Abstract
This research was designed to determine the association of serum lipid peroxidation products with disease severity in patients with abdominal aortic aneurysm (AAA). In total, 76 pairs of AAA cases as well as matched controls were enrolled in our research using propensity score matching (PSM). And their malondialdehyde (MDA), lipid hydroperoxide (LPO), and glutathione peroxidase (GSH-Px) levels were also detected through enzyme-linked immunosorbent assay (ELISA). Additionally, the relative clinical data of enrolled participants were extracted. The serum biomarker concentrations were measured in 76 patients with AAAs (diameter between 30 and 54 mm, n = 54; diameter ≥55 mm, n = 22) and 76 control patients from observational cohort study. After PSM adjustment for clinical variables, including age, gender, heart ratio, body mass index, smoking, hypertension, diabetes mellitus, coronary heart disease, and stroke, the serum MDA and LPO among AAA cases were remarkably increased compared with those from the normal patients. Inversely, serum GSH-Px was significantly decreased in patients with AAA compared to the control group. Besides, the serum levels of MDA and LPO were independently associated with AAA risk. Typically, there was significantly positive correlation between MDA level and LPO level (R = 0.358) but negative correlation of MDA level with GSH-Px (R = -0.203) level in patients with AAA. Meanwhile, the area under the receiver operating characteristic curve was 0.965 when MDA was used to diagnose AAA, and the optimal threshold value was 0.242 nmol/mL. Moreover, serum MDA level was significantly increased in cases with rupture AAA compared to those in selective AAA cases. Logistic regression analysis suggested that a higher serum MDA level indicated an elevated risk of AAA rupture (odds ratio = 2.536; 95% CI: 1.037-6.203; P =0.041). Our present findings suggest that serum peroxidation contents were evidently changed among AAA cases. Serum MDA and LPO concentrations could be used to predict disease severity in patients with AAA. Moreover, serum MDA may serve as the candidate biomarker for diagnosis of AAA and accurate identification of increased risks of AAA rupture.
Keywords: abdominal aortic aneurysm, lipid oxidative stress, malondialdehyde, glutathione peroxidase
Introduction
Abdominal aortic aneurysm (AAA) is a common vascular disorder featured by abnormal dilation and degeneration of focal aortaventralis. Abdominal aortic aneurysm is commonly asymptomatic before its cataclysmic presentation when ruptured, including acute abdominal pain and hemorrhagic shock.1,2 The prevalence of AAA ranges from 4% to 7%, with over 175 000 deaths due to the rupture of AAA worldwide.3,4 The diameter of aneurysm is a crucial determinant of rupture. Despite the accessible endovascular aneurysm repair as well as open surgical repair, the mortality of rupture AAA (rAAA) is as high as 80%.5,6 Therefore, the prevention of AAA is of significance due to the extremely high mortality caused by its rupture. Nevertheless, there is no therapeutic agent to prevent the progression and rupture of AAA.7,8 Moreover, there is an increasing appreciation that growth of AAA is nonlinear, and the risk of rupture varies with time.9 Therefore, it will be meaningful to detect the reasons for AAA rupture and to subsequently decrease its incidence.
The pathological progression in the aortic wall is associated with the generation and involvement of various biomarkers, with extensive investigations on these biomarkers worldwide.10,11 Biomarkers, especially those related to pathophysiological processes of inflammation and aortic wall degradation, are attractive potential candidates.12,13 However, no biomarker has yet been sufficiently validated with additional prognostic value on AAA diameter for clinical practice.14,15 Inflammation and tissue degeneration play vital roles in the pathogenesis of AAA formation and rupture.16,17 Elevated production of free radicals could trigger endothelial injury, phenotype from a contractile to an inflammatory phenotype in smooth muscle cells (SMCs), ultimately causing apoptosis. More importantly, free radicals can also mediate lipid peroxidation, causing atherosclerosis, thereby contributing to hemodynamic stress as well as hypertensive pathology, all of which are considered as integral elements of aneurysm development.18,19 This process is mostly correlated with cellular damage due to oxidative stress, and the formation of various aldehydes and malondialdehyde (MDA) would occur in the case of breakdown of lipid hydroperoxides (LPO) in biological systems. Inversely, glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) are20 free radical cleaning enzymes, with essential roles in cleaning these radicals against tissue defect.21,22 To our knowledge, few studies are accessible on detailed comparison of serum lipid oxidative stress product levels in AAA, and it remains unclear of the performance of serum peroxidation products to identify aortic aneurysm.
The present study was designed to examine the performance of personalized biomarkers in AAA diagnostics to correlate the AAA diameter or rAAA and selected biomarkers, followed by multivariate analysis to construct multiparameter model for stratification in patients with AAA. This model could be utilized as a diagnostic tool for the identification of potential risk aneurysms, which would facilitate in the individualized therapeutic approach. Therefore, evidence gathering on risk factors of developing AAA would enhance the cost-effectiveness of screening programs and detection rate of AAA.
Materials and Methods
Abdominal Aortic Aneurysm Population
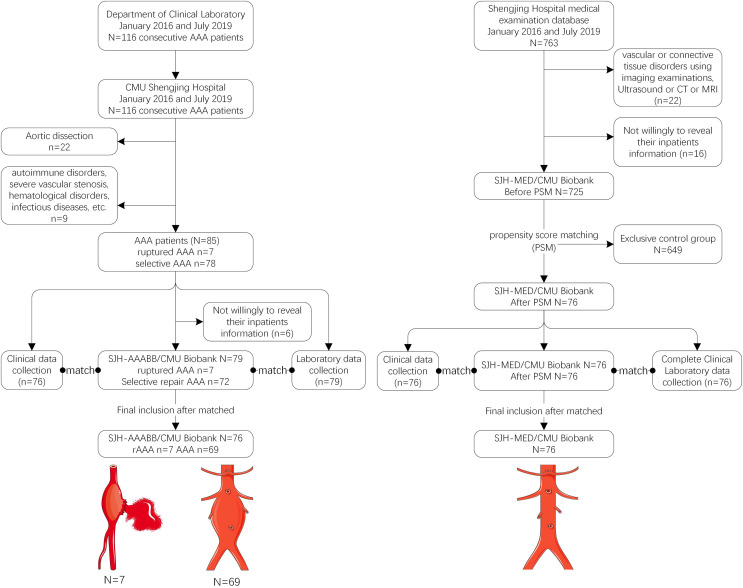
A hospital-based, cross-sectional, observational, case–control study was carried out in this study, aiming to analyze the serum lipid peroxidation contents among patients with AAA and the matched control patients at the Shengjing Hospital of China Medical University (CMU). Peripheral blood specimens were collected from 116 consecutive AAA cases from Department of Clinical Laboratory (N = 116) at Shengjing Hospital of CMU from January 2016 to July 2019. The clinical data were extracted from patients upon admission from the Department of Health Management (n = 76). Seven and 69 out of these 76 cases were diagnosed with ruptured AAA and selective AAA, respectively, during hospitalization (Figure 1). Patients were diagnosed with AAA on the basis of imaging findings (including echocardiography, magnetic resonance imaging and computed tomography), and the classification of AAA was based on the clinical practice guidelines of the European Society for Vascular Surgery (ESVS).23 Our study protocol gained approval from the Ethics Committee (No. 2016PS085K) at Shengjing Hospital of CMU. Written informed consent was provided by all patients.
Figure 1.
Flowchart of patient selection and blood specimen selection from patients with AAA and healthy control group from the Shengjing Hospital of CMU Abdominal Aortic Aneurysm Blood sample Biobank (SJH-AAABB/CMU) and Shengjing Hospital of CMU Medical Examination Blood sample Biobank (SJH-MED/CMU).
