Abstract
There is growing evidence that the neuropeptide oxytocin (OT) modulates fear and extinction in humans and rodents through actions in corticolimbic circuits including the central amygdala (CeA). Prior studies have, however, been limited to subjects that exhibit intact basal extinction, rather than extinction-impaired populations that could potentially therapeutically benefit from viable OT-targeting treatments. Here, we assessed the effects of pre-extinction training infusion of OT into the CeA, or basolateral amygdala (BLA), on extinction in an inbred mouse strain (S1) model of impaired extinction. We found that intra-CeA OT, at a dose of 0.01 μg, enabled extinction memory formation, as evidenced by lesser freezing as compared to vehicle-infused controls on a drug-free retrieval test. Conversely, infusion of a higher, 1.0 μg OT dose, markedly reduced freezing and increased grooming during extinction training and produced elevated freezing on drug-free retrieval. Infusion of the 0.01 μg dose into the BLA was without behavioral effects. Together, our data show that OT acts in a dose-dependent manner within the CeA to promote extinction in otherwise extinction-deficient mice. These findings provide further support for the potential utility of OT as an adjunctive treatment to extinction-based therapies for trauma and anxiety disorders.
Keywords: Oxytocin, basolateral amygdala, central amygdala, fear extinction, mice, impaired extinction, microinfusion
Fear extinction is a learning process in which repeated experience with a fear-associated cue or context leads to the diminution of fear responses to that cue or context. As a form of ‘safety learning,’ fear is of significant translational relevance because it can work to counter the persistent fear and anxiety evident in trauma-related disorders. Recent studies aimed at deciphering neural circuits and neuromodulators underlying fear extinction [1, 2] have implicated oxytocin (OT), a neuropeptide known for its role in maternal and social behavior [3–8] in extinction.
Intranasal delivery of OT to healthy volunteers prior to extinction training (with or without a fear memory reminder) has been shown to promote extinction and produce an associated reduction in amygdala BOLD activity [9–11]. Along similar lines, microinfusion of OT or the OT receptor (OTR) agonist, TGOT, directly into the rat basolateral amygdala (BLA) [12] or dorsolateral striatum [13] facilitated extinction. Additionally, OT delivered intracerebroventricularly (icv) or directly into the infralimbic cortex (IL) before fear conditioning facilitated extinction, though, conversely, pre-extinction icv administration either increased fear or impaired extinction [14–17].
The central amygdala (CeA) is a key node region within the fear and extinction-mediating corticolimbic circuitry in which OT could act to promote extinction. OT is produced in neurons located in the paraventricular nucleus of the hypothalamus (PVN) that project to the CeA [18, 19]. Once released therein, OT acts on OTR expressed in the lateral subdivision of CeA (CeL) [20, 21], where it increases the activity of ‘fear off’ cells that send inhibitory projections to fear-promoting neurons located in the medial division of the CeA (CeM) [19, 20, 22]. Consequently, previous studies have found that intra-CeA delivering of OT or TGOT prior to conditioning [12, 14] or retrieval testing [22, 23] (but see [12]) reduces fear, and that pre-test infusion of an OT antagonist into the CeA blocks the decreases in fear attributed to optogenetically stimulated OT release in this region [19].
Together, these prior findings suggest that OT acting in the CeA could have pro-extinction properties. However, an earlier study found null effects of TGOT infusion into the CeA on contextual fear extinction [14], while another showed that intra-CeA infusion of OT (equivalent to ~0.25 μg/μl) or TGOT either pre or post extinction training impaired within-session extinction and drug-free extinction retrieval [12]. One potential explanation for these mixed findings could be the dose infused. High doses of OT delivered icv (0.25 μg/μl equivalent) produce hyperactivity in mice [15] [see also 24], while OTR-dependent ‘compulsive’ self-grooming is produced when OT is infused into the rat CeA (5 μg/μl equivalent) [25]. These behavioral effects could interfere with OT’s effects on fear extinction, for example by causing inattention to non-reinforced cue presentations. Another potentially important factor is that while extinction studies typically employ rodent strains and healthy human volunteers normally exhibiting robust extinction, beneficial effects of OT and other interventions may be more readily evident in extinction-deficient populations. In this context, fear-suppressing [22] and fear-augmenting [12] effects of intra-CeA OT on contextual fear, were evident against a relatively high and low level of fear respectively, [for discussion, see 12].
