate-risk PC (4220 total deaths), the 10-year OS for men who initiated RT 0 to 60 days before ADT initiation was 59.2%, RT 1 to 60 days after ADT initiation was 57.9%, RT 61 to 120 days after ADT initiation was 62.3%, and RT 121 to 180 days after ADT initiation was 58.9%. No OS difference was found for groups receiving later RT compared with those receiving RT 0 to 60 days before ADT initiation (1-60 days after: HR, 1.03; 95% CI, 0.90-1.19; P = .64; 61-120 days after: HR, 0.95; 95% CI, 0.82-1.08; P = .42; and 121-180 days after: HR, 0.99; 95% CI, 0.85-1.16; P = .90).
Table. Selected Demographic Characteristics of Men Diagnosed With Prostate Cancer From 2004 to 2014 by Risk Group.
| Characteristic | No. (%) | |
|---|---|---|
| Unfavorable intermediate-risk prostate cancer (n = 19 258) | High-risk prostate cancer (n = 44 600) | |
| Median (IQR) follow-up, y | 6.3 (3.6-9.0) | 5.8 (3.4-8.6) |
| Race | ||
| White | 15 407 (80.0) | 35 174 (78.9) |
| Black | 3132 (16.3) | 7583 (17.0) |
| Native American | 44 (0.2) | 94 (0.2) |
| Asian American | 377 (2.0) | 1038 (2.3) |
| Othera | 298 (1.5) | 711 (1.6) |
| Age, y | ||
| ≤50 | 209 (1.1) | 515 (1.2) |
| 51-60 | 2171 (11.3) | 5473 (12.3) |
| 61-70 | 7186 (37.3) | 15 759 (35.3) |
| 71-80 | 8510 (44.2) | 19 035 (42.7) |
| ≥81 | 1182 (6.1) | 3818 (8.6) |
| Charlson-Deyo comorbidity score | ||
| 0 | 16 461 (85.5) | 38 062 (85.3) |
| 1 | 2270 (11.8) | 5335 (12.0) |
| 2 | 414 (2.1) | 909 (2.0) |
| ≥3 | 113 (0.6) | 294 (0.7) |
| Zip code–wide median annual household income, $ | ||
| <38 000 | 3561 (18.5) | 8318 (18.7) |
| 38 000-47 999 | 4425 (23.0) | 10 648 (23.9) |
| 48 000-62 999 | 5151 (26.7) | 11 815 (26.5) |
| ≥63 000 | 6121 (31.8) | 13 819 (31.0) |
Abbreviation: IQR, interquartile range.
Other included individuals who did not identify as any of the other race/ethnicity categories.
Among men with high-risk or very high-risk PC (10 959 total deaths), the 10-year OS for men who initiated RT 0 to 60 days before ADT initiation was 58.9%, RT 1 to 60 days after ADT initiation was 51.7%, RT 61 to 120 days after ADT initiation was 54.8%, and RT 121 to 180 days after ADT initiation was 52.4%. No OS difference was found for groups receiving later RT compared with RT before ADT initiation (1-60 days after: HR, 1.07; 95% CI, 0.98-1.17; P = .12; 61-120 days after: HR, 1.04; 95% CI, 0.96-1.14; P = .36; and 121-180 days after: HR, 1.07; 95% CI, 0.97-1.18; P = .17).
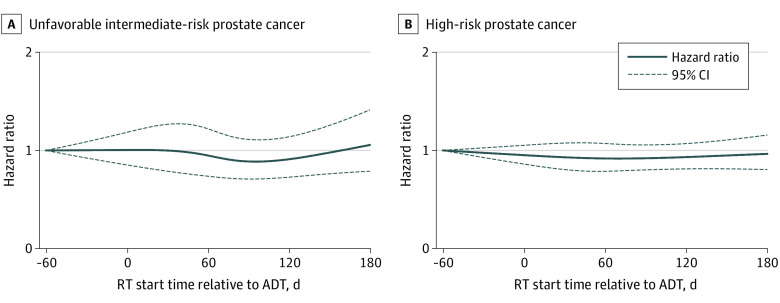
Date of RT initiation, modeled continuously and nonlinearly, was not associated with OS (Figure; 95% confidence bands include HR = 1).
Figure. Hazard Ratios for Overall Survival Among Men With Prostate Cancer as a Function of Radiotherapy (RT) Initiation Time Relative to Androgen Deprivation Therapy (ADT) Initiation.
Curves were generated by modeling RT start time using restricted cubic splines with 4 knots, located at the 5th, 35th, 65th, and 95th percentiles of relative start times for each risk subgroup.
Discussion
Based on analysis of a large database of patients with unfavorable intermediate-risk, high-risk, or very high-risk PC, later RT initiation up to 6 months after ADT initiation was not associated with worse OS compared with initiating RT before ADT. These results validate the findings of 2 prior randomized trials2,3 and possibly justify the delay of prostate RT for patients currently receiving ADT until COVID-19 infection rates in the community and hospitals are lower. Limitations of this study included the short follow-up period, retrospective design, lack of information about ADT duration, and possible data entry errors in the database. Nonetheless, if COVID-19 outbreaks continue to occur sporadically during the coming months to years, these data could allow future flexibility about the timing of RT initiation.
References
- 1.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. Published online March 25, 2020. doi: 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malone S, Roy S, Eapen L, et al. Sequencing of androgen-deprivation therapy with external-beam radiotherapy in localized prostate cancer: a phase III randomized controlled trial. J Clin Oncol. 2020;38(6):593-601. doi: 10.1200/JCO.19.01904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roach M, Moughan J, Lawton CAF, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): long-term results of a randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1504-1515. doi: 10.1016/S1470-2045(18)30528-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winchester DP, Stewart AK, Phillips JL, Ward EE. The National Cancer Data Base: past, present, and future. Ann Surg Oncol. 2010;17(1):4-7. doi: 10.1245/s10434-009-0771-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(5):479-505. doi: 10.6004/jnccn.2019.0023 [DOI] [PubMed] [Google Scholar]
- 6.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551-561. doi: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]