Definitions
In all participants, abdominal aortic diameter was defined as the maximal anteroposterior inner wall to inner wall diameter of the infrarenal aorta.24 Patients with expanded aortic diameter between 40 mm and 54 mm were categorized as small abdominal aortic aneurysms,25 and rAAA is indicated by the triad of sudden-onset mid-abdominal or flank pain (possible radiation into the scrotum), shock, and a pulsatile abdominal mass.26,27 Additionally, hypertension was diagnosed according to the diastolic blood pressure (BP) ≥90 mm Hg and/or the systolic BP ≥140 mm Hg, followed by administration of antihypertensive agents. Meanwhile, diabetes mellitus was diagnosed when the glycosylated hemoglobin A1c level was ≥6.5%, the fasting blood glucose was ≥7.0 mmol/L, and insulin or oral hypoglycemic agents were administered. Further, status of current smoker was determined according to the self-report by patients. Additionally, lower and upper serum lipid peroxidation contents were determined according to the threshold based on the receiver operating characteristic (ROC) curve in this study.
Exclusion Standards
In this study, 31 cases were excluded due to the acute, chronic, or traumatic artic dissection (n = 22), Marfan syndrome, intramural aortic hematoma, thoracic aortic aneurysm or the involvement of additional connective tissues, and cases who received steroids or non-steroidal anti-inflammatory drugs (n = 9). A previous history of cardiac surgery, myocardial infarction, valvular heart disease, infection, or other inflammatory diseases was known to affect the serum lipid peroxidation levels. Thus, patients with these above conditions, who were examined by echocardiography, laboratory tests, imaging examinations, angiography diagnosis, and additional medical examinations based on patients’ medical history and clinical presentations, were also excluded from this study. Eventually, 85 patients were potentially identified as AAA and were potentially suitable for inclusion in this study.
Control Group (Ctrl)
Our controls were negative to systemic or topical application of drugs, such as anti-inflammatory drugs, anticonvulsants, steroids, or antifungals, and had no concurrent cutaneous or systemic disorders. The Medical Examination Database of Shengjing Hospital of CMU was utilized to identify normal patients by thoroughly retrieving each control patients between 2016 and 2019. The control population was identified from 725 originally healthy individuals undergoing comprehensive routine health examination at outpatient department. Among them, 76 were confirmed to have normal findings on physical and echocardiographic examinations, with matched age and gender with those in the study group. Moreover, patients diagnosed with connective tissue or vascular diseases upon admission based on imaging examinations were also eliminated from control group. Further, patients with immune-associated diseases, history of drug application, infection, or malignant cancer were also excluded from the control group. This study was carried out in line with the Declaration of Helsinki. The participant selection and inclusion process are summarized in Figure 1.
Aortic Aneurysm and Medical Examination Blood Biobank
According to the exclusion criteria of unqualified patients, 6 cases were further excluded due to their unwillingness to offer the inpatient records for publication, blood samples, or clinical data (Figure 1). Eventually, the blood samples were collected from 76 eligible AAA cases (including 7 with rAAA and 69 with selective AAA) to perform additional analyses for the blood Biobank. Typically, the Biobank was registered as the Shengjing Hospital of CMU Abdominal Aortic Aneurysm Blood sample Biobank (SJH-AAABB/CMU). Meanwhile, blood samples were also collected from 76 healthy controls, and registered as the Shengjing Hospital of CMU Medical Examination Blood sample Biobank (SJH-MED/CMU). Intravenous puncture was performed in all eligible cases upon admission, followed by extraction of blood samples into the anticoagulant ethylenediaminetetraacetic acid plastic tubes (5.0 mL, BD Vacutainer lavender) as well as the silica/gel plastic tubes (5.0 mL, SST BD Vacutainer gold). In addition, the blood samples were subjected to centrifugation to extract serum, which was further preserved at −80 °C prior to test (as long as 1 year). Moreover, peripheral blood mononuclear cells were collected from these eligible AAA cases using the density gradient centrifugation of Ficoll-sodium diatrizoate according to prior description.28 This Biobank study also gained approval from Ethics Committee of Shengjing Hospital of CMU and was carried out according to the Declaration of Helsinki.
Serum Lipid Peroxidation Contents
The Human malondialdehyde (Human MDA) ELISA Kit (ml062874, mlBio; Shanghai Enzyme-linked Biotechnology Co, Ltd) was purchased to detect the serum MDA levels according to the enzyme-linked immunosorbent assay (ELISA) strictly following manufacturer’s protocols. The Human lipid hydroperoxides (Human LPO) ELISA Kit (ml320104, mlBio; Shanghai Enzyme-linked Biotechnology Co, Ltd) was purchased to assess the serum LPO. Human serum GSH-Px levels were also measured by ELISA using GSH-Px ELISA kits (ml065582, mlBio; Shanghai Enzyme-linked Biotechnology Co, Ltd) in line with the manufacturer’s protocol. Additionally, those raw standards provided by the kits were utilized to construct standard curves after proper dilution, and the biomarker concentrations within samples were calculated using standard curves. Each sample was tested in duplicate.
Laboratory Examinations
The Department of Clinical Laboratory of Shengjing Hospital of CMU was responsible for each blood test in SJH-MED/CMU Biobank and SJH-AAABB/CMU Biobank. Afterward, the relevant clinical information was extracted, the corresponding blood Biobank was established, and laboratory tests were conducted as previously described.28 In total, 76 qualified patients were eventually enrolled based on the patient inclusion criteria. The patient screening and inclusion procedures were summarized in Figure 1.
Lipid panel
The selective solubilization approach (Determiner L HDL or LDL-C test Kit, Kyowa Medex) was utilized to directly detect the plasma contents of high-density lipid cholesterol and low-density lipid cholesterol. Besides, enzymatic approaches were used to detect triglyceride and total cholesterol (TC) contents. The lipid profiles were produced using the automatic biochemistry analyzer (ARCHIRECT ci16200, Abbott Laboratories).
Other biochemical tests
The method proposed by the International Federation of Clinical Chemistry (Abbott Laboratories) was used to determine the levels of glutamic oxalacetic transaminase (aspartate aminotransferase [AST]) and alanine aminotransferase. Additionally, the biuret approach (FUJIFILM Wako Pure Chemical industries Ltd) was adopted to determine the total protein (TP) level in plasma. The glucose oxidase and urease glutamate dehydrogenase approaches (DiaSys Diagnostic Systems GmbH) were utilized to determine fasting plasma glucose level.
Statistical Analysis
The propensity score matching (PSM) method was adopted to decrease selection bias between cases and controls, and to reduce the potential clinical confounders using the 1:1 matching protocol in the current observational case–control study. Besides, the Hosmer-Lemeshow degree of fitting test was used to evaluate the model, to carry out logistic regression analysis and the C-statistic test. Before PSM, Student t test was adopted to compare clinical characteristics of patients with those in control group (for continuous variables), whereas χ2 test was adopted to compare categorical variables. After PSM was performed, nonparametric Mann-Whitney test was conducted to detect the differences between both groups. Thereafter, those categorical variables were shown as numbers and percentages, followed by χ2 test to determine the differences of these 2 groups (for clinical and biochemical factors). Linear regression and Spearman correlation were performed to assess possible correlations. Moreover, partial correlation analyses regarding smoking status, gender, and age were carried out to analyze the correlations across those continuous variables. Multiple logistic regression analysis was performed to assess the predictive significance of serum lipid peroxidation products in AAA or rAAA risk after adjusting for potential confounding factors. The relevant threshold values were determined using the logistic models as well as the ROC curves with corresponding area under curve (AUC) values. Youden index was used to determine the optimal cutoff for lipid peroxidation contents (sensitivity + specificity-1). SPSS version 25.0 software (SPSS Inc) was employed to all statistical analysis. A P value <.05 was considered to be statistically significant.
Results
Baseline Clinical Characteristics for PSM
The clinical characteristics for 76 patients with AAA and 725 control patients are shown in Table 1. Five clinical variables, including age, gender, body mass index, heart ratio, and hypertension, were statistically significant between both groups before PSM, which was used to reduce the selection bias and potential clinical confounders. Using PSM, those 76 matched pairs of AAA cases and control patients were compared through logistic regression analysis. Moreover, the Hosmer–Lemeshow degree of fit test revealed P = .72, C-statistic test indicated P = .85. Following PSM, the differences in clinical characteristics between the 2 groups were eliminated. Among the 76 cases in AAA group, 54 patients were diagnosed with small AAA (<55 mm), while the remaining 22 were identified as AAA (diameter more than 55 mm) based on guidelines of the ESVS.23 The clinical characteristics for patients with AAA are elaborated in Table 1.