To clarify and extend the literature on OT and extinction in the current study, we assessed the extinction-related effects of OT microinfused into either the CeA or BLA in a mouse inbred strain, 129S1SvImJ (S1), that exhibits deficits in extinction [26] and associated functional and structural abnormalities in the amygdala [27, 28]. We obtained male S1 mice from The Jackson Laboratory (Bar Harbor, ME, USA) and housed them 2 per cage prior to surgery (singly thereafter to prevent cage-mate cannula damage) in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle (lights on 0630h, testing in light phase). An important limitation of this study was that due to its preliminary nature there was no examination of potential sex differences in OT’s effects. Using stereotaxic surgery, we chronically implanted guide cannula to bilaterally target the CeA (coordinates: −1.30 mm anterior-posterior, ±3.20 mm mediolateral, −4.15 mm ventral to Bregma) or BLA (coordinates: −1.40 mm anterior-posterior, ±3.30 mm mediolateral, −3.90 mm ventral to Bregma), under isoflurane anesthesia, and fixed the cannula in place using dental cement.
After at least 1 week after surgery, mice underwent cued fear conditioning and extinction using our standard procedures (see schematic in Figure 1A and B) [28–30]. Briefly, on day 1 mice were conditioned to associate, via 3 pairings, a white noise conditioned stimulus (CS) that co-terminated with a 0.6 mA footshock (separated by 60 and 90 second intervals, with a 120 second period after the final pairing). On day 2, 15 minutes prior to extinction training, entailing 50 CS presentations (separated by 5-second intervals), 0.5 μl/hemisphere of phosphate buffered saline (PBS) vehicle, 0.01 μg or 1.0 μg OT (for CeA, vehicle or 0.01 μg for BLA) was infused over 2 minutes (injectors stayed in place for a further 3 minutes to ensure diffusion) using a syringe pump (Harvard Apparatus PHD 22/2000; Harvard Apparatus, Holliston, MA). The dosing of OT and the timing of administration was based on previously published studies [15, 23]. On day 3, extinction retrieval entailed 3 CS presentations (5-second interval) without additional drug-infusion. Each day’s session began with a 180-second no-stimulus period.
Figure 1: Experimental schematic for assessing fear extinction-related effects of intra-amygdala oxytocin (OT).
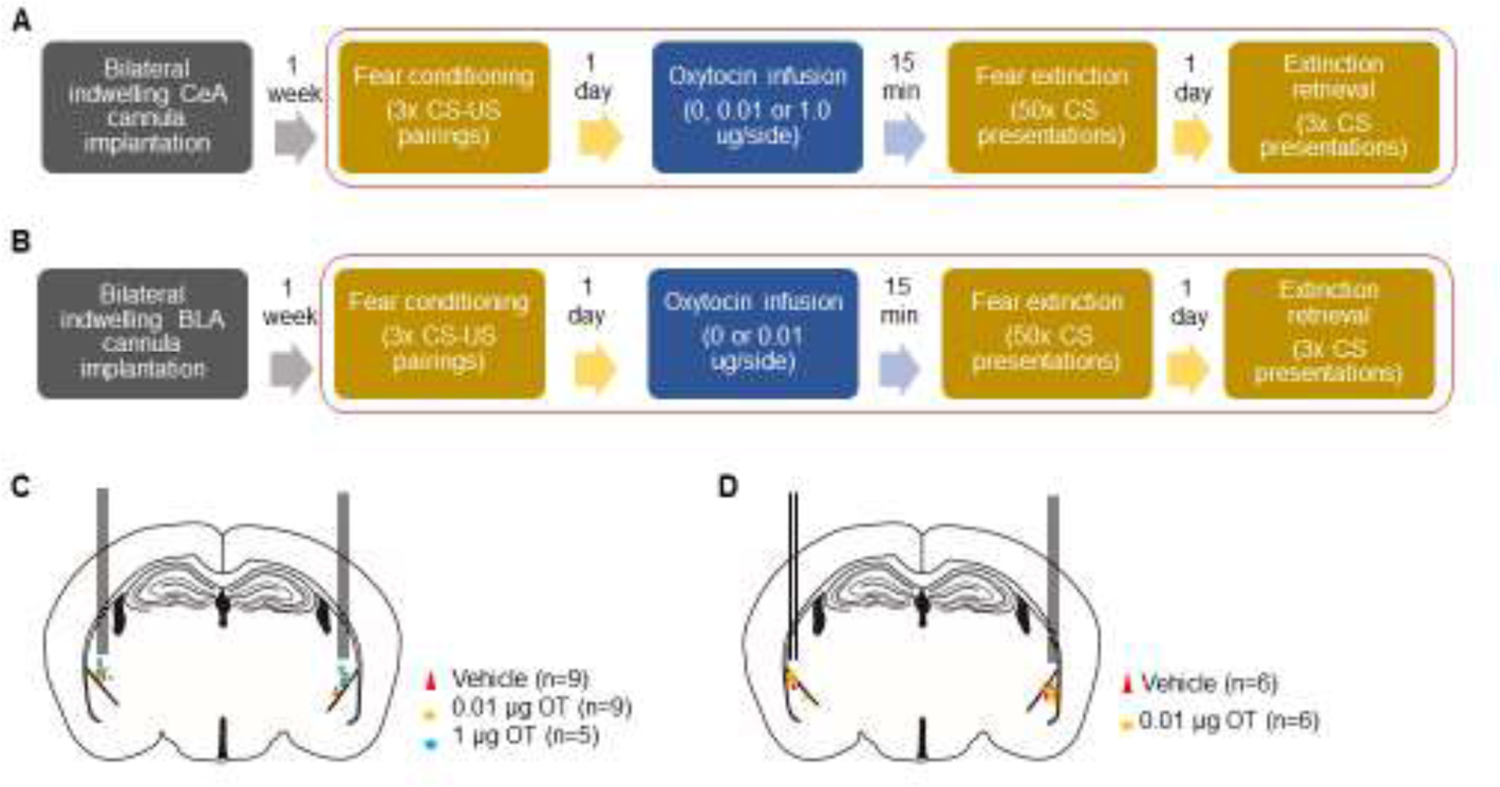
(A) Following implantation of guide cannulae directed at the CeA, mice underwent fear conditioning via 3 tone-shock pairings (Day 1). On experimental Day 2, vehicle, 0.01 or 1.0 μg OT infused into the CeA prior to extinction training, entailing 50 tone-CS presentations. On Day 3, extinction retrieval was tested via 3 tone-CS presentations. (B) After implantation of guide cannulae directed at the BLA, mice underwent testing as in A, but without the inclusion of the 1.0 μg OT dose. (C) Estimated cannula tip location for bilateral CeA infusions of vehicle (red triangles, n=9), 0.01 µg OT (orange circles, n=9) and 1 µg OT (blue circles, n=5). (D) Estimated cannula tip location for bilateral BLA OT infusions for vehicle (red triangles, n=6), 0.01 µg OT (orange circles, n=6).
Throughout testing, an observer blind to the treatment group manually scored freezing (cessation of movement except breathing) and grooming (washing, scratching, licking any part of the body with the forepaws) every 5 seconds. These dependent variables were analyzed for the effects of trial (i.e., CS-US pairing during conditioning) or trial-block (5-CS average during extinction training and between training and retrieval) and drug-group via analysis of variance (ANOVA), with repeated measures for trial/trial-block followed by Dunnett’s test when comparing between groups and Sidak test when comparing across trial-blocks within groups. Student’s t-test was used to compare average grooming during CS and inter-CS periods. The threshold for statistical significance was set at P<0.05. The number of mice used in each group is indicated in the figure legends and Figure 1C and D. Experimental procedures were approved by the NIAAA Animal Care and Use Committee and followed the NIH guidelines outlined in Using Animals in Intramural Research and the local Animal Care and Use Committees.
At the completion of testing, mice were deeply anaesthetized with a ketamine/xylazine cocktail and transcardially perfused with ice cold phosphate buffered saline (PBS, pH 7.4) followed by ice cold 4% paraformaldehyde (PFA). Brains were removed and 50 µm coronal sections cut on a vibratome (Leica VT1000 S, Leica Biosystems Inc, Buffalo Grove, IL, USA) and stored free floating in phosphate buffer (PB) 0.1M at 4°C for no longer than 1 week prior to visual inspection of cannula tracts using an Olympus BX-40 light microscope (Olympus, Center Valley, PA, USA). Mice with mistargeted cannulae were removed from the analysis see cartoon for cannulae placements Figure 1C and D).
In the intra-CeA infusion experiment, freezing significantly increased from the first to third CS-US pairing of conditioning irrespective of drug group (effect of trial F(1,20)=61.40, P<0.01, dose F(2,20)=0.24, P=0.79, trial × dose interaction F(2,20)=0.26, P=0.78) (Figure 2A). On extinction training, there was an overall effect of increased freezing over trial-blocks, but freezing in the 1.0 μg OT group was lower than in vehicle controls (effect of trial-block F(1,20)=5.20, P<0.05, dose F(2,20)=8.57, P<0.05, trial-block × dose interaction F(2,20)=0.05, P=0.46) (Figure 2B). During extinction retrieval, the OT 0.01 μg group froze less as compared to vehicle controls (effect of dose F(2,20)= 7.89, P<0.01) (Figure 2C). Grooming was negligible during conditioning (effect of trial F(1,20)=2.37, P=0.14, dose F(2,20)=1.96, P=0.17, trial × dose interaction F(2,20)=1.96, P=0.17) and there was no grooming on extinction retrieval. During extinction training grooming was higher in the 1.0 μg OT group than vehicle controls (effect of trial-block F(1,20)=12.02, P<0.01, dose F(2,20)=9.95, P<0.01, trial-block × dose interaction F(2,20)=10.65, P<0.01) (Figure 2D–F).