Table 1.
Clinical Characteristics in Patients With AAA and Control Patients Before and After PSM.a
| Characteristics | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | AAA | P | Control | AAA | P | AAA diameter (30-54 mm) | AAA diameter ≥55mm | P | |
| N = 725 | N = 76 | N = 76 | N = 76 | n = 54 | n = 22 | ||||
| Age, years | 54.80 ± 13.34 | 63.59 ± 11.74 | <.001 | 63.64 ± 13.42 | 63.59 ± 11.74 | .859 | 61.13 ± 11.69 | 69.50 ± 9.76 | .004 |
| Men, n (%) | 371 (51.17%) | 65 (8.97%) | <.001 | 65 (85.53%) | 65 (85.53%) | 1.000 | 46 (85.19%) | 19 (86.36%) | 1.000 |
| BMI | 24.66 ± 5.27 | 23.31 ± 5.22 | .034 | 23.09 ± 5.18 | 23.31 ± 5.22 | .796 | 23.20 ± 4.62 | 23.59 ± 6.57 | .770 |
| HR, bmp | 78.63 ± 14.21 | 89.42 ± 12.67 | <.001 | 90.15 ± 12.39 | 89.42 ± 12.67 | .723 | 88.93 ± 10.91 | 90.64 ± 16.47 | .597 |
| Current smoker, n (%) | 144 (19.86%) | 18 (23.68%) | .453 | 18 (23.68%) | 18 (23.68%) | 1.000 | 11 (20.37%) | 7 (31.82%) | .373 |
| Hypertension, n (%) | 313 (43.17%) | 49 (64.47%) | <.001 | 47 (61.84%) | 49 (64.47%) | .867 | 32 (59.26%) | 17 (77.27%) | .188 |
| Type 2 diabetes mellitus, n (%) | 81 (11.17%) | 11 (14.47%) | .448 | 10 (13.16%) | 11 (14.47%) | .811 | 8 (14.81%) | 3 (13.64%) | 1.000 |
| Prior CHD, n (%) | 154 (21.24%) | 21 (27.63%) | .242 | 20 (26.32%) | 21 (27.63%) | 1.000 | 14 (25.93%) | 7 (31.82%) | .587 |
| Prior stroke, n (%) | 55 (7.59%) | 5 (6.58%) | 1.000 | 5 (6.58%) | 5 (6.58%) | 1.000 | 4 (7.41%) | 1 (4.55%) | 1.000 |
Abbreviations: AAA, abdominal aortic aneurysm; BMI, body mass index; CHD, coronary heart disease; HR, heart rate; LPO, lipid hydroperoxides; PSM, propensity score matching.
aValues are mean ± SD or n (%).
Serum Levels of Lipid Peroxidation Products Among AAA Cases
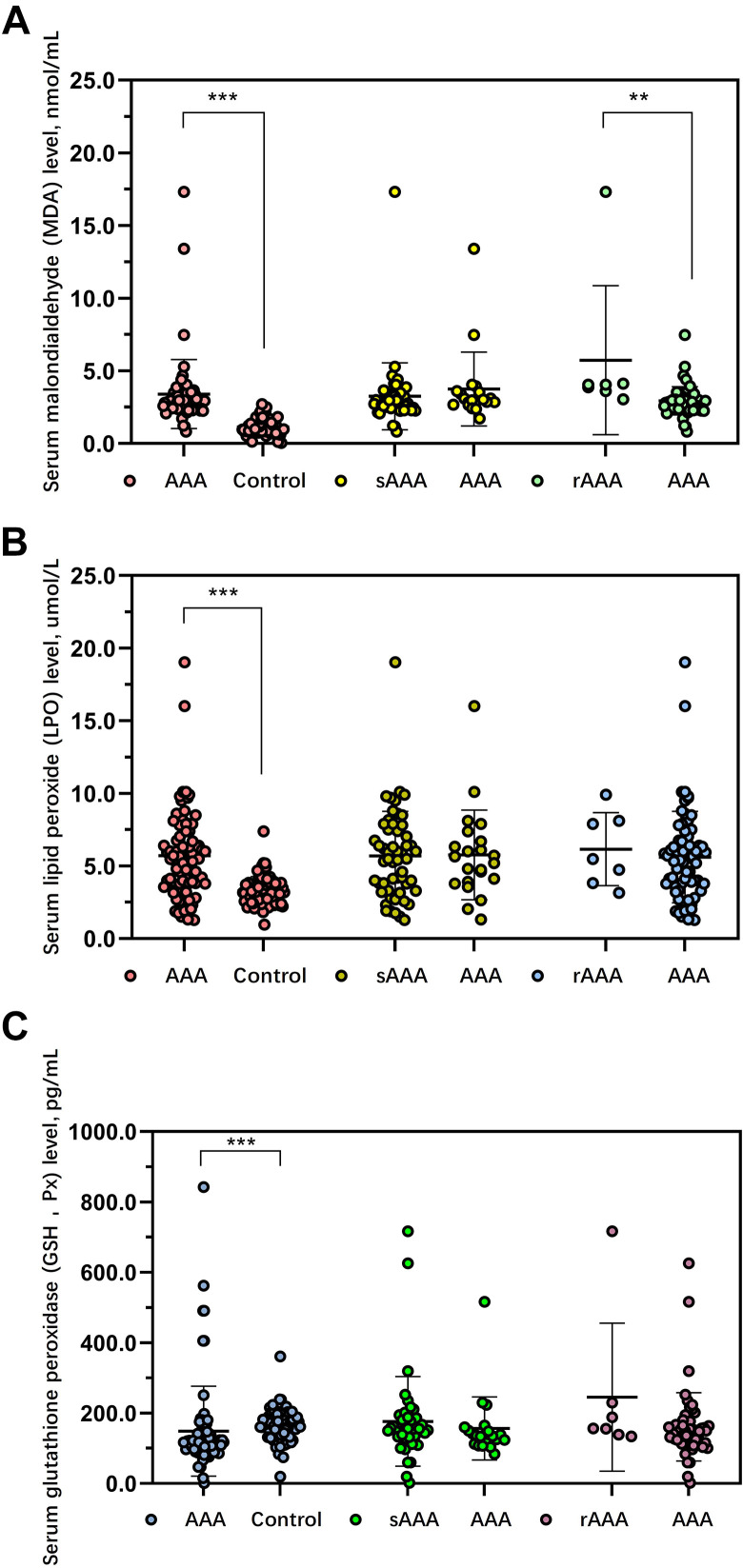
Serum MDA levels were significantly increased among AAA cases in comparison to those in control group (3.40 ± 2.37 vs 1.23 ± 0.69 nmol/mL, P < .001; Figure 2A). Serum MDA level was not significantly different between small AAA and AAA (3.12 ± 0.95 vs 3.75 ± 2.54 nmol/mL; P = .276). Interestingly, a significant difference was observed in the serum MDA level between non-rAAA and rupture patients with AAA (2.94 ± 0.97 vs 5.73 ± 5.12 nmol/mL, P = .001; Table 2).
Figure 2.
Analysis of lipid peroxidation levels in different groups. A comparison of malondialdehyde (A), lipid hydroperoxides (B), and glutathione peroxidase (C) levels among controls and AAA and subgroup analysis of lipid peroxidation levels between small AAA and patients with AAA, between ruptured AAA and selective AAA. *P < .05, **P < .01, ***P < .001, ns indicates no significance.
Table 2.
Comparisons of Blood Parameters Between Different Subgroups in AAA Patient Group.
| Serum lipid peroxidation products | AAA diameter 30-54 mm | AAA diameter >55 mm | P | Non-rupture | Rupture | P | |
|---|---|---|---|---|---|---|---|
| Subgroup | n = 54 | n = 22 | n = 7 | n = 69 | |||
| MDA, nmol/mL | 3.12 ± 0.95 | 3.75 ± 2.54 | .276 | 2.94 ± 0.97 | 5.73 ± 5.12 | .001a | |
| LPO, μmmol/L | 5.33 ± 3.35 | 5.76 ± 3.08 | .643 | 5.61 ± 3.15 | 6.16 ± 2.51 | .656 | |
| GSH-Px, pg/mL | 131.52 ± 87.67 | 153.88 ± 169 | .548 | 133.9 ± 90.59 | 193.89 ± 164.41 | .132 | |
| Basic statistics | |||||||
| Age, year | 63.89 ± 11.39 | 69.5 ± 9.76 | .072 | 63.94 ± 10.97 | 57.33 ± 18.38 | .188 | |
| BMI | 22.77 ± 4.21 | 23.59 ± 6.57 | .594 | 23.45 ± 5.33 | 21.06 ± 3.11 | .251 | |
| HR, bmp | 88.5 ± 10.3 | 90.64 ± 16.47 | .577 | 89.01 ± 12.26 | 93.57 ± 18.19 | .375 | |
| Routine blood indexes, leukocyte | |||||||
| WBC, ×109/L | 7.92 ± 3.07 | 6.33 ± 2.12 | .043b | 7.62 ± 3.07 | 8.14 ± 2.97 | .719 | |
| BA, ×109/L | 0.07 ± 0.14 | 0.08 ± 0.19 | .858 | 0.08 ± 0.16 | 0.09 ± 0.07 | .852 | |
| EO, ×109/L | 0.34 ± 0.47 | 0.2 ± 0.16 | .197 | 0.25 ± 0.33 | 0.33 ± 0.54 | .615 | |
| LY, ×109/L | 1.7 ± 0.68 | 1.8 ± 0.69 | .605 | 1.63 ± 0.66 | 1.7 ± 0.61 | .825 | |
| MO, ×109/L | 0.76 ± 0.99 | 0.8 ± 1.36 | .919 | 0.69 ± 0.89 | 1.65 ± 2.23 | .052 | |
| NE, ×109/L | 5.45 ± 3.05 | 3.79 ± 1.69 | .027b | 5.2 ± 3.1 | 5.76 ± 2.76 | .700 | |
| Routine blood indexes, erythrocyte | |||||||
| RBC, ×1012/L | 4.34 ± 0.78 | 4.33 ± 0.55 | .943 | 4.31 ± 0.7 | 4.19 ± 0.41 | .704 | |
| HGB, g/L | 132.11 ± 25.76 | 130.5 ± 20.64 | .813 | 131.55 ± 23.15 | 123 ± 15.43 | .424 | |
| HCT, L/L | 0.4 ± 0.07 | 0.4 ± 0.06 | .934 | 0.4 ± 0.07 | 0.38 ± 0.04 | .596 | |
| MCH, pg | 30.31 ± 2.24 | 30.1 ± 2.11 | .740 | 30.49 ± 2.03 | 29.36 ± 2.4 | .242 | |
| MCHC, g/L | 327.82 ± 12.98 | 325.32 ± 10.65 | .468 | 328.04 ± 11.42 | 319.6 ± 9.04 | .115 | |
| MCV, fL | 92.45 ± 5.67 | 92.48 ± 4.93 | .986 | 92.93 ± 5.13 | 91.88 ± 6.32 | .668 | |
| RDW, % | 11.69 ± 1.94 | 10.92 ± 1.3 | .213 | 11.28 ± 1.55 | 12.83 ± 2.26 | .078 | |
| Routine blood indexes, thrombocyte | |||||||
| PLT, ×109/L | 208.45 ± 87.16 | 225.38 ± 94.33 | .594 | 212.97 ± 89.05 | 197.25 ± 62.55 | .734 | |
| MPV, fL | 10.24 ± 1.00 | 9.83 ± 0.57 | .187 | 10.02 ± 0.78 | 10.85 ± 1.18 | .060 | |
| PCT, L/L | 0.22 ± 0.08 | 0.24 ± 0.10 | .558 | 0.22 ± 0.09 | 0.23 ± 0.06 | .927 | |
| PDW, 10GSD | 12.8 ± 1.09 | 13.3 ± 1.58 | .271 | 12.92 ± 1.28 | 12.98 ± 0.79 | .930 | |
| Liver function | |||||||
| ALT, U/L | 17.32 ± 7.50 | 18.08 ± 9.20 | .792 | 19.78 ± 12.91 | 15.5 ± 5.97 | .520 | |
| AST, U/L | 17.70 ± 3.16 | 19.15 ± 3.63 | .232 | 18.70 ± 3.80 | 14.75 ± 2.63 | .047b | |
| ALB, g/L | 38.36 ± 4.67 | 37.15 ± 5.85 | .504 | 37.78 ± 4.54 | 37.55 ± 8.28 | .929 | |
| GGT, U/L | 27.55 ± 19.58 | 26.62 ± 16.30 | .886 | 31.25 ± 23.51 | 26.00 ± 18.07 | .669 | |
| ALP, U/L | 74.32 ± 17.76 | 69.85 ± 20.16 | .498 | 75.17 ± 21.21 | 68.5 ± 8.43 | .541 | |
| BChE, 1000U/L | 7.35 ± 2.10 | 7.32 ± 2.02 | .968 | 7.21 ± 2.08 | 7.18 ± 2.99 | .980 | |
| PA, mg/dL | 21.92 ± 5.92 | 22.65 ± 7.14 | .765 | 21.79 ± 7.09 | 21.13 ± 5.62 | .878 | |
| TP, g/L | 64.63 ± 5.85 | 65.22 ± 6.71 | .793 | 64.37 ± 5.33 | 67.48 ± 7.11 | .291 | |
| TBIL, μmol/L | 11.99 ± 4.85 | 11.36 ± 5.13 | .719 | 11.71 ± 4.35 | 17.00 ± 8.70 | .045 | |
| DBIL, μmol/L | 6.16 ± 1.51 | 7.5 ± 0.57 | .298 | 7.23 ± 2.17 | 7.10 ± 0.01 | .958 | |
| Renal function and serum electrolyte | |||||||
| Crea, mmol/L | 83.6 ± 15.24 | 76.5 ± 16.26 | .606 | 90.29 ± 22.21 | 73.00 ± 21.21 | .361 | |
| Urea, mmol/L | 6.83 ± 2.47 | 8.16 ± 3.61 | .205 | 7.32 ± 2.93 | 5.80 ± 1.78 | .318 | |
| FPG, mmol/L | 5.58 ± 1.74 | 5.53 ± 0.74 | .920 | 5.77 ± 1.58 | 5.38 ± 0.34 | .623 | |
| CYSC, mg/L | 1.08 ± 0.29 | 1.54 ± 0.99 | .066 | 1.26 ± 0.65 | 0.95 ± 0.31 | .437 | |
| Ca, mmol/L | 2.21 ± 0.13 | 2.21 ± 0.16 | .907 | 2.20 ± 0.14 | 2.21 ± 0.08 | .883 | |
| K, mmol/L | 4.10 ± 0.45 | 3.86 ± 0.33 | .100 | 4.01 ± 0.44 | 3.74 ± 0.38 | .241 | |
| CL, mmol/L | 104.61 ± 3.36 | 105.48 ± 2.53 | .425 | 104.87 ± 3.1 | 104.05 ± 2.43 | .615 | |
| Na, mmol/L | 141.03 ± 3.79 | 140.94 ± 1.48 | .936 | 140.73 ± 3.18 | 141.6 ± 1.93 | .598 | |
| P, mmol/L | 1.12 ± 0.25 | 1.12 ± 0.19 | .997 | 1.1 ± 0.23 | 1.27 ± 0.10 | .147 | |
| HCO3, mmol/L | 24.69 ± 2.48 | 23.78 ± 2.00 | .268 | 23.95 ± 2.10 | 25.2 ± 4.36 | .319 | |
| Serum lipid profile | |||||||
| TC, mmol/L | 4.43 ± 0.87 | 4.86 ± 0.91 | .216 | 4.63 ± 0.90 | 4.19 ± 0.24 | .409 | |
| TG, mmol/L | 2.04 ± 2.38 | 1.48 ± 0.81 | .462 | 1.71 ± 1.88 | 1.86 ± 0.72 | .910 | |
| LDL-C, mmol/L | 2.80 ± 0.83 | 3.26 ± 0.76 | .140 | 2.97 ± 0.82 | 2.74 ± 0.37 | .639 | |
| HDL-C, mmol/L | 1.02 ± 0.24 | 1.16 ± 0.34 | .192 | 1.11 ± 0.34 | 1.02 ± 0.06 | .628 | |
| Cardiovascular injury-related parameters | |||||||
| CK, U/L | 74.38 ± 31.20 | 72.18 ± 35.70 | .867 | 71.54 ± 29.30 | 78.67 ± 49.94 | .709 | |
| LDH, U/L | 212.06 ± 46.01 | 210.73 ± 71.05 | .953 | 204.32 ± 39.3 | 268.00 ± 122.99 | .044b | |
| Blood coagulation function | |||||||
| PT, s | 13.55 ± 0.78 | 13.54 ± 1.52 | .970 | 13.47 ± 1.06 | 14.06 ± 1.32 | .249 | |
| APTT, s | 38.49 ± 5.35 | 38.53 ± 4.35 | .981 | 38.40 ± 5.16 | 40.60 ± 4.82 | .362 | |
| PTA, s | 91.68 ± 8.41 | 92.09 ± 12.83 | .892 | 92.38 ± 9.65 | 87.60 ± 12.22 | .303 | |
| INR | 1.06 ± 0.07 | 1.07 ± 0.14 | .732 | 1.06 ± 0.09 | 1.10 ± 0.12 | .395 | |
| D-Dimer, μg/mL | 2.10 ± 2.32 | 3.89 ± 3.85 | .105 | 3.26 ± 3.80 | 2.37 ± 2.71 | .696 |