Figure 2: Dose-dependent effects of intra-CeA oxytocin (OT) on fear extinction.
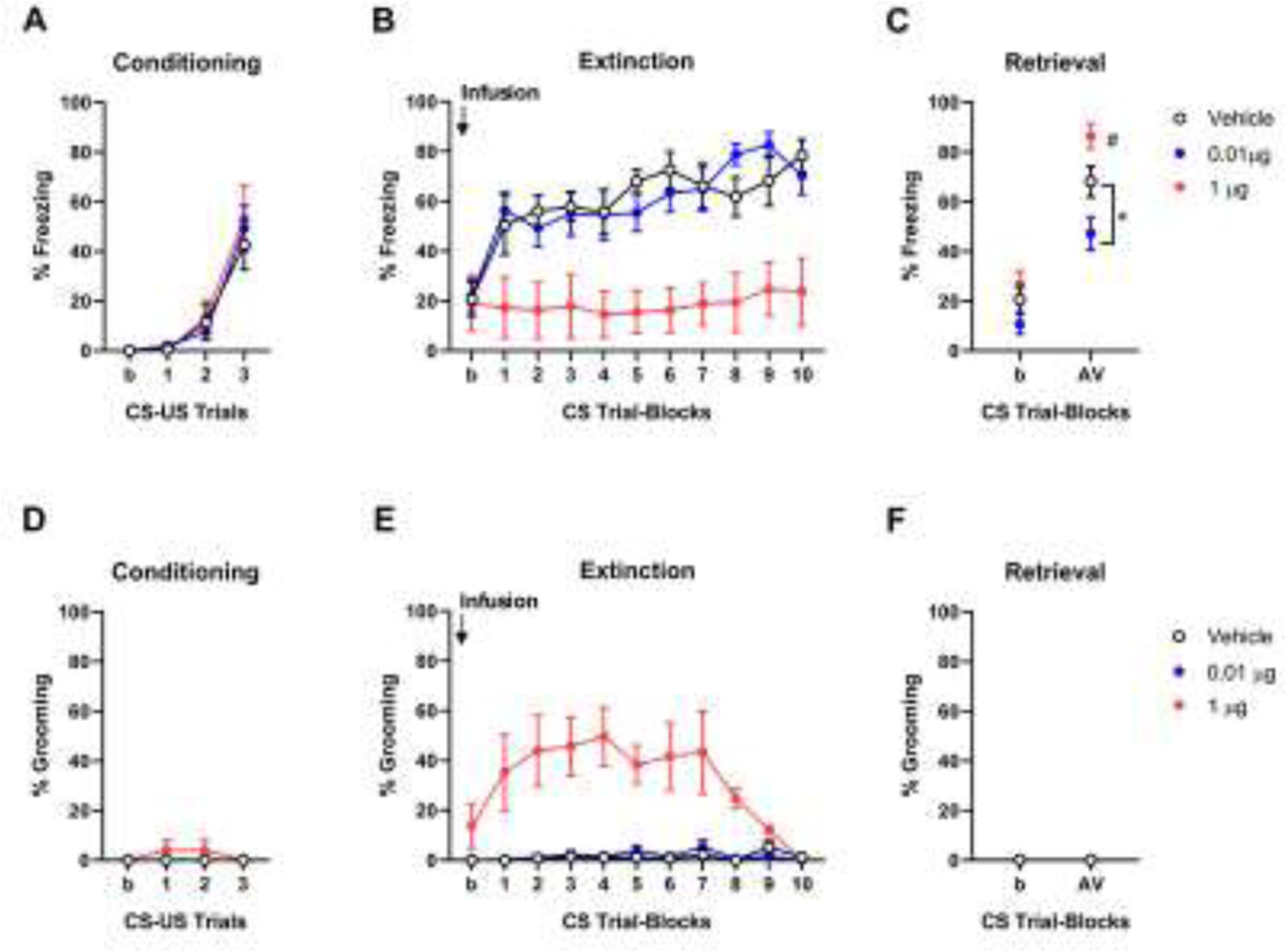
(A) Freezing increased from the first to third CS-US pairing during conditioning. (B) Intra-CeA infusion of 1.0 μg OT (n=5, red circles) decreased freezing during extinction training and increased freezing on (drug-free) extinction retrieval, relative to vehicle (n=9, black empty circles). (C) Intra-CeA infusion of 0.01 μg OT (n=9 blue circles) prior to extinction training produced decreased freezing on (drug-free) extinction retrieval, relative to vehicle. (D) Grooming levels were very low during conditioning. Intra-CeA infusion of 1.0 μg, but not 0.01 μg, OT prior to extinction training increased grooming during extinction training (E) and decreased freezing on (drug-free) extinction retrieval (F), relative to vehicle controls. *P<0.05 versus vehicle. # P<0.05 versus first CS block in extinction. Data are means ± SEM. Abbreviations: CS: conditioned stimulus, US: unconditioned stimulus, b: baseline, AV: Average.