Abbreviations: AAA, abdominal aortic aneurysm; ALT, alanine aminotransferase; ALB, albumin; ALP, alkaline phosphatase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BChE, cholinesterase; BA, basophil; BMI, body mass index; Ca, serum calcium; CK, creatine kinase; CL, serum chlorine; Crea, Creatinine; CYSC, Cystatin C; DBIL, direct bilirubin; EO, eosinophil; FPG, fasting plasma glucose; GSH-Px, glutathione peroxidase; GGT, gamma glutamyl transpeptidase; HCT, hematocrit; HDL-C, high-density lipoprotein-cholesterol; HGB, hemoglobin; HR, heart rate; INR, international normalized ratio; K, serum kalium; LDH, lactate dehydrogenase; LY, lymphocyte; LPO, lipid hydroperoxides; LDL-C, low-density lipoprotein-cholesterol; MO, monocyte; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MDA, malondialdehyde; MPV, mean platelet volume; Na, serum sodium; NE, neutrophil; RBC, red blood cells; RDW, red blood cell volume distribution width; P, serum phosphorus; PCT, platelet hematocrit; PDW, platelet distribution width; PLT, platelet; PA, pre-albumin; PT, prothrombin time; PTA, prothrombin activity; TP, total protein; TBIL, total bilirubin; Urea, Carbamide; TC, total cholesterol; TG, triglycerides; WBC, white blood cells.
a P < .01.
b P < .05.
Serum LPO levels were significantly elevated among AAA cases than those in control patients (5.71 ± 3.06 vs 3.29 ± 0.97 μmol/L, P < .001; Figure 2B). Serum MDA level was not significantly different between small AAA and AAA (5.33 ± 3.35 vs 5.76 ± 3.08 μmol/L; P = .643), or between non-rAAA and rupture patients with AAA (5.61 ± 3.15 vs 6.61 ± 2.51, P = .656; Table 2).
As expected, the level of GSH-Px was significantly decreased in patients with AAA than that in control group (148.61 ± 127.72 vs 162.42 ± 56.08 pg/mL, P < .001; Figure 2C). Serum GSH-Px level was not significantly different between small AAA and AAA (131.52 ± 87.67 vs 153.88 ± 169.99 pg/mL; P = .548), or between non-rAAA and rupture patients with AAA (133.90 ± 90.59 vs 193.89 ± 164.41, P = .132; Table 2).
The subgroup analyses for AAA cases are summarized in Table 2. A significant difference was observed in the white blood cell counts (7.92 ± 3.07 vs 6.33 ± 2.12 × 109/L, P = .043) and neutrophil counts (5.45 ± 3.05 vs 3.79 ± 1.69 × 109/L, P = .027) between small AAA and AAA (Table 2). Consequently, AST concentrations were significantly decreased and serum lactate dehydrogenase (LDH) levels were markedly increased among rAAA cases in comparison with those in nonrupture patients with AAA (Table 2).
Associations Between Serum Lipid Peroxidation Products and Clinical Characteristics
The possible associations between serum lipid peroxidation content and clinical characteristics were evaluated in patients with AAA. As a result, MDA level was positively correlated with TP (R = .381), LPO level was positively correlated with red cell volume distribution width (RDW, R = 0.353) and prothrombin time (PT, R = 0.323). Additionally, GSH-Px level was negatively associated with serum TC (R = −0.348) in patients with AAA (Table 3). The association of lipid peroxidation products with clinical features is shown in Table 3.
Table 3.
The Correlations Between Individual Serum Peroxidation Products and Clinical Features in Patients With AAA.
| Characterization | Indices | MDA (nmol/mL) | LPO (umol/L) | GSH-Px (pg/mL) |
|---|---|---|---|---|
| Basic statistics | ||||
| BMI | −0.040 | 0.037 | −0.009 | |
| HR, bmp | −0.132 | −0.014 | −0.051 | |
| Maximum diameter, mm | 0.238 | 0.050 | 0.137 | |
| Routine blood indexes, leukocyte | ||||
| WBC, ×109/L | 0.004 | −0.034 | 0.020 | |
| BA, ×109/L | −0.001 | −0.177 | −0.036 | |
| EO, ×109/L | 0.041 | −0.074 | −0.040 | |
| LY, ×109/L | 0.211 | −0.037 | 0.257a | |
| MO, ×109/L | 0.075 | −0.074 | 0.016 | |
| NE, ×109/L | −0.053 | −0.048 | −0.050 | |
| Routine blood indexes, erythrocyte | ||||
| RBC, ×1012/L | 0.118 | −0.244 | 0.127 | |
| HGB, g/L | 0.036 | −0.198 | 0.091 | |
| HCT, L/L | 0.082 | −0.251 | 0.115 | |
| MCH, pg | −0.168 | 0.061 | −0.067 | |
| MCHC, g/L | −0.169 | 0.045 | −0.068 | |
| MCV, fL | −0.108 | 0.037 | −0.038 | |
| RDW, % | −0.076 | 0.353a | −0.109 | |
| Routine blood indexes, thrombocyte | ||||
| PLT, ×109/L | 0.010 | 0.005 | 0.017 | |
| MPV, fL | −0.050 | −0.080 | −0.142 | |
| PCT, L/L | 0.029 | −0.185 | 0.027 | |
| PDW, 10GSD | −0.023 | −0.089 | −0.142 | |
| Liver function | ||||
| ALT, U/L | 0.027 | −0.153 | −0.023 | |
| AST, U/L | 0.280 | −0.091 | 0.252 | |
| ALB, g/L | 0.209 | −0.148 | 0.145 | |
| GGT, U/L | −0.182 | −0.135 | −0.127 | |
| ALP, U/L | −0.083 | −0.142 | −0.089 | |
| BChE, 1000U/L | 0.283 | -0.082 | 0.058 | |
| PA, mg/dL | −0.097 | −0.256 | −0.085 | |
| TP, g/L | 0.381a | −0.066 | 0.232 | |
| TBIL, μmol/L | 0.135 | −0.014 | 0.132 | |
| DBIL, μmol/L | 0.707 | −0.117 | 0.284 | |
| Renal function and serum electrolyte | ||||
| Crea, mmol/L | −0.027 | −.667a | 0.206 | |
| Urea, mmol/L | −0.148 | 0.035 | −0.086 | |
| FPG, mmol/L | 0.154 | −0.116 | 0.010 | |
| CYSC, mg/L | −0.230 | 0.077 | −0.205 | |
| Ca, mmol/L | 0.176 | −0.066 | 0.063 | |
| K, mmol/L | −0.021 | −0.021 | 0.038 | |
| CL, mmol/L | 0.110 | 0.109 | 0.049 | |
| Na, mmol/L | 0.132 | −0.019 | 0.034 | |
| P, mmol/L | −0.130 | 0.010 | −0.208 | |
| HCO3, mmol/L | 0.325 | −0.052 | 0.271 | |
| Serum lipid profile | ||||
| TC, mmol/L | −0.116 | 0.150 | −.348a | |
| TG, mmol/L | 0.177 | −0.159 | −0.203 | |