These data show that the 0.01 μg dose of OT produced a pattern of effects consistent with the facilitation of extinction memory formation (i.e., decreased freezing on retrieval, but not training), possibly by promoting memory consolidation. Conversely, the 1.0 μg dose of OT acutely increased grooming and led to a subsequent impairment in extinction memory expression on retrieval. These findings make two important points. Firstly, OT acts within the CeA, likely in the OTR rich CeL region, to enable the formation of fear extinction memory in a mouse model of impaired extinction. This finding is broadly consistent with previous rodent studies showing fear-attenuating effects of intra-CeA OT in rats and other mouse strains [19, 22, 23] and may be explained by the activation of ‘fear-off’ cells in the CeL [19].
Second, our data are in line with earlier reports that high doses of OT infused into CeA produce marked levels of grooming (‘hyper-grooming’) in rats [25]. The relevance of this increase in grooming to the animal’s emotional state is unclear. Grooming has been reported in mice exposed to anxiety-provoking situations, and interpreted therein as a form of anxiety-related ‘displacement’ activity [31]. The expression of grooming in such conditions is typically much lower than that we observed, suggesting the response produced by intra-CeA OT is more akin to a motor stereotypy. However, the fact that grooming was higher during CS presentations than intervening CS-free periods (CS= 33.4 ± 5.1 SEM, inter-CS period = 14.8 ± 3.2 SEM, t(15)=3.80, P<0.01), does suggest the motor response is exacerbated by the fear-related stimulus. Nonetheless, this effect on grooming appears to ‘compete’ with the expression of freezing and account for the low levels of freezing we observed during extinction training. High grooming could also explain the subsequent deficit in (drug-free) extinction retrieval if the engagement in this behavior served as a distractor interfering with attention to the CS during training.
We next examined whether the extinction-facilitating effects of the 0.01 μg dose of intra-CeA OT were also produced by infusion into the neighboring BLA. Regardless of group, freezing increased over conditioning trials (effect of trial F(1,10)=34.17, P<0.01, dose F(1,10)=0.74, P=0.41, trial × dose interaction F(1,10)=0.74, P=0.41) (Figure 3A). Freezing was unchanged across trial-blocks on extinction training and did not differ between groups either during training (effect of trial-block F(1,10)=2.71, P=0.13, dose F(1,10)=2.38, P=0.15, trial-block × dose interaction F(1,10)=1.47, P=0.24) or retrieval (effect of dose F(1,10)=0.02, P=0.89) (Figure 3B and C). Grooming was unaltered by trial or group during conditioning (effect of trial F(1,10)=0.00 P>0.99, dose F(1,10)=0.00 P>0.99, trial × dose interaction F(1,10)=0.00, P>0.99), extinction training (effect of trial-block F(1,10)=0.31, P=0.59, dose F(1,10)=0.61, P=0.45, trial-block × dose interaction F(1,10)=1.83, P=0.22) or retrieval (effect of dose F(1,10)=1.0, P=0.34) (Figure 3D–F). The absence of effects produced by OT infusion into the BLA contrast with the pro-extinction effects of intra-CeA OT infusion (~0.25 μg/μl equivalent) and also disagree with a prior study [12]. The current data are, however, limited to one concentration and it remains to be shown whether positive behavioral effects would be evident at other doses. It should also be noted that retrieval freezing levels in the vehicle control group for the BLA experiment were visibly lower (although not significantly so t(9)=1.22, P=0.25) than those in the CeA experiment’s vehicle group. This could be due the normal variation between experiments or could stem from residual damage to the BLA from the cannula, given BLA inactivation impairs extinction memory formation [32], and may have limited the range to detect a further decrease in freezing in the OT group. Finally, it is possible that the mechanism through which intra-BLA OT facilitates extinction (e.g., activation of BLA ‘extinction’ cells [4]) is so severely deficient, and therefore difficult to rescue, in S1 mice. For example, given divalent cations effect OTR affinity for OT [33] and manipulation of certain cations has been shown to profoundly alter extinction in S1 mice [27], it is possible that cation-OT interactions are disrupted in the BLA of these mice.
Figure 3: Lack of effects of a single dose of intra-BLA oxytocin (OT) on fear extinction.
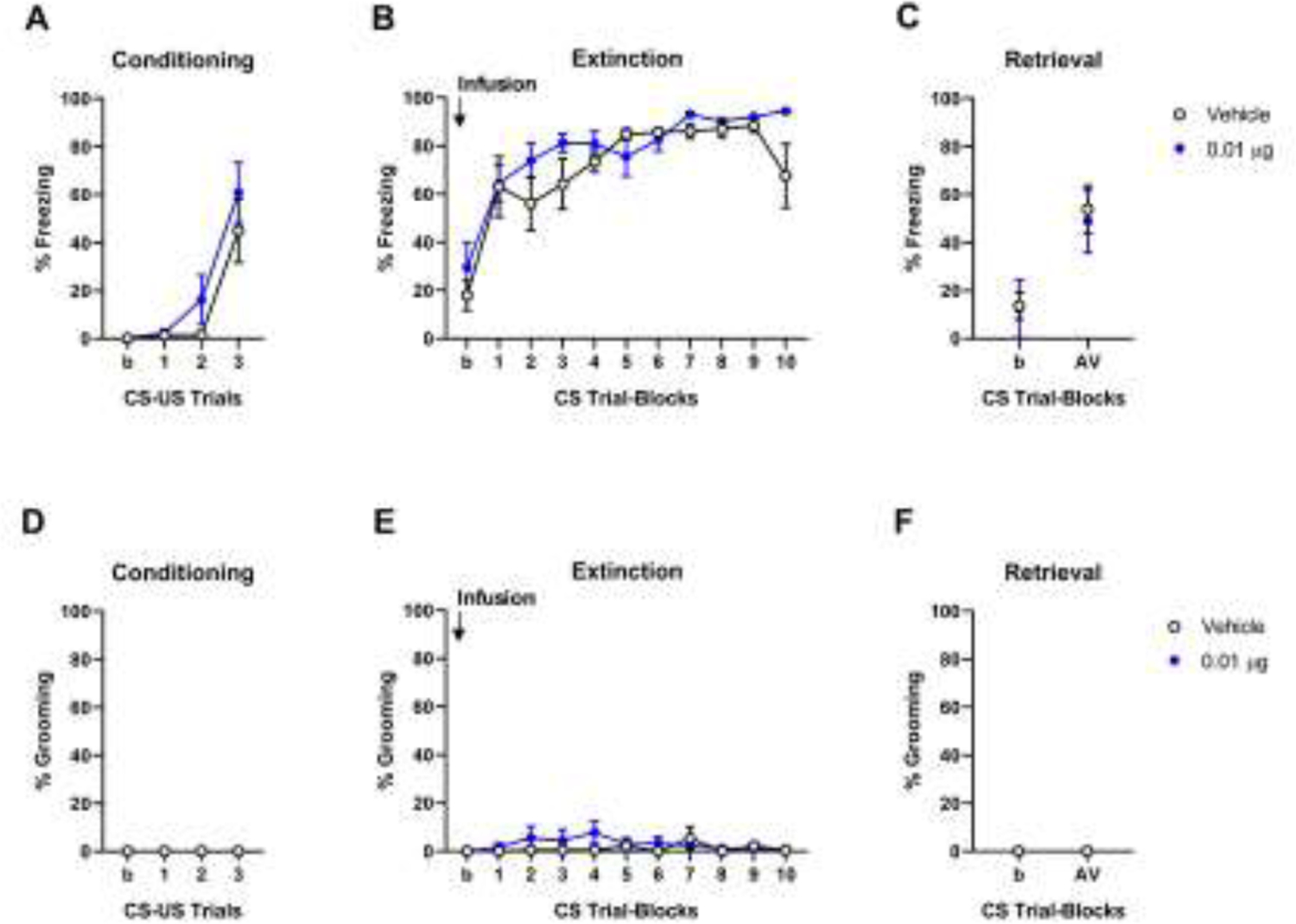
(A) Freezing increased from the first to third CS-US pairing during conditioning. (B) Intra-BLA infusion of 0.01 μg (n=6, blue circles) OT prior to extinction training did not affect freezing on extinction training (C) or (drug-free) extinction retrieval, as compared to vehicle controls (n=6, black empty circles). (D) Grooming was absent during conditioning. Intra-BLA infusion of 0.01 μg OT prior to extinction training did not affect grooming on extinction training (E) or extinction retrieval (F) compared to vehicle. Data are means ± SEM. Abbreviations: CS: conditioned stimulus, US: unconditioned stimulus, b: baseline, AV: Average.