| LDL-C, mmol/L | −0.101 | 0.105 | −0.194 | |
| HDL-C, mmol/L | −0.257 | 0.203 | −0.150 | |
| Cardiovascular injury-related parameters | ||||
| CK, U/L | 0.063 | −0.198 | 0.121 | |
| LDH, U/L | 0.238 | −0.181 | 0.217 | |
| Blood coagulation function | ||||
| PT, s | −0.013 | 0.323a | −0.003 | |
| APTT, s | 0.137 | 0.230 | −0.019 | |
| PTA, s | −0.004 | −0.061 | −0.025 | |
| INR | −0.021 | 0.161 | −0.001 | |
| D-Dimer, μg/mL | −0.155 | 0.089 | −0.131 |
Abbreviations: AAA, abdominal aortic aneurysm; ALT, alanine aminotransferase; ALB, albumin; ALP, alkaline phosphatase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BChE, cholinesterase; BA, basophil; BMI, body mass index; Ca, serum calcium; CK, creatine kinase; CL, serum chlorine; Crea, Creatinine; CYSC, Cystatin C; DBIL, direct bilirubin; EO, eosinophil; FPG, fasting plasma glucose; GSH-Px, glutathione peroxidase; GGT, gamma glutamyl transpeptidase; HCT, hematocrit; HDL-C, high-density lipoprotein-cholesterol; HGB, hemoglobin; HR, heart rate; INR, international normalized ratio; K, serum kalium; LDH, lactate dehydrogenase; LY, lymphocyte; LPO, lipid hydroperoxides; LDL-C, low-density lipoprotein-cholesterol; MO, monocyte; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MDA, malondialdehyde; MPV, mean platelet volume; Na, serum sodium; NE, neutrophil; RBC, red blood cells; RDW, red blood cell volume distribution width; P, serum phosphorus; PCT, platelet hematocrit; PDW, platelet distribution width; PLT, platelet; PA, pre-albumin; PT, prothrombin time; PTA, prothrombin activity; TP, total protein; TBIL, total bilirubin; Urea, Carbamide; TC, total cholesterol; TG, triglycerides; WBC, white blood cells.
a P < .05.
Correlations between individual lipid peroxidation products were evaluated by Spearman rank correlation coefficient (as shown in Table 4). The results demonstrated that LPO level was significantly and positively related to MDA (R = 0.358, P < .001), while negatively correlated with GSH-Px level (R = −0.203, P = .032).
Table 4.
The Correlations Between Corresponding Serum Peroxidation Products Within AAA Patients’ Group.
| MDA (nmol/mL) | LPO (ummol/L) | GSH-Px (pg/mL) | ||
|---|---|---|---|---|
| MDA (nmol/mL) | Coefficient | - | 0.358a | −0.021 |
| P value | - | <.001 | .835 | |
| N | - | 76 | 76 | |
| LPO (ummol/L) | Coefficient | - | −0.203 | |
| P value | - | .032 | ||
| N | - | 76 | ||
| GSH-Px (pg/mL) | Coefficient | - | ||
| P value | - | |||
| N | - | |||
Abbreviations: AAA, abdominal aortic aneurysm; GSH-Px, glutathione peroxidase; LPO, lipid hydroperoxides; MDA, malondialdehyde.
aP<0.05
Predictive and Diagnostic Significance of Serum Lipid Peroxidation Content for AAA
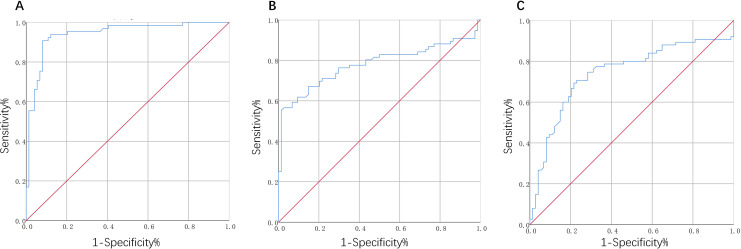
Receiver operating characteristic analysis was used to determine the threshold lipid peroxidation content, in order to evaluate AAA (as shown in Table 5). The AUC for AAA was 0.965, and the optimal threshold was 2.242 nmol/mL, while the sensitivity was 90.8% and the specificity was 91.9% for MDA level (Figure 3A). Furthermore, the values of AUC were 0.780 and 0.741 for LPO and GSH-Px, respectively, and the optimal thresholds were 5.185 μmol/L and 129.38 pg/mL, respectively. The sensitivity value in diagnosis was 56.6% for LPO (Figure 3B) and 69.3% for GSH-Px (Figure 3C ), respectively, and the values of specificity were 97.3% and 78.4%, respectively.
Table 5.
Diagnostic Value of Individual Lipid Peroxidation Products for Patients With AAA.
| AUC | 95% CI | P value | Cutoff value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|
| MDA (nmol/mL) | 0.965 | 0.934-0.995 | <.001 | 2.242 nmol/mL | 90.8 | 91.9 |
| LPO (μmmol/L) | 0.780 | 0.701-0.859 | <.001 | 5.185 umol/L | 56.6 | 97.3 |
| GSH-Px (pg/mL) | 0.741 | 0.658-0.825 | <.001 | 129.38 pg/mL | 69.3 | 78.4 |
Abbreviations: AUC, area under curve; GSH-Px, glutathione peroxidase; LPO, lipid hydroperoxides; MDA, malondialdehyde.
Figure 3.
Receiver operating characteristic curve for serum malondialdehyde (A), lipid hydroperoxides (B), and glutathione peroxidase (C) levels to predict AAA.
As shown in Table 6, multiple logistic regression analysis was further carried out to assess the prediction value of serum lipid peroxidation content as a risk factor for AAA under different adjustment models. After adjusting all possible confounding factors, serum MDA level remained significant correlation with AAA risk (odds ratio [OR] = 13.706 per unit increase, 95% CI = 5.888-31.909, P < .01) and serum LPO was significantly associated with AAA risk (OR: 1.891 per unit increase, 95% CI = 1.451-2.464, P < .001).
Table 6.
Multiple Logistic Regression Analysis of MDA, LOP, and GSH-Px Levels for AAA Risk.a
| Adjusted variable | MDA (nmol/mL) | LPO (ummol/L) | GSH-Px (pg/mL) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Model 1 | 13.308 (6.056-29.241) | <.001 | 2.022 (1.551-2.636) | <.001 | 0.998 (0.995-1.002) | .388 |
| Model 2 | 12.870 (5.734-28.885) | <.001 | 1.895 (1.456-2.467) | <.001 | 0.998 (0.994-1.002) | .246 |
| Model 3 | 13.706 (5.888-31.909) | <.001 | 1.891 (1.451-2.464) | <.001 | 0.998 (0.994-1.002) | .236 |
Abbreviations: GSH-Px, glutathione peroxidase; LPO, lipid hydroperoxides; MDA, malondialdehyde; OR, odds ratio.
aModel 1: age and gender were adjusted; Model 2: Model 1 plus heart rate, BMI and smoking status; Model 3: Model 2 plus hypertension, type 2 diabetes, prior coronary heart disease and prior stroke status.