In summary, we demonstrate that OT promotes the formation of fear extinction memory when delivered directly into the CeA of mice that normally exhibit profound extinction deficits. However, we also find that these effects are restricted to a dose-window, with a higher OT concentration producing a sustained grooming response and augmented levels of fear during drug-free retrieval. Finally, we show that at least one dose of OT fails to alter fear behavior when infused into the BLA, suggesting the pro-extinction effects of OT are, within the amygdala, specific to the CeA. Promoting fear extinction via treatment with cognitive enhancers represents a tractable approach to therapeutically alleviating the symptoms associated with traumatic memory, especially if it can be delivered intranasally, a promising route of administration for peptides [2, 34].
The results of the current study add to growing evidence that the neuropeptide OT could provide a beneficial pharmacological adjunct to extinction-based (i.e., exposure) therapies; that is OT given during exposure could potentially strengthen the formation of extinction memory. By showing extinction facilitating effects in a mouse model of impaired extinction, our data suggest efficacy in extinction-deficient populations, including those with anxiety and trauma-related disorders who would be the most likely recipients of OT for therapeutic purposes. In this context, given the higher rates of anxiety disorders in women [35] which means women would be more likely recipients of such treatment, together with known sex differences in the OT system [36, 37], it will be essential to extend the current data to female subjects. However, our findings also caution that the extinction-promoting effects of OT may be brain region specific and require careful calibration to a narrow effective dose-window.
Acknowledgements
Research supported by the NIAAA Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bukalo O, Pinard CR, Holmes A, Mechanisms to medicines: elucidating neural and molecular substrates of fear extinction to identify novel treatments for anxiety disorders, Br J Pharmacol 171(20) (2014) 4690–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ, Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders, Pharmacol Ther 149 (2015) 150–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maroun M, Wagner S, Oxytocin and Memory of Emotional Stimuli: Some Dance to Remember, Some Dance to Forget, Biol Psychiatry 79(3) (2016) 203–12. [DOI] [PubMed] [Google Scholar]
- [4].Triana-Del Rio R, van den Burg E, Stoop R, Hegoburu C, Acute and long-lasting effects of oxytocin in cortico-limbic circuits: consequences for fear recall and extinction, Psychopharmacology (Berl) 236(1) (2019) 339–354. [DOI] [PubMed] [Google Scholar]
- [5].Neumann ID, Landgraf R, Tracking oxytocin functions in the rodent brain during the last 30 years: From push-pull perfusion to chemogenetic silencing, J Neuroendocrinol 31(3) (2019) e12695. [DOI] [PubMed] [Google Scholar]
- [6].Cilz NI, Cymerblit-Sabba A, Young WS, Oxytocin and vasopressin in the rodent hippocampus, Genes Brain Behav 18(1) (2019) e12535. [DOI] [PubMed] [Google Scholar]
- [7].Neumann ID, Slattery DA, Oxytocin in General Anxiety and Social Fear: A Translational Approach, Biol Psychiatry 79(3) (2016) 213–21. [DOI] [PubMed] [Google Scholar]
- [8].van den Burg EH, Hegoburu C, Modulation of expression of fear by oxytocin signaling in the central amygdala: From reduction of fear to regulation of defensive behavior style, Neuropharmacology 173 (2020) 108130. [DOI] [PubMed] [Google Scholar]
- [9].Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, Grinevich V, Kendrick KM, Maier W, Hurlemann R, Oxytocin facilitates the extinction of conditioned fear in humans, Biol Psychiatry 78(3) (2015) 194–202. [DOI] [PubMed] [Google Scholar]
- [10].Hu J, Wang Z, Feng X, Long C, Schiller D, Post-retrieval oxytocin facilitates next day extinction of threat memory in humans, Psychopharmacology (Berl) 236(1) (2019) 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Acheson D, Feifel D, de Wilde S, McKinney R, Lohr J, Risbrough V, The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample, Psychopharmacology (Berl) 229(1) (2013) 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Campbell-Smith EJ, Holmes NM, Lingawi NW, Panayi MC, Westbrook RF, Oxytocin signaling in basolateral and central amygdala nuclei differentially regulates the acquisition, expression, and extinction of context-conditioned fear in rats, Learn Mem 22(5) (2015) 247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zoicas I, Slattery DA, Neumann ID, Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum, Neuropsychopharmacology 39(13) (2014) 3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lahoud N, Maroun M, Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction, Psychoneuroendocrinology 38(10) (2013) 2184–95. [DOI] [PubMed] [Google Scholar]
- [15].Toth I, Neumann ID, Slattery DA, Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner, Psychopharmacology (Berl) 223(2) (2012) 149–58. [DOI] [PubMed] [Google Scholar]
- [16].Kritman M, Lahoud N, Maroun M, Oxytocin in the amygdala and not the prefrontal cortex enhances fear and impairs extinction in the juvenile rat, Neurobiol Learn Mem 141 (2017) 179–188. [DOI] [PubMed] [Google Scholar]
- [17].Brill-Maoz N, Maroun M, Extinction of fear is facilitated by social presence: Synergism with prefrontal oxytocin, Psychoneuroendocrinology 66 (2016) 75–81. [DOI] [PubMed] [Google Scholar]
- [18].Palkovits M, Young WS 3rd, Kovacs K, Toth Z, Makara GB, Alterations in corticotropin-releasing hormone gene expression of central amygdaloid neurons following long-term paraventricular lesions and adrenalectomy, Neuroscience 85(1) (1998) 135–47. [DOI] [PubMed] [Google Scholar]
- [19].Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V, Evoked axonal oxytocin release in the central amygdala attenuates fear response, Neuron 73(3) (2012) 553–66. [DOI] [PubMed] [Google Scholar]
- [20].Huber D, Veinante P, Stoop R, Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala, Science 308(5719) (2005) 245–8. [DOI] [PubMed] [Google Scholar]
- [21].Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ, Genetic dissection of an amygdala microcircuit that gates conditioned fear, Nature 468(7321) (2010) 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R, Oxytocin selectively gates fear responses through distinct outputs from the central amygdala, Science 333(6038) (2011) 104–7. [DOI] [PubMed] [Google Scholar]
- [23].Rickenbacher E, Perry RE, Sullivan RM, Moita MA, Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother-pup interactions, Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID, Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice, Psychoneuroendocrinology 42 (2014) 225–36. [DOI] [PubMed] [Google Scholar]
- [25].Marroni SS, Nakano FN, Gati CD, Oliveira JA, Antunes-Rodrigues J, Garcia-Cairasco N, Neuroanatomical and cellular substrates of hypergrooming induced by microinjection of oxytocin in central nucleus of amygdala, an experimental model of compulsive behavior, Mol Psychiatry 12(12) (2007) 1103–17. [DOI] [PubMed] [Google Scholar]
- [26].Singewald N, Holmes A, Rodent models of impaired fear extinction, Psychopharmacology (Berl) 236(1) (2019) 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N, Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction, J Neurosci 30(41) (2010) 13586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Camp MC, Macpherson KP, Lederle L, Graybeal C, Gaburro S, Debrouse LM, Ihne JL, Bravo JA, O’Connor RM, Ciocchi S, Wellman CL, Luthi A, Cryan JF, Singewald N, Holmes A, Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes, Neuropsychopharmacology 37(6) (2012) 1534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sengupta A, Holmes A, A Discrete Dorsal Raphe to Basal Amygdala 5-HT Circuit Calibrates Aversive Memory, Neuron 103(3) (2019) 489–505 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gunduz-Cinar O, Brockway E, Lederle L, Wilcox T, Halladay LR, Ding Y, Oh H, Busch EF, Kaugars K, Flynn S, Limoges A, Bukalo O, MacPherson KP, Masneuf S, Pinard C, Sibille E, Chesler EJ, Holmes A, Identification of a novel gene regulating amygdala-mediated fear extinction, Mol Psychiatry 24(4) (2019) 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Holmes A, Rodgers RJ, Responses of Swiss-Webster mice to repeated plus-maze experience: further evidence for a qualitative shift in emotional state?, Pharmacol Biochem Behav 60(2) (1998) 473–88. [DOI] [PubMed] [Google Scholar]
- [32].Sierra-Mercado D Jr., Corcoran KA, Lebron-Milad K, Quirk GJ, Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction, Eur J Neurosci 24(6) (2006) 1751–8. [DOI] [PubMed] [Google Scholar]
- [33].Jurek B, Neumann ID, The Oxytocin Receptor: From Intracellular Signaling to Behavior, Physiol Rev 98(3) (2018) 1805–1908. [DOI] [PubMed] [Google Scholar]
- [34].Lee MR, Shnitko TA, Blue SW, Kaucher AV, Winchell AJ, Erikson DW, Grant KA, Leggio L, Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques, Nat Commun 11(1) (2020) 2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, Wittchen HU, Anxiety disorders, Nat Rev Dis Primers 3 (2017) 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Love TM, The impact of oxytocin on stress: the role of sex, Curr Opin Behav Sci 23 (2018) 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dumais KM, Veenema AH, Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior, Front Neuroendocrinol 40 (2016) 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