Univariate and Multivariate Logistic Regression Analysis for AAA Rupture
The following 4 variables had been previously found to be correlated with AAA rupture, MDA level, AST concentration, TBIL, and serum LDH concentration (P < .05). Additional 3 variables, including monocyte counts, red cell volume distribution width, and mean platelet volume, were indicated to be potentially associated with rAAA (all P values were approximately .05 in univariate analysis; Table 2). As a result, the above 7 variables, together with the maximum diameter of AAA, hypertension, smoker, and pulsating sensations in the abdomen, were incorporated into the multivariate logistic regression analyses. The results suggested that, only serum MDA level (OR = 2.536; 95% CI: 1.037-6.203; P = .041) was significantly correlated with AAA rupture (Table 7) after adjusting for confounding factors. Nevertheless, rAAA showed no correlation with any variable included in the current study.
Table 7.
Univariate and Multivariate Logistic Regression Analyses for AAA Rupture.a
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| MDA, nmol/mL | 2.010 (0.951-4.251) | .068 | 2.536 (1.037-6.203) | .041b |
| Maximum diameter, mm | 1.010 (0.960-1.062) | .707 | 1.030 (0.962-1.103) | .392 |
| Hypertension, % | 3.571 (0.406-31.409) | .251 | 2.949 (0.253-34.407) | .388 |
| Current smoker, % | 0.490 (0.055-4.368) | .523 | 1.069 (0.091-12.605) | .958 |
| Pulsating sensations in the abdomen, % | 1.400 (0.177-11.083) | .750 | 1.724 (0.109-27.366) | .700 |
| AST, U/L | 0.703 (0.481-1.029) | .070 | 0.120 (0.009-1.635) | .112 |
| TBIL, μmol/L | 1.184 (0.983-1.427) | .075 | 1.153 (0.882-1.507) | .299 |
| LDH, U/L | 1.015 (0.998-1.033) | .093 | 1.331 (0.001-47.916) | .996 |
| MO, ×109/L | 1.533 (0.910-2.584) | .109 | 1.452 (0.739-2.856) | .279 |
| RDW, % | 1.039 (0.459-2.351) | .927 | 2.582 (0.449-14.839) | .288 |
| MPV, fL | 3.139 (0.876-11.24) | .079 | 1.651 (0.405-6.727) | .484 |
Abbreviations: AAA, abdominal aortic aneurysm; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; LDH, lactate dehydrogenase; MDA, malondialdehyde; MO, monocyte; MPV, mean platelet volume; OR, odds ratio; RDW, red blood cell volume distribution width; TBIL, total bilirubin.
aModel 1 no adjustments, Model 2 adjusted for age, gender, BMI
b P < .05.
Discussion
Oxidative stress is widely acknowledged to play a major role in the development of aneurysmal diseases.29,30 Oxidative burden has been reported to potentially worsen the cellular degeneration. Certain molecules, such as MDA, are well investigated to be able to enhance oxidative stress through lipid peroxidation.31 The indirect detection of oxygen free radicals is possible by measuring their oxidative attack on lipids and proteins, which lead to products such as aldehydes, hydroperoxides, and conjugated dienes. Our present findings suggested higher MDA and LPO levels in either AAA or rAAA group than those in the control group. Inversely, GSH-Px level was significantly decreased in patients with AAA than that in the control group. Intriguingly, patients with rAAA were also more likely to have higher MDA levels than patients with AAA overall, possibly depending on their different wall mechanics and arterial hemodynamics. This outcome supports the contributive role of enhanced lipid oxidative stress to the pathogenesis of AAA.19
Subgroup comparisons further revealed that patients with rAAA had higher serum MDA level compared with controls in univariate analysis. In addition, serum MDA level was found to be significantly related to AAA rupture after adjusting for confounding factors. Malondialdehyde, one of the most common and harmful products of lipid peroxidation, would cause cell damaging, react with the free amino groups of proteins and nucleic acids, with the target mutagenic activity at guanine site in DNA sequence.32,33 Thus, the intensity of oxidative stress or lipid peroxidation-triggered damage could be assessed by determination of MDA. This study is similar with a previous study concentrating on determining oxidative stress based on aortic media tissue. Billaud et al determined the levels of superoxide anion and lipid peroxidation marker MDA, and activity of peroxidase and SOD in aortic media tissue, revealing that elevated hemodynamic stress caused by bicuspid aortic valve could result in increased oxidative stress parameters.34 Their findings indicate that the characteristic absence of SMCs in bicuspid aortic valve aortopathy might be a result of superoxide-mediated cell damage. However, there is barely any information about the biochemical alterations in blood or their correlation with clinical stage of the AAA disease.
In the present study, we also found that serum LPO level was significantly elevated among AAA cases than those in control patients. The levels of F2-isoprostane, specific markers of LPO, revealed that rAAAs populations were burdened with 2 phases of oxidative injury, including before arrival at hospital and postoperation.35 Moreover, the significant correlation of postoperatively increased level of F2-isoprostane with neutrophil oxidant production indicates the occurrence of neutrophils in the oxidative injury after rAAA. Therefore, novel therapeutic interventions by decreasing neutrophil-mediated oxidant injury during reperfusion might relieve organ failure, thereby ultimately decreasing mortality in rAAAs patients.35 However, there are still certain limitations in the predictive models in consideration of the incomplete understanding of precise molecular mechanisms underlying AAA rupture. Novel understanding of the pathomechanism of aneurysm rupture would definitely assist decision-making in clinical management and further enhance the development of new noninvasive therapeutic strategies.
Intracellular decreased GSH is converted into oxidized glutathione by GSH-Px, which catalyzes the reduction of peroxides.36 GSH-Px eliminates H2O2 by using glutathione as a hydrogen donor and converts organic hydroperoxides to the corresponding alcohol.37,38 Nevertheless, there are only limited studies on glutathione system and GSH-Px levels of aneurysm, and no investigations concerning the specific region of the above systems for rAAA group and small AAA.
Nevertheless, certain limitations should be noted in the current research. To begin with, in this single-centered study, the sample size was relatively small. Therefore, multicenter cohort study with a large sample size should be conducted by enrolling a large number of patients with AAA, to investigate the relationships of serum lipid peroxidation content with AAA. Secondly, the lipid peroxidation contents were only measured in some transverse time periods. However, lipid peroxidation at different stage and in-hospital or postoperative period during the identical AAA pathological process remains unclear. Therefore, future study should be performed to dynamically detect MDA, LPO, and GSH-Px levels at various AAA stages, which would contribute to more accurate judgment of the lipid peroxidation contents/changes among patients with AAA. Additionally, few women were included in our study, typical of studies in patients with AAA. This is particularly important because being female is an important predictor of AAA rupture,39 and a third of all deaths caused by AAA rupture are in women.40 Additionally, histological specimens and lipid peroxidation biomarker tissue expression were not examined in this study.
Conclusion
Collectively, in the present study, we demonstrated a positive correlation between serum MDA, LPO, and AAA. Serum MDA and LPO concentrations could be used to predict disease severity in patients with AAA. Moreover, serum MDA may serve as the candidate biomarker for diagnosis of AAA and accurate identification of increased risks of AAA rupture. These findings indicated that lipid peroxidation might be critically involved in the initiation and progression of AAA, while the prognostic significance of lipid peroxidation requires further investigation. In addition, all these speculations can be important issues topics in lipid peroxidation biology and AAA pathobiology, which deserve further investigation.
Footnotes
Authors’ Note: The data used in this study are accessible from the corresponding author upon reasonable request. Feng Shi and Yanshuo Han conducted the experiment. Feng Shi and Chao Ji performed the analysis and drafted the manuscript. Changcheng Ma collected the laboratory data. Feng Shi collected the clinical data. Feng Shi and Yanshuo Han designed the study and revised the manuscript. All authors read and approved the final manuscript. The study protocol was approved by the Ethics Committee of Shengjing Hospital of China Medical University (CMU) according to the Declaration of Helsinki.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The current study was funded by the Fundamental Research Funds for the Central Universities (grant: DUT19RC(3)076), the National Natural Science Foundation of China (grant number: 81600370), and China Postdoctoral Science Foundation (grant number: 2018M640270).
ORCID iD: Yanshuo Han  https://orcid.org/0000-0002-4897-2998
https://orcid.org/0000-0002-4897-2998
References
- 1. Force USPST, Owens DK, Davidson KW, et al. Screening for abdominal aortic aneurysm: US preventive services task force recommendation statement. JAMA. 2019;322(22):2211–2218. [DOI] [PubMed] [Google Scholar]
- 2. Ali MU, Fitzpatrick-Lewis D, Miller J, et al. Screening for abdominal aortic aneurysm in asymptomatic adults. J Vasc Surg. 2016;64(6):1855–1868. [DOI] [PubMed] [Google Scholar]
- 3. Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ. 2005;330(7494):750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howard DP, Banerjee A, Fairhead JF, et al. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. Br J Surg. 2015;102(8):907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK small aneurysm trial participants. Ann Surg. 1999;230(3):289–296; discussion 296-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schermerhorn ML, Bensley RP, Giles KA, et al. Changes in abdominal aortic aneurysm rupture and short-term mortality, 1995-2008: a retrospective observational study. Ann Surg. 2012;256(4):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis FM, Rateri DL, Daugherty A. Abdominal aortic aneurysm: novel mechanisms and therapies. Curr Opin Cardiol. 2015;30(6):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg AX, Devereaux PJ, Hill A, et al. Oral curcumin in elective abdominal aortic aneurysm repair: a multicentre randomized controlled trial. CMAJ. 2018;190(43):E1273–E1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurvers H, Veith FJ, Lipsitz EC, et al. Discontinuous, staccato growth of abdominal aortic aneurysms. J Am Coll Surg. 2004;199(5):709–715. [DOI] [PubMed] [Google Scholar]
- 10. Ijaz T, Tilton RG, Brasier AR. Cytokine amplification and macrophage effector functions in aortic inflammation and abdominal aortic aneurysm formation. J Thorac Dis. 2016;8(8):E746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forsythe RO, Newby DE, Robson JM. Monitoring the biological activity of abdominal aortic aneurysms beyond ultrasound. Heart. 2016;102(11):817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molacek J, Treska V, Zeithaml J, et al. Blood biomarker panel recommended for personalized prediction, prognosis, and prevention of complications associated with abdominal aortic aneurysm. EPMA J. 2019;10(2):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yokoyama U, Arakawa N, Ishiwata R, et al. Proteomic analysis of aortic smooth muscle cell secretions reveals an association of myosin heavy chain 11 with abdominal aortic aneurysm. Am J Physiol Heart Circ Physiol. 2018;315(4):H1012–H1018. [DOI] [PubMed] [Google Scholar]
- 14. Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008;118(23):2382–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moris DN, Georgopoulos SE. Circulating biomarkers for abdominal aortic aneurysm: what did we learn in the last decade? Int Angiol. 2013;32(3):266–280. [PubMed] [Google Scholar]
- 16. The MA3RS Investigators. Aortic wall inflammation predicts abdominal aortic aneurysm expansion, rupture, and need for surgical repair. Circulation. 2017;136(9):787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H, Bai S, Ao Q, et al. Modulation of immune-inflammatory responses in abdominal aortic aneurysm: emerging molecular targets. J Immunol Res. 2018;2018:7213760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papalambros E, Sigala F, Georgopoulos S, et al. Malondialdehyde as an indicator of oxidative stress during abdominal aortic aneurysm repair. Angiology. 2007;58(4):477–482. [DOI] [PubMed] [Google Scholar]
- 19. Miller FJ, Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol. 2002;22(4):560–565. [DOI] [PubMed] [Google Scholar]
- 20. Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. [DOI] [PubMed] [Google Scholar]
- 21. Bulkley GB. Free radicals and other reactive oxygen metabolites: clinical relevance and the therapeutic efficacy of antioxidant therapy. Surgery. 1993;113(5):479–483. [PubMed] [Google Scholar]
- 22. Bast A, Haenen GR, Doelman CJ. Oxidants and antioxidants: state of the art. Am J Med. 1991;91(3C):2S–13S. [DOI] [PubMed] [Google Scholar]
- 23. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41(suppl 1): S1–S58. [DOI] [PubMed] [Google Scholar]
- 24. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577–1589. [DOI] [PubMed] [Google Scholar]
- 25. Mussa FF. Screening for abdominal aortic aneurysm. J Vasc Surg. 2015;62(3):774–778. [DOI] [PubMed] [Google Scholar]
- 26. Soares Ferreira R, Gomes Oliveira N, Oliveira-Pinto J, et al. Review on management and outcomes of ruptured abdominal aortic aneurysm in women. J Cardiovasc Surg (Torino). 2018;59(2):195–200. [DOI] [PubMed] [Google Scholar]
- 27. Schmitz-Rixen T, Keese M, Hakimi M, et al. Ruptured abdominal aortic aneurysm-epidemiology, predisposing factors, and biology. Langenbecks Arch Surg. 2016;401(3):275–288. [DOI] [PubMed] [Google Scholar]
- 28. Han Y, Ma Y, Liu Y, et al. Plasma cholinesterase is associated with Chinese adolescent overweight or obesity and metabolic syndrome prediction. Diabetes, Metab Syndr Obes. 2019;12:685–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Memon AA, Zarrouk M, Agren-Witteschus S, Sundquist J, Gottsater A, Sundquist K. Identification of novel diagnostic and prognostic biomarkers for abdominal aortic aneurysm. Eur J Prev Cardiol. 2020;27(2):132–142. [DOI] [PubMed] [Google Scholar]
- 30. Meital LT, Windsor MT, Perissiou M, et al. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci Rep. 2019;9(1):12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43(5):550–557. [DOI] [PubMed] [Google Scholar]
- 32. Salzman R, Pacal L, Tomandl J, et al. Elevated malondialdehyde correlates with the extent of primary tumor and predicts poor prognosis of oropharyngeal cancer. Anticancer Res. 2009;29(10):4227–4231. [PubMed] [Google Scholar]
- 33. Singh M, Kapoor A, Bhatnagar A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chem Biol Interact. 2015;234:261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Billaud M, Phillippi JA, Kotlarczyk MP, et al. Elevated oxidative stress in the aortic media of patients with bicuspid aortic valve. J Thorac Cardiovasc Surg. 2017;154(5):1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindsay TF, Luo XP, Lehotay DC, et al. Ruptured abdominal aortic aneurysm, a “two-hit” ischemia/reperfusion injury: evidence from an analysis of oxidative products. J Vasc Surg. 1999;30(2):219–228. [DOI] [PubMed] [Google Scholar]
- 36. Comporti M. Glutathione depleting agents and lipid peroxidation. Chem Phys Lipids. 1987;45(2-4):143–169. [DOI] [PubMed] [Google Scholar]
- 37. Chiou TJ, Chu ST, Tzeng WF. Protection of cells from menadione-induced apoptosis by inhibition of lipid peroxidation. Toxicology. 2003;191(2-3):77–88. [DOI] [PubMed] [Google Scholar]
- 38. Ristoff E, Larsson A. Oxidative stress in inborn errors of metabolism: lessons from glutathione deficiency. J Inherit Metab Dis. 2002;25(3):223–226. [DOI] [PubMed] [Google Scholar]
- 39. Sweeting MJ, Thompson SG, Brown LC, Powell JT, collaborators R. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99(5):655–665. [DOI] [PubMed] [Google Scholar]
- 40. Nelissen BG, Herwaarden JA, Pasterkamp G, Moll FL, Vaartjes I. Shifting abdominal aortic aneurysm mortality trends in The Netherlands. J Vasc Surg. 2015;61(3):642–647. e642. [DOI] [PubMed] [Google Scholar